Abstract
Coxiella burnetii is an obligate intracellular bacterial pathogen that directs biogenesis of a lysosome-like, parasitophorous vacuole in mammalian cells. We recently reported that C. burnetii inhibits apoptotic cell death in macrophages, presumably as a mechanism to sustain the host for completion of its lengthy infectious cycle. In the current study, we further investigated C. burnetii manipulation of host cell signaling and apoptosis by examining the effect of C. burnetii infection on activation of 15 host proteins involved in stress responses, cytokine production, and apoptosis. C. burnetii infection of THP-1 human macrophage-like cells caused increased levels of phosphorylated c-Jun, Hsp27, Jun N-terminal protein kinase, and p38 at 2 h postinfection (hpi), and this activation rapidly decreased to near basal levels by 24 hpi. The prosurvival kinases Akt and Erk1/2 (extracellular signal-regulated kinases 1 and 2) were also activated at 2 to 6 hpi; however, the phosphorylation of these proteins increased coincident with C. burnetii replication through at least 72 hpi. Sustained phosphorylation of Akt and Erk1/2 was abolished by treatment of infected cells with rifampin, indicating their activation is a C. burnetii-directed event requiring pathogen RNA synthesis. Moreover, pharmacological inhibition of Akt or Erk1/2 significantly decreased C. burnetii antiapoptotic activity. Collectively, these results indicate the importance of C. burnetii modulation of host signaling and demonstrate a critical role for Akt and Erk1/2 in successful intracellular parasitism and maintenance of host cell viability.
A common property of intracellular bacterial pathogens is their ability to manipulate host cell signaling cascades to promote biogenesis and maintenance of a privileged vacuolar niche for replication. In particular, intracellular pathogens are especially adept at regulating host cell death and cytokine/chemokine production to ensure a growth compartment that is protected from the host innate immune response (5, 12, 42). Elucidation of these host-pathogen interactions has provided insight into novel mechanisms of signaling pathway regulation and enhanced our understanding of the complex interplay between intracellular pathogens and their host (4).
Coxiella burnetii is an obligate intracellular bacterium and the etiological agent of human Q fever. The pathogen is transmitted by aerosols and initially infects alveolar mononuclear phagocytes in vivo. Q fever normally manifests as an acute, debilitating, influenza-like illness, but chronic infection can result in severe endocarditis (27). Environmental stability (15), aerosol transmission (27), and a low infectious dose that approaches one organism (32) have resulted in classification of C. burnetii as a Centers for Disease Control and Prevention category B select agent with potential for illegitimate use.
In the host cell, C. burnetii directs maturation of a parasitophorous vacuole (PV) that displays lysosomal characteristics, including moderately acidic pH (pH ∼ 5), active acid hydrolases, and lysosome-associated membrane proteins (2, 16, 45). The PV membrane is also cholesterol rich and decorates with lipid raft proteins, which are predicted to influence the stability and fusogenicity of the vacuole (17). In the PV, C. burnetii converts from an environmentally stable, nonreplicative small-cell variant (SCV) morphological form to a replicatively proficient large-cell variant (LCV) form (7, 15). LCVs replicate to high numbers with a doubling time of 12 h during an exponential growth phase lasting ∼4 days (7). At ∼1 week postinfection, LCVs begin to transition back to SCVs, presumably as a mechanism to ensure a stable population of infectious organisms following demise of the host cell.
During C. burnetii's lengthy infectious cycle, the organism actively regulates host cell processes to ensure development of a stable niche for growth. C. burnetii protein synthesis is needed for early interactions between the PV and autophagosomes, a process that potentially delivers nutrients that may influence transition of dormant SCVs to replicative LCVs (37). C. burnetii also directs PV fusion with lysosomes, which provides the moderately acidic conditions required for pathogen metabolism (14). Finally, C. burnetii proteins inhibit apoptotic death of epithelial cells and macrophages (24, 46). In macrophages, we recently demonstrated that C. burnetii antagonizes cell death triggered by both staurosporine and tumor necrosis factor α, inducers of intrinsic (mitochondrial mediated) and extrinsic (death receptor mediated) apoptosis, respectively (46). C. burnetii antiapoptotic activity results in dramatically reduced processing of caspase-3, caspase-9, and poly-ADP(ribose) polymerase (PARP), hallmarks of the apoptotic process (20). Along with reduced activation of the caspase cascade, C. burnetii-infected cells also display an antiapoptotic transcriptional program that results in increased production of A1/Bfl-1, an antiapoptotic mitochondrial protein, and c-IAP2, a caspase inhibitor. Intracellular pathogens that inhibit cell death typically block several components of the apoptotic pathway (1, 3, 23, 31); however, the critical early step(s) antagonized by C. burnetii is currently unknown.
Bacterial pathogens commonly manipulate host prosurvival phosphorylation cascades that inhibit caspase-mediated apoptosis (4). To better understand the regulation of host signaling by C. burnetii, we examined the phosphorylation state of 15 mammalian signaling proteins during infection. The prosurvival proteins Akt and Erk1/2 (extracellular signal-regulated kinases 1 and 2) showed sustained activation while c-Jun, Hsp27, Jun N-terminal protein kinase (JNK), and p38 were transiently phosphorylated early during infection. Inhibition of Akt or Erk1/2 antagonized C. burnetii antiapoptotic activity. Thus, C. burnetii modulates multiple host cell signaling pathways to inhibit apoptosis and establish a protected replication niche.
MATERIALS AND METHODS
Mammalian cell culture and C. burnetii.
Virulent C. burnetii Nine Mile phase I (RSA493) and avirulent phase II (clone 4; RSA439) isolates were propagated in African green monkey kidney (Vero) cells (CCL-81; ATCC, Manassas, VA) and purified as previously described (6, 39). Human monocyte-like (THP-1) cells (TIB-202; ATCC) were maintained in RPMI 1640 medium (Gibco, Carlsbad, CA) supplemented with 10% fetal calf serum (Gibco) at 37°C in 5% CO2. All cells were used between passages 10 and 20. Prior to infection, THP-1 cells were incubated in the presence of 200 nM phorbol 12-myristate 13-acetate (PMA; EMD Biosciences, San Diego, CA) for 24 h to induce differentiation into an adherent, macrophage-like cell. Before C. burnetii infection, PMA-containing medium was removed from cell cultures, and cultures were replenished with fresh medium lacking PMA. Alveolar macrophages from cynomolgus macaques (Macaca fascicularis) were isolated by bronchial lavage and cultured in Dulbecco's minimal essential medium as previously described (46). In all experiments, cells were infected with C. burnetii phase I or phase II isolates at a multiplicity of infection (MOI) of 250 or 25, respectively, by addition of organisms to cell culture medium. (This time point was considered 0 h postinfection [hpi].) These MOIs result in a high percentage of infected THP-1 cells (9, 10, 46), with the lower MOI of phase II organisms employed because they are roughly 10-fold more infectious for cultured cells than phase I C. burnetii cells (45). When needed, rifampin (final concentration, 10 μg/ml) was added to cell cultures along with the C. burnetii inoculum.
Bio-Plex assay.
C. burnetii-infected or uninfected THP-1 cells were lysed by a freeze-thaw cycle in Bio-Plex lysis buffer (Bio-Rad, Hercules, CA). Lysates were incubated overnight with Bio-Plex beads coupled to phospho-specific antibodies. Beads were washed, incubated with biotin-labeled secondary antibodies for 30 min, and then incubated with a streptavidin reporter for 10 min. All steps were performed at room temperature. Internal bead fluorescence, indicative of each distinct signaling protein, and fluorescence intensity were determined using a Bio-Plex Array Reader (Bio-Rad).
Immunoblot analysis.
C. burnetii-infected cells cultured in six-well plates were directly lysed in buffer containing 50 mM Tris, 5 mM EDTA, 1% sodium dodecyl sulfate, and a phosphatase inhibitor cocktail (Sigma, St. Louis, MO) followed by 10 passages through a 26-gauge syringe needle. The protein concentration of each sample was determined using a detergent-compatible protein assay (Bio-Rad). Total protein (10 μg/lane) was separated by 10% or 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to an Immobilon-P membrane (Millipore, Bedford, MA). After membranes were blocked for 1 h at room temperature in Tris-buffered saline (TBS; 150 mM NaCl, 100 mM Tris-HCl, pH 7.6) containing 0.1% Tween 20 and 5% nonfat milk, they were incubated overnight at 4°C in TBS-Tween 20 containing primary antibodies directed against the phosphorylated and nonphosphorylated forms of Akt, Erk1/2, c-Jun, Hsp27, JNK, p38, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Cell Signaling Technology, Danvers, MA). Membranes were then washed and incubated for 1 h at room temperature in TBS-Tween 20 containing anti-rabbit or anti-mouse immunoglobulin G secondary antibodies conjugated to horseradish peroxidase (Cell Signaling Technology). Reacting proteins were detected via enhanced chemiluminescence using ECL Pico reagent (Pierce, Rockford, IL).
Signaling pathway inhibition and apoptosis induction.
Akt was inhibited by incubating infected cells with the class I phosphatidylinositol-3-kinase (PI3K) antagonists LY294002 (10 μM) or wortmannin (150 nM). Erk1/2 was inhibited by treatment of infected cells with the mitogen-activated protein kinase kinase 1 and 2 (MEK1/2) antagonist U0126 (20 μM). Inhibitors were added along with the C. burnetii inoculum and were present throughout the time course of infection. Intrinsic apoptosis was induced by addition of staurosporine (500 nM) to cell cultures at 48 hpi (48). All inhibitors and staurosporine were obtained from EMD Biosciences.
Cell death assay.
Cell viability was assessed using a colorimetric Cell Counting Kit-8 (Dojindo Laboratories, Gaithersburg, MD), as previously described (46). Briefly, infected or uninfected THP-1 cells in 96-well plates with or without LY294002 or U0126 treatments were incubated with staurosporine for 4 or 8 h. WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] reagent was then added to wells, and incubation continued for an additional 4 h at 37°C. Following measurement of the A450 of cell cultures, cell viability was calculated using the following formula: [(Atest − Abackground)/(Acontrol − Abackground)] × 100, where A is the absorbance at 450 nm, the background value represents medium alone, and the control value represents untreated cells.
Fluorescence microscopy.
THP-1 cells on 12-mm glass coverslips were infected with C. burnetii in the presence or absence of LY294002 or U0126 as described above. Following a 2-h treatment with staurosporine, cells were fixed and permeabilized with 100% ice-cold methanol for 3 min and then blocked for 1 h in phosphate-buffered saline (PBS; 1 mM KH2PO4, 155 mM NaCl, 3 mM Na2HPO4, pH 7.4) containing 5% bovine serum albumin. Cells were then incubated with rabbit anti-cleaved PARP (Cell Signaling Technology) and guinea pig anti-C. burnetii antibodies. Following a 1-h incubation, cells were washed three times in PBS and then incubated in PBS containing AlexaFluor-488 anti-rabbit and AlexaFluor-594 anti-guinea pig immunoglobulin G antibodies (Molecular Probes, Carlsbad, CA) for 1 h. Host and bacterial DNA were stained with DRAQ5 (Alexa Corporation, Lausen, Switzerland). Confocal fluorescence microscopy was conducted with a modified Perkin-Elmer UltraView spinning disk confocal system connected to a Nikon Eclipse TE2000-S microscope. Images were acquired with a 60× (1.4 numerical aperture) oil immersion objective (Nikon, Melville, NY) and a Photometrics Cascade II:512 digital camera (Princeton Instruments, Trenton, NJ) using Metamorph software (Molecular Devices, Downingtown, PA). A total of 100 cells were examined to determine the percentage of cleaved PARP-positive (apoptotic) cells in each cell culture.
RESULTS
C. burnetii infection causes transient phosphorylation of c-Jun, Hsp27, JNK, and p38 and sustained activation of Akt and Erk1/2.
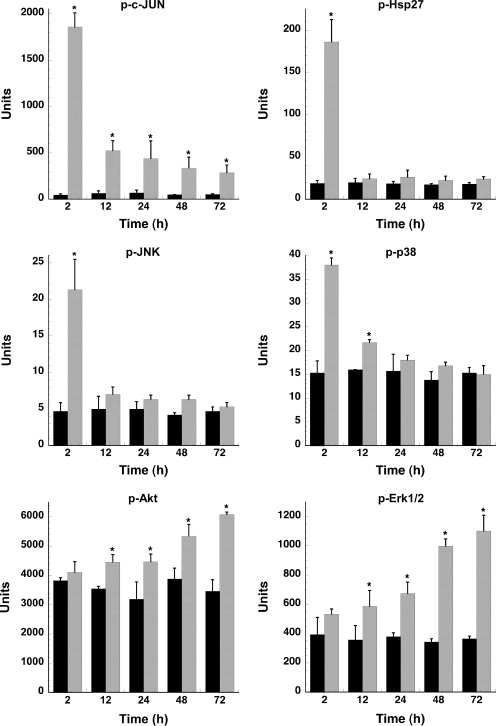
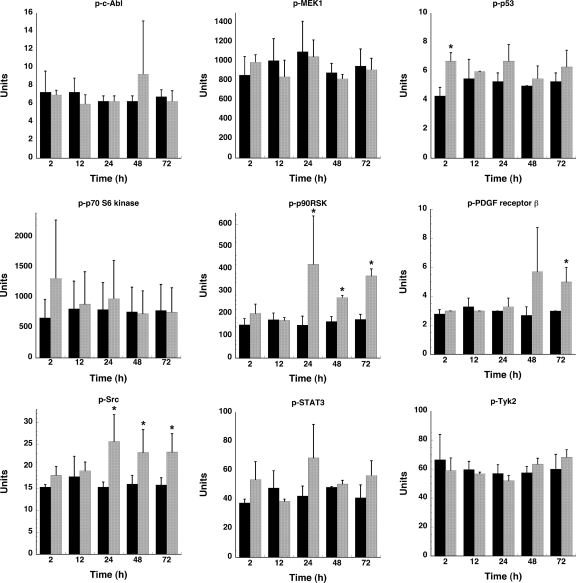
To analyze the effect of C. burnetii infection on host cell signaling, a Bio-Plex assay was performed. This multiplex system can determine the phosphorylation state of multiple proteins in a single well of a 96-well plate. The 15 proteins analyzed in this study and their respective phosphorylated amino acid residues are listed in Table 1. PMA-differentiated THP-1 cells, which biologically mimic monocyte-derived macrophages (22), were used in these experiments. This cell line has been used to model the interaction between macrophages and intracellular pathogens, including Mycobacterium tuberculosis and C. burnetii (22, 46). As shown in Fig. 1, lysates of THP-1 cells infected with C. burnetii for 2 h contained phosphorylated c-Jun, Hsp27, JNK, and p38. This activation was transient as phosphorylation in most cases returned to basal levels by 24 hpi. c-Jun was an exception: although levels of phosphorylated protein decreased dramatically by 12 hpi, they remained higher than levels in uninfected cells through 72 hpi. These short-lived responses are likely associated with the initial phagocytic uptake of C. burnetii. C. burnetii infection also induced Akt and Erk1/2 activation at 12 hpi (Fig. 1); however, these proteins remained activated through 72 hpi, with phosphorylation levels increasing coincident with C. burnetii replication. Sustained activation suggests that downstream signaling by these proteins occurs throughout the C. burnetii infectious process. The phosphorylation status of c-Abl, MEK1, p53, p70 S6 kinase, platelet-derived growth factor receptor β, STAT3, and Tyk2 remained unchanged throughout the 72-h course of infection, while p90RSK and Src showed slightly elevated phosphorylation levels at 24 to 72 hpi (Fig. 2).
TABLE 1.
Host signaling proteins in the Bio-Plex assay
| Protein | Phosphorylated residue(s) |
|---|---|
| Akt | S473 |
| c-Abl | Y245 |
| c-Jun | S63 |
| Erk1/2 | T202/Y204, T185/Y187 |
| Hsp27 | S78 |
| JNK | T183/Y185 |
| MEK1 | S217/S221 |
| p38 | T180/Y182 |
| p53 | S15 |
| p70 S6 kinase | T421/S424 |
| p90RSK | T359/S363 |
| PDGF receptor βa | Y751 |
| Src | Y416 |
| Stat3 | S727 |
| Tyk2 | Y1054/Y1055 |
PDGF, platelet-derived growth factor.
FIG. 1.
C. burnetii infection induces phosphorylation of host proteins involved in cell survival. Lysates of mock- or C. burnetii phase II-infected THP-1 cells were harvested at the indicated times and processed for the Bio-Plex assay as described in Materials and Methods. Protein phosphorylation levels of infected cell lysates (gray bars) were compared to those of uninfected cell lysates (black bars). Phosphorylation of c-Jun, Hsp27, JNK, and p38 was transient, while phosphorylation of Akt and Erk1/2 increased through 72 hpi. Samples were analyzed in triplicate, and results are representative of those found in two independent experiments. Error bars represent the standard deviation from the mean. An asterisk indicates a P value of <0.05 in comparison to uninfected control cells as determined by a Student's t test. p, phosphorylated.
FIG. 2.
Phosphorylation levels of seven proteins involved in cell survival responses are relatively unchanged during C. burnetii infection. Lysates of mock- and C. burnetii phase II-infected THP-1 cells were harvested at the indicated times postinfection and processed for the Bio-Plex assay as described in Materials and Methods. Protein phosphorylation levels of infected cell lysates (gray bars) were compared to those of uninfected cell lysates (black bars). Only p90RSK and Src showed slightly elevated phosphorylation at 24 to 72 hpi. p, phosphorylated. Samples were analyzed in triplicate, and results are representative of those found in two independent experiments. Error bars represent the standard deviation from the mean. An asterisk indicates a P value of <0.05 in comparison to uninfected control cells as determined by a Student's t test.
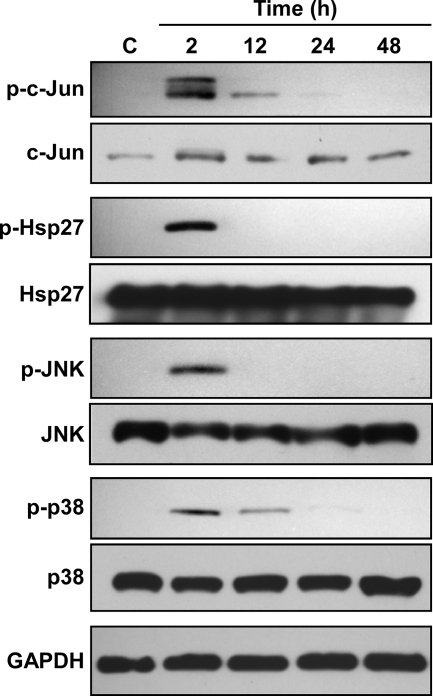
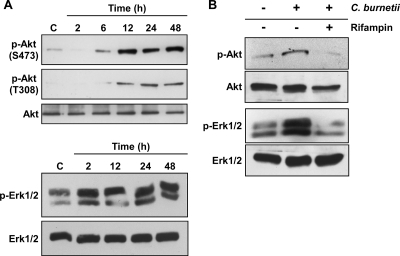
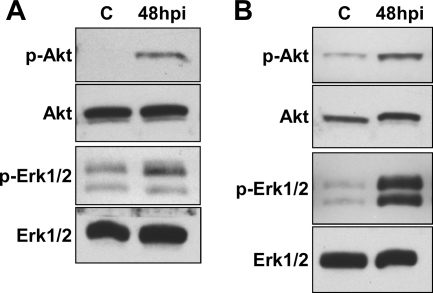
Consistent with the Bio-Plex results, immunoblotting demonstrated that c-Jun, Hsp27, JNK, and p38 phosphorylation levels were dramatically increased at 2 hpi, followed by a decline to near basal levels by 24 hpi (Fig. 3). Total levels of c-Jun, Hsp27, JNK, and p38 remained constant during the same time course. In contrast to this transient activation, Akt was phosphorylated at both S473 and T308 at 6 to 12 hpi, and phosphorylation was sustained through 48 hpi (Fig. 4A). Phosphorylation of both S473 and T308 is required for full Akt activity (26), indicating complete activation of the kinase. Erk1/2 was also activated early after C. burnetii infection (2 hpi), with phosphorylation levels sustained through 48 hpi (Fig. 4A). The total levels of both Akt and Erk1/2 remained constant throughout the 48-h infection. These results are consistent with the Bio-Plex data indicating that multiple host cell signaling pathways are differentially activated during C. burnetii infection. To determine whether de novo C. burnetii protein synthesis is required for Akt and Erk1/2 activation, infected THP-1 cells were treated with rifampin to inhibit bacterial RNA synthesis. As shown in Fig. 4B, increased levels of phosphorylated Akt and Erk1/2 were not observed at 48 hpi in infected THP-1 cells treated with rifampin. These results indicate that Akt and Erk1/2 activation are C. burnetii-driven processes. Akt and Erk1/2 phosphorylation levels were also elevated at 48 hpi in THP-1 cells infected with virulent C. burnetii Nine Mile phase I, indicating that activation is not strictly associated with avirulent organisms (Fig. 5A), and during C. burnetii Nine Mile phase II infection of monkey primary alveolar macrophages, indicating activation also occurs in primary cells (Fig. 5B).
FIG. 3.
Immunoblotting confirms transient phosphorylation of c-Jun, Hsp27, JNK, and p38 during C. burnetii infection. Lysates were harvested from mock- and C. burnetii phase II-infected THP-1 cells at the indicated times postinfection and subjected to immunoblot analysis. A representative immunoblot is shown for each protein. Mock-infected THP-1 cell lysates were used as a negative control (lane C). Phosphorylation levels of c-Jun, Hsp27, JNK, and p38 increased at 2 hpi, followed by a decrease to levels near those of uninfected cells by 12 to 24 hpi. In contrast, total levels of c-Jun, Hsp27, JNK, and p38 remained constant across the same time course. GAPDH levels were probed as a loading control. p, phosphorylated.
FIG. 4.
Sustained C. burnetii activation of Akt and Erk1/2 is dependent on bacterial protein synthesis. Lysates were harvested from mock- and C. burnetii phase II-infected THP-1 cells at the indicated times postinfection and subjected to immunoblot analysis. In each panel, a representative immunoblot is shown for each protein. Mock-infected THP-1 cell lysates were used as a negative control (lanes C). (A) Akt and Erk1/2 were phosphorylated early after infection and remained activated through 48 hpi. (B) THP-1 cells were infected for 48 h in the presence or absence of rifampin to inhibit C. burnetii RNA synthesis. Cell lysates from mock- and C. burnetii-infected THP-1 cells were subsequently analyzed for phosphorylated Akt and Erk1/2 by immunoblotting. Phosphorylation of each protein was substantially reduced in rifampin-treated, infected cells. p, phosphorylated.
FIG. 5.
Akt and Erk1/2 activation occurs irrespective of C. burnetii isolate virulence and is observed during infection of monkey primary alveolar macrophages. (A) THP-1 cells were infected with virulent phase I C. burnetii for 48 h, and then lysates were probed for phosphorylated Akt and Erk1/2 by immunoblotting. Similar to cells infected with avirulent phase II C. burnetii, cells infected with virulent phase I organisms also showed increased phosphorylation of both kinases at 48 hpi compared to uninfected cells. (B) Monkey primary alveolar macrophages isolated by bronchial lavage were infected for 48 h with phase II C. burnetii. Lysates were collected and probed by immunoblotting for the phosphorylated forms of Akt and Erk1/2. Similar to experiments using THP-1 cells, primary alveolar macrophages showed increased phosphorylation of Akt and Erk1/2 at 48 hpi. Lanes C, mock-infected control cells; p, phosphorylated.
Akt and Erk1/2 activation are required for C. burnetii inhibition of cell death.
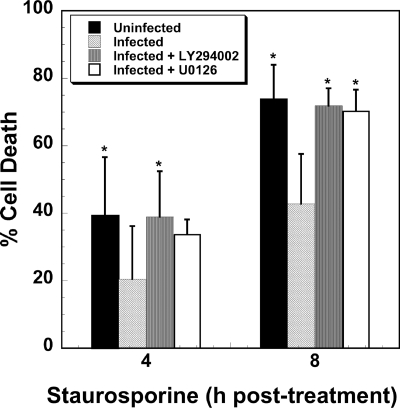
Akt directs eukaryotic cell survival responses via kinase activity that regulates at least seven different apoptosis-related components (28). Erk1/2 is commonly involved in antiapoptotic events, including phosphorylation and inactivation of proapoptotic Bad (29). To test whether either pathway is involved in C. burnetii's ability to inhibit cell death, THP-1 cells were infected for 48 h in the presence of LY294002 or U0126 and then treated with staurosporine to induce intrinsic apoptosis. Uninfected cells treated with staurosporine for 4 and 8 h displayed 40% and 74% death, respectively. In contrast, infected cells treated with staurosporine for 4 and 8 h showed 20% and 42% death, respectively. Infected cells incubated in the presence of LY294002, which inhibits PI3K and consequently Akt (43), displayed 39% and 72% death when treated with staurosporine for 4 and 8 h, respectively (Fig. 6). A similar effect was observed when infected cells were incubated with the PI3K antagonist wortmannin (data not shown). Infected cells incubated in the presence of U0126, which inhibits MEK1/2 and consequently Erk1/2 (43), showed 34% and 70% death when treated with staurosporine for 4 and 8 h, respectively. Thus, all inhibitor treatments resulted in levels of death that were similar to those of uninfected cells treated with staurosporine (Fig. 6). Akt and Erk1/2 inhibitor treatments did not demonstrably affect C. burnetii entry or growth as PVs containing replicating organisms were indistinguishable between treated and untreated THP-1 cells (Fig. 7).
FIG. 6.
C. burnetii requires Akt and Erk1/2 activation to protect mammalian cells from staurosporine-induced death. THP-1 cells were infected with phase II C. burnetii for 48 h in the presence or absence of LY294002 (class I PI3K inhibitor) or U0126 (MEK1/2 inhibitor) and then treated with staurosporine for 4 or 8 h. Cell death was assessed by WST-8 staining. In the presence of either inhibitor, C. burnetii was unable to prevent cell death induced by staurosporine. Experiments were performed in triplicate, and error bars represent the standard deviation from the mean. An asterisk indicates a P value of <0.05 in comparison to infected, staurosporine-treated cells as determined by a Student's t test.
FIG. 7.
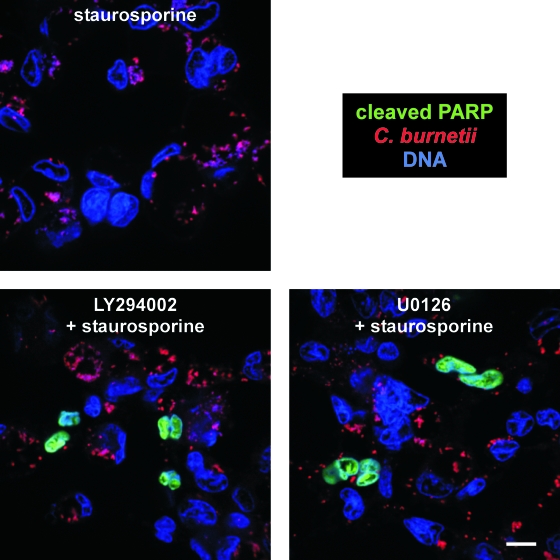
C. burnetii requires Akt and Erk1/2 activation to protect mammalian cells from apoptosis. THP-1 cells were infected with phase II C. burnetii in the presence or absence of LY294002 or U0126 for 48 h and then treated with staurosporine for 2 h to induce apoptosis. Cells were fixed and immunostained for confocal fluorescence microscopy using antibodies directed against C. burnetii (red) and cleaved PARP (green). THP-1 and bacterial DNA (blue) were stained with DRAQ5. LY294002- or U0126-treated cells showed substantially increased levels of cleaved PARP compared to untreated cells. Bar, 10 μM.
To confirm that Akt and Erk1/2 activation by C. burnetii protects host cells from apoptotic death, we assessed levels of cleaved PARP, an indicator of apoptosis, in THP-1 cells infected for 48 h in the presence of LY294002 or U0126 and then treated with staurosporine for 2 h. Approximately 4% of infected cells were positive for cleaved PARP in the absence of inhibitors. Conversely, ∼10% of cells treated with LY294002 or U0126 were positive for cleaved PARP, roughly half the percentage (∼20%) observed with uninfected cells (Fig. 7 and data not shown). Collectively, these results indicate that both Akt and Erk1/2 contribute to C. burnetii's antiapoptotic activity.
DISCUSSION
C. burnetii actively modulates Akt and Erk1/2 prosurvival host signaling pathways during intracellular growth in THP-1 cells and primary alveolar macrophages. Sustained activation of each kinase is observed for at least 3 days following infection, with levels of phosphorylated protein increasing concomitantly with C. burnetii replication. Each of these signaling proteins is a well-established regulator of mammalian cell survival responses (28, 29). We previously showed that C. burnetii infection potently inhibits apoptosis associated with caspase inhibition and a prosurvival transcriptional program (46). Here, we demonstrate that Akt and Erk1/2 cascades are integral components of C. burnetii-directed antiapoptotic signaling.
Sustained prosurvival signaling by Akt and Erk1/2 contributes to decreased host cell apoptosis during C. burnetii infection as specific inhibition of either of these kinases antagonizes the ability of C. burnetii to protect macrophages from staurosporine-induced apoptotic cell death. Akt is a prosurvival kinase that directly or indirectly inhibits activation of proapoptotic mitochondrial proteins (e.g., Bad) and transcription factors (e.g., p53). Additionally, Akt regulates activation of antiapoptotic transcription factors (e.g., FOXO) and caspase inhibitors (e.g., X-linked inhibitor of apoptosis protein), leading to a prosurvival cellular response (28). Erk1/2 regulates numerous transcription factors, such as Elk-1 and CREB, that enhance expression of antiapoptotic components (29). In addition to C. burnetii, Akt- and Erk1/2-mediated cell survival is observed during infection by other intracellular pathogenic bacteria. Activation of Akt by the Salmonella enterica type III effector SopB leads to host cell protection from caspase-mediated apoptosis during early stages of intracellular growth in epithelial cells (21). Chlamydia trachomatis-directed Akt phosphorylation leads to Bad inactivation and subsequent inhibition of mitochondrial-mediated apoptosis (44). Activation of Erk1/2 by Helicobacter pylori and Neisseria gonorrhoea results in increased production of antiapoptotic Mcl-1 (30) and degradation of the proapoptotic mitochondrial proteins Bad and Bim (18), respectively. The downstream effects of Akt and Erk1/2 activation in C. burnetii-infected cells are unknown. However, slightly elevated levels of p90RSK, an Erk1/2 effector that mediates numerous transcriptional events including NF-κB activation (13), are observed at 24 to 72 hpi.
The sustained activation of Akt and Erk1/2 during C. burnetii infection is unusual as the pathways regulating these proteins are tightly controlled by the host. Indeed, the kinases are generally rapidly dephosphorylated within minutes of performing their inherent functions (28). For example, S. enterica activates Akt only upon entry into nonphagocytic cells (21). Similarly, activation of Akt by M. tuberculosis surface-associated lipoarabinomannan is strictly associated with uptake of the pathogen by macrophages (25). Only C. trachomatis is reported to sustain phosphorylation of Akt during infection, with an elevated level at 24 hpi that decreases to basal level by 36 hpi (44). With respect to Erk1/2, the kinase is activated by Legionella pneumophila, Mycobacterium avium, and S. enterica at 0.5 to 1 hpi; however, phosphorylation returns to basal levels within 5 hpi, suggesting that Erk1/2 activity is only critical for early infection events (33, 36, 47). In contrast, Mycobacterium leprae and C. trachomatis induce prolonged Erk1/2 phosphorylation for 30 and 1 day(s) postinfection (dpi), respectively (5, 41), indicating that this pathway is utilized by a subset of intracellular pathogens for postentry infection processes.
While differential host cell interactions have been proposed for virulent phase I and avirulent phase II C. burnetii organisms (45), we demonstrate that Akt and Erk1/2 activation occurs irrespective of the virulence of C. burnetii isolates. This result is consistent with the similar antiapoptotic effects induced by phase variants during infection of THP-1 cells (46). In contrast, virulent and avirulent isolates of some other pathogens, such as M. tuberculosis, have been shown to differentially activate Erk1/2 (36).
Shortly following infection (2 hpi), c-Jun, JNK, Hsp27, and p38 are activated in response to C. burnetii. However, phosphorylation of these proteins rapidly decreases to nearly basal levels by 24 hpi, suggesting that C. burnetii quickly escapes a host response that could potentially alert the immune system to the presence of the pathogen (8, 34, 38, 40). For example, p38 contributes to the normal inflammatory response by regulating interleukin-1 and tumor necrosis factor α production by cells stimulated with endotoxin (8). Transient activation of these proteins is presumably a host cell response associated with phagocytosis of a foreign particle and not specifically directed by C. burnetii as significant pathogen metabolism at this time postinfection would not be expected (14). Moreover, similar events are observed early after infection of monocyte-derived macrophages by L. pneumophila, indicating that a common set of proteins is activated during initial host cell interactions with intracellular pathogens (47).
Multiple pathways are commonly manipulated by intracellular pathogens to inhibit apoptosis. For example, L. pneumophila activates an NF-κB-dependent transcriptional program early during infection (1, 23) and also secretes Dot/Icm type IV secretion system effectors that directly antagonize proapoptotic BNIP3 and Bcl-rambo (3). M. tuberculosis activates Akt, which leads to inactivation of proapoptotic Bad and induction of antiapoptotic NF-κB (11, 25). Chlamydia spp. are perhaps the most versatile apoptosis-antagonizing pathogens with NF-κB activation, Akt phosphorylation, and degradation of multiple proapoptotic mitochondrial proteins all occurring during infection of various cell types (31, 44). In addition to sustained phosphorylation of Akt and Erk1/2, C. burnetii also activates NF-κB during infection (2 to 24 hpi) (D. E. Voth and R. A. Heinzen, unpublished results). Indeed, NF-κB-dependent transcriptional responses, such as upregulation of the A1/Bfl-1 and c-IAP2 genes, are observed in C. burnetii-infected cells that likely potentiate Akt and Erk1/2 control of apoptosis-related genes (46).
Most intracellular pathogens promote a lytic release from host cells within 1 to 3 dpi (19). However, C. burnetii has a lengthy infectious cycle, with stationary phase occurring ∼6 dpi, after which the pathogen is presumably released to the extracellular milieu following demise of the host cell. Thus, C. burnetii has necessarily evolved efficient methods of regulating host survival, including the sustained activation of the Akt and Erk1/2 pathways described here. C. burnetii protein synthesis is required for sustained phosphorylation of these proteins; thus, pathogen effectors of activation are likely constitutively produced during infection. Although the C. burnetii proteins directing these responses are unknown, they are presumably delivered to the host cytosol via the organism's Dot/Icm type IV secretion system. Indeed, our laboratory along with other investigators has recently identified multiple C. burnetii proteins that are translocated into the host cytosol in a Dot/Icm-dependent manner (35; also D. E. Voth et al., unpublished data). Further characterization of C. burnetii's manipulation of host signaling pathways and of the bacterial proteins responsible will provide insight into virulence mechanisms exploited by this obligate intracellular bacterial pathogen.
Acknowledgments
We thank Leigh Knodler, Sonja Best, and Shelly Robertson for critical reading of the manuscript and Gary Hettrick for graphic illustrations.
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 3 November 2008.
REFERENCES
- 1.Abu-Zant, A., S. Jones, R. Asare, J. Suttles, C. Price, J. Graham, and Y. A. Kwaik. 2007. Anti-apoptotic signalling by the Dot/Icm secretion system of L. pneumophila. Cell. Microbiol. 9246-264. [DOI] [PubMed] [Google Scholar]
- 2.Akporiaye, E. T., J. D. Rowatt, A. A. Aragon, and O. G. Baca. 1983. Lysosomal response of a murine macrophage-like cell line persistently infected with Coxiella burnetii. Infect. Immun. 401155-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banga, S., P. Gao, X. Shen, V. Fiscus, W. X. Zong, L. Chen, and Z. Q. Luo. 2007. Legionella pneumophila inhibits macrophage apoptosis by targeting pro-death members of the Bcl2 protein family. Proc. Natl. Acad. Sci. USA 1045121-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhavsar, A. P., J. A. Guttman, and B. B. Finlay. 2007. Manipulation of host-cell pathways by bacterial pathogens. Nature 449827-834. [DOI] [PubMed] [Google Scholar]
- 5.Buchholz, K. R., and R. S. Stephens. 2007. The extracellular signal-regulated kinase/mitogen-activated protein kinase pathway induces the inflammatory factor interleukin-8 following Chlamydia trachomatis infection. Infect. Immun. 755924-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cockrell, D. C., P. A. Beare, E. R. Fischer, D. Howe, and R. A. Heinzen. 2008. A method for purifying obligate intracellular Coxiella burnetii that employs digitonin lysis of host cells. J. Microbiol. Methods 72321-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman, S. A., E. R. Fischer, D. Howe, D. J. Mead, and R. A. Heinzen. 2004. Temporal analysis of Coxiella burnetii morphological differentiation. J. Bacteriol. 1867344-7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuenda, A., and S. Rousseau. 2007. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta 17731358-1375. [DOI] [PubMed] [Google Scholar]
- 9.Dellacasagrande, J., C. Capo, D. Raoult, and J. L. Mege. 1999. IFN-gamma-mediated control of Coxiella burnetii survival in monocytes: the role of cell apoptosis and TNF. J. Immunol. 1622259-2265. [PubMed] [Google Scholar]
- 10.Dellacasagrande, J., E. Ghigo, D. Raoult, C. Capo, and J. L. Mege. 2002. IFN-gamma-induced apoptosis and microbicidal activity in monocytes harboring the intracellular bacterium Coxiella burnetii require membrane TNF and homotypic cell adherence. J. Immunol. 1696309-6315. [DOI] [PubMed] [Google Scholar]
- 11.Dhiman, R., M. Raje, and S. Majumdar. 2007. Differential expression of NF-κB in mycobacteria infected THP-1 affects apoptosis. Biochim. Biophys. Acta 1770649-658. [DOI] [PubMed] [Google Scholar]
- 12.Faherty, C. S., and A. T. Maurelli. 2008. Staying alive: bacterial inhibition of apoptosis during infection. Trends Microbiol. 16173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frödin, M., and S. Gammeltoft. 1999. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol. Cell. Endocrinol. 15165-77. [DOI] [PubMed] [Google Scholar]
- 14.Hackstadt, T., and J. C. Williams. 1981. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc. Natl. Acad. Sci. USA 783240-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinzen, R. A., T. Hackstadt, and J. E. Samuel. 1999. Developmental biology of Coxiella burnetii. Trends Microbiol. 7149-154. [DOI] [PubMed] [Google Scholar]
- 16.Heinzen, R. A., M. A. Scidmore, D. D. Rockey, and T. Hackstadt. 1996. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect. Immun. 64796-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howe, D., and R. A. Heinzen. 2006. Coxiella burnetii inhabits a cholesterol-rich vacuole and influences cellular cholesterol metabolism. Cell. Microbiol. 8496-507. [DOI] [PubMed] [Google Scholar]
- 18.Howie, H. L., S. L. Shiflett, and M. So. 2008. Extracellular signal-regulated kinase activation by Neisseria gonorrhoeae downregulates epithelial cell proapoptotic proteins Bad and Bim. Infect. Immun. 762715-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hybiske, K., and R. S. Stephens. 2008. Exit strategies of intracellular pathogens. Nat. Rev. Microbiol. 699-110. [DOI] [PubMed] [Google Scholar]
- 20.Jin, Z., and W. S. El-Deiry. 2005. Overview of cell death signaling pathways. Cancer Biol. Ther. 4139-163. [DOI] [PubMed] [Google Scholar]
- 21.Knodler, L. A., B. B. Finlay, and O. Steele-Mortimer. 2005. The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J. Biol. Chem. 2809058-9064. [DOI] [PubMed] [Google Scholar]
- 22.Loeuillet, C., F. Martinon, C. Perez, M. Munoz, M. Thome, and P. R. Meylan. 2006. Mycobacterium tuberculosis subverts innate immunity to evade specific effectors. J. Immunol. 1776245-6255. [DOI] [PubMed] [Google Scholar]
- 23.Losick, V. P., and R. R. Isberg. 2006. NF-κB translocation prevents host cell death after low-dose challenge by Legionella pneumophila. J. Exp. Med. 2032177-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luhrmann, A., and C. R. Roy. 2007. Coxiella burnetii inhibits activation of host cell apoptosis through a mechanism that involves preventing cytochrome c release from mitochondria. Infect. Immun. 755282-5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maiti, D., A. Bhattacharyya, and J. Basu. 2001. Lipoarabinomannan from Mycobacterium tuberculosis promotes macrophage survival by phosphorylating Bad through a phosphatidylinositol 3-kinase/Akt pathway. J. Biol. Chem. 276329-333. [DOI] [PubMed] [Google Scholar]
- 26.Manning, B. D., and L. C. Cantley. 2007. AKT/PKB signaling: navigating downstream. Cell 1291261-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurin, M., and D. Raoult. 1999. Q fever. Clin. Microbiol. Rev. 12518-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCubrey, J. A., L. S. Steelman, S. L. Abrams, J. T. Lee, F. Chang, F. E. Bertrand, P. M. Navolanic, D. M. Terrian, R. A. Franklin, A. B. D'Assoro, J. L. Salisbury, M. C. Mazzarino, F. Stivala, and M. Libra. 2006. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv. Enzyme Regul. 46249-279. [DOI] [PubMed] [Google Scholar]
- 29.McCubrey, J. A., L. S. Steelman, W. H. Chappell, S. L. Abrams, E. W. Wong, F. Chang, B. Lehmann, D. M. Terrian, M. Milella, A. Tafuri, F. Stivala, M. Libra, J. Basecke, C. Evangelisti, A. M. Martelli, and R. A. Franklin. 2007. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation, and drug resistance. Biochim. Biophys. Acta 17731263-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mimuro, H., T. Suzuki, S. Nagai, G. Rieder, M. Suzuki, T. Nagai, Y. Fujita, K. Nagamatsu, N. Ishijima, S. Koyasu, R. Haas, and C. Sasakawa. 2007. Helicobacter pylori dampens gut epithelial self-renewal by inhibiting apoptosis, a bacterial strategy to enhance colonization of the stomach. Cell Host Microbe. 2250-263. [DOI] [PubMed] [Google Scholar]
- 31.Miyairi, I., and G. I. Byrne. 2006. Chlamydia and programmed cell death. Curr. Opin. Microbiol. 9102-108. [DOI] [PubMed] [Google Scholar]
- 32.Moos, A., and T. Hackstadt. 1987. Comparative virulence of intra- and interstrain lipopolysaccharide variants of Coxiella burnetii in the guinea pig model. Infect. Immun. 551144-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mynott, T. L., B. Crossett, and S. R. Prathalingam. 2002. Proteolytic inhibition of Salmonella enterica serovar Typhimurium-induced activation of the mitogen-activated protein kinases ERK and JNK in cultured human intestinal cells. Infect. Immun. 7086-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishina, H., T. Wada, and T. Katada. 2004. Physiological roles of SAPK/JNK signaling pathway. J. Biochem. 136123-126. [DOI] [PubMed] [Google Scholar]
- 35.Pan, X., A. Luhrmann, A. Satoh, M. A. Laskowski-Arce, and C. R. Roy. 2008. Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science 3201651-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roach, S. K., and J. S. Schorey. 2002. Differential regulation of the mitogen-activated protein kinases by pathogenic and nonpathogenic mycobacteria. Infect. Immun. 703040-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romano, P. S., M. G. Gutierrez, W. Beron, M. Rabinovitch, and M. I. Colombo. 2007. The autophagic pathway is actively modulated by phase II Coxiella burnetii to efficiently replicate in the host cell. Cell. Microbiol. 9891-909. [DOI] [PubMed] [Google Scholar]
- 38.Schmitt, E., M. Gehrmann, M. Brunet, G. Multhoff, and C. Garrido. 2007. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J. Leukoc. Biol. 8115-27. [DOI] [PubMed] [Google Scholar]
- 39.Shannon, J. G., and R. A. Heinzen. 2008. Infection of human monocyte-derived macrophages with Coxiella burnetii. Methods Mol. Biol. 431189-200. [DOI] [PubMed] [Google Scholar]
- 40.Takeda, K., T. Noguchi, I. Naguro, and H. Ichijo. 2008. Apoptosis signal-regulating kinase 1 in stress and immune response. Annu. Rev. Pharmacol. Toxicol. 48199-225. [DOI] [PubMed] [Google Scholar]
- 41.Tapinos, N., and A. Rambukkana. 2005. Insights into regulation of human Schwann cell proliferation by Erk1/2 via a MEK-independent and p56Lck-dependent pathway from leprosy bacilli. Proc. Natl. Acad. Sci. USA 1029188-9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tse, H. M., S. I. Josephy, E. D. Chan, D. Fouts, and A. M. Cooper. 2002. Activation of the mitogen-activated protein kinase signaling pathway is instrumental in determining the ability of Mycobacterium avium to grow in murine macrophages. J. Immunol. 168825-833. [DOI] [PubMed] [Google Scholar]
- 43.Tsurutani, J., K. A. West, J. Sayyah, J. J. Gills, and P. A. Dennis. 2005. Inhibition of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway but not the MEK/ERK pathway attenuates laminin-mediated small cell lung cancer cellular survival and resistance to imatinib mesylate or chemotherapy. Cancer Res. 658423-8432. [DOI] [PubMed] [Google Scholar]
- 44.Verbeke, P., L. Welter-Stahl, S. Ying, J. Hansen, G. Hacker, T. Darville, and D. M. Ojcius. 2006. Recruitment of Bad by the Chlamydia trachomatis vacuole correlates with host cell survival. PLoS Pathog. 2e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voth, D. E., and R. A. Heinzen. 2007. Lounging in a lysosome: the intracellular lifestyle of Coxiella burnetii. Cell. Microbiol. 9829-840. [DOI] [PubMed] [Google Scholar]
- 46.Voth, D. E., D. Howe, and R. A. Heinzen. 2007. Coxiella burnetii inhibits apoptosis in human THP-1 cells and monkey primary alveolar macrophages. Infect. Immun. 754263-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welsh, C. T., J. T. Summersgill, and R. D. Miller. 2004. Increases in c-Jun N-terminal kinase/stress-activated protein kinase and p38 activity in monocyte-derived macrophages following the uptake of Legionella pneumophila. Infect. Immun. 721512-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu, H., H. O. Fearnhead, and G. M. Cohen. 1995. An ICE-like protease is a common mediator of apoptosis induced by diverse stimuli in human monocytic THP.1 cells. FEBS Lett. 374303-308. [DOI] [PubMed] [Google Scholar]