Abstract
Meningitis and meningoencephalitis caused by Escherichia coli are associated with high rates of mortality. When an infection occurs, Toll-like receptors (TLRs) expressed by microglial cells can recognize pathogen-associated molecular patterns and activate multiple steps in the inflammatory response that coordinate the brain's local defense, such as phagocytosis of invading pathogens. An upregulation of the phagocytic ability of reactive microglia could improve the host defense in immunocompromised patients against pathogens such as E. coli. Here, murine microglial cultures were stimulated with the TLR agonists Pam3CSK4 (TLR1/TLR2), lipopolysaccharide (TLR4), and CpG oligodeoxynucleotide (TLR9) for 24 h. Upon stimulation, levels of tumor necrosis factor alpha and the neutrophil chemoattractant CXCL1 were increased, indicating microglial activation. Phagocytic activity was studied after adding either E. coli DH5α or E. coli K1 strains. After 60 and 90 min of bacterial exposure, the number of ingested bacteria was significantly higher in cells prestimulated with TLR agonists than in unstimulated controls (P < 0.01). Addition of cytochalasin D, an inhibitor of actin polymerization, blocked >90% of phagocytosis. We also analyzed the ability of microglia to kill the ingested E. coli strains. Intracellularly surviving bacteria were quantified at different time points (90, 150, 240, and 360 min) after 90 min of phagocytosis. The number of bacteria killed intracellularly after 6 h was higher in cells primed with the different TLR agonists than in unstimulated microglia. Our data suggest that microglial stimulation by the TLR system can increase bacterial phagocytosis and killing. This approach could improve central nervous system resistance to infections in immunocompromised patients.
Mammals have two main forms of inducible immune defense systems against infectious agents that are sequentially activated: the innate and the adaptive immunity. The brain, previously considered as an immuno-privileged site, shows a well-organized innate immune reaction in response to bacteria in blood and cerebrospinal fluid (1, 15, 32). Microglial cells, the resident phagocytes of the central nervous system (CNS), survey their microenvironment and notice any pathological event disturbing the brain homeostasis (10). Microglia express Toll-like receptors (TLRs) that can recognize pathogen-associated molecular patterns (PAMPs) (27, 30). The coding sequences, function, and signaling pathways of the vertebrate TLRs are highly conserved (22). Up to now, 12 different TLRs have been identified in mice (10 in humans), each one mediating the immune response to different PAMPs. Lipopeptides from gram-positive bacteria are recognized by TLR2 as a heterodimer with TLR1 or TLR6 (30). Lipopolysaccharide (LPS) from gram-negative bacteria activates TLR4 (21), and bacterial DNA containing CpG motifs triggers signaling through TLR9 (13). The receptor-ligand interaction activates microglia to undergo morphological transformation as well as functional changes, such as production of proinflammatory cytokines, chemokines and reactive oxygen species, phagocytic activity, and antigen presentation (10, 12, 28). In addition, reactive microglia are also involved in clearance of toxic cellular debris and promotion of tissue repair (10). While the release of proinflammatory compounds by TLR-mediated signaling has been widely documented, the role of TLRs in the phagocytosis and destruction of invading pathogens remains unclear. Here, we hypothesized that enhanced TLR signaling could ameliorate bacterial phagocytosis by increasing protection against some infections especially in immunocompromised patients. Escherichia coli strains are frequent gram-negative bacilli causing urinary tract, intra-abdominal, and soft-tissue infections, pneumonia, sepsis, and meningitis (24). Bacteremia and meningitis caused by E. coli are still associated with high rates of long-term sequelae and mortality in infants and immunocompromised and elderly persons as well as substantial healthcare costs despite advances in antimicrobial therapy (23). The presence of the antiphagocytic capsule K1 confers invasiveness to the strains (26). Recent studies have shown that the activation of microglia occurs in both cerebral and systemic infections, probably as a mechanism to increase the resistance of the brain against infections (15, 32). Therefore, the aims of this study were (i) to investigate whether PAMPs may stimulate microglia, thereby increasing their ability to phagocytose nonencapsulated and K1-encapsulated E. coli strains, and (ii) to compare the intracellular bacterial killing in unstimulated and prestimulated microglial cells.
MATERIALS AND METHODS
Primary mouse microglial cell cultures.
Primary cultures of microglial cells were prepared from brains of newborn C57BL/6 mice (1 to 3 days). After the removal of the meninges, cells were mechanically dissociated and suspended in Dulbecco's modified Eagle medium (DMEM) with Glutamax I supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco, Karlsruhe, Germany). Cells were plated at a density of two brains per T75 culture flask (Corning Costar, Wiesbaden, Germany) and maintained at 37°C in a humidified atmosphere with 5% CO2. Culture medium was changed twice a week. After 10 to 14 days of culture, microglial cells were isolated from the mixed glial (astrocytes and microglia) cultures by shaking 200 times/min for 30 min, and cells in the supernatant were replated at a density of 60,000 cells/well in both 96-well plates (for phagocytosis assay) and in 24-well plates (for intracellular survival assay). Additionally, some microglia were plated on poly-l-lysine-coated coverslips in 12-well plates at the same density for subsequent staining and confocal microscopy. To test for the purity of the cultures, Griffonia simplicifolia isolectin B4 staining was performed. For this purpose, microglial cells were plated on poly-l-lysine-coated coverslips, fixed in 4% formaldehyde, treated with Triton X-100 (0.1% in phosphate-buffered saline [PBS]) for 30 min, and then incubated with biotinylated isolectin B4 (5 μg/ml, diluted in PBS, containing 1% [wt/vol] bovine serum albumin; Sigma, Taufkirchen, Germany) for 90 min. Thereafter, cells were treated with avidin-biotin complex (Vector, Burlingame, CA) for 30 min, and diaminobenzidine was used for visualization, resulting in a brown staining of the somata of microglial cells. The staining showed that the cultures contained >98% microglia.
Microglial stimulation with TLR agonists.
Microglial cells plated in 24-well and 96-well plates were cultured in medium containing 10% FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin for 20 h. Then, microglial cultures were exposed to one of the TLR agonists for 24 h. Tripalmitoyl-S-glyceryl-cysteine (Pam3CSK4; 910.5 Da; EMC Microcollections, Tübingen, Germany) was used as a specific agonist of TLR1/TLR2 (TLR1/2). For activation of TLR4, microglial cells were exposed to LPS from E. coli serotype 026:B6 (Sigma, Taufkirchen, Germany). CpG oligodesoxynucleotide 1668 (TCC ATG ACG TTC CTG ATG CT; 6,383 Da; TIB Molbiol, Berlin, Germany) was used as a specific ligand of TLR9. A control group with unstimulated microglial cells was included in all experiments.
Our group had previously shown that Pam3CSK4, LPS, and CpG induce NO release by microglial cells in a dose-dependent manner. All dose-response curves reached a plateau of maximum NO release (7). In the current work, TLR agonists were used at the lowest concentrations inducing maximum stimulation of microglia in terms of NO release. Pam3CSK4 was tested at 0.1 μg/ml (110 nM), LPS at 0.01 μg/ml (1 nM), and CpG at 1 μg/ml (157 nM).
Supernatants from stimulated microglial cultures and unstimulated controls were collected after 24 h of incubation and stored at −80°C until measurement of cytokine and chemokine levels. Microglial cells were used in bacterial phagocytosis assays or intracellular survival assays or stained and subsequently examined by confocal microscopy.
Cytokine and chemokine release.
Tumor necrosis factor alpha (TNF-α) and CXCL1 (KC, the mouse equivalent of GROα) levels were determined using DuoSet ELISA Development Kits (R&D Systems, Wiesbaden, Germany). Procedures were performed according to the manufacturer's instructions. The color reaction was measured at 450 nm in a microplate reader (Bio-Rad, Munich, Germany). Total protein content was determined using a MicroBCA protein assay (Pierce, Rockford, IL).
Bacterial strains.
E. coli DH5α, a strain apathogenic for immunocompetent animals, was transformed with plasmids encoding the red fluorescent protein DSRed (29). The plasmids were gifts from D. Bumann (Max Planck Institut für Infektionsbiologie, Berlin, Germany).
An E. coli strain with the antiphagocytic capsule K1, isolated from a child with neonatal meningitis (gift of G. Zysk, Institute of Medical Microbiology, Düsseldorf, Germany) was also tested.
E. coli strains were suspended in a medium consisting of DMEM supplemented with 10% FCS. In order to preserve the plasmid encoding E. coli DH5α fluorescence emission, 100 μg/ml ampicillin (Sigma-Aldrich, St. Louis, MO) was added to the respective growth medium. The bacterial inoculum concentration was determined as the number of CFU for each assay by quantitative plating on sheep blood agar plates.
Phagocytosis assay.
Phagocytosis assays were performed as previously described (19, 25). Microglial cells were exposed to bacteria for different times to study ingestion of bacteria. Then, the supernatants were removed and gentamicin (Sigma-Aldrich, St. Louis, MO), an antibiotic that does not penetrate eukaryotic cells, was added to kill extracellular bacteria. Subsequently, cell monolayers were lysed, and the number of intracellular bacteria was counted.
After 24 h of stimulation with a TLR agonist, microglial cells were washed twice with PBS and then infected with either E. coli DH5α or E. coli K1 by adding 0.25 ml of bacterial suspension in each well. In a first set of experiments, different amounts of bacteria (106, 107, and 108 CFU/ml) were used. A multiplicity of infection of about 100 bacteria per microglial cell (final concentration, approximately 6 × 106 CFU/ml) was found to be optimal and was used for all following experiments. Phagocytosis was left to proceed for 30, 60, or 90 min at 37°C and 5% CO2. In phagocytosis inhibition studies, cytochalasin D (Sigma-Aldrich, St. Louis, MO), an inhibitor of actin polymerization, was added to the cell monolayers (final concentration, 10 μM) (see below) prior to the addition of bacteria and was present throughout the experiment (19). After 4 h of exposure, cytochalasin D at 10 μM had no cytotoxic effects on microglial cells (data not shown). After bacterial exposure, cell monolayers were washed twice with warm PBS and incubated for 1 h in culture medium containing gentamicin (final concentration, 200 μg/ml). After antibiotic incubation, microglial cells were washed twice with warm PBS and lysed with distilled water. The intracellular bacteria were enumerated by quantitative plating of the lysates on sheep blood agar plates. Each test was done four times in independent experiments.
During the phagocytosis assay, we verified extracellular E. coli replication by quantitative plating. We determined the bacterial concentration in the inoculum and in the supernatants after 30, 60, and 90 min of exposure; samples were then washed, and medium containing gentamicin was added. Additionally, we confirmed gentamicin activity by plating supernatants in each experiment after 1 h of incubation with gentamicin. The number of CFU was below the level of detection (10 CFU/well) in all cell culture supernatants.
Cytochalasin D was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 4 mM and stored at −20°C. Cytochalasin D was added to microglial cells in a final concentration of 10 μM. In order to rule out an inhibitory effect of DMSO on phagocytosis of E. coli, microglial cells were stimulated with TLR agonists for 24 h, and then prior to the addition of bacteria, the same volume of either culture medium (DMEM with 10% FCS), 10 μM cytochalasin D (in 0.25% DMSO in culture medium), or 0.25% DMSO (in culture medium) was added to different wells. Treatment with DMSO did not alter the bacterial uptake.
Intracellular survival assay.
To monitor intracellular survival and replication inside microglia, E. coli DH5α and E. coli K1 strains were allowed to infect the cells for 90 min. Thereafter, cells were washed twice with PBS and incubated in culture medium containing gentamicin (200 μg/ml) for 6 h. At various times (90, 150, 240, and 360 min), the monolayers were washed with PBS and lysed with distilled water. The amounts of intracellular bacteria were determined by quantitative plating on sheep blood agar plates. Each test was done at least four times.
Measurement of microglial cell viability.
Microglial cell viability was determined using the WST-1 cell proliferation reagent (Roche Applied Science, Mannheim, Germany). The assay is based on the cleavage of the tetrazolium salt WST-1 by active mitochondria, producing a soluble formazan. This conversion occurs only in viable cells. Cells were incubated with WST-1 for 2 h. Then, the formazan dye formed was quantified by measuring the optical density at 490 nm using a Genios multiplate reader (Tecan, Crailsheim, Germany). The absorbance was directly correlated with the metabolic activity of the cells.
Staining and confocal laser scanning microscopy of microglial cells.
Confocal laser scanning microscopy was used to confirm intracellular localization of E. coli DH5α encoding the red fluorescent protein DSRed in microglia. Cells were plated on coverslips in 12-well plates and exposed to one of the TLR agonists for 24 h. Afterwards, the cell monolayers were washed three times with warm PBS and then incubated with Vybrant DiO cell-labeling solution (VybrantCell labeling solution kit; Molecular Probes, Leiden, Netherlands) for 30 min at 37°C according to the manufacturer's instructions. DiO bright green fluorescent lipophilic carbocyanine dye selectively labels the plasma membrane and is not cytotoxic. Subsequently, cells were washed twice with warm PBS, and bacteria were added for 90 min. For phagocytosis inhibition studies, cytochalasin D was added (final concentration, 10 μM) prior to the addition of bacteria and remained present throughout the experiment. Thereafter, cells were washed and incubated with gentamicin for 1 h. Finally, cells were washed twice with warm PBS and fixed in 4% formaldehyde in PBS. Then, confocal laser scanning microscopy measurements were performed using a scanning confocal microscope (Zeiss LSM 510) attached to an inverted microscope (Zeiss Axiovert 100 M). A 15-mW argon ion and a 1-mW helium/neon laser were used as light sources. DiO and DSRed E. coli DH5α cells were excited at 488 and 546 nm, respectively. A series of optical sections were imaged at intervals of 0.5 to 1.0 μm. From the three-dimensional (3D) image stacks, isosurfaces were calculated using self-written Matlab programs. The threshold values were obtained as background intensity plus 10 times the background standard deviation to localize the cell border and clearly separate cells from the background signal. The animated 3D isosurface reconstructions are provided in Fig. S1 to S5 in the supplemental material.
Statistical analysis.
GraphPad Prism software (GraphPad Software, San Diego, CA) was used to perform statistical analysis and graphical presentation. Analysis of variance (ANOVA) followed by Bonferroni's multiple comparison test was used to perform normally distributed data comparisons among all groups. Data from the intracellular survival assays among unstimulated and prestimulated microglia were not normally distributed and were analyzed by a Kruskal-Wallis test followed by Dunn's multiple comparison test to correct for repeated testing. A P value of <0.05 was considered significant.
RESULTS
Stimulation of microglia by TLR agonists induced cytokine and chemokine release.
Activated microglia release chemokines and cytokines including TNF-α and CXCL1 (9). TNF-α (also cachectin, a member of the TNF family) acts as a proinflammatory cytokine but has a plethora of cellular, organ, and systemic effects. It is essential for host responses and tissue remodeling. However, overshooting production can cause multiorgan failure, fever, hypotension, and tissue damage. CXCL1 (also KC or GROα) is largely known for its neutrophil-attracting feature. More recently, other functions emerged as well, but the ability to organize for the early infiltration of afflicted tissues by neutrophils places CXCL1 among the essential chemokines also in infection. In order to confirm microglial activation by different TLR agonists, we measured the amount of TNF-α and CXCL1 in the supernatants of microglial cultures (n ≥ 12 per group) incubated with Pam3CSK4, LPS, or CpG for 24 h. In all experiments, a group of unstimulated cells was included for comparison. Microglial cells remained viable after 24 h of exposure to TLR2, TLR4, and TLR9 agonists.
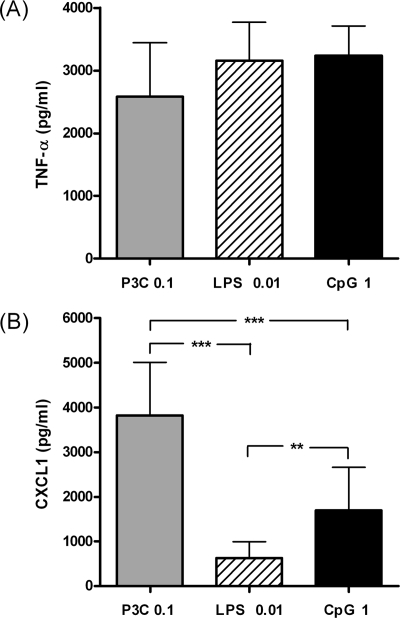
The supernatants of unstimulated microglia were devoid of measurable amounts of both CXCL1 and TNF-α. Incubation of microglia with the different TLR agonists induced a comparable release of TNF-α, indicating that the tested concentrations had a similar potency to activate microglial cells (Fig. 1A). The concentrations of TNF-α (mean ± standard deviation) were 2,585 ± 860, 3,154 ± 620, and 3,232 ± 475 pg/ml after 24 h of stimulation with 0.1 μg/ml Pam3CSK4, 0.01 μg/ml LPS, and 1 μg/ml CpG, respectively (P > 0.05).
FIG. 1.
TNF-α (A) and CXCL1 (B) concentrations (in pg/ml) in the supernatants of microglial cell cultures after 24 h of stimulation with 0.1 μg/ml Pam3CSK4 (P3C), 0.01 μg/ml LPS, and 1 μg/ml CpG. The supernatants of unstimulated microglia were devoid of measurable amounts of both TNF-α and CXCL1 (data not shown). Data are given as means ± standard deviations (error bars). Data were analyzed using one-way ANOVA followed by Bonferroni's multiple comparison test (**, P < 0.01; ***, P < 0.001).
The stimulation of microglia with TLR agonists resulted in different secretion levels of CXCL1 among experimental groups (Fig. 1B). Treatment of microglia with 0.1 μg/ml Pam3CSK4 resulted in higher CXCL1 levels (3,821 ± 1,190 pg/ml) than those found after stimulation with 0.01 μg/ml LPS (642 ± 364 pg/ml; P < 0.001) and 1 μg/ml CpG (1,697 ± 964; P < 0.001). We also found significant differences in CXCL1 release between microglia stimulated with 1 μg/ml CpG and 0.01 μg/ml LPS (P < 0.01).
Phagocytosis of E. coli DH5α and E. coli K1 by microglial cells increased after TLR stimulation.
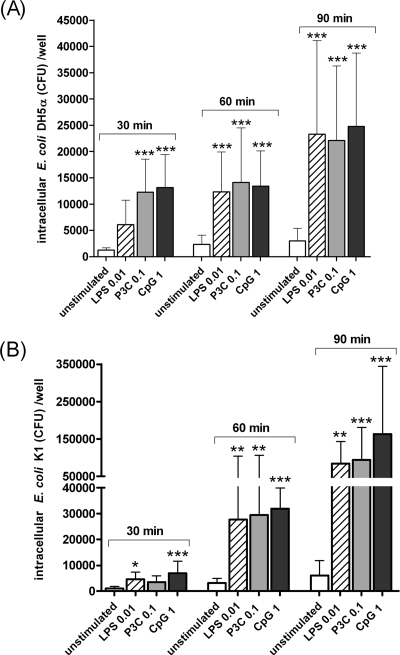
Microglial cells remained viable after 3 h of exposure to E. coli strains (multiplicity of infection, 100 bacteria per cell). The phagocytosis of E. coli strains was compared quantitatively after incubation for 30, 60, and 90 min in unstimulated cell cultures (control group) and in microglia that were previously stimulated with the TLR2, TLR4, or TLR9 agonists (n ≥ 12 wells per time point). The intracellular bacterial density increased over time in all tested groups for both strains (Fig. 2). After 90 min of exposure, the amount of phagocytosed E. coli DH5α was approximately two times higher than the uptake at 30 min. The number of ingested E. coli K1 cells after 90 min of infection was sixfold higher than at the 30-min time point.
FIG. 2.
Phagocytosis of E. coli DH5α (A) and E. coli K1 (B) strains by microglial cells after 24 h of stimulation with the TLR agonists: 0.1 μg/ml Pam3CSK4 (P3C), 0.01 μg/ml LPS, and 1 μg/ml CpG. After stimulation, cells were washed, and bacteria were added for different time periods (30, 60, and 90 min). Then, gentamicin (200 μg/ml) was added for 1 h to kill extracellular bacteria. The number of ingested bacteria was determined by quantitative plating of the cell lysates after the different incubation intervals. Data are shown as recovered bacteria (CFU) per well (means ± standard deviations [error bars]). Prestimulation with TLR agonists increased the number of bacteria ingested by microglia in comparison to unstimulated cells (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Data were analyzed using one-way ANOVA followed by Bonferroni's multiple comparison test.
In phagocytosis assays involving E. coli DH5α, unstimulated cells ingested bacteria at a low rate (mean ± standard deviation) with 1,245 ± 442, 2,371 ± 1,733, and 3,019 ± 2,388 CFU/well after 30, 60, and 90 min of infection, respectively. In contrast, the number of phagocytosed bacteria after 30 min was higher in microglia previously stimulated with 0.01 μg/ml Pam3CSK4 (12,283 ± 6,254 CFU/well; P < 0.001), 0.01 μg/ml LPS (6,086 ± 4,683 CFU/well; P > 0.05), or with 1 μg/ml CpG (13,143 ± 6,298 CFU/well; P < 0.001) than in unstimulated cells. At 60 and 90 min postinfection, microglia prestimulated with TLR agonists had still phagocytosed higher amounts of bacteria than the control group (P < 0.001). After 60 and 90 min, cells prestimulated by any of the TLRs had ingested approximately equal numbers of bacteria (P > 0.05).
In phagocytosis assays with E. coli K1, unstimulated cells phagocytosed small amounts of bacteria (mean ± standard deviation): 966 ± 809, 3,097 ± 1,855, and 6,073 ± 5,738 CFU/well after 30, 60, and 90 min of infection, respectively. The phagocytosis rates were higher in microglia pretreated with TLR ligands. At 30 min, the amounts of ingested bacteria were 3,498 ± 2,393, 4,609 ± 2,795, and 6,946 ± 4,652 CFU/well in cells stimulated with 0.01 μg/ml Pam3CSK4 (P > 0.05), 0.01 μg/ml LPS (P < 0.05), or 1 μg/ml CpG (P < 0.001), respectively. After 60 and 90 min postinfection, the ingestion of the K1-encapsulated strain was also higher in microglia prestimulated with TLR agonists than in unstimulated cells (P < 0.01).
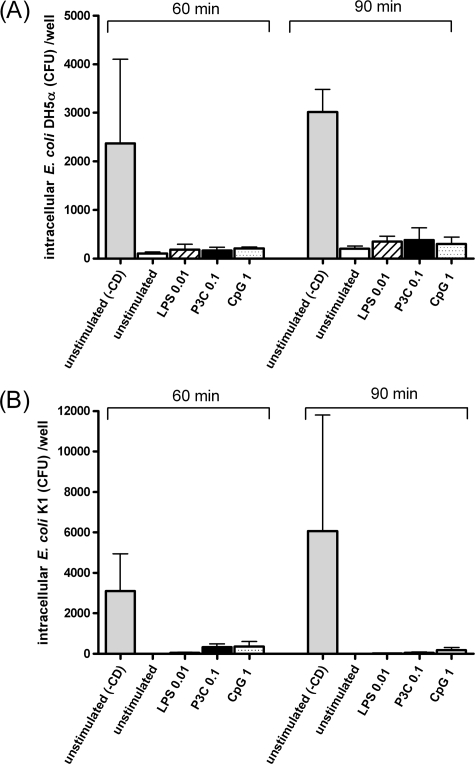
In phagocytosis inhibition studies, microglial cells treated with 10 μM cytochalasin D ingested very low numbers of bacteria (Fig. 3). Cytochalasin D blocked the entry of E. coli DH5α by >95% at 60 min and >93% at 90 min of infection. Cytochalasin D inhibited the uptake of E. coli K1 by >98% at 60 min and >99% at 90 min of infection. The inhibitory effect of cytochalasin D demonstrated that the phagocytosis of both E. coli strains by microglia requires rearrangement of the actin cytoskeleton.
FIG. 3.
Effect of 10 μM cytochalasin D (CD) on phagocytosis of E. coli DH5α (A) and E. coli K1 (B) strains by microglial cells after 60 and 90 min of infection. First, microglial cells were stimulated for 24 h with TLR agonists: 0.1 μg/ml Pam3CSK4 (P3C), 0.01 μg/ml LPS, or 1 μg/ml CpG. A control group with unstimulated microglial cells was also included. Cytochalasin D was added to the cell monolayers prior to the addition of bacteria and was present throughout the experiment. The bacterial uptake was determined by quantitative plating of the lysates after 1 h of incubation with gentamicin. Data are shown as the number of CFU per well (means ± standard deviations [error bars]). In order to show the inhibitory effect of cytochalasin D, a group with unstimulated cells that was not treated with cytochalasin D (−CD) is included in the figure.
Extracellular bacterial replication was tested by quantifying the number of bacteria in the supernatants before the addition of gentamicin. The extracellular concentration of E. coli strains did not differ significantly throughout the 90 min of incubation either in experiments studying phagocytosis or in experiments involving cytochalasin D (P > 0.05). Differences between the bacterial inoculum added to the wells and the bacterial concentration after 90 min of incubation were less than 0.5 log CFU/ml. Therefore, the increasing number of internalized bacteria throughout the observation period was not due to a higher concentration of extracellular bacteria.
Intracellular killing of E. coli DH5α and E. coli K1 by microglial cells increased after TLR stimulation.
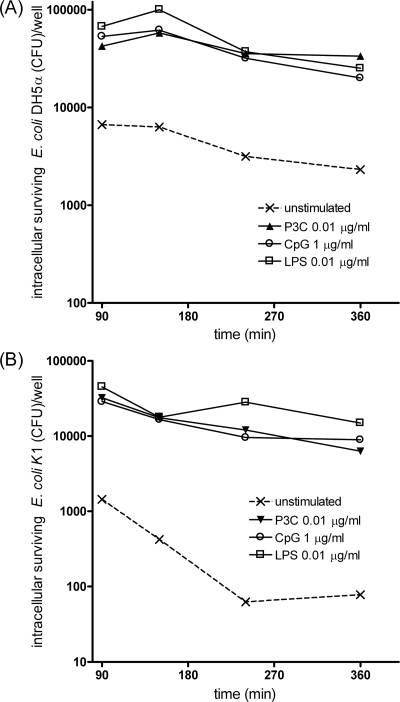
We next addressed the question whether the TLR-stimulated increase of phagocytosis would also combine with an enhanced intracellular neutralization of bacteria. In order to monitor the ability of microglia to kill E. coli strains, intracellular survival assays were performed as described in Materials and Methods. After 90 min, medium was changed, and gentamicin was added to kill selectively extracellular bacteria. The viable intracellular bacteria were quantified at various times (90, 150, 240, and 360 min), and counts are shown in Fig. 4.
FIG. 4.
Time course of bacterial killing of E. coli DH5α (A) and E. coli K1 (B) by murine microglial cells. After 24 h of stimulation with TLR agonists (0.1 μg/ml Pam3CSK4 [P3C], 0.01 μg/ml LPS, or 1 μg/ml CpG), cells were washed and allowed to ingest bacteria for 90 min. Then, gentamicin was added for 1 h, and the amount of intracellular bacteria was quantified by plating cell lysates at several postinfection times up to 360 min. For each group, intracellular survival is expressed as the number of recovered bacteria at the different time points expressed in logarithmic scale.
In unstimulated microglia, the number of intracellular E. coli DH5α slowly but steadily decreased from the 150-min time point, and 4,350 CFU/well were killed within 6 h. In microglia prestimulated with TLR agonists, the number of intracellular E. coli DH5α also declined from the 150-min postinfection time. The decrease was also relatively slow and comparable among the different TLR-stimulated groups. Importantly, at 6 h, the absolute amounts of neutralized E. coli DH5α CFU were higher in TLR-stimulated microglia than in unstimulated cells. After treatment with TLR2, TLR4, and TLR9 agonists, 20,500, 41,900, and 33,000 CFU/well, respectively, were killed.
The time course of intracellular killing of E. coli K1 was similar to the nonencapsulated strain. Microglia prestimulated with TLR agonists were able to kill larger absolute amounts of intracellular bacteria up to 6 h (26,000, 30,400, and 22,100 CFU/well in cells pretreated with TLR2, TLR4, and TLR9 ligands, respectively) than unstimulated cells (1,370 CFU/well killed).
Taken together, these results indicate that TLR-stimulated microglia were not only able to phagocytose more bacteria but also to clear approximately 1 order of magnitude more bacteria from the intracellular space than unstimulated cells. In other words, the TLR agonist pretreatment boosted the total clearance capacity.
Confocal laser imaging confirmed the intracellular localization of E. coli DH5α.
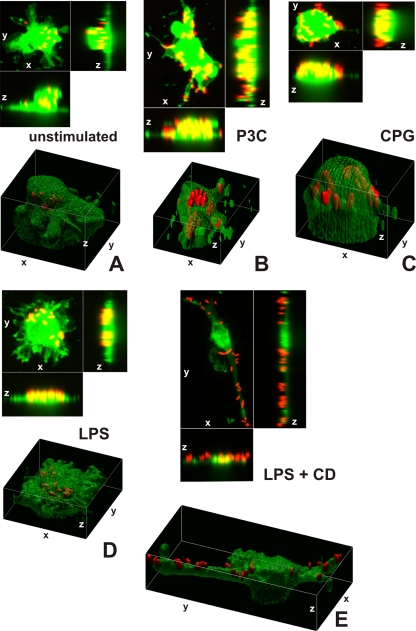
Images projected in xz and xy axes and isosurface reconstructions enabled us to differentiate clearly between red fluorescent bacteria localized intracellularly and those attached to the cell membrane of microglial cells (labeled by green Vybrant DiO) (Fig. 5A to D). The animated 3D isosurface reconstructions are provided as separate figures in the supplemental material. Microglial cells prestimulated with a TLR agonist showed higher bacterial internalization (Fig. 5B, C, and D) than unstimulated cells (Fig. 5A), supporting the results found in the phagocytosis assay.
FIG. 5.
3D confocal images of microglial cells (green; stained with Vybrant DiO prior to the addition of bacteria) ingesting fluorescent E. coli DH5α (red) are shown in the xy maximum projection as well as the xz and yz maximum projections for unstimulated cells (A) and for microglia stimulated for 24 h with Pam3CSK4 (P3C, 0.1 μg/ml) (B), CpG (1 μg/ml) (C), and LPS (0.01 μg/ml) (D) after 90 min of bacterial exposure. (E) The addition of cytochalasin D (CD; final concentration, 10 μM) blocked the phagocytosis of E. coli DH5α by microglial cells. Τhe inhibitory effect of cytochalasin D on phagocytosis is shown in LPS-stimulated cells. The surface reconstructions in each of the shown examples clearly enabled us to differentiate between intracellular bacteria and those attached to the cell membrane. Lengths of the axes are as follows (x, y, and z, respectively, in μm): 46.41, 46.64, and 37.20 (A); 36.81, 48.90, and 20.23 (B); 32.12, 38.91, and 24.45 (C); 42.33, 42.89, and 23.19 (D); 40.84, 77.50, and 30.04 (E).
Addition of 10 μM cytochalasin D prior to the addition of bacteria inhibited the internalization of E. coli DH5α by unstimulated microglia and those pretreated with a TLR agonist. Figure 5E shows the inhibitory effect of cytochalasin D on LPS-stimulated cells.
DISCUSSION
The CNS shows various defense mechanisms against the invasion by bacterial pathogens: (i) the blood-brain barrier, (ii) meningeal and perivascular macrophages, and (iii) parenchymal microglia. When the first two lines of defense fail, activated microglia can mount an early innate immune response (10). Reactive microglia develop a phagocytic phenotype engulfing and killing microbes and present antigens to recruit other cells to the site of infection, thereby initiating the adaptative immune response. Data from experimental studies strongly suggest that reactive microglia participate in the prevention of the spread of systemic infections into the CNS. Either the intravenous or intraperitoneal administration of LPS led to an upregulation of the expression of major histocompatibility complex class I (MHC-I) and MHC-II structures on microglial cells in postnatal rat brains (32). The intravenous injection of immunostimulants such as LPS, interleukins, or TNF-α caused a profound NF-κB activation within parenchymal microglia, indicating signaling through a pathway which is essential for TLR-induced responses (15). A recent study directly reported on activated microglia in human brain tissue from patients who died of sepsis (16).
Also in vitro, microglia can sense microorganisms and their products through a variety of pattern recognition receptors, including TLRs expressed on their surfaces and in endosomal compartments (7, 17). Immunocompromised individuals are at risk of developing CNS infections (2). One cause of this increased susceptibility to CNS infections might be a decreased local immune defense. The TLR system in microglia, which is highly efficient in immunocompetent individuals, is probably compromised during immunosuppression. Stimulation of the TLR system may therefore be a valuable therapeutic approach in immunocompromised patients. For this reason, we hypothesized that TLR agonists would stimulate microglia, thereby increasing their ability to phagocytose bacteria, such as E. coli. In our experimental setting, we chose the concentrations of microglial stimulators according to our previous findings of NO release in murine cell cultures; we used the lowest concentrations found to induce a maximal response of NO release (7). With this experimental design, we ensured that TLR1/2, TLR4, and TLR9 agonists were tested at comparable potency in terms of microglial stimulation.
In our study, microglia were exposed to a TLR ligand for 24 h, and TNF-α and CXCL1 were released, confirming microglial activation. TNF-α levels were comparable among the different TLR agonist-tested groups, whereas CXCL1 levels were more strongly increased after stimulation with 0.1 μg/ml Pam3CSK4 than after stimulation with 1 μg/ml CpG or 0.01 μg/ml LPS. In this respect, TNF-α should be considered as a proinflammatory cytokine that is similarly regulated among different stimuli while CXCL1 release apparently is regulated differentially by different TLRs (4, 14). Indeed, we have previously shown that TNF-α and CXCL1 are differentially controlled by the Th1 cytokine IFN-γ in microglia and that their induction is independently organized in macrophage-like cells and cell lines, respectively (12).
Our results indicate that prestimulation of microglial cells through TLR1/2, TLR4, or TLR9 increases their ability to phagocytose both the apathogenic E. coli DH5α and the pathogenic E. coli K1 strains. There was a strong correlation between the duration of bacterial exposure and the number of ingested bacteria. After 30 min of bacterial exposure, we found minor divergences between cells stimulated with LPS, Pam3CSK4, or CpG. At later time points, stimulation of TLR1/2, TLR4, and TLR9 uniformly increased bacterial phagocytosis.
The internalization of E. coli strains by murine microglial cells requires intact actin filaments since this process was blocked by >90% in the presence of 10 μM cytochalasin D. The phagocytosis of E. coli strains did not require either opsonization by antibodies or complement since our experiments were performed in medium supplemented with heat-inactivated serum of fetal bovine origin. Confocal microscopy methods were employed for visualization and confirmation of the intracellular localization of bacteria within microglial cells.
Few reports have focused on the role of TLRs in bacterial clearance. Upon microbial exposure, the TLR system modulated the phagocytic activity of macrophages at different steps, including internalization and phagosome maturation (3, 20). Exposure to TLR ligands upregulated the bacterial uptake in murine macrophage cells and in THP-1 human monocytes (6, 18). Our results demonstrate the enhancement of bacterial phagocytosis by TLR-mediated signaling in microglial cells. To our knowledge, the present report is the first addressing TLR-dependent phagocytosis in phagocytic cells of the nervous system.
Once bacteria are engulfed, they are incorporated into phagolysosomes and exposed to reactive oxygen species and other bacteriotoxic compounds which eventually will result in bacterial lysis. The efficiency of the phagocytic activity of reactive microglia depends not only on their ability to ingest bacteria but also on the pathogen's ability to modulate phagocyte signaling. Intracellular survival of E. coli within neutrophils has been previously described (8, 19). It is believed that bacterial aggregation might be the reason for bacterial resistance to neutrophil-mediated intracellular killing.
In our study, the killing rate represented by plotting the logarithmic intracellular bacterial concentration-versus-time curve revealed that prestimulated and unstimulated microglial cells killed intracellular E. coli strains relatively slowly. However, starting from higher numbers of phagocytosed bacteria, absolute killing rates were clearly enhanced after TLR stimulation.
The possible benefit of an enhancement of the TLR-induced response has been studied in other infectious and noninfectious diseases. To date, the most successful TLR therapeutic is imiquimod. This synthetic agonist of TLR7 is used to treat superficial basal cell carcinoma and genital warts caused by human papillomavirus (31). Other applications use TLR agonists as vaccine adjuvants. In this setting, CpG DNA has shown good results (5, 11).
In conclusion, stimulation of various TLRs can increase the phagocytic properties of microglial cells. This may be a promising therapeutic approach to improve the efficiency of the local immune system of the CNS against invading pathogens. Further studies in immunocompromised mice are in progress in order to assess whether the resistance of the brain against infections can be increased by preconditioning of microglial cells with TLR agonists.
Supplementary Material
Acknowledgments
This work was supported by grants from Else Kröner Fresenius Stiftung (R. Nau) and the Deutsche Forschungsgemeinschaft (SFB-TRR43; to U.-K.H.). S.R. was a recipient of a fellowship from the Departament d'Educació i Universitats de la Generalitat de Catalunya. A.Z. was supported by the Deutsche Forschungsgemeinschaft through the Research Center Molecular Physiology of the Brain (FZT 103 and EXC 171).
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 3 November 2008.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Aravalli, R. N., P. K. Peterson, and J. R. Lokensgard. 2007. Toll-like receptors in defense and damage of the central nervous system. J. Neuroimmune Pharmacol. 2297-312. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, D. 1984. Central nervous system infections in the immunocompromised host. Infection 12(Suppl. 1)S58-S64. [DOI] [PubMed] [Google Scholar]
- 3.Blander, J. M., and R. Medzhitov. 2004. Regulation of phagosome maturation by signals from Toll-like receptors. Science 3041014-1018. [DOI] [PubMed] [Google Scholar]
- 4.Dalpke, A. H., M. Frey, S. Morath, T. Hartung, and K. Heeg. 2002. Interaction of lipoteichoic acid and CpG-DNA during activation of innate immune cells. Immunobiology 206392-407. [DOI] [PubMed] [Google Scholar]
- 5.Daubenberger, C. A. 2007. TLR9 agonists as adjuvants for prophylactic and therapeutic vaccines. Curr. Opin. Mol. Ther. 945-52. [PubMed] [Google Scholar]
- 6.Doyle, S. E., R. M. O'Connell, G. A. Miranda, S. A. Vaidya, E. K. Chow, P. T. Liu, S. Suzuki, N. Suzuki, R. L. Modlin, W. C. Yeh, T. F. Lane, and G. Cheng. 2004. Toll-like receptors induce a phagocytic gene program through p38. J. Exp. Med. 19981-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebert, S., J. Gerber, S. Bader, F. Muhlhauser, K. Brechtel, T. J. Mitchell, and R. Nau. 2005. Dose-dependent activation of microglial cells by Toll-like receptor agonists alone and in combination. J. Neuroimmunol. 15987-96. [DOI] [PubMed] [Google Scholar]
- 8.Fexby, S., T. Bjarnsholt, P. O. Jensen, V. Roos, N. Hoiby, M. Givskov, and P. Klemm. 2007. Biological Trojan horse: antigen 43 provides specific bacterial uptake and survival in human neutrophils. Infect. Immun. 7530-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanisch, U. K. 2002. Microglia as a source and target of cytokines. Glia 40140-155. [DOI] [PubMed] [Google Scholar]
- 10.Hanisch, U. K., and H. Kettenmann. 2007. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 101387-1394. [DOI] [PubMed] [Google Scholar]
- 11.Harris, T. H., J. M. Mansfield, and D. M. Paulnock. 2007. CpG oligodeoxynucleotide treatment enhances innate resistance and acquired immunity to African trypanosomes. Infect. Immun. 752366-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Häusler, K. G., M. Prinz, C. Nolte, J. R. Weber, R. R. Schumann, H. Kettenmann, and U. K. Hanisch. 2002. Interferon-gamma differentially modulates the release of cytokines and chemokines in lipopolysaccharide- and pneumococcal cell wall-stimulated mouse microglia and macrophages. Eur. J. Neurosci. 162113-2122. [DOI] [PubMed] [Google Scholar]
- 13.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408740-745. [DOI] [PubMed] [Google Scholar]
- 14.Jones, B. W., T. K. Means, K. A. Heldwein, M. A. Keen, P. J. Hill, J. T. Belisle, and M. J. Fenton. 2001. Different Toll-like receptor agonists induce distinct macrophage responses. J. Leukoc. Biol. 691036-1044. [PubMed] [Google Scholar]
- 15.Laflamme, N., and S. Rivest. 1999. Effects of systemic immunogenic insults and circulating proinflammatory cytokines on the transcription of the inhibitory factor κB alpha within specific cellular populations of the rat brain. J. Neurochem. 73309-321. [DOI] [PubMed] [Google Scholar]
- 16.Lemstra, A. W., J. C. Groen in't Woud, J. J. Hoozemans, E. S. van Haastert, A. J. Rozemuller, P. Eikelenboom, and W. A. van Gool. 2007. Microglia activation in sepsis: a case-control study. J. Neuroinflammation 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lotz, M., S. Ebert, H. Esselmann, A. I. Iliev, M. Prinz, N. Wiazewicz, J. Wiltfang, J. Gerber, and R. Nau. 2005. Amyloid beta peptide 1-40 enhances the action of Toll-like receptor-2 and -4 agonists but antagonizes Toll-like receptor-9-induced inflammation in primary mouse microglial cell cultures. J. Neurochem. 94289-298. [DOI] [PubMed] [Google Scholar]
- 18.Mae, M., M. Iyori, M. Yasuda, H. M. Shamsul, H. Kataoka, K. Kiura, A. Hasebe, Y. Totsuka, and K. Shibata. 2007. The diacylated lipopeptide FSL-1 enhances phagocytosis of bacteria by macrophages through a Toll-like receptor 2-mediated signalling pathway. FEMS Immunol. Med. Microbiol. 49398-409. [DOI] [PubMed] [Google Scholar]
- 19.Nazareth, H., S. A. Genagon, and T. A. Russo. 2007. Extraintestinal pathogenic Escherichia coli survives within neutrophils. Infect. Immun. 752776-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neal, M. D., C. Leaphart, R. Levy, J. Prince, T. R. Billiar, S. Watkins, J. Li, S. Cetin, H. Ford, A. Schreiber, and D. J. Hackam. 2006. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J. Immunol. 1763070-3079. [DOI] [PubMed] [Google Scholar]
- 21.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 2822085-2088. [DOI] [PubMed] [Google Scholar]
- 22.Roach, J. C., G. Glusman, L. Rowen, A. Kaur, M. K. Purcell, K. D. Smith, L. E. Hood, and A. Aderem. 2005. The evolution of vertebrate Toll-like receptors. Proc. Natl. Acad. Sci. USA 1029577-9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo, T. A., and J. R. Johnson. 2003. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect. 5449-456. [DOI] [PubMed] [Google Scholar]
- 24.Russo, T. A., and J. R. Johnson. 2000. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J. Infect. Dis. 1811753-1754. [DOI] [PubMed] [Google Scholar]
- 25.Segura, M. A., P. Cleroux, and M. Gottschalk. 1998. Streptococcus suis and group B Streptococcus differ in their interactions with murine macrophages. FEMS Immunol. Med. Microbiol. 21189-195. [DOI] [PubMed] [Google Scholar]
- 26.Silver, R. P., W. Aaronson, and W. F. Vann. 1988. The K1 capsular polysaccharide of Escherichia coli. Rev. Infect. Dis. 10(Suppl. 2)S282-S286. [DOI] [PubMed] [Google Scholar]
- 27.Simard, A. R., and S. Rivest. 2005. Do pathogen exposure and innate immunity cause brain diseases? Neurol. Res. 27717-725. [DOI] [PubMed] [Google Scholar]
- 28.Smith, M. E., K. van der Maesen, and F. P. Somera. 1998. Macrophage and microglial responses to cytokines in vitro: phagocytic activity, proteolytic enzyme release, and free radical production. J. Neurosci. Res. 5468-78. [DOI] [PubMed] [Google Scholar]
- 29.Sorensen, M., C. Lippuner, T. Kaiser, A. Misslitz, T. Aebischer, and D. Bumann. 2003. Rapidly maturing red fluorescent protein variants with strongly enhanced brightness in bacteria. FEBS Lett. 552110-114. [DOI] [PubMed] [Google Scholar]
- 30.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21335-376. [DOI] [PubMed] [Google Scholar]
- 31.Tyring, S. K., I. I. Arany, M. A. Stanley, M. H. Stoler, M. A. Tomai, R. L. Miller, M. L. Owens, and M. H. Smith. 1998. Mechanism of action of imiquimod 5% cream in the treatment of anogenital warts. Prim. Care Update Ob. Gyns. 5151-152. [DOI] [PubMed] [Google Scholar]
- 32.Xu, J., and E. A. Ling. 1994. Upregulation and induction of surface antigens with special reference to MHC class II expression in microglia in postnatal rat brain following intravenous or intraperitoneal injections of lipopolysaccharide. J. Anat. 184285-296. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.