Abstract
Production of reactive oxygen species (ROS) is an important aspect of phagocyte-mediated host responses. Since phagocytes play a crucial role in the host response to Candida albicans, we examined the ability of Candida to modulate phagocyte ROS production. ROS production was measured in the murine macrophage cell line J774 and in primary phagocytes using luminol-enhanced chemiluminescence. J774 cells, murine polymorphonuclear leukocytes (PMN), human monocytes, and human PMN treated with live C. albicans produced significantly less ROS than phagocytes treated with heat-killed C. albicans. Live C. albicans also suppressed ROS production in murine bone marrow-derived macrophages from C57BL/6 mice, but not from BALB/c mice. Live C. albicans also suppressed ROS in response to external stimuli. C. albicans and Candida glabrata suppressed ROS production by phagocytes, whereas Saccharomyces cerevisiae stimulated ROS production. The cell wall is the initial point of contact between Candida and phagocytes, but isolated cell walls from both heat-killed and live C. albicans stimulated ROS production. Heat-killed C. albicans has increased surface exposure of 1,3-β-glucan, a cell wall component that can stimulate phagocytes. To determine whether surface 1,3-β-glucan exposure accounted for the difference in ROS production, live C. albicans cells were treated with a sublethal dose of caspofungin to increase surface 1,3-β-glucan exposure. Caspofungin-treated C. albicans was fully able to suppress ROS production, indicating that suppression of ROS overrides stimulatory signals from 1,3-β-glucan. These studies indicate that live C. albicans actively suppresses ROS production in phagocytes in vitro, which may represent an important immune evasion mechanism.
Although most people are colonized with the pathogenic yeast Candida albicans (8), the majority never develop invasive C. albicans disease. In contrast, patients with decreased numbers of phagocytes are at increased risk for invasive disease from Candida, indicating that the phagocyte response to Candida is critical in prevention of disease (34). One important host response generated by phagocytes is production of reactive oxygen species (ROS), through activity of the NADPH oxidase complex.
A wide variety of bacterial pathogens possess mechanisms for preventing generation of or avoiding contact with ROS, including Francisella tularensis, Salmonella enterica USA, Anaplasma phagocytophilum, and Helicobacter pylori (1, 5, 11, 20). Through these mechanisms, these pathogenic organisms evade or modulate host immune responses. Likewise, the fungal pathogen Aspergillus fumigatus produces gliotoxin, a molecule that inhibits multiple steps in the formation of active NADPH oxidase complexes (33). In contrast to Aspergillus, Candida does not produce gliotoxin (16, 17). Little is known about Candida immune evasion mechanisms that might subvert phagocyte functions. Therefore, we investigated ROS production in phagocytes exposed to Candida. We found that live C. albicans, unlike heat-killed or UV-inactivated Candida, suppressed production of ROS.
Because the cell wall is the initial point of contact in Candida-phagocyte interactions, we also investigated the role of the cell wall in suppression of ROS production. One important component of the cell wall is 1,3-β-glucan, which is typically exposed only at bud scars (21). C. albicans 1,3-β-glucan is recognized by phagocyte receptors CR3 and dectin-1. Activation of dectin-1 has been shown to be immunostimulatory, including stimulation of ROS production in murine bone marrow-derived macrophages (BMDM) (12). In contrast, we found that the suppressive effect of live C. albicans on ROS production overrode stimulatory signals from isolated cell walls and from 1,3-β-glucan. Thus, these studies suggest that live C. albicans suppresses phagocyte production of ROS, even in the face of stimulatory signals provided by the cell wall.
MATERIALS AND METHODS
Yeast strains and growth conditions.
Candida albicans strain SC5314 was used in these studies (15). Candida glabrata strain MR084-R was obtained from a collection of clinical isolates maintained at the University of Rochester (4). Both Candida strains were grown overnight at 37°C in yeast extract-peptone-dextrose medium. Saccharomyces cerevisiae strain BY4741 (Invitrogen, Carlsbad, CA) was grown in yeast extract-peptone-dextrose at 30°C.
For use in ROS assays, 1 ml of overnight culture was washed three times in Hanks balanced salt solution (HBSS; Invitrogen) and diluted 1:2 in HBSS. Samples were then sonicated in a water bath sonicator (FS30; Thermo Fisher Scientific, Inc., Waltham, MA) to disrupt clumps, using two 60-s cycles of sonication separated by a 1-minute incubation on ice. Sonication is routinely performed in our laboratory prior to enumeration of yeast cells; no differences in ROS production have been observed between sonicated and nonsonicated yeast (data not shown). Yeast were counted in a hemocytometer and adjusted to the desired organism density prior to use.
For preparation of heat-killed (HK) yeast cells, 1 ml of overnight culture was washed three times in HBSS, diluted 1:10 in HBSS, and incubated at 65°C for 90 min. After incubation, yeast were washed three times in HBSS, sonicated, and quantified as above. UV-inactivated yeast were prepared as described previously (37), using four 0.1-J/cm doses of UV irradiation to yeast in a six-well microtiter dish. UV-inactivated yeast were washed and sonicated as above prior to use. Control plating experiments confirmed that this treatment resulted in nonviable cells.
Phagocyte preparation.
The murine macrophage cell line J774.A1 (American Type Culture Collection, Manassas, VA) was cultured, passaged, and harvested as recommended by the American Type Culture Collection. Prior to assays, cells were harvested from tissue culture flasks by scraping and washed once in HBSS. An aliquot of cells was suspended in trypan blue (0.4% in phosphate-buffered saline [PBS]) and live cells were counted using a hemocytometer.
Human peripheral blood mononuclear cells (hPBMC) and neutrophils (hPMN) were obtained from healthy adult blood donors using a protocol approved by the Institutional Review Board at the University of Rochester Medical Center, Rochester, NY. After consent was obtained, blood was drawn into heparin-containing Vacutainer tubes (Becton, Dickinson and Company, Franklin Lakes, NJ). For hPBMC, blood was diluted 1:2 in sterile 0.9% sodium chloride or HBSS to a total volume of 40 ml. Ficoll-Paque Plus (10 ml; GE Healthcare, Piscataway, NJ) was layered under the diluted blood sample. The samples were centrifuged at 400 × g for 40 min at room temperature with the brake off, and hPBMC were harvested from the plasma-Ficoll interface. Cells were washed three times with sterile HBSS, counted in a hemocytometer, and adjusted to the appropriate concentration.
hPMN were isolated using density centrifugation (as above), after which the pellet was suspended in HBSS and mixed with an equal volume of 2% dextran (Fisher Scientific, Fair Lawn, NJ) in HBSS. Samples were allowed to sediment at room temperature for 30 min; after sedimentation, the top, leukocyte-rich layer was harvested. The cells were washed once in HBSS, the pellet was resuspended in PBS, and red blood cells were lysed by addition of sterile water. After mixing for 20 s, isotonicity was restored by addition of PBS. The hPMN were then centrifuged for 5 min at 500 × g and resuspended in HBSS.
Murine BMDM were isolated from 6- to 8-week-old BALB/c or C57BL/6 mice (National Cancer Institute, Frederick, MD). Bone marrow was flushed from the femurs and tibias using PBS. Mononuclear cells were isolated by density gradient centrifugation using Lymphoprep medium (Axis-Shield, Norton, MA). Cells were washed and maintained overnight in 40 ng/ml macrophage colony-stimulating factor (M-CSF; Peprotech, Inc., Rocky Hill, NJ). After 24 h, nonadherent cells were harvested and cultured in M-CSF for 7 days, with addition of fresh M-CSF on day 4. BMDM were harvested from flasks using 0.02% EDTA in PBS, after which they were washed in HBSS and counted in a hemocytometer.
Murine peritoneal PMN were elicited by intraperitoneal administration of 1 ml of sterile 3% Brewer's thioglycolate medium (Becton Dickinson) to 6- to 8-week-old BALB/c mice. Cells were harvested from euthanized animals 4 hours after thiogylcolate injection by washing the peritoneal cavity with 5 to 8 ml of ice-cold RPMI 1640 supplemented with penicillin-streptomycin, 55 μM 2-mercaptoethanol, 10 mM HEPES, 1 mM sodium pyruvate, and 10% fetal bovine serum. Cells were centrifuged for 10 min at 200 × g at 4°C, washed once, resuspended in HBSS, and counted in a hemocytometer.
Measurement of ROS production.
ROS production was measured by luminol-enhanced chemiluminescence using the Superluminol kit (World Precision Instruments, Inc., Sarasota, FL; additional luminol was obtained from Sigma-Aldrich, St. Louis, MO). A mixture containing luminol, signal enhancer, and either HBSS or phorbol myristate acetate (PMA, diluted in HBSS; Alexis Biochemicals, San Diego, CA) was prewarmed to 37°C. To minimize preactivation of primary phagocytes, the wells of a white, opaque-bottom, 96-well microtiter plate (Corning Incorporated, Corning, NY) were treated with 0.05% bovine serum albumin in PBS (200 μl/well) for 1 hour and washed three times with HBSS prior to use. This step was omitted for experiments using J774 cells. A 5 × 106 phagocyte/ml suspension (100 μl) was placed in each well. Yeast cells (in 25 μl of HBSS) were added to each well, and the yeast and phagocytes were brought together by centrifugation at 500 × g for 2 min. The prewarmed luminol solution (42.5 μl) was added immediately prior to quantification. Luminescence was measured using a 1-s integration time at intervals of 30 to 45 s over a 3-hour incubation at 37°C using a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA). Final concentrations were 90 μM luminol, 3% signal enhancer, and 750 ng/ml PMA. The Candida/phagocyte ratio was 5:1 unless noted otherwise.
To measure the viability of phagocytes during this assay, phagocytes and yeast were combined with the detection reagents in a 96-well plate, as described above. The plate was then incubated at 37°C for 1 hour, which corresponded to the approximate peak in ROS production. After incubation, 67.5 μl of the supernatant was removed and replaced with an equal volume of trypan blue (0.4% in PBS). Samples were mixed by pipetting, and an aliquot was examined by light microscopy. Phagocyte viability was determined as the percentage of phagocytes that excluded trypan blue.
ROS production was also measured using chloromethyl-dichlorodihydrofluorescein diacetate (CM-H2DCFDA; EMD Biosciences, Inc., San Diego, CA). Phagocytes were incubated in 8 μM CM-H2DCFDA at room temperature for 30 min and washed twice in HBSS prior to exposure to C. albicans. Assay conditions were as above, with the exception that the luminol solution was replaced with HBSS. Background fluorescence (excitation, 485 nm; emission, 530 nm) was measured prior to incubation; these values were subtracted from fluorescence measurements after an incubation of 150 min at 37°C.
XTT metabolism assay.
Measurement of Candida metabolic activity was performed based on metabolism of the tetrazolium dye 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT; Research Organics, Cleveland, OH), as described in reference 36. A freshly prepared solution containing 0.5 mg/ml XTT, 4.5 mg/ml glucose (Mallinckrodt Baker, Inc., Phillipsburg, NJ), and 40 μg/ml coenzyme Q (Sigma-Aldrich) in PBS was filter sterilized and kept in the dark until use. Yeast were washed, counted, and diluted as above and added to a 96-well plate. XTT solution (100 μl) was added to each well, and the plate was incubated at room temperature for 20 min in the dark. Absorbance was measured at 450 nm with subtraction of background absorbance at 570 nm.
ROS scavenging assay.
Stock solutions of xanthine oxidase (670 mU/ml in HBSS; Sigma-Aldrich), hypoxanthine (25 mM in 30 mM NaOH; Sigma-Aldrich), and superoxide dismutase (SOD; 22,350 U/ml; Calbiochem-EMD Chemicals, Inc.) were diluted in HBSS prior to use. A 25-μl aliquot of live or HK yeast or HBSS was placed into each well of a 96-well microtiter plate. A xanthine oxidase solution (25 μl) and luminol/enhancer solution (42.5 μl; prepared as described above) were then added to each well. The reaction was started by adding a hypoxanthine solution (75 μl), and the plate was immediately placed in the plate reader. Luminescence was measured using a 1-s integration time and intervals of 90 to 105 s over a 1-hour incubation at 25°C. As a positive control for ROS scavenging, SOD was added to the xanthine oxidase solution of control wells. Final reaction concentrations were 8 mU/ml xanthine oxidase, 150 μM hypoxanthine, 681 U/ml SOD, 90 μM luminol, and 3% signal enhancer.
1,3-β-Glucan immunofluorescence.
Surface-exposed 1,3-β-glucan was detected by indirect immunofluorescence. Overnight cultures of yeast were grown as above, or with the addition of 1.25 ng/ml caspofungin or vehicle alone (0.1% dimethyl sulfoxide) to the medium. They were then washed, sonicated, and counted as above. Yeast that had neither been fixed nor permeabilized were treated with 3% bovine serum albumin in PBS to block nonspecific binding, after which they were stained for 2 h at 4°C with anti-1,3-β-glucan antibody diluted 1:200 (Biosupplies Australia Pty. Ltd., Parkville, Australia). Samples were then washed in PBS and treated with 15 μg/ml Texas red-conjugated goat anti-mouse immunoglobulin G (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 60 min at 4°C. After secondary antibody treatment, samples were washed with PBS. Fluorescence microscopy was performed using a Nikon TE2000-E inverted fluorescence microscope with a 100×, 1.4 numerical aperture oil immersion objective lens; images were captured using a CoolSnap HQ camera (Photometrics, Tucson, AZ) and MetaVue imaging software (Molecular Devices, Sunnyvale, CA).
Preparation of cell wall ghosts.
HK or live C. albicans yeast cells were washed twice in water, counted using a hemocytometer, and resuspended in 1 mM phenylmethylsulfonyl fluoride (EMD Chemicals, Inc). Soda lime glass beads were added to each sample, and yeast cells were disrupted using a BioSpec Mini8 bead beater (BioSpec Products, Inc., Bartlesville, OK) in 20-s bursts for six cycles with 1-minute incubations on ice between each cycle. Cell disruption was monitored by light microscopy. Cell wall material was washed from the beads using 1 mM phenylmethylsulfonyl fluoride and collected by centrifugation (3,000 × g). The pellet was washed twice with HBSS, and the resulting cell wall ghosts were stored at 4°C until use. Just prior to use, cell wall ghosts were diluted to the appropriate concentration based on the density of yeast in the initial sample.
Analysis of the effect of secreted factors on ROS production.
To determine whether factors secreted by yeast during the assay inhibit production of ROS, J774 cells (600 μl of a 5 × 106 cell/ml suspension) were placed into the wells of a 24-well tissue culture plate. HBSS or HK or live yeast were then added (stimulus A; 150 μl of a 1 × 108 yeast cells/ml suspension) to the J774 cells and the samples were incubated for 1 hour at 37°C. The supernatants were then removed and filtered through a 0.45-μm filter. A new sample of J774 cells was suspended in the filtered medium; these cells were assayed for ROS production as above (using HK or live yeast [stimulus B]).
Statistical analysis.
For individual pair-wise comparisons, Student's t tests were performed using Microsoft Excel. For analyses involving more than two comparisons, data were analyzed using a one- or two-way analysis of variance (ANOVA), depending on the number of conditions, using SigmaStat software (Systat Software, Inc., San Jose, CA). Post hoc analyses using Student's t tests were performed only when the ANOVA results indicated a significant difference.
RESULTS
Live C. albicans suppresses production of ROS by phagocytes.
Production of ROS was assayed in real time using luminol-enhanced chemiluminescence. Luminol-enhanced chemiluminescence can detect both intracellular and extracellular ROS, most likely through interactions with superoxide (7). Although luminol enhanced chemiluminescence may be affected by the products of nitric oxide synthase (6), this technique is widely used as a quantitative measure of the phagocyte oxidative burst.
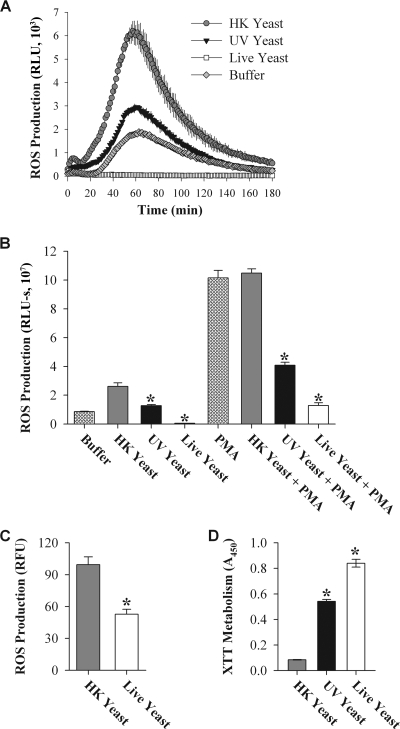
Cells of the murine macrophage cell line J774 produced ROS in response to HK C. albicans, with a peak at approximately 60 min (Fig. 1A and B). However, almost no ROS were produced in response to live C. albicans. A decrease in production of ROS in response to live C. albicans was also observed when ROS production was measured using the oxidation-dependent fluorophore dichloro-dihydrofluorescein diacetate (Fig. 1C). This technique measures the intracellular production of many different oxidant species, but it is frequently used for characterization of ROS produced by phagocytes (7).
FIG. 1.
Live C. albicans suppresses production of ROS by J774 cells. (A) ROS production by unstimulated J774 cells was measured by luminol-enhanced chemiluminescence. Data represent mean RLU of three identical samples collected with 1-second integrations over 3 hours; error bars represent the standard deviations (SD) of the three measurements. J774 cells were treated with C. albicans at a yeast/phagocyte ratio of 5:1. (B) Bar heights indicate the mean area under the curve (AUC) of the data presented in panel A; error bars are the SD of the three AUC results. Data for J774 cells stimulated with PMA were acquired as for panel A, with the exception that PMA was added with the luminol mixture. (C) ROS production was measured using CM-H2DCFDA. Bar heights represent the mean fluorescence intensity after 150 min of incubation, after background fluorescence was subtracted (error bars represent the SD). (D) Metabolic activities of live, UV yeast, and HK yeast were measured as the ability to metabolize the tetrazolium dye XTT. Bar heights indicate mean absorbances; error bars indicate SD. All experiments were performed at least three times (with the exception of the CM-H2DCFDA experiment, which was performed twice), with the same trend observed each time. A representative experiment of each type is shown. Asterisks indicate results significantly different from those obtained with HK yeast (Student's t test; P < 0.005).
Viability of phagocytes was evaluated by exclusion of trypan blue; there was no difference in viability after exposure to live or HK yeast (percentages of viable cells: no yeast, 85 ± 5; HK yeast, 84 ± 3; live yeast, 85 ± 3). Thus, the decreased production of ROS was not due to increased death of phagocytes.
To determine whether ROS production stimulated by HK C. albicans was dependent on the manner of killing, we also exposed J774 cells to C. albicans that had been UV inactivated (UV C. albicans). UV C. albicans is incapable of replication but has an intact cell wall, whereas HK C. albicans has cell wall alterations (37). When unstimulated J774 cells were exposed to UV C. albicans (Fig. 1A and B), ROS production was 1.3 × 107 relative light units-seconds (RLU-s), an intermediate level of production compared to HK (2.6 × 107 RLU-s) and live C. albicans (4.9 × 105 RLU-s). Although it is not viable with respect to growth in culture, UV C. albicans exhibits metabolic activity at approximately 65% of that measured with live C. albicans (Fig. 1D). The finding that UV C. albicans has an intermediate level of metabolism and an intermediate effect on ROS production suggests that suppression of ROS production may be dependent on Candida metabolic activity.
To determine whether the lack of ROS production in response to live C. albicans was due to active suppression or, alternatively, a failure to stimulate the phagocyte, J774 cells were treated with PMA, a strong stimulant for ROS production. PMA-stimulated J774 cells generated approximately fourfold more ROS than unstimulated cells when exposed to HK C. albicans (Fig. 1B). Nevertheless, exposure to live C. albicans still resulted in a significant decrease in ROS production, indicating that live C. albicans suppresses production of ROS. As with unstimulated J774 cells, PMA-stimulated production of ROS in the presence of UV C. albicans was intermediate to HK and live C. albicans.
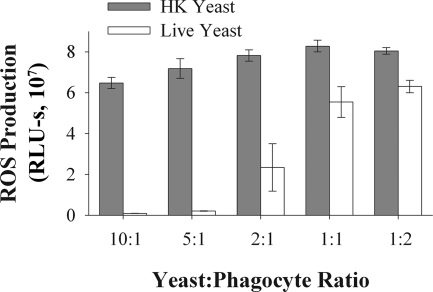
Suppression of ROS production varied with the Candida/phagocyte ratio (Fig. 2), with maximal suppression occurring at ratios of 5:1 and 10:1 and intermediate suppression at a 2:1 ratio. This may reflect the fact that at higher Candida/phagocyte ratios, a larger proportion of the phagocytes are in contact with at least one yeast cell. Because C. albicans exists in both yeast and filamentous forms at sites of infection (23), we also investigated production of ROS in response to germ tubes (nascent filamentous growth). Both live yeast and live germ tubes suppressed ROS production; however, HK germ tubes induced less ROS production than HK yeast (see Fig. S1 in the supplemental material).
FIG. 2.
Live C. albicans suppresses ROS production in a dose-dependent manner. ROS production by J774 cells was measured in response to live or HK yeast at the indicated yeast/phagocyte ratio. Each experiment was performed with triplicate samples; the bar heights indicates the mean values and error bars represent the standard deviations. A representative experiment is presented; the experiment was repeated three times with the same trend observed each time. Live yeast resulted in significantly less ROS production than HK yeast at each yeast/phagocyte ratio (two-way ANOVA, P < 0.001 overall; for all individual yeast/phagocyte ratios, post hoc Student's t test comparisons yielded P values <0.001).
A variety of phagocyte responses are regulated by signaling pathways that are triggered by ingestion of pathogens. To determine if the process of phagocytosis was necessary for suppression of ROS production, J774 cells were treated with cytochalasin D to block phagocytosis. No difference in ROS production between vehicle-treated or cytochalasin D-treated cells was observed, indicating that phagocytosis is not necessary for suppression of ROS production (see Fig. S2 in the supplemental material).
Suppression of ROS production in primary phagocytes.
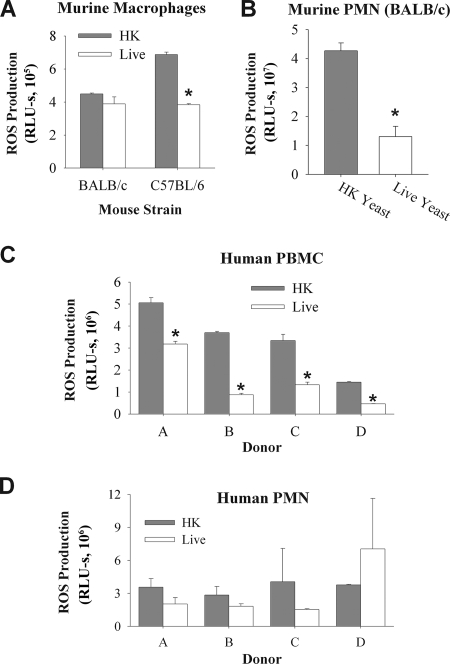
Phagocyte cell lines are selected for their ability to function under tissue culture conditions and do not always behave the same as primary phagocytes. To determine whether C. albicans yeast suppresses ROS production in primary phagocytes, we measured ROS generated by murine BMDM and elicited peritoneal PMN in response to Candida (Fig. 3). Interestingly, BMDM from C57BL/6 mice underwent suppression, whereas BMDM from BALB/c mice did not (Fig. 3A). In contrast, ROS production by elicited peritoneal PMN from BALB/c mice was suppressed by live C. albicans (Fig. 3B), with HK stimulating threefold more ROS than live C. albicans.
FIG. 3.
Live C. albicans suppresses production of ROS in primary phagocytes. ROS production by murine BMDM derived from BALB/c and C57BL/6 mice (A), murine elicited peritoneal PMN (B), hPBMC (C), or hPMN (D) was determined as for Fig. 1. Experiments using murine phagocytes were performed at least three times, with the same trend observed each time. A representative experiment is shown. Experiments using human phagocytes were performed using four different donors, as indicated. Donors for hPBMC and hPMN were not the same. For each donor, measurements were made in triplicate (with the exception of hPBMC donor B, for which results were measured in duplicate). Asterisks in panels A and B indicate results significantly different from those obtained with HK yeast (Student's t test; P < 0.001). For panels C and D, a one-way ANOVA of the entire data set indicated that there was significantly lower ROS production with live yeast for both hPBMC and hPMN (P < 0.001 for PBMC; P = 0.015 for PMN). Asterisks indicate that subsequent Student's t test comparisons for individual donors demonstrated a significant difference between HK and live yeast for all hPBMC donors (P < 0.01) but for none of the hPMN donors.
To determine if suppression of ROS production occurred in human phagocytes, we tested the effect of live Candida on ROS production in hPBMC and hPMN. On average, ROS production by hPBMC was suppressed by live C. albicans 2.9-fold (range, 1.6- to 4.2-fold) (Fig. 3C). The ROS production by hPMN from three out of four donors was lower with live C. albicans compared to HK (average decrease, 1.6-fold; range, 0.5- to 2.7-fold) (Fig. 3D). The donor-to-donor variation is consistent with other studies with PMN which have documented donor-to-donor variability in areas as diverse as granzyme production (35), cytokine production (18), and adhesion (19). When the results from hPMN were analyzed as a whole, significantly less ROS was produced in response to live C. albicans. Thus, in vitro, primary human phagocytes, primary murine PMN, and BMDM derived from C57BL/6 mice undergo suppression of ROS production by live C. albicans.
Candida albicans and non-albicans Candida spp. suppress ROS production, while Saccharomyces cerevisiae does not.
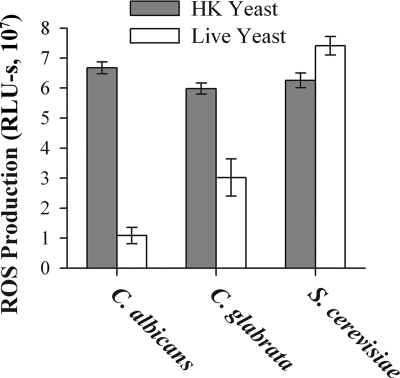
To investigate the generality of ROS suppression by live yeast, we examined ROS production in J774 cells exposed to C. glabrata and S. cerevisiae (Fig. 4). Like C. albicans, C. glabrata suppressed ROS suppression, although by only twofold, rather than the sixfold suppression seen with C. albicans. In contrast, S. cerevisiae did not suppress ROS production. Thus, suppression of ROS production seems to be associated with pathogenic yeast, since S. cerevisiae did not suppress ROS production.
FIG. 4.
Candida species suppress ROS production but S. cerevisiae does not. ROS production by J774 cells in response to HK or live yeast was measured as for Fig. 1. A representative experiment is presented; the experiment was repeated three times, with the same trend observed each time. Each experiment was performed with triplicate samples; the bar heights indicates the mean values and error bars represent the standard deviations. Results were analyzed using a two-way ANOVA; ROS production in response to live S. cerevisiae was significantly different than with either of the Candida species (P < 0.001 for all comparisons). Live yeast induced less ROS than HK for both Candida species (P < 0.001 for both comparisons).
Suppression of ROS production versus scavenging of ROS.
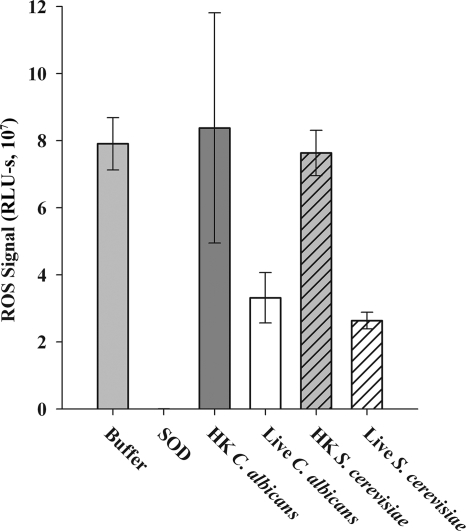
Both C. albicans and S. cerevisiae produce multiple ROS-scavenging enzymes, including SOD and catalase (13, 14, 32). To determine whether suppression of ROS production by live C. albicans was the result of ROS scavenging, we used a cell-free in vitro assay (24) in which ROS is produced enzymatically by xanthine oxidase. The presence of a ROS scavenger in the system would result in decreased levels of ROS, as demonstrated by the significant decrease in signal when exogenous SOD was included in the assay (Fig. 5). Live C. albicans scavenged more ROS than HK C. albicans. However, scavenging of ROS by live and HK S. cerevisiae was similar to that observed with C. albicans. Because S. cerevisiae does not mediate suppression of ROS production by J774 cells, these data indicate that scavenging alone cannot account for the observed suppression of ROS production.
FIG. 5.
Live C. albicans and S. cerevisiae scavenge ROS at similar levels. Superoxide was produced in a cell-free system by the action of xanthine oxidase on hypoxanthine (as described in reference 24). The amount of ROS signal was evaluated using luminol-enhanced chemiluminescence. A decrease in ROS signal relative to that observed with buffer alone indicates ROS is being scavenged. As a positive control for ROS scavenging, SOD was added to some samples. The mean signal in SOD-containing samples was 2.68 × 104; this value was too low for the bar to be visible on the graph. The experiment was performed three times; a representative experiment is shown. There was no difference in ROS signal between live C. albicans and S. cerevisiae (Student's t test, P = 0.21).
Suppression of ROS production is not mediated by C. albicans cell wall-phagocyte interactions.
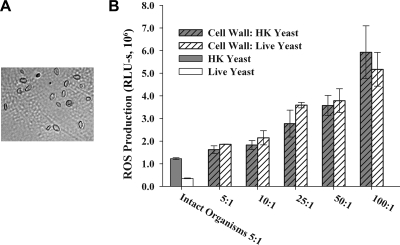
The C. albicans cell wall serves as the initial point of contact between yeast and phagocytes. To investigate the role of the cell wall in suppression of ROS, we treated J774 cells with isolated cell wall fractions prepared from HK or live C. albicans. Cell wall ghosts, which consist of roughly intact cell walls without cytoplasmic contents, were prepared using a physical disruption method that does not rely on heating or chemical denaturation (25). Using this method, cell wall ghosts were produced that are microscopically similar to intact C. albicans (Fig. 6A). At the equivalent of a Candida/phagocyte ratio of 5:1, cell wall ghosts from both HK and live C. albicans stimulated ROS production to 1.6 × 106 and 1.9 × 106 RLU-s, respectively, compared with 1.2 × 106 RLU-s for intact HK organisms (Fig. 6B). As the dose of the cell wall preparation increased, ROS production also increased, regardless of whether the cell walls were prepared from HK or live C. albicans. These results suggest that live C. albicans overrides the stimulatory effects of the cell wall and suppresses ROS production.
FIG. 6.
Cell wall ghosts prepared from either HK or live C. albicans yeast induce ROS production. Cell wall ghosts were isolated from live or HK yeast using a nondenaturing protocol. J774 cells were exposed to intact organisms or cell wall preparations at a concentration equivalent to the indicated Candida/phagocyte ratio. ROS production was measured as described for Fig. 1. A representative experiment is presented; the experiment was repeated three times with similar results. Bar heights indicate the means of three replicate samples; error bars indicate the standard deviations.
Heat killing of C. albicans results in increased exposure of cell wall 1,3-β-glucan, which is typically only exposed at bud scars in live C. albicans (21, 37). To specifically investigate the role of 1,3-β-glucan in ROS production, live C. albicans cells were treated with a nonlethal dose of caspofungin to expose surface 1,3-β-glucan (37). At the concentration used in this experiment, caspofungin-treated C. albicans has normal viability as measured by propidium iodide staining (data not shown) but has exposed surface 1,3-β-glucans (Fig. 7).
FIG. 7.
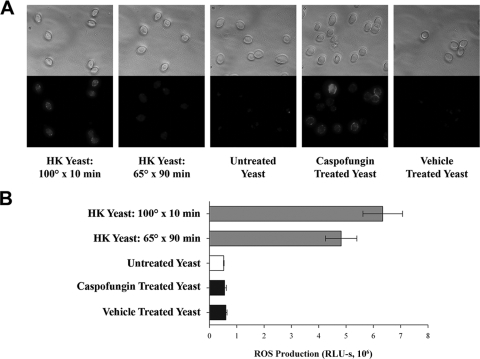
Exposure of 1,3-β-glucan by treatment with caspofungin does not prevent suppression of ROS production. (A) Bright-field (top panel) and epifluorescence (bottom panel) photomicrographs of yeast stained with anti-1,3-β-glucan antibody. Yeast were boiled for 10 min, HK, or treated during overnight growth with 1.25 ng/ml caspofungin or vehicle alone (0.1% dimethyl sulfoxide), as indicated. All epifluorescence images were captured using the same exposure time. (B) ROS production by J774 cells measured by luminol-enhanced chemiluminescence as for Fig. 1. A representative experiment is presented; the experiment was repeated three times with similar results. Bar lengths represent the means of triplicate values; error bars indicate the standard deviations. There was no significant difference in ROS production among untreated, caspofungin-treated, and vehicle-treated yeast as analyzed by one-way ANOVA (P > 0.80 for all comparisons). There were significant differences between HK yeast and all other groups (one-way ANOVA, P < 0.005); the comparison between yeast killed at 100°C versus 65°C was significant upon post hoc analysis (Student's t test, P = 0.046).
For these studies, two protocols for heat killing of Candida were used. First, Candida was incubated at 65°C for 90 min. This protocol was used throughout these studies, as it minimizes the effects of heat killing on cell wall components. The second protocol, incubation at 100°C for 10 min, was reported to yield significant surface exposure of 1,3-β-glucan (37) and was used as a positive control for the immunofluorescence assays.
As determined in an immunofluorescence assay for 1,3-β-glucan (Fig. 7A), both C. albicans incubated at 100°C and C. albicans treated with caspofungin displayed significant surface exposure of 1,3-β-glucan. In contrast, C. albicans cells that were killed at 65°C had less exposed 1,3-β-glucan and untreated C. albicans had almost none. J774 cells exposed to live C. albicans produced less ROS than HK C. albicans, whether 1,3-β-glucan was exposed by caspofungin treatment or not. This suggests that the mechanism of ROS suppression overrides any stimulatory signals provided by 1,3-β-glucan-based recognition of C. albicans. Interestingly, C. albicans killed at 100°C shows a modest, but statistically significant, increase in ROS production compared with C. albicans killed at 65°C (6.3 × 106 RLU-s versus 4.8 × 106 RLU-s) (Fig. 7B), suggesting that 1,3-β-glucan exposure may be somewhat stimulatory for ROS production in this system.
Suppression of ROS production does not appear to be mediated by a stable secreted factor.
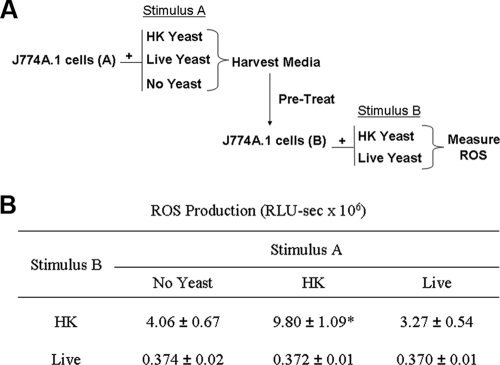
In all of these studies, yeast were washed prior to phagocyte exposure to remove medium components and/or yeast metabolic by-products that might affect phagocyte performance. However, it remains possible that ROS suppression is mediated by a product secreted by live yeast during the experiment. No suppression of ROS production occurred when J774 cells were treated with filtered medium from overnight cultures of C. albicans (grown in RPMI) (data not shown). To investigate the possibility that suppressive products are produced by Candida only when in contact with phagocytes, a two-step experiment was performed (Fig. 8A). First, J774 cells were treated with HK or live C. albicans, or buffer only (no yeast), under conditions employed in the ROS assays described above (this treatment is referred to as stimulus A in Fig. 8A). After treatment with stimulus A, the supernatant from these samples was collected and filtered to remove cells and organisms. The filtered supernatants were then used to pretreat a second set of J774 cells, which were subsequently exposed to either HK or live C. albicans (stimulus B in Fig. 8A). If a stable suppressive factor were released into the medium during stimulus A, pretreatment of the second set of cells with the medium from stimulus A would be predicted to suppress ROS production and result in lower responses to stimulus B. Such an effect was not observed.
FIG. 8.
Secreted factors do not appear to suppress ROS production. (A) A schematic of the experiment is shown. Medium was harvested from J774 cells that had been exposed to HK yeast, live yeast, or no stimulus (stimulus A) under the same conditions used for an ROS assay. The medium was harvested, filtered, and then used to pretreat a second set of J774 cells; these cells were exposed to HK or live yeast (stimulus B) and ROS production was measured. (B) Results of the above experiment, presented as means ± standard deviations from a representative experiment. The experiment was repeated three times, with similar results. As analyzed by a two-way ANOVA, significantly more ROS was produced when stimulus A was HK compared with buffer (*, P < 0.05).
When stimulus A contained no yeast (buffer) and stimulus B was HK yeast, ROS production was 4.06 × 106 RLU-s (Fig. 8B) and did not differ significantly from ROS produced when stimulus A was live yeast (3.27 × 106 RLU-s). Thus, there was no suppression of ROS production associated with supernatants from cells treated with live yeast (stimulus A), suggesting that suppression of ROS production is not mediated by a stable secreted factor. It is possible that such a factor is produced, but the concentration in the total assay volume was insufficient to affect ROS production.
In contrast, when stimulus A and stimulus B were both HK C. albicans, significantly more ROS was produced than when stimulus A was buffer (9.8 × 106 RLU-s for stimulus A, HK yeast). Since the HK yeast are not likely to produce secreted factors, this experiment suggests that contact with HK C. albicans stimulates J774 cells to produce a stable secreted factor, possibly a cytokine, which then enhances ROS production in other J774 cells. This effect demonstrates that this assay is capable of detecting secreted factors. It also suggests that phagocyte responses to C. albicans may be cooperative with respect to ROS production.
DISCUSSION
We have demonstrated that live C. albicans suppresses ROS production by phagocytes in vitro. This suppression did not occur with S. cerevisiae, which is infrequently pathogenic. Suppression of ROS production was not dependent on phagocytosis and overrode the stimulatory signals of the cell wall, including 1,3-β-glucan. Many recent studies of phagocyte interactions with Candida have focused on the cell wall and phagocyte pathogen recognition receptors, such as dectin, mannose receptor, and Toll-like receptors (22, 27, 37). Given the topology of Candida-phagocyte interactions, phagocyte recognition of the cell wall is clearly important in modulating the host response to yeast. However, our data demonstrate that live Candida suppressed production of ROS in a manner that appeared to override the stimulatory effects of the cell wall and thus may represent a novel immune evasion strategy for Candida.
One possible mechanism through which live C. albicans could decrease ROS in these assays is by scavenging of ROS by SOD, catalase, or other redox-reactive molecules, such as thiols. To investigate this, we compared ROS scavenging by HK and live C. albicans, as well as by S. cerevisiae, which does not suppress ROS production. We found that both types of yeast were more effective at ROS scavenging when alive compared to HK. However, both live C. albicans and live S. cerevisiae scavenged ROS at similar levels. Thus, while scavenging of ROS may be active in these assays, there appears to be an additional mechanism through which C. albicans actively suppresses ROS production by the phagocytes.
ROS production in BMDM from BALB/c mice was not suppressed by live C. albicans, whereas ROS production was suppressed in BMDM from C57BL/6 mice, primary murine PMN, and human phagocytes. The differences observed with BMDM from the two mouse strains raise interesting questions. Responses in BALB/c and C57BL/6 mice have often been compared because of their preponderance to Th2 or Th1 responses, respectively, as demonstrated by the classic experiments in which C57BL/6 mice are more resistant to leishmaniasis than are BALB/c mice (26). This is thought to be due, in part, to the ability of macrophages from C57BL/6 mice to more effectively stimulate a Th1 response (28). Thus, it was not wholly unexpected that the BMDM from these strains would respond differently to C. albicans. In fact, it has been demonstrated that BALB/c and C57BL/6 mice, while both susceptible to gastric candidiasis, produce different cytokine profiles after Candida infection (3). Despite these differences, both strains of mice are considered to be similarly resistant to systemic challenge with Candida (2).
These considerations highlight a limitation of these studies: in analyzing these interactions in vitro, we do not know how well our findings model host-pathogen interactions in vivo. It is possible that, in an immunocompetent host, other components of the immune system can activate phagocytes and allow them to overcome suppression of ROS production, or that ROS is not necessary for the clearance of C. albicans. Nevertheless, the overall finding that suppression occurs in hPBMC and hPMN suggests this process may be important for Candida in some fashion, perhaps by providing an immune evasion mechanism that is important in maintenance of its commensal state.
Several studies have investigated phagocyte ROS production in response to C. albicans. Smail et al. described the release of a soluble inhibitory product from hyphae treated with UV light (31). This product, later identified as adenosine (30), suppressed production of ROS in PMN stimulated by the chemoattractant peptide fMLP, but not by PMA. In contrast, we found that suppression of ROS production occurred for both PMA-stimulated and unstimulated phagocytes. Thus, it seems unlikely that adenosine mediates suppression of ROS production in our system.
Donini et al. investigated production of ROS in dendritic cells (DC) exposed to C. albicans (9); consistent with our results, they found that live C. albicans suppressed PMA-stimulated production of ROS by DC. They also found that treatment of DC with dectin-1 agonists stimulated production of ROS, whereas treatment with agonists for both dectin-1 and the mannose receptor (CD206) resulted in lower production of ROS. Thus, they postulated that activation of the mannose receptor inhibited dectin-1-dependent ROS production.
Our finding that caspofungin treatment of yeast suppresses ROS production is consistent with the postulated inhibition of dectin-1 by mannose receptor signals, because caspofungin-treated Candida have both mannans and 1,3-β-glucan exposed on the surface. However, we found that UV-inactivated Candida, with an intact surface mannoprotein layer, only partially suppressed ROS production. If ROS suppression were solely due to mannose receptor activation, we would expect UV-inactivated yeast to fully suppress ROS production. Furthermore, we found that cell wall ghosts prepared from live Candida stimulated ROS production. The cell wall ghosts were prepared under nondenaturing conditions designed to preserve the native cell wall structure. Thus, it is unlikely that the suppression of ROS production we observed is due to stimulation of the mannose receptor, as it did not occur in the presence of cell wall ghosts, which should have an intact mannoprotein layer. It is possible that the physical disruption used to produce the cell wall ghosts resulted in the loss of some key mannoprotein constituent that might be responsible for suppression of ROS production. However, the bulk mannose/mannoprotein state of the cell wall ghosts should resemble that of live, intact cells. Thus, our data are more consistent with the possibility that suppression of ROS production in phagocytes is mediated by a pathway unrelated to the mannose receptor.
Given the intermediate suppression of ROS production seen with UV-inactivated Candida, which was paralleled by a similar decrease in metabolic activity, it is possible that the mechanism of ROS suppression requires metabolic activity, such as the production of a small metabolite that could function as a phagocyte toxin. Thus, we investigated whether a secreted product might be responsible. We did not find ROS-suppressive activity in spent culture medium or in medium harvested from phagocytes exposed to live Candida. If a secreted product is involved, it is either not stable or is present at a low concentration in the supernatant. The production of a secreted compound that would only function at relatively high concentrations is an interesting possibility: such a compound might be expected to be in high concentration at the site of Candida-phagocyte interactions but be diluted below the active concentration after diffusion into the environment. In this way, Candida could inhibit ROS production in closely associated phagocytes, in a paracrine-like fashion.
Phagocyte-generated ROS are well-known to kill many microbial pathogens; suppression of ROS production might thus represent an important mechanism for Candida to evade phagocytic killing. However, data to suggest that ROS also carry out important signaling functions are accumulating. For example, in some cell lines, activation of the proinflammatory transcription factor NF-κB is dependent on ROS (29). ROS may also be important in regulation of tyrosine phosphorylation as well as activation of mitogen-activated protein kinases, protein kinase C, and phospholipase A2 in leukocytes (10). As these signaling pathways are important in activation of host immune responses, suppression of ROS production by Candida may result in significant modulation of anti-Candida inflammatory responses.
An immunomodulatory role for ROS is supported by studies in which mice deficient in production of both ROS and reactive nitrogen intermediates were inoculated with Candida via the gastrointestinal tract (3). Although all of these mice died after inoculation, the cause of death appeared to be an exaggerated immune response rather than overwhelming fungal infection. Furthermore, phagocytes from normal mice and ROS/reactive nitrogen intermediate-deficient mice were equally able to kill C. albicans in vitro. These data suggest that production of ROS in response to C. albicans may not be important for direct killing of Candida. This is consistent with our finding that Candida suppresses ROS production in phagocytes. Thus, ROS production in response to Candida infection may be more important for regulation of inflammatory responses than for direct anti-Candida effects.
In summary, we have demonstrated that live Candida suppresses production of ROS by phagocytes. Suppression of ROS production does not occur with S. cerevisiae, suggesting that it may be important in the pathogenesis of Candida disease. Suppression of ROS production appears to override stimulatory signals from the cell wall, including 1,3-β-glucan. Thus, the ability of Candida to control host production of ROS may represent an important factor in host-Candida interactions.
Supplementary Material
Acknowledgments
This publication was made possible by grant number UL1 RR024160 from the National Center for Research Resources, a component of the National Institutes of Health, and the NIH Roadmap for Medical Research.
Editor: A. Casadevall
Footnotes
Published ahead of print on 3 November 2008.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Allen, L. A., B. R. Beecher, J. T. Lynch, O. V. Rohner, and L. M. Wittine. 2005. Helicobacter pylori disrupts NADPH oxidase targeting in human neutrophils to induce extracellular superoxide release. J. Immunol. 1743658-3667. [DOI] [PubMed] [Google Scholar]
- 2.Ashman, R. B. 1998. Candida albicans: pathogenesis, immunity and host defence. Res. Immunol. 149281-288. [DOI] [PubMed] [Google Scholar]
- 3.Balish, E., T. F. Warner, P. J. Nicholas, E. E. Paulling, C. Westwater, and D. A. Schofield. 2005. Susceptibility of germfree phagocyte oxidase- and nitric oxide synthase 2-deficient mice, defective in the production of reactive metabolites of both oxygen and nitrogen, to mucosal and systemic candidiasis of endogenous origin. Infect. Immun. 731313-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bliss, J. M., K. P. Basavegowda, W. J. Watson, A. U. Sheikh, and R. M. Ryan. 2008. Vertical and horizontal transmission of Candida albicans in very low birth weight infants using DNA fingerprinting techniques. Pediatr. Infect. Dis. J. 27231-235. [DOI] [PubMed] [Google Scholar]
- 5.Carlyon, J. A., D. Abdel-Latif, M. Pypaert, P. Lacy, and E. Fikrig. 2004. Anaplasma phagocytophilum utilizes multiple host evasion mechanisms to thwart NADPH oxidase-mediated killing during neutrophil infection. Infect. Immun. 724772-4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catz, S. D., M. C. Carreras, and J. J. Poderoso. 1995. Nitric oxide synthase inhibitors decrease human polymorphonuclear leukocyte luminol-dependent chemiluminescence. Free Radic. Biol. Med. 19741-748. [DOI] [PubMed] [Google Scholar]
- 7.Dahlgren, C., A. Karlsson, and J. Bylund. 2007. Measurement of respiratory burst products generated by professional phagocytes, p. 349-363. In M. Quinn, F. DeLeo, and G. Bokoch (ed.), Methods in molecular biology: neutrophil methods and protocols, vol. 412. Humana Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 8.Dignani, M. C., J. S. Solomkin, and E. J. Anaissie. 2003. Candida, p. 195-239. In E. J. Anaissie, M. R. McGinnis, and M. A. Pfaller (ed.), Clinical mycology. Churchill Livingstone, Philadelphia, PA.
- 9.Donini, M., E. Zenaro, N. Tamassia, and S. Dusi. 2007. NADPH oxidase of human dendritic cells: role in Candida albicans killing and regulation by interferons, dectin-1 and CD206. Eur. J. Immunol. 371194-1203. [DOI] [PubMed] [Google Scholar]
- 10.Fialkow, L., Y. Wang, and G. P. Downey. 2007. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic. Biol. Med. 42153-164. [DOI] [PubMed] [Google Scholar]
- 11.Gallois, A., J. R. Klein, L. A. Allen, B. D. Jones, and W. M. Nauseef. 2001. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J. Immunol. 1665741-5748. [DOI] [PubMed] [Google Scholar]
- 12.Gantner, B. N., R. M. Simmons, and D. M. Underhill. 2005. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 241277-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant, C. M., G. Perrone, and I. W. Dawes. 1998. Glutathione and catalase provide overlapping defenses for protection against hydrogen peroxide in the yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 253893-898. [DOI] [PubMed] [Google Scholar]
- 14.Jamieson, D. J., D. W. Stephen, and E. C. Terriere. 1996. Analysis of the adaptive oxidative stress response of Candida albicans. FEMS Microbiol. Lett. 13883-88. [DOI] [PubMed] [Google Scholar]
- 15.Jones, T., N. A. Federspiel, H. Chibana, J. Dungan, S. Kalman, B. B. Magee, G. Newport, Y. R. Thorstenson, N. Agabian, P. T. Magee, R. W. Davis, and S. Scherer. 2004. The diploid genome sequence of Candida albicans. Proc. Natl. Acad. Sci. USA 1017329-7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosalec, I., O. Puel, M. Delaforge, N. Kopjar, R. Antolovic, D. Jelic, B. Matica, P. Galtier, and S. Pepeljnjak. 2008. Isolation and cytotoxicity of low-molecular-weight metabolites of Candida albicans. Front. Biosci. 136893-6904. [DOI] [PubMed] [Google Scholar]
- 17.Kupfahl, C., T. Ruppert, A. Dietz, G. Geginat, and H. Hof. 2007. Candida species fail to produce the immunosuppressive secondary metabolite gliotoxin in vitro. FEMS Yeast Res. 7986-992. [DOI] [PubMed] [Google Scholar]
- 18.Marucha, P. T., R. A. Zeff, and D. L. Kreutzer. 1990. Cytokine regulation of IL-1 beta gene expression in the human polymorphonuclear leukocyte. J. Immunol. 1452932-2937. [PubMed] [Google Scholar]
- 19.Matsui, M. S., N. Muizzuddin, S. Arad, and K. Marenus. 2003. Sulfated polysaccharides from red microalgae have antiinflammatory properties in vitro and in vivo. Appl. Biochem. Biotechnol. 10413-22. [DOI] [PubMed] [Google Scholar]
- 20.McCaffrey, R. L., and L. A. Allen. 2006. Francisella tularensis LVS evades killing by human neutrophils via inhibition of the respiratory burst and phagosome escape. J. Leukoc. Biol. 801224-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Netea, M. G., G. D. Brown, B. J. Kullberg, and N. A. Gow. 2008. An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 667-78. [DOI] [PubMed] [Google Scholar]
- 22.Netea, M. G., N. A. Gow, C. A. Munro, S. Bates, C. Collins, G. Ferwerda, R. P. Hobson, G. Bertram, H. B. Hughes, T. Jansen, L. Jacobs, E. T. Buurman, K. Gijzen, D. L. Williams, R. Torensma, A. McKinnon, D. M. MacCallum, F. C. Odds, J. W. Van der Meer, A. J. Brown, and B. J. Kullberg. 2006. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Investig. 1161642-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odds, F. C. 1988. Candida and candidosis. Baillière Tindall, London, England.
- 24.Quick, K. L., J. I. Hardt, and L. L. Dugan. 2000. Rapid microplate assay for superoxide scavenging efficiency. J. Neurosci. Methods 97139-144. [DOI] [PubMed] [Google Scholar]
- 25.Ram, A. F., E. H. Van den, and F. M. Klis. 1998. Green fluorescent protein-cell wall fusion proteins are covalently incorporated into the cell wall of Saccharomyces cerevisiae. FEMS Microbiol. Lett. 162249-255. [DOI] [PubMed] [Google Scholar]
- 26.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13151-177. [DOI] [PubMed] [Google Scholar]
- 27.Roeder, A., C. J. Kirschning, R. A. Rupec, M. Schaller, and H. C. Korting. 2004. Toll-like receptors and innate antifungal responses. Trends Microbiol. 1244-49. [DOI] [PubMed] [Google Scholar]
- 28.Rossi-Bergmann, B., I. Muller, and E. B. Godinho. 1993. TH1 and TH2 T-cell subsets are differentially activated by macrophages and B cells in murine leishmaniasis. Infect. Immun. 612266-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoonbroodt, S., and J. Piette. 2000. Oxidative stress interference with the nuclear factor-kappa B activation pathways. Biochem. Pharmacol. 601075-1083. [DOI] [PubMed] [Google Scholar]
- 30.Smail, E. H., B. N. Cronstein, T. Meshulam, A. L. Esposito, R. W. Ruggeri, and R. D. Diamond. 1992. In vitro, Candida albicans releases the immune modulator adenosine and a second, high-molecular weight agent that blocks neutrophil killing. J. Immunol. 1483588-3595. [PubMed] [Google Scholar]
- 31.Smail, E. H., D. A. Melnick, R. Ruggeri, and R. D. Diamond. 1988. A novel natural inhibitor from Candida albicans hyphae causing dissociation of the neutrophil respiratory burst response to chemotactic peptides from other post-activation events. J. Immunol. 1403893-3899. [PubMed] [Google Scholar]
- 32.Steinman, H. M. 1980. The amino acid sequence of copper-zinc superoxide dismutase from bakers' yeast. J. Biol. Chem. 2556758-6765. [PubMed] [Google Scholar]
- 33.Tsunawaki, S., L. S. Yoshida, S. Nishida, T. Kobayashi, and T. Shimoyama. 2004. Fungal metabolite gliotoxin inhibits assembly of the human respiratory burst NADPH oxidase. Infect. Immun. 723373-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vazquez-Torres, A., and E. Balish. 1997. Macrophages in resistance to candidiasis. Microbiol. Mol. Biol. Rev. 61170-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner, C., C. Iking-Konert, B. Denefleh, S. Stegmaier, F. Hug, and G. M. Hansch. 2004. Granzyme B and perforin: constitutive expression in human polymorphonuclear neutrophils. Blood 1031099-1104. [DOI] [PubMed] [Google Scholar]
- 36.Wellington, M., K. Dolan, and C. G. Haidaris. 2007. Monocyte responses to Candida albicans are enhanced by antibody in cooperation with antibody-independent pathogen recognition. FEMS Immunol. Med. Microbiol. 5170-83. [DOI] [PubMed] [Google Scholar]
- 37.Wheeler, R. T., and G. R. Fink. 2006. A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog. 2e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.