Abstract
Enterotoxigenic Escherichia coli (ETEC) is a common cause of travelers' and postweaning diarrhea in humans and swine, respectively. The extent to which ETEC damages host cells is unclear. Experiments are presented that probe the ability of porcine ETEC isolates to induce apoptosis and cell death in porcine intestinal epithelial cells. Quantification of host phosphatidylserine exposure following ETEC infection suggested that ETEC induced changes in plasma membrane asymmetry, independent of the expression of the heat-labile enterotoxin. Significant host cell death was not observed. ETEC infection also caused a drastic inhibition of host esterase activity, as measured by calcein fluorescence. While ETEC infection resulted in activation of host caspase 3, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling of DNA double-strand breakage, indicative of late stages of apoptosis, was not observed. Camptothecin-induced apoptosis markedly increased subsequent ETEC adherence. Transfer of cell-free supernatants from apoptotic cells to bacterial inocula prior to infection of naïve cells increased the transcriptional activity of the regulatory region upstream of the K88ac operon and promoted subsequent adherence to host cells.
Enterotoxigenic Escherichia coli (ETEC) causes childhood mortality and travelers' diarrhea in humans (36) and postweaning diarrhea in pigs (32). ETEC adherence to intestinal cells is mediated by proteinaceous, species-specific colonization factors (25). ETEC induces water and electrolyte loss from the intestine due to the activities of several enterotoxins, including the heat-labile enterotoxin (LT), the heat-stable toxins (ST), and the enteroaggregative E. coli heat-stable enterotoxin 1 (32).
Bacterial pathogens often induce host cell apoptosis to promote their survival and dissemination (16, 30). While several E. coli serotypes (1, 4, 9, 15) and toxins (3, 19) have been shown to induce apoptosis of epithelial cells, the extent to which ETEC damages host cells is unclear. The prototypic ETEC isolate H10407 was cytotoxic to but failed to induce apoptosis in the macrophage cell line J774 (as measured by DNA fragmentation) (24). The extent to which LT induces apoptosis has also received considerable attention due to interest in the receptor-binding subunit (LT-B) as a mucosal adjuvant. LT-B induces apoptosis of CD8+ but not CD4+ T cells (37). Some studies indicate that binding of LT-B to GM1 may be sufficient for triggering apoptosis of CD8+ T cells (33), while others suggest that modified LT-B constructs (e.g., H57S) still able to bind GM-1 may fail to trigger apoptosis (14). Yet other studies suggest that LT-mediated apoptosis requires the ADP-ribosylation activity of the LT-A subunit (41).
Overall, the roles of ETEC and LT in mediating apoptosis have not been defined clearly and remain somewhat controversial. The goals of this study were therefore to quantify the ability of ETEC to damage porcine intestinal epithelial cells, to clarify the role of LT in these processes, and to measure any resultant increases in ETEC adherence.
MATERIALS AND METHODS
Chemicals.
All chemicals were obtained from Sigma-Aldrich, unless otherwise indicated.
Bacterial strains.
Bacterial plasmids and strains used in this study are described in Table 1.
TABLE 1.
Bacterial strains used in this study
| Strain | Relevant genotype or characteristics | Source or reference |
|---|---|---|
| G58-1 | O101:K28:NM | 13 |
| 2534-86 | WAM2317 (O8:K87:NM) K88ac LT STb | 2 |
| 2534-86 ΔeltAB | WAM2317 ΔeltAB | 2 |
| 2534-86 ΔeltAB/pLT | Complemented version of 2534-86 ΔeltAB | 2 |
| 2534-86/pKK232-8 | Promoterless CAT plasmid | 38 |
| 2534-86/pK88ac-CAT | K88ac promoter-CAT fusion | This study |
| 3030-2 | K88ac LT STb | 13 |
| B41M | F41 K99 STa | 31 |
| 06-10167 | Unknown | M. Zhao |
| 06-10457 | Unknown | M. Zhao |
| 06-14529 | K88ac LT STb | M. Zhao |
| NADC 1477 | 987P K88ac LT STa STb | M. Zhao |
| NADC 2456 | F18 STa STb Stx2e | M. Zhao |
| 06-7728 | K88ac LT STb | M. Zhao |
| 06-6988 | F18 LT STb | M. Zhao |
| 06-547 | F18 STa STb Stx2e | M. Zhao |
| 06-641 | K88ac LT STb | M. Zhao |
Mammalian cell culture.
IPEC-J2 cells are undifferentiated porcine intestinal epithelial cell lines derived from the small intestines of 1-day-old piglets (35) and were maintained in a humidified incubator in an atmosphere of 5% CO2 at 37°C and cultured in Dulbecco's modified Eagle's medium-F12 supplemented with 5% fetal bovine serum (Atlanta Biologicals), insulin (5 μg/ml), transferrin (5 μg/ml), selenium (5 ng/ml), and epidermal growth factor (5 ng/ml).
Purification of LT.
LT was extracted from E. coli C600, a derivative of E. coli K-12 containing the EWD299 plasmid (6), and purified by one-step chromatography with an immobilized d-galactose column, using the protocol described by Uesaka et al. (43).
Purification of OMVs.
Outer membrane vesicles (OMVs) were harvested from culture supernatants of bacteria grown overnight in 50 ml LB at 37°C with shaking (150 rpm) according to the method of Kesty et al. (21). Bacteria were pelleted by centrifugation (10,000 × g, 10 min, 4°C), and the supernatant was decanted and passed through a 0.2-μm filter. OMVs were collected by ultracentrifugation (150,000 × g, 3 h, 4°C) and resuspended to 1.0 mg/ml in water.
Quantification of IPEC-J2 PS exposure.
Exposure of phosphatidylserine (PS) on the outer leaflet of IPEC-J2 cells was quantified by staining with Alexa fluor 488-annexin V, using a Vybrant apoptosis assay kit as described by the manufacturer (Invitrogen). Propidium iodide (PI) uptake was quantified as a measure of cell death. Where indicated, cells were infected for 4 h with ETEC (multiplicity of infection [MOI], ∼10) or treated with 100 ng/ml LT, 1 μg/ml OMVs (21), or 100 μM camptothecin (7), with or without 100 μM Ac-DEVD-CHO (39). For all flow cytometric analyses, 20,000 events were collected and analyzed with a FACScan flow cytometer (Becton Dickinson).
Quantification of IPEC-J2 esterase activity.
Intracellular esterase activity was quantified by staining cells with calcein-acetoxymethyl ester (calcein-AM) and measuring the resultant conversion to fluorescent calcein, using a Live/Dead viability/cytotoxicity kit as described by the manufacturer (Invitrogen). Where indicated, cells were treated with 100 μg/ml gentamicin to remove extracellular bacteria.
Quantification of IPEC-J2 DNA fragmentation.
DNA fragmentation resulting from ETEC infection was quantified by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining, using an in situ cell death detection kit as described by the manufacturer (Roche), at different time intervals for up to 24 h postinfection.
Immunoblot analyses.
IPEC-J2 cells were seeded in six-well plates (∼3.0 × 105 cells/well) and infected with the indicated ETEC strains at an MOI of ∼10 for 4 h. After infection, cells were washed with phosphate-buffered saline (PBS), scraped into 1 ml PBS, pelleted by centrifugation (8,000 × g, 2 min, 4°C), and lysed in 1% sodium dodecyl sulfate, 10% glycerol, 10% β-mercaptoethanol, 0.01% bromophenol blue, 50 mM Tris, pH 6.8. Equivalent amounts of proteins were resolved through sodium dodecyl sulfate-12% polyacrylamide gels, transferred to nitrocellulose, blocked for 1 h in Odyssey blocking buffer (Li-Cor Biosciences), and incubated overnight with a rabbit anti-active-caspase-3 primary antibody (1:1,000; BD Biosciences). Secondary anti-rabbit IRDye 680 was used at a 1:15,000 dilution in Odyssey blocking buffer containing 0.2% Tween 20 (vol/vol) (Li-Cor) for 30 min at room temperature. After washing of the samples in PBS, images were obtained using an Odyssey infrared imaging system (Li-Cor).
CAT assays.
Chloramphenicol acetyltransferase (CAT) assays were performed as described previously (38), using ETEC 2534-86 grown to an optical density at 600 nm of 0.4 to 0.6 in CFA medium (1% Casamino Acids, pH 7.4, 0.08% yeast extract, 0.4 mM MgSO4, 0.04 mM MnCl2), supplemented where indicated with cell-free supernatants (3% [vol/vol]) derived from donor cells treated with 100 μM camptothecin, with or without 100 μM Ac-DEVD-CHO, for 1 h.
Quantitative bacterial adherence assays.
IPEC-J2 cells were seeded in 24-well plates (∼1.0 × 104 cells/well), grown overnight, and infected with ∼1.0 × 105 CFU/well (MOI, ∼10). After 4 h of incubation, samples were processed for enumeration of adherent bacteria by being washed extensively with PBS and treated with 0.25% trypsin (5 min, 37°C). Trypsinized cells and adherent ETEC were centrifuged (1,000 × g, 5 min), resuspended in 1 ml PBS, serially diluted, plated on LB, and incubated overnight at 37°C. The number of CFU was measured, and data were normalized to the CFU/ml of the bacterial inoculum. Where indicated, cells were treated with 100 μM camptothecin, with or without 100 μM Ac-DEVD-CHO, for 1 h prior to ETEC infection.
For experiments involving pretreatment of ETEC prior to adherence assays, bacteria were grown overnight, subcultured 1:20, and grown for 2 h in the presence of filtered cell supernatants obtained from cells treated with 100 μM camptothecin, with or without 100 μM Ac-DEVD-CHO. Naïve cells were then infected for 4 h with ∼1.0 × 105 CFU/well.
Statistical methods.
Experiments were performed in duplicate on at least three separate occasions. Flow cytometry data are presented as means ± standard deviations (SD) and were analyzed with unpaired Student's t tests. Bacterial adherence assays were analyzed by calculating the median number of CFU in each treatment group and were compared with the Mann-Whitney test to determine significant differences among treatments. P values of <0.05 were considered significant.
RESULTS AND DISCUSSION
ETEC induces an increase in PS exposure.
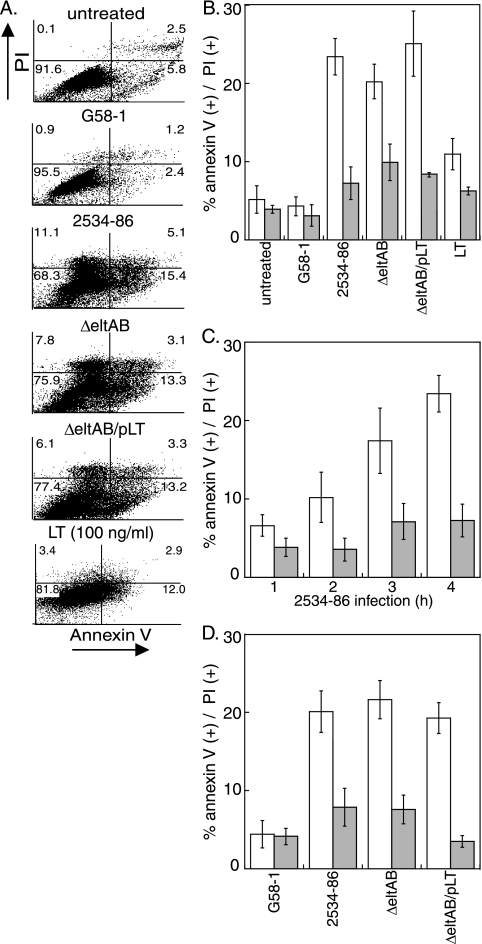
We tested the hypothesis that ETEC alters host membrane asymmetry in intestinal epithelial cells by quantifying changes in annexin V staining (a measure of PS outer leaflet exposure) and PI staining (a measure of cell death) following infection or intoxication of IPEC-J2 cells (Fig. 1A). Infection with wild-type (wt) ETEC 2534-86 (2) significantly increased PS exposure (23.4% ± 2.3% versus 5.2% ± 1.7%; P = 0.02) (Fig. 1B, open bars). Infection with both 2534-86 ΔeltAB (the ΔeltAB strain), an isogenic mutant deficient in LT expression, and a mutant complemented by plasmid-based eltAB expression (the ΔeltAB/pLT strain) also increased PS exposure to similar magnitudes (20.2% ± 2.2% and 25.1% ± 4.2%; P = 0.05 and 0.04, respectively). In contrast, infection with G58-1, an E. coli strain originally isolated from swine feces that lacks any known plasmids, produces no known enterotoxins (12), and is considered to be a commensal strain (5), did not significantly alter PS exposure (4.3% ± 1.2%; P = 0.58). Administration of 100 ng/ml LT only modestly increased PS exposure relative to that in untreated cells (11.8% ± 2.0%; P = 0.20) (Fig. 1B).
FIG. 1.
ETEC induces PS exposure in IPEC-J2 cells. (A) Flow cytometry analysis of PI (y axis) versus annexin V (x axis) staining of IPEC-J2 cells following infection (4 h) with the bacterial strains G58-1, wt 2534-86, 2534-86 ΔeltAB, and 2534-86 ΔeltAB/pLT or intoxication with 100 ng/ml LT. (B) Quantification (% positive cells) (means ± SD; n = 4) of annexin V (open bars) and PI (shaded bars) staining from flow cytometry experiments. (C) Quantification (means ± SD; n = 4) of annexin V (open bars) and PI (shaded bars) staining as a function of time (1 to 4 h) following inoculation of IPEC-J2 cells with wt 2534-86. (D) Quantification (means ± SD; n = 4) of annexin V (open bars) and PI (shaded bars) staining following intoxication of IPEC-J2 cells (4 h) with OMVs (100 ng/ml) purified from the indicated bacterial strains.
Infection with wt ETEC slightly, but statistically insignificantly, increased cell death (as inferred by PI uptake) compared to that of uninfected cells (7.2% ± 2.1% versus 3.9% ± 0.5%; P = 0.21) (Fig. 1B, shaded bars). Infection with both the ΔeltAB and ΔeltAB/pLT strains also modestly increased cell death.
To determine the time course over which PS exposure occurred during ETEC infection, we quantified annexin V and PI staining as a function of time following inoculation with wt 2534-86. The percentage of IPEC-J2 cells that stained positive for annexin V increased as a function of time, from 6.6% ± 1.4% (1 h) to 23.4% ± 2.3% (4 h). The extent of PI staining was not significantly different as a function of time (Fig. 1C).
To determine if PS exposure was due to a secreted factor versus dependent upon ETEC binding to host cells, we intoxicated host cells with OMVs purified from ETEC possessing or lacking LT. LT is highly enriched in OMVs when ETEC is grown to high density in liquid culture (18). Incubation of IPEC-J2 cells with 1.0 μg/ml OMVs purified from 2534-86 significantly increased PS exposure, irrespective of the presence of LT (19% to 23%; P = 0.03) (Fig. 1D). Neither purified LT (Fig. 1B) (P = 0.17) nor OMVs (Fig. 1D) (P = 0.09) significantly increased cell death. These data suggest that porcine ETEC induces alterations in intestinal epithelial cell membrane structure commonly associated with apoptosis (8) and further suggest that LT is unlikely the primary determinant of this activity.
ETEC inhibits host calcein-AM degradation.
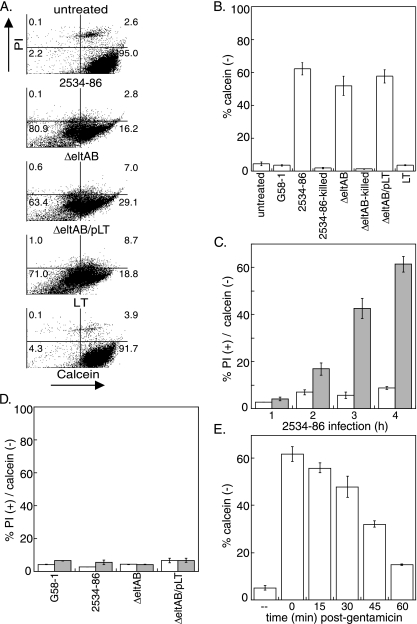
We next sought to determine if ETEC might also alter the metabolic activity of infected cells. We therefore quantified host calcein fluorescence following ETEC infection and subsequent incubation with calcein-AM, a nonfluorescent lipophilic ester that penetrates cellular membranes. Rapid degradation of calcein-AM by cytosolic esterases generates calcein, a fluorescent, non-membrane-permeative molecule (42). Quantification of calcein fluorescence following calcein-AM uptake therefore provides a convenient assay of cell viability and metabolic activity (11).
ETEC infection, irrespective of eltAB expression, significantly inhibited the conversion of calcein-AM to fluorescent calcein compared to that in uninfected cells (80.9% versus 2.2%; P = 0.004) (Fig. 2A, lower left quadrants). We observed a particularly intriguing phenotype, as a large percentage of infected cells failed to stain with PI yet had only limited calcein fluorescence (Fig. 2A). We gated these cells to quantify calcein fluorescence in live (able to exclude PI) cells. Only 4.4% ± 1.1% of live, uninfected cells failed to exhibit intense green fluorescence, whereas 62.0% ± 3.8% of cells infected with the wt strain were markedly reduced in calcein fluorescence (Fig. 2B) (P = 0.001), suggesting a loss of cell viability and/or esterase activity. Infection with both the ΔeltAB (P = 0.003) and ΔeltAB/pLT (P = 0.01) strains also significantly inhibited calcein fluorescence. Neither intoxication with LT (Fig. 2B) or guanylin, an agonist of the guanylyl cyclase C (GC-C) receptor targeted by ST (10; data not shown), nor infection with G58-1 or heat-killed ETEC significantly altered calcein fluorescence (Fig. 2B). To determine the time course over which host calcein fluorescence was diminished as a result of ETEC infection, we quantified calcein fluorescence as a function of time following inoculation with wt 2534-86. The percentage of calcein-negative cells increased from 4.2% ± 0.8% to 62.0% ± 3.8% from 1 to 4 h postinoculation (Fig. 2C). In contrast to the ability of OMVs to induce PS exposure, incubation of IPEC-J2 cells with OMVs purified from ETEC possessing or lacking LT did not alter calcein fluorescence (Fig. 2D).
FIG. 2.
ETEC infection inhibits calcein-AM conversion in IPEC-J2 cells. (A) Flow cytometry analysis of PI staining (y axis) versus calcein fluorescence (x axis) in IPEC-J2 cells following infection with the bacterial strains G58-1, wt 2534-86, 2534-86 ΔeltAB, and 2534-86 ΔeltAB/pLT or intoxication with 100 ng/ml LT. (B) Quantification (means ± SD; n = 4) of the percentages of calcein-negative cells from flow cytometry experiments. (C) Quantification (means ± SD; n = 4) of the percentages of PI-positive (open bars) and calcein-negative (shaded bars) cells as a function of time (1 to 4 h) following inoculation of IPEC-J2 cells with wt 2534-86. (D) Quantification of the percentages of PI-positive (open bars) and calcein-negative (shaded bars) cells following intoxication of IPEC-J2 cells (4 h) with 100 ng/ml OMVs purified from the indicated bacterial strains. (E) Quantification of calcein fluorescence as a function of time after gentamicin treatment (0 to 60 min) of IPEC-J2 cells infected with wt 2534-86.
To determine if host calcein fluorescence would recover following removal of ETEC, we treated host cells with gentamicin to kill any extracellular ETEC and then quantified calcein fluorescence as a function of time after gentamicin treatment. The percentage of calcein-negative cells decreased from 62.0% ± 3.8% to 14.3% ± 0.6% over 1 h following the addition of gentamicin (Fig. 2E), suggesting that host metabolic activity recovers rapidly following removal of ETEC.
Together, these data indicate that porcine ETEC profoundly modulates the membrane asymmetry and metabolic activity of infected host cells. Notably, the observed phenotypes are unlikely to be attributable to either CD14 or the lipopolysaccharide-binding protein (40) because OMVs (rich in lipopolysaccharide) from a nonpathogenic strain (G58-1) had no measurable activity in annexin V assays. In addition, heat-killed ETEC did not alter PS exposure or calcein fluorescence.
ETEC does not induce host DNA double-strand breaks.
To determine if ETEC and/or LT induces double-strand DNA breakage, which typically occurs in the late stages of apoptosis, we employed a TUNEL assay to quantify DNA strand breakage at the single-cell level (24). As shown in Table 2, no significant difference in TUNEL staining was observed as a function of ETEC infection, even at incubation times of up to 24 h postinfection, in agreement with earlier studies of ETEC H10407 infection of J774 macrophages (24), or following incubation with LT. Together, these data suggest that while ETEC may induce changes to host cells that are commonly associated with the early stages of apoptosis, subsequent signal transduction events may be inhibited at a later point in the pathway.
TABLE 2.
ETEC does not induce DNA double-strand breaks in IPEC-J2 cells
| Treatment | % TUNEL-positive cellsa |
|---|---|
| No treatment | 2.7 ± 0.4 |
| DNase | 88.9 ± 2.7 |
| wt 2534-86 | 2.9 ± 0.5 |
| ΔeltAB strain | 5.9 ± 0.7 |
| ΔeltAB/pLT strain | 6.0 ± 0.5 |
| LT (1 μg/ml) | 1.0 ± 0.2 |
The percentage of cells staining positive by TUNEL (mean ± SD; n = 3) was quantified by flow cytometry.
Diversity in porcine ETEC propensity to induce host cell damage.
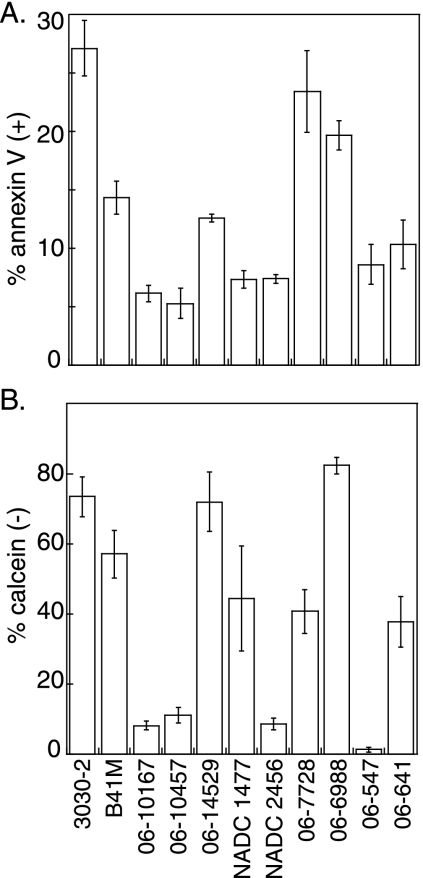
To determine if other ETEC isolates of proven significance to porcine postweaning diarrheal disease are able to induce host cell damage, we infected IPEC-J2 cells with 11 other ETEC strains with diverse toxin and adhesin profiles (Table 1) and then quantified PS exposure and calcein fluorescence. These strains were selected from recent porcine diarrheal disease isolates characterized by the South Dakota Animal Disease Research & Diagnostic Laboratory and/or because they had been examined in experiments probing ETEC virulence in livestock (13, 31). Three strains, 3030-2, 06-7728, and 06-6988, induced PS exposure in >20% of IPEC-J2 cells (Fig. 3A), a level equivalent to that for 2534-86 (Fig. 1B). Two strains, B41M and 06-14529, induced PS exposure in 10 to 15% of IPEC-J2 cells, whereas the remaining six strains did not induce significant host PS exposure.
FIG. 3.
Diversity of porcine ETEC strains in inducing host cell damage. (A) Quantification of annexin V-positive cells (y axis) (means ± SD; n = 4) following a 4-h infection with the indicated bacterial strains (x axis). (B) Quantification of calcein-negative cells (means ± SD; n = 4).
Similarly, significant diversity was observed in the ability of porcine ETEC strains to alter host calcein fluorescence. Four strains (3030-2, B41M, 06-14529, and 06-6988) reduced host calcein fluorescence to levels (≥60% of cells were calcein negative) equivalent to that in 2534-86 (Fig. 3B). Three strains (NADC 1477, 06-7728, and 06-641) had an intermediate phenotype (∼40% of cells were calcein negative), whereas the remaining four strains did not induce significant changes in host calcein fluorescence.
All ETEC strains that induced significant PS exposure and reduced calcein fluorescence, except for 06-6988, display either K88ac or F41 fimbriae and efficiently bind to IPEC-J2 cells (23). The strains that failed to induce significant changes in PS exposure and host calcein fluorescence display F18 or unknown fimbria phenotypes yet still possess various enterotoxins. This observation suggests that ETEC enterotoxins do not play a significant role in mediating early apoptotic changes in these cells and that efficient adherence to host cells is required.
Host cell damage increases ETEC adherence.
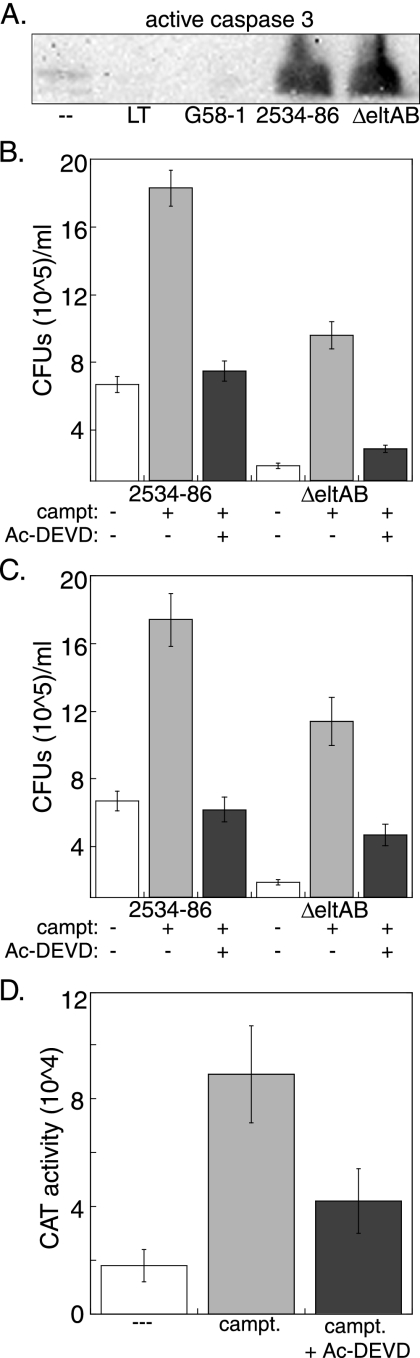
To determine if host caspases are activated in response to ETEC infection, we assayed for the presence of active caspase 3 in IPEC-J2 cells following infection with ETEC or intoxication with LT. Both the wt and ΔeltAB strains activated host caspase 3, whereas infection with G58-1 or intoxication with LT did not (Fig. 4A).
FIG. 4.
Host cell damage promotes ETEC adherence. (A) Immunoblot of IPEC-J2 cells after 1 h of treatment with 100 ng/ml LT or after 4 h of infection with E. coli G58-1 or wt or ΔeltAB ETEC at an MOI of 10. Blots were probed with rabbit polyclonal antisera against active caspase 3. (B) ETEC adherence (mean CFU/ml ± SD) following treatment of IPEC-J2 cells with 100 μM camptothecin (campt), with or without 100 μM Ac-DEVD-CHO, for 1 h prior to ETEC infection. (C) ETEC adherence (mean CFU/ml ± SD) to naïve host cells following prestimulation of bacterial inocula with sterile cell-free supernatants derived from donor cells treated with camptothecin, with or without Ac-DEVD-CHO. (D) Relative expression of K88ac-CAT in the presence of supernatants derived from host cells treated with camptothecin, with or without Ac-DEVD-CHO. Data are plotted as relative CAT activities versus that of the bacterial culture additive.
We next tested the hypothesis that inducing host cell apoptosis would increase ETEC adherence. IPEC-J2 cells were treated with 100 μM camptothecin for 1 h, a concentration and time previously shown to promote significant levels of apoptosis (7). Cells were then infected with ETEC, and subsequent adherence was quantified. Pretreatment of host cells with camptothecin significantly increased adherence of both the wt and ΔeltAB strains (Fig. 4B, gray bars) (P = 0.02), suggesting that the observed phenotype is independent of LT expression.
To determine if adherence promotion was dependent upon activation of host caspases, we cotreated cells with camptothecin and Ac-DEVD-CHO, an inhibitor of caspase 3-dependent pathways (39). Treatment of cells with Ac-DEVD-CHO restored subsequent bacterial adherence to near basal levels (Fig. 4B, black bars). These data indicate that inducing apoptosis in IPEC-J2 cells is sufficient to promote subsequent ETEC adherence and that this phenomenon may require the activity of caspase 3 and/or the downstream effectors of this enzyme.
Since chemical induction of apoptosis increased ETEC adherence, we also tested the hypothesis that ETEC might sense a factor secreted from apoptotic cells to upregulate processes associated with adherence to host cells. We treated IPEC-J2 cells with camptothecin, with or without Ac-DEVD-CHO, obtained and filtered the supernatants from these donor cells, and then added these supernatants to log-phase ETEC cultures. After 2 h of incubation, these ETEC inocula were used in bacterial adherence assays on naïve host cells.
Both the wt and ΔeltAB ETEC strains incubated with supernatants from apoptotic IPEC-J2 cells were enhanced in their subsequent adherence to naïve cells (Fig. 4C, gray bars) (P = 0.02). Cotreatment of donor cells with camptothecin and Ac-DEVD-CHO abolished the adherence-promoting ability of these supernatants (Fig. 4C, black bars). These data raise the possibility that a factor released from host cells following the induction of caspase 3-dependent apoptotic pathways may play a role in regulating gene expression of pathways associated with ETEC adherence. We did not observe an increase in the binding of heat-killed ETEC to apoptotic host cells (data not shown).
To determine if ETEC gene expression was altered in the presence of supernatants from apoptotic IPEC-J2 cells, we measured the transcriptional activity of the regulatory region upstream of the K88ac operon by constructing a fusion to CAT and performing CAT activity assays with ETEC 2534-86 grown in CFA medium supplemented with supernatants derived from IPEC-J2 cells that had been treated with camptothecin, with or without Ac-DEVD-CHO. Notably, transfer of supernatants (3% [vol/vol]) from IPEC-J2 cells treated with camptothecin (Fig. 4D, gray bars), but not those treated with camptothecin plus Ac-DEVD-CHO (Fig. 4D, black bars), resulted in a significant increase in CAT activity.
Concluding remarks.
We have described the preliminary characterization of changes to intestinal cell pathways associated with apoptosis induced by porcine ETEC isolates. ETEC infection, independent of LT, rapidly induces the loss of plasma membrane asymmetry (as measured by annexin V staining) and results in a significant reduction in host metabolic activity (as measured by calcein fluorescence). We found no evidence for host cell DNA fragmentation, in agreement with earlier studies of ETEC infection of J774 macrophages (22), despite our observation of host caspase 3 activation. In contrast to the ability of purified OMVs to stimulate PS exposure, bacterial binding was necessary to reduce calcein fluorescence. While it is likely that this intriguing phenotype is due to reduced intracellular esterase activity, it is also possible that activation of the multidrug resistance transporter (17) or a depletion of intracellular ATP associated with epithelial hyperpermeability (29) might also contribute to these observations.
We did not observe a role for guanylin in inducing apoptosis of IPEC-J2 cells. Others have examined the GC-C receptor, which is activated by ST (27). The addition of exogenous guanylin causes apoptosis in T84 cells (26), and the absence of GC-C in a mouse model of intestinal neoplasia results in increased apoptosis (27). However, other studies have shown that ST causes a delay in cell cycle progression in the absence of apoptosis (34).
It is remarkable that both inducing apoptosis in IPEC-J2 cells and the transfer of apoptotic cell supernatants to ETEC promote subsequent bacterial adherence. Invasive enteric pathogens induce apoptosis in human colon epithelial cells, but with a significant delay (12 to 18 h) (22) compared to the time in our studies. While apoptosis may provide the host with a mechanism to delete damaged epithelial cells (22), it may also benefit pathogens by allowing more time for adaptation to host defenses and by promoting penetration of the protective glycocalyx (28) and sampling of limited receptor epitopes (20). Our observation that ETEC may promote changes in host cells typically associated with the initial but not later stages of apoptosis raises the intriguing possibility that ETEC may modulate epithelial cell viability to promote its adherence and dissemination.
Acknowledgments
We thank Rodney Moxley (University of Nebraska) and Mojun Zhao (South Dakota State University) for generous contributions of bacterial strains.
This work was supported by a grant to P.R.H. from the South Dakota Agricultural Experiment Station (SD00H177-06IHG) and was conducted in part using the South Dakota State University Functional Genomics Core Facility, which receives support from the National Science Foundation/EPSCoR grant 0091948 and from the State of South Dakota.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 20 October 2008.
REFERENCES
- 1.Barnett Foster, D., M. Abul-Milh, M. Huesca, and C. A. Lingwood. 2000. Enterohemorrhagic Escherichia coli induces apoptosis, which augments bacterial binding and phosphatidylethanolamine exposure on the plasma membrane outer leaflet. Infect. Immun. 683108-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berberov, E. M., Y. Zhou, D. H. Francis, M. A. Scott, S. D. Kachman, and R. A. Moxley. 2004. Relative importance of heat-labile enterotoxin in the causation of severe diarrheal disease in the gnotobiotic piglet model by a strain of enterotoxigenic Escherichia coli that produces multiple enterotoxins. Infect. Immun. 723914-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bielaszewska, M., B. Sinha, T. Kuczius, and H. Karch. 2005. Cytolethal distending toxin from Shiga toxin-producing Escherichia coli O157 causes irreversible G2/M arrest, inhibition of proliferation, and death of human endothelial cells. Infect. Immun. 73552-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blomgran, R., L. Zheng, and O. Stendahl. 2004. Uropathogenic Escherichia coli triggers oxygen-dependent apoptosis in human neutrophils through the cooperative effect of type 1 fimbriae and lipopolysaccharide. Infect. Immun. 724570-4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler, J. E., P. Weber, M. Sinkora, D. Baker, A. Schoenherr, B. Mayer, and D. Francis. 2002. Antibody repertoire development in fetal and neonatal piglets. VIII. Colonization is required for newborn piglets to make serum antibodies to T-dependent and type 2 T-independent antigens. J. Immunol. 1696822-6830. [DOI] [PubMed] [Google Scholar]
- 6.Dallas, W. S., D. M. Gill, and S. Falkow. 1979. Cistrons encoding Escherichia coli heat-labile toxin. J. Bacteriol. 139850-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng, W., L. Balazs, D. A. Wang, L. Van Middlesworth, G. Tigyi, and L. R. Johnson. 2002. Lysophosphatidic acid protects and rescues intestinal epithelial cells from radiation- and chemotherapy-induced apoptosis. Gastroenterology 123206-216. [DOI] [PubMed] [Google Scholar]
- 8.Fadok, V. A., D. R. Voelker, P. A. Campbell, J. J. Cohen, D. L. Bratton, and P. M. Henson. 1992. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1482207-2216. [PubMed] [Google Scholar]
- 9.Flynn, A. N., and A. G. Buret. 2008. Caspases-3, -8, and -9 are required for induction of epithelial cell apoptosis by enteropathogenic E. coli but are dispensable for increased paracellular permeability. Microb. Pathog. 44311-319. [DOI] [PubMed] [Google Scholar]
- 10.Forte, L. R., R. M. London, W. J. Krause, and R. H. Freeman. 2000. Mechanisms of guanylin action via cyclic GMP in the kidney. Annu. Rev. Physiol. 62673-695. [DOI] [PubMed] [Google Scholar]
- 11.Fortenberry, J. D., M. L. Owens, M. R. Brown, D. Atkinson, and L. A. Brown. 1998. Exogenous nitric oxide enhances neutrophil cell death and DNA fragmentation. Am. J. Respir. Cell Mol. Biol. 18421-428. [DOI] [PubMed] [Google Scholar]
- 12.Francis, D. H., J. E. Collins, and J. R. Duimstra. 1986. Infection of gnotobiotic pigs with an Escherichia coli O157:H7 strain associated with an outbreak of hemorrhagic colitis. Infect. Immun. 51953-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francis, D. H., and J. A. Willgohs. 1991. Evaluation of a live avirulent Escherichia coli vaccine for K88+, LT+ enterotoxigenic colibacillosis in weaned pigs. Am. J. Vet. Res. 521051-1055. [PubMed] [Google Scholar]
- 14.Fraser, S. A., L. de Haan, A. R. Hearn, H. K. Bone, R. J. Salmond, A. J. Rivett, N. A. Williams, and T. R. Hirst. 2003. Mutant Escherichia coli heat-labile toxin B subunit that separates toxoid-mediated signaling and immunomodulatory action from trafficking and delivery functions. Infect. Immun. 711527-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guignot, J., J. Breard, M. F. Bernet-Camard, I. Peiffer, B. J. Nowicki, A. L. Servin, and A. B. Blanc-Potard. 2000. Pyelonephritogenic diffusely adhering Escherichia coli EC7372 harboring Dr-II adhesin carries classical uropathogenic virulence genes and promotes cell lysis and apoptosis in polarized epithelial Caco-2/TC7 cells. Infect. Immun. 687018-7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guiney, D. G. 2005. The role of host cell death in Salmonella infections. Curr. Top. Microbiol. Immunol. 289131-150. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton, G., E. P. Cosentini, B. Teleky, T. Koperna, J. Zacheri, M. Riegler, W. Feil, R. Schiessel, and E. Wenzi. 1993. The multidrug-resistance modifiers verapamil, cyclosporine A and tamoxifen induce an intracellular acidification in colon carcinoma cell lines in vitro. Anticancer Res. 132059-2063. [PubMed] [Google Scholar]
- 18.Horstman, A. L., and M. J. Kuehn. 2000. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J. Biol. Chem. 27512489-12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, N. L., A. Islur, R. Haq, M. Mascarenhas, M. A. Karmali, M. H. Perdue, B. W. Zanke, and P. M. Sherman. 2000. Escherichia coli Shiga toxins induce apoptosis in epithelial cells that is regulated by the Bcl-2 family. Am. J. Physiol. Gastrointest. Liver Physiol. 278G811-819. [DOI] [PubMed] [Google Scholar]
- 20.Karlsson, K. A. 1998. Meaning and therapeutic potential of microbial recognition of host glycoconjugates. Mol. Microbiol. 291-11. [DOI] [PubMed] [Google Scholar]
- 21.Kesty, N. C., K. M. Mason, M. Reedy, S. E. Miller, and M. J. Kuehn. 2004. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 234538-4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, J. M., L. Eckmann, T. C. Savidge, D. C. Lowe, T. Witthoft, and M. F. Kagnoff. 1998. Apoptosis of human intestinal epithelial cells after bacterial invasion. J. Clin. Investig. 1021815-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koh, S. Y., S. George, V. Brozel, R. Moxley, D. Francis, and R. S. Kaushik. 2008. Porcine intestinal epithelial cell lines as a new in vitro model for studying adherence and pathogenesis of enterotoxigenic Escherichia coli. Vet. Microbiol. 130191-197. [DOI] [PubMed] [Google Scholar]
- 24.Lai, X. H., J. G. Xu, S. Melgar, and B. E. Uhlin. 1999. An apoptotic response by J774 macrophage cells is common upon infection with diarrheagenic Escherichia coli. FEMS Microbiol. Lett. 17229-34. [DOI] [PubMed] [Google Scholar]
- 25.Levine, M. M. 1987. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J. Infect. Dis. 155377-389. [DOI] [PubMed] [Google Scholar]
- 26.Liu, L., H. Li, T. Underwood, M. Lloyd, M. David, G. Sperl, R. Pamukcu, and W. J. Thompson. 2001. Cyclic GMP-dependent protein kinase activation and induction by exisulind and CP461 in colon tumor cells. J. Pharmacol. Exp. Ther. 299583-592. [PubMed] [Google Scholar]
- 27.Mann, E. A., K. A. Steinbrecher, C. Stroup, D. P. Witte, M. B. Cohen, and R. A. Giannella. 2005. Lack of guanylyl cyclase C, the receptor for Escherichia coli heat-stable enterotoxin, results in reduced polyp formation and increased apoptosis in the multiple intestinal neoplasia (Min) mouse model. Int. J. Cancer 116500-505. [DOI] [PubMed] [Google Scholar]
- 28.Mantis, N. J., A. Frey, and M. R. Neutra. 2000. Accessibility of glycolipid and oligosaccharide epitopes on rabbit villus and follicle-associated epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 278G915-G923. [DOI] [PubMed] [Google Scholar]
- 29.Menconi, M. J., A. L. Salzman, N. Unno, R. M. Ezzell, D. M. Casey, D. A. Brown, Y. Tsuji, and M. P. Fink. 1997. Acidosis induces hyperpermeability in Caco-2BBe cultured intestinal epithelial monolayers. Am. J. Physiol. 272G1007-G1021. [DOI] [PubMed] [Google Scholar]
- 30.Monack, D., and S. Falkow. 2000. Apoptosis as a common bacterial virulence strategy. Int. J. Med. Microbiol. 2907-13. [DOI] [PubMed] [Google Scholar]
- 31.Morris, J. A., C. Thorns, A. C. Scott, W. J. Sojka, and G. A. Wells. 1982. Adhesion in vitro and in vivo associated with an adhesive antigen (F41) produced by a K99 mutant of the reference strain Escherichia coli B41. Infect. Immun. 361146-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagy, B., and P. Z. Fekete. 2005. Enterotoxigenic Escherichia coli in veterinary medicine. Int. J. Med. Microbiol. 295443-454. [DOI] [PubMed] [Google Scholar]
- 33.Nashar, T. O., N. A. Williams, and T. R. Hirst. 1996. Cross-linking of cell surface ganglioside GM1 induces the selective apoptosis of mature CD8+ T lymphocytes. Int. Immunol. 8731-736. [DOI] [PubMed] [Google Scholar]
- 34.Pitari, G. M., M. D. Di Guglielmo, J. Park, S. Schulz, and S. A. Waldman. 2001. Guanylyl cyclase C agonists regulate progression through the cell cycle of human colon carcinoma cells. Proc. Natl. Acad. Sci. USA 987846-7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhoads, J. M., W. Chen, P. Chu, H. M. Berschneider, R. A. Argenzio, and A. M. Paradiso. 1994. l-Glutamine and l-asparagine stimulate Na+-H+ exchange in porcine jejunal enterocytes. Am. J. Physiol. 266G828-G838. [DOI] [PubMed] [Google Scholar]
- 36.Rowe, B., J. Taylor, and K. A. Bettelheim. 1970. An investigation of traveller's diarrhoea. Lancet i1-5. [DOI] [PubMed] [Google Scholar]
- 37.Salmond, R. J., R. Williams, T. R. Hirst, and N. A. Williams. 2003. Selective induction of CD8+CD4− thymocyte apoptosis mediated by the B-subunit of Escherichia coli heat-labile enterotoxin. Immunol. Lett. 8843-46. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-SanMartin, C., V. H. Bustamante, E. Calva, and J. L. Puente. 2001. Transcriptional regulation of the orf19 gene and the tir-cesT-eae operon of enteropathogenic Escherichia coli. J. Bacteriol. 1832823-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simizu, S., M. Takada, K. Umezawa, and M. Imoto. 1998. Requirement of caspase-3(-like) protease-mediated hydrogen peroxide production for apoptosis induced by various anticancer drugs. J. Biol. Chem. 27326900-26907. [DOI] [PubMed] [Google Scholar]
- 40.Stein, T., J. S. Morris, C. R. Davies, S. J. Weber-Hall, M. A. Duffy, V. J. Heath, A. K. Bell, R. K. Ferrier, G. P. Sandilands, and B. A. Gusterson. 2004. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 6R75-R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamayo, E., R. Merino, J. Gonzalez-Rojas, R. Marquina, I. Santiuste, J. A. Amado, R. Rappuoli, G. Del Giudice, and J. Merino. 2005. The Escherichia coli heat-labile enterotoxin induces apoptosis of immature lymphocytes in vivo via a glucocorticoid-dependent pathway. Eur. J. Immunol. 353505-3515. [DOI] [PubMed] [Google Scholar]
- 42.Tenopoulou, M., T. Kurz, P. T. Doulias, D. Galaris, and U. T. Brunk. 2007. Does the calcein-AM method assay the total cellular ‘labile iron pool’ or only a fraction of it? Biochem. J. 403261-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uesaka, Y., Y. Otsuka, Z. Lin, S. Yamasaki, J. Yamaoka, H. Kurazono, and Y. Takeda. 1994. Simple method of purification of Escherichia coli heat-labile enterotoxin and cholera toxin using immobilized galactose. Microb. Pathog. 1671-76. [DOI] [PubMed] [Google Scholar]