Abstract
Streptococcus pneumoniae has been shown to bind to erythrocytes via a process called immune adherence. This adherence and the subsequent transfer of pneumococci from erythrocytes to macrophages are both dependent on complement C3 deposition onto the pneumococcal surface. The observation that anti-capsule antibody increases C3 deposition on the pneumococcal capsule indicated that anti-capsule antibody may also facilitate the clearance of pneumococci through immune adherence. Using pneumococcal strain WU2 (capsule type 3) and its nonencapsulated mutant JD908, we found that monoclonal antibody (MAb) to type 3 capsule increases complement C3, C1q, and C4 deposition on WU2 and enhanced the immune adherence of WU2 to erythrocytes. The MAb to type 3 capsule also enhanced the transfer of WU2 from erythrocytes to macrophages. Moreover, the transfer reaction was inhibited by preincubating macrophages with anti-CR3 or anti-FcγRIII/II MAb, indicating that CR3 and FcγRIII/II on macrophages mediate this process. The transfer reactions of JD908 (opsonized with complement) and WU2 (opsonized with complement plus MAb to type 3 capsule) were similarly inhibited by anti-CR3 MAb, but only the latter was inhibited by anti-FcγRIII/II MAb. This finding indicates that although complement and the macrophage receptor CR3 are essential for the transfer reaction, if antibody is present it can further enhance the transfer reaction through a process dependent on FcγRIII/II. Using pre- and postvaccination sera of people immunized with the 23-valent pneumococcal polysaccharide vaccine, we confirmed that human anti-capsule antibodies are also able to increase the immune adherence of pneumococci and their transfer to macrophages.
Streptococcus pneumoniae (pneumococci) is a major human pathogen that causes pneumonia, bacteremia, meningitis, otitis media, and sinusitis, especially in children, the elderly, and immunocompromised patients (36). All of the natural strains of pneumococci are encapsulated by polysaccharide. According to the different constituents of their capsular polysaccharide, 91 serotypes of pneumococci are known (39). Among these, types 14, 6B, 19F, and 18C are most prevalent in small children and types 4, 14, 9V, and 23F are more frequently isolated from adults with invasive pneumococcal diseases (29). The 23-valent polysaccharide vaccine and a protein conjugate vaccine are recommended for adults and children, respectively (3).
Pneumococci are able to activate both the classical and alternative pathways of complement (12, 41). The thick and rigid cell wall of pneumococci can protect them from being lysed by the complement membrane attack complex (28), and therefore opsonophagocytosis, mediated by surface-bound C3b, is thought to be essential for the elimination of pneumococci from the bloodstream (5, 9). The ability of complement to effectively opsonize pneumococci is dependent on the location and orientation of C3b bound to the bacterial surface, as this determines the accessibility of C3b to phagocytic cell C3b receptors (10). Although capsular polysaccharide, the outermost layer of pneumococci, is not an efficient activator of complement, the underlying cell wall teichoic acid has been reported to activate complement via the alternative pathway (45). Being sheltered by capsular polysaccharide, however, C3b deposited on the pneumococcal cell wall cannot interact efficiently with complement receptors (CR) on phagocytic cells. As a result, antibody to the pneumococcal cell wall is much less opsonic and less protective than antibody to pneumococcal capsular polysaccharides (6, 7, 10).
S. pneumoniae adheres to erythrocytes in a complement- and antibody-dependent process called immune adherence (IA), which enhances the phagocytosis of pneumococci by polymorphonuclear leukocytes (23, 38). Studies using soluble immune complexes have shown that IA is mediated by complement C3b, C1q, C4b, and MBL interacting with CR type 1 (CR1) on human erythrocytes (21, 22, 43). The IA of pneumococci to human erythrocytes, as well as their subsequent transfer from erythrocytes to macrophages for clearance, depends on complement C3 deposition onto the pneumococcal surface (31). The known ability of antibody to pneumococcal capsular polysaccharide to enhance complement activation and C3 deposition led us to hypothesize that anti-capsule antibody might facilitate the IA and transfer reaction of pneumococci.
In this study, a capsular type 3 pneumococcal strain and its capsule-negative isogenic mutant were used to investigate the effects mediated by anti-capsule antibody. We found that deposition of complement C3b, C1q, and C4b was associated with elevated IA of pneumococci in the presence of anti-capsule antibody. Moreover, anti-capsule antibody increases the transfer of pneumococci from erythrocytes to macrophages by promoting interaction with both CR3 and Fcγ receptors.
MATERIALS AND METHODS
Pneumococcal strains.
Capsule type 3 pneumococcal strain WU2 (Cps3+) and its nonencapsulated mutant JD908 (Cps3−) (17, 18) were used. Pneumococcal strains of capsular type 3 (A66.1), capsular type 4 (TIGR4), capsular type 6B (STREP6B), and capsular type 23F (STREP23F) were also used (13). The bacteria were grown on blood agar plates at 37°C for 16 to 18 h in a candle jar and subcultured in Todd-Hewitt broth supplemented with 0.5% yeast extract. The bacteria were grown to an optical density of 0.45 at 600 nm and washed twice with pH 7.4 phosphate-buffered saline (PBS). A portion of the bacteria was frozen at −80°C in Hanks' balanced salt solution supplemented with 0.25% bovine serum albumin (0.25% BSA/HBSS) with 10% glycerol or labeled with fluorescein isothiocyanate (FITC) as described previously (31). The remaining bacteria were quantified by serial dilution and plating on blood agar. To maintain the inactivating insert in its cap3 gene, JD908 was grown in culture medium containing erythromycin (0.3 μg/ml).
Cells.
Erythrocytes were separated from human venous blood drawn from healthy volunteers (with informed consent and Institutional Review Board approval) with Ficoll-Paque PLUS (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) according to the manufacturer's instructions. The purity of the erythrocytes was >99% as checked with a hemocytometer. Purified erythrocytes were preserved in Alsever's solution (MP Biomedicals Inc., Aurora, OH) and stored at 4°C. The J774A.1 murine macrophage cell line was cultured as an adherent monolayer in Dulbecco modified Eagle medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (HyClone, Logan, UT) and 1% gentamicin (10 mg/ml; Invitrogen, Carlsbad, CA). The cells were split every 3 days to maintain a viability of no less than 90% as judged by trypan blue exclusion.
Sera.
Normal human serum (NHS) was obtained from blood drawn to purify erythrocytes. Human sera were also obtained from adults before and 1 month after vaccination with a 23-valent polysaccharide vaccine. Mouse immunoglobulin G3 (IgG3) monoclonal antibody (MAb) 16.3 to type 3 capsule (6) was obtained from mouse ascites fluid and heat inactivated by incubation at 56°C for 30 min. MAbs to CR3 and FcγRIII/II (2.4G2) were both purchased from BD Pharmingen. MAb to keyhole limpet hemocyanin was kindly provided by Mary Ann Accavitti-Loper (Genetics Research Division, University of Alabama at Birmingham). Complement-deficient mouse serum was obtained from animals with a genetically determined complete deficiency of C1q or C3 (4, 14). All sera were stored at −80°C as single-use aliquots of 50 to 100 μl.
Complement deposition assay.
Pneumococci were dispersed in 5% BSA/HBSS to a concentration of 1 × 109 CFU/ml. A volume of 200 μl of the pneumococcal dispersion was incubated with 10 μl of human serum and 20 μl of MAb to type 3 capsule at 37°C for 30 min. The bacteria were then washed with PBS and resuspended in 200 μl of biotin-labeled goat IgG antibodies reactive with human C3, C1q, or C4 (1:100 dilutions in PBS; ICN Biomedicals, Aurora, OH). Each antibody was biotinylated with a biotin-labeling kit according to the manufacturer's instructions (Roche Diagnostics GmbH, Mannheim, Germany). As a control, bacteria were incubated with 5% BSA/HBSS (instead of human serum) and exposed to biotin-labeled antiserum. After 30 min of incubation at 37°C, the bacteria were washed and incubated with 200 μl of Alexa Fluor 488-conjugated streptavidin (10 μg/ml in PBS; Molecular Probes, Eugene, OR) on ice for 30 min. After washing, the bacteria were fixed in 300 μl of 1% paraformaldehyde. Bacterial surface-bound C3, C1q, or C4 was measured by flow cytometry on a FACScalibur machine with CellQuest software (Becton Dickinson, Mountain View, CA). The mean fluorescence (MF) was calculated for each sample.
Erythrocyte adherence assay.
Erythrocytes were prepared by washing with isotonic (2.37%) sodium iodide (Sigma, St. Louis, MO) to elute adsorbed serum proteins and then resuspended in 5% BSA/HBSS to a concentration of 2 × 108/ml. A volume of 200 μl of FITC-labeled bacteria (2 × 108/ml) was incubated with 10 μl of NHS, alone or along with different percentages of MAb to type 3 capsule, at 37°C for 30 min while shaking. Thereafter, 200 μl of erythrocytes was added and the incubation was continued for 30 min. After washing with 0.1% BSA/HBSS to remove unbound bacteria, the erythrocytes and adherent bacteria were fixed with 1% paraformaldehyde for flow cytometry. Erythrocytes were gated, and 20,000 events were counted. The MF of erythrocytes was calculated for each sample. To measure the erythrocyte adherence mediated by human anti-capsule antibody, bacteria were incubated with 10 μl of normal mouse serum as a common source of complement, alone or along with 10 μl of heat-inactivated (56°C for 30 min) human pre- or postvaccination serum. Erythrocyte adherence was calculated by subtracting the erythrocyte adherence exhibited in normal mouse serum from that exhibited in normal mouse serum plus pre- or postvaccination serum.
Transfer reaction (transfer of opsonized pneumococci from erythrocytes to macrophages).
Transfer reaction experiments were conducted exactly as in the erythrocyte adherence assay described above, except that after the free bacteria were washed from the erythrocytes (and prior to flow cytometry), 200 μl of J774A.1 macrophages (2 × 106/ml) was added and the mixture was incubated at 37°C for 30 min while shaking. The erythrocytes were then lysed with BD FACS lysing solution (BD Biosciences, San Jose, CA) for 10 min at room temperature. After washing with 0.1% BSA/HBSS, the macrophages were fixed with 1% paraformaldehyde and analyzed by flow cytometry. Macrophages were gated, and 15,000 events were collected. The MF of macrophages was used to measure the transfer reaction. The natural fluorescence of macrophages was subtracted from each sample. To evaluate the involvement of CR3 and FcγRIII/II in mediating the transfer reaction, the macrophages were preincubated with rat anti-mouse CD11b (CR3) or rat anti-mouse CD16/CD32 (FcγRIII/II) MAb at 37°C for 30 min, after which the macrophages were washed and added to the erythrocytes as described above. To evaluate the transfer reaction mediated by human anti-capsule antibody, the transfer reaction was conducted with normal mouse serum as a common source of complement, alone or along with heat-inactivated human pre- or postvaccination serum. The transfer reaction was calculated by subtracting the MF of macrophages obtained in normal mouse serum from that obtained in normal mouse serum plus pre- or postvaccination human serum.
RESULTS
Anti-capsule 3 MAb increases complement C3, C1q, and C4 deposition onto type 3 pneumococcus.
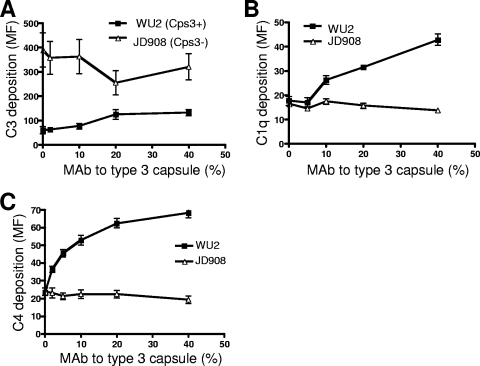
To determine the effects of a mouse MAb to type 3 capsule on complement C3, C1q, and C4 deposition onto the pneumococcal surface, type 3 pneumococcal strain WU2 (Cps3+) and its nonencapsulated mutant JD908 (Cps3−) were opsonized in NHS alone or along with different concentrations of MAb to type 3 capsule. The bacterial surface-bound C3, C1q, and C4 were then detected by flow cytometry. We found that in the absence of MAb to type 3 capsule, complement C3 deposition onto Cps3+ strain WU2 was much lower than that onto the Cps3− isogenic mutant JD908 (Fig. 1A), although similar amounts of C1q and C4 were deposited on WU2 and JD908 (Fig. 1B and C). These findings suggest that the classical pathway was equally activated on WU2 and JD908. The more robust C3 deposition onto Cps3− mutant JD908 versus WU2 might have been via the alternative or mannose pathway C3 activation that may have been stimulated because of exposure of the cell wall.
FIG. 1.
Effects of heat-inactivated MAb to type 3 capsule (16.3, ascites fluid) on C3 (A), C1q (B), and C4 (C) deposition on WU2 (Cps3+) and its nonencapsulated mutant JD908 (Cps3−). Pneumococci were opsonized in NHS supplemented with different concentrations of MAb to type 3 capsule at 37°C for 30 min. FITC-conjugated goat-anti-human C3, C1q, or C4 antibody was added. After incubation on ice for 30 min, the bacteria were washed and fixed for FACS analysis. Complement deposition was measured by MF of the gated bacteria. Error bars indicate the standard errors of triplicate samples. The amount of MAb to capsule is presented as the percentage of MAb-containing ascites fluid in the final mixture of pneumococci, serum (complement source), and ascites fluid. The amounts of pneumococci and complement remained constant.
The amount of C3, C1q, and C4 deposited onto WU2 increased with increasing amounts of MAb to type 3 capsule, whereas no increase in complement deposition was observed with Cps3− strain JD908 (Fig. 1A to C). Maximal C3 deposition onto WU2 was achieved with 2% MAb (Fig. 1A). The increased C1q and C4 deposition onto WU2 in the presence of MAb to type 3 capsule suggested that MAb to type 3 capsule could enhance the activation of the classical pathway, which contributed to the increased C3 deposition on WU2 when MAb to type 3 capsule was added. Therefore, although the type 3 capsule of WU2 inhibits C3 deposition generated via the alternative pathway, addition of MAb to type 3 capsule overcomes this by promoting classical pathway activation, which increases C3 deposition.
Anti-capsule 3 MAb increases the adherence of type 3 pneumococci to erythrocytes.
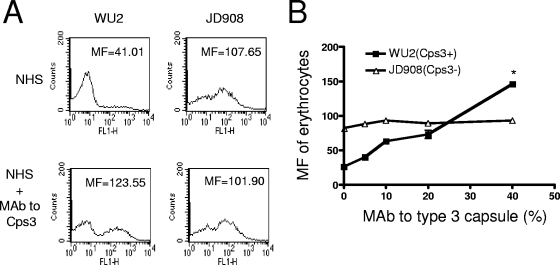
The erythrocyte adherence assay was conducted by flow cytometry. The Cps3+ strain (WU2) opsonized in NHS exhibited a much lower adherence to erythrocytes than the Cps3− mutant (JD908), as measured by the MF of erythrocytes after incubation with the FITC-labeled bacteria (Fig. 2A). However, when the concentration of MAb to type 3 capsule ascites fluid was 4%, the adherence of WU2 to erythrocytes increased to double that observed with NHS alone and reached a level higher than the adherence of JD908 to erythrocytes. As expected, the level of adherence of Cps3− mutant JD908 to erythrocytes was not affected by the addition of MAb to type 3 capsule, reinforcing our conclusions that the increase in the adherence of WU2 to erythrocytes was mediated by the MAb to the type 3 capsule in the diluted ascites fluid.
FIG. 2.
Effects of MAb to type 3 capsule on the adherence of WU2 (Cps3+) and JD908 (Cps3−) to erythrocytes. (A) Erythrocyte adherence of WU2 and JD908 in the presence of NHS alone or supplemented with 4% MAb to type 3 capsule. (B) Adherence of WU2 and JD908 to erythrocytes in the presence of NHS in relation to the concentration of MAb to type 3 capsule. The adherence of pneumococci to erythrocytes was measured by MF of erythrocytes. The asterisk indicates that the adherence of WU2 to erythrocytes was significantly higher than that of JD908 opsonized in NHS plus 4% heat-inactivated MAb to type 3 capsule (16.3, ascites fluid) (P = 0.0001, Student's two-tailed t test). Error bars indicate the standard errors of triplicate samples.
The adherence of WU2 to erythrocytes in the presence of (4%) MAb to type 3 capsule was significantly higher than that of JD908 (P = 0.0001, Student's two-tailed t test) (Fig. 2B). The adherence of JD908 to erythrocytes was essentially unchanged by the addition of MAb to the type 3 capsule, which is consistent with our observation in Fig. 1 that complement deposition on JD908 was not influenced by adding of MAb to type 3 capsule.
Anti-capsule 3 MAb increases the adherence of type 3 pneumococci to erythrocytes by promoting complement activation.
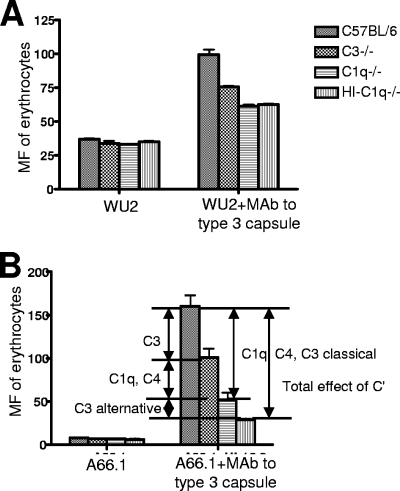
To decipher which complement components were responsible for the increase in the adherence of WU2 to erythrocytes in the presence of MAb to type 3 capsule, the erythrocyte adherence assay was conducted with WU2 and another type 3 pneumococcal strain A66.1, opsonized in mouse sera that were deficient in specific complement components. Both WU2 and A66.1 exhibited higher adherence to erythrocytes in the presence of MAb to type 3 capsule than in its absence (Fig. 3A and B). The absence of C3, C1q, or all complement activity (heat-inactivated sera lacking C1q) depressed IA with both strains, with the absence of C1q or the absence of all complement having the largest effect (Fig. 3A and B).
FIG. 3.
Adherence of type 3 pneumococci to erythrocytes in the presence of mouse serum. Sera from a wild-type mouse (C57BL/6), a C3-deficient mouse (C3−/−), a C1q-deficient mouse (C1q−/−), and a heat-inactivated C1q-deficient mouse (HI-C1q−/−) were used to opsonize type 3 pneumococcal strains WU2 and A66.1. The adherence of WU2 (A) and A66.1 (B) to erythrocytes in various mouse sera with or without supplementation with heat-inactivated MAb to type 3 capsule (16.3, ascites fluid) was measured by MF of erythrocytes. The portions of adherence of pneumococci to erythrocytes mediated by different complement components are indicated in panel B.
The difference in adherence obtained with C3−/− serum versus normocomplementemic (C57BL/6) serum is attributable to the inability of C3−/− serum to support C3b-mediated opsonization (Fig. 3B). Although complement components downstream of C3 cannot be activated in C3−/− serum, C1 and C4, which are upstream of C3 in the classical pathway, can still serve as opsonins (43). Therefore, it is likely that C1q and C4b are able to promote some erythrocyte adherence of pneumococci even in the absence of C3. To eliminate adherence mediated by C1q and classical pathway-generated C3b and C4b, the erythrocyte adherence assay was conducted with C1q-deficient (C1q−/−) mouse serum. In this instance, adherence was reduced even further with both WU2 and A66.1. In C1q−/− serum, C3b can still be generated through activation of the alternative pathway. To eliminate this source of opsonization, we heat inactivated C1q−/− serum. When heat-inactivated C1q−/− serum was used, there was no additional reduction of the erythrocyte adherence of WU2 but a further reduction of the adherence of A66.1 was observed. This result is consistent with those shown in Fig. 1, wherein WU2 completely inhibited complement C3 deposition mediated by the alternative pathway.
Overall, the difference in adherence observed with heat-inactivated C1q−/− serum versus normocomplementemic (C57BL/6) serum represents the total adherence mediated by complement. It appears that MAb to capsule type 3 polysaccharide increases the adherence of WU2 to erythrocytes by increasing complement C1q, C4b, and C3b deposition through the classical pathway, whereas for A66.1 both the classical and alternative pathways are involved in the elevated complement deposition mediated by MAb to capsular polysaccharide.
Erythrocyte adherence exhibited in heat-inactivated C1q−/− mouse serum presumably is mediated by noncomplement components. The greater adherence mediated by MAb to the type 3 capsule in the absence of complement, compared to the adherence in the absence of complement and no antibody, may be due to a direct effect of the MAb. The absolute size of this effect was greater with WU2 than with A66.1, but the percentage of increase over no antibody was greater with A66.1.
Capsule type 3 strains A66.1 and WU2 differ greatly in virulence but are both readily protected against by specific antibody (8). They were both included in this study to help allow us to generalize the results obtained.
Anti-capsule 3 MAb facilitates the transfer of type 3 pneumococcus from erythrocytes to macrophages.
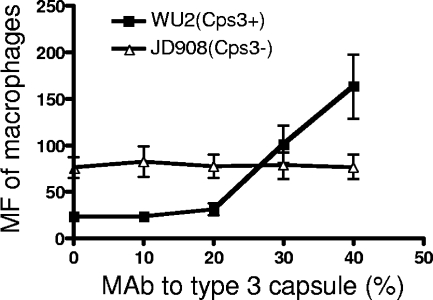
To determine whether the adherence of WU2 to erythrocytes promoted by MAb to type 3 capsule in the presence of complement is biologically relevant, transfer reactions were conducted with erythrocyte-bound WU2 or JD908 and various concentrations of MAb to type 3 capsule. In the absence of added MAb to type 3 capsule, there were more JD908 (Cps3−) than WU2 (Cps3+) pneumococci transferred from erythrocytes to macrophages (Fig. 4), which is in agreement with the observed higher adherence of JD908 to erythrocytes in NHS (Fig. 2).
FIG. 4.
Influence of MAb to type 3 capsule on the transfer reaction of WU2 (Cps+) and JD908 (Cps3−). FITC-labeled pneumococci were opsonized in NHS supplemented with different concentrations of MAb to type 3 capsule. Erythrocytes were added and incubated at 37°C for 30 min. After washing, J774A.1 macrophages were added and the incubation was repeated. Erythrocytes in the samples were then lysed and washed off. The macrophages were fixed for FACS analysis. The MF of macrophages was used to measure the transfer reaction of pneumococci. The natural fluorescence of macrophages was subtracted from each sample. The subtracted values were never more than 3% of the total MF of macrophages observed after the transfer reaction.
Similar amounts of JD908 were transferred to macrophages regardless of the amounts of MAb to type 3 capsule added. In contrast, significantly more WU2 was transferred to macrophages when more than 2% MAb to type 3 capsule was added. With the addition of 4% MAb to type 3 capsule, the transfer reaction of WU2 reached a higher level than that of JD908, which resembled the erythrocyte adherence of WU2 and JD908 in the presence of MAb to type 3 capsule (Fig. 2B). These data indicate that the increased erythrocyte adherence of WU2 mediated by MAb to type 3 capsule also promotes transfer of WU2 to macrophages, suggesting that MAb to type 3 capsule may facilitate the clearance of type 3 pneumococci through IA. We also conducted this study with a MAb to keyhole limpet hemocyanin. This MAb did not enhance either IA or transfer of bacteria to macrophages (data not shown).
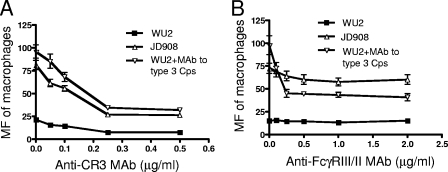
The transfer reaction of pneumococci is mediated by CR3 and Fcγ receptors on macrophages.
To determine whether CR3 is involved in the transfer reaction of opsonized pneumococci, macrophages were pretreated with various concentrations of MAb to CR3 before incubation with erythrocyte-bound pneumococci. The transfer reactions of both WU2 and JD908 were inhibited by anti-CR3 MAb (Fig. 5A). When WU2 was preincubated with 4% MAb to type 3 capsule, although the transfer reaction was elevated to a higher level than that of JD908, the transfer reaction was still inhibited by anti-CR3 MAb. The maximal inhibition was achieved with 0.25 μg/ml anti-CR3 MAb for all three preparations of pneumococci. The transfer reactions of JD908 in NHS and WU2 in NHS plus MAb to type 3 capsule were similarly inhibited by anti-CR3 MAb, suggesting that the elevated C3b deposited on WU2 upon the addition of MAb to type 3 capsule functions in a manner similar to that of C3b on JD908 in mediating the transfer reaction.
FIG. 5.
Inhibition of macrophage transfer reaction of pneumococci by anti-CR3 MAb (A) and anti-FcγRIII/II MAb (B). The macrophages were preincubated with different concentrations of anti-CR3 MAb or anti-FcγRIII/II MAb before the transfer reaction was conducted. Error bars indicate the standard errors of triplicate samples.
The contribution of Fcγ receptors to the transfer reaction was similarly determined by pretreating macrophages with various concentrations of MAb to FcγRIII/II (Fig. 5B).
Anti-FcγRIII/II MAb caused little, if any, change in the transfer reactions of WU2 and JD908, suggesting that FcγRIII/II may not play a major role in mediating the transfer of WU2 and JD908 from erythrocytes to macrophages in NHS, in which the antipneumococcal antibody titers are low. In contrast, the transfer reaction of WU2 opsonized with MAb to type 3 capsule was significantly inhibited by anti-FcγRIII/II MAb at concentrations as low as 0.125 μg/ml (Fig. 5B). Moreover, the transfer reaction of WU2 opsonized with MAb to type 3 capsule dropped to a level lower than that of JD908 when macrophages were pretreated with 0.25 μg/ml anti-FcγRIII/II MAb. Higher concentrations of anti-FcγRIII/II MAb did not yield any further inhibition of the transfer reaction, suggesting that 0.25 μg/ml anti-FcγRIII/II MAb was sufficient to block the FcγRIII/II that mediates the transfer reaction. Together, these data show that CR3 is a primary mediator of the transfer reaction of the type 3 pneumococcus independent of the antipneumococcal antibodies, whereas FcγRIII/II on macrophages plays a supplemental role in that it mediates the transfer reaction only when antipneumococcal antibodies are present.
Human anti-capsule antibody is able to increase the IA of pneumococci and promote their transfer to macrophages.
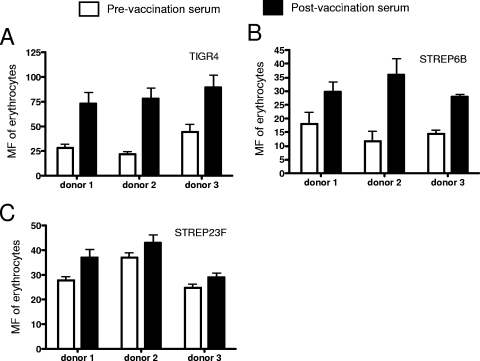
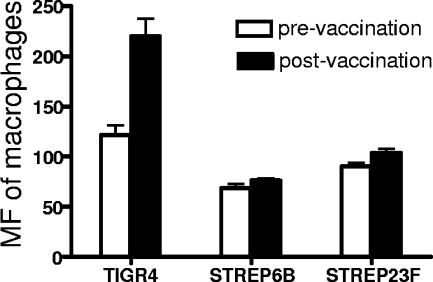
To determine whether human anti-capsule antibodies facilitate the IA and transfer reaction of pneumococci, an erythrocyte adherence assay and a transfer reaction were conducted with pre- and postvaccination sera collected from people immunized with the 23-valent pneumococcal polysaccharide vaccine. Erythrocyte adherence was measured in three types of pneumococci (types 4, 6B, and 23F) opsonized in pre- and postvaccination sera of three people (donors 1, 2, and 3) (Fig. 6). For all three strains, the erythrocyte adherence exhibited in postvaccination sera was higher than that in prevaccination sera. The differences in erythrocyte adherence between pre- and postvaccination sera were greatest with the type 4 strain. The pattern of erythrocyte adherence exhibited in three serum samples for each strain (Fig. 6A to C) was in agreement with the pattern of opsonophagocytosis exhibited in these sera with each strain (data not shown). Of the three strains, TIGR4 was the one that showed the greatest increase in erythrocyte adherence in comparisons of the pre- and postimmune sera from donor 1. For strain TIGR4, the transfer reaction exhibited with the postimmune serum from donor 1 was almost twice that seen with the preimmune serum (Fig. 7). No significant difference between the transfer reactions obtained with pre- and postimmune sera from donor 1 was observed. The transfer reaction was not examined with any of the strains with sera from donors 2 and 3.
FIG. 6.
Adherence of pneumococci to erythrocytes opsonized with pre- and postvaccination sera of people immunized with the 23-valent pneumococcal polysaccharide vaccine. Type 4 (A), 6B (B), and 23F (C) pneumococci were examined. Error bars indicate the standard errors of triplicate samples.
FIG. 7.
Macrophage transfer reaction of pneumococci obtained with pre- and postvaccination sera. Type 4, 6B, and 23F pneumococci were opsonized with pre- and postvaccination sera of a person (donor 1, as in Fig. 6) immunized with the 23-valent pneumococcal polysaccharide vaccine. Error bars indicate the standard errors of triplicate samples.
The failure of the immune versus the preimmune sera to cause a similar increase in erythrocyte adherence for each capsular type and the failure of the different capsular types to be affected similarly by any one serum (Fig. 6) are undoubtedly due to differences in the amounts of complement-fixing antibody to the different capsular types elicited in the different donors. Similarly, the failure of donor 1 serum to cause the transfer reaction with all three capsular types is probably related to differences in complement-fixing antibody to the different types in donor serum 1. In this regard, it should be noted that donor serum 1 caused the strongest IA for capsular type 4, the same capsular type for which the same donor serum caused the greatest increase in IA.
DISCUSSION
The phenomenon of IA, recognized long ago, has been the topic of renewed interest in recent years. Several pathogens are now known to attach to erythrocytes through IA (19, 26, 40). In the case of human immunodeficiency virus (HIV), IA might play a role in disseminating the infection, because in the presence of complement, free HIV type 1, as well as HIV type 1/anti-HIV immune complexes, could attach to erythrocytes through IA (26). In addition, immunotherapeutic methods using heteropolymers to target circulating immune complexes have been developed based on the process of IA (15, 42, 44).
Previous studies done in our laboratory indicated that the IA of pneumococci and the transfer of pneumococci from erythrocytes to macrophages are dependent on C3 deposition onto the pneumococcal surface (31), suggesting that molecules that increase C3 deposition on the pneumococcal surface may possibly enhance both the IA and the transfer reaction of pneumococci. In the present study, we have shown that antibody to type 3 pneumococcal capsular polysaccharide facilitates the IA of pneumococci by increasing complement C3b, C1q, and C4b deposition, and the increased erythrocyte-bound pneumococci could be transferred to macrophages through interaction with CR3 and FcγRIII/II of macrophages.
Our study supports the previous findings that the pneumococcal capsule interferes with the recognition of cell wall-bound C3b molecules by the complement receptors on erythrocytes and phagocytic cells (10, 11). Furthermore, we showed that the type 3 capsule of pneumococci may directly inhibit complement activation via the alternative pathway. The lower level of C3 deposition on the Cps3+ strain compared to the Cps3− mutant opsonized in NHS (Fig. 1A) was likely not due to a failure to detect C3 on the cell wall, since C1q and C4 were detected on the Cps3+ strain at a level similar to that on the Cps3− mutant (Fig. 1B and C). In consideration of the equally activated classical pathway on the Cps3+ strain and the Cps3− mutant, the elevated C3 deposition on the Cps3− mutant suggested that the presence of type 3 capsule may inhibit the activation of the alternative pathway.
Earlier studies found that C3 deposition on WU2 was three times less than on its Cps3− mutant JD611 (1). Although the absence of capsule in JD611 was conferred by stop mutations in cps3D (18), in contrast to the insertions between cps3D and cps3S that eliminated the capsule production in JD908 (17, 18), the inhibition of C3 deposition by type 3 capsule was manifested in both studies. When the type 3 capsule of WU2 was switched with the type 2 capsule of strain D39, the level of C3 deposition on the capsule switch mutant was intermediate between the levels observed with WU2 and D39, which suggested that the capsular type of pneumococci affects the amount of C3 deposition (1). Moreover, pneumococcal capsule may influence the proportions of C3b, iC3b, and C3d attached in ester linkage to capsular polysaccharides (27), which could eventually influence the IA and the subsequent transfer reaction of pneumococci.
The mechanisms by which immune complexes are transferred from erythrocytes to phagocytic cells remain controversial. Some in vitro models suggested that C3b, which mediates the IA, could be degraded into iC3b and then C3dg by the combined action of CR1 and factor I (34). The degradation products do not bind to CR1, thus releasing complement-opsonized immune complexes from erythrocyte CR1 back into the plasma for downstream clearance (25, 35). Some studies have suggested that the transfer reaction requires Fc recognition of erythrocyte-bound complexes by fixed tissue macrophages, followed by proteolysis of CR1 (32, 37). Still other studies have suggested that the transfer of soluble immune complexes from erythrocytes to monocytes is driven by the greater number of immune complex binding sites available on monocytes relative to erythrocytes and that the transfer reaction is not dependent on factor I or other enzymatic processing of immune complexes (20).
Our study showed that both CR3 and FcγRIII/II are involved in the transfer reaction of type 3 pneumococci and that CR3 plays a fundamental role in this process while FcγR is supplemental. These results are consistent with the findings of Hepburn et al. on the transfer reaction of soluble immune complexes, although in their study the transfer reaction was considered as a series of reactions (24). The complexity of pneumococcal surface components could make the transfer reaction of pneumococci more complicated than in the case of soluble immune complexes. Pneumococci have been shown to interact with several macrophage receptors other than complement and Fc receptors, such as Toll-like receptors 2 and 4, scavenger receptor SR-AI/II, and SIGN-R1 (2, 16, 30, 33), which could participate in the transfer reaction as well.
In summary, the present study shows that the type 3 capsule of pneumococci inhibits C3 deposition through the alternative pathway. However, in the presence of anti-capsule antibody, the deposition of C3, C1q, and C4 through the classical pathway is increased, which enhances the IA of pneumococci and the transfer of pneumococci from erythrocytes to macrophages. In addition, we found that CR3 plays a major role in mediating the transfer reaction and that FcγRIII/II is supplemental.
Demonstrating a role for IA in the in vivo clearance of pneumococci is a difficult problem. We are hopeful, however, that we will be able to address these issues in the future by studies that will include comparisons of immune blood clearance between transgenic mice expressing human CR-1 on their erythrocytes (42) and wild-type mice which lack CR-1 expression on their erythrocytes.
Acknowledgments
We thank Janice King and Robert Burton for technical assistance.
This work was supported by National Institutes of Health grant AI21548 (to D.E.B.).
Editor: J. N. Weiser
Footnotes
Published ahead of print on 10 November 2008.
REFERENCES
- 1.Abeyta, M., G. G. Hardy, and J. Yother. 2003. Genetic alteration of capsule type but not PspA type affects accessibility of surface-bound complement and surface antigens of Streptococcus pneumoniae. Infect. Immun. 71218-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arredouani, M. S., Z. Yang, A. Imrich, Y. Ning, G. Qin, and L. Kobzik. 2006. The macrophage scavenger receptor SR-AI/II and lung defense against pneumococci and particles. Am. J. Respir. Cell Mol. Biol. 35474-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barocchi, M. A., S. Censini, and R. Rappuoli. 2007. Vaccines in the era of genomics: the pneumococcal challenge. Vaccine 252963-2973. [DOI] [PubMed] [Google Scholar]
- 4.Botto, M. 1998. C1q knock-out mice for the study of complement deficiency in autoimmune disease. Exp. Clin. Immunogenet. 15231-234. [DOI] [PubMed] [Google Scholar]
- 5.Braconier, J. H., H. Odeberg, and A. G. Sjoholm. 1983. Granulocyte phagocytosis of Streptococcus pneumoniae in properdin-deficient serum. Infect. Immun. 40219-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briles, D. E., J. L. Claflin, K. Schroer, and C. Forman. 1981. Mouse IgG3 antibodies are highly protective against infection with Streptococcus pneumoniae. Nature 29488-90. [DOI] [PubMed] [Google Scholar]
- 7.Briles, D. E., C. Forman, J. C. Horowitz, J. E. Volanakis, W. H. Benjamin, Jr., L. S. McDaniel, J. Eldridge, and J. Brooks. 1989. Antipneumococcal effects of C-reactive protein and monoclonal antibodies to pneumococcal cell wall and capsular antigens. Infect. Immun. 571457-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briles, D. E., J. D. King, M. A. Gray, L. S. McDaniel, E. Swiatlo, and K. A. Benton. 1996. PspA, a protection-eliciting pneumococcal protein: immunogenicity of isolated native PspA in mice. Vaccine 14858-867. [DOI] [PubMed] [Google Scholar]
- 9.Brown, E. J., S. W. Hosea, and M. M. Frank. 1983. The role of antibody and complement in the reticuloendothelial clearance of pneumococci from the bloodstream. Rev. Infect. Dis. 5(Suppl. 4)S797-S805. [DOI] [PubMed] [Google Scholar]
- 10.Brown, E. J., S. W. Hosea, C. H. Hammer, C. G. Burch, and M. M. Frank. 1982. A quantitative analysis of the interactions of antipneumococcal antibody and complement in experimental pneumococcal bacteremia. J. Clin. Investig. 6985-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown, E. J., K. A. Joiner, R. M. Cole, and M. Berger. 1983. Localization of complement component 3 on Streptococcus pneumoniae: anti-capsular antibody causes complement deposition on the pneumococcal capsule. Infect. Immun. 39403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown, J. S., T. Hussell, S. M. Gilliland, D. W. Holden, J. C. Paton, M. R. Ehrenstein, M. J. Walport, and M. Botto. 2002. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc. Natl. Acad. Sci. USA 9916969-16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton, R. L., and M. H. Nahm. 2006. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin. Vaccine Immunol. 131004-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Circolo, A., G. Garnier, W. Fukuda, X. Wang, T. Hidvegi, A. J. Szalai, D. E. Briles, J. E. Volanakis, R. A. Wetsel, and H. R. Colten. 1999. Genetic disruption of the murine complement C3 promoter region generates deficient mice with extrahepatic expression of C3 mRNA. Immunopharmacology 42135-149. [DOI] [PubMed] [Google Scholar]
- 15.Craig, M. L., M. L. Reinagel, E. N. Martin, R. Schlimgen, A. Nardin, and R. P. Taylor. 1999. Infusion of bispecific monoclonal antibody complexes into monkeys provides immunologic protection against later challenge with a model pathogen. Clin. Immunol. 92170-180. [DOI] [PubMed] [Google Scholar]
- 16.Dessing, M. C., S. Florquin, J. C. Paton, and T. van der Poll. 2008. Toll-like receptor 2 contributes to antibacterial defence against pneumolysin-deficient pneumococci. Cell. Microbiol. 10237-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillard, J. P., M. W. Vandersea, and J. Yother. 1995. Characterization of the cassette containing genes for type 3 capsular polysaccharide biosynthesis in Streptococcus pneumoniae. J. Exp. Med. 181973-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dillard, J. P., and J. Yother. 1994. Genetic and molecular characterization of capsular polysaccharide biosynthesis in Streptococcus pneumoniae type 3. Mol. Microbiol. 12959-972. [DOI] [PubMed] [Google Scholar]
- 19.Domínguez, M., and A. Torano. 2001. Leishmania immune adherence reaction in vertebrates. Parasite Immunol. 23259-265. [DOI] [PubMed] [Google Scholar]
- 20.Emlen, W., V. Carl, and G. Burdick. 1992. Mechanism of transfer of immune complexes from red blood cell CR1 to monocytes. Clin. Exp. Immunol. 898-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fearon, D. T. 1980. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J. Exp. Med. 15220-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghiran, I., S. F. Barbashov, L. B. Klickstein, S. W. Tas, J. C. Jensenius, and A. Nicholson-Weller. 2000. Complement receptor 1/CD35 is a receptor for mannan-binding lectin. J. Exp. Med. 1921797-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hament, J. M., H. van Dijk, A. Fleer, P. C. Aerts, M. Schoenmakers, M. W. de Snoo, B. H. Dekker, J. L. Kimpen, and T. F. Wolfs. 2003. Pneumococcal immune adherence to human erythrocytes. Eur. J. Clin. Investig. 33169-175. [DOI] [PubMed] [Google Scholar]
- 24.Hepburn, A. L., J. C. Mason, S. Wang, C. J. Shepherd, O. Florey, D. O. Haskard, and K. A. Davies. 2006. Both Fcγ and complement receptors mediate transfer of immune complexes from erythrocytes to human macrophages under physiological flow conditions in vitro. Clin. Exp. Immunol. 146133-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hess, C., and J. A. Schifferli. 2003. Immune adherence revisited: novel players in an old game. News Physiol. Sci. 18104-108. [DOI] [PubMed] [Google Scholar]
- 26.Horakova, E., O. Gasser, S. Sadallah, J. M. Inal, G. Bourgeois, I. Ziekau, T. Klimkait, and J. A. Schifferli. 2004. Complement mediates the binding of HIV to erythrocytes. J. Immunol. 1734236-4241. [DOI] [PubMed] [Google Scholar]
- 27.Hostetter, M. K. 1986. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J. Infect. Dis. 153682-693. [DOI] [PubMed] [Google Scholar]
- 28.Joiner, K., E. Brown, C. Hammer, K. Warren, and M. Frank. 1983. Studies on the mechanism of bacterial resistance to complement-mediated killing. III. C5b-9 deposits stably on rough and type 7 S. pneumoniae without causing bacterial killing. J. Immunol. 130845-849. [PubMed] [Google Scholar]
- 29.Kalin, M. 1998. Pneumococcal serotypes and their clinical relevance. Thorax 53159-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang, Y. S., J. Y. Kim, S. A. Bruening, M. Pack, A. Charalambous, A. Pritsker, T. M. Moran, J. M. Loeffler, R. M. Steinman, and C. G. Park. 2004. The C-type lectin SIGN-R1 mediates uptake of the capsular polysaccharide of Streptococcus pneumoniae in the marginal zone of mouse spleen. Proc. Natl. Acad. Sci. USA 101215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, J., D. T. Glover, A. J. Szalai, S. K. Hollingshead, and D. E. Briles. 2007. PspA and PspC minimize immune adherence and transfer of pneumococci from erythrocytes to macrophages through their effects on complement activation. Infect. Immun. 755877-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindorfer, M. A., C. S. Hahn, P. L. Foley, and R. P. Taylor. 2001. Heteropolymer-mediated clearance of immune complexes via erythrocyte CR1: mechanisms and applications. Immunol. Rev. 18310-24. [DOI] [PubMed] [Google Scholar]
- 33.Malley, R., P. Henneke, S. C. Morse, M. J. Cieslewicz, M. Lipsitch, C. M. Thompson, E. Kurt-Jones, J. C. Paton, M. R. Wessels, and D. T. Golenbock. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. USA 1001966-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medof, M. E., K. Iida, C. Mold, and V. Nussenzweig. 1982. Unique role of the complement receptor CR1 in the degradation of C3b associated with immune complexes. J. Exp. Med. 1561739-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medof, M. E., and G. M. Prince. 1983. Immune complex alterations occur on the human red blood cell membrane. Immunology 5011-18. [PMC free article] [PubMed] [Google Scholar]
- 36.Musher, D. M. 1992. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin. Infect. Dis. 14801-807. [DOI] [PubMed] [Google Scholar]
- 37.Nardin, A., M. A. Lindorfer, and R. P. Taylor. 1999. How are immune complexes bound to the primate erythrocyte complement receptor transferred to acceptor phagocytic cells? Mol. Immunol. 36827-835. [DOI] [PubMed] [Google Scholar]
- 38.Nelson, R. A., Jr. 1953. The immune-adherence phenomenon; an immunologically specific reaction between microorganisms and erythrocytes leading to enhanced phagocytosis. Science 118733-737. [DOI] [PubMed] [Google Scholar]
- 39.Park, I. H., D. G. Pritchard, R. Cartee, A. Brandao, M. C. Brandileone, and M. H. Nahm. 2007. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 451225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pilsczek, F. H., A. Nicholson-Weller, and I. Ghiran. 2005. Phagocytosis of Salmonella montevideo by human neutrophils: immune adherence increases phagocytosis, whereas the bacterial surface determines the route of intracellular processing. J. Infect. Dis. 192200-209. [DOI] [PubMed] [Google Scholar]
- 41.Ren, B., A. J. Szalai, S. K. Hollingshead, and D. E. Briles. 2004. Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect. Immun. 72114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Repik, A., S. E. Pincus, I. Ghiran, A. Nicholson-Weller, D. R. Asher, A. M. Cerny, L. S. Casey, S. M. Jones, S. N. Jones, N. Mohamed, L. B. Klickstein, G. Spitalny, and R. W. Finberg. 2005. A transgenic mouse model for studying the clearance of blood-borne pathogens via human complement receptor 1 (CR1). Clin. Exp. Immunol. 140230-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tas, S. W., L. B. Klickstein, S. F. Barbashov, and A. Nicholson-Weller. 1999. C1q and C4b bind simultaneously to CR1 and additively support erythrocyte adhesion. J. Immunol. 1635056-5063. [PubMed] [Google Scholar]
- 44.Taylor, R. P., E. N. Martin, M. L. Reinagel, A. Nardin, M. Craig, Q. Choice, R. Schlimgen, S. Greenbaum, N. L. Incardona, and H. D. Ochs. 1997. Bispecific monoclonal antibody complexes facilitate erythrocyte binding and liver clearance of a prototype particulate pathogen in a monkey model. J. Immunol. 1594035-4044. [PubMed] [Google Scholar]
- 45.Winkelstein, J. A., and A. Tomasz. 1978. Activation of the alternative complement pathway by pneumococcal cell wall teichoic acid. J. Immunol. 120174-178. [PubMed] [Google Scholar]