Abstract
As the disease caused by Mycobacterium tuberculosis continues to be a burden, there is a concerted effort to find new vaccines to combat this problem. One of the important vaccine strategies is whole bacterial vaccines. This approach relies on multiple antigens and built-in adjuvanticity. Other mycobacterial strains which share cross-reactive antigens with M. tuberculosis have been considered as alternatives to M. bovis for vaccine use. One such strain, “Mycobacterium w”, had been evaluated for its immunomodulatory properties in leprosy. A vaccine against leprosy based on killed M. w is approved for human use, where it has resulted in clinical improvement, accelerated bacterial clearance, and increased immune responses to Mycobacterium leprae antigens. M. w shares antigens not only with M. leprae but also with M. tuberculosis, and initial studies have shown that vaccination with killed M. w induces protection against tuberculosis in Mycobacterium bovis BCG responder, as well as BCG nonresponder, strains of mice. Hence, we further studied the protective potential of M. w and the underlying immune responses in the mouse model of tuberculosis. We analyzed the protective efficacy of M. w immunization in both live and killed forms through the parenteral route and by aerosol immunization, compared with that of BCG. Our findings provide evidence that M. w has potential protective efficacy against M. tuberculosis. M. w activates macrophage activity, as well as lymphocytes. M. w immunization by both the parenteral route and aerosol adminstration gives higher protection than BCG given by the parenteral route in the mouse model of tuberculosis.
The current vaccine against tuberculosis (TB), Mycobacterium bovis BCG, fails to protect against the most prevalent disease form, pulmonary TB in adults. It is generally assumed that active TB occurs because of a weakening of the immune system, which keeps Mycobacterium tuberculosis in check as long as it is fully competent. M. tuberculosis does not induce the optimum protection because the pathogen is not eradicated, and it has now been shown that exogenous reinfection does occur, suggesting that natural immunity is insufficient (26) and fails to control the pathogen in the long run. Hence, other mycobacterial strains which share cross-reactive antigens (Ags) with M. tuberculosis have also been considered as alternatives to M. bovis for vaccine use. One strain, “Mycobacterium w,” had been evaluated for its immunomodulatory properties in leprosy. M. w is a nonpathogenic, cultivable mycobacterium (18) which has been found to improve immunity to leprosy (30). A vaccine against leprosy based on M. w is approved for human use, where it has resulted in clinical improvement, accelerated bacterial clearance, and increased immune responses to Mycobacterium leprae Ags (13, 21, 25). M. w shares Ags not only with M. leprae but also with M. tuberculosis (29), and initial studies have shown that vaccination with killed M. w induces protection against TB in animal models (22, 23) and also resulted in early sputum conversion in TB patients (17). Recently it has been suggested that M. w be referred to as Mycobacterium indicus pranii to avoid confusion with M. tuberculosis-W (Beijing strain) (24). It is generally known that live bacteria impart greater protection than killed bacteria. It may be that persistence of live bacteria in the host for some time results in a robust memory response (12). Another important factor is that secretory proteins which are absent in the killed bacterial vaccines have been shown to play an important role in protection. In this study, we analyzed the M. tuberculosis-specific immune response induced in mice immunized with live or killed M. w and compared it with the BCG-induced immune response and also compared the protective efficacies of the two mycobacteria.
As the lung is the primary target organ of this disease, immunization potential by the aerogenic route was also studied. Inhalation of aerosols provides a noninvasive delivery system that physically targets the lung as the desired site of the pharmacological effect. This route of immunization has emerged a very attractive route of vaccine delivery, inducing both local and systemic immunity (7, 10). The immune response induced by live M. w given by aerosol was studied and compared with those of other groups of immunized mice.
Establishment of a protective immune response during the course of M. tuberculosis infection requires successful recruitment of T lymphocytes and activation of macrophages. In our present study, we demonstrate that immunization with M. w activates macrophage activity, as well as lymphocytes, as indicated by gamma interferon (IFN-γ) secretion and cytotoxic activity toward infected macrophages. Activated macrophages could inhibit the intracellular growth and multiplication of M. tuberculosis. M. w immunization by both the parenteral route and aerosol immunization gave higher protection than BCG given by the parenteral route in the mouse model of TB.
MATERIALS AND METHODS
Animals.
Inbred C57BL/6 mice at 6 weeks of age were obtained from the animal facility of the National Institute of Immunology, New Delhi, India, where animals are bred and housed in agreement with the guidelines of the Institute's Animal Ethics Committee.
Mycobacteria.
M. w maintained on Lowenstein-Jensen medium (LJ) slants (BD Difco) and kept at −70°C was grown in Middlebrook 7H9 medium (BD Difco) with 0.02% glycerol, 0.05% Tween 80, and 10% albumin-dextrose complex enrichment (BD Difco) as a shake flask culture. Bacteria were harvested in the log growth phase by centrifugation at 840 × g for 15 min, washed twice by the centrifugal washing method, and suspended in saline at the desired concentration for immunization. Some of the bacteria were inactivated by autoclaving for 20 min at a pressure of 15 lb/in2.
Immunization.
Five groups of C57BL/6 mice at 6 weeks of age were used. Two groups were immunized with killed and live M. w bacteria, respectively, through the subcutaneous (s.c.) route each at a dose of 107 bacteria in 100 μl of saline (preliminary experiments were done to find the optimum dose). The third group was immunized with live M. w aerosol by an aerosol immunization device (3) as described earlier. Respirable aerosol of the bacterial suspension was made by passing compressed air through a nebulizer holding the bacterial suspension. The concentration of the bacterial suspension in the nebulizer and the time of exposure of mice to aerosol were standardized, which resulted in the deposition of about 1,000 bacteria in one pair of lungs, as determined by CFU counting at 24 h after immunization. The fourth group was immunized with 107 BCG (Danish 1331 strain) bacteria per mouse by the s.c. route. The fifth group was given a saline injection by the s.c. route (control group). Three weeks after primary immunization, a booster immunization was given with the same Ag and by the same route as in the primary immunization. Three weeks after the booster immunization, mice were sacrificed and different assays were performed.
Infection of mice.
Mice were challenged with live M. tuberculosis H37Rv by the aerosol route with an inhalation exposure system (Glas-Col, Terre Haute, IN). About 300 to 400 M. tuberculosis bacilli per lung were delivered with the above inhalation exposure system. The protective efficacy of vaccination in different groups was evaluated by plating serial 10-fold dilutions of lung, spleen, and liver homogenates in quadruplicate on LJ plates. Plates were incubated inside semisealed plastic bags at 37°C for 3 to 4 weeks, and colonies were counted. Bacterial loads in the lungs, spleens, and livers of different groups of immunized and control mice were determined at 8 and 12 weeks after a challenge with M. tuberculosis. Bacterial loads in four individual mice per group were determined at each time point. Two mice were sacrificed for histopathological studies.
Lymphocyte proliferation assay.
Spleens were aseptically removed and crushed with sterile blunt forceps. A single-cell suspension was prepared, and red blood cells were lysed with Gey's solution. Cells were washed, and viability was assessed by the trypan blue exclusion method after plating at a concentration of 4 × 105 per well in RPMI 1640 medium supplemented with 10% fetal calf serum in a 96-well plate. These cells were stimulated in vitro with M. w Ag, M. tuberculosis Ag, or no Ag (control) in triplicate. After incubation for 72 h at 37°C and 5% CO2, cultures were pulsed with 1 μCi of [3H]thymidine. At 18 h later, cells were harvested and [3H]thymidine uptake was measured with a beta counter. The stimulation index was calculated by dividing the mean counts per minute of Ag-stimulated cells by the mean counts per minute of corresponding unstimulated cells.
Cytokine measurement.
In the lymphocyte culture for proliferation, a duplicate set was prepared for cytokine assay. Cell culture supernatant was collected after 48 h of Ag stimulation and kept at −20°C until further use. The levels of IFN-γ and interleukin-4 (IL-4) were measured with a mouse-specific enzyme-linked immunosorbent assay kit (BD Pharmingen).
Bronchoalveolar lavage (BAL) to collect alveolar macrophages.
Animals were euthanized, and the thoracic cavity was exposed. The trachea was canulated with a 26-gauge butterfly canula and secured with a silk thread. Lungs were lavaged three times with a 1-ml aliquot of ice-cold saline to collect alveolar macrophages.
In vitro assessment of inhibition of mycobacterial growth in alveolar macrophages.
Alveolar macrophages were harvested by centrifuging BAL fluid at 200 × g for 5 min at 4°C. The pellet was suspended in 1 ml of complete medium consisting of RPMI 1640 medium with 10% fetal calf serum and antibiotic antimycotic solution. Cell counts were taken with a hemocytometer, and viability was determined with trypan blue. Macrophages were taken from four mice per group, and 5 × 104 macrophages per well were seeded in 500 μl of complete medium and allowed to adhere. Lymphocytes were prepared from spleen samples, and 5 × 105 lymphocytes per well were stimulated in vitro with 2 μg of soluble Ag of M. w for 48 h. Alveolar macrophages were infected with live M. tuberculosis H37Ra at a 20:1 multiplicity of infection overnight. Infected macrophages were washed thrice with RPMI medium to remove the extracellular bacteria after infection. Infected macrophages were then cocultured with stimulated lymphocytes for 72 h, after which supernatants were aspirated and macrophages were lysed with 0.2% saponin for 2 min to release the intracellular bacteria. Viable bacteria present in the lysate were determined by plating different dilutions of the lysate on 7H11 agar plates supplemented with 10% oleic acid-albumin-dextrose-catalase (BD Difco).
Frequency of IFN-γ-producing cells.
To evaluate the lung response further, M. tuberculosis Ag-specific IFN-γ-secreting lymphocytes were quantitated by enzyme-linked immunospot (ELISPOT) assay. Briefly, macrophages were collected from BAL fluid as described earlier. Lungs were taken from four mice in each group and cut into small pieces. These were suspended in RPMI medium and treated with collagenase type IA-S (Sigma) for 30 min at 37°C and homogenized briefly with a homogenizer, and the resulting cell suspension was passed through a sterile nylon wool column to get a single-cell suspension of lymphocytes. We plated 3 × 104 macrophages and 3 × 105 lymphocytes per well of 96-well nitrocellulose-backed plates along with M. tuberculosis Ag and incubated the plates for 48 h. The plates were processed by following the instructions given with the kit, and the spots in the air-dried plates were counted in an ELISPOT reader with KS Elispot software.
Cell-mediated cytotoxicity assay.
The CD8+ T-cell-mediated cytotoxicity of different groups of immunized mice toward infected and uninfected syngeneic macrophages collected from BAL fluid was studied by flow cytometry. This method is considered a promising alternative to the use of radioactive isotopes for these analyses (9). Briefly, CD8+ T cells were isolated from lymphocytes with CD8a antibody conjugated with magnetic nanoparticles (BD Biosciences, Pharmingen). Target cells were labeled with the fluorescent cell membrane marker PKH-67 (Sigma). Effector (CD8+ T cells) and target (M. tuberculosis-infected or uninfected macrophages) cells were cocultured at a 2:1 ratio for 8 h in a six-well tissue culture plate. The nuclear marker 7-aminoactinomycin D (Molecular Probes; Invitrogen) was added to distinguish between live and dead cells. Samples were run in a flow cytometer (BD LSR). A minimum of 10,000 events were collected and analyzed with WinMDI software.
Statistical analysis.
Results are shown as the mean ± the standard error of the mean (SEM). Comparisons between groups were performed by analysis of variance. P values of <0.05 were considered statistically significant.
RESULTS
Immune response to M. w.
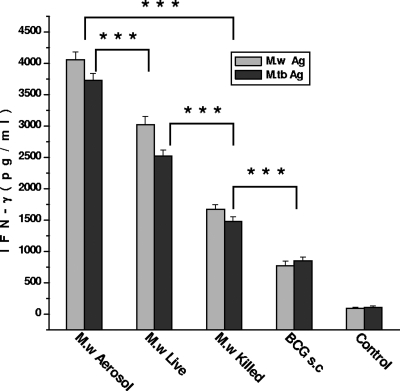
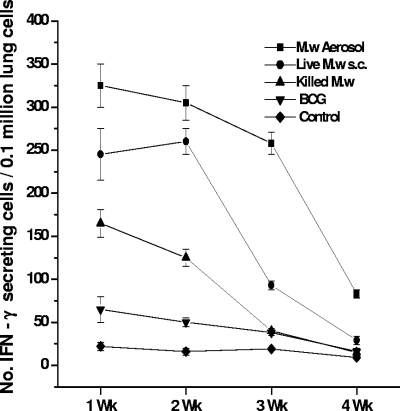
As IFN-γ is a crucial component of antimycobacterial immunity, the induction of this cytokine by in vitro restimulation of lymphocytes was assessed. It was observed that M. w aerosol immunization induced significantly higher IFN-γ compared to immunization by the s.c. route. Live M. w by the s.c. route, in turn, induced a significantly higher IFN-γ response compared to killed M. w (Fig. 1). IL-4 secretion was also checked. None of the immunized groups showed a detectable amount of IL-4 (data not shown).
FIG. 1.
IFN-γ secretion by lymphocytes from different groups of immunized mice on restimulation with M. w Ag or M. tuberculosis (M.tb) Ag in vitro. Sonicate supernatant of M. w or M. tuberculosis at a concentration of 2 μg per well was used as the Ag for in vitro restimulation. Data are expressed as the mean ± SEM (n = 4 mice per group). ***, P < 0.001.
Cell-mediated cytotoxicity.
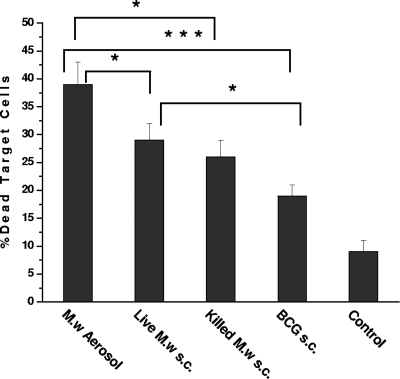
It has been shown convincingly that CD8+ T cells have a role in protection against M. tuberculosis. Hence, cell-mediated cytotoxicity of CD8+ T cells isolated from different groups of immunized mice were studied in Ag-pulsed and unpulsed syngeneic macrophages from BAL fluid. The percentage of dead target cells was significantly higher in M. w aerosol-immunized mice compared to the group of mice immunized by the s.c. route with live or killed M. w (Fig. 2). The cell-mediated cytotoxicity of M. w in the aerosol group and the live M. w s.c. group was significantly higher than that in BCG-immunized mice and control mice. These results provide evidence that M. w immunization also activates a CD8+ T-cell response.
FIG. 2.
Cell-mediated cytotoxicity of CD8+ T cells isolated from different groups of immunized or unimmunized mice toward M. tuberculosis Ag-pulsed syngeneic macrophages from BAL fluid. Target cells were labeled with PKH-67. The nuclear marker 7-aminoactinomycin D was added to distinguish between live and dead cells. Cells were taken from four mice per group for this study. The results shown here are representative of two similar experiments. *, P < 0.05; ***, P < 0.001.
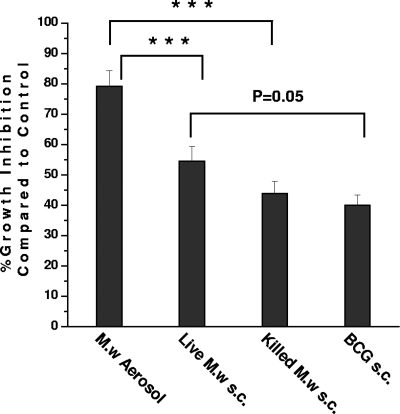
Intracellular growth inhibition activity of macrophages.
Multiple mechanisms are important for inhibition of intracellular mycobacteria. To further study the lung-specific immune response, the intracellular growth inhibition activity of alveolar macrophages was studied. There was an about 80% reduction in the number of viable bacteria in alveolar macrophages collected from the M. w aerosol-immunized group compared to the control group (Fig. 3). The percent reduction in this group was significantly higher than in the other three groups. An about 55% reduction in intracellular bacteria was observed in the live M. w s.c. group, which was higher than in the BCG group (P = 0.05), where an about 38% reduction was observed.
FIG. 3.
Intracellular growth inhibition activity of alveolar macrophages. Macrophages collected from the BAL fluid of all groups of immunized or control mice were infected in vitro with M. tuberculosis H37Ra and cocultured with lymphocytes for 72 h. The number of viable bacilli present inside macrophages after 72 h were determined by CFU counting. Cells were taken from four mice per group for this study. The results shown here are representative of two similar experiments. ***, P < 0.001.
Cocultures of alveolar macrophages and lymphocytes were analyzed for nitric oxide and reactive oxygen, as these are implicated in the killing of intracellular bacteria. It was observed that after 72 h of coculture, reactive oxygen and nitric oxide levels were significantly higher in the M. w aerosol-immunized group than in the control group (data not shown).
Local lung immune response.
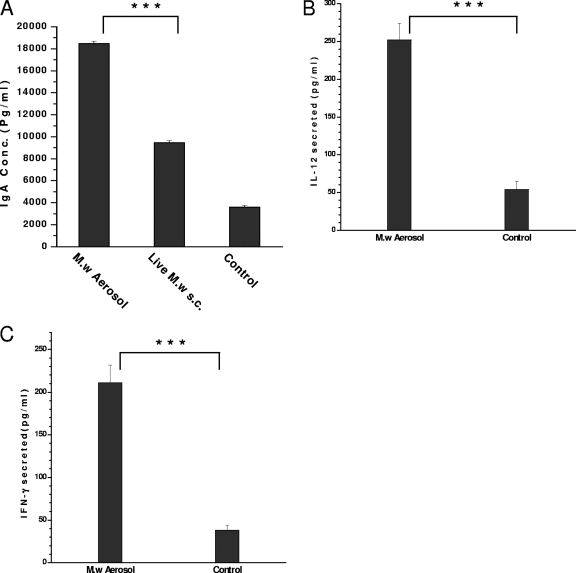
BAL fluid collected from M. w aerosol-immunized mice, as well as control mice, was analyzed for secretory immunoglobulin A (IgA) antibody. A significantly higher IgA antibody response was induced in aerosol-immunized mice than in those parenterally injected with M. w and in control mice (Fig. 4A). These studies indicate that immunization with M. w aerosol results in the activation of a local lung immune response.
FIG. 4.
(A) Secreted IgA antibody in BAL fluid supernatant. (B and C) Cytokines IL-12 and IFN-γ, respectively, in BAL fluid supernatant. ***, P < 0.001.
The cytokines IL-12 and IFN-γ, as markers of activation of macrophages and lymphocytes, were quantitated in BAL fluid by enzyme-linked immunosorbent assay. Moderate amounts of both cytokines were present in the BAL fluid supernatant of M. w aerosol-immunized mice, but these were significantly higher than those in control mice (Fig. 4B and C).
Kinetics of immune response.
The kinetics of the immune responses in the lungs, spleens, and lymph nodes of all five groups of mice were studied at different time points after booster immunization by the s.c. or aerosol route.
Ag-specific IFN-γ-secreting cells in the lungs were quantitated by ELISPOT, as lymphocyte proliferation of lung cells was low (data not shown). We have checked the response in the lungs until 4 weeks after a booster immunization. Significantly higher numbers of IFN-γ-secreting cells could be seen in the lungs of all of the groups of mice immunized with M. w than in control mice at 1 week after a booster immunization (Fig. 5). The number of IFN-γ-secreting cells decreased gradually in all of the groups until 4 weeks, but in M. w aerosol-immunized mice, the number was higher at all time points than in the other groups.
FIG. 5.
IFN-γ-secreting lymphocytes from the lungs of all groups of immunized or control mice on in vitro restimulation with M. tuberculosis Ag as determined by ELISPOT assay. Lymphocytes taken from four mice per group at each time point were used for this assay. Data are expressed as the mean ± SEM.
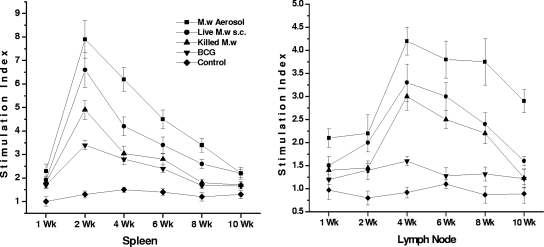
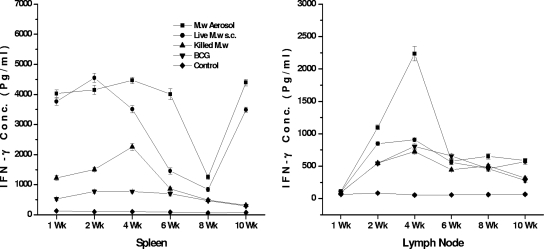
Ag-specific lymphocyte proliferation and IFN-γ secretion in the supernatant were quantified at different time points for lymphocytes collected from the spleen and lymph nodes. We separated the brachial lymph nodes of mice immunized by the aerosol route and the inguinal lymph nodes of those immunized by the s.c. route. We found that the Ag-specific proliferative response in the spleen peaked at 2 to 3 weeks after the booster immunization while the immune response in the lymph nodes peaked at 3 to 4 weeks after immunization (Fig. 6). Until 10 weeks, a specific immune response was observed, although quantitatively it decreased gradually from 4 weeks onward. When we evaluated the IFN-γ secretion in the supernatant of in vitro-restimulated lymphocytes from the spleen, it was observed that until 6 weeks there was a very high level of IFN-γ secretion in the M. w aerosol group (Fig. 7). Then again at 10 weeks there was an increase in IFN-γ secretion both in the M. w aerosol group and in the live M. w s.c. group. This is an interesting observation, which could be related to the persistence of live M. w in mice for about 6 to 7 weeks, during which activated effector T cells which secrete IFN-γ are generated. But once the live bacteria are cleared, there is a surge of memory T cells which produce IFN-γ. (The persistence of live M. w in mice was checked by plating organ homogenates on LJ plates at different time points after immunization [data not shown].) Further analysis and experiments are necessary to define the phenotype of T cells secreting IFN-γ at different time points. These studies provide evidence that M. w immunization of mice leads to activation of T cells and macrophages in lymphoid organs.
FIG. 6.
Kinetics of immune responses in the spleen and lymph nodes. M. tuberculosis Ag-specific lymphocyte proliferation at different time points is shown. Data are expressed as the mean ± SEM (n = 4 mice per group at each time point). The stimulation index is the counts per minute of Ag-stimulated cells divided by the counts per minute of unstimulated cells.
FIG. 7.
Kinetics of immune responses in the spleen and lymph nodes. IFN-γ secreted in the supernatant of in vitro restimulated lymphocytes is shown. Data are expressed as the mean ± SEM (n = 4 mice per group at each time point).
Protection against M. tuberculosis challenge.
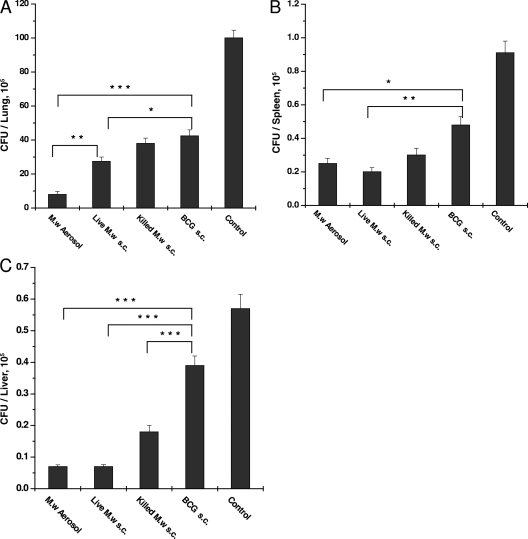
The bacterial loads in different organs were determined. It was observed that 8 weeks after challenge, M. w aerosol immunization reduced the lung bacterial load about eight times compared to the control while M. w immunization by the s.c. route reduced the bacterial load about five times (data not shown). The reduction in the bacterial load produced by M. w immunization was significantly greater than that in the BCG-immunized group and the control group. The splenic load was about 50 to 100 times less than the lung bacterial load in all of the groups.
At 12 weeks postinfection, the lung bacterial load was 12 times less in the M. w aerosol-immunized group than in the control group and was also significantly less than in all of the other immunized groups (Fig. 8). The second best protection was given by the live M. w s.c. group, which was higher than that of the killed M. w s.c. group in all of the organs studied. One important observation was that in the M. w aerosol-immunized group, the bacterial load in the lungs decreased from 8 to 12 weeks while in the rest of the groups, there was some increase in the number of bacteria in the lungs, although it was not significant. At 12 weeks postinfection, in both the live M. w aerosol and s.c. groups, the bacterial loads in the liver and spleen were similar. In the liver, there was an about eight times reduction in both of the groups while in spleen there was a four to five times reduction in both of the groups compared to the control. This observation suggests that the systemic protection given by two different routes of immunization is similar while the protection afforded by aerosol immunization in the lung is greater.
FIG. 8.
Bacterial loads in the lungs (A), spleens (B), and livers (C) of different groups of immunized and control mice at 12 weeks after challenge with M. tuberculosis H37Rv. Bacterial loads in four individual mice per group were determined, and the results of one of two similar experiments are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Mice were checked for body weight at different time intervals after infection, as it correlates with the extent of disease. In the control group of mice, there was an about 15 to 20% weight loss at 8 weeks after infection (data not shown). But interestingly, there was a 5 to 8% gain in weight in M. w aerosol-immunized mice at this time, which reflects protection in M. w-immunized mice.
Histopathological observation.
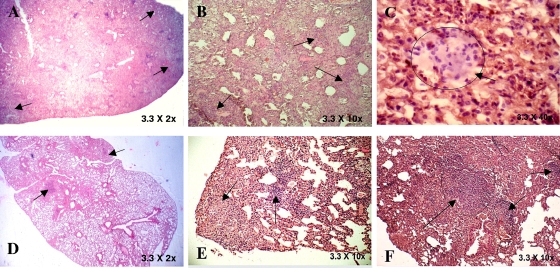
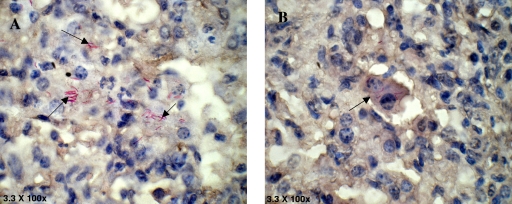
In keeping with significant immune protection in M. w-immunized mice, there was much reduced lung histopathology in these mice compared to that in control, unimmunized mice. After a challenge with M. tuberculosis H37Rv, unimmunized control mice developed a grossly evident number of nodular lung lesions and hemorrhagic spots, which were quite large compared to those of M. w-immunized mice (data not shown). When sections from immunized and unimmunized groups of mice were compared at 8 and 12 weeks postchallenge with M. tuberculosis, it was found that the number and size of granulomas in the M. w-immunized mice were smaller and they were surrounded by healthy alveolar architecture. These granulomas consisted primarily of tight aggregates of lymphocytes and were more organized in immunized mice and there was less parenchymal inflammation and slower progression of lung pathology than in control unimmunized mice, where diffused granulomas were seen and areas of nongranulomatous infiltrating portions were quite evident (Fig. 9). Distended alveolar spaces (edematic lung) were also seen in unimmunized mice, suggesting a relative loss of lung function. This was not seen in M. w-immunized mice. Large numbers of extracellular and intracellular bacilli were seen in sections of lungs of control mice, compared to very rare extracellular bacilli in M. w-immunized mice (Fig. 10).
FIG. 9.
Hematoxylin-eosin-stained sections of M. tuberculosis-infected lungs of control mice (A, B, and C) and M. w-immunized mice (D, E, and F) at 12 weeks postinfection. The arrows in panels A and B indicate lymphocyte infiltration and aggregates of lymphocytes. An extensive inflammatory reaction and infiltration were seen in the sections. Frequent edema formation was also seen in the lungs of control mice (C). In contrast to those of controls, sections of M. w-immunized mice had localized areas of infiltration surrounded by healthy alveolar structure (D and E). Small, organized granulomas were seen, and there was less parenchymal inflammation (F).
FIG. 10.
Lung sections of control mice (A) and M. w-immunized mice (B) stained with carbolfuchsin and counterstained with hematoxylin. Large numbers of extracellular and intracellular bacilli were seen in the sections of control mice, compared to very rare extracellular and few intracellular bacilli in M. w-immunized mice.
DISCUSSION
TB can occur at any tissue site, but the lung is the primary target organ of this disease. Inhaled bacteria are engulfed by alveolar macrophages. Macrophages are the effector cells, while T lymphocytes are the specific mediators of resistance to TB. IFN-γ produced by T cells activates antimycobacterial activities in macrophages and has been shown to be crucial for protection against TB (14, 15). Although it is not perfect, at present IFN-γ is considered the most attractive correlate of protective immunity. Hence, we started our study by evaluating the lymphocyte proliferation- and IFN-γ secretion-inducing capacities of M. w.
In order to accelerate the control of initial M. tuberculosis infection, vaccine-induced immunity in the lung must be further enhanced. It has been shown that M. tuberculosis is considerably more virulent when administrated by the respiratory route compared to the intravenous route, largely because of the delayed expression of immunity in the lung (4, 5). In this study, we have observed that M. w induces a Th1 type of response whether given in killed or live form. Aerosol immunization with M. w induced the greatest T-cell response compared to all of the other groups studied, especially the local lung response. Increasing evidence suggests that vaccination at the mucosal site is superior to vaccination at other sites in eliciting protection from mucosal infectious diseases (16). This is partially explained by the observation that memory T and B cells generated upon mucosal vaccination acquire mucosa-homing receptors and preferentially accumulate at the mucosal site of induction (4, 5). However, few mucosally delivered TB vaccines, except replicating mycobacteria, could successfully trigger protective immune responses in the lung (7, 10). Vaccination of humans by the nasal route could also offer practical advantages for vaccine administration, as this is a noninvasive delivery system.
In our study, aerosol immunization induced both an Ag-specific IgA response and local and systemic T-cell immune responses. Secretory IgA antibody has been shown to play a major role in host protection against bacterial and viral pathogens by blocking pathogen entrance and/or by modulating the proinflammatory responses (19, 27).
To achieve significant protection, it is necessary to induce different components of the immune response. CD8+ T cells are required to achieve significant protection against TB (11, 20). Hence, the cell-mediated cytotoxicity of CD8+ T cells was studied. This assay potentially measures one of the most relevant biological parameters. Our findings indicate that besides IFN-γ, M. w also induces significant CD8 T-cell cytotoxic activity.
Mycobacterial survival and replication depend on a complicated interplay between various host and mycobacterial factors. Most studies of mycobacterial immunity have focused on T-cell proliferation, cytokine production, and cytolytic activity, but the effects of these in vitro responses on the intracellular viability and growth of mycobacteria are also crucial (28). Cytolytic assays demonstrate specific lysis of infected monocytes by immune cells, but intracellular growth inhibition is not demonstrated by these assays. The mechanisms responsible for growth inhibition could be the activation of macrophage effector functions, viz., production of toxic effector molecules like reactive nitrogen and reactive oxygen (6), limiting the availability of iron to mycobacteria, or apoptosis. Paradoxically, macrophages are also the main host cells of M. tuberculosis (15). Results of intracellular growth inhibition activity of alveolar macrophages from M. w-immunized mice provide evidence that macrophage effector functions are activated by M. w immunization. This assay provides a more direct measure of in vivo resistance to M. tuberculosis. The panel of assays done in this study provides information about different aspects of mycobacterial immunity.
It has been proposed that persistent Ags which provide strong T-cell stimuli over extended periods of time activate effector T cells efficiently but exhaust memory (1, 2). What is required is a vaccine that can persist long enough to generate memory T cells but still gradually gets cleared by the host immune response without generating adverse pathology (8). It is probable that the immune response induced by a viable vaccine which persists for only a restricted time period best fulfills these requirements. In this study, the presence of M. w in the lungs of immunized mice was checked at different time points after immunization. At 6 to 7 weeks after immunization, no viable bacteria were detected, as determined by CFU counting on LJ plates (data not shown). This provides evidence that infection with M. w is self-limiting and short-lived, which is important for the induction of a memory response and also from a safety point of view.
In summary, our findings provide evidence that M. w has potential protective efficacy against M. tuberculosis. The lung bacterial load was lowest in the M. w aerosol-immunized group, while the liver and splenic loads were similar after immunization by both routes. These results support the notion that the immune system is sort of compartmentalized and responses are often strongest in compartments proximal to the site of vaccine application. M. w activates macrophage activity, as well as lymphocytes, as indicated by IFN-γ secretion and cytotoxic activity toward infected macrophages. Activated macrophages could inhibit the intracellular growth and multiplication of M. tuberculosis. M. w immunization by both the parenteral and aerosol routes gives higher protection than BCG given by the s.c. route in the mouse model of TB. Our current findings warrant further evaluation with guinea pigs and other large animals, along with comparison of long-term protection potential in different immunized groups.
Acknowledgments
This work was supported by a research grant (BT/PR/5340/Med/14/619/2005) from the Department of Biotechnology of the Indian Ministry of Science and Technology.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 3 November 2008.
REFERENCES
- 1.Barber, D. L., E. J. Wherry, D. Masopust, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439682-687. [DOI] [PubMed] [Google Scholar]
- 2.Bevan, M. J. 2002. Immunology: remembrance of things past. Nature 420748-749. [DOI] [PubMed] [Google Scholar]
- 3.Bhaskar, S., and P. Upadhyay. 2003. Design and evaluation of an aerosol infection chamber for small animals. Int. J. Pharm. 25543-48. [DOI] [PubMed] [Google Scholar]
- 4.Brandtzaeg, P., I. N. Farstad, and G. Haraldsen. 1999. Regional specialization in the mucosal immune system: primed cells do not always home along the same track. Immunol. Today 20267-277. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, D. J., and E. C. Butcher. 2002. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 195135-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, J., Y. Xing, R. S. Majliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 1751111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, L., J. Wang, A. Zganiacz, and Z. Xing. 2004. Single intranasal mucosal Mycobacterium bovis BCG vaccination confers improved protection compared to subcutaneous vaccination against pulmonary tuberculosis. Infect. Immun. 72238-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins, D. M. 2000. New tuberculosis vaccines based on attenuated strains of the Mycobacterium tuberculosis complex. Immunol. Cell Biol. 78342-348. [DOI] [PubMed] [Google Scholar]
- 9.Derby, E., V. Reddy, M. Baseler, and A. Malyguine. 2001. Flow cytometric assay for the simultaneous analysis of cell-mediated cytotoxicity and effector cell phenotype. BioTechniques 31660-665. [DOI] [PubMed] [Google Scholar]
- 10.Falero-Diaz, G., S. Challacombe, D. Banerjee, G. Douce, and J. Ivanyi. 2000. Intranasal vaccination of mice against infection with Mycobacterium tuberculosis. Vaccine 183223-3229. [DOI] [PubMed] [Google Scholar]
- 11.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 1993-129. [DOI] [PubMed] [Google Scholar]
- 12.Kamath, A. T., U. Fruth, M. J. Brennan, K. B. Walker, and M. Liu. 2005. New live mycobacterial vaccines: the Geneva consenses on essential steps towards clinical development. Vaccine 233753-3761. [DOI] [PubMed] [Google Scholar]
- 13.Katoch, K., and V. M. Katoch. 1995. Treatment of bacilliferrous BL/LL cases with combined chemotherapy and immunotherapy. Int. J. Lepr. 63202-212. [PubMed] [Google Scholar]
- 14.Kaufmann, S. H. 2001. How can immunology contribute to the control of tuberculosis? Nat. Rev. Immunol. 120-30. [DOI] [PubMed] [Google Scholar]
- 15.Leemans, J. C., T. Thepen, S. Weijer, S. Florquin, and T. V. D. Poll. 2005. Macrophages play a dual role during pulmonary tuberculosis in mice. J. Infect. Dis. 19165-74. [DOI] [PubMed] [Google Scholar]
- 16.McGhee, J. R., C. Czerkinsky, and J. Mestecky. 1999. Mucosal vaccines: an overview, p. 741-757. In P. L. Ogra, M. E. Lamm, J. Bienenstock, J. Mestecky, and J. R. McGhee (ed.), Mucosal immunology, 2nd ed. Academic, London, United Kingdom.
- 17.Patel, N., M. M. Deshpande, and M. Shah. 2002. Effect of an immunomodulator containing Mycobacterium w on sputum conversion in pulmonary tuberculosis. J. Indian Med. Assoc. 100191-193. [PubMed] [Google Scholar]
- 18.Reddi, P. P., A. G. Amin, P. S. Khandekar, and G. P. Talwar. 1994. Molecular definition of unique species status of Mycobacterium w; a candidate leprosy vaccine strain. Int. J. Lepr. Other Mycobact. Dis. 62229-236. [PubMed] [Google Scholar]
- 19.Rodríguez, A., A. Tjarnlund, J. Ivanji, M. Singh, M. T. Blomberg, and C. Fernandez. 2005. Role of IgA in the defense against respiratory infections IgA deficient mice exhibited increased susceptibility to intranasal infection with Mycobacterium bovis BCG. Vaccine 232565-2572. [DOI] [PubMed] [Google Scholar]
- 20.Serbina, N. V., C. C. Liu, C. A. Scanga, and J. L. Flynn. 2000. CD8+ CTL from lungs of Mycobacterium tuberculosis-infected mice express perforin in vivo and lyse infected macrophages. J. Immunol. 165353-363. [DOI] [PubMed] [Google Scholar]
- 21.Sharma, P., R. Mukherjee, G. P. Talwar, K. G. Sarathchandra, and P. Singh. 2005. Immunoprophylactic effects of the anti-leprosy Mw vaccine in household contacts of leprosy patients: clinical field trials with a follow up of 8-10 years. Lepr. Rev. 76127-143. [PubMed] [Google Scholar]
- 22.Singh, I. G., R. Mukherjee, and G. P. Talwar. 1991. Resistance to intravenous inoculation of Mycobacterium tuberculosis H37Rv in mice of different inbred strains following immunization with a leprosy vaccine based on Mycobacterium w. Vaccine 910-13. [DOI] [PubMed] [Google Scholar]
- 23.Singh, I. G., R. Mukherjee, G. P. Talwar, and S. H. E. Kaufmann. 1992. In vitro characterization of T cells from Mycobacterium w-vaccinated mice. Infect. Immun. 60257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talwar, G. P., N. Ahmed, and V. Saini. 2007. The use of the name Mycobacterium w for the leprosy immunotherapeutic bacillus creates confusion with M. tuberculosis-W (Beijing strain): a suggestion. Infect. Genet. Evol. 8100-101. [DOI] [PubMed] [Google Scholar]
- 25.Talwar, G. P., S. A. Zaheer, R. Mukherjee, R. Walia, and N. R. Suresh. 1990. Immunotherapeutic effects of a vaccine based on a saprophytic cultivable mycobacterium, Mycobacterium w in multibacillary leprosy patients. Vaccine 8121-129. [DOI] [PubMed] [Google Scholar]
- 26.van Rie, A., R. Warren, M. Richardson, T. C. Victor, R. P. Gie, D. A. Enarson, N. Beyers, and P. D. van Helden. 1999. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N. Engl. J. Med. 3411174-1179. [DOI] [PubMed] [Google Scholar]
- 27.Williams, A., R. Reljic., I. Naylor., P. D. Marsh., and J. Ivanyi. 2004. Passive protection with immunoglobulin A antibodies against tuberculous early infection of the lungs. Immunology 111328-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Worku, S., and D. F. Hoft. 2003. Differential effects of control and antigen-specific T cells on intracellular mycobacterial growth. Infect. Immun. 711763-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yadava, A., and R. Mukherjee. 1993. An immunodominant 30-kDa antigen of a candidate anti-leprosy vaccine, Mycobacterium w, shares T and B cell determinants with M. leprae and M. tuberculosis. Med. Microbiol. Immunol. 182243-253. [DOI] [PubMed] [Google Scholar]
- 30.Zaheer, S. A., R. Mukherjee, B. Ramkumar, R. S. Misra, A. K. Sharma, H. K. Kar, H. Kaur, S. Nair, A. Mukherjee, and G. P. Talwar. 1993. Combined multidrug and Mycobacterium w vaccine therapy in patients with multibacillary leprosy. J. Infect. Dis. 167401-410. [DOI] [PubMed] [Google Scholar]