Abstract
Available vaccines against Brucella spp. are live attenuated Brucella strains. In order to engineer a better vaccine to be used in animals and humans, our laboratory aims to develop an innocuous subunit vaccine. Particularly, we are interested in the outer membrane proteins (OMPs) of B. abortus: Omp16 and Omp19. In this study, we assessed the use of these proteins as vaccines against Brucella in BALB/c mice. Immunization with lipidated Omp16 (L-Omp16) or L-Omp19 in incomplete Freund's adjuvant (IFA) conferred significant protection against B. abortus infection. Vaccination with unlipidated Omp16 (U-Omp16) or U-Omp19 in IFA induced a higher degree of protection than the respective lipidated versions. Moreover, the level of protection induced after U-Omp16 or U-Omp19 immunization in IFA was similar to that elicited by live B. abortus S19 immunization. Flow cytometric analysis showed that immunization with U-Omp16 or U-Omp19 induced antigen-specific CD4+ as well as CD8+ T cells producing gamma interferon. In vivo depletion of CD4+ or CD8+ T cells in mice immunized with U-Omp16 or U-Omp19 plus IFA resulted in a loss of the elicited protection, indicating that both cell types are mediating immune protection. U-Omp16 or U-Omp19 vaccination induced a T helper 1 response, systemic protection in aluminum hydroxide formulation, and oral protection with cholera toxin adjuvant against B. abortus infection. Both immunization routes exhibited a similar degree of protection to attenuated Brucella vaccines (S19 and RB51, respectively). Overall these results indicate that U-Omp16 or U-Omp19 would be a useful candidate for a subunit vaccine against human and animal brucellosis.
Brucellae are facultative intracellular bacteria that infect animals, thereby provoking abortion and infertility and leading to important economic losses. The main pathogenic species for domestic animals are the following: Brucella abortus, responsible for bovine brucellosis; Brucella melitensis, the major etiologic agent of small ruminant brucellosis; and Brucella suis, responsible for swine brucellosis (19). Brucellosis is also a human disease with minimal mortality. However, human brucellosis is one of the commonest zoonotic diseases worldwide, with more than 500,000 new cases annually (28), and it is a weakening disease that requires prolonged antibiotic treatment, often leaving permanent and disabling aftereffects (27, 37). In association with animal infection, human brucellosis has been attributed to at least four of the six recognized Brucella species in terrestrial mammals, with Brucella ovis and Brucella neotomae as the exceptions. Human brucellosis has also been attributed to some marine mammal strains recently (27). Control and eradication of brucellosis in domestic animals have important public health and economic implications. Test-and-slaughter programs in conjunction with vaccination are the most important methods of control of animal brucellosis. Thus, prevention of human brucellosis depends predominantly on the control of the disease in animals (19).
Currently, B. abortus S19 or B. abortus RB51 is used to immunize cattle, whereas the B. melitensis Rev 1 strain is used to immunize goats and sheep. No other vaccines are licensed for other animals, and a human brucellosis vaccine does not exist (33).
In general, the use of live attenuated organisms as vaccines, though a tried and true approach, possesses some problems in terms of safety during vaccine production (e.g., the potential of the organism to revert to its original virulent condition and the shedding of the organism into the environment with a danger to immunocompromised recipients) (10, 22). In fact, attenuated Brucella vaccines have many disadvantages (26, 33). For these reasons, different strategies are being sought that provide safe, nonreplicating vaccines that are easy to reproduce for quality assurance (10, 22). In this context, in our laboratory we have been working on the development of a subunit vaccine against Brucella. In particular we aim to develop a recombinant subunit vaccine that can be more versatile and meet the following criteria: (i) to be applicable to any host, (ii) to elicit immunity at different sites of infection by different application methods, and (iii) to be protective against any species of Brucella. We envision that this vaccine would have to be made of different Brucella proteins.
In the search of novel vaccine targets, we have focused on the outer membrane proteins (OMPs) of B. abortus, Omp16 and Omp19. It has been established by molecular cloning and sequencing that these OMPs have structural features of bacterial lipoproteins. Physicochemical and functional analyses have verified that Omp16 and Omp19 are indeed lipoproteins and that they are surface exposed (34). It has also been reported that these lipoproteins are present in all six Brucella species and all their biovars (18). In addition, it has been demonstrated that monoclonal antibodies (MAbs) against Omp16 or Omp19 can protect mice against a B. ovis challenge (9). We cloned Omp16 or Omp19, expressed them in Escherichia coli, purified recombinant Omp16 and Omp19, and used them as model stimulants. We have shown that they are important mediators of the proinflammatory response elicited by heat-killed B. abortus (18).
Therefore, vaccines based upon recombinant Omp16 and Omp19 lipoproteins could probably elicit a cellular immune response and provide the host protection against Brucella infection. We test this hypothesis in the present work.
MATERIALS AND METHODS
Mice.
Female BALB/c mice (8 to 9 weeks old) obtained from the University of La Plata, Argentina, were acclimated and randomly distributed into experimental groups. The mice were kept in conventional animal facilities and received water and food ad libitum. After inoculation with B. abortus, mice were kept in biosafety level 2 animal facilities.
Bacterial strains.
B. abortus 544, B. abortus 2308 (wild type; smooth, virulent strains), B. abortus S19 (vaccine strain; smooth), B. abortus RB51 (vaccine strain; rough), B. ovis REO 198 (wild type; rough, virulent strain) and B. melitensis H38 (wild type; smooth, virulent strain) were obtained from our own laboratory collection (12, 15, 35). Bacteria were grown, and inocula were prepared as described previously (12, 15, 21, 35). Brucella strain manipulations were performed in biosafety level 3 facilities.
Antigen (Ag) production.
Methods for cloning, expression in E. coli, and purification of recombinant lipidated Omp16 (L-Omp16) and L-Omp19 and unlipidated Omp16 (U-Omp16) and U-Omp19 from B. abortus have been previously described (18). Briefly, the complete sequence information of both proteins was previously reported by Tibor et al. (34). Specific primers for the entire sequence of L-Omp16 and of L-Omp19 were designed. U-Omp16 and U-Omp19 were cloned using different forward primers, flanking the complete gene of the protein and avoiding the putative signal peptide for lipidation. B. abortus 544 genomic DNA was used as a template for PCR. The products were cloned into the pET 22b+ vector (Novagen, Madison, WI), resulting in the plasmids pET-L-Omp16, pET-U-Omp16, pET-L-Omp19, and pET-U-Omp19 containing the genes with a COOH-terminal six-histidine tag. The recombinant OMPs were successfully expressed in E. coli BL21(DE3). Recombinant L-Omp16 and L-Omp19 were isolated from bacterial membranes by sonication and selective extraction by phase partitioning with 2% Triton X-114. U-Omp16 and U-Omp19 were isolated from bacterial cytoplasm by sonication. These preparations were further purified by affinity chromatography with a Ni-agarose resin (Qiagen, Dorking, United Kingdom). Expression and purification of the recombinant proteins were monitored by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by silver staining. The identities of the OMPs were confirmed by Western blotting with anti-Omp16 and anti-Omp19 MAbs as described previously (18). To eliminate lipopolysaccharide (LPS) contamination, OMPs were adsorbed with Sepharose-polymyxin B (Sigma-Aldrich, St. Louis, MO). They contained <0.25 endotoxin units/μg of protein, as assessed by Limulus amebocyte assay (Associates of Cape Cod, Woods Hole, MA). Hot saline (HS) extract from B. ovis REO 198 was obtained as described previously (21). Protein concentration was determined by the bicinchoninic acid method (Pierce, Rockford, IL).
Immunization of mice.
Groups of 5 to 8 mice were anesthetized with methoxyfuorane (Mallinckrodt, Phillipsburg, NJ) and immunized using the following protocols. (i) Mice were immunized by the intraperitoneal (i.p.) route at days 0 and 15 with 30 μg of L-Omp16, L-Omp19, or phosphate-buffered saline ([PBS] negative control) in incomplete Freund's adjuvant (IFA; Sigma), and as a positive control, another group of mice was immunized once subcutaneously at day 15 with 8 × 108 formalin-killed B. melitensis H38 CFU in IFA. (ii) Mice were immunized i.p. at days 0 and 15 with 10 μg of L-Omp16, U-Omp16, L-Omp19, U-Omp19, U-Omp16 plus U-Omp19, or PBS in IFA. For the protection experiments other mice were immunized i.p at day 15 with 1 × 104 live B. abortus S19 CFU (positive control). (iii) Mice were immunized i.p. at days 0 and 15 with 10 μg of L-Omp16, U-Omp16, L-Omp19, U-Omp19, or PBS adsorbed in aluminum hydroxide gel or i.p at day 15 with 1 × 104 live B. abortus S19 CFU. Aluminum hydroxide was kindly provided by Instituto Biológico Argentino S.A.I.C. An aluminum hydroxide suspension (0.6 mg/ml) was mixed with U-Omp16, L-Omp16, U-Omp19, or L-Omp19 and incubated for 30 min at room temperature. The aluminum hydroxide-adsorbed Ag was washed, and the final pellet was suspended in PBS. (iv) Mice were immunized intragastrically with 100 μg of U-Omp16 or U-Omp19 mixed with 5 μg of cholera toxin (CT) or CT with PBS in 200 μl of 0.1 M bicarbonate buffer (pH 8) as described in Delpino et al. (16). Animals received three consecutive weekly immunizations. Mice used as positive controls in these protection experiments were intragastrically immunized once at day 21 with 1.2 × 109 CFU of live B. abortus S19 or 0.5 × 109 CFU of live B. abortus RB51.
In all cases, serum was obtained at 15, 30, 45, and 75 days after the first immunization by retro-orbital bleeding under anesthesia. Thirty days after the last immunization, mice were challenged with virulent Brucella organisms or were sacrificed by cervical dislocation to perform immunity tests. All experiments were conducted at least twice.
Protection experiments.
Protection experiments were performed as described previously (15, 16, 35). Briefly, 30 days after the last immunization, mice from each group were challenged i.p. with 4 × 104 CFU of B. abortus 544 (immunized groups i, ii, and iii) or intragastrically with 1.3 × 1010 CFU of B. abortus 544 or 3 × 108 CFU of B. abortus 2308 (immunized group iv). Four weeks later, the infected mice were sacrificed by cervical dislocation, their spleens were removed aseptically and homogenized, and dilutions were plated and incubated as previously described (15, 35) to determine the number of Brucella CFU per spleen. To differentiate the B. abortus 544 or B. abortus 2308 CFU (challenging strains) from attenuated vaccine strains, the CFU number was calculated by subtraction of numbers obtained under nonselective and selective conditions and then logarithmically transformed as described by Delpino et al. (15). Units of protection were obtained by subtracting the mean log10 CFU of the experimental group from the mean log10 CFU of the corresponding negative control group.
ELISA.
The titers of serum immunoglobulin G (IgG), IgG1, and IgG2a isotypes with specificity to Omp16 or Omp19 were determined by enzyme-linked immunosorbent assay (ELISA). Polystyrene plates (immunoplate with MaxiSorp surface Nunclon; Nunc, Roskilde, Denmark) were coated with purified recombinant U-Omp16 or U-Omp19 (0.3 or 0.5 μg/well, respectively) in PBS. After a 1-h incubation at room temperature, plates were washed four times in PBS with 0.05% Tween 20 (PBS-T) and blocked overnight at 4°C with 200 μl of PBS containing 3% skim milk per well. Then, plates were incubated with serial dilutions of the serum for 1 h at room temperature and washed four times. Serum was diluted in PBS-T containing 1.5% skim milk. Isotype-specific goat anti-mouse horseradish peroxidase conjugates (Santa Cruz Biotechnology, Santa Cruz, CA) were added (50 μl/well) at appropriate dilutions. After 1 h of incubation at room temperature, plates were washed four times, and 50 μl/well of substrate solution (200 μmol of o-phenylenediamine and 0.04% H2O2) was added to each well. After 20 min of incubation at room temperature, the enzyme reaction was stopped by the addition H2SO4, and the absorbance was measured at 492 nm. The cutoff value for the assay was calculated as the mean specific optical density plus 3 standard deviations (SD) from 20 sera from nonimmunized mice assayed at a 1:100 dilution. The specific titer of serum was calculated as the reciprocal of the highest serum dilution with a higher optical density than the cutoff value.
The presence of serum B. abortus-binding IgG antibodies (Abs) was determined as explained before with some changes. In this case, polystyrene plates were coated overnight at 4°C with 100 μl with 3.33 × 108 CFU of heat-killed B. abortus strain 544 or RB51 in 0.1 M sodium carbonate buffer, pH 9.5, and blocked for 2 h at room temperature with 200 μl of PBS containing 3% skim milk per well. Then, plates were incubated with a 1:500 dilution of serum in PBS-T containing 1.5% skim milk for 1 h at room temperature. The ELISA was developed as described for the ELISA of specific Omp16 or Omp19 Abs.
Determination of cytokine production.
Spleen cells from immunized and control mice were homogenized and suspended in RPMI 1640 medium (Gibco BRL, Life Technologies, Grand Island, NY) supplemented with 10% fetal calf serum (Gibco), 1 mM sodium pyruvate, 2 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml (complete medium). Cells were cultured at 4 × 106 cells/ml in duplicate with HS extract (20 μg/ml), concanavalin A (ConA) (5 μg/ml) (Sigma), or complete medium alone. Cultures were incubated at 37°C in a humidified atmosphere (5% CO2 and 95% air) for 72 h. At the end of the incubation, cell culture supernatants were collected, aliquoted, and frozen at −70°C until analyzed for gamma interferon (IFN-γ), interleukin-2 (IL-2), IL-4, IL-5, and IL-10 production by sandwich ELISA using paired cytokine-specific MAbs, according to the manufacturer's instructions (PharMingen, San Diego, CA).
Intracellular IFN-γ production.
Splenocytes from vaccinated and control mice were cultured for 5 days with mitomycin C-treated A20J cells (American Type Culture Collection, Manassas, VA) that were preincubated for 16 h with 10 μg/ml of U-Omp16 (A20J-Omp16) or U-Omp19 (A20J-Omp19). After primary stimulation cells were extensively washed, plated at 4 × 106 cells/ml, and restimulated for 6 h with mitomycin C-treated A20J-Omp16 and U-Omp16 (10 μg/ml) or mitomycin C-treated A20J-Omp19 cells and U-Omp19 (10 μg/ml) or complete medium. As a positive control for the assay, ionomycin (750 ng/ml; Sigma) plus phorbol myristate acetate (20 ng/ml; Sigma) was added in parallel (data not shown). Brefeldin A (10 μg/ml; Sigma) was added to the cells for the last 4 h of culture. The cells were then harvested, incubated with Cy-chrome-conjugated anti-CD8+ (clone 53-6.7; PharMingen) and fluorescein isothiocyanate-conjugated anti-CD4+ (clone GK1.5; PharMingen) and washed with PBS-2% fetal calf serum. To determine IFN-γ production, cells were additionally labeled with phycoerythrin-conjugated anti-IFN-γ (clone XMG1.2) (PharMingen) after treatment with a Fix & Perm kit (PharMingen), according to the manufacturer's instructions. Irrelevant isotype-matched Abs were incubated in parallel with all experimental samples. For each sample at least 300,000 events were analyzed on a FACScan flow cytometer (BD Biosciences, Mountain View, CA).
In vivo T-cell depletion.
For depletion of CD4+ or CD8+ T cells, vaccinated mice were injected i.p. with 200 μg of purified GK1.5 or GK2.43 (American Type Culture Collection) MAbs, respectively, on days −2, 1, 4, 7, and 10 after bacterial challenge. The efficacy of cell depletion was determined by flow cytometric analysis of splenocytes and was greater than 98% (data not shown). Nonspecific rat IgG-purified MAb was used as an isotype control.
DTH test.
Two weeks after the last intragastric immunization, delayed-type hypersensitivity (DTH) tests were performed as an index of cell-mediated immunity. Thirty micrograms of U-Omp16 or U-Omp19 was injected into one footpad, and PBS was injected into the contralateral footpad as a negative control. The DTH reaction was quantified 72 h later by using a digital caliper with a precision of 0.01 mm to measure the difference between the thicknesses of footpads. The mean increase in footpad thickness (expressed in mm) was calculated according to the following formula: thickness of left footpad challenged with Ag − thickness of right footpad challenged with saline.
Statistical analysis.
The CFU data were logarithmically transformed, and statistical analysis was conducted using analysis of variance followed by Dunnett's posthoc test. A nonparametric Mann-Whitney U test was also used to compare humoral and cellular responses. DTH was evaluated by one-way analysis of variance, followed by Bonferroni's multiple comparison test. The analysis was performed using the InStat program (Graphpad software, version 4; San Diego, CA).
RESULTS
L-Omp16 or L-Omp19 immunization in IFA induces specific humoral immune responses and protection against B. abortus infection.
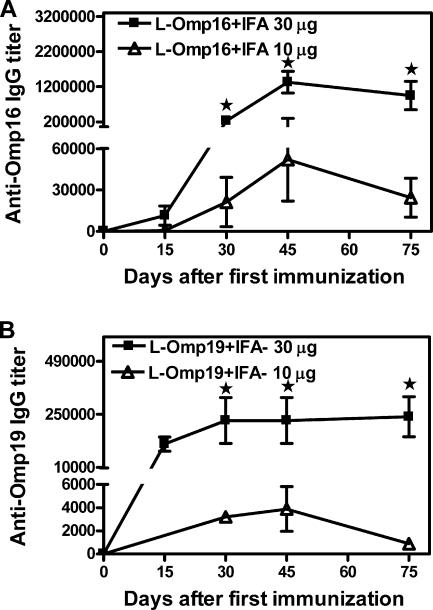
BALB/c mice were vaccinated with purified recombinant L-Omp16 or L-Omp19 protein plus IFA adjuvant and then challenged i.p. with B. abortus 544. Other groups of mice were immunized with the adjuvant preparation plus PBS or with whole Brucella as negative and positive controls, respectively. As a first approach, we evaluated the efficacy of both preparations using 30 μg of each purified lipidated protein. At this dose, immunized mice developed a strong specific humoral immune response, reaching an anti-Omp16 IgG mean titer of 1,331,200 and an anti-Omp19 IgG mean titer of 221,800 at the day of challenge (Fig. 1). Immunization with both recombinant lipoproteins conferred significant (P < 0.01, versus PBS) protection against B. abortus infection (1.78 and 1.72 units of protection with L-Omp16 and L-Omp19, respectively) (Table 1). The positive control, H38 in IFA, induced 2.02 units of protection (Table 1). Obtaining large amounts of the purified recombinant lipidated OMPs is very hard and time-consuming work; thus, we considered trying a dose of immunization lower than 30 μg. Immunization with 10 μg of each OMP induced lower levels of specific Abs than the 30-μg dose (anti-Omp16 IgG mean titer of 52,000 and anti-Omp19 IgG mean titer of 3,887 at the day of challenge) (Fig. 1). However, the degrees of protection afforded when the immunization dose was 10 μg (L-Omp16, 1.44 units of protection; L-Omp19, 1.38 units of protection) (Table 1) were still significant (P < 0.01, versus PBS). Both L-Omp16 and L-Omp19 Ags induced a lower (P < 0.05) degree of protection than the attenuated S19 vaccine (2.13 units of protection) (Table 1).
FIG. 1.
Kinetics of the humoral immune response elicited after immunizations with L-Omp16 (A) or L-Omp19 (B). BALB/c mice were immunized with 10 μg (▵) or 30 μg (▪) of L-Omp16 or L-Omp19 protein in IFA i.p. at days 0 and 15, and bled retro-orbitally at the indicated days after the first immunization. Anti-Omp16 or anti-Omp19 IgG titers were determined by ELISA. Each point represents the mean Ab titer from 5 mice in each group ± standard error of the mean. Data are representative of two separate experiments. Star, significantly different from the 10-μg immunized group (P < 0.05).
TABLE 1.
Protection against B. abortus 544 in BALB/c mice immunized with L-Omp16 or L-Omp19 protein in IFA
| Vaccine | Protection against B. abortus 544 with the indicated vaccine dosea
|
|||
|---|---|---|---|---|
| 30 μg
|
10 μg
|
|||
| Log10 CFU of bacteriaa | Protection (U) | Log10 CFU of bacteriaa | Protection (U) | |
| H38+IFA | 2.88 ± 0.17b | 2.02 | ND | ND |
| B. abortus S19 | ND | ND | 2.90 ± 0.21b | 2.13 |
| L-Omp16+IFA | 3.12 ± 0.16b | 1.78 | 3.59 ± 0.36b,d | 1.44 |
| L-Omp19+IFA | 3.18 ± 0.12b | 1.72 | 3.65 ± 0.63b,d | 1.38 |
| PBS+IFA | 4.90 ± 0.40c | 0 | 5.03 ± 0.38d | 0 |
The content of bacteria in spleens is represented as the mean log CFU ± standard deviation per group (n = 5 mice per group). ND, not done.
Significantly different from PBS-immunized mice (P < 0.01, estimated by Dunnett's test).
Significantly different from H38-immunized mice (P < 0.01, estimated by Dunnett's test).
Significantly different from S19-immunized mice (P < 0.05 estimated by Dunnett's test).
U-Omp16 and U-Omp19 vaccination in IFA also confers protection against B. abortus infection.
It is important to bear in mind that the purification yield of the lipidated version of the recombinant proteins is much lower than that obtained with U-Omp16 or U-Omp19. Moreover, extra LPS depletion rounds are needed to ensure that the recombinant lipoproteins are LPS free (unpublished results). With this concern in mind, we thought that it would be interesting to evaluate whether the unlipidated versions of both proteins also conferred protection against Brucella infection. For this purpose, animals were immunized i.p. with U-Omp16 or U-Omp19 protein plus IFA. Auspiciously, immunization with U-Omp16 or U-Omp19 conferred significant protection against B. abortus infection (P < 0.01 versus PBS). Of note, vaccination with the unlipidated versions induced slightly higher degrees of protection than the respective lipidated versions (1.97 units of protection for U-Omp16 versus 1.50 for L-Omp16 and 1.85 units of protection for U-Omp19 versus 1.48 for L-Omp19) (Table 2). Furthermore, the degree of protection induced after U-Omp16 or U-Omp19 immunization, both in IFA, was similar (P > 0.05) to that elicited by immunization with the attenuated control vaccine S19 (2.18 units of protection). In contrast, the degree of protection elicited by the lipidated versions in IFA was statistically different (P<0.05) from that elicited by S19 (Table 2).
TABLE 2.
Protection against B. abortus 544 in BALB/c mice immunized with L-Omp16, U-Omp16, L-Omp19, or U-Omp19 protein with IFA
| Vaccine (dose [μg]) | Adjuvant | Log10 CFU of B. abortus 544a | Protection (U) |
|---|---|---|---|
| B. abortus S19 | None | 2.90 ± 0.18b | 2.18 |
| L-Omp16 (10) | IFA | 3.58 ± 0.39b,c | 1.50 |
| U-Omp16 (10) | IFA | 3.11 ± 0.23b | 1.97 |
| L-Omp19 (10) | IFA | 3.65 ± 0.64b,d | 1.43 |
| U-Omp19 (10) | IFA | 3.23 ± 0.65b | 1.85 |
| PBS | IFA | 5.08 ± 0.43d | 0 |
The content of bacteria in spleens is represented as the mean log CFU ± standard deviation per group (n = 8 mice per group).
Significantly different from PBS-immunized mice (P < 0.01 estimated by Dunnett's test).
Significantly different from S19-immunized mice (P < 0.05 estimated by Dunnett's test).
Significantly different from S19-immunized mice (P < 0.01 estimated by Dunnett's test).
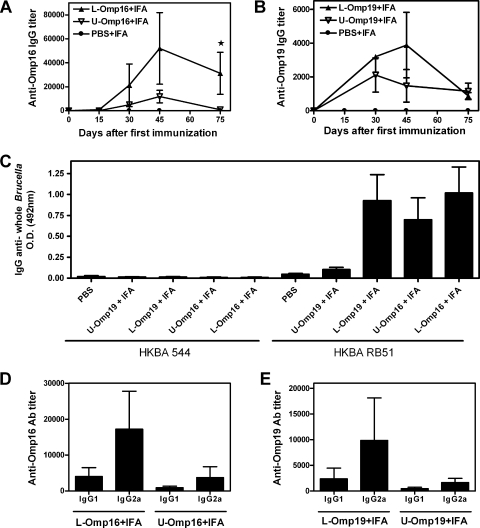
As seen in Fig. 2A, immunization with L-Omp16 in IFA induced a higher (P < 0.05) specific IgG response than immunization with U-Omp16 plus IFA at day 45 after the first immunization and thereafter. A similar pattern was observed in the serum of mice immunized with L-Omp19 plus IFA versus U-Omp19 plus IFA, but in this case, the differences were not statistically different (Fig. 2B). The elicited Abs recognized whole heat-killed B. abortus RB51 organisms (rough phenotype) but not the heat-killed B. abortus 544 (smooth phenotype) (Fig. 2C). These results could be explained by the known steric hindrance of LPS on smooth strains (8). In relation to this concern, Abs against these OMPs are not expected to confer protection against a smooth strain like B. abortus; however, we tested the profile of the elicited humoral responses as a marker of the evoked cellular immune responses (IgG1, T helper 2 [Th2]; IgG2a, Th1) (1). In all cases (after vaccination with lipidated or unlipidated versions) specific anti-OMP IgG2a titers were greater than those of IgG1 (Fig. 2D and E).
FIG. 2.
Comparison of specific humoral immune responses induced after immunization with L-Omp16, U-Omp16, L-Omp19, or U-Omp19. Mice were immunized with 10 μg of lipidated (▴) or unlipidated (▿) Omp16 or Omp19 in IFA i.p. at days 0 and 15 and bled retro-orbitally at the indicated days after the first immunization. Anti-Omp16 (A) or anti-Omp19 (B) IgG titers were evaluated by ELISA. Each point represents the mean Ab titer from 5 mice in each group ± standard error of the mean. Star, significantly different from U-Omp-immunized group (P < 0.05). IgG Abs against HKBA 544 (smooth strain) or HKBA RB51 (rough strain) were determined at the day of challenge (C). Each bar represents the mean absorbance (optical density at [OD] at 492 nm) measured from 5 mice in each group ± standard error of the mean. IgG1 and IgG2a isotype profiles of mice inoculated with 10 μg of Omp16 (D) or Omp19 (E). Specific isotype titers were evaluated by ELISA. Data are representative of two separate experiments. Each bar represents the mean Ab titer ± standard error of the mean of five animals.
U-Omp16 or U-Omp19 in IFA vaccination induces specific CD8+ as well as CD4+ T cells that produce IFN-γ and mediate protection against B. abortus infection.
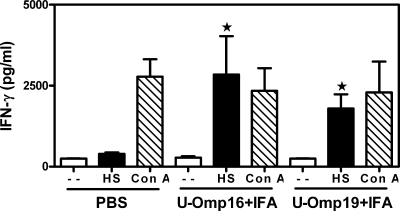
It is well known that cellular immune responses are important in vaccine-mediated protection against B. abortus infection; in particular, Th1 responses are considered crucial (4). We first studied the IFN-γ production of splenocytes from mice immunized with PBS plus IFA, U-Omp16 plus IFA, or U-Omp19 plus IFA in response to a known Brucella membrane extract that contains native OMPs (HS extract). As shown in Fig. 3, spleen cells from U-Omp16- or U-Omp19-immunized mice secreted IFN-γ in response to HS extract. In contrast, these cells did not produce IL-2, IL-4, IL-5, or IL-10 after in vitro stimulation with HS (data not shown). IFN-γ production from the splenocytes of mice vaccinated with U-Omp16 plus IFA or with U-Omp19 plus IFA in response to the Brucella extract was statistically different (P < 0.01) from the amount secreted by the splenocytes of PBS-vaccinated mice. ConA induced the production of the corresponding cytokines in all groups (Fig. 3).
FIG. 3.
IFN-γ production in spleen cells from mice immunized with PBS or with U-Omp16, or U-Omp19 protein plus IFA. Spleen cells were cultured at 4 × 106 cells/ml with HS extract (20 μg/ml), ConA (5 μg/ml), or complete medium alone (−) for 72 h. Each sample was assayed in duplicate wells. IFN-γ in these culture supernatants was measured by sandwich ELISA. Data represent the mean ± standard error of the mean from each group of five mice. Data are representative of two separate experiments. Star, significantly different from the same stimulus in PBS-immunized mice (P < 0.05).
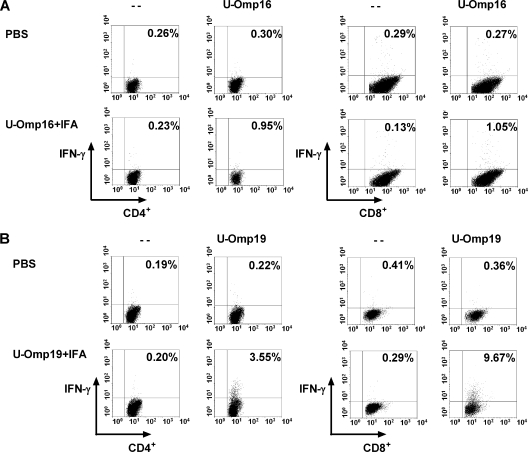
To further determine the contribution of CD8+ and CD4+ T cells in the Ag-specific IFN-γ production, intracellular IFN-γ production was determined at the single-cell level by three-color flow cytometric analysis. Omp16 stimulation of splenocytes from mice immunized with U-Omp16 plus IFA induced intracellular IFN-γ production in both CD4+ (0.95%) and CD8+ T cells (1.05%) (Fig. 4A). In a similar way, vaccination with U-Omp19 plus IFA induced IFN-γ-producing CD4+ T cells (3.55%) and CD8+ T cells (9.67%) in the spleen in response to Omp19 (Fig. 4B). Vaccination with PBS plus IFA induced only background levels of intracellular IFN-γ production in both CD4+ and CD8+ T cells (0.26 to 0.36%) (Fig. 4).
FIG. 4.
Flow cytometric analysis of intracellular IFN-γ production by CD4+ or CD8+ T cells. Spleen cells from mice immunized with PBS or with U-Omp16 or U-Omp19 protein plus IFA were cultured with mitomycin C-treated A20J-Omp16 or mitomycin C-treated A20J-Omp19 cells (stimulator cells) for 5 days. Then cells were restimulated for 6 h with U-Omp16 (10 μg/ml) and stimulator cells or with U-Omp19 (10 μg/ml) and stimulator cells or complete medium alone (−). Brefeldin A was added for the last 4 h, and then cells were stained for CD4+ and CD8+. Cells were then fixed, permeabilized, and stained for intracellular IFN-γ. Numbers in the upper quadrant represent the percentage of CD4+ or CD8+ T cells positive for IFN-γ.
Given these results, we sought to determine if vaccination would induce CD4+ as well as CD8+ T cells that may contribute in protection in vivo. For this purpose mice vaccinated with U-Omp16 plus IFA or U-Omp19 plus IFA were injected i.p. with MAb 2.43 to deplete CD8+ T cells, with GK1.5 to deplete CD4+ T cells, or with the isotype MAb as a control. When treated with the isotype control MAb, mice given U-Omp16 plus IFA or U-Omp19 plus IFA exhibited a significant (P < 0.01 versus PBS) degree of protection against B. abortus (1.38 or 1.58 units of protection, respectively) (Table 3). Vaccination with U-Omp16 plus IFA induced 0.66 units of protection in mice depleted of CD4+ T cells (P > 0.05, versus PBS) and 0.79 units of protection in CD8+-depleted mice (P > 0.05, versus PBS). Similarly, vaccination with U-Omp19 plus IFA induced 0.68 units of protection in mice depleted of CD4+ T cells (P > 0.05, versus PBS) and 0.65 units of protection in CD8+-depleted mice (P > 0.05, versus PBS) (Table 3). Therefore, depletion of CD4+ T or CD8+ T cells in mice immunized with U-Omp16 plus IFA or U Omp19 plus IFA resulted in a loss of the vaccine-elicited protection.
TABLE 3.
Contribution of CD4+ and CD8+ T lymphocytes, induced by immunization with U-Omp16 or U-Omp19 protein in IFA, to protection against B. abortus 544 in BALB/c mice
| Vaccine | Treatment | Log10 CFU of B. abortus 544a | Protection (U) |
|---|---|---|---|
| B. abortus S19 | None | 3.86 ± 0.46b | 2.00 |
| U-Omp16+IFA | IgG | 4.48 ± 0.55b | 1.38 |
| U-Omp16+IFA | Anti-CD4+ | 5.20 ± 0.51c | 0.66 |
| U-Omp16+IFA | Anti-CD8+ | 5.07 ± 0.46c | 0.79 |
| U-Omp19+IFA | IgG | 4.28 ± 0.48b | 1.58 |
| U-Omp19+IFA | Anti-CD4+ | 5.18 ± 0.59c | 0.68 |
| U-Omp19+IFA | Anti-CD8+ | 5.21 ± 0.54c | 0.65 |
| PBS+IFA | None | 5.86 ± 0.06c | 0 |
The content of bacteria in spleens is represented as the mean log CFU ± standard deviation per group (n = 5 mice per group).
Significantly different from PBS-immunized mice (P < 0.01 estimated by Dunnett's test).
Significantly different from S19-immunized mice (P < 0.01 estimated by Dunnett's test).
As both proteins induced protection, we decided to study if the combination of these proteins would induce greater levels of protection than the administration of a single Ag, giving a synergic or an additive effect. For this purpose, animals were immunized with U-Omp16 plus IFA, with U-Omp19 plus IFA, or with U-Omp16 plus U-Omp19 and IFA, and the levels of protection afforded were evaluated. As seen in Table 4, coimmunization with both proteins induced slightly greater protection than immunization with each individual protein, but the differences were not statistically supported. Again, all the subunit vaccines evaluated conferred similar (P > 0.05) levels of protection against B. abortus S19.
TABLE 4.
Protection against B. abortus 544 in BALB/c mice immunized with U-Omp16, U-Omp19, or U-Omp16 plus U-Omp19 antigen in IFA
| Vaccine (dose [μg]) | Adjuvant | Log10 CFU of B. abortus 544a | Protection (U) |
|---|---|---|---|
| B. abortus S19 | None | 4.18 ± 0.19b | 2.27 |
| U-Omp16 (10) | IFA | 4.84 ± 0.50b | 1.61 |
| U-Omp19 (10) | IFA | 4.67 ± 0.59b | 1.78 |
| U-Omp16+U-Omp19 (10 each) | IFA | 4.56 ± 0.15b | 1.89 |
| PBS | IFA | 6.45 ± 0.14c | 0 |
The content of bacteria in spleens is represented as the mean log CFU ± standard deviation per group (n = 5 mice per group).
Significantly different from PBS-immunized mice (P < 0.01 estimated by Dunnett's test).
Significantly different from S19-immunized mice (P < 0.01 estimated by Dunnett's test).
U-Omp16 or U-Omp19 vaccination also induces systemic protection in aluminum hydroxide and oral protection with CT adjuvant against B. abortus infection.
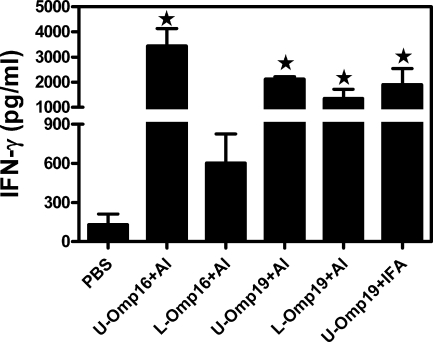
As stated in the introduction, a human Brucella vaccine does not exist, and the only adjuvants authorized for human vaccines are aluminum compounds (5). For this reason we sought to evaluate the protection afforded when the Ags were adsorbed in aluminum hydroxide. Therefore, animals were immunized with L-Omp16, U-Omp16, L-Omp19, U-Omp19 adsorbed in aluminum hydroxide, or U-Omp19 in IFA for comparison purposes. As a control, other groups of mice were immunized with PBS in aluminum hydroxide or with live B. abortus S19. Promisingly, all the studied vaccines in aluminum hydroxide induced a degree of protection (P > 0.05) similar to that of the control attenuated B. abortus S19 or to U-Omp19 in IFA (Table 5). Likewise, immunization with these vaccines induced in vitro IFN-γ production in response to the Brucella extract (Fig. 5).
TABLE 5.
Protection against B. abortus 544 in BALB/c mice immunized with L-Omp16, U-Omp16, L-Omp19, or U-Omp19 protein adsorbed in aluminum compounds
| Vaccine (dose [μg]) | Adjuvant | Log10 CFU of B. abortus 544a | Protection (U) |
|---|---|---|---|
| B. abortus strain 19 | None | 3.71 ± 0.17b | 2.04 |
| L-Omp16 (10) | Al | 4.35 ± 0.52b | 1.40 |
| U-Omp16 (10) | Al | 4.27 ± 0.66b | 1.48 |
| L-Omp19 (10) | Al | 4.44 ± 1.00b | 1.31 |
| U-Omp19 (10) | Al | 4.48 ± 0.59b | 1.27 |
| U-Omp19 (10) | IFA | 4.05 ± 0.55b | 1.70 |
| PBS | Al | 5.75 ± 0.15c | 0 |
The content of bacteria in spleens is represented as the mean log CFU ± standard deviation per group (n = 6 mice per group).
Significantly different from PBS-immunized mice (P < 0.01 estimated by Dunnett's test).
Significantly different from S19-immunized mice (P < 0.01 estimated by Dunnett's test).
FIG. 5.
IFN-γ production in spleen cells from mice immunized with PBS, U-Omp16 plus aluminum hydroxide (Al), L-Omp16 plus aluminum hydroxide, U-Omp19 plus aluminum hydroxide, L-Omp19 plus aluminum hydroxide, or U-Omp19 plus IFA. Spleen cells were cultured for 72 h as described in the legend of Fig. 3. Each sample was assayed in duplicate wells. IFN-γ in these culture supernatants was measured by sandwich ELISA. Spontaneous (unstimulated) cytokine levels have been subtracted. Data represent the mean ± standard error of the mean of each group of five mice and are representative of two separate experiments. Star, significantly different from the PBS-immunized mice (P < 0.05).
On the other hand, while Brucella species do not reside in the gut, oral infection is one of the principal ways in which the disease is acquired both in humans and animals (16). Hence, we decided to test if immunization with U-Omp16 or U-Omp19 induced protection against an oral Brucella challenge. Mice were immunized intragastrically with recombinant U-Omp16 or U-Omp19 mixed with CT, and they were challenged by the same route with live B. abortus 544. Mice receiving CT and PBS served as controls. Brucella CFU were counted in the spleen 1 month after the oral challenge. Encouragingly, mice immunized orally with U-Omp16 or U-Omp19 exhibited a degree of protection similar (P > 0.05) to that of B. abortus S19 vaccination when challenged by the oral route (Table 6). At this moment only B. abortus RB51 has been used by the oral route as a positive vaccine control when animals were challenged with B. abortus 2308 (29). For comparison purposes with other published oral Brucella vaccine candidates, we decided to conduct in this case another experiment using these particular strains. Again, oral immunization with U-Omp16 or U-Omp19 protein plus CT exhibited a degree of protection similar (P > 0.05) to that of B. abortus RB51 when animals were orally challenged with B. abortus 2308 (Table 6).
TABLE 6.
Protection against B. abortus 544 or B. abortus 2308 in BALB/c mice immunized orally with U-Omp16 or U-Omp19 protein with CT
| Vaccine | Adjuvant | Protection against oral B. abortus 544
|
Protection against oral B. abortus 2308
|
||
|---|---|---|---|---|---|
| Log10 CFU of bacteriaa | Protection (U) | Log10 CFU of bacteriaa | Protection (U) | ||
| B. abortus strain 19 | None | 4.27 ± 0.22b | 1.20 | ND | |
| B. abortus RB51 | None | ND | 3.90 ± 0.80b | 1.51 | |
| U-Omp16 | CT | 4.32 ± 0.21b | 1.15 | 4.17 ± 0.61d | 1.24 |
| U-Omp19 | CT | 3.81 ± 0.52b | 1.66 | 4.07 ± 0.47d | 1.34 |
| PBS | CT | 5.47 ± 0.02c | 0.00 | 5.41 ± 0.16e | 0.00 |
The content of bacteria in spleens is represented as the mean log CFU ± standard deviation per group (n = 5 mice per group). ND, not done.
Significantly different from PBS-immunized mice (P < 0.01 estimated by Dunnett's test).
Significantly different from strain 19 -immunized mice (P < 0.01 estimated by Dunnett's test).
Significantly different from PBS-immunized mice (P < 0.05 estimated by Dunnett's test).
Significantly different from RB51-immunized mice (P < 0.01 estimated by Dunnett's test).
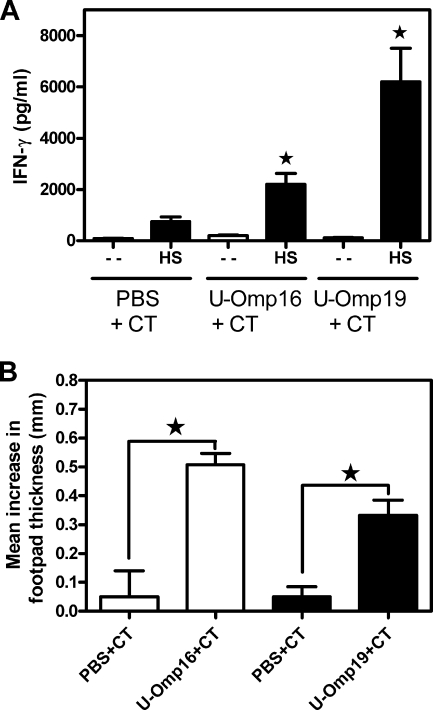
Oral immunization of U-Omp16 or U-Omp19 protein with CT did not induce detectable levels of anti-Omp IgA in fecal extracts or specific IgG in serum (data not shown). In contrast, oral delivery of U-Omp16 or U-Omp19 protein plus CT induced a specific cellular immune response in vitro, as evidenced by the secretion of IFN-γ in response to HS extract (Fig. 6A), nor was IL-4 or IL-2 secretion detected in the same cell supernatants (data not shown). Furthermore, animals immunized by the oral route induced a specific cellular immune response in vivo as demonstrated by a significant (P < 0.001) increase in footpad thickness in mice immunized with U-Omp16 or U-Omp19 compared to controls that received PBS (Fig. 6B).
FIG. 6.
(A) IFN-γ production in spleen cells from mice immunized orally with PBS, U-Omp16, or U-Omp19 plus CT. Spleen cells were cultured at 4 × 106 cells/ml with HS extract (20 μg/ml) or complete medium alone (−) for 72 h. Each sample was assayed in duplicate wells. IFN-γ in these culture supernatants was measured by sandwich ELISA. Data represent the mean ± standard error of the mean from each group of five mice. Data are representative of two separate experiments. Star, significantly different from the same stimulus in PBS-immunized mice (P < 0.05). (B) Induction of the DTH response after intragastric immunizations with U-Omp16, U-Omp19, or PBS with CT. Bars represent the mean increase in footpad thickness (mm) ± standard error of the mean at 72 h after the injection of U-Omp16 or U-Omp19 into the left footpad and PBS into the right footpad. Star, significantly different from mice immunized with PBS plus CT injected with the same Ag (U-Omp16 or U-Omp19) (P < 0.01).
Taken together, these results indicate that the unlipidated versions of Omp16 or Omp19 would be useful candidates for a subunit vaccine against human and animal brucellosis.
DISCUSSION
The OMPs of Brucella spp. have been broadly characterized as immunogenic and protective Ags. Yet studies have been focused on the major OMPs (13), and investigations on the protective capacity of minor OMPs have been scant. A previous report demonstrated that inoculation with a recombinant virus expressing an 18-kDa OMP of B. abortus produced a Th1 in vitro response and 18-kDa protein-specific antibodies in BALB/c mice (36). These authors indicated that the 18-kDa protein they were studying was identical to the previously described Omp19 lipoprotein from B. abortus strain 544. However, this Ag preparation did not protect against a challenge with the virulent strain B. abortus 2308 (36). In contrast, in the present work we have established that immunization with purified lipidated Omp19 in adjuvant elicits protection against B. abortus infection. Altogether these results highlight the importance of testing different delivery systems in the case of this particular Ag.
On the other hand, a divalent fusion DNA vaccine encoding both the B. abortus L7/L12 protein and the Omp16 protein elicited a Th1-dominated immune response in vitro and a significant level of protection against challenge with the virulent strain B. abortus 544 in BALB/c mice (23). In this work, Omp16 as a recombinant lipoprotein in adjuvant elicited protection against B. abortus infection, further confirming that Omp16 is an important candidate for a vaccine against Brucella.
The fact that immunization with purified recombinant L-Omp16 or L-Omp19 protein plus IFA induced protection against B. abortus infection prompted us to study in further detail different aspects that we consider important at the moment of choosing an Ag for further commercial use as a vaccine. In this regard, the capacity to manufacture an Ag that is molecularly defined and pure is highly beneficial in terms of safety, effectiveness, and large-scale production. This is an important issue for mass population vaccine delivery in rural areas of developing countries (11). We determined that i.p administration of 10 μg of each OMP induced protection against B. abortus infection similar to the 30-μg dose. This is advantageous since the purification yield of L-Omp16 or L-Omp19 is very low (data not shown) (see below for details).While lipoproteins and lipopeptides have many advantages for use as vaccines (6), we have encountered a major drawback: in our hands acylation of Omp16 or Omp19 dramatically decreased the level of expression in E. coli after induction with isopropyl-β-d-thiogalactopyranoside (data not shown). When we expressed L-Omp16 or L-Omp19 in E. coli, the yield was 30 times less than the amount with the respective unlipidated versions of the proteins. Moreover, extra LPS depletion rounds are required to obtain recombinant lipoproteins that are LPS free. In addition, it has been reported by other authors that the purification of lipopeptides by reverse-phase high-pressure liquid chromatography induced line-broadening due to fatty acids. Furthermore, multiple chromatographic runs are required to purify commercial quantities due to limited column loadings (7). Overall, these results and ours led us to investigate further the usefulness of the unlipidated versions as vaccines against Brucella. U-Omp16 or U-Omp19 vaccination in IFA also conferred protection against B. abortus infection. Moreover, the degrees of protection elicited were slightly higher than the ones obtained by the lipidated versions and remarkably similar to the S19 control attenuated vaccine.
As Brucella species are facultative intracellular pathogens that reside mainly in macrophages, cellular immune responses are considered central in the mediated immune protection (4). IFN-γ is generally the most essential effector cytokine for activating macrophages for more efficient killing and inhibition of replication of intracellular microbial pathogens (4). In the present work, we demonstrated that vaccination with U-Omp16 or U-Omp19 protein in IFA induces specific CD8+ as well as CD4+ T cells that produce IFN-γ. Furthermore, in vivo depletion of CD4+ or CD8+ T cells in mice vaccinated with U-Omp16 or U-Omp19 resulted in a loss of the vaccine-mediated protection. These results indicate that both specific Omp16 or Omp19 T-cell types, CD4+ as well as CD8+ T cells, are mediating in vivo protection against B. abortus challenge. Immunization with both proteins induced slightly greater protection than the immunization with each individual Ag, but the differences were not statistically different. As both OMPs individually induced a similar degree of protection to that of S19, we speculate that in this particular case to obtain a higher degree of protection than S19, we will have to make a chimeric protein between them to avoid possible immune interference or combine these vaccines with Ags expressed at different stages of the pathogen life cycle to ensure that the organism is adequately confronted by the immune effectors during different stages of infection.
To date, there have been various attempts to develop a vaccine against Brucella spp. utilizing numerous proteins (2, 12, 15, 25, 36). Most of the studies have shown satisfying results in animal models but no clear prospect for further experimentation in humans. Aluminum in the form of aluminum hydroxide or aluminum phosphate has been commonly used as an adjuvant in many human vaccines licensed by the U.S. Food and Drug Administration (5). Auspiciously, vaccinations with U-Omp16 or U-Omp19 in aluminum hydroxide formulation also induced a Th1 response in vitro and systemic protection against B. abortus infection. Importantly, the degree of protection was similar to that elicited by the same Ags in IFA and similar to the control vaccine S19. Generally, water in oil emulsions (e.g., IFA) are recommended for bovines, small ruminants, poultry, and fish when long-term immunity is required (3). Therefore, we have demonstrated that U-Omp16 or U-Omp19 in conjunction with adjuvants that would be suitable for use in humans or animals is protective in the mouse model of brucellosis.
Oral infection is one of the principal ways in which the disease is acquired. Animals usually sniff and lick fetal and placental tissues from abortions, most of which are caused by brucellae (16). Transmission of brucellosis to humans occurs through the consumption of infected, unpasteurized animal milk products, through direct contact with infected animal parts (such as the placenta by inoculation through ruptures of skin and mucous membranes), and through the inhalation of infected aerosolized particles (27). Oral delivery of vaccines is an attractive mode of immunization because it would induce both systemic and mucosal immunity, conferring protection at the site of infection; moreover, oral delivery has no requirement for needle administration, and it can be readily administered (i.e., oral vaccines combined with feed) (17, 24). Furthermore, intragastric immunizations with different purified Ags and appropriate adjuvants were able to induce protective responses against different pathogens (20, 30, 32). Even though there are a significant number of recent studies evaluating oral Brucella challenge (14, 29, 31), to our knowledge there is only one report describing the use of a recombinant purified protein as an oral vaccine against Brucella (16). Therefore, a subunit vaccine that would also prevent oral infection would be of great value in the brucellosis field. Encouragingly, U-Omp16 or U-Omp19 oral administration with CT adjuvant induced a Th1 response in vitro and a cellular immune response in vivo as well as protection against an oral B. abortus challenge. Again, a degree of protection similar to that of the control attenuated vaccine administered by the same route was obtained. As the oral delivery did not induce detectable levels of anti-OMP IgA in fecal extracts or specific IgG in serum, oral protection conferred by U-Omp16 or U-Omp19 may be due to the elicited T-cell response.
As stated, we were interested in developing a recombinant subunit vaccine that is more adaptable to any situation than the currently used attenuated Brucella vaccines without their disadvantages, which include the following: inducing abortion when administered to pregnant cattle, complicating differentiation between infected and vaccinated animals by standard serological tests, causing brucellosis in humans, and possessing antibiotic resistance (33). We expect that the use of U-Omp16 and U-Omp19 in a multisubunit vaccine composed of different proteins of Brucella spp. will not have all of the mentioned disadvantages because these recombinant proteins are well defined Ags and are easy to produce. Moreover, in this work we have shown that recombinant U-Omp16 or U-Omp19 in adjuvant would accomplish important requirements for a vaccine against brucellosis: (i) it could be administered to any host with different adjuvants; (ii) it could be delivered by different administration routes that would elicit immunity at different sites of infection; and (iii) because both proteins are present in all Brucella species, it could be protective against any species of Brucella.
Acknowledgments
This work was supported in part by grants from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT-Argentina), CONICET (to G.H.G. and to J.C.), and from Centro Argentino Brasileño de Biotecnología (to C.A.F.). K.A.P., A.Z., and C.G.S. are recipients of doctoral fellowships from CONICET (Argentina). S.M.E., P.B., C.A.F., G.H.G., and J.C. are members of the Research Career of CONICET. C.A.F. is also a member of the Facultad de Ciencias Exactas, Universidad Nacional de La Plata.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 3 November 2008.
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383787-793. [DOI] [PubMed] [Google Scholar]
- 2.Al-Mariri, A., A. Tibor, P. Mertens, X. De Bolle, P. Michel, J. Godefroid, K. Walravens, and J. J. Letesson. 2001. Protection of BALB/c mice against Brucella abortus 544 challenge by vaccination with bacterioferritin or P39 recombinant proteins with CpG oligodeoxynucleotides as adjuvant. Infect. Immun. 694816-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aucouturier, J., L. Dupuis, and V. Ganne. 2001. Adjuvants designed for veterinary and human vaccines. Vaccine 192666-2672. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin, C. L., and R. Goenka. 2006. Host immune responses to the intracellular bacteria Brucella: does the bacteria instruct the host to facilitate chronic infection? Crit. Rev. Immunol. 26407-442. [DOI] [PubMed] [Google Scholar]
- 5.Baylor, N. W., W. Egan, and P. Richman. 2002. Aluminum salts in vaccines-US perspective. Vaccine 20(Suppl. 3)S18-S23. [DOI] [PubMed] [Google Scholar]
- 6.BenMohamed, L., S. L. Wechsler, and A. B. Nesburn. 2002. Lipopeptide vaccines—yesterday, today, and tomorrow. Lancet Infect. Dis. 2425-431. [DOI] [PubMed] [Google Scholar]
- 7.Bourel-Bonnet, L., D. Bonnet, F. Malingue, H. Gras-Masse, and O. Melnyk. 2003. Simultaneous lipidation of a characterized peptide mixture by chemoselective ligation. Bioconjug. Chem. 14494-499. [DOI] [PubMed] [Google Scholar]
- 8.Bowden, R. A., A. Cloeckaert, M. S. Zygmunt, S. Bernard, and G. Dubray. 1995. Surface exposure of outer membrane protein and lipopolysaccharide epitopes in Brucella species studied by enzyme-linked immunosorbent assay and flow cytometry. Infect. Immun. 633945-3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowden, R. A., S. M. Estein, M. S. Zygmunt, G. Dubray, and A. Cloeckaert. 2000. Identification of protective outer membrane antigens of Brucella ovis by passive immunization of mice with monoclonal antibodies. Microbes Infect. 2481-488. [DOI] [PubMed] [Google Scholar]
- 10.Brown, L. E., and D. C. Jackson. 2005. Lipid-based self-adjuvanting vaccines. Curr. Drug Deliv. 2383-393. [DOI] [PubMed] [Google Scholar]
- 11.Cassataro, J., S. M. Estein, K. A. Pasquevich, C. A. Velikovsky, S. de la Barrera, R. Bowden, C. A. Fossati, and G. H. Giambartolomei. 2005. Vaccination with the recombinant Brucella outer membrane protein 31 or a derived 27-amino-acid synthetic peptide elicits a CD4+ T helper 1 response that protects against Brucella melitensis infection. Infect. Immun. 738079-8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassataro, J., K. A. Pasquevich, S. M. Estein, D. A. Laplagne, C. A. Velikovsky, S. de la Barrera, R. Bowden, C. A. Fossati, G. H. Giambartolomei, and F. A. Goldbaum. 2007. A recombinant subunit vaccine based on the insertion of 27 amino acids from Omp31 to the N terminus of BLS induced a similar degree of protection against B. ovis than Rev. 1 vaccination. Vaccine 254437-4446. [DOI] [PubMed] [Google Scholar]
- 13.Cloeckaert, A., N. Vizcaino, J. Y. Paquet, R. A. Bowden, and P. H. Elzer. 2002. Major outer membrane proteins of Brucella spp.: past, present and future. Vet. Microbiol. 90229-247. [DOI] [PubMed] [Google Scholar]
- 14.Delpino, M. V., S. M. Estein, C. A. Fossati, and P. C. Baldi. 2007. Partial protection against Brucella infection in mice by immunization with nonpathogenic alphaproteobacteria. Clin. Vaccine Immunol. 141296-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delpino, M. V., S. M. Estein, C. A. Fossati, P. C. Baldi, and J. Cassataro. 2007. Vaccination with Brucella recombinant DnaK and SurA proteins induces protection against Brucella abortus infection in BALB/c mice. Vaccine 256721-6729. [DOI] [PubMed] [Google Scholar]
- 16.Delpino, M. V., M. I. Marchesini, S. M. Estein, D. J. Comerci, J. Cassataro, C. A. Fossati, and P. C. Baldi. 2007. A bile salt hydrolase of Brucella abortus contributes to the establishment of a successful infection through the oral route in mice. Infect. Immun. 75299-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerdts, V., G. K. Mutwiri, S. K. Tikoo, and L. A. Babiuk. 2006. Mucosal delivery of vaccines in domestic animals. Vet. Res. 37487-510. [DOI] [PubMed] [Google Scholar]
- 18.Giambartolomei, G. H., A. Zwerdling, J. Cassataro, L. Bruno, C. A. Fossati, and M. T. Philipp. 2004. Lipoproteins, not lipopolysaccharide, are the key mediators of the proinflammatory response elicited by heat-killed Brucella abortus. J. Immunol. 1734635-4642. [DOI] [PubMed] [Google Scholar]
- 19.Godfroid, J., A. Cloeckaert, J. P. Liautard, S. Kohler, D. Fretin, K. Walravens, B. Garin-Bastuji, and J. J. Letesson. 2005. From the discovery of the Malta fever's agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet. Res. 36313-326. [DOI] [PubMed] [Google Scholar]
- 20.Harada, H., F. Nishikawa, N. Higashi, and E. Kita. 2002. Development of a mucosal complex vaccine against oral Salmonella infection in mice. Microbiol. Immunol. 46891-905. [DOI] [PubMed] [Google Scholar]
- 21.Jimenez de Bagues, M. P., P. H. Elzer, J. M. Blasco, C. M. Marin, C. Gamazo, and A. J. Winter. 1994. Protective immunity to Brucella ovis in BALB/c mice following recovery from primary infection or immunization with subcellular vaccines. Infect. Immun. 62632-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liljeqvist, S., and S. Stahl. 1999. Production of recombinant subunit vaccines: protein immunogens, live delivery systems and nucleic acid vaccines. J. Biotechnol. 731-33. [DOI] [PubMed] [Google Scholar]
- 23.Luo, D., B. Ni, P. Li, W. Shi, S. Zhang, Y. Han, L. Mao, Y. He, Y. Wu, and X. Wang. 2006. Protective immunity elicited by a divalent DNA vaccine encoding both the L7/L12 and Omp16 genes of Brucella abortus in BALB/c mice. Infect. Immun. 742734-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitragotri, S. 2005. Immunization without needles. Nat. Rev. Immunol. 5905-916. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira, S. C., and G. A. Splitter. 1996. Immunization of mice with recombinant L7/L12 ribosomal protein confers protection against Brucella abortus infection. Vaccine 14959-962. [DOI] [PubMed] [Google Scholar]
- 26.Olsen, S. C., and W. S. Stoffregen. 2005. Essential role of vaccines in brucellosis control and eradication programs for livestock. Expert Rev. Vaccines 4915-928. [DOI] [PubMed] [Google Scholar]
- 27.Pappas, G., N. Akritidis, M. Bosilkovski, and E. Tsianos. 2005. Brucellosis. N. Engl. J. Med. 3522325-2336. [DOI] [PubMed] [Google Scholar]
- 28.Pappas, G., P. Papadimitriou, N. Akritidis, L. Christou, and E. V. Tsianos. 2006. The new global map of human brucellosis. Lancet Infect. Dis. 691-99. [DOI] [PubMed] [Google Scholar]
- 29.Pasquali, P., A. Rosanna, C. Pistoia, P. Petrucci, and F. Ciuchini. 2003. Brucella abortus RB51 induces protection in mice orally infected with the virulent strain B. abortus 2308. Infect. Immun. 712326-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pechine, S., C. Janoir, H. Boureau, A. Gleizes, N. Tsapis, S. Hoys, E. Fattal, and A. Collignon. 2007. Diminished intestinal colonization by Clostridium difficile and immune response in mice after mucosal immunization with surface proteins of Clostridium difficile. Vaccine 253946-3954. [DOI] [PubMed] [Google Scholar]
- 31.Pontes, D. S., F. A. Dorella, L. A. Ribeiro, A. Miyoshi, Y. Le Loir, A. Gruss, S. C. Oliveira, P. Langella, and V. Azevedo. 2003. Induction of partial protection in mice after oral administration of Lactococcus lactis producing Brucella abortus L7/L12 antigen. J. Drug Target. 11489-493. [DOI] [PubMed] [Google Scholar]
- 32.Rosas, G., G. Fragoso, N. Ainciart, F. Esquivel-Guadarrama, A. Santana, R. J. Bobes, O. Ramirez-Pliego, A. Toledo, C. Cruz-Revilla, G. Meneses, P. Berguer, F. A. Goldbaum, and E. Sciutto. 2006. Brucella spp. lumazine synthase: a novel adjuvant and antigen delivery system to effectively induce oral immunity. Microbes Infect. 81277-1286. [DOI] [PubMed] [Google Scholar]
- 33.Schurig, G. G., N. Sriranganathan, and M. J. Corbel. 2002. Brucellosis vaccines: past, present and future. Vet. Microbiol. 90479-496. [DOI] [PubMed] [Google Scholar]
- 34.Tibor, A., B. Decelle, and J. J. Letesson. 1999. Outer membrane proteins Omp10, Omp16, and Omp19 of Brucella spp. are lipoproteins. Infect. Immun. 674960-4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velikovsky, C. A., F. A. Goldbaum, J. Cassataro, S. Estein, R. A. Bowden, L. Bruno, C. A. Fossati, and G. H. Giambartolomei. 2003. Brucella lumazine synthase elicits a mixed Th1-Th2 immune response and reduces infection in mice challenged with Brucella abortus 544 independently of the adjuvant formulation used. Infect. Immun. 715750-5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vemulapalli, R., S. Cravero, C. L. Calvert, T. E. Toth, N. Sriranganathan, S. M. Boyle, O. L. Rossetti, and G. G. Schurig. 2000. Characterization of specific immune responses of mice inoculated with recombinant vaccinia virus expressing an 18-kilodalton outer membrane protein of Brucella abortus. Clin. Diagn. Lab. Immunol. 7114-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young, E. J. 1995. An overview of human brucellosis. Clin. Infect. Dis. 21283-289, 290. [DOI] [PubMed] [Google Scholar]