Abstract
Interleukin-10 (IL-10)-deficient mice are resistant to several pathogens, including Yersinia pestis. Surprisingly, we observed that heterozygous IL-10+/− mice also survive high-dose intravenous infection with Y. pestis KIM5 (Pgm−). Analysis of commercial IL-10−/− mice revealed that at least 30 cM of genomic DNA from the original 129 strain remains, including a functional Slc11a1 (Nramp1) gene. Interestingly, two substrains of 129 mice were resistant to high-dose Y. pestis KIM5. Resistance does not appear to be recessive, as F1 mice (C57BL/6J × 129) also survived a high-dose challenge. A QTL-based genetic scan of chromosome 1 with 35 infected F1 backcrossed mice revealed that resistance to KIM5 maps to a region near IL-10. Two novel IL-10+/+ mouse strains which each possess most of the original 30-cM stretch of 129 DNA maintained resistance to high-dose infection with Y. pestis KIM5 even in a heterozygous state. Conversely, a novel IL-10−/− mouse strain in which most of the 129 DNA has been crossed out exhibited intermediate resistance to KIM5, while the corresponding IL-10+/− strain was completely susceptible. Taken together, these results demonstrate that 129-derived genomic DNA near IL-10 confers resistance to Yersinia pestis KIM5 and contributes to the observed resistance of IL-10−/− mice.
The etiological agent for bubonic and pneumonic plague, Yersinia pestis (14), is one of three pathogenic Yersinia species. In its bubonic form plague is at least 50% lethal if left untreated, while pneumonic plague is nearly 100% fatal. The other two species, Y. enterocolitica and Y. pseudotuberculosis, are enteric pathogens which usually cause self-limiting gastroenteritis. All three species possess a 70-kb virulence plasmid (pCD1 in Y. pestis, pYV in Y. enterocolitica and Y. pseudotuberculosis) encoding a type III secretion system (TTSS) and six effector proteins called Yops (Yersinia outer membrane proteins) that are injected into the mammalian host cell (5). One component of the injectisome of the TTSS is LcrV, also known as V antigen. LcrV along with the capsular protein Caf1, also known as F1 antigen, has been shown to be highly effective as a vaccine against plague (6), and a phase III clinical trial is currently under way.
LcrV has been implicated in suppression of host inflammation through a mechanism independent of the TTSS. In 1995 Nakajima et al. found that treatment with a recombinant LcrV protein suppressed the levels of gamma interferon and tumor necrosis factor alpha during infection with an LcrV-deficient Y. pestis strain, resulting in in vivo survival of the bacteria (11). Nedialkov et al. went on to show that LcrV could protect against a lethal injection of lipopolysaccharide by inducing production of interleukin-10 (IL-10) (12). Ex vivo exposure of macrophages to purified Yersinia LcrV has been shown to induce IL-10 production. IL-10 is a cytokine with immunosuppressive properties and inactivates phagocytes through inhibition of nuclear factor (NF)-κB (2). Certain viruses are known to harbor IL-10 gene homologues, and many pathogens appear to upregulate IL-10 and thereby evade host immune defenses by suppressing inflammatory responses. Consistent with the notion that IL-10 plays a role in enabling Yersinia to evade immune destruction, IL-10-deficient mice have been found to be resistant to Y. enterocolitica (21) and Y. pestis (15).
In work toward defining an underlying mechanism for resistance to Yersinia, LcrV was subsequently reported to induce IL-10 production through engagement of the Toll-like receptor 2 (TLR2) complex (22). Consistent with this mechanism, TLR2−/− mice on a C57BL/6 background were reported to be resistant to infection with Y. enterocolitica (21). The biological role of the interaction between Yersinia LcrV and TLR2 has come under considerable scrutiny, however. For example, different regions of LcrV responsible for TLR2 activation have been defined by different groups (13, 20). Moreover, IL-10 levels are repressed until the late stages of Y. pseudotuberculosis infection through YopJ (1), an inhibitor of NF-κB and mitogen-activated protein kinase signaling (24). Most strikingly, Sing et al. reported that TLR2−/− mice backcrossed on a C3H background exhibit a reversed phenotype in which the receptor-deficient animals were more susceptible to Y. enterocolitica oral infection than their wild-type counterparts (23). More recent studies have shown that TLR2−/− mice are as susceptible to Y. pseudotuberculosis as wild-type mice and that IL-10 induction in macrophages occurs independently of both LcrV and TLR2 (1). Finally, two studies have recently reported that TLR2 deficiency has no effect on the course of disease, levels of IL-10, degree of inflammation in infected tissue, or overall resistance of mice to fully virulent Y. pestis (16, 17).
In this study we report that although IL-10−/− mice are resistant to Y. pestis KIM5, heterozygous IL-10+/− animals also survive a high-dose infection with this pathogen. This surprising observation suggested that an IL-10-independent mechanism of resistance to Y. pestis may exist. During our investigation of this phenomenon, we discovered that a large segment of DNA, derived from 129 embryonic stem cells used to generate the knockout, is present in these IL-10-deficient mice. We report that this 129-derived DNA confers resistance to Y. pestis KIM5 in a nonrecessive manner. Elimination of this DNA from the original IL-10−/− strain does not render these mice fully susceptible but reduces their resistance to Y. pestis.
MATERIALS AND METHODS
Mice.
All experimental mice were between 6 and 10 weeks of age. C57BL/6J, IL-10−/−, 129S1/SvImJ, and 129P3/J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The IL-10−/− mice (B6.129P2-Il10tm1Cgn/J, catalog number 002251) are reported by the vendor to have been backcrossed for 10 generations. C3H/HeN mice were purchased from Charles River Laboratories (Wilmington, MA). All intercrosses were performed in our Animal Research Facility at the University of Illinois at Urbana-Champaign. All experiments were conducted with female mice and were approved by the University of Illinois at Urbana-Champaign Institutional Animal Care and Use Committee.
Bacteria.
The bacterial strain used in this study is Y. pestis KIM5 (Pgm−) (26). Bacteria were grown overnight in heart infusion (HI) broth at 23°C. After overnight growth, the bacteria were diluted 1:10 in HI broth and grown to mid-exponential phase (optical density at 600 nm of ∼0.4). Bacteria were collected by centrifugation, washed twice, and serially diluted in phosphate-buffered saline (0.067 M phosphate, 150 mM NaCl) to the appropriate concentration. For all experiments, mice were intravenously infected via the caudal tail vein with a 200-μl inoculum. For survival experiments, animals were monitored at least twice daily and culled in a timely fashion upon reaching a moribund state. Significance was determined by the log rank test where the P value was <0.05. For enumeration of bacterial titer, infected mice were sacrificed at the indicated time points by CO2 asphyxiation followed by cervical dislocation. Spleens and livers were removed aseptically and weighed. Organs were transferred to a sterile stomacher bag containing phosphate-buffered saline and homogenized. The bacterial burden was determined by plating the dilutions onto HI agar plates and incubating at room temperature for 2 days. Significance was determined by Student's t test.
Genetic screening.
Interval mapping of chromosome 1 was performed with 35 F1 backcrossed (C57BL/6J × B6129SF1/J) mice using QTL Cartographer (http://statgen.ncsu.edu/qtlcart). Prior to infection, DNA samples were obtained and the mice were genotyped for several markers on chromosome 1. The markers used were D1Mit211, D1Mit303, D1Mit46, D1Mit187, D1Mit265, and D1Mit362. The markers cover a range from 15.0 to 106.3 cM. Mice were then infected with 500 CFU Y. pestis. Total splenic CFU at day 4 postinfection was used as the phenotype for analysis. The data were analyzed in 1-cM steps and presented as logarithmic odds (base 10) (LOD) scores.
RESULTS
Both IL-10−/− and IL-10+/− mice are resistant to Y. pestis KIM5.
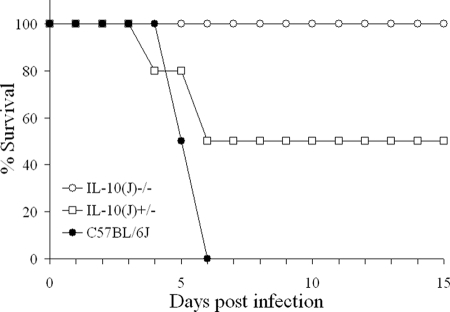
To verify that IL-10−/− mice are resistant to Y. pestis KIM5, groups of 10 C57BL/6J and IL-10−/− mice were infected with 105 CFU and monitored for survival (Fig. 1). As an additional control, 10 IL-10+/− mice were also included. As expected, all C57BL/6J mice succumbed to infection, while all IL-10−/− animals survived. Surprisingly, 5 of 10 IL-10+/− (IL-10−/− × C57BL/6J) mice also survived. Both the IL-10−/− and IL-10+/− groups were significantly different from the C57BL/6J mice. We have verified this result several times using different doses and found that at least 50% of IL-10+/− mice survive a dose of 105 CFU and are even more resistant at lower doses. These findings led us to explore the genotype of the IL-10−/− mice in more detail. Using microsatellite markers, we found that these mice possess at least 30 cM of DNA, which includes a functional Slc11a1 gene, from the 129P2/OlaHsd-derived embryonic stem cells used in the generation of the knockout.
FIG. 1.
IL-10−/− and IL-10+/− mice are resistant to Y. pestis KIM5. Groups of 10 C57BL/6J, IL-10−/−, and IL-10+/− (C57BL/6J × IL-10−/−) mice were infected with 105 CFU Y. pestis KIM5 and monitored for survival for 15 days (P < 0.001).
A functional Slc11a1 (Nramp1) gene does not render C57BL/6J mice resistant to Y. pestis.
Slc11a1 is a cationic transporter present in macrophages and neutrophils and is necessary for protection against many intracellular pathogens (3). It is well established that certain inbred mouse strains possess nonfunctional variants of this gene that can render them susceptible to infection in certain models (8). In this regard, 129 and C57BL/6J mice possess functional and nonfunctional Slc11a1 variants. Due to its proximity to IL-10, we examined the Slc11a1 gene in IL-10−/− mice and found that they possess a functional copy from the 129 genome.
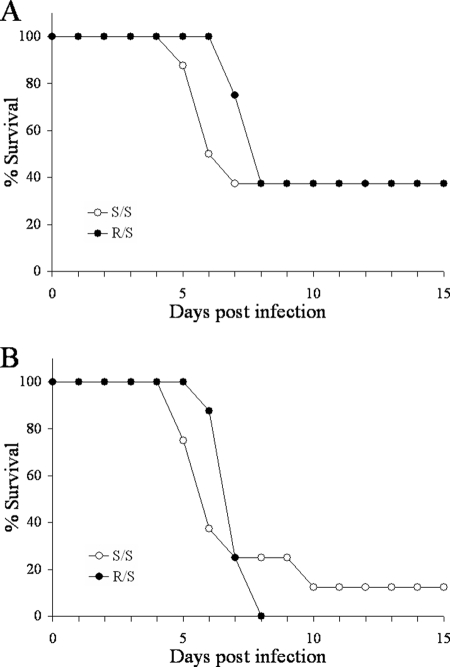
As the presence of a functional Slc11a1 gene could provide a straightforward explanation for the resistance phenotype of IL-10+/− mice, we wanted to examine its role in resistance to Y. pestis. To this end, a functional Slc11a1 gene from C3H/HeN mice was backcrossed onto the C57BL/6J background for nine generations. Progeny from these mice either contained one copy (R/S) or zero copies (S/S) of the functional gene. Groups of eight mice were infected with 50 (Fig. 2A) or 300 (Fig. 2B) CFU Y. pestis KIM5. At both doses, there was no significant difference between the two groups, indicating that a functional Slc11a1 gene is not associated with resistance to Y. pestis.
FIG. 2.
Slc11a1 does not confer resistance to Y. pestis KIM5. C3H/HeN mice were backcrossed with C57BL/6J mice, maintaining the functional Slc11a1 gene (R) for nine generations. Groups of eight mice containing either one copy (R/S) or zero copies (S/S) of functional Slc11a1 were infected with 50 (A) or 300 (B) CFU Y. pestis KIM5 and monitored for survival for 15 days (P = 0.43 and 0.74 for panels A and B, respectively).
129 substrains are resistant to Y. pestis KIM5 and clear bacteria within 6 days.
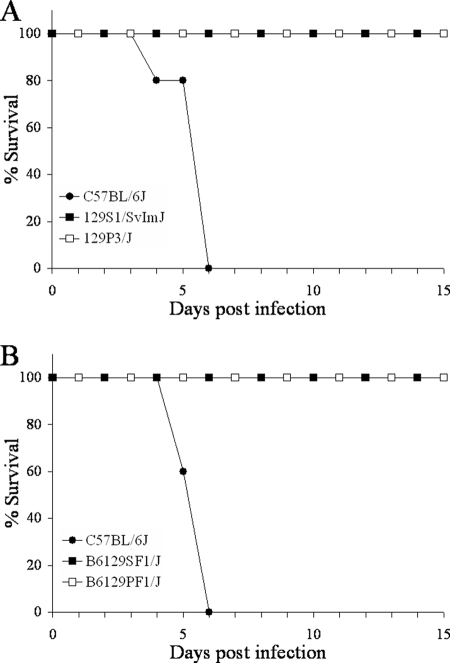
After evaluating the genotype of the IL-10−/− mice, we wanted to determine if 129 mouse substrains are resistant to Y. pestis. To this end, groups of 10 C57BL/6J, 129S1/SvImJ, and 129P3/J mice were infected with 105 CFU Y. pestis KIM5 and monitored for survival (Fig. 3A). All C57BL/6J mice succumbed to infection, while all 129S1/SvImJ and 129P3/J animals survived. These results show that two 129 substrains are significantly resistant to Y. pestis compared to susceptible C57BL/6J mice. A recent paper has reported several 129 substrains that are similarly resistant (4).
FIG. 3.
129 substrain mice are resistant to Y. pestis KIM5. Groups of 10 C57BL/6J, 129S1/SvImJ, and 129P3/J mice (A) or groups of 10 C57BL/6J, B6129SF1/J, and B6129PF1/J mice (B) were infected with 105 CFU Y. pestis KIM5 and monitored for survival for 15 days (P < 0.001 for both panels).
IL-10+/− mice are resistant to Y. pestis KIM5 despite possessing only one copy of the 129 DNA. To verify that only one copy of 129 DNA is required for resistance, we examined the susceptibility of F1 (C57BL/6J × 129) mice. To this end, 10 C57BL/6J, B6129SF1/J, and B6129PF1/J mice were infected with 105 CFU Y. pestis KIM5 (Fig. 3B). Again, all C57BL/6J mice succumbed to infection, while both sets of F1 mice survived. These results show that two different 129 substrain-derived F1 mice are significantly resistant to a high-dose Y. pestis infection compared to susceptible C57BL/6J animals, indicating that resistance is not recessive. Presently, we do not know if the trait is completely dominant.
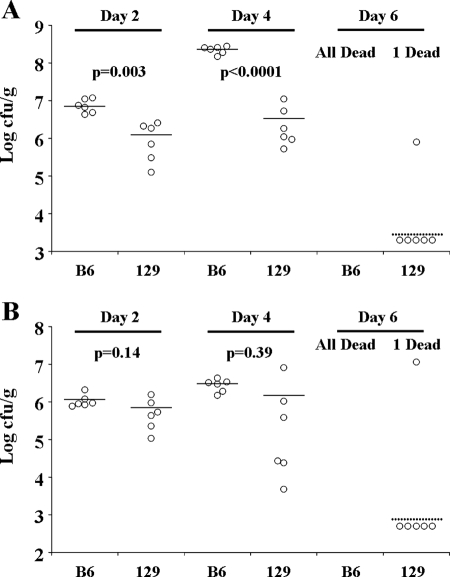
To examine whether innate or adaptive immune responses are responsible for resistance, we compared the course of infection in groups of C57BL/6J and 129S1/SvImJ mice infected with 500 CFU Y. pestis KIM5. This lower dose was used to enable C57BL/6J mice to survive until at least day 4 postinfection. At days 2 (n = 6), 4 (n = 6), and 6 (n = 7), surviving mice were sacrificed and the total bacterial burdens in the spleen (Fig. 4A) and liver (Fig. 4B) were determined. The 129S1/SvImJ mice had significantly lower bacterial titers in the spleen at days 2 and 4, as determined by Student's t test. While the splenic bacterial titers in C57BL/6J mice increased more than 10-fold between days 2 and 4, they did not significantly change in 129S1/SvImJ mice during this period. Similar trends were observed in the liver but were more subtle and not statistically significant. By day 6, all seven C57BL/6J mice had succumbed to infection. Conversely, of the seven infected 129S1/SvImJ mice, five had cleared all bacteria, one had measurable bacteria in the spleen and liver, and one had succumbed to infection. Although not conclusive, these results suggest that an initial innate immune response may enable 129S1/SvImJ mice to control Y. pestis KIM5 infection. However, clearance of the bacteria between days 4 and 6 suggests that an adaptive immune response may be required for full resistance.
FIG. 4.
Growth kinetics of Y. pestis in the spleen and liver. Mice were infected with 500 CFU Y. pestis KIM5. At days 2 (n = 6), 4 (n = 6), and 6 (n = 7), the surviving mice were euthanized and bacterial burdens in the spleen (A) and liver (B) were determined. Solid lines indicate the average, and dashed lines indicate the limit of detection. B6, C57BL/6J; 129, 129S1/SvImJ.
Resistance of 129 mice to Y. pestis KIM5 maps to a region near IL-10 on chromosome 1.
We have found that C57BL/6J transgenic IL-10−/− mice possess at least 30 cM of DNA from the original 129 line used in their generation. As we suspected that this region of DNA was responsible for the resistance of the IL-10+/− mice to Y. pestis KIM5, a genetic screen of chromosome 1 was performed using 35 backcrossed (C57BL/6J × B6129SF1/J) mice. Backcrossed mice were used because the trait is not recessive and would likely provide clearer results than an F2 screen. The mice were genotyped using six microsatellite markers on chromosome 1 and then infected with 500 CFU Y. pestis KIM5. At day 4 postinfection, total splenic CFU was determined, and the resulting data were analyzed using QTL Cartographer. Interval mapping of chromosome 1 shows a broad region with high LOD scores that approach zero on either side. The maximum LOD score of 1.8 is located at D1Mit187 (62.0 cM) (P = 0.0036) (Fig. 5). These results indicate that a gene(s) near IL-10 is associated with resistance to Y. pestis KIM5 in 129 mice.
FIG. 5.
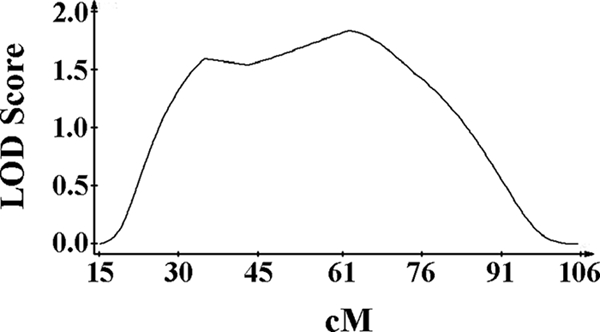
Genetic screen of F1 backcrossed mice on chromosome 1. Interval mapping of 35 F1 backcrossed (C57BL/6J × B6129SF1/J) mice was performed using total splenic CFU at day 4 postinfection as the phenotype. QTL Cartographer was used to analyze the data. A maximum LOD score of 1.8 at D1Mit187 (62.0 cM, P = 0.0036) was revealed.
IL-10-associated DNA from 129 mice confers resistance to Y. pestis KIM5.
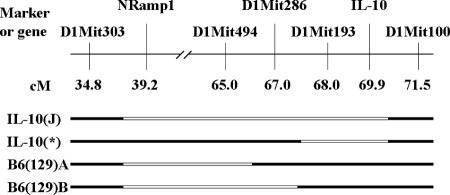
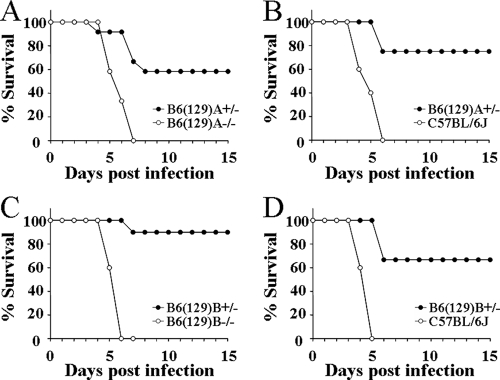
To assess whether the 129 DNA near the IL-10 knockout allele can independently confer resistance to Y. pestis, we crossed IL-10+/− mice with C57BL/6J and screened for novel crossovers in this region. Through this approach, we were able to generate a novel chromosome, named B6(129)A, that contains at least 25 cM of DNA from the 129 genome but possesses a functional IL-10 gene from the C57BL/6J strain (Fig. 6). This line was crossed with C57BL/6J such that the resulting progeny possessed either one copy [B6(129)A+/−] or zero copies [B6(129)A−/−] of the novel chromosome. All the progeny from this cross possess two functional IL-10 genes. A second novel chromosome, B6(129)B, was similarly obtained from an independent crossover event stemming from the cross between IL-10+/− mice and C57BL/6J mice. B6(129)B+/− and B6(129)B−/− progeny were derived by crossing these mice to C57BL/6J as described for the B6(129)A strain. The first line, B6(129)A, was infected with 103 and 104 CFU Y. pestis KIM5 (Fig. 7A and B, respectively), while the second line, B6(129)B, was infected with 104 and 105 CFU (Fig. 7C and D, respectively). Different doses were used to determine the contribution of the 129 DNA to resistance. In these four independent experiments, utilizing 4 to 12 mice per group, more than half of the B6(129)+/− mice survived, while their littermate controls [B6(129)−/−] or the C57BL/6J control animals all succumbed to infection. In all four experiments, the experimental group was found to be significantly different from the corresponding control group (P < 0.02 in all cases). These results verify that this region of 129 DNA, distinct from the IL-10 knockout allele, contains a gene(s) that is associated with resistance to Y. pestis KIM5.
FIG. 6.
Physical map of chromosome 1 of the mouse strains used in this study. The remaining 129 DNA (white) of chromosome 1 from the original IL-10−/− mouse, as well as from the three novel mouse strains, is shown. All other chromosomes theoretically possess less than 1% of the 129 genome. The original IL-10−/− mice available from The Jackson Laboratory are referred to as IL-10(J)−/−. The IL-10 mice possessing less flanking 129 DNA are referred to as IL-10(*)−/−. The two IL-10+/+ mouse strains that possess 129 DNA are referred to as B6(129)A and B6(129)B.
FIG. 7.
B6(129) mice are resistant to Y. pestis KIM5. Groups of 4 to 12 mice were infected with 103 CFU (A) or 104 CFU (B) Y. pestis KIM5. (P = 0.003 and 0.02 for panels A and B, respectively). Groups of 5 to 10 mice were infected with 104 CFU (C) or 105 CFU (D) Y. pestis KIM5, respectively (P < 0.001 and P = 0.001 for panels C and D, respectively). All mice were monitored for survival for 15 days postinfection.
Removal of 129 DNA from commercial IL-10−/− mice reduces their resistance to Y. pestis KIM5.
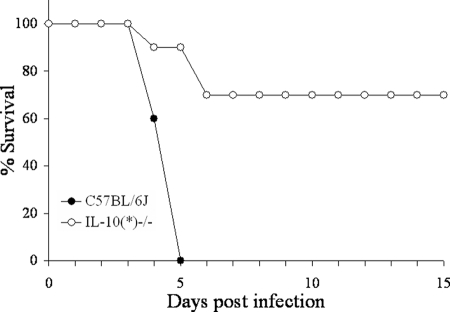
We next wanted to assess the resistance of IL-10-deficient mice in the absence of the 129 DNA. By screening progeny of a cross between the original IL-10+/− mice and C57BL/6J mice, an IL-10-deficient chromosome [designated IL-10(*)] that contains less that 3 cM of the original surrounding 129 DNA was identified (Fig. 6). After intercrossing, an IL-10(*)−/− mouse line was established. Groups of 5 C57BL/6J and 10 IL-10(*)−/− mice were infected with 105 CFU Y. pestis KIM5 and monitored for survival. While all C57BL/6J mice succumbed to infection, only 7 of 10 IL-10(*)−/− animals survived (Fig. 8). Despite losing the 129 DNA, the IL-10(*)−/− mice were still significantly more resistant than the C57BL/6J animals. However, they were less resistant than the original IL-10(J)−/− strain that previously displayed complete resistance to a challenge of 105 CFU (Fig. 1). Taken together, we conclude that even though IL-10 deficiency renders mice resistant to Y. pestis, resistance is not as complete as previously observed by us and reported by others (15).
FIG. 8.
IL-10(*)−/− mice are less resistant to a high-dose infection. Groups of 5 C57BL/6J and 10 IL-10(*)−/− mice were infected with 105 CFU Y. pestis KIM5 and monitored for survival for 15 days (P = 0.001).
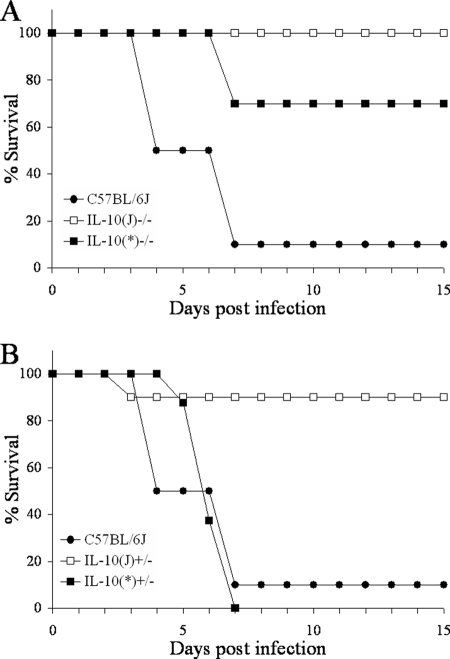
Our final goal was to verify the above results in a low-dose infection and assess the gene dosage effect of IL-10. Figure 9 shows the results of a low-dose infection experiment. Groups of 8 to 10 mice were infected with 100 CFU Y. pestis KIM5. All 10 IL-10(J)−/− mice, but only 7 of 10 IL-10(*)−/− mice, survived (P = 0.067). These results are identical to those in Fig. 8, despite a 1,000-fold difference in dose, and demonstrate that 129 DNA contributes to the observed resistance of the original IL-10(J)−/− strain to Y. pestis KIM5. Additionally, while 9 of 10 IL-10(J)+/− [IL-10(J)−/− × C57BL/6J] mice survived, all of the IL-10(*)+/− [IL-10(*)−/− × C57BL/6J] animals succumbed to infection (P < 0.001). These data demonstrate that a single copy of the flanking 129 DNA, and not IL-10 haploinsufficiency, contributes to resistance to Y. pestis KIM5.
FIG. 9.
KIM5 resistance is linked to the IL-10 knockout allele. Groups of 8 to 10 mice were infected with 100 CFU Y. pestis KIM5 and monitored for survival for 15 days. Panels A and B represent results obtained from the same infection experiment.
DISCUSSION
We have observed that serum IL-10 levels do not increase in response to Y. pestis KIM5 at 24 h postinfection in either C57BL/6J or BALB/cJ mice (25). We have also observed that TLR2−/− mice are as susceptible as C57BL/6J animals to the attenuated KIM5 strain, even at a relatively low dose (data not shown). In support of these observations, two recent studies have reported that TLR2 deficiency has no effect on the course of disease, level of IL-10, degree of inflammation in infected tissue, or overall resistance of mice to fully virulent Y. pestis (16, 17). Given these findings, it appears unlikely that the interaction between LcrV and TLR2 toward the induction of IL-10 constitutes a bona fide virulence mechanism for Y. pestis. Nevertheless, it is clear from our results and those published by others (15) that IL-10-deficient mice maintain a greater resistance to Y. pestis than their wild-type counterparts.
During our study of the role of IL-10 in plague, we were surprised to find that in addition to IL-10−/− mice, IL-10+/− mice were also highly resistant to Y. pestis KIM5. After examining the DNA of the IL-10−/− mice, however, we discovered that more than 30 cM of 129 DNA remained on the centromeric side of the IL-10 gene. Accordingly, we found that both 129S1/SvImJ and 129P3/J mice survive a high-dose Y. pestis infection. During the course of infection, the mice have slightly ruffled fur, but they are not hunched over and are nearly as active as uninfected mice. Another recent report identified three 129 substrains that are resistant to Y. pestis KIM5 (4), suggesting that resistance is a general phenomenon of all 129 substrains with a common genetic cause. As both of our F1 strains survive a high-dose infection, the gene(s) responsible for resistance appears to act in a nonrecessive manner.
An examination of bacterial load revealed that at 48 h postinfection, 129 mice have significantly fewer bacteria in the spleen than the C57BL/6J animals, with a similar trend observed in the liver. By day 6, all C57BL/6J mice succumbed to infection, while, in contrast, bacteria were cleared in the spleens and livers of most 129 mice. As significant differences in bacterial load are not observed earlier, at 1 day postinfection (data not shown), it is unlikely that the lack of essential nutrients, such as iron, or immediate innate immune killing underlies the resistance of 129 mice to KIM5. However, unlike susceptible mice, in which bacterial titers increased, bacterial titers did not increase from day 2 to 4 in resistant animals. This indicates that resistance of 129 mice may be due to a relatively early adaptive immune response such as a T-independent B-cell response to the bacteria. The bacterial clearance observed between days 4 and 6 is consistent with this notion, but further experimentation is required to verify it.
We have generated a novel IL-10-deficient mouse line [IL-10(*)−/−] in which all but 3 cM of 129 DNA was eliminated from the original IL-10(J)−/− strain. These mice are only partially resistant to both high- and low-dose infections with Y. pestis KIM5, and in fact, only 70% of animals survive these infections. These findings contrast with those for the original IL-10(J)−/− mice, in which all mice survive at these doses. Moreover, heterozygous mice derived from this strain are as susceptible as C57BL/6J animals, indicating that the resistance of heterozygous IL-10(J)+/− mice to KIM5 is not due to a gene dosage effect. Taken together, these findings clearly establish that an additional gene(s) near IL-10 confers resistance to Y. pestis KIM5.
We were initially concerned that the presence of a functional Slc11a1 (Nramp1) gene could be responsible for the observed resistance of IL-10(J)−/− mice. However, a genetic screen using 35 backcrossed (C57BL/6J × B6129SF1/J) mice and day 4 splenic CFU as a phenotype revealed a genomic region quite distant from Slc11a1. Moreover, we have found that a functional Slc11a1 gene from C3H/HeN mice does not significantly contribute to resistance to Y. pestis KIM5 in a C57BL/6J background. Although a minor delay in death is consistently observed at lower doses, this effect cannot account for the resistance of the B6(129) mice, which occurs at a much higher dose. Nevertheless, we cannot rule out that another gene from 129 mice in this region interacts with Slc11a1 and that resistance occurs when both genes are present.
The presence of a functional Slc11a1 gene in the IL-10−/− mouse line from The Jackson Laboratory presents a dilemma for work with other pathogens in assessing the role of IL-10 in response to infection. For example, Matsumoto, et al. showed that IL-10-deficient mice are able to clear Helicobacter pylori infection more quickly than C57BL/6J mice (9). It is possible that this was due to a functional Slc11a1 gene rather than a lack of IL-10. Studies with other intracellular pathogens such as Listeria, Mycobacterium, and Leishmania could all possibly be affected by the presence of a functional Slc11a1 gene in these IL-10-deficient mice (10).
The problem of genetic background and flanking genes affecting phenotype is not new and has been documented in numerous studies, especially in the areas of neuroscience and autoimmunity. One highly cited example in the area of immunology involved the discovery that protection from diabetes in NOD.129 IFNgr−/− mice was not to due to the lack of gamma interferon receptor as originally thought. Instead, the phenotype was due to a linked 129 gene that was retained despite 10 generations of backcrosses (7). Fortunately, detailed knowledge of genetic differences between mouse strains has enabled a better assessment of the genetic purity of backcrossed animals (18). Moreover, the availability of embryonic stem cells from a greater diversity of mouse strains, including C57BL/6J, allows transgenic animals to be created directly within the strain of interest. Conditional knockouts as well as a number of breeding strategies provide additional ways to overcome the flanking gene problem (19, 27).
We have generated two independent mouse lines, both referred to as B6(129), that contain approximately 30 cM of DNA from the 129 embryonic stem cells used to create the IL-10−/− mice. This chromosomal region confers resistance to KIM5 in a heterozygous state, indicating some form of genetic dominance. We have not analyzed whether resistance will increase if the region is in a homozygous state, and therefore we do not know if there is a gene dosage effect. One interesting observation is that resistance of B6(129) mice does not significantly change with the bacterial dose given, and at doses ranging from 102 to 105, 50 to 80% of mice survive. The B6(129) mice are not as resistant as either of the two F1 strains we tested. This indicates that another gene(s) in the 129 substrains likely plays at least a minor role in resistance to KIM5. Although this region is relatively small compared to the entire mouse genome, we cannot exclude the possibility that more than one gene is responsible for resistance, as nearly 200 genes are present. We are currently generating crossovers in the B6(129) mice in order to genetically map and ultimately identify the gene(s) responsible for resistance to Y. pestis KIM5.
Acknowledgments
We thank the Animal Core Facility at the University of Illinois for their excellent assistance in animal husbandry.
This work was supported by NIH grants AI056148 and AI057153 (to J.L.X.) and AI056148 (to R.I.T.).
Editor: J. B. Bliska
Footnotes
Published ahead of print on 27 October 2008.
REFERENCES
- 1.Auerbuch, V., and R. R. Isberg. 2007. Growth of Yersinia pseudotuberculosis in mice occurs independently of Toll-like receptor 2 expression and induction of interleukin-10. Infect. Immun. 753561-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brubaker, R. R. 2003. Interleukin-10 and inhibition of innate immunity to yersiniae: roles of Yops and LcrV (V antigen). Infect. Immun. 713673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cellier, M. F., P. Courville, and C. Campion. 2007. Nramp1 phagocyte intracellular metal withdrawal defense. Microbes Infect. 91662-1670. [DOI] [PubMed] [Google Scholar]
- 4.Congleton, Y. H., C. R. Wulff, E. J. Kerschen, and S. C. Straley. 2006. Mice naturally resistant to Yersinia pestis Δpgm strains commonly used in pathogenicity studies. Infect. Immun. 746501-6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornelis, G. R. 2002. The Yersinia Ysc-Yop ‘type III’ weaponry. Nat. Rev. Mol. Cell Biol. 3742-752. [DOI] [PubMed] [Google Scholar]
- 6.Cornelius, C., L. Quenee, D. Anderson, and O. Schneewind. 2007. Protective immunity against plague. Adv. Exp. Med. Biol. 603415-424. [DOI] [PubMed] [Google Scholar]
- 7.Kanagawa, O., G. Xu, A. Tevaarwerk, and B. A. Vaupel. 2000. Protection of nonobese diabetic mice from diabetes by gene(s) closely linked to IFN-gamma receptor loci. J. Immunol. 1643919-3923. [DOI] [PubMed] [Google Scholar]
- 8.Malo, D., K. Vogan, S. Vidal, J. Hu, M. Cellier, E. Schurr, A. Fuks, N. Bumstead, K. Morgan, and P. Gros. 1994. Haplotype mapping and sequence analysis of the mouse Nramp gene predict susceptibility to infection with intracellular parasites. Genomics 2351-61. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto, Y., T. G. Blanchard, M. L. Drakes, M. Basu, R. W. Redline, A. D. Levine, and S. J. Czinn. 2005. Eradication of Helicobacter pylori and resolution of gastritis in the gastric mucosa of IL-10-deficient mice. Helicobacter 10407-415. [DOI] [PubMed] [Google Scholar]
- 10.Mege, J. L., S. Meghari, A. Honstettre, C. Capo, and D. Raoult. 2006. The two faces of interleukin 10 in human infectious diseases. Lancet Infect. Dis. 6557-569. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima, R., V. L. Motin, and R. R. Brubaker. 1995. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect. Immun. 633021-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nedialkov, Y. A., V. L. Motin, and R. R. Brubaker. 1997. Resistance to lipopolysaccharide mediated by the Yersinia pestis V antigen-polyhistidine fusion peptide: amplification of interleukin-10. Infect. Immun. 651196-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overheim, K. A., R. W. Depaolo, K. L. Debord, E. M. Morrin, D. M. Anderson, N. M. Green, R. R. Brubaker, B. Jabri, and O. Schneewind. 2005. LcrV plague vaccine with altered immunomodulatory properties. Infect. Immun. 735152-5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis: etiologic agent of plague. Clin. Microbiol. Rev. 1035-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philipovskiy, A. V., C. Cowan, C. R. Wulff-Strobel, S. H. Burnett, E. J. Kerschen, D. A. Cohen, A. M. Kaplan, and S. C. Straley. 2005. Antibody against V antigen prevents Yop-dependent growth of Yersinia pestis. Infect. Immun. 731532-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pouliot, K., N. Pan, S. Wang, S. Lu, E. Lien, and J. D. Goguen. 2007. Evaluation of the role of LcrV-Toll-like receptor 2-mediated immunomodulation in the virulence of Yersinia pestis. Infect. Immun. 753571-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reithmeier-Rost, D., J. Hill, S. J. Elvin, D. Williamson, S. Dittmann, A. Schmid, G. Wilharm, and A. Sing. 2007. The weak interaction of LcrV and TLR2 does not contribute to the virulence of Yersinia pestis. Microbes Infect. 9997-1002. [DOI] [PubMed] [Google Scholar]
- 18.Ridgway, W. M., B. Healy, L. J. Smink, D. Rainbow, and L. S. Wicker. 2007. New tools for defining the ‘genetic background’ of inbred mouse strains. Nat. Immunol. 8669-673. [DOI] [PubMed] [Google Scholar]
- 19.Silva, A., E. Simpson, J. Takahashi, H. Lipp, S. Nakanishi, J. Wehner, K. Giese, T. Tully, T. Abel, P. Chapman, K. Fox, S. Grant, S. Itohara, R. Lathe, M. Mayford, J. McNamara, R. Morris, M. Picciotto, J. Roder, H. Shin, P. Slesinger, D. Storm, M. Stryker, S. Tonegawa, Y. Wang, and D. Wolfer. 1997. Mutant mice and neuroscience: recommendations concerning genetic background. Banbury Conference on genetic background in mice. Neuron 19755-759. [DOI] [PubMed] [Google Scholar]
- 20.Sing, A., D. Reithmeier-Rost, K. Granfors, J. Hill, A. Roggenkamp, and J. Heesemann. 2005. A hypervariable N-terminal region of Yersinia LcrV determines Toll-like receptor 2-mediated IL-10 induction and mouse virulence. Proc. Natl. Acad. Sci. USA 10216049-16054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sing, A., A. Roggenkamp, A. M. Geiger, and J. Heesemann. 2002. Yersinia enterocolitica evasion of the host innate immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10-deficient mice. J. Immunol. 1681315-1321. [DOI] [PubMed] [Google Scholar]
- 22.Sing, A., D. Rost, N. Tvardovskaia, A. Roggenkamp, A. Wiedemann, C. J. Kirschning, M. Aepfelbacher, and J. Heesemann. 2002. Yersinia V-antigen exploits toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 1961017-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sing, A., N. Tvardovskaia, D. Rost, C. J. Kirschning, H. Wagner, and J. Heesemann. 2003. Contribution of Toll-like receptors 2 and 4 in an oral Yersinia enterocolitica mouse infection model. Int. J. Med. Microbiol. 293341-348. [DOI] [PubMed] [Google Scholar]
- 24.Sweet, C. R., J. Conlon, D. T. Golenbock, J. Goguen, and N. Silverman. 2007. YopJ targets TRAF proteins to inhibit TLR-mediated NF-kappaB, MAPK and IRF3 signal transduction. Cell. Microbiol. 92700-2715. [DOI] [PubMed] [Google Scholar]
- 25.Turner, J. K., M. M. McAllister, J. L. Xu, and R. I. Tapping. 2008. Resistance of BALB/cJ mice to Yersinia pestis maps to the major histocompatibility complex of chromosome 17. Infect. Immun. [DOI] [PMC free article] [PubMed]
- 26.Une, T., and R. R. Brubaker. 1984. In vivo comparison of avirulent Vwa− and Pgm− or Pstr phenotypes of yersiniae. Infect. Immun. 43895-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfer, D. P., W. E. Crusio, and H. P. Lipp. 2002. Knockout mice: simple solutions to the problems of genetic background and flanking genes. Trends Neurosci. 25336-340. [DOI] [PubMed] [Google Scholar]