Abstract
Many proteins secreted by the type V secretion system (autotransporters) have been linked to virulence in gram-negative bacteria. Several putative conventional autotransporters are present in the Yersinia pestis genome, but only one, YapE, is conserved in the other pathogenic Yersinia species. Here, we introduce YapE and demonstrate that it is secreted via a type V mechanism. Inactivation of yapE in Y. pestis results in decreased efficiency in colonization of tissues during bubonic infection. Coinfection with wild-type bacteria only partially compensates for this defect. Analysis of the host immune response suggests that YapE is required for either efficient colonization at the inoculation site or dissemination to draining lymph nodes. YapE also demonstrates adhesive properties capable of mediating interactions with bacteria and eukaryotic cells. These findings support a role for YapE in modulating host-pathogen interactions that are important for colonization of the mammalian host.
Yersinia pestis is a gram-negative zoonotic pathogen that causes bubonic and pneumonic plague in humans (44). The bacterium is primarily transmitted via the bite of an infected flea and proceeds to colonize the proximal lymph node (5). Bacterial growth and host inflammation at these sites lead to the development of a swollen, painful bubo, the hallmark manifestation of bubonic plague. Without treatment, Y. pestis escapes the immune response in the lymph nodes and disseminates through the bloodstream to colonize other tissues such as the spleen, liver, and lungs (33). Colonization of the lungs can lead to the development of a pneumonic infection (secondary pneumonic plague) and person-to-person transmission (primary pneumonic infection) when patients aerosolize the bacteria during coughing (43). Infection progresses rapidly, with mortality rates approaching 100% in untreated pneumonic patients.
Y. pestis recently evolved from Y. pseudotuberculosis but still shares virulence factors with its enteric progenitor, including the pCD1 (pYV) virulence plasmid essential for successful colonization of the mammalian host (1, 12). Encoded on pCD1 are the Ysc type III secretion apparatus and the Yop effector proteins that are translocated by this system (for a review, see reference 54). During infection, the Yops are translocated directly into host cells and disrupt normal cellular functions. The Yops have been shown to inhibit phagocytosis by disrupting actin polymerization, suppress host cytokine responses, and trigger apoptosis in macrophages (3, 9, 18, 31, 45). The combination of these actions allows Y. pestis to circumvent the early innate immune response and rapidly disseminate throughout the host. Mutational analysis in Y. pseudotuberculosis emphasizes the importance of the Ysc/Yop system by demonstrating that many of the Yops encode redundant functions, suggesting selection for an efficient, multifaceted disregulation of the innate immune response (39).
During the evolution from an enteric to a vector-borne pathogen, Y. pestis lost factors important for Y. pseudotuberculosis virulence, including inactivation of the adhesins inv and yadA (12). However, other adhesins have been identified in Y. pestis that appear to be important for virulence. The psa locus encodes components of a fimbrial structure that promotes binding to respiratory epithelial cells (15, 38, 57). Furthermore, Y. pestis lacking the fimbrial subunit PsaA is deficient in dissemination during bubonic infection and, to a lesser degree, during pneumonic plague (11). A second potential adhesin, Pla, is encoded on the pPCP1 plasmid. Pla is a member of the omptin surface protease family and cleaves host plasminogen and components of the complement pathway (53). Independent of this protease activity, Pla binds to the extracellular matrix component laminin and promotes invasion of endothelial cells (35). Inactivation of pla severely attenuates Y. pestis during bubonic infection (47, 52); however, a pla mutant is still lethal during intranasal or intravascular infection (37). In addition to PsaA and Pla, there is evidence that Y. pestis has other adhesins. Liu at el. demonstrated that in the absence of PsaA and the Caf1 capsule, Y. pestis still bound to epithelial cells and induced invasion (38). The Y. pestis protein(s) responsible for these interactions has yet to be identified, but any cryptic adhesin may prove to be important in Y. pestis pathogenesis.
Autotransporters are a family of secreted proteins found in gram-negative bacteria that encode a variety of virulence functions (for reviews, see references 28 and 30). Proteins that use this system for secretion (type V secretion) are unique in that the secreted protein encodes all of the necessary information to mediate translocation across the bacterial membranes. All autotransporters share a conserved domain structure: an N-terminal signal peptide, a central domain encoding the function of the mature protein (referred to as the passenger domain), and a C-terminal beta domain that mediates translocation of the passenger domain across the outer membrane.
The importance of the trimeric autotransporter YadA in the pathogenesis of Y. pseudotuberculosis and Y. enterocolitica has been evident through several studies. YadA has been implicated in autoaggregation, host cell binding, and serum resistance and is required for enteric infection (8, 21, 42); however, YadA does not appear to contribute to Y. pestis virulence, since it is a pseudogene in all of the Y. pestis biovars (51). Recently, at least 10 additional putative autotransporters have been identified in the genomes of Y. pestis (58, 29; M. B. Lawrenz, J. D. Lenz, and V. L. Miller, unpublished data). These novel autotransporters have been named Yaps for Yersinia autotransporter proteins. The Yaps demonstrate limited sequence similarity to other characterized proteins, but three appear to have some adherence properties (23, 58). Escherichia coli expressing YapN and YapK agglutinate red blood cells, and YapC demonstrates autoagglutination and adherence to epithelial cells. Although many autotransporters from other bacteria have been implicated as important virulence factors, it has yet to be determined whether the Yaps contribute to Y. pestis virulence.
In this study, we introduce the YapE autotransporter of Y. pestis and demonstrate its importance in efficient colonization of the mammalian host. In addition, we analyzed the cytokine response of infected animals to help decipher possible defects in ΔyapE colonization. Finally, we demonstrate that YapE can mediate binding to eukaryotic cells in an apparent cell-type-specific manner.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and animals.
Wild-type (WT) Y. pestis strain CO92 was provided by the U.S. Army, Fort Dietrich, MD (19). The Δpla, ΔyapE, ΔlacZ, and ΔpsaA Δcaf1 mutants have in-frame deletions in the indicated genes generated using the lambda red recombinase method essentially as described previously (11, 16, 37). The ΔyapE mutant was complemented with yapE plus 600 bp of upstream DNA at the attTn7 site using the mini-Tn7 system developed by Choi et al. (14). The kanamycin resistance cassette was removed from the mutant strains using the FRT recombinase provided by a version of pLH29 which has been modified by replacing the chloramphenicol resistance gene with an ampicillin resistance gene. Ampicillin and kanamycin sensitivity was confirmed prior to passage in animals. A tetracycline-inducible yapE strain was generated in the avirulent WT pCD1(−), WT pCD1(−)Δpla, and WT pCD1(−) ΔpsaA Δcaf1 strains using the mini-Tn7-based system described by Lathem et al. (37). For adherence assays using E. coli, yapE was introduced between the KpnI and HindIII sites of the plasmid pLP-PROTet-6xHN (Clontech, Palo Alto, CA) downstream of the tetracycline promoter and transformed into E. coli DH5αPRO (Clontech) or the ΔompT strain UT5600 (New England Biolabs, Ipswich, MA) containing the plasmid pVM1286 harboring the tetracycline repressor (Clontech). Y. pestis and E. coli were cultivated on brain heart infusion (BHI) or Luria-Bertani (LB) (Difco, Sparks, MD) plates or broth, respectively, with the addition of kanamycin (50 μg ml−1), ampicillin (50 μg ml−1), spectinomycin (100 μg ml−1) or X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; 100 μg ml−1) when needed. No alterations in the in vitro growth characteristics of the Y. pestis mutant strains compared to the parental strain were observed.
All animal experiments were approved by the Animal Studies Committee of Washington University (protocol 20050189). Four- to six-week-old female C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) were maintained in a barrier facility and allowed free access to sterilized food and water. Animals were anesthetized prior to infection with a mixture of ketamine-HCl (2 mg per mouse) and xylazine-HCl (0.32 mg per mouse) by intraperitoneal injection. Mice were inoculated with Y. pestis subcutaneously (∼102 CFU) or intranasally (∼105 CFU) as described previously (11, 36). At the indicated time points after inoculation, mice were euthanized by an intraperitoneal injection of sodium pentobarbital at a dose of 75 mg/kg of body weight. Tissues were aseptically harvested and macerated, and the bacterial load of each tissue was determined by plating serial dilutions.
RNA isolation and reverse transcription-PCR (RT-PCR).
An overnight culture of WT Y. pestis was subcultured into BHI to an optical density at 600 nm (OD600) of 0.2 and grown for ∼8 h at 26°C to an OD600 of 3.5. RNA was isolated by using the RiboPure-Bacteria kit (Ambion, Austin, TX). Contaminating DNA was removed from the samples with the DNA-free DNase kit (Ambion), and cDNA was generated from 2.0 μg of total DNase-treated RNA by using Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA).
Surface proteolysis and Western blot analysis.
To determine the surface localization of YapE, E. coli cultures were grown overnight, diluted 1:50 in fresh media, and grown for 2 h at 37°C. Y. pestis cultures were grown overnight, diluted 1:25 in fresh media, and grown for 3 h at 26°C. YapE expression was induced by the addition of anhydrous tetracycline (Sigma, St. Louis, MO) at 10 ng ml−1 (E. coli) or 100 ng ml−1 (Y. pestis), and cultures were grown for an additional 2 h. Samples from induced cultures equivalent to an OD600 of 1.0 were harvested, washed twice with 1× phosphate-buffered saline (PBS)-5 mM MgCl2 (pH 7.5) (resuspension buffer), and resuspended in 450 μl of the same buffer. Then, 50 μl of proteinase K (4 mg ml−1) was added to each sample, followed by incubation on ice for 30 min, and 50 μl of 50 mM phenylmethylsulfonyl fluoride was added to inhibit further proteolysis. Samples were washed twice with resuspension buffer, and cell pellets were resuspended in Laemmli buffer containing 10% β-mercaptoethanol. Samples were boiled for 10 min, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to Immobilon-P membranes (Millipore, Billerica, MA) for Western blot analysis. Anti-YapE serum was generated in rabbits (Cocalico Biological, Reamstown, PA) against the passenger domain (amino acids 42 to 709) of YapE, preabsorbed against E. coli lysates, and used at a concentration of 1:1,000. Anti-MalE rabbit serum (New England Biolabs) was used at a dilution of 1:10,000. Proteins from culture supernatants were isolated from induced cultures as described previously (55, 59).
Host cytokine response.
To determine host cytokine response, superficial cervical lymph nodes from either naive or mice infected with WT or the ΔyapE mutant were harvested at 24, 36, and 48 h postinfection (n = 5 for each time point), directly immersed into RNAlater (Ambion), and stored at 4°C. RNA was isolated from the lymph nodes by using the RiboPure RNA isolation system (Ambion). Contaminating DNA was removed from the samples and cDNA generated as described above. Quantitative RT-PCR was performed to determine cytokine transcript levels using SYBR Green (Qiagen, Valencia, CA) and 900 nM gene-specific primers (26), and data were normalized to the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) transcript. The relative fold change was calculated by using the ΔΔCT method (4) comparing infected to naive lymph nodes.
Competitive index.
ΔlacZ (fully virulent) and ΔyapE strains were grown in BHI for 15 h at 26°C. A 1:1 mixture of ΔlacZ and ΔyapE strains was made and diluted to ∼103 CFU/ml in 1× PBS. Next, 100 μl of the diluted mixture was plated on BHI agar containing X-Gal to differentiate between the ΔlacZ strain (white colonies) and the ΔyapE strains (blue colonies), and the ratio between the two strains was determined (CFU of ΔyapE/CFU of ΔlacZ = input ratio). Mice were challenged with 100 μl of the mixture subcutaneously, and tissues were harvested as described above. Serial dilutions of macerated tissues were plated on BHI agar containing X-Gal to determine the recovered ratio (CFU of ΔyapE per tissue/CFU of ΔlacZ per tissue). A competitive index score was generated by dividing the recovered ratio by the input ratio. A score of less than zero indicates that the recovered ratio differs from the input ratio and, specifically, fewer ΔyapE bacteria are present in the tissues. Similar infections were performed with a mixed infection of the ΔlacZ and yapE complemented ΔyapE strains.
Bacterial settling and adherence assays.
To analyze settling of E. coli expressing YapE in broth cultures, bacteria were grown overnight, diluted 1:50 in fresh medium, and grown for 2 h at 37°C on a roller drum. YapE expression was induced by the addition of 10 ng of anhydrous tetracycline ml−1, and cultures were grown for an additional 2 h. Cultures were removed from the rotator and allowed to sit statically, and samples were harvested at a specific depth to determine the changes in absorbance at 600 nm over time.
For adherence assays, wells of a 24-well plate were seeded with 4 × 105 or 2 × 105 A549 human lung or HEp-2 human epithelial cells, respectively, and grown overnight at 37°C with 5% CO2 to obtain ca. 80% confluent monolayers. YapE expression was induced in E. coli as described above with either 10 or 20 ng of anhydrous tetracycline ml−1. For Y. pestis, YapE expression was induced as described above with 100 ng of anhydrous tetracycline ml−1. Bacteria were diluted to an OD600 of 0.3 (E. coli) or 0.003 (Y. pestis) ml−1 in fresh tissue culture medium, and 1 ml of diluted culture was added to each well. Plates were centrifuged at 200 × g for 5 min to initiate bacterium-cell interactions and incubated at 37°C for either 2 h (E. coli) or 30 min (Y. pestis). To remove nonadherent bacteria, the culture medium was removed, and wells were washed four times with PBS. Adherent bacteria were recovered by treatment with 0.02% trypsin for 10 min and 1% Triton for 5 min (to lyse the eukaryotic cells). Serial dilutions of recovered bacteria were plated on agar. To calculate the percentage of adherent bacteria, the number of adherent bacteria was divided by the number of total bacteria as determined from an independent well. For microscopy, cells were fixed and stained by using the Diff Quick Stain kit (IMEB, Inc., San Marcos, CA).
RESULTS
Characterization of the Yersinia autotransporter YapE.
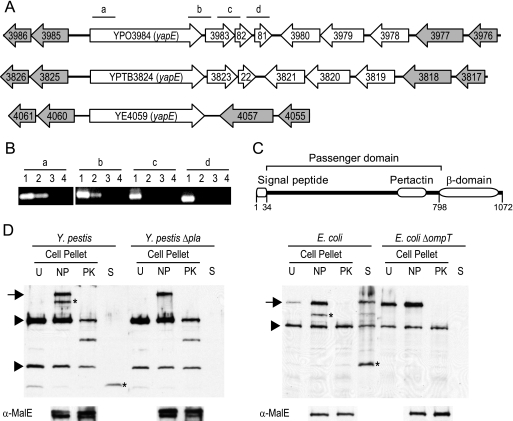
The Y. pestis CO92 genome encodes 10 conventional autotransporters (Lawrenz et al., unpublished data) but only yapE (YPO3984) is conserved in the enteric pathogens Y. pseudotuberculosis IP32953 (YPTB3824, 97% amino acid identity) and Y. enterocolitica 8081(YE4059, 65% amino acid identity). YapE orthologs are also found in the other Y. pestis biovars, Y. pestis subspecies Microtus and Pestoides F (100% amino acid identity for both), Y. mollaretii (62% amino acid identity), and Y. bercovieri (60% amino acid identity). Outside the Yersinia genus, YapE has little similarity to other proteins, with the highest degree of similarity occurring between the C-terminal end of the protein and β-domains of other putative autotransporters. A comparison between the yapE loci of the human pathogenic species is shown in Fig. 1A. The presence of conserved open reading frames (ORFs) flanking yapE in all three species suggests the gene was acquired prior to the divergence of Y. pseudotuberculosis and Y. enterocolitica. In the case of the Y. pseudotuberculosis lineage, five ORFs are present between yapE and the 3′ flanking conserved gene YPTB3818 (in Y. pestis an extra ORF is present due to a duplication of YPO3982). These ORFs do not have homologs in the Y. enterocolitica genome and are likely a more recent acquisition by Y. pseudotuberculosis or loss by Y. enterocolitica. In Y. pestis, there are only 92 bp separating yapE from YPO3983, suggesting yapE may be in an operon with the downstream ORFs.
FIG. 1.
Characterization of yapE. (A) yapE and surrounding genes from Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica (top to bottom, respectively). Gray arrows represent flanking genes that are conserved in all three species. Bars with lowercase letters represent regions amplified by RT-PCR. (B) RT-PCR to determine whether yapE is in an operon. Lowercase letters correspond to regions in panel A amplified by RT-PCR. Lanes: 1, DNA; 2, RNA; 3, No RT; 4, no template. (C) Diagram showing the conserved domains in YapE predicted by Pfam. Numbers indicate amino acid positions of predicted domains within YapE. (D) Western blot analysis demonstrating the localization of YapE in E. coli and Y. pestis. The bottom panel demonstrates the stability of MalE during protease digestions. Protein samples from cell pellets (U, uninduced; NP, induced, no proteinase K; PK, induced, + proteinase K) and culture supernatants from induced, no-proteinase-K samples (S) were probed with anti-YapE serum. Arrows denote surface-exposed mature-length YapE. Asterisks denote omptin-processed YapE fragments. Arrowheads denote nonspecific proteins that are recognized by the anti-YapE serum.
To determine whether yapE is transcribed in an operon with these ORFs, we isolated RNA from WT CO92 and performed RT-PCR with primers bridging the genes of the putative operon (Fig. 1B). We obtained PCR products for the region spanning YapE and YPO3983 but not for the regions spanning YPO3983-YPO3982 or YPO3982-YPO3981. This suggests that yapE is transcribed monocistronically with YPO3983 but not the two downstream ORFs. However, it is possible that YPO3982 and YPO3981 are also part of the operon, but our RT-PCRs yielded negative results due to decreased stability of the transcript at the 3′ end.
Sequence analysis of YapE reveals the presence of three putative domains that are important for autotransporter translocation. Computational analysis predicts an N-terminal signal peptide, an autotransporter conserved pertactin domain (Pfam ID PF03212), and a C-terminal β-domain, which is required for translocation of all autotransporters (Fig. 1C) (25). The presence of these domains, particularly the signal peptide and the β-domain, strongly suggests that YapE is an autotransporter. To demonstrate experimentally that YapE is a secreted autotransporter, we determined the accessibility of the protein to proteinase K. Proteinase K is unable to diffuse across the outer membrane of gram-negative bacteria and can only cleave outer surface proteins in intact bacteria. Therefore, if YapE is a functional autotransporter, it should be accessible to proteolysis by proteinase K. E. coli and Y. pestis containing inducible yapE were incubated with or without proteinase K as described in Materials and Methods. An inducible system was used because we were unable to visualize YapE protein expressed from its native promoter by Western blotting (data not shown). Induction of YapE resulted in the appearance of two additional proteins that are recognized by the anti-YapE antibody (Fig. 1D). The largest protein migrated near the predicted molecular mass (∼108 kDa) for YapE. We did not observe the presence of reactive bands indicating trimerization of YapE. Within 30 min of incubation with proteinase K, both proteins were completely digested in E. coli, and a truncated protein, likely representing the β-domain (protected from digestion) and a small region of the passenger domain, remained in the Y. pestis samples. As a control, we determined the stability of the periplasmic protein MalE after proteinase K treatment. The levels of MalE did not change after protease treatment, indicating that the bacteria remained intact throughout the experiment.
The presence of two proteins upon induction of YapE expression suggests that YapE is processed after translocation. Protein processing has been observed for several autotransporters and is a mechanism to secrete portions of the passenger domain into the environment. Many autotransporters require outer surface proteases such as omptins for processing, but a select group have autocatalytic mechanisms for release (17). To determine whether the smaller protein in the induced samples resulted from cleavage of the larger protein, we harvested culture supernatant from YapE induced cultures and looked for the presence of YapE in these samples by Western blotting. In both E. coli and Y. pestis cultures, we discovered an ∼37-kDa reactive protein (Fig. 1D). This protein was not present in uninduced cultures (data not shown), indicating that, at least in vitro, YapE can be processed after translocation and secreted. Furthermore, deletion of the omptins ompT in E. coli or pla in Y. pestis resulted in the loss of both the smaller protein in the cell pellet and the ∼37-kDa protein from the supernatant (Fig. 1D). Taken together with the surface proteolysis results, these data confirm that YapE is a functional, translocated autotransporter that can be processed by members of the omptin family of proteases.
YapE is required for Y. pestis virulence during bubonic plague.
The presence of YapE in all three human pathogenic Yersinia suggests that it may be a conserved virulence factor. To determine the contribution of YapE to Y. pestis virulence, we generated an in-frame deletion of the yapE gene in the fully virulent CO92 strain and compared its ability to colonize mice to the WT parental strain. Compared to the WT, the ΔyapE mutant was defective in efficiently colonizing the proximal lymph nodes, spleen, and lungs via the bubonic route of infection. We observed a noticeable delay in the colonization of the lymph nodes by the ΔyapE mutant beginning as early as 36 h postinfection (Fig. 2A). Significantly more WT-infected animals had detectable bacteria present in their cervical lymph nodes at 36 and 48 h postinfection. Furthermore, the median bacterial load was consistently lower in the lymph nodes of ΔyapE strain-infected animals, with a >106-fold difference in the median recovered CFU at 60 h postinfection.
FIG. 2.
Dissemination of the ΔyapE mutant during bubonic infection. Mice were infected subcutaneously in the neck with ∼102 CFU of the ΔyapE mutant (open circles), WT (gray circles), or the yapE complemented mutant (open squares). Mice were sacrificed at 36, 48, and 60 h postinfection, and the colonization of the superficial cervical lymph nodes (A), spleen (B), and lungs (C) was determined. Each symbol represents an individual animal. Black bars correspond to the median CFU/tissue for each group. The dashed line indicates the limit of detection. Asterisks denote significant differences between the median CFU/tissue (Mann-Whitney t test with a two-tailed nonparametric analysis: *, P ≤ 0.05; **, P ≤ 0.005; ***, P ≤ 0.0005). The data represent the composite of three independent experiments. The number (n) of tissues harvested for each indicated time point is given in parentheses in the format “time point = (n)ΔyapE, (n)WT, (n)complemented mutant” as follows: lymph nodes (36 h = 17, 24, 6; 48 h = 17, 22, 6; 60 h = 7, 14, 7), spleen (36 h = 17, 24, 7; 48 h = 17, 24, 7; 60 h = 17, 24, 7), and lungs (60 h = 17, 24, 7).
We also observed a difference in the ability of the mutant to efficiently disseminate or survive in the spleens and lungs of infected animals. By 60 h, all mice infected with WT had bacteria in their spleens, but 35% of the ΔyapE-infected mice had spleens without detectable bacteria (Fig. 2B). As observed in the lymph nodes, bacterial loads were also significantly lower in spleens of ΔyapE-infected mice at 60 h, with >103-fold more bacteria in tissues of WT-infected animals. A similar delay in colonization by the mutant was observed in the lungs, with more organs colonized, and to higher levels, by WT at 60 h (Fig. 2C). Virulence could be restored to the ΔyapE mutant by complementing the mutant with a functional copy of the yapE gene, indicating that attenuation was specific for the yapE mutation.
While Y. pestis is primarily a vector-borne pathogen, pneumonic infection is a secondary route of transmission associated with rapid progression of disease and severe mortality rates. To determine whether YapE contributes to pathogenesis during pneumonic plague, we inoculated mice intranasally with ∼105 CFU of the ΔyapE mutant and compared colonization of the lungs and dissemination to the spleen with a WT infection. Unlike the bubonic route, deletion of yapE did not significantly alter the ability of CO92 to efficiently colonize mice (Fig. 3A). ΔyapE strain-infected mice had bacterial counts in their lungs by 12 h postinfection that were similar to WT-infected counterparts. The mutant also appeared to replicate effectively in the lungs, reaching levels comparable to those of the WT throughout the course of the experiment. By 48 h postinfection, the spleens of animals infected with both strains of Y. pestis were colonized, with a slightly lower bacterial load in mutant-infected animals (P = 0.0078), but similar levels were reached by 60 h postinfection (Fig. 3B). These results suggest YapE has a greater impact on the virulence of Y. pestis during bubonic infection than intranasal inoculation. Interestingly, the mutant still caused a lethal infection and had a 50% lethal dose similar to that of the parental WT strain (<10 CFU) during bubonic infection.
FIG. 3.
Dissemination of the ΔyapE mutant during pneumonic infection. Mice were infected intranasally with ∼105 CFU of ΔyapE mutant (open circles), WT (gray circles), or the yapE complemented mutant (open squares). Mice were sacrificed at 12, 24, 48, and 60 h postinfection, and colonization of the lungs (A) and spleen (B) was determined. Each symbol represents an individual animal, and black bars correspond to the median CFU/tissue for each group. The dashed line indicates the limit of detection. Symbols on the dashed line represent animals with CFU below the limit of detection, and symbols on the x-axis represent animals that succumbed to infection. The number (n) of tissues harvested for each time point is given in parentheses in the format “time point = (n)ΔyapE, (n)WT, (n)complemented mutant” as follows: lungs (12 h = 18, 17, 11; 24 h = 8, 8, 0; 48 h = 21, 20, 14; 60 h = 13, 13, 13) and spleen (24 h = 8, 8, 0; 48 h = 21, 21, 14; 60 h = 12, 14, 12).
ΔyapE mutant does not stimulate an earlier immune response in infected lymph nodes.
Attenuation in colonization of the lymph nodes by the ΔyapE mutant during bubonic infection could be a result of either poor dissemination from the inoculation site or a reduced ability to survive the immune response in the lymph nodes. WT Y. pestis is able to delay early recognition by the innate immune system, but as the infection progresses the host eventually mounts a strong inflammatory response (10, 36, 46; M. B. Lawrenz and V. L. Miller, unpublished data). We postulated that monitoring cytokine and chemokine expression in the lymph nodes may provide insight into the reason(s) that the ΔyapE mutant is attenuated in lymph node colonization. In WT-infected lymph nodes, expression of the proinflammatory cytokines interleukin-6 (IL-6), IL-1α, and IL-1β was low at 24 h postinfection, but by 48 h all three were dramatically induced compared to uninfected controls (Fig. 4A). In contrast, during the ΔyapE mutant infection transcription of these cytokines remained low through 36 h postinfection. When cytokine levels began to increase in the ΔyapE mutant-infected mice at 48 h, expression was dramatically lower than that seen in WT-infected tissues. These differences correlated with less bacteria in the lymph nodes of ΔyapE mutant-infected animals at these time points (Fig. 4C) and suggest that a similar immune response occurred in both cases but was delayed in ΔyapE mutant-infected mice.
FIG. 4.
Cytokine response in the lymph nodes during bubonic infection of mice. Mice were infected subcutaneously in the neck with ∼102 CFU of WT (black circles) or the ΔyapE mutant (open circles), and RNA was harvested from the superficial cervical lymph nodes of five mice at 24, 36, and 48 h postinfection. The expression of IL-6, IL-1α, and IL-1β (A) or IFN-γ, TNF-α, and IL-12 (B) was determined by quantitative RT-PCR and is represented as the fold difference over levels from uninfected lymph nodes. (C) Two mice were sacrificed at each time point to determine the approximate CFU/tissue.
Previous work suggests that gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and IL-12 are protective against Y. pestis infection (6, 40, 41). We hypothesized that the ΔyapE mutant may be attenuated in lymph node colonization because it stimulates the expression of these cytokines more effectively than does the WT. Earlier induction could in turn lead to a more effective immune response and increased killing of the mutant. However, we did not observe a significant difference in the expression of IFN-γ, TNF-α, and IL-12 transcripts in the lymph nodes of mice infected with the ΔyapE mutant compared to uninfected samples (Fig. 4B). These data suggest that the ΔyapE mutant does not stimulate an earlier immune response in the lymph nodes.
Coinfection with WT does not fully complement attenuation of the ΔyapE mutant.
Competitive infections are often used to better understand the impact of factors on virulence. In many cases, coinfection can enhance the attenuation of the mutant (7, 39); however, it has been speculated that WT bacteria could complement the defects of some mutants in trans, especially mutants in secreted virulence factors (13). To determine the effect of the WT on the ability of the ΔyapE mutant to cause disease, we infected mice subcutaneously with a 1:1 mixture of a ΔlacZ derivative of WT and the ΔyapE mutant and monitored the colonization of the lymph nodes, spleen, and lungs by determining the competitive index (Fig. 5). In contrast to the phenotype we observed in single infections, the ΔyapE mutant efficiently colonized the lymph nodes during coinfection to levels comparable to WT, represented by a competitive index score of ≥1.0 at all time points. However, we recovered fewer ΔyapE bacteria in the spleens and lungs of coinfected mice at 60 h postinfection (median competitive index scores of 0.006 and 0.012, respectively), indicating that the mutant is still attenuated in the colonization of these tissues. Complementation of the ΔyapE strain with yapE in the Tn7 att site restored virulence and resulted in no differences in colonization of tissues during coinfection. These results indicate that the WT is able to complement the virulence defect of the ΔyapE mutant in the lymph nodes but not during dissemination to and/or colonization of the spleen or lungs of coinfected mice.
FIG. 5.
Colonization by the ΔyapE mutant during mixed infection with WT CO92. Mice were infected subcutaneously in the neck with a 1:1 mixture of the ΔyapE mutant and ΔlacZ WT (open symbols) or the yapE complemented mutant and ΔlacZ WT (gray symbols). Lymph nodes (circles), spleen (squares), and lungs (triangles) were harvested at 24, 36, 48, and 60 h postinfection. Serial dilutions were plated on agar containing X-Gal to differentiate between the test and ΔlacZ WT strains, and CFU of each were determined. The ratio of recovered mutant bacteria to ΔlacZ WT was compared to the equivalent ratio of the inocula to generate a competitive index (C.I.) score. Median competitive index scores are represented by black bars. Scores less than one indicate attenuation in colonization by the test strain.
YapE mediates autoaggregation and adherence to eukaryotic cells.
During our localization analyses, we observed that induction of YapE resulted in the formation of a ring at the fluid-air interphase on test tubes and settling of E. coli to the bottom of the cultures (data not shown). Similar phenotypes have been reported for other autotransporters and are indicative of protein-protein interactions between bacteria (27, 49, 50). To determine whether YapE mediates autoaggregation, YapE expression was induced in E. coli for 2 h, and samples were harvested from static cultures to monitor the bacterial settling (Fig. 6A). We observed that while the absorbance of YapF expressing and vector control strains did not decrease significantly over the course of the assay, the absorbance of the YapE-expressing culture decreased quickly and was ca. 50% of the starting OD600 within 3 h. We observed no differences in the number of recovered viable CFU between any of the strains at 8 h, indicating that protein induction was not harming the YapE-expressing bacteria (data not shown). These data indicate that YapE mediates autoaggregation in E. coli and suggest that YapE has adhesive properties.
FIG. 6.
Autoaggregation and adherence to eukaryotic cells by YapE-expressing bacteria. (A) YapE expression was induced in E. coli (open circles), and changes in the absorbance of static cultures were monitored over time and compared to vector only (gray circles)- and YapF (black circles)-expressing E. coli cultures. The data represent the mean percentage of the absorbance ± the standard error of the mean at 0 h (n = 6). The standard errors of the mean for YapE data were calculated but are smaller than the symbols. (B and C) YapE expression was induced in bacterial cultures, and bacteria were incubated with eukaryotic cells. Nonadherent bacteria were washed away, and the percentage of Y. pestis ΔpsaA Δcaf1 that adhered to A549 cells was determined (B). (C) Microscopy demonstrating YapE-mediated binding to A549 but not HEp-2 cells. White arrows denote bacteria.
Since other autotransporters demonstrating autoaggregation also interact with eukaryotic cells (34), we hypothesized that YapE may promote adherence of Y. pestis to eukaryotic cells. Previous work demonstrated that Y. pestis adheres to various eukaryotic cells in vitro and that some of these interactions are mediated by cryptic adhesins (23, 35, 38). To determine whether YapE mediates adherence to epithelial cells, we incubated either E. coli or E. coli expressing YapE with two epithelial cell lines (Fig. 6C). E. coli poorly adhered to both cell lines; however, expression of YapE resulted in specific adherence to A549 cells but not HEp-2 cells. To demonstrate in Y. pestis that YapE promotes adherence to A549 cells, we generated a Y. pestis strain with an inducible copy of yapE that lacked PsaA (a known major adhesin) and Caf1 (shown to mask other adhesins) (38). YapE expression in Y. pestis resulted in adherence to A549 but not to HEp-2 cells (Fig. 6B and C). These data suggest YapE promotes adherence to eukaryotic cells and that these interactions occur through a receptor present on A549 but not HEp-2 cells.
DISCUSSION
Autotransporters have been associated with virulence in many gram-negative pathogens (28). Analysis of the Y. pestis CO92 genome revealed the presence of several putative conventional autotransporters. YapE is unique in this group since it is the only one that is also found in Y. pseudotuberculosis and Y. enterocolitica and appears to have been acquired prior to the divergence of these species. Its conservation in other Yersinia species suggests that YapE provides a selective advantage for the genus, perhaps during infection. Furthermore, because the vector-borne lifestyle of Y. pestis differs considerably from its enteric counterparts, the functions of YapE may not be specific to an enteric lifestyle.
One advantage of the type V secretion mechanism is that while all autotransporters use the same basic mechanism to migrate across the bacterial cell envelope, the system imposes little restriction on the final localization of the passenger domain (17). Some autotransporters remain associated with the bacterium, either as intact polypeptides or through noncovalent interactions with the outer membrane after proteolytic processing. Others are released after translocation and are free to diffuse away from the bacterium. In the case of YapE, we demonstrated that the passenger domain primarily remains associated with the bacterium, but some polypeptides are processed, resulting in the release of a portion of the passenger domain into the supernatant. YapE does not appear to autoproteolytically cleave its passenger domain, as has been shown for several members of the SPATE family of autotransporters (reviewed in reference 17), but is processed by outer membrane omptins (Pla in Y. pestis and OmpT in E. coli). Omptin cleavage is not unique to YapE; autotransporters from other bacteria are processed by omptins (32). It is unclear at this time how omptin processing affects YapE. Pla processing may be part of the normal maturation process of YapE, resulting in a functional protein. Conversely, cleavage may allow Y. pestis to release itself from YapE-mediated interactions with eukaryotic cells. A similar release mechanism has been proposed for the Hap adhesin of Haemophilus influenzae, although Hap relies on autoproteolysis for cleavage (24).
Omptin processing may also determine the surface localization of YapE. In Shigella flexneri cleavage of the autotransporter IcsA by the omptin SopA is required for polar localization of the protein and actin-based intracellular motility (20, 48). Location-specific cleavage could explain the presence of both full-length and processed versions of YapE in Y. pestis. Finally, it is possible that increased expression of YapE with our inducible system may artificially promote omptin cleavage. However, other Yersinia autotransporters expressed by this system are not sensitive to OmpT cleavage (unpublished data). Therefore, we believe that omptin processing of YapE is not a result of overexpression.
We have demonstrated that a yapE-null mutant is significantly attenuated in colonizing tissues during bubonic infection compared to WT. Attenuation is first observed in the draining lymph nodes. Three scenarios may explain this delay in colonization: (i) the ΔyapE mutant is defective in colonization of the subcutaneous tissue, (ii) the mutant is defective for dissemination to the proximal lymph node, or (iii) the mutant disseminates to the tissue at the same rate but is less successful at surviving in the tissue. These scenarios are not necessarily mutually exclusive, but at this time, our data support either of the first two as more likely to be occurring. First, in animals with detectable bacteria in the lymph nodes, the mutant colonizes the tissue to levels comparable to the WT. Second, if the mutant was killed more efficiently in the lymph nodes, one might expect that antigen presentation and activation of the innate system would be stimulated earlier. In contrast to this hypothesis, we did not observe an earlier or more intense immune response in mutant-infected lymph nodes. In fact, the proinflammatory response was delayed in mutant-infected mice, likely because the mutant arrives at the lymph node later than the WT. Furthermore, once initiated, the inflammatory response to the ΔyapE mutant appears similar to the WT infection.
In addition to delayed lymph node colonization, we observed that the ΔyapE mutant was attenuated in dissemination to the spleen and lungs. Colonization of the lymph nodes is thought to be a prerequisite for infection of other tissues; therefore, the defect in dissemination to deeper tissues could be a direct consequence of delayed colonization of the lymph nodes. However, mixed infections demonstrated that the ΔyapE mutant was still attenuated in efficient colonization of spleens and lungs even when the lymph nodes were colonized. Similarly, we did not observe differences in colonization of the initial tissue during intranasal inoculation but detected subtle defects in colonization of the spleens by the ΔyapE mutant. We also observed that some mice succumbed by 60 h to infections with WT and yapE complemented strains but not with the ΔyapE mutant. Together, these results suggest that YapE may contribute independently both to colonization of the lymph nodes and to dissemination to deeper tissues, possibly through distinct functions; it is not unprecedented for autotransporters to be multifunctional (2, 28).
Dissemination defects similar to those we observed for the ΔyapE mutant have been previously reported for another Y. pestis virulence factor, the surface protein Pla (52, 56). Inactivation of pla does not alter colonization of the inoculation site during subcutaneous infection but results in delayed spleen colonization. It has been speculated that Pla activates host plasminogen to degrade fibrin deposits, allowing Y. pestis to disseminate from the inoculation site. We have shown that Pla processes YapE and, due to similar defects in dissemination by the two mutants, it is possible that lack of the processed form of YapE in the Δpla mutant may contribute to the attenuated dissemination phenotype of the Δpla mutant. However, we believe that Pla likely also contributes to virulence independent of YapE. This is evidenced by the significantly higher 50% lethal dose of the Δpla mutant (52) and its decreased ability to colonize the lungs during intranasal infection (37). In the future, we will determine the effect of yapE inactivation on colonization of the subcutaneous tissue during bubonic infection. Comparing the abilities of the ΔyapE, Δpla, and ΔyapE Δpla mutants to colonize the inoculation site will help clarify the role of Pla processing of YapE in virulence.
The passenger domain of YapE shares little similarity to other described autotransporters, making it difficult to predict the function(s) of the protein. We have demonstrated that YapE mediates interactions between bacteria and with eukaryotic cells. Adhesins are common virulence factors in many bacteria, suggesting that an adherence defect in the YapE mutant could be responsible for attenuation in the mouse model. Identification of a novel adhesin in Y. pestis is of particular interest due to its lack of the major enteric Yersinia adhesins. In Y. pestis, the adhesins inv and yadA are both pseudogenes, containing naturally occurring mutations. It has been proposed for Y. enterocolitica and Y. pseudotuberculosis that YadA and Inv binding promotes efficient translocation of Yop effectors into mammalian cells (22, 45). If similar intimate interactions are required for Yop translocation by Y. pestis, then other adhesins must be responsible. In addition to YapE, pH6 antigen (PsaA), Pla, and YapC have been shown to contribute to adherence in Y. pestis (23, 35, 38). It is unclear at this time whether these adhesins promote Yop translocation, but one could envision that decreased Yop translocation could be responsible for the attenuation we observed for the YapE mutant. Furthermore, the specificity of YapE binding suggests that YapE mediates interactions with specific cells and/or tissues. Our future studies will seek to determine the YapE receptor on eukaryotic cells and define the role of YapE in Yop translocation in the context of the other known Y. pestis adhesins.
Mixed infections demonstrated that attenuation in the ΔyapE mutant can be at least partially complemented by WT bacteria, restoring colonization of the lymph nodes by the mutant. To our knowledge, this is the first report of trans-complementation in bacterial coinfections. More surprising was that the WT could complement a mutation in a bacterium-associated adhesin. However, as stated earlier, autotransporters can encode multiple functions. For example, YadA has been shown to mediate adherence, invasion, and resistance to killing by complement (21). We have not ruled out the possibility that YapE may also be multifunctional. Furthermore, the secreted and bacterial associated proteins may contribute independent virulence functions. It is possible that the secreted portion of YapE is responsible for the delayed colonization of the lymph nodes, while systemic dissemination is more dependent on the cell surface form of YapE. This would explain why WT bacteria can trans-complement colonization of the lymph node but not later stages of infection by the mutant. As we begin to better understand the interactions of YapE with the mammalian host, new functions for YapE may be revealed.
Acknowledgments
This study was supported by funds from National Institutes of Health grant AI064313 (V.L.M.).
We thank Greer Kaufman for help with intranasal infections. The ΔpsaA Δcaf1 mutant was generated by Jason Cathelyn. The ΔlacZ mutant was generously provided by Joshua Fisher and William Goldman.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 20 October 2008.
REFERENCES
- 1.Achtman, M., K. Zurth, G. Morelli, G. Torrea, A. Guiyoule, and E. Carniel. 1999. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 9614043-14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alamuri, P., and H. L. Mobley. 2008. A novel autotransporter of uropathogenic Proteus mirabilis is both a cytotoxin and an agglutinin. Mol. Microbiol. 68997-1017. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, K., N. Carballeira, K. E. Magnusson, C. Persson, O. Stendahl, H. Wolf-Watz, and M. Fallman. 1996. YopH of Yersinia pseudotuberculosis interrupts early phosphotyrosine signalling associated with phagocytosis. Mol. Microbiol. 201057-1069. [DOI] [PubMed] [Google Scholar]
- 4.Applied Biosytems. 1997. User bulletin no. 2: ABI Prism 7700 sequence detection system. Applied Biosystems, Foster City, CA.
- 5.Bacot, A., and C. Martin. 1914. Observations on the mechanism of transmission of plague by fleas. J. Hyg. 13423-439. [PMC free article] [PubMed] [Google Scholar]
- 6.Barton, E. S., D. W. White, J. S. Cathelyn, K. A. Brett-McClellan, M. Engle, M. S. Diamond, V. L. Miller, and H. W. Virgin IV. 2007. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 447326-329. [DOI] [PubMed] [Google Scholar]
- 7.Beuzon, C. R., and D. W. Holden. 2001. Use of mixed infections with Salmonella strains to study virulence genes and their interactions in vivo. Microbes Infect. 31345-1352. [DOI] [PubMed] [Google Scholar]
- 8.Bliska, J. B., M. C. Copass, and S. Falkow. 1993. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into Hep-2 cells. Infect. Immun. 613914-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boland, A., and G. R. Cornelis. 1998. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect. Immun. 661878-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bubeck, S. S., A. M. Cantwell, and P. H. Dube. 2007. Delayed inflammatory response to primary pneumonic plague occurs in both outbred and inbred mice. Infect. Immun. 75697-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cathelyn, J. S., S. D. Crosby, W. W. Lathem, W. E. Goldman, and V. L. Miller. 2006. RovA, a global regulator of Yersinia pestis, specifically required for bubonic plague. Proc. Natl. Acad. Sci. USA 10313514-13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chain, P. S., E. Carniel, F. W. Larimer, J. Lamerdin, P. O. Stoutland, W. M. Regala, A. M. Georgescu, L. M. Vergez, M. L. Land, V. L. Motin, R. R. Brubaker, J. Fowler, J. Hinnebusch, M. Marceau, C. Medigue, M. Simonet, V. Chenal-Francisque, B. Souza, D. Dacheux, J. M. Elliott, A. Derbise, L. J. Hauser, and E. Garcia. 2004. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 10113826-13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiang, S. L., J. J. Mekalanos, and D. W. Holden. 1999. In vivo genetic analysis of bacterial virulence. Annu. Rev. Microbiol. 53129-154. [DOI] [PubMed] [Google Scholar]
- 14.Choi, K. H., J. B. Gaynor, K. G. White, C. Lopez, C. M. Bosio, R. R. Karkhoff-Schweizer, and H. P. Schweizer. 2005. A Tn7-based broad-range bacterial cloning and expression system. Nat. Methods 2443-448. [DOI] [PubMed] [Google Scholar]
- 15.Cowan, C., H. A. Jones, Y. H. Kaya, R. D. Perry, and S. C. Straley. 2000. Invasion of epithelial cells by Yersinia pestis: evidence for a Y. pestis-specific invasin. Infect. Immun. 684523-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dautin, N., and H. D. Bernstein. 2007. Protein secretion in gram-negative bacteria via the autotransporter pathway. Annu. Rev. Microbiol. 6189-112. [DOI] [PubMed] [Google Scholar]
- 18.Denecker, G., W. Declercq, C. A. Geuijen, A. Boland, R. Benabdillah, M. van Gurp, M. P. Sory, P. Vandenabeele, and G. R. Cornelis. 2001. Yersinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of Bid. J. Biol. Chem. 27619706-19714. [DOI] [PubMed] [Google Scholar]
- 19.Doll, J. M., P. S. Zeitz, P. Ettestad, A. L. Bucholtz, T. Davis, and K. Gage. 1994. Cat-transmitted fatal pneumonic plague in a person who traveled from Colorado to Arizona. Am. J. Trop. Med. Hyg. 51109-114. [DOI] [PubMed] [Google Scholar]
- 20.Egile, C., H. d'Hauteville, C. Parsot, and P. J. Sansonetti. 1997. SopA, the outer membrane protease responsible for polar localization of IcsA in Shigella flexneri. Mol. Microbiol. 231063-1073. [DOI] [PubMed] [Google Scholar]
- 21.El Tahir, Y., and M. Skurnik. 2001. YadA, the multifaceted Yersinia adhesin. Int. J. Med. Microbiol. 291209-218. [DOI] [PubMed] [Google Scholar]
- 22.Falgarone, G., H. S. Blanchard, F. Virecoulon, M. Simonet, and M. Breban. 1999. Coordinate involvement of invasin and Yop proteins in a Yersinia pseudotuberculosis-specific class I-restricted cytotoxic T cell-mediated response. J. Immunol. 1622875-2883. [PubMed] [Google Scholar]
- 23.Felek, S., M. B. Lawrenz, and E. S. Krukonis. 2008. The Yersinia pestis autotransporter YapC mediates host cell binding, autoaggregation and biofilm formation. Microbiology 1541802-1812. [DOI] [PubMed] [Google Scholar]
- 24.Fink, D. L., and J. W. St. Geme III. 2003. Chromosomal expression of the Haemophilus influenzae Hap autotransporter allows fine-tuned regulation of adhesive potential via inhibition of intermolecular autoproteolysis. J. Bacteriol. 1851608-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finn, R. D., J. Mistry, B. Schuster-Bockler, S. Griffiths-Jones, V. Hollich, T. Lassmann, S. Moxon, M. Marshall, A. Khanna, R. Durbin, S. R. Eddy, E. L. Sonnhammer, and A. Bateman. 2006. Pfam: clans, web tools, and services. Nucleic Acids Res. 34D247-D251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Handley, S. A., and V. L. Miller. 2007. General and specific host responses to bacterial infection in Peyer's patches: a role for stromelysin-1 (matrix metalloproteinase-3) during Salmonella enterica infection. Mol. Microbiol. 6494-110. [DOI] [PubMed] [Google Scholar]
- 27.Hasman, H., T. Chakraborty, and P. Klemm. 1999. Antigen-43-mediated autoaggregation of Escherichia coli is blocked by fimbriation. J. Bacteriol. 1814834-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henderson, I. R., and J. P. Nataro. 2001. Virulence functions of autotransporter proteins. Infect. Immun. 691231-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson, I. R., J. P. Nataro, R. Cappello, and C. Stein. 2000. Evolutionary origins of the autotransporter proteins. Direct submission. NCBI, NIH, Bethesda, MD.
- 30.Henderson, I. R., F. Navarro-Garcia, M. Desvaux, R. C. Fernandez, and D. Ala'Aldeen. 2004. Type V protein secretion pathway: the autotransporter story. Microbiol. Mol. Biol. Rev. 68692-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iriarte, M., and G. R. Cornelis. 1998. YopT, a new Yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol. Microbiol. 29915-929. [DOI] [PubMed] [Google Scholar]
- 32.Jacob-Dubuisson, F., R. Fernandez, and L. Coutte. 2004. Protein secretion through autotransporter and two-partner pathways. Biochim. Biophys. Acta 1694235-257. [DOI] [PubMed] [Google Scholar]
- 33.Janssen, W. A., G. M. Fukui, and M. J. Surgalla. 1958. A study of the fate of Pasteurella pestis following intracardial injection into guinea pigs. J. Infect. Dis. 103183-187. [DOI] [PubMed] [Google Scholar]
- 34.Klemm, P., R. M. Vejborg, and O. Sherlock. 2006. Self-associating autotransporters, SAATs: functional and structural similarities. Int. J. Med. Microbiol. 296187-195. [DOI] [PubMed] [Google Scholar]
- 35.Lahteenmaki, K., M. Kukkonen, and T. K. Korhonen. 2001. The Pla surface protease/adhesin of Yersinia pestis mediates bacterial invasion into human endothelial cells. FEBS Lett. 50469-72. [DOI] [PubMed] [Google Scholar]
- 36.Lathem, W. W., S. D. Crosby, V. L. Miller, and W. E. Goldman. 2005. Progression of primary pneumonic plague: a mouse model of infection, pathology, and bacterial transcriptional activity. Proc. Natl. Acad. Sci. USA 10217786-17791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lathem, W. W., P. A. Price, V. L. Miller, and W. E. Goldman. 2007. A plasminogen-activating protease specifically controls the development of primary pneumonic plague. Science 315509-513. [DOI] [PubMed] [Google Scholar]
- 38.Liu, F., H. Chen, E. M. Galvan, M. A. Lasaro, and D. M. Schifferli. 2006. Effects of Psa and F1 on the adhesive and invasive interactions of Yersinia pestis with human respiratory tract epithelial cells. Infect. Immun. 745636-5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Logsdon, L. K., and J. Mecsas. 2003. Requirement of the Yersinia pseudotuberculosis effectors YopH and YopE in colonization and persistence in intestinal and lymph tissues. Infect. Immun. 714595-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lukaszewski, R. A., D. J. Kenny, R. Taylor, D. G. Rees, M. G. Hartley, and P. C. Oyston. 2005. Pathogenesis of Yersinia pestis infection in BALB/c mice: effects on host macrophages and neutrophils. Infect. Immun. 737142-7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakajima, R., and R. R. Brubaker. 1993. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect. Immun. 6123-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pepe, J. C., M. R. Wachtel, E. Wagar, and V. L. Miller. 1995. Pathogenesis of defined invasion mutants of Yersinia enterocolitica in a BALB/c mouse model of infection. Infect. Immun. 634837-4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis: etiologic agent of plague. Clin. Microbiol. Rev. 1035-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prentice, M. B., and L. Rahalison. 2007. Plague. Lancet 3691196-1207. [DOI] [PubMed] [Google Scholar]
- 45.Rosqvist, R., A. Forsberg, M. Rimpilainen, T. Bergman, and H. Wolf-Watz. 1990. The cytotoxic protein YopE of Yersinia obstructs the primary host defense. Mol. Microbiol. 4657-667. [DOI] [PubMed] [Google Scholar]
- 46.Sebbane, F., D. Gardner, D. Long, B. B. Gowen, and B. J. Hinnebusch. 2005. Kinetics of disease progression and host response in a rat model of bubonic plague. Am. J. Pathol. 1661427-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sebbane, F., C. O. Jarrett, D. Gardner, D. Long, and B. J. Hinnebusch. 2006. Role of the Yersinia pestis plasminogen activator in the incidence of distinct septicemic and bubonic forms of flea-borne plague. Proc. Natl. Acad. Sci. USA 1035526-5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shere, K. D., S. Sallustio, A. Manessis, T. G. D'Aversa, and M. B. Goldberg. 1997. Disruption of IcsP, the major Shigella protease that cleaves IcsA, accelerates actin-based motility. Mol. Microbiol. 25451-462. [DOI] [PubMed] [Google Scholar]
- 49.Sherlock, O., M. A. Schembri, A. Reisner, and P. Klemm. 2004. Novel roles for the AIDA adhesin from diarrheagenic Escherichia coli: cell aggregation and biofilm formation. J. Bacteriol. 1868058-8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherlock, O., R. M. Vejborg, and P. Klemm. 2005. The TibA adhesin/invasin from enterotoxigenic Escherichia coli is self recognizing and induces bacterial aggregation and biofilm formation. Infect. Immun. 731954-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skurnik, M., and H. Wolf-Watz. 1989. Analysis of the yopA gene encoding the Yop1 virulence determinants of Yersinia spp. Mol. Microbiol. 3517-529. [DOI] [PubMed] [Google Scholar]
- 52.Sodeinde, O. A., Y. V. Subrahmanyam, K. Stark, T. Quan, Y. Bao, and J. D. Goguen. 1992. A surface protease and the invasive character of plague. Science 2581004-1007. [DOI] [PubMed] [Google Scholar]
- 53.Suomalainen, M., J. Haiko, P. Ramu, L. Lobo, M. Kukkonen, B. Westerlund-Wikstrom, R. Virkola, K. Lahteenmaki, and T. K. Korhonen. 2007. Using every trick in the book: the Pla surface protease of Yersinia pestis. Adv. Exp. Med. Biol. 603268-278. [DOI] [PubMed] [Google Scholar]
- 54.Viboud, G. I., and J. B. Bliska. 2005. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 5969-89. [DOI] [PubMed] [Google Scholar]
- 55.Walker, K. A., and V. L. Miller. 2004. Regulation of the Ysa type III secretion system of Yersinia enterocolitica by YsaE/SycB and YsrS/YsrR. J. Bacteriol. 1864056-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Welkos, S. L., A. M. Friedlander, and K. J. Davis. 1997. Studies on the role of plasminogen activator in systemic infection by virulent Yersinia pestis strain CO92. Microb. Pathog. 23211-223. [DOI] [PubMed] [Google Scholar]
- 57.Yang, Y., J. J. Merriam, J. P. Mueller, and R. Isberg. 1996. The psa locus is responsible for thermoinducible binding of Yersinia pseudotuberculosis to cultured cells. Infect. Immun. 642483-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yen, Y. T., A. Karkal, M. Bhattacharya, R. C. Fernandez, and C. Stathopoulos. 2007. Identification and characterization of autotransporter proteins of Yersinia pestis KIM. Mol. Membr. Biol. 2428-40. [DOI] [PubMed] [Google Scholar]
- 59.Young, B. M., and G. M. Young. 2002. YplA is exported by the Ysc, Ysa, and flagellar type III secretion systems of Yersinia enterocolitica. J. Bacteriol. 1841324-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]