Abstract
The association between extreme-prematurity births and intrauterine infection emphasizes the importance of understanding the host immune responses against uterine-invading microbes during early pregnancy to the prevention of preterm births. Listeria monocytogenes, a clinically relevant intracellular bacterium, has a predilection for replication at the maternofetal interface during pregnancy. Here, using mice carrying the recessive null osteopetrotic mutation in the colony-stimulating factor-1 (CSF-1) gene, we show that CSF-1-dependent macrophage functions are required for the maternal decidua immune responses against L. monocytogenes infections during early gestation in mice. In the absence of CSF-1, pregnant mice were more susceptible to uterine infection by L. monocytogenes; their inability to control the expansion of colonized bacteria in the pregnant uterus led to decidual cell death, tissue disintegration, and resorption of the developing embryo. However, CSF-1-deficient mice were able to produce significant levels of both Th1 cytokines and neutrophil chemoattractants and to recruit neutrophils to the decidual tissue in response to Listeria infection. Depletion of macrophages in hormonally induced pseudopregnant mice resulted in higher uterine bacterial levels after L. monocytogenes infection. These data suggest that the anti-Listeria responses in the maternal decidual tissue are dependent on CSF-1-regulated macrophages.
In the United States, the preterm delivery rate is 12 to 13% (38), which accounts for 75% of perinatal mortality and more than 50% of long-term morbidity (39); among these preterm births, 65 to 70% are spontaneous preterm births (22). Intrauterine infection has played a frequent and important role in causing preterm births, estimated to account for at least 25 to 40% of all cases (23), and it is associated with extreme-prematurity births (41). Therefore, effective anti-intrauterine infection mechanisms are important in maintaining a viable pregnancy. However, the use of antimicrobial therapy for the prevention of preterm delivery has remained ineffective, begging for better understanding of the molecular mechanisms of the immune regulation at the maternofetal interface (56). On the other hand, pregnancy also presents a major challenge to the maternal immune system, due to the existence of paternal alloantigens in the fetus that are foreign to the mother. Thus, strict regulation of the maternal immune system during pregnancy to induce immune tolerance of the developing fetus is crucial to a successful pregnancy (21), and an immunosuppressive environment at the maternofetal interface has been suggested (40). Nevertheless, the immune system of the pregnant mother has to balance the need to provide protection against microbial infections without compromising the viability of the allogeneic fetus.
Listeria monocytogenes is a clinically relevant microbe during human pregnancy, as this facultative intracellular bacterium has the ability to replicate at the maternofetal interface in the decidua basalis and the placenta (48, 49), and is a significant cause of fetal morbidity and mortality (37). Additionally, because of its ability to provoke a strong innate immune response, numerous studies have used L. monocytogenes to establish the importance of macrophages, neutrophils, and cytotoxic T cells in the systemic immune responses against this intracellular microbe (54).
Macrophages and cytotoxic T cells are normally excluded from the maternofetal interface; thus, the host anti-Listeria responses in the placenta are characterized by neutrophil dominance (49). Previous studies in our laboratory have established the role of trophoblasts as a component of the innate immune system during pregnancy to organize the placental defense against L. monocytogenes. Using mice carrying a mutation in the colony-stimulating factor-1 (CSF-1) gene, we have shown that trophoblastic tissue is the source of CSF-1-induced production of neutrophil chemoattractants, KC and macrophage inflammatory protein-2, which result in the recruitment of neutrophils to the placenta after L. monocytogenes infection (26). Moreover, our studies have also indicated important roles for tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) at the maternofetal interface (6).
However, at the early stage of pregnancy, when the placenta has not been formed, it is unclear how the bacterial infection at the maternofetal interface is resolved. Recent reports from studies with human tissue have suggested the expression of functional Toll-like receptors in the decidua (10, 33) and the production of antimicrobial peptides in the first-trimester decidua (32) and the choriodecidua (9), suggesting that decidual tissue might possibly play a role in the maternal defense against bacterial infection during early pregnancy. To further test this possibility, we infected pregnant mice that were homozygous or heterozygous for the mutation in the CSF-1 gene with intravenous (i.v.) injection of L. monocytogenes and studied the pregnancy outcome and host immune responses at times prior to placentation. Here we report that CSF-1 regulates the maternal decidual tissue in the defense against L. monocytogenes infections during early pregnancy, and in the absence of this macrophage growth factor, mice infected during early gestation are more prone to pregnancy loss.
(Data in this paper are from a thesis to be submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate Division of Medical Sciences, Albert Einstein College of Medicine, Yeshiva University.)
MATERIALS AND METHODS
Animals.
All procedures involving mice were conducted in accordance with the National Institutes of Health regulations concerning the use and care of experimental animals and under institutional animal care guidelines and an approved protocol at the Albert Einstein College of Medicine (AECOM). Mice carrying the CSF-1 null mutation osteopetrotic (op) were used in this study. Homozygous (Csf1op/op) and heterozygous (Csf1op/+) mice were maintained on a C3H/C57BL/6 background in a closed, randomly bred colony under pathogen-free conditions in a barrier facility at AECOM (Bronx, NY). Detailed descriptions of the origin, care, and identification of Csf1op/op mice and their heterozygote controls have been given previously (13). Both age-matched (8- to 12-week-old) and weight-matched (18- to 20-g) females were mated with Csf1op/+ males, and the day the vaginal plug was observed was considered day 1 of gestation (GD1). Heterozygous mice were chosen as control mice since their systemic concentrations of CSF-1 and tissue macrophage densities were not different from those of homozygous wild-type mice (11).
Transgenic mouse expressing the human diphtheria toxin (DT) receptor (DTR) driven by the CD11b promoter [tg(CD11b-DTR)Llan] were kept on the FVB background (18). Homozygous males were used as bone marrow donors to generate chimeras with lethally irradiated 3-week-old FVB females (Charles River, MA). DT or the mutant and inactive toxin Glu52-DT (Sigma-Aldrich, MO) was given via intraperitoneal injections at a dose of 50 ng per gram of body weight in phosphate-buffered saline (PBS) 1 day prior to and on the day of L. monocytogenes infection (see below). The specificity of the depletion of CD11b-expressing cells in the chimeras was confirmed in our laboratory in another study, which showed an ∼80% depletion of CD11b+ cells in the blood and liver without effects on CD11b+ GR1+ or Cd11c+ Cd11b− cells (B. Qian, and J. W. Pollard, unpublished results).
Oil induction of decidualization.
Age-matched (8- to 12-week-old) and weight-matched (18- to 20-g) female mice were ovariectomized under anesthesia and rested for 2 weeks. Hormones (Sigma, St. Louis, MO) were dissolved in sterile peanut oil (Sigma, St. Louis, MO) and injected subcutaneously in a total volume of 0.1 ml according to the regimen developed by Finn and Martin (19) with modifications (46). Briefly, mice were primed with two consecutive daily injections of 100 ng estradiol-17β (E2), followed by 3 days of rest, and three consecutive daily injections of 10 ng E2 plus 1 mg progesterone. Six hours after the last E2-progesterone dose, animals were anesthetized and sterile peanut oil was injected into the uterine lumina in a total volume of 10 μl per uterine horn with a Hamilton syringe. Mice continued to receive daily injections of 10 ng E2 plus 1 mg progesterone until the day of sacrifice.
Bacteria and infections of mice.
L. monocytogenes strain EGD was grown to log phase in Bacto tryptic soy broth (BD Diagnostic Systems, Sparks, MD) and collected by centrifugation. The pellet was resuspended with 20% glycerol-PBS and stored in aliquots at −80°C. Bacterial titers of the stock were determined by plating duplicate serial 10-fold dilutions on tryptose phosphate agar plates and counting colonies after a 24-hour incubation at 37°C.
Pregnant female mice were infected i.v. on GD5, -6, or -7 (as indicated in Results) through the lateral tail veins with 104 CFU of L. monocytogenes and sacrificed after 24, 48, or 72 h postinfection (p.i.). Blood was collected, and the livers, spleens, and pregnant uteri were harvested using aseptic techniques and homogenized separately in sterile ice-cold PBS by use of a tissue homogenizer (IKA, Wilmington, NC). Additionally, in oil-induced decidualization experiments, mice were infected i.v. the day after intrauterine oil injection (see above), and organs were obtained after 60 to 72 h p.i. (also see Results); this was followed by homogenization in sterile ice-cold PBS.
Bacterial titers were determined for blood and tissue homogenates as described for bacterial stock aliquots. Uninfected tissue homogenates were spiked with known amounts of L. monocytogenes and assayed by serial dilution, and the detection limit was determined to be 25 CFU/ml. In cases where no colonies could be recovered in an organ, an arbitrary value of 1 colony/gram of tissue was assigned for graphing purposes.
In one experiment, pregnant mice were infected i.v. via the lateral tail veins with 104 CFU of L. monocytogenes on GD5, the mice were followed for pregnancy outcomes, and the number of pups born to a litter was recorded.
Antibodies and reagents.
The following antibodies were used for immunohistochemistry: anti-mouse antibody against neutrophils (clone 7/4; Caltag Laboratories, Burlingame, CA), anti-F4/80 (a kind gift from David Hume, University of Edinburgh), anti-CSF-1 receptor (anti-CSF-1R) (Upstate Biotechnology Inc., Lake Placid, NY), anti-CD3ɛ (Santa Cruz Biotechnology Inc., Santa Cruz, CA), anti-keratin (clone AE1/AE3; Thermo Fisher Scientific Inc., Fremont, CA), and anti-Listeria O poly (BD Diagnostic Systems, Sparks, MD). Biotinylated secondary antibodies and Vectastain ABC kits were purchased from Vector Laboratories Inc., Burlingame, CA. 3′3′-Diaminobenzidine substrate kits were purchased from Pierce Biotechnology of Thermo Fisher Scientific Inc., Rockford, IL.
The following antibodies were used for flow cytometric and fluorescence-activated cell sorting studies: anti-CD45-phycoerythrin (BD Pharmingen of BD Biosciences, San Jose, CA), anti-Gr-1-allophycocyanin (Biolegend, San Diego, CA), anti-F4/80-fluorescein isothiocyanate (AbD Serotec, Raleigh, NC), and 7-aminoactinomycin (Molecular Probes under Invitrogen, Carlsbad, CA).
Flow cytometric analysis, fluorescence-activated cell sorting, and cytospinning.
Pregnant mice were sacrificed on GD8 and their uteri were harvested and slit open to expose each decidual swelling. The decidual swellings of each animal were collected in 2 ml of ice-cold 0.5% bovine serum albumin (BSA) (Sigma, St. Louis, MO) in PBS and pressed with a 3-ml syringe plunger through a cell strainer with a 40-μm nylon mesh (Fisher Scientific, Pittsburgh, PA) to obtain a single-cell suspension. Red blood cells were removed using RBC-lysis buffer (eBiosciences, San Diego, CA). Cells were resuspended in 100 μl 0.5% BSA-PBS and blocked using anti-mouse CD16/CD32 antibody (eBiosciences, San Diego, CA), followed by antibody staining for 30 min on ice. Cells were washed briefly with cold 0.5% BSA-PBS, regained by centrifugation, and resuspended in cold 0.5% BSA-PBS containing 7-aminoactinomycin. This cell suspension was passed through a 40-μm nylon mesh again and analyzed with flow cytometry or fluorescence-activated cell sorting.
All scanning was performed on a FACSCalibur (Becton Dickinson, Inc., Franklin Lakes, NJ), and data were analyzed using the Flowjo software (Tree Star Inc., Ashland, OR). All sorting was performed on a Dakocytomation MoFlo high-speed fluorescence-activated cell sorter (Dako, Denmark). Sorted cells were collected onto glass slides by cytospinning and stained with Accustain Giemsa stain (Sigma, St. Louis, MO) per the manufacturer's protocol.
Histological analysis.
Mice were sacrificed either on GD8, or at 72 h p.i. in cases of oil-induced decidualization. The pregnant or decidualized uterus from each animal was collected and fixed in 10% buffered formalin overnight, transferred into 70% ethanol overnight, and then processed for paraffin embedding. Five-micrometer sections were stained with hematoxylin and eosin (Sigma, St. Louis, MO) or immunostained with the antibodies described above according to specific instructions from the suppliers, followed by incubation with the appropriate biotinylated secondary antibodies. Immunoreactive material was detected using a peroxidase detection system described above. Nuclei were counterstained with hematoxylin (Sigma, St. Louis, MO).
Images were taken using a Zeiss upright microscope and an AxioCam. Scale bars were added to digitized images by comparison to a reference ruler under the same magnification by use of ImageJ (imaging analysis software downloaded from the National Institute of Health website [http://rsb.info.nih.gov/ij]).
Cytokine analysis.
Pregnant mice were infected with L. monocytogenes as described above. The gravid uteri were collected and homogenized by use of a tissue homogenizer (IKA, Wilmington, NC) in 2.5 ml of cold PBS containing protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Tissue homogenates were centrifuged at 1,000 × g for 10 min at 4°C. Supernatants were collected and stored as frozen aliquots. On the day of the experiments, aliquots were thawed and centrifuged again to remove any precipitate, and the supernatants were used to determine the levels of cytokines and chemokines by use of a sandwich enzyme-linked immunosorbent assay with matched antibody pairs. Interleukin-6 (IL-6), IL-10, IL-17A, TNF-α, and IFN-γ were measured using the Ready-SET-Go! enzyme-linked immunosorbent assay kits from eBiosciences (San Diego, CA). KC was measured using the Quantikine kits from R&D Systems (Minneapolis, MN). IL-4 and IL-5, together with the above-mentioned cytokines and chemokines, were also measured with LINCOplex, a multiplex cytokine/chemokine kit by Linco Research (St. Charles, MO).
Statistical analysis and data presentation.
The numbers of CFU in tissues were compared between different groups of mice by use of the Mann-Whitney U test. The statistical significance of differences in values for cytokines and chemokines and of neutrophil percentages in total hematopoietic cells from the pregnant uterine deciduomas between different groups of mice were analyzed by Student's t test.
RESULTS
CSF-1R is expressed in decidual cells.
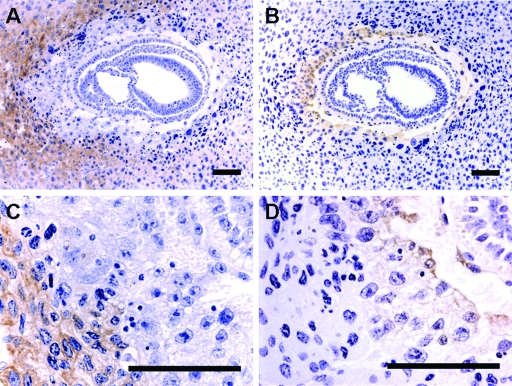
It was previously reported that CSF-1 is synthesized at high concentrations in the murine uterine epithelium during pregnancy (46), whereas its receptor, CSF-1R, is expressed in decidual cells and trophoblastic cells (2). However, since the previous study identified the mRNA for CSF-1R expression only by in situ hybridization, we examined the expression of CSF-1R in the cross sections of GD8 gravid uteri of both Csf1op/op (Fig. 1) and Csf1op/+ (data not shown) mice by immunohistochemistry, using an antibody against CSF-1R. An adjacent section was immunostained using an antibody against the trophoblastic cell marker cytokeratin (17). In the section stained with CSF-1R antibody, we observed that the receptor-bearing cells were localized to the maternal decidual tissue, with the strongest staining in the primary decidual zone and clearly demarcated from the invasion front of the trophoblasts (Fig. 1A and C). Cells staining positive for cytokeratin were not stained with CSF-1R antibody (Fig. 1B and D). These results suggest that on GD8, although the message for CSF-1R is present in both decidual cells and trophoblastic cells, only decidual cells express significant quantities of the receptor protein, and these are more likely the cells to respond to CSF-1 signaling.
FIG. 1.
CSF-1R is expressed in decidual cells. (A and C) Representative transverse sections of the GD8 uterus of a Csf1op/op mouse immunostained using an antibody against CSF-1R. (B and D) An adjacent section immunostained using an antibody against the trophoblastic cell marker, cytokeratin. Positive cells are stained brown with the antibodies. CSF-1R-positive decidual cells do not colocalize with the cytokeratin-positive trophoblast. Bars = 100 μm.
CSF-1-deficient mice are unable to carry a litter to term when infected with L. monocytogenes early during early pregnancy.
It is known that L. monocytogenes replicates in decidual cells (49); thus, the decidualized uterus is susceptible to infection during early pregnancy. Therefore, we first studied how this susceptibility might affect the pregnancy outcomes in Csf1op/op and Csf1op/+ animals. Pregnant mice homozygous or heterozygous for the null Csf1 allele were infected i.v. on GD5 with 104 CFU of L. monocytogenes and followed until either moribundity, when the animal would be euthanized, or parturition, when the number of pups born per litter was recorded. As shown in Table 1, no pups were born to the CSF-1-deficient mothers infected with L. monocytogenes on GD5; in contrast, after the same inoculation dose on GD5, 10 out of 11 Csf1op/+ mothers gave birth to live pups, with litters that were slightly small but reasonably sized compared to those of uninfected heterozygous animals described previously (47). These data strongly suggested that in the absence of CSF-1, mice are more susceptible to pregnancy loss than wild-type mice when infected with L. monocytogenes during early gestation. These data, taken together with the localization of CSF-1R on decidual cells (Fig. 1), prompted us to hypothesize that CSF-1 might regulate the decidual responses against L. monocytogenes infection during early pregnancy.
TABLE 1.
Pregnancy outcomes for Csf1op/op versus Csf1op/+ mice upon infection with 104 CFU of L. monocytogenes on GD5
| Mouse genotype (no. of mice) | No. of litters born | Total no. of pups born | Avg. litter size |
|---|---|---|---|
| Csf1op/op (10) | 0 | N/Aa | N/Aa |
| Csf1op/+ (11) | 10 | 74 | 7.4 ± 0.81 |
N/A, not applicable.
CSF-1 is required for resistance to L. monocytogenes infection during early gestation, and this resistance is maternal in origin.
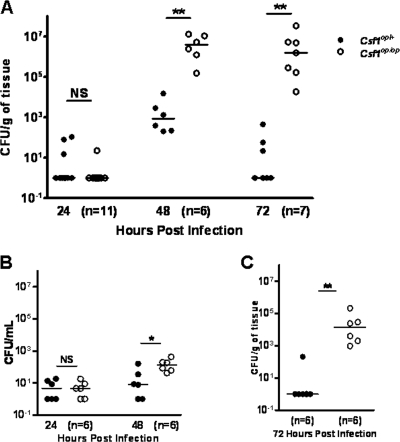
Csf1op/+ and Csf1op/op mice were infected i.v. on GD5, -6, or -7, and the pregnant uteri were taken on GD8 (at 24, 48, or 72 h p.i.) to determine the numbers of CFU of L. monocytogenes per gram of tissue (Fig. 2A). At 24 h p.i., organ titers in GD8 pregnant uteri of both Csf1op/+ and Csf1op/op mice were similar, indicating that the initial colonizations by the bacteria were comparable for the two genotypes. However, at 48 h p.i., Csf1op/op mice had a median organ titer in their pregnant uteri 3 orders of magnitude higher than that for Csf1op/+ mice (P = 0.002; Mann-Whitney U test). When the infection time was prolonged to 72 h, the median organ titer in Csf1op/op uteri held at 1.55 × 106 CFU/gram of tissue compared with 3.86 × 106 CFU/gram of tissue from 48 h p.i., whereas Csf1op/+ animals reduced their uterine titers to the levels comparable to those at 24 h p.i., and there were no recoverable CFU detected in four out of seven uteri examined.
FIG. 2.
Csf1op/op mice are susceptible to L. monocytogenes infection during early gestation compared to Csf1op/+ mice. (A) Pregnant mice (both Csf1op/op and Csf1op/+) were infected i.v. on GD5, -6, or -7 with 104 CFU of L. monocytogenes. Mice were sacrificed on GD8 (at 24, 48, or 72 h p.i.), and the numbers of CFU of L. monocytogenes per gram of tissue recovered from the pregnant uteri were determined by plating serial dilutions of tissue homogenate on tryptic soy agar plates. Each data point represents one animal; data represent results from at least five independent experiments. (B) Similar to what was done for panel A, pregnant mice were infected i.v. on GD6 or -7 with 104 CFU of L. monocytogenes and sacrificed on GD8 (at 24 or 48 h p.i.), and the numbers of CFU of L. monocytogenes in circulation (represented as CFU per ml of blood) were determined. Each data point represents one animal; data represent results from at least five independent experiments. (C) Ovariectomized mice (both Csf1op/op and Csf1op/+) were hormonally treated and injected with intrauterine oil to induce the formation of deciduomas, followed by i.v. injection of 104 CFU of L. monocytogenes. Mice were sacrificed 72 h p.i., and the numbers of CFU of L. monocytogenes per gram of tissue recovered from the induced deciduomas were determined. Each data point represents one animal; data represent results from at least three independent experiments. Symbols: open circles, Csf1op/op; filled circles, Csf1op/+; horizontal bars, median titers (NS, not significant; ** in panel A, P = 0.002; * in panel B, P = 0.02; ** in panel C, P = 0.004; Mann-Whitney U test).
Despite the markedly elevated median organ titers in the uteri, the median systemic organ titers in the spleens and livers of Csf1op/op mice were 6.4- and 19.2-fold, respectively, higher than to those in the Csf1op/+ animals at 48 h p.i. (data not shown). Listeria is cleared from the blood within 10 min after i.v. injection. Consistent with this, maternal blood cultures at 24 h p.i. showed virtually undetectable levels of circulating bacteria (median titer of <10 CFU/ml) in both genotypes, which increased to median titers of 8 and 133 CFU/ml at 48 h p.i. for Csf1op/+ and Csf1op/op, respectively (Fig. 2B). These results suggest that it takes approximately 24 to 48 h p.i. for L. monocytogenes to colonize and expand in the decidualized uteri, a period when there were few circulating bacteria, and that this colonization and expansion can be reduced after 72 h p.i. However, in the absence of CSF-1, the infection by L. monocytogenes cannot be cleared.
It has been established that trophoblasts from the placenta, in response to CSF-1 signaling, become a component of the innate immune system during pregnancy against L. monocytogenes infection in response to CSF-1 signaling (26), and we observed that CSF-1R was expressed only in decidual cells during early pregnancy (Fig. 1); therefore, we hypothesized that before placentation, the maternal tissue was responsible for bacterial clearance during L. monocytogenes infections. To test this hypothesis, we induced decidualization with intrauterine oil injection in hormonally treated, ovariectomized mice (both Csf1op/op and Csf1op/+) based on a well-established method (19). One day after oil injection, the mice were infected i.v. with 104 CFU L. monocytogenes. The mice were sacrificed 72 h p.i., and only the decidualized uteri were taken to determine organ titers (Fig. 2C). We observed that in the deciduomas of Csf1op/op mice, the median bacterial titer was approximately 4 orders of magnitude higher than that in Csf1op/+ deciduomas (P = 0.004, Mann-Whitney U test), although this difference was not as pronounced as in the pregnant animals (6 orders of magnitude difference) (Fig. 2A). Therefore, our results suggest that the CSF-1-dependent anti-Listeria responses are maternal in origin.
Absence of CSF-1 is associated with decidual tissue destruction and fetal loss after L. monocytogenes challenge during early gestation.
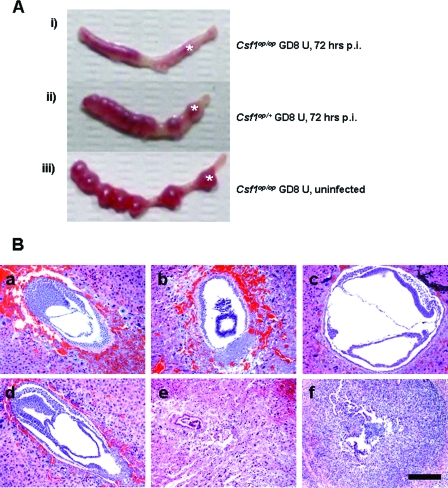
Pregnant mice (both Csf1op/op and Csf1op/+) were infected with 104 CFU L. monocytogenes i.v. on GD5. The pregnant uteri were harvested 72 h p.i., and their gross morphologies were compared with that of an uninfected GD8 pregnant uterus of a Csf1op/op animal (Fig. 3A). i.v. infection of the heterozygous animal led to mild edema of the pregnant uterus (Fig. 3Aii); however, the individual implantation sites were intact, and their sizes were comparable to those seen for the uninfected animal (Fig. 3Aiii). In contrast, the homozygous mutant animal exhibited edematous and hemorrhagic remnants of implantation sites that were barely identified, indicating resorption of the implanted fetuses (Fig. 3Ai). These observations were consistently found in all the other experiments done in this study.
FIG. 3.
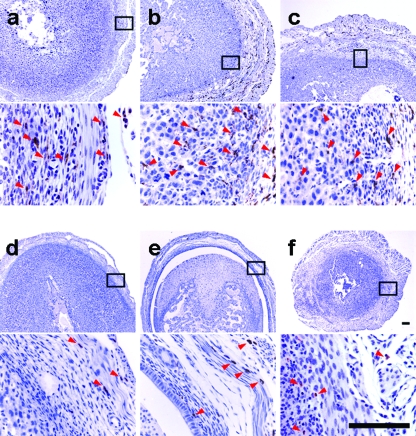
Absence of CSF-1 results in decidual tissue destruction and fetal loss after L. monocytogenes challenge during early gestation. (A) Representative photographs of GD8 pregnant uteri (U) either uninfected (iii [Csf1op/op]) or challenged with 104 CFU of L. monocytogenes i.v. on GD5 and harvested at 72 h p.i. (i [Csf1op/op] and ii [Csf1op/+]). * marks an example of an implantation site, which is resorbed in the Csf1op/op uterus at 72 h p.i. (B) Pregnant mice (both Csf1op/op and Csf1op/+) were infected i.v. on GD5 or -6 with 104 CFU of L. monocytogenes. Mice were sacrificed on GD8 (at 48 or 72 h p.i.), and cross sections at the implantation sites were stained with hematoxylin and eosin. (Top) Csf1op/+, either uninfected (a) or 48 (b) and 72 (c) h p.i.; (bottom) Csf1op/op, either uninfected (d) or 48 (e) and 72 (f) h p.i. Note decidual tissue necrosis and leukocyte infiltration in the Csf1op/op animal (e) and resorption of the fetal tissue at 72 h p.i. (f). Bar = 100 μm.
To further study the tissue structure and integrity at the implantation sites, GD8 pregnant uteri from both genotypes at 48 and 72 h p.i., or without infection, were processed for paraffin embedding, and cross sections at the implantation sites were stained with hematoxylin and eosin and examined by light microscopy (Fig. 3B). In heterozygous animals, infection by i.v. L. monocytogenes did not cause decidual tissue disintegration at either 48 (Fig. 3Bb) or 72 (Fig. 3Bc) h p.i. compared to what was seen for the uninfected animal (Fig. 3Ba). The developing embryos at both time points were also intact. However, in the homozygous mutant animals, although the tissue morphology was normal at 24 h p.i. (data not shown), the well-organized decidual tissue seen in the uninfected uteri (Fig. 3Bd) began to deteriorate and appeared necrotic at 48 h p.i. (Fig. 3Be). The large polyploidy decidual cells eventually disappeared and were replaced by dense, leukocytic infiltrate at 72 h p.i.; additionally, at the center of the cross section where the developing embryo should be, there was no embryonic structure observed, but only a small aggregation of cells which might be the remnant of the resorbed fetal tissue (Fig. 3Bf). These results suggest that wild-type mice are able to maintain their tissue structural integrity during L. monocytogenes infection at the maternofetal interface, while in the absence of CSF-1, pregnant mice fail to maintain decidual tissue integrity and fetal loss becomes inevitable.
Neutrophils are recruited normally to the decidual tissue after L. monocytogenes infection during early gestation in the absence of CSF-1.
In the case of placental immunity against L. monocytogenes infections, our laboratory previously reported that the trophoblastic cells of CSF-1-deficient mice were unable to produce the neutrophil chemoattractants, KC and macrophage inflammatory protein-2, and neutrophils were not found in their placenta (26). To test whether a similar mechanism underlies the pregnancy loss in Csf1op/op animals with L. monocytogenes infections during early gestation, we examined neutrophil recruitment in the decidual tissue by both immunohistochemical (Fig. 4A) and flow cytometric (Fig. 4B) analyses.
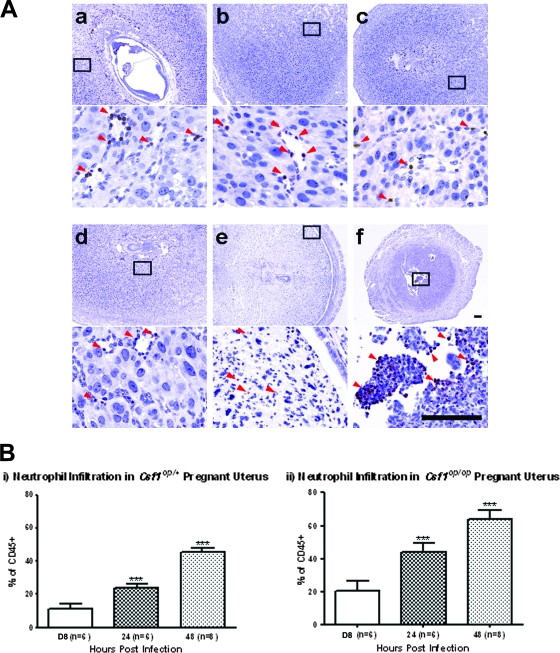
FIG. 4.
Both Csf1op/op and Csf1op/+ mice recruit neutrophils to their decidual tissue after L. monocytogenes infection during early gestation. (A) Pregnant mice (both Csf1op/op and Csf1op/+) were infected i.v. on GD5 or -6 with 104 CFU of L. monocytogenes. Mice were sacrificed on GD8 (at 48 or 72 h p.i.), and cross sections at the implantation sites were immunostained using a neutrophil-specific antibody, clone 7/4; positive cells (arrowheads) were stained brown with the antibody. (Top) Csf1op/+, either uninfected (a) or 48 (b) and 72 (c) h p.i. (respective insets [boxes] are shown below); (bottom) Csf1op/op, either uninfected (d) or 48 (e) and 72 (f) h p.i. (respective insets [boxes] are shown below). Bars = 100 μm. (B) Pregnant mice (both Csf1op/op and Csf1op/+) were infected i.v. on GD6 or -7 with 104 CFU of L. monocytogenes. Mice were sacrificed on GD8 (at 24 or 48 h p.i.), and the deciduomas of the animals were pooled to prepare single-cell suspensions and analyzed by fluorescence-activated cell sorting. The neutrophil percentage of total hematopoietic cells for each animal was determined. Data represent mean ± standard deviation from at least five independent experiments (***, P < 0.0001; Student's t test). Compared to uninfected deciduomas, infected Csf1op/+ deciduomas had increased neutrophil percentages with time, until peaking at 48 h p.i.; a similar trend was also seen for Csf1op/op animals, although at every time point postinfection, the neutrophil percentage was higher than that for the Csf1op/+ animals.
We immunostained the paraffin-embedded cross sections from the implantation sites of gravid uteri from both Csf1op/op and Csf1op/+ mice that were harvested on GD8 (at 24, 48, or 72 h p.i.) with a neutrophil-specific antibody, clone 7/4 (27). Neutrophils were found in similar numbers and distributions in the uninfected decidual tissue sections for both genotypes, mostly clustering around endothelial spaces resembling the developing vasculature (Fig. 4Aa and 4d). In the presence of infection in Csf1op/+ animals, at this level of resolution there was no obvious change in neutrophil number or distribution in the decidual tissue at either 24 (data not shown), 48 (Fig. 4Ab), or 72 (Fig. 4Ac) h p.i. compared to what was seen for uninfected animals (Fig. 4Aa). In Csf1op/op animals, although neutrophil number and distribution did not change significantly in the decidual tissue of infected animals at 24 h p.i. (data not shown), a dramatic increase in clone 7/4-positive cells in the uterine sections was observed at 72 h p.i. (Fig. 4Af) compared to what was seen for an uninfected animal (Fig4Ad). However, at 48 h p.i. (Fig. 4Ae), despite massive cell death and disintegration of the decidual tissue, few neutrophils could be identified with the antibody, possibly due to cell debris and cell death interfering with immunostaining of this cell surface marker.
To use a method more sensitive and quantitative than the relatively imprecise immunohistochemistry, we performed flow cytometric analysis to examine neutrophil recruitment in the GD8 gravid uteri after infection. To this effect, pregnant mice (both Csf1op/op and Csf1op/+) were infected with i.v. injection of L. monocytogenes and sacrificed at 24, 48, or 72 h p.i., and their uteri were slit open to collect the deciduomas. Single-cell suspensions from the pooled deciduoma of each animal were prepared and analyzed by flow cytometry after staining with fluorochrome-conjugated cell lineage markers. A total of 100,000 to 150,000 cells per animal were collected for analysis by the Flowjo software. After gating for the hematopoietic-lineage CD45+ cells (35) from the live cells, we used anti-Gr-1 to identify the neutrophil population (20). We also used the anti-F4/80 antibody as an exclusion marker because it was reported that the anti-Gr-1 antibody would also stain monocytes (34) and eosinophils (53), which are positive for F4/80 (24). Indeed, fluorescence-activated cell sorting and cytospin results confirmed that, according to the use of a combination of the two antibodies, Gr-1-positive and F4/80-negative cells were neutrophils with less than 4% contamination from monocytes (data not shown).
Using this method, we determined the neutrophil percentage of total hematopoietic cells for each animal. As shown in Fig. 4Bi, the average neutrophil percentage of total CD45+ cells in the Csf1op/+ deciduomas increased to 24.3% at 24 h p.i. compared to 11.3% in uninfected animals (P < 0.0001; Student's t test). This percentage continued to increase at 48 h p.i. to 45.3% (P < 0.0001; Student's t test) but decreased slightly to 35.4% at 72 h p.i. (P < 0.0001; Student's t test; data not shown). Csf1op/op animals also had increased decidual neutrophil percentage in the presence of infection (Fig. 4Bii). At 24 h p.i., the neutrophil percentage increased to 44.0% from 21.0% in the uninfected animals, and this percentage increased to 63.8% at 48 h p.i. (P < 0.0001; Student's t test). We were unable to collect enough cells from the infected deciduomas of Csf1op/op animals at 72 h p.i., due to massive cell death and tissue resorption at the implantation sites (also see Fig. 3). Of note is that at each time point postinfection when we were able to collect enough cells for flow cytometric analysis, we observed a higher neutrophil percentage for Csf1op/op animals than for heterozygous animals. This was also true for the uninfected mice. Taken together, our results indicate that CSF-1 deficiency does not cause a failure of neutrophil recruitment in the decidual tissue of the Csf1op/op mice after L. monocytogenes infection during early pregnancy, in contrast to what was found for the placenta (26).
T cells are not recruited to the decidual tissue after L. monocytogenes infection during early gestation.
T cells have been implicated in the sterile eradication of L. monocytogenes during systemic infections (5). Previous studies by us (6) and others (49) have shown that T cells are excluded from the maternofetal interface in the murine placenta. In this study, we also examined the presence and localization of T cells in the decidual tissue sections with or without L. monocytogenes infection during early pregnancy in the Csf1op/+ and Csf1op/op mice, by use of an antibody against the T-cell surface marker CD-3ɛ (12). In the uninfected GD8 uterus, T cells were found in both genotypes in low numbers in the myometriums and the secondary decidual zones, but never at the maternofetal interfaces, where the invading trophoblastic cells were found (data not shown). Moreover, the number and distribution of T cells did not significantly change in the presence of L. monocytogenes infection in either genotype (data not shown), indicating that up to 72 h p.i., T cells were not recruited in large numbers in the pregnant uterus. Taken together, our results suggest that T cells may not be required for the early phase of anti-Listeria responses in the decidual tissue.
CSF-1 is not required for uterine production of Th1 and Th2 cytokines or neutrophil chemoattractants after L. monocytogenes infection during early gestation.
Effective immune responses against L. monocytogenes infections require the production of cytokines and chemokines to mobilize immune cells to the sites of infection and mount their effector functions (42). Therefore, we measured the uterine production of cytokines and chemokines in the GD8 pregnant Csf1op/op and Csf1op/+ mice with or without infection, up until 48 h after L. monocytogenes infection. As shown in Table 2, considerable levels of IL-6 were detected in the uninfected uteri, but they were not significantly different between the two genotypes. After 24 h p.i., the homozygously null mutant mice were as capable of producing a higher uterine amount of IL-6 as were the heterozygous mice, until it reached a level significantly higher than those found for the heterozygous uteri at 48 h p.i. (P = 0.03; Student's t test). Similarly, Csf1op/op mice produced high levels of IFN-γ in their pregnant uteri in the absence of an infection; these levels were higher than those found for the Csf1op/+ pregnant uteri (P = 0.01; Student's t test); when the animals were infected with L. monocytogenes, the IFN-γ levels in the uteri from both genotypes did not differ significantly. TNF-α levels were low in the two genotypes in the uninfected pregnant uteri and increased nearly ninefold at 48 h p.i. in the Csf1op/op animals from 24 h p.i., but this marked elevation was not observed for the heterozygous animals. The Th2 cytokines IL-4 and IL-5 were not detectable. IL-10 was present at high levels in the uninfected uteri, with the Csf1op/op mice producing higher amounts than the heterozygous mice (P = 0.03; Student's t test). Infection did not significantly change the uterine IL-10 levels in either genotype.
TABLE 2.
Cytokine levels in uterine homogenates of GD8 Csf1op/op and Csf1op/+ mice following L. monocytogenes infections
| Cytokine/chemokine | Level (pg/g of tissue) in mice of indicated genotype and infection statusa
|
|||||
|---|---|---|---|---|---|---|
| Uninfected
|
24 h p.i.
|
48 h p.i.
|
||||
| Csf1op/+ | Csf1op/op | Csf1op/+ | Csf1op/op | Csf1op/+ | Csf1op/op | |
| Th1 cytokines | ||||||
| IL-6 | 248.5 ± 30.4 | 420.6 ± 151.0 | 661.9 ± 172.6 | 1,445.5 ± 521.7 | 513.6 ± 237.9 | 1,812.3 ± 641.8* |
| IFN-γ | 581.9 ± 129.6 | 2,615.7 ± 796.8* | 2,222.1 ± 648.1 | 3,954.2 ± 1,395.1 | 1,775.2 ± 493.4 | 2,040.2 ± 591.6 |
| TNF-α | 43.9 ± 16.1 | 81.7 ± 15.9* | 61.3 ± 8.1 | 185.2 ± 117.9 | 117.2 ± 27.1 | 1,662.5 ± 383.7** |
| Th2 cytokines | ||||||
| IL-4 | ND | ND | ND | ND | ND | ND |
| IL-5 | ND | ND | ND | ND | ND | ND |
| IL-10 | 5,664.5 ± 1,270.1 | 13,259.5 ± 3,811.0* | 4,892.1 ± 1,532.9 | 10,620.2 ± 3,760.7 | 6,902.0 ± 559.1 | 8,900.6 ± 2,427.4 |
| Chemokines | ||||||
| KC | 733.7 ± 328.9 | 600.9 ± 106.7 | 1,456.1 ± 174.8 | 1,967.1 ± 650.6 | 1,517.2 ± 615.0 | 5,493.9 ± 1,642.6* |
| IL-17A | 391.5 ± 108.8 | 607.9 ± 133.1 | 244.0 ± 68.5 | 688.1 ± 226.2* | 504.4 ± 123.6 | 497.2 ± 143.5 |
*, P < 0.05; **, P = 0.002 (Student's t test); ND, not detectable.
In addition, we also determined the levels of neutrophil chemoattractants. As expected from the lack of a defective neutrophil recruitment in the decidual tissue in the CSF-1-deficient mice (Fig. 4B), quantification of KC and IL-17A in the pregnant uteri during infection indicated that both genotypes produced comparable and significant levels (Table 2), with the Csf1op/op mice producing more KC at 48 h p.i. (P = 0.02), or more IL-17A at 24 h p.i. (P = 0.03; Student's t test), than the heterozygous animals.
CSF-1 is required for macrophage recruitment to the decidual tissue after L. monocytogenes infection during early gestation.
In the mouse placenta, macrophages normally are excluded from the maternofetal interface, even in the presence of L. monocytogenes infections (26, 49). To examine the presence and localization of macrophages during L. monocytogenes infections in early gestation, pregnant mice (both Csf1op/op and Csf1op/+) were infected on GD5 or -6 with i.v. injection of 104 CFU of L. monocytogenes. Pregnant uteri were harvested on GD8 and processed for paraffin embedding. Five-micrometer cross sections at the implantation sites were immunostained using an antibody against the macrophage-specific surface marker F4/80 (3, 30).
Deficiency in CSF-1 led to a reduction in uterine F4/80-positive cell numbers in Csf1op/op (Fig. 5d) compared with heterozygous (Fig. 5a) animals in the unperturbed uteri. In the presence of L. monocytogenes infection, Csf1op/+ mice recruited large numbers of F4/80-positive cells to the pregnant uterus at 48 and 72 h p.i. (Fig. 5b and c). These recruited cells had multiple projections from their cell body, indicating an activated state, and they accumulated mainly in the myometrium, with a few invading into the secondary decidual zone (Fig. 5b and c, inset). Similar to previous reports on macrophage localization in the decidual basalis (47), the invasion front of the cells extended to a few layers of decidual cells into the secondary decidual zone but they failed to migrate further into the tissue near the maternofetal interface. CSF-1-deficient mice had very few F4/80-positive cells at 48 (Fig. 5e) or 72 (Fig. 5f) h p.i. in the uterine stromata compared to heterozygous littermates (Fig. 5b and c), with no apparent increase in number despite high bacterial titers (Fig. 2A). Furthermore, the change in the F4/80-positive cells to a more dendritic morphology was not observed for the Csf1op/op mice. These results demonstrate that macrophages are recruited to the uterine stroma, but not to the maternofetal interface, during L. monocytogenes infection in early gestation, and that CSF-1 deficiency leads to the failure of macrophage recruitment.
FIG. 5.
Csf1op/op mice fail to recruit macrophages to their decidual tissue after L. monocytogenes infection during early gestation. Pregnant mice (both Csf1op/op and Csf1op/+) were infected i.v. on GD5 or -6 with 104 CFU of L. monocytogenes. Mice were sacrificed on GD8 (at 48 or 72 h p.i.), and cross sections at the implantation sites were immunostained using an antibody against the macrophage-specific surface marker F4/80; positive cells (arrowheads) were stained brown with the antibody. (Top), Csf1op/+, either uninfected (a) or 48 (b) and 72 (c) h p.i. (respective insets [boxes] are shown below); (bottom), Csf1op/op, either uninfected (d) or 48 (e) and 72 (f) h p.i. (respective insets [boxes] are shown below). Note the increased number and the change into a more dendritic morphology of macrophages in the Csf1op/op mice (b and c) after Listeria infection and the absence of such changes in the Csf1op/op mice (e and f). Bars = 100 μm.
Macrophage depletion results in susceptibility of decidual tissue to L. monocytogenes infection.
Since CSF-1R is present on macrophages (51) and decidual cells (Fig. 1), we sought to identify which one of the two cell types was responsible for the CSF-1-regulated maternal anti-Listeria responses during early gestation, by depleting macrophages in the decidualized uterus followed by i.v. infection with L. monocytogenes. To this effect, we utilized the transgenic mouse expressing the human DTR driven by the CD11b promoter [tg(CD11b-DTR)Llan], which is a previously reported animal model for in vivo transient CD11b-expressing macrophage depletion (18). Due to the complication of lethality after DT treatments in the homozygous animals carrying the CD11b-DTR transgene (our unpublished results), bone marrow transplantation was performed using homozygous males as donors to generate chimeras with lethally irradiated 3-week-old FVB females.
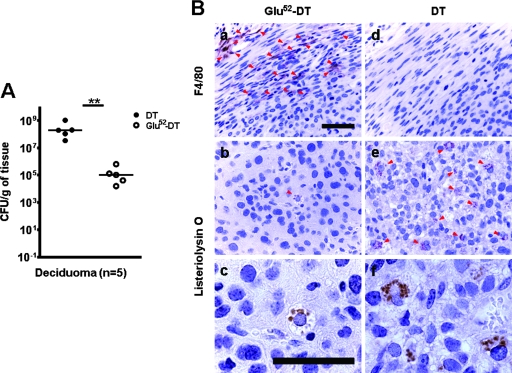
At least 5 weeks after bone marrow transplantation, female FVB mice were oil induced to decidualize as described above. Intraperitoneal injection of DT or a mutant and inactive form of the toxin (Glu52-DT) was performed 1 day prior to, and on the day of, the i.v. injection of 104 CFU of L. monocytogenes. This regimen reduced the circulating F4/80+ CD11b+ cells from 9.9% to 1.6% in the chimeric FVB mice (Qian et al., unpublished). Infected mice with DT treatment started to die after 60 h p.i.; therefore, they were sacrificed at either 60 or 72 h p.i. In contrast, none of the infected mice treated with Glu52-DT showed signs of being moribund, and they were sacrificed at 72 h p.i. The decidualized uterine bacteria titers were determined for both treatment groups (Fig. 6A). The median bacterial titer in the deciduomas of the DT-treated animals was more than 3 orders of magnitude higher than that of the Glu52-DT-treated animals (P = 0.008; Mann-Whitney U test). In another experiment where we treated pregnant Csf1op/+ mice with i.v. injection of liposome-encapsulated dichloromethylene diphosphonate (liposomal clodronate) to deplete macrophages (55) prior to L. monocytogenes infections, all the animals succumbed to the infection before 72 h p.i., whereas pregnant Csf1op/+ animals treated with liposomal clodronate without concomitant Listeria infections carried their litters to term (data not shown).
FIG. 6.
Macrophage depletion results in susceptibility of the decidual tissue to L. monocytogenes infection in oil-induced decidualized animals. (A) Ovariectomized FVB mice with CD11b-DTR bone marrow transplants were hormonally treated and injected with intrauterine oil to induce the formation of deciduomas. Intraperitoneal injection of DT or a mutant form of the toxin (Glu52-DT) was performed 1 day prior to and on the day of i.v. injection of 104 CFU of L. monocytogenes. Mice were sacrificed 60 to 72 h p.i., and the numbers of CFU of L. monocytogenes per gram of tissue recovered from the induced deciduomas were determined. Each data point represents one animal; data represent results from at least three independent experiments. Symbols: filled circles, DT-treated animals; open circles, Glu52-DT-treated animals; **, P = 0.008 (Mann-Whitney U test). (B) Cross sections of the decidualized uteri of the animals in panel A were immunostained using an antibody against the macrophage-specific surface marker F4/80 (a and d) or an antibody against the L. monocytogenes-specific antigen listeriolysin O (b, c, e, and f); positive cells or bacteria (arrowheads) were stained brown with the antibodies. Note the absence of F4/80-positive cells in the DT-treated uterus (d), which also contains a higher number of listeriolysin O-positive cells (e and f). Bars = 50 μm; panels a, b, d, and e are shown at the same magnification; panels c and f are shown at the same higher magnification.
We also performed immunohistochemical analysis on the cross sections of the decidualized uteri to confirm the effectiveness of DT treatment in macrophage depletion and to identify the location of L. monocytogenes infection. As shown in Fig. 6Ba, numerous F4/80-positive cells were found in the uterine stromata of animals treated with the mutant toxin Glu52-DT. In contrast, DT treatment dramatically reduced the number of F4/80-positive cells in the oil-induced decidualized uterus to an almost undetectable level (Fig. 6Bd). Immunostaining with anti-listeriolysin O antibody revealed that in the mutant toxin-treated animals, very few decidual cells contained intracellular L. monocytogenes (Fig. 6Bb and 6Bc); on the other hand, DT-treated mice had many infected cells with intracellular bacteria (Fig. 6Be and 6Bf). These results indicate that CSF-1-regulated maternal immune responses against L. monocytogenes are mediated through macrophages, not the CSF-1R-bearing decidual cells, despite the fact that the bacteria replicate in the decidual cells where macrophages do not have direct access (Fig. 5b).
DISCUSSION
As early as the late 1970s, it was postulated that intrauterine infection may be a cause of preterm births. However, the lack of benefits from antimicrobial therapy during pregnancy in the prevention of preterm delivery suggests a more complex picture (56). Indeed, studies have found that the presence of intrauterine bacteria alone is not sufficient (50); rather, a combination of microbes and microbial products that trigger the activation of the innate immune system and the production of proinflammatory cytokines, prostaglandins, inflammatory mediators, and matrix-degrading enzymes ultimately leads to preterm births (22).
Our understanding of the immune regulations during pregnancy has been greatly enhanced through studies using Listeria monocytogenes as an important tool, especially in delineating the differences between systemic and placental responses, as during pregnancy this bacterium has a predilection for replication at the maternofetal interface (49). In the early phase of systemic infection, L. monocytogenes is largely controlled by CSF-1-dependent macrophages (25, 31) and neutrophils (54), whereas natural killer cells are not required (7); genetic ablation studies have established the importance of IL-12 (28), TNF-α (43-45), and IFN-γ (14, 16, 29) in the host responses. Sterile eradication of the bacterium requires the activation of cytotoxic T cells (5).
In pregnant mice, the placental anti-Listeria responses are characterized by neutrophil dominance due to the exclusion of macrophages and T cells at the maternofetal interface (26, 49). Based on our previous studies, a chronology of the placental immune response has been proposed, suggesting that in the presence of Listeria infection, the trophoblasts synthesize the neutrophil chemoattractants in response to uterine CSF-1 and become a part of the innate immune system to organize the maternal anti-Listeria responses at early times postinfection (26). The recruited neutrophils resolve most, but not all, of the infection; at 48 and 72 h p.i., maternally synthesized proinflammatory cytokines, namely, TNF-α and IFN-γ, are required in the placental immune responses against Listeria infection, in which context the synthesis of these cytokines is induced without promoting abortion (6).
These studies have focused mainly on the problem of placental infection. Under most normal circumstances where placental infection is under check, the minimally infected placenta is discarded after parturition as long as the fetus is sufficiently protected to be born (6). This may also explain why a high percentage of women undergoing elective cesarean section at term are found to have bacteria in the membranes (52). However, at the early stage of pregnancy, when the placenta has not been formed, the pregnant uterus is still susceptible to L. monocytogenes infection (49), and it is unclear how the bacterial infection at the maternofetal interface is resolved. Studies of humans also highlight the relationship between infection and extremely early preterm births (23), emphasizing that understanding the mechanisms of host defense against microbial invasion during early pregnancy may help to prevent preterm births more effectively.
In this study, we examined the anti-Listeria responses in the maternal decidual tissue in early pregnancy. The availability of mutant mice carrying the recessive null osteopetrotic mutation in the CSF-1 gene (58, 59) allowed us to study these responses in the presence or absence of the macrophage CSF-1. The poor pregnancy outcome for CSF-1-deficient mice (Table 1), the decidual and fetal tissue damage associated with infections in the absence of CSF-1 (Fig. 3), and the markedly increased uterine bacterial titers in oil-induced decidual tissue of Csf1op/op animals (Fig. 2) all underscore the importance of this cytokine in the maternal anti-Listeria responses. The elevation in the uterine bacterial levels is unlikely a result of seeding from systemic organs, as when the median uterine titer for the Csf1op/op animals was (4.52 × 103)-fold higher than that for the Csf1op/+ animals at 48 h p.i. (Fig. 2A), the median systemic organ titers were less than 20-fold higher than those for the Csf1op/+ animals (data not shown). Moreover, the median level of circulating bacteria for the Csf1op/op animals was increased only by less than 30-fold between 24 and 48 h p.i. (Fig. 2B), whereas the median uterine titer was increased by (3.86 × 106)-fold between those two time points (Fig. 2A). This discordance between the systemic and the uterine Listeria titers and the low circulating bacterial levels in the blood strongly suggests that failed local control of bacteria replication, rather than the influx and seeding from systemic infection, is responsible for the highly elevated bacterial levels in the pregnant uterus in the Csf1op/op mouse. Similarly, it has been reported that in a Listeria infection model using pregnant guinea pigs, the uncontrolled Listeria infection in the placenta is a result of local replication rather than influx from circulation, because between 24 and 48 h p.i., the estimated number of bacteria that traffic to the placenta from the maternal organs was fewer than 10 based on a mathematical simulation of the infection kinetics in all the organ compartments (4).
Unlike in the placental responses, where CSF-1 deficiency led to the inability of the trophoblasts to recruit neutrophils for the local suppression of the invading pathogen (26), the decidual tissue produced high levels of neutrophil chemoattractants (Table 2) and neutrophils were recruited to this site during L. monocytogenes infection at early gestation even in the absence of CSF-1 (Fig. 4). Therefore, CSF-1 does not seem to regulate the mobilization of neutrophils to the maternal decidua following an infection. However, despite neutrophil accumulation at the site of the infection, uterine Listeria titers remained high in the pregnant Csf1op/op mice, and this susceptibility was independent of the developing embryo (Fig. 2). The failure for the recruited neutrophils to effectively clear the pathogen could be due to the intracellular localization of the bacterium (8). As a result, uncontrolled bacterial replication in the decidual cells eventually led to apoptosis and secondary necrosis of the maternal decidual tissue, and the development of the embryo was no longer sustained (Fig. 3).
Further dissection of the immune responses revealed that macrophage functions were important in the maternal decidual anti-Listeria responses, as depletion of macrophages in the CD11b-DTR (18) chimeric animals led to increased susceptibility to L. monocytogenes in the oil-induced deciduoma compared to what was seen for macrophage-sufficient deciduoma (Fig. 6). Examination of the infected tissue showed that in control mice, macrophage recruitment and activation were limited to the uterine stroma at the outer rim of the secondary decidual zone (Fig. 5), whereas the location of the L. monocytogenes bacterium was in the decidual cells (Fig. 6). This discrepancy between the localization of the seemingly responsible effector cell and the localization of the invading pathogen suggested that a link between the two was required to explain the host defense against the microbe. It is therefore reasonable to hypothesize that decidual cells, the site of the bacterial replication, might carry out the anti-Listeria functions in response to CSF-1-dependent macrophage signaling. This requirement for CSF-1 might also explain the rapid rise in uterine CSF-1 under hormonal control at these early periods postimplantation for all mammalian species examined (15, 36, 46) as a preemptive mechanism to stop bacterial infection.
The increase in neutrophil influx in the Csf1op/op decidua somewhat resembles the systemic responses against Listeria infection in the mutant mice (25). However, the decidua of Csf1op/op mice were able to produce levels of Th1 cytokines and the Th2 cytokine IL-10, implicated in the macrophage-dependent efflux of neutrophils in systemic infection (1, 25), that were similar to or even higher than those seen for the heterozygous control mice. These results suggested that the defect in controlling the local infection was not a result of defective cytokine production, and in the context of the decidua, the production of these cytokines was not dependent on CSF-1. Moreover, Th2 cytokines known to antagonize the effects of IFN-γ, an important anti-Listeria cytokine, have been found to be at very low levels in the infected mouse placenta (6, 26), despite the previously proposed hypothesis that pregnancy favors toward a Th2 cytokine environment to limit cytotoxic T-cell responses that may reject the allogeneic fetus (57). Similarly, we were unable to detect the Th2 cytokines IL-4 or IL-5 in the mouse decidual tissue from either genotype in the presence or absence of infection (Table 2). The relative scarcity of T cells in the decidual tissue was also consistent with the findings from our previous studies (6) and others (49) supporting the hypothesis that cytotoxic T cells are excluded from the maternofetal interface to prevent fetal rejection.
Recent reports from studies of human tissue have suggested that functional Toll-like receptors are expressed in the decidua (10, 33) and that antimicrobial peptides are produced in the first-trimester decidua (32) and the choriodecidua (9), which provided a basis for the decidual tissue to detect microbes and play a role in the maternal defense against bacterial infection during early pregnancy. It is interesting to observe by light microscopy that Listeria seems to be restricted to endocytic/lysosomal vesicles in decidual cells (Fig. 6). This suggests that these decidual cells may have some intrinsic defense against such a pathogen. However, how these functions are regulated by CSF-1 remains to be determined through further studies.
Acknowledgments
We acknowledge expert assistance from the Cancer Center's Histopathology, Analytical Imaging, and fluorescence-activated cell sorting facilities for histology, microscopy, and flow cytometric studies. We thank Richard Lang (University of Cincinnati) for the CD11b-DTR MacTerminator mice.
This work was supported by a Public Health Services grant to J.W.P. from the National Institute of Child Health and Disease (HD30820) and to the Cancer Center from the National Cancer Institute (P30-13330). X.Q. was supported by the Medical Scientist Training Program Grant (T32 GM07288) from the National Institutes of Health. J.W.P. is the Louis Goldstein Swan Chair in Women's Cancer Research.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 13 October 2008.
REFERENCES
- 1.Ajuebor, M. N., A. M. Das, L. Virag, R. J. Flower, C. Szabo, and M. Perretti. 1999. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. J. Immunol. 1621685-1691. [PubMed] [Google Scholar]
- 2.Arceci, R. J., S. Pampfer, and J. W. Pollard. 1992. Role and expression of colony stimulating factor-1 and steel factor receptors and their ligands during pregnancy in the mouse. Reprod. Fertil. Dev. 4619-632. [Google Scholar]
- 3.Austyn, J. M., and S. Gordon. 1981. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 11805-815. [DOI] [PubMed] [Google Scholar]
- 4.Bakardjiev, A. I., J. A. Theriot, and D. A. Portnoy. 2006. Listeria monocytogenes traffics from maternal organs to the placenta and back. PLoS Pathogens 2e66. doi: 10.1371/journal.ppat.0020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bancroft, G. J., M. J. Bosma, G. C. Bosma, and E. R. Unanue. 1986. Regulation of macrophage Ia expression in mice with severe combined immunodeficiency: induction of Ia expression by a T cell-independent mechanism. J. Immunol. 1374-9. [PubMed] [Google Scholar]
- 6.Barber, E. M., M. Fazzari, and J. W. Pollard. 2005. Th1 cytokines are essential for placental immunity to Listeria monocytogenes. Infect. Immun. 736322-6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barber, E. M., and J. W. Pollard. 2003. The uterine NK cell population requires IL-15 but these cells are not required for pregnancy nor the resolution of a Listeria monocytogenes infection. J. Immunol. 17137-46. [DOI] [PubMed] [Google Scholar]
- 8.Brinkmann, V., and A. Zychlinsky. 2007. Beneficial suicide: why neutrophils die to make NETs. Nat. Rev. Microbiol. 5577-582. [DOI] [PubMed] [Google Scholar]
- 9.Buhimschi, I. A., M. Jabr, C. S. Buhimschi, A. P. Petkova, C. P. Weiner, and G. M. Saed. 2004. The novel antimicrobial peptide beta3-defensin is produced by the amnion: a possible role of the fetal membranes in innate immunity of the amniotic cavity. Am. J. Obstet. Gynecol. 1911678-1687. [DOI] [PubMed] [Google Scholar]
- 10.Canavan, T. P., and H. N. Simhan. 2007. Innate immune function of the human decidual cell at the maternal-fetal interface. J. Reprod. Immunol. 7446-52. [DOI] [PubMed] [Google Scholar]
- 11.Cecchini, M. G., M. G. Dominguez, S. Mocci, A. Wetterwald, R. Felix, H. Fleisch, O. Chisholm, W. Hofstetter, J. W. Pollard, and E. R. Stanley. 1994. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development 1201357-1372. [DOI] [PubMed] [Google Scholar]
- 12.Clevers, H., B. Alarcon, T. Wileman, and C. Terhorst. 1988. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu. Rev. Immunol. 6629-662. [DOI] [PubMed] [Google Scholar]
- 13.Cohen, P. E., O. Chisholm, R. J. Arceci, E. R. Stanley, and J. W. Pollard. 1996. Absence of colony-stimulating factor-1 in osteopetrotic (csfmop/csfmop) mice results in male fertility defects. Biol. Reprod. 55310-317. [DOI] [PubMed] [Google Scholar]
- 14.Dai, W. J., W. Bartens, G. Kohler, M. Hufnagel, M. Kopf, and F. Brombacher. 1997. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-gamma receptor-deficient mice. J. Immunol. 1585297-5304. [PubMed] [Google Scholar]
- 15.Daiter, E., S. Pampfer, Y. G. Yeung, D. Barad, E. R. Stanley, and J. W. Pollard. 1992. Expression of colony-stimulating factor-1 in the human uterus and placenta. J. Clin. Endocrinol. Metab. 74850-858. [DOI] [PubMed] [Google Scholar]
- 16.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 2591739-1742. [DOI] [PubMed] [Google Scholar]
- 17.de Souza, P. C., and S. G. Katz. 2001. Coexpression of cytokeratin and vimentin in mice trophoblastic giant cells. Tissue Cell 3340-45. [DOI] [PubMed] [Google Scholar]
- 18.Duffield, J. S., S. J. Forbes, C. M. Constandinou, S. Clay, M. Partolina, S. Vuthoori, S. Wu, R. Lang, and J. P. Iredale. 2005. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Investig. 11556-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finn, C. A., and L. Martin. 1972. Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol. Reprod. 782-86. [DOI] [PubMed] [Google Scholar]
- 20.Fleming, T. J., M. L. Fleming, and T. R. Malek. 1993. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J. Immunol. 1512399-2408. [PubMed] [Google Scholar]
- 21.Gaunt, G., and K. Ramin. 2001. Immunological tolerance of the human fetus. Am. J. Perinatol. 18299-312. [DOI] [PubMed] [Google Scholar]
- 22.Goldenberg, R. L., J. F. Culhane, J. D. Iams, and R. Romero. 2008. Epidemiology and causes of preterm birth. Lancet 37175-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldenberg, R. L., J. C. Hauth, and W. W. Andrews. 2000. Intrauterine infection and preterm delivery. N. Engl. J. Med. 3421500-1507. [DOI] [PubMed] [Google Scholar]
- 24.Gouon-Evans, V., M. E. Rothenberg, and J. W. Pollard. 2000. Postnatal mammary gland development requires macrophages and eosinophils. Development 1272269-2282. [DOI] [PubMed] [Google Scholar]
- 25.Guleria, I., and J. W. Pollard. 2001. Aberrant macrophage and neutrophil population dynamics and impaired Th1 response to Listeria monocytogenes in colony-stimulating factor 1-deficient mice. Infect. Immun. 691795-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guleria, I., and J. W. Pollard. 2000. The trophoblast is a component of the innate immune system during pregnancy. Nat. Med. 6589-593. [DOI] [PubMed] [Google Scholar]
- 27.Hirsch, S., and S. Gordon. 1983. Polymorphic expression of a neutrophil differentiation antigen revealed by monoclonal antibody 7/4. Immunogenetics 18229-239. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh, C. S., S. E. Macatonia, C. S. Tripp, S. F. Wolf, A. O'Garra, and K. M. Murphy. 1993. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260547-549. [DOI] [PubMed] [Google Scholar]
- 29.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-gamma receptor. Science 2591742-1745. [DOI] [PubMed] [Google Scholar]
- 30.Hume, D. A., and S. Gordon. 1983. Mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Identification of resident macrophages in renal medullary and cortical interstitium and the juxtaglomerular complex. J. Exp. Med. 1571704-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufmann, S. H. 1993. Immunity to intracellular bacteria. Annu. Rev. Immunol. 11129-163. [DOI] [PubMed] [Google Scholar]
- 32.King, A. E., H. O. Critchley, and R. W. Kelly. 2000. Presence of secretory leukocyte protease inhibitor in human endometrium and first trimester decidua suggests an antibacterial protective role. Mol. Hum. Reprod. 6191-196. [DOI] [PubMed] [Google Scholar]
- 33.Krikun, G., C. J. Lockwood, V. M. Abrahams, G. Mor, M. Paidas, and S. Guller. 2007. Expression of Toll-like receptors in the human decidua. Histol. Histopathol. 22847-854. [DOI] [PubMed] [Google Scholar]
- 34.Lagasse, E., and I. L. Weissman. 1996. Flow cytometric identification of murine neutrophils and monocytes. J. Immunol. Methods 197139-150. [DOI] [PubMed] [Google Scholar]
- 35.Ledbetter, J. A., and L. A. Herzenberg. 1979. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol. Rev. 4763-90. [DOI] [PubMed] [Google Scholar]
- 36.Lee, R. S., N. Li, A. M. Ledgard, and J. W. Pollard. 2003. Dynamic regulation of expression of colony-stimulating factor 1 in the reproductive tract of cattle during the estrous cycle and in pregnancy. Biol. Reprod. 69518-528. [DOI] [PubMed] [Google Scholar]
- 37.Lorber, B. 1997. Listeriosis. Clin. Infect. Dis. 241-9. [DOI] [PubMed] [Google Scholar]
- 38.Martin, J. A., B. E. Hamilton, P. D. Sutton, S. J. Ventura, F. Menacker, S. Kirmeyer, and M. L. Munson. 2007. Births: final data for 2005. Natl. Vital Stat. Rep. 561-103. [PubMed] [Google Scholar]
- 39.McCormick, M. C. 1985. The contribution of low birth weight to infant mortality and childhood morbidity. N. Engl. J. Med. 31282-90. [DOI] [PubMed] [Google Scholar]
- 40.Mellor, A. L., and D. H. Munn. 2000. Immunology at the maternal-fetal interface: lessons for T cell tolerance and suppression. Annu. Rev. Immunol. 18367-391. [DOI] [PubMed] [Google Scholar]
- 41.Mueller-Heubach, E., D. N. Rubinstein, and S. S. Schwarz. 1990. Histologic chorioamnionitis and preterm delivery in different patient populations. Obstet. Gynecol. 75622-626. [PubMed] [Google Scholar]
- 42.Pamer, E. G. 2004. Immune responses to Listeria monocytogenes. Nat. Rev. Immunol. 4812-823. [DOI] [PubMed] [Google Scholar]
- 43.Pasparakis, M., L. Alexopoulou, V. Episkopou, and G. Kollias. 1996. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 1841397-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peschon, J. J., D. S. Torrance, K. L. Stocking, M. B. Glaccum, C. Otten, C. R. Willis, K. Charrier, P. J. Morrissey, C. B. Ware, and K. M. Mohler. 1998. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J. Immunol. 160943-952. [PubMed] [Google Scholar]
- 45.Pfeffer, K., T. Matsuyama, T. M. Kundig, A. Wakeham, K. Kishihara, A. Shahinian, K. Wiegmann, P. S. Ohashi, M. Kronke, and T. W. Mak. 1993. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73457-467. [DOI] [PubMed] [Google Scholar]
- 46.Pollard, J. W., A. Bartocci, R. Arceci, A. Orlofsky, M. B. Ladner, and E. R. Stanley. 1987. Apparent role of the macrophage growth factor, CSF-1, in placental development. Nature 330484-486. [DOI] [PubMed] [Google Scholar]
- 47.Pollard, J. W., J. S. Hunt, W. Wiktor-Jedrzejczak, and E. R. Stanley. 1991. A pregnancy defect in the osteopetrotic (op/op) mouse demonstrates the requirement for CSF-1 in female fertility. Dev. Biol. 148273-283. [DOI] [PubMed] [Google Scholar]
- 48.Redline, R. W., and C. Y. Lu. 1987. Role of local immunosuppression in murine fetoplacental listeriosis. J. Clin. Investig. 791234-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Redline, R. W., and C. Y. Lu. 1988. Specific defects in the anti-listerial immune response in discrete regions of the murine uterus and placenta account for susceptibility to infection. J. Immunol. 1403947-3955. [PubMed] [Google Scholar]
- 50.Romero, R., F. Gotsch, B. Pineles, and J. P. Kusanovic. 2007. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr. Rev. 65S194-S202. [DOI] [PubMed] [Google Scholar]
- 51.Stanley, E. R., L. J. Guilbert, R. J. Tushinski, and S. H. Bartelmez. 1983. CSF-1—a mononuclear phagocyte lineage-specific hemopoietic growth factor. J. Cell. Biochem. 21151-159. [DOI] [PubMed] [Google Scholar]
- 52.Steel, J. H., S. Malatos, N. Kennea, A. D. Edwards, L. Miles, P. Duggan, P. R. Reynolds, R. G. Feldman, and M. H. Sullivan. 2005. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr. Res. 57404-411. [DOI] [PubMed] [Google Scholar]
- 53.Tepper, R. I., R. L. Coffman, and P. Leder. 1992. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science 257548-551. [DOI] [PubMed] [Google Scholar]
- 54.Unanue, E. R. 1997. Inter-relationship among macrophages, natural killer cells and neutrophils in early stages of Listeria resistance. Curr. Opin. Immunol. 935-43. [DOI] [PubMed] [Google Scholar]
- 55.Van Rooijen, N., and A. Sanders. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 17483-93. [DOI] [PubMed] [Google Scholar]
- 56.Vidaeff, A. C., and S. M. Ramin. 2006. From concept to practice: the recent history of preterm delivery prevention. Part II. Subclinical infection and hormonal effects. Am. J. Perinatol. 2375-84. [DOI] [PubMed] [Google Scholar]
- 57.Wegmann, T. G., H. Lin, L. Guilbert, and T. R. Mosmann. 1993. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol. Today 14353-356. [DOI] [PubMed] [Google Scholar]
- 58.Wiktor-Jedrzejczak, W., A. Bartocci, A. W. Ferrante, Jr., A. Ahmed-Ansari, K. W. Sell, J. W. Pollard, and E. R. Stanley. 1990. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl. Acad. Sci. USA 874828-4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshida, H., S. Hayashi, T. Kunisada, M. Ogawa, S. Nishikawa, H. Okamura, T. Sudo, L. D. Shultz, and S. Nishikawa. 1990. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345442-444. [DOI] [PubMed] [Google Scholar]