Abstract
The role of Toll-like receptor 9 (TLR9) in antifungal responses in the immunodeficient and allergic host is unclear. We investigated the role of TLR9 in murine models of invasive aspergillosis and fungal asthma. Neutrophil-depleted TLR9 wild-type (TLR9+/+) and TLR9-deficient (TLR9−/−) mice were challenged with resting or swollen Aspergillus fumigatus conidia and monitored for survival and lung inflammatory responses. The absence of TLR9 delayed, but did not prevent, mortality in immunodeficient mice challenged with resting or swollen conidia compared to TLR9+/+ mice. In a fungal asthma model, TLR9+/+ and TLR9−/− mice were sensitized to soluble A. fumigatus antigens and challenged with resting or swollen A. fumigatus conidia, and both groups of mice were analyzed prior to and at days 7, 14, and 28 after the conidium challenge. When challenged with resting conidia, TLR9−/− mice exhibited significantly lower airway hyper-responsiveness compared to the TLR9+/+ groups. In contrast, A. fumigatus-sensitized TLR9−/− mice exhibited pulmonary fungal growth at days 14 and 28 after challenge with swollen conidia, a finding never observed in their allergic wild-type counterparts. Increased fungal growth in allergic TLR9−/− mice correlated with markedly decreased dectin-1 expression in whole lung samples and isolated dendritic cell populations. Further, whole lung levels of interleukin-17 were lower in allergic TLR9−/− mice compared to similar TLR9+/+ mice. Together, these data suggest that TLR9 modulates pulmonary antifungal immune responses to swollen conidia, possibly through the regulation of dectin-1 expression.
Aspergillus fumigatus is a ubiquitous but generally benign mold in immunocompetent individuals (18, 28, 43). Immunocompromised patients experiencing solid organ or hematopoietic stem cell transplant are at extreme risk for the development of invasive aspergillosis, and the lung is a major target organ due to its constant exposure to airborne A. fumigatus spores or conidia (47, 57). Further, a developing body of evidence suggests that A. fumigatus spores or conidia play a role in the exacerbation of allergic and asthmatic disease (25, 39). Current evidence suggests the underlying mechanisms behind this phenomenon might lie in the pulmonary host immune response induced by this pathogen in individuals with underlying allergic airway disease (28). Experimental and clinical data show that the pulmonary immune response to A. fumigatus conidia in the immunocompromised and the allergic host is skewed toward Th2-type immunity instead of a protective Th1-type immune response (30). Therefore, therapeutic redirection of the immune response toward a protective Th1-type immunity is an attractive option for containment of invasive fungal growth in the immunodeficient host with fungal exacerbation of allergic airway disease and asthma.
The relatively frequent incidence of invasive pulmonary aspergillosis (IPA) in immunocompromised and neutropenic individuals combined with the dearth of knowledge about its immunopathogenesis has made IPA an important target of study. Allergic bronchopulmonary aspergillosis (ABPA) is a disease in which the characteristic Th2 immune activation that occurs produces asthmalike pathology complicated by fungal growth (18). Investigations in the area of innate immune receptors and soluble factors that inhibit or enhance immune activation during IPA or ABPA have led to considerable progress in understanding its underlying mechanisms. In particular, inflammatory cytokines and chemokines such as tumor necrosis factor alpha (TNF-α) (36, 40, 46), CCL2 (6), and CXCL10 (38) play critical roles in the activation and recruitment of immune cells such as dendritic cells and macrophages that further act to regulate inflammation at the site of Aspergillus infection. More recently, interleukin-17 (IL-17) has also been demonstrated to promote inflammation and infection during Aspergillus infection (58). Specifically, IL-17 neutralization has been demonstrated to increase fungal clearance, reduce inflammation, and restore protective Th1 antifungal responses (49).
Studies of the innate immune mechanisms, which recognize and eliminate fungi such as A. fumigatus, have demonstrated the importance of the Toll-like receptors (TLRs) in innate immune signaling. In particular, it has been demonstrated that TLR2 and TLR4 provide critical antifungal immune signals in macrophages, neutrophils, and dendritic cells (2, 3), due in part to their collaboration with the essential antifungal β-glucan receptor dectin-1 (17, 20, 23, 34). Dectin-1 signals through a Syk- and CARD-9-dependent pathway that leads to the induction of Th cells producing IL-17 (33). Dectin-1 is highly expressed on dendritic cells and macrophages and functions to recognize fungal β-glucans. Three forms of Aspergillus conidia are recognized, and they include the resting form, the swollen form, and the hyphal form. Each of these forms is antigenically distinct. β-Glucans are strongly expressed by swollen conidia (29, 54). TLR9, a MyD88 adapter protein-dependent receptor, has also been shown to contribute to the inflammatory pathology of the lung and early pulmonary responses during Aspergillus infection (3, 9), but the manner in which it regulates immune responses during aspergillosis have not been previously investigated. TLR9 detects A. fumigatus DNA, resulting in the secretion of proinflammatory cytokines, which might contribute to the immune response to the pathogen (45). Also, recent studies of TLR9 polymorphisms have suggested that a single nucleotide polymorphism in TLR9 coincides with increased risk for clinical ABPA (13), an allergic hypersensitivity to bronchial colonization by Aspergillus. Previous studies have demonstrated that fungal clearance of Cryptococcus neoformans is decreased in TLR9−/− mice (41). We have previously observed that the therapeutic targeting of CCR1 promoted an increase in TLR9 expression in a chronic fungal asthma model, thus establishing a role for CCR1 in fungal asthma (11).
In the present study, we evaluated the role of TLR9 in the innate immune responses elicited by A. fumigatus in murine models of IPA and chronic fungal asthma. We observed that TLR9 was induced in the lung after the introduction of either resting or swollen conidia into immunocompromised and allergic mice and that it was particularly important for appropriate protective immune responses against swollen A. fumigatus conidia in the respiratory tracts of both. Surprisingly, we observed that the absence of TLR9 also adversely affected the expression of dectin-1 and IL-17 in the lung in both the immunocompromised and the allergic host. Thus, TLR9 appears to modulate the innate immune response directed against swollen A. fumigatus conidia, and this modulation appears to be specific for dectin-1 expression and the generation of IL-17.
MATERIALS AND METHODS
Mice.
Homozygous, female TLR9 gene-deficient (TLR9−/−) mice on a BALB/c background were bred at the University of Michigan. Female, wild-type BALB/c (TLR9+/+) mice at 6 to 8 weeks of age were purchased from The Jackson Laboratory (Bar Harbor, ME). Both groups of mice were maintained in a specific-pathogen-free facility for the duration of the study. Prior approval for mouse usage was obtained from the University Laboratory of Animal Medicine facility at the University of Michigan Medical School.
A. fumigatus growth and isolation.
Lyophilized A. fumigatus strain 13073 conidia (American Type Culture Collection, Manassas, VA) stored in phosphate-buffered saline (PBS) with 0.1% bovine serum albumin and glycerol were aseptically inoculated on Sabouraud dextrose agar plates (Teknova, Hollister, CA) and cultured for 10 days at 37°C until mature conidia cultures were visually apparent (i.e., dark green coloration). Conidia were washed from culture plates by using 50 ml of sterile PBS with 0.1% Tween 80, strained through sterile gauze to remove hyphal contamination, centrifuged, and finally reconstituted to a concentration of 1.7 × 108 conidia/ml. Conidia were then either used immediately (designated throughout as resting conidia) for intratracheal (i.t.) injections or incubated for 6 h in sterile PBS with 0.1% Tween 80 at 37°C to allow for germination (designated as swollen conidia) prior to i.t. injection. All visible conidia were swollen after 6 h of incubation at 37°C; hyphal growth was not detectable until after at least 8 to 12 h of incubation at 37°C and was minimal at this time point.
Invasive aspergillosis model.
A well-described model of invasive aspergillosis was used in the present study (12). To induce neutropenia, mice were injected with α-GR-1 antibody (100 μg in PBS) 24 h prior to i.t. challenge. Briefly, mice were anesthetized with a mixture of ketamine and xylazine, the trachea was exposed by blunt dissection of adjacent muscle tissue, and 5.0 × 106 A. fumigatus resting or swollen conidia suspended in 30 μl of PBS with 0.1% Tween 80 were administered by i.t. injection. For survival studies, mice were monitored every 12 to 24 h after the A. fumigatus conidium challenge and euthanized if they appeared moribund. Whole-lung samples were processed for proteomic or histological analysis (see below) at either day 2 or day 4 after conidium injection. In our studies, the extent of neutropenia was ca. 90%, and rebound of neutrophil numbers began to occur 24 to 48 h after injection of anti-GR-1 antibody. Full reconstitution of neutrophil numbers occurred by 3 to 5 days after anti-GR-1 injection.
Chronic fungal asthma model.
TLR9+/+ and TLR9−/− mice were sensitized with single 100-μl intraperitoneal and subcutaneous injections of Aspergillus antigen (500 μg of antigen diluted in 5 ml of incomplete Freund adjuvant and 5 ml of sterile saline). These were followed 1 week later by three intranasal treatments (20 μl of a 1-μg/μl solution in sterile saline) with Aspergillus antigen, each spaced 1 week apart. A commercially available preparation of soluble A. fumigatus antigens as has been previously described in detail was used (27). Seven days after a third intranasal challenge, each mouse received 5 × 106 resting or swollen conidia suspended in 30 μl of PBS-Tween 80 (0.1% [vol/vol]) via i.t. administration. Immediately prior to and at days 7, 14, and 28 after an i.t. A. fumigatus resting or swollen conidium challenge, bronchial hyper-responsiveness was assessed in a Buxco plethysmograph (Buxco, Troy, NY). Briefly, sodium pentobarbital (Butler Co., Columbus, OH; 0.04 mg/g of mouse body weight) was used to anesthetize mice prior to their intubation and ventilation with a Harvard pump ventilator (Harvard Apparatus, Reno, NV). Once baseline airway resistance was established, 50 μg of methacholine/ml was nebulized, and airway hyper-responsiveness was monitored for approximately 5 min. The peak increase in airway resistance was then recorded. Once airway responsiveness had returned to baseline, each mouse was challenged again with a higher dose of nebulized methacholine (80 μg/ml). Again, airway hyper-responsiveness was monitored for approximately 5 min, and the peak resistance was recorded. After the assessment of airway hyper-responsiveness, blood was removed for immunoglobulin analysis (see below), and whole lungs were dissected from each mouse and snap-frozen in liquid nitrogen for genomic and proteomic analysis or fixed in 10% formalin for histological analysis (see below). Leg bones were also dissected for the retrieval of bone marrow and the culture of bone marrow-derived dendritic cells.
Serum IgE analysis.
Serum levels of immunoglobulin E (IgE) at days 14 and 28 after conidia in TLR9+/+ and TLR9−/− groups were analyzed by using complementary capture and detection antibody pairs for IgE (Pharmingen, San Diego, CA). Immunoglobulin enzyme-linked immunosorbent assays (ELISAs) were performed according to the manufacturer's directions. Duplicate sera samples were diluted 1:20 for IgE determination. Immunoglobulin levels were then calculated from optical density readings at 492 nm, and immunoglobulin concentrations were calculated from a standard curve generated by using recombinant IgE or IgG2a (the standard curves ranged from 0 to 1,000 ng/ml).
Whole-lung RNA isolation and TaqMan analysis.
Total RNA was isolated from homogenized mouse lungs by using TRIzol reagent (Invitrogen/Life Technologies, Carlsbad, CA). Purified RNA was treated with DNase and reverse transcribed into cDNA by using TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA). For quantitative TaqMan analysis, a total of 0.2 μg of total RNA was reverse transcribed to yield cDNA, and predeveloped TaqMan gene expression assays were used to quantify IL-10, CXCL10, CCL5, CCL2, CCL3, dectin-1, IL-23p19, IL-12p35, IL-12p40, IL-25, and IL-13 according to the manufacturer's instructions (Applied Biosystems). GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was analyzed as an internal control and gene expression was normalized to GAPDH before the fold change in gene expression was calculated. The fold changes in transcript expression were calculated via comparison of the gene expression in naive whole-lung samples using the formula 2−ΔΔCT, which were assigned a value of 1 to that in whole-lung samples from mice with chronic fungal asthma.
Whole lung proteomic analysis.
Murine IL-4, IL-5, IL-10, IL-13, gamma interferon (IFN-γ), transforming growth factor β (TGF-β), TNF-α, IL-12, CXCL10, CCL11, CXCL9, CCL21, CCL6, CCL2, CCL3, CCL5, CCL22, and CCL17 were determined in 50-μl samples from whole-lung homogenates using a standardized sandwich ELISA technique previously described in detail or through a bead-based multiple target sandwich ELISA system (Bioplex; Bio-Rad Laboratories, Hercules, CA). Recombinant murine cytokines and chemokines (R&D Systems, Rochester, MN) were used to generate the standard curves from which the sample concentrations were derived. The limit of ELISA detection for each cytokine was consistently greater than 50 pg/ml for sandwich ELISA and 1 pg/ml for Bioplex. The cytokine and chemokine levels in each sample were normalized to total protein levels measured by using the Bradford assay.
Whole-lung histological analysis.
Whole lungs from A. fumigatus-sensitized TLR9+/+ and TLR9−/− mice prior to and at various times after A. fumigatus conidium challenge were fully inflated with 10% formalin, dissected, and placed in fresh 10% formalin for 24 h. Routine histological techniques were used to paraffin embed the entire lung, and 5-μm sections of the whole lung were stained with hematoxylin-eosin or Gomori methenamine silver (GMS) to detect Aspergillus conidia.
Bone marrow-derived dendritic cell culture and isolation.
Myeloid dendritic cells were isolated and prepared by culture of bone marrow obtained from allergic TLR9+/+ and TLR9−/− mice at various times after conidium challenge and cultured for 6 days with murine granulocyte-macrophage colony-stimulating factor (20 ng/ml; R&D Systems), followed by selection sorting for CD11c+ cells through magnetic activated cell sorting purification (Miltenyi Biotech, Bergisch Gladbach, Germany). Cells were cultured for 6 or 24 h for RNA or protein determination, respectively, with medium only, CpG (2 nM), Pam3Cys (2.5 μg/ml), or poly(I-C) (50 μg/ml), and analysis was performed according to the TaqMan and proteomic analysis protocol described earlier.
Statistical analysis.
All results are expressed as mean ± the standard error of the mean (SEM). A Student t test or analysis of variance and a Student-Newman-Keuls multiple-comparison test were used to determine statistical significance between TLR9+/+ and TLR9−/− mice at various times after the conidium challenge; a P value of <0.05 was considered statistically significant.
RESULTS
Neutrophil-depleted TLR9−/− mice were modestly protected from invasive aspergillosis compared to TLR9+/+ mice.
It has been previously established that MyD88-dependent innate immune mechanisms, particularly those involving TLR2, TLR4, and TLR9, are critical in the recognition of fungal components during Aspergillus infection (3). For example, neutrophil-mediated antifungal responses rely on these TLR pathways (2, 3). In a previous study, C57BL/6J TLR9−/− mice exhibited enhanced fungal clearance after exposure to resting conidia (3). Survival responses in neutrophil-depleted TLR9−/− and TLR9+/+ mice on a BALB/c background after challenge with resting conidia and swollen conidia are shown in Fig. 1A and B, respectively. Overall, a significant difference in survival between TLR9+/+ and TLR9−/− mice was observed in a model of invasive aspergillosis initiated by the administration of resting conidia into neutrophil-depleted mice (Fig. 1A). i.t. challenge of TLR9+/+ and TLR9−/− mice with swollen conidia led to faster mortality in the TLR9+/+ group, but the overall survival in both groups was similar at day 4 (Fig. 1B). Thus, these data suggested that TLR9 delayed but did not prevent mortality due to invasive aspergillosis in neutrophil-depleted mice.
FIG. 1.
Neutrophil-depleted TLR9−/− mice are significantly less susceptible to resting conidia and succumb more slowly to swollen conidia compared to TLR9+/+ mice. (A and B) Unstained bright-field micrographs of resting (A) and swollen (B) A. fumigatus conidia. (C and D) Kaplan-Meier survival curve analysis of α-GR-1 neutrophil-depleted TLR9+/+ and TLR9−/− mice after i.t. challenge with 5 × 106 resting (C) or swollen (D) conidia (n = 10). To induce neutropenia, all mice were injected with 100 μg of α-GR-1 antibody 24 h prior to conidial challenge. The data are representative of two separate experiments. P ≤ 0.05 for comparison between resting versus swollen conidia. Original magnification, ×1,000 for all micrographs.
Neutrophil-depleted TLR9−/− mice exhibited slower fungal growth compared to TLR9+/+ mice after challenge with resting or swollen conidia.
GMS-stained lung sections from TLR9+/+ and TLR9−/− mice at day 2 after i.t. challenge with resting conidia revealed increased Aspergillus growth in the TLR9+/+ (Fig. 2A) versus the TLR9−/− (Fig. 2B) group. However, increased fungal growth was observed in TLR9−/− mice at day 4 after challenge with swollen conidia relative to TLR9+/+ mice (Fig. 2E and F). Together, these data indicate the lack of TLR9 allowed neutrophil-depleted mice to slow but not eliminate the growth of resting Aspergillus conidia in the lung, but TLR9-deficient mice exhibit increased fungal growth with swollen-conidium challenge in neutrophil-depleted mice.
FIG. 2.
Neutrophil-depleted TLR9−/− mice exhibited less fungal growth and less tissue inflammation at day 2 after challenge with resting conidia. TLR9+/+ (A) and TLR9−/− (B) mouse whole-lung tissue sections from neutropenic mice processed via GMS staining at 2 days after i.t. challenge with 5 × 106 resting conidia. Aspergillus conidia and hyphae stain black using the GMS staining procedure. (C and D) Representative hematoxylin-and-eosin-stained TLR9+/+ (C) and TLR9−/− (D) neutropenic mouse whole-lung sections obtained 2 days after i.t. challenge with 5 × 106 resting conidia. (E and F) TLR9+/+ (E) and TLR9−/− (F) mouse whole-lung tissue sections from neutropenic mice processed via GMS staining 4 days after i.t. challenge with 5 × 106 swollen conidia. Original magnification, ×200 for all photomicrographs.
Whole-lung cytokine and chemokine changes were apparent in TLR9+/+ and TLR9−/− mice during invasive aspergillosis induced by resting and swollen conidia.
Next, we examined whole-lung cytokine and chemokine levels to determine significant mediators of the immune response to resting versus swollen Aspergillus conidia. All mediators were assessed via ELISA and Bioplex analysis of whole-lung homogenates, which were taken from TLR9+/+ and TLR9−/− mice at 2 days after challenge with resting conidia. Representative examples of these analyses are shown in Fig. 3. A decrease in the levels of TNF-α (Fig. 3A) and significant decreases in CCL2 (Fig. 3B) and CXCL10 (Fig. 3C) were observed in the knockout group compared to the wild-type group. Similar analysis of TLR9+/+ and TLR9−/− mice 4 days after challenge with swollen conidia demonstrated significant increases in the levels of TNF-α (Fig. 3D), CCL2 (Fig. 3E), and CXCL10 (Fig. 3F) in TLR9−/− mice compared to TLR9+/+ mice. In fact, whole-lung homogenates from TLR9+/+ mice appeared to express little, if any, of these proteins. Thus, these data show that the presence of TLR9 markedly altered the whole-lung cytokine and chemokine response to A. fumigatus in the immunocompromised host; this receptor appeared to be required for the full initiation of immune activation to resting conidia, but its presence appeared not to be required for expression of TNF-α (Fig. 3D), CCL2 (Fig. 3E), and CXCL10 (Fig. 3F) in response to swollen conidia.
FIG. 3.
Dynamic changes in whole-lung cytokines and chemokines were apparent in TLR9+/+ and TLR9−/− mice during invasive aspergillosis induced by resting and swollen conidia. (A to C) ELISA or Bioplex analysis of TNF-α (A), CXCL10 (B), and CCL2 (C), comparing TLR9+/+ to TLR9−/− whole-lung protein levels from neutrophil-depleted mice at day 2 after challenge with 5 × 106 resting Aspergillus conidia. (D to F) ELISA or Bioplex analysis of TNF-α (D), CXCL10 (E), and CCL2 (F), comparing TLR9+/+ to TLR9−/− whole-lung protein levels from neutrophil-depleted mice challenged with 5 × 106 swollen Aspergillus conidia. To induce neutropenia, mice were injected with α-GR-1 antibody 24 h prior to i.t. The data are expressed as means ± the SEM. *, P ≤ 0.05.
Sustained whole-lung TLR9 transcript expression was observed in A. fumigatus-sensitized mice that received swollen conidia.
Because of the marked differences in the cytokine and chemokine responses to resting versus swollen conidia in neutrophil-depleted TLR9−/− mice, we next assessed the role of this TLR in a model of chronic fungal asthma. We have extensively characterized a model of chronic fungal asthma in which defective clearance of resting Aspergillus conidia introduced by i.t. injection into A. fumigatus-sensitized mice occurs (27, 52). We found that this defect is due to the inhibitory effect of allergic Th2-type immune response on the antifungal innate immune response (27, 52). The effect of resting and swollen conidia on whole-lung TLR9 transcript expression in A. fumigatus-sensitized TLR9+/+ mice is shown in Fig. 4. At day 14, TLR9 transcript expression was increased ∼2-fold in both groups of TLR9+/+ mice. At day 28, only the group of TLR9+/+ mice that received swollen conidia showed a persistent twofold increase in TLR9 transcript expression. These data indicate that whole-lung TLR9 levels were maintained in wild-type mice, which were challenged with swollen conidia, suggesting a role for TLR9 during allergic airway responses to this form of conidia.
FIG. 4.
Sustained whole-lung TLR9 transcript expression was observed in A. fumigatus-sensitized mice that received swollen conidia. A TaqMan analysis of whole-lung TLR9 mRNA levels from Aspergillus-sensitized mice receiving 5 × 106 resting (□) or swollen (▪) conidia i.t. was performed. The data are expressed as means ± the SEM; n = 3/group/time point. *, P ≤ 0.05.
Swollen conidia grow in A. fumigatus-sensitized TLR9−/− mice.
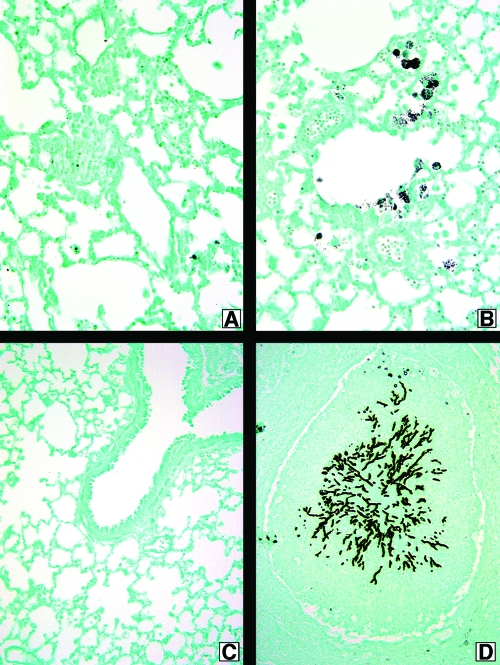
Previous studies have not explored the role of TLR9 during chronic fungal asthma responses. In the present study, we observed that fungal material was present upon GMS staining of whole-lung samples from A. fumigatus-sensitized TLR9+/+ and TLR9−/− mice challenged with resting conidia, and no differences were observed between these groups at any time after conidium challenge (not shown). However, marked differences were observed when A. fumigatus-sensitized TLR9+/+ and TLR9−/− mice were challenged with swollen conidia (Fig. 5). A. fumigatus-sensitized TLR9+/+ mice challenged with swollen conidia exhibited small amounts of fungal material at day 14 (Fig. 5A), which was gone by day 28 (Fig. 5C). Surprisingly and unexpectedly, A. fumigatus-sensitized TLR9−/− mice challenged with swollen conidia exhibited fungal growth at both times after conidium challenge (Fig. 5B and D). At day 28, large masses of fungus were observed in the lungs of TLR9−/− mice (Fig. 5D). Together, these histological analyses suggested that A. fumigatus-sensitized TLR9−/− mice were unable to contain the growth of swollen conidia.
FIG. 5.
Swollen conidia grow in A. fumigatus-sensitized TLR9−/− mice. TLR9+/+ (A and C) and TLR9−/− (B and D) mouse whole-lung tissue sections from sensitized mice challenged i.t. with 5 × 106 swollen Aspergillus conidia are shown. Slides were processed using GMS, which stains Aspergillus conidia and hyphae black. Lungs were harvested at day 14 (A and B) and at day 28 (C and D). Original magnification, ×200 for all photomicrographs.
Conidia significantly augmented circulating total IgE levels in A. fumigatus-sensitized TLR9−/− mice regardless of germination status, whereas TLR9−/− mice showed significantly lower airway hyper-responsiveness after challenge with resting conidia.
Elevated serum IgE levels characterize atopy and asthma in individuals (31, 42). Sensitized TLR9−/− mice that received an i.t. challenge with resting conidia showed a significant increase in serum IgE levels at day 28 after conidium challenge compared to TLR9+/+ mice (Fig. 6A). Likewise, A. fumigatus-sensitized mice that received swollen conidia showed a significant sixfold increase in serum IgE levels relative to TLR9+/+ mice (Fig. 6B). Importantly, there was a 100-fold difference in serum IgE levels between groups of mice receiving resting versus swollen conidia. These data indicated that TLR9 regulated the IgE response in A. fumigatus-sensitized mice and that swollen conidia provoked a more robust IgE response in A. fumigatus-sensitized mice.
FIG. 6.
Conidia significantly augmented circulating total IgE levels in A. fumigatus-sensitized TLR9−/− mice regardless of germination status, but TLR9−/− mice showed significantly lower airway hyper-responsiveness after challenge with resting conidia. ELISAs for serum IgE from TLR9+/+ and TLR9−/− sensitized mice challenged with 5 × 106 resting (A) or swollen (B) Aspergillus conidia were performed. Airway hyper-responsiveness TLR9+/+ and TLR9−/− sensitized mice infected with resting (C) or swollen (D) Aspergillus conidia was also assessed. Peak increases in airway resistance or hyper-responsiveness (units = cm H2O/ml/s) were determined at each time point after the intravenous injection of methacholine. The data are expressed as means ± the SEM; n = 3/group/time point. *, P ≤ 0.05; ***, P ≤ 0.001.
Allergic responses to A. fumigatus are characterized by airway hyper-responsiveness following a methacholine stimulus (27). This response differed significantly between A. fumigatus TLR9+/+ and TLR9−/− mice given resting conidia (Fig. 6C). At day 28 after challenge with resting conidia, TLR9−/− mice showed significantly less airway hyper-responsiveness than did TLR9+/+ mice. In contrast, airway hyper-responsiveness was similar in both groups of sensitized mice challenged with swollen conidia (Fig. 6D). Thus, these data indicated that the absence of TLR9 dampened the airway allergic response to resting but not to swollen conidia.
Divergent effects on whole-lung cytokine and chemokine levels were observed in A. fumigatus-sensitized TLR9−/− mice after resting or swollen conidium challenge.
Next, we examined whole-lung cytokine and chemokine levels to assess the nature of the cytokine/chemokine response elicited in allergic TLR9+/+ and TLR9−/− mice after challenge with resting or swollen conidia. ELISA and Bioplex analysis of whole-lung homogenates taken from Aspergillus-sensitized TLR9+/+ and TLR9−/− mice challenged with either resting or swollen conidia revealed divergent effects on whole-lung cytokine and chemokine levels. Many of the cytokines and chemokines measured in whole-lung samples did not differ between sensitized TLR9+/+ and TLR9−/− mice at day 14 and day 28 after challenge with resting conidia (Table 1) . Significant differences between the two groups were noted, although these were observed in IL-13 (at day 14) and CXCL9 (at both times after conidium challenge) levels after challenge with resting conidia. After challenge with swollen conidia, significant differences in numerous whole-lung cytokines and chemokine levels, including IL-5, IL-13, CXCL10, CCL6, CCL11, CXCL9, and CCL21, were observed at both times after conidium challenge between sensitized TLR9+/+ and TLR9−/− mice (Table 1). Most cytokine and chemokines were significantly lower in the TLR9−/− mice than in the TLR9+/+ group. However, both IL-13 and CCL6 were significantly higher in the TLR9−/− mice compared to the TLR9+/+ group at day 14 after challenge with swollen conidia. Other examples of whole-lung cytokine and chemokine levels in these groups relevant to fungal asthma are shown in Fig. 7. Whole-lung CCL2 levels were significantly decreased at day 28 in TLR9−/− mice challenged with resting conidia (Fig. 7A), whereas in TLR9−/− mice, which received swollen conidia (Fig. 7B), whole-lung CCL2 levels were significantly increased. Whole-lung CXCL10 levels were significantly increased at day 14 after challenge with resting conidia in TLR9−/− mice but significantly decreased at day 28 in this group (Fig. 7C). Conversely, whole-lung CXCL10 levels were significantly lower in sensitized TLR9−/− mice at both times after challenge with swollen conidia compared to the TLR9+/+ groups (Fig. 7D). Finally, whole-lung IL-17 levels were not significantly different between TLR9+/+ and TLR9−/− mice at days 14 and 28 after conidium challenge with either resting or swollen conidia, but the levels of this cytokine were consistently lower in the TLR9−/− group than in the TLR9+/+ group (Fig. 7E and F). These data showed that the cytokine responses evoked by swollen conidia in particular were markedly different in the absence of TLR9, suggesting that this receptor had an important role in the regulation of immune responses to swollen A. fumigatus conidia.
TABLE 1.
Whole-lung cytokine and chemokine levels at day 14 and 28 after challenge with resting or swollen conidia in A. fumigatus-sensitized TLR9+/+ and TLR9−/− micea
| Cytokine or chemokine | Mean cytokine or chemokine level (ng/mg of total protein) ± SD
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Resting conidia
|
Swollen conidia
|
|||||||
| Day 14
|
Day 28
|
Day 14
|
Day 28
|
|||||
| TLR9+/+ | TLR9−/− | TLR9+/+ | TLR9−/− | TLR9+/+ | TLR9−/− | TLR9+/+ | TLR9−/− | |
| IL-4 | ND | ND | ND | ND | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00* |
| IL-5 | ND | ND | ND | ND | 0.16 ± 0.01 | 0.13 ± 0.01* | 0.11 ± 0.01 | 0.04 ± 0.01† |
| IL-10 | ND | ND | ND | ND | 0.31 ± 0.01 | 0.25 ± 0.02* | 0.21 ± 0.04 | 0.09 ± 0.00† |
| IL-12 | ND | ND | ND | ND | 0.07 ± 0.00 | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.02 ± 0.00 |
| IL-13 | 0.04 ± 0.01 | 0.02 ± 0.00* | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.00 ± 0.02 | 0.10 ± 0.01† | 0.08 ± 0.01 | 0.04 ± 0.00* |
| IFN-γ | ND | ND | ND | ND | 0.18 ± 0.01 | 0.15 ± 0.02 | 0.12 ± 0.02 | 0.06 ± 0.01* |
| TGF-β | ND | ND | ND | ND | 0.08 ± 0.01 | 0.05 ± 0.00* | 0.04 ± 0.00 | 0.03 ± 0.00* |
| CCL11 | ND | ND | ND | ND | 0.26 ± 0.02 | 0.18 ± 0.03* | 0.15 ± 0.02 | 0.11 ± 0.01* |
| CXCL9 | 0.07 ± 0.01 | 0.04 ± 0.00* | 0.06 ± 0.01 | 0.02 ± 0.01* | 0.08 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.00 | 0.03 ± 0.00* |
| CCL21 | ND | ND | ND | ND | 1.12 ± 0.07 | 1.49 ± 0.11* | 0.91 ± 0.06 | 0.53 ± 0.04* |
| CCL6 | ND | ND | ND | ND | 0.25 ± 0.06 | 0.49 ± 0.08† | 0.17 ± 0.03 | 0.23 ± 0.05 |
| CCL3 | ND | ND | ND | ND | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| CCL5 | ND | ND | ND | ND | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.02 ± 0.00 | 0.04 ± 0.00* |
| CCL22 | ND | ND | ND | ND | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| CCL17 | 0.03 ± 0.00 | 0.03 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.04 ± 0.00 | 0.03 ± 0.00* | 0.03 ± 0.00 | 0.03 ± 0.00 |
*, P ≤ 0.05; †, P ≤ 0.01 (compared to TLR9+/+ mice at the same time after challenge with resting or swollen conidia). ND, not detected.
FIG. 7.
Divergent effects on whole-lung cytokine and chemokine levels were observed in A. fumigatus-sensitized TLR9−/− mice after challenge with resting or swollen conidia. (A, C, and E) ELISA or Bioplex analyses of CCL2 (A), CXCL10 (C), and IL-17 (E), comparing TLR9+/+ to TLR9−/− whole-lung protein levels from sensitized mice challenged i.t. with resting Aspergillus conidia. (B, D, and F) ELISA or Bioplex analyses of CCL2 (B), CXCL10 (D), and IL-17 (F), comparing TLR9+/+ to TLR9−/− whole-lung protein levels from sensitized mice infected with swollen Aspergillus conidia. The data are expressed as means ± the SEM; n = 3/group/time point. *, P ≤ 0.05; **, P ≤ 0.01.
Nonsensitized and A. fumigatus-sensitized TLR9−/− mice exhibit lower whole-lung dectin-1 transcript expression relative to TLR9+/+ mice.
Fungal β-glucans expressed on the surface of Aspergillus conidia are recognized through the innate immune receptor dectin-1, and this binding results in the generation of a Th17 response (29). Our TaqMan analysis of whole-lung samples from the IPA model suggested that dectin-1 expression was lower in the TLR9−/− group than in the TLR9+/+ group (data not shown). These data prompted us to analyze dectin-1 levels in this asthma model. The data indicated that lung dectin-1 expression was significantly decreased at day 28 after conidium challenge in TLR9−/− mice receiving resting conidia (Fig. 8A). In TLR9−/− mice receiving swollen conidia, decreased expression of dectin-1 was observed at both days 14 and 28 after conidium challenge (Fig. 8B). These data indicated that TLR9 was involved in the regulation of dectin-1 expression in the lung, particularly after challenge with swollen conidia.
FIG. 8.
Nonsensitized and A. fumigatus-sensitized TLR9−/− mice exhibit lower whole-lung dectin-1 expression relative to TLR9+/+ mice. TaqMan analysis of TLR9+/+ and TLR9−/− mouse lung dectin-1 levels from Aspergillus-sensitized mice infected i.t. with 5 × 106 resting (A) or swollen (B) Aspergillus conidia was performed. The data are expressed as means ± the SEM; n = 3/group/time point. *, P ≤ 0.05.
Bone marrow dendritic cells exhibit significantly lower dectin-1 expression at day 14 after challenge with swollen conidia in TLR9−/− mice compared to TLR9+/+ mice.
To further explore the role of TLR9 on dectin-1 expression, we assessed how this β-glucan receptor was regulated in dendritic cells from A. fumigatus-sensitized TLR9+/+ and TLR9−/− mice at days 14 and 28 after challenge with conidia. TaqMan quantitative PCR analysis of bone marrow-derived dendritic cells stimulated with CpG revealed that dectin-1 expression was significantly lower at day 14 after conidium challenge in sensitized TLR9−/− mice than in TLR9+/+ mice, but the levels of this scavenging receptor were similar to the wild-type levels at day 28 (Fig. 9). In TLR9+/+ sensitized mice, dectin-1 levels increased in dendritic cells compared to unsensitized wild-type mice and were maintained at increased levels at day 28. Thus, these data revealed that TLR9 is an important factor for dectin-1 transcript expression.
FIG. 9.
Bone marrow dendritic cells exhibit significantly lower dectin-1 expression at day 14 after challenge with swollen conidia in TLR9−/− mice than in TLR9+/+ mice. TaqMan analysis of dectin-1 expression by TLR9+/+ and TLR9−/− mouse bone marrow dendritic cells from Aspergillus-sensitized mice infected i.t. with 5 × 106 swollen Aspergillus conidia. The data are expressed as means ± the SEM; n = 3/group/time point. *, P ≤ 0.05.
DISCUSSION
In the present study, we characterized the altered immune responses in neutrophil-depleted and A. fumigatus-sensitized TLR9−/− mice after their exposure to either resting or swollen conidia. Although the absence of TLR9 had a minor albeit significant protective effect in invasive aspergillosis (i.e., survival) and allergic asthma (i.e., airway hyper-responsiveness) following challenge of neutrophil-depleted and A. fumigatus-sensitized mice, respectively, with resting conidia, it was apparent that TLR9−/− mice were susceptible to A. fumigatus growth when given swollen conidia. This effect was most profound when TLR9−/− mice were sensitized to this fungus and challenged with swollen conidia. Interestingly, we observed that unsensitized and A. fumigatus-sensitized TLR9−/− mice expressed significantly less dectin-1 in whole lung and isolated dendritic cells, and we speculate that the lower expression of dectin-1 contributed to the impaired ability of these mice to respond to swollen conidia. Another indication that dectin-1 expression was lower in TLR9−/− mice was revealed by the lower IL-17 levels present in these mice. Thus, these data demonstrated that the absence of TLR9 markedly impaired the immune responses required to contain the growth of swollen conidia in allergic mice.
Much of the pathology associated with invasive aspergillosis is due to disregulated inflammatory processes (48). Inflammation is a necessary component of an effective immune response against fungus during invasive pulmonary aspergillosis. Properly regulated, inflammation can effectively contain Aspergillus conidia in the lung and promote their clearance, whereas inadequate or excessive inflammation can lead to IPA and/or damage of the lung tissues (8). In the present study we found significant changes in the levels of TNF-α, CXCL10, and CCL2, all of which are significant mediators of inflammation during IPA infection. TNF-α plays an important role in the recruitment of neutrophils, CXCL10 promotes dendritic cell and macrophage recruitment, and CCL2 acts as a chemoattractant for macrophages (38, 50). In our studies, we found that all three of these factors were decreased in TLR9−/− mice at day 2 after challenge with resting conidia but increased in TLR9−/− mice at day 4 after challenge with swollen conidia. Previous reports involving conidium challenge in neutrophil-depleted mice have demonstrated an early elevation in inflammatory mediators such as TNF-α by day 2 after conidium challenge and normalization of mediator levels by day 4 after challenge (36). Our studies indicate the induction of TNF-α, CXCL10, and CCL2 is greatest at day 4 after challenge with swollen conidia in TLR9−/− mice. We interpret these data to indicate that temporal changes in cytokines and chemokines are TLR9 dependent during IPA. TLR9-dependent effects on cytokine and chemokine generation appeared to provide significant protection during immune responses to resting conidia but not during immune responses to swollen conidia. Further, these results indicate a role for TLR9 in reigning in the immune response to swollen conidia since TLR9−/− mice exhibit elevated levels of inflammatory cytokines at day 4 after conidium challenge.
Appropriate fungal recognition leads to a balanced immune response against Aspergillus conidia during pulmonary responses to Aspergillus. For example, TLR9 recognizes fungal DNA (45), while dectin-1 receptors have been demonstrated to recognize β-glucan components from the swollen conidium cell wall (10, 53). In addition to TLR9, the innate immune receptors TLR2, TLR4, and the adaptor protein MyD88 are also required for efficient conidial phagocytosis and immune responses to Aspergillus (14, 24, 34). MyD88-dependent signaling on dendritic cells is crucial for priming antifungal Th1 responses (2). Polymorphisms in the TLR2 gene have been identified as important factors for susceptibility to development of allergic diseases and/or IPA (1, 13, 19, 32). TLR4-deficient mice possess neutrophils that are deficient in conidium-killing activity but phagocytose conidia normally (2, 3). Since β-glucans are abundant on swollen Aspergillus conidia, the dectin-1 pathway appears to be fundamentally important for the recognition of metabolically active conidia and the control of fungal infection (16, 29). The functional equivalence of dectin-1 in human cells and in murine models has been well characterized (56). Further, it has been established that fungal components such as chitin can stimulate IL-17A and induce acute inflammation through TLR2 and a MyD88-dependent pathway (15). During IPA, IL-17A and IL-23 are rapidly produced at sites of infection (8, 58). IL-17 and IL-23 control the immune response to fungal infection and excessive IL-17 acts to enhance inflammation through polymorphonuclear leukocytes. The Th17 pathway appears to directly contribute to defective pathogen clearance and the failure to resolve inflammation during Aspergillus infection (49). Thus, the TLRs and dectin-1 appear to cooperate in the recognition and elimination of A. fumigatus from the respiratory system.
Our studies indicate that TLR9 activation contributed to the susceptibility of neutropenic mice to challenge with resting or swollen conidia. Two key observations in this IPA model included the following: (i) fungal growth was slower in TLR9−/− mice challenged with either resting or swollen conidia, and (ii) the inflammatory response appeared to develop slower in the neutropenic TLR9−/− mouse after conidium challenge. Our data agree, in part, with those of Bellocchio et al. (2), who showed that TLR9−/− mice on a C57BL/6 background were significantly protected from a challenge with multiple conidia. In that study it was not clear as to the activation state of the conidia, but if the conidia were in a resting state our present data and theirs coincide. Our data also suggest this protective effect could be due in part to lower tissue inflammation. However, neutropenic TLR9−/− mice were not protected from invasive aspergillosis due to swollen conidia. One explanation for this susceptibility might lie in the importance of dectin-1 during the response of the lung to swollen A. fumigatus (53). Indeed, targeting dectin-1 appears to render mice more susceptible to IPA (29), whereas activating dectin-1 via dectin-1-Fc fusion protein demonstrated increased mouse survival in a model of IPA (35). Examination of whole-lung samples from TLR9+/+ and TLR9−/− mice revealed that dectin-1 transcript expression was lower in the knockout group compared to the wild-type group. Corresponding with lower dectin-1 expression were lower IL-17 levels. Together, these data suggest that the lack of TLR9 is associated with lower dectin-1 expression, which might permit swollen conidia to grow in neutropenic TLR9−/− mice.
Allergic asthma is a Th2 cytokine-dominated disease (11). During ABPA and fungal asthma, persistence of conidia drives airway hyper-responsiveness and airway remodeling. Our observations of these phenomena have led us to pose two major questions. (i) Why do immune cells hold onto conidia and yet are unable to kill them? (ii) What factor(s) drives the clearance of fungus from the lungs of asthmatic mice? Several novel observations arose from the present study, which suggest that TLR9 has a major role in controlling fungal growth in the allergic lung. We observed that the asthmatic response to resting conidia was largely similar between TLR9+/+ and TLR9−/− mice, although the latter group exhibited decreased airway hyper-responsiveness compared to the former group. This decrease in the TLR9−/− group was coincident with decreased whole-lung IL-13 levels. IL-13 acts to improve B-cell production of IgE, one observation we did note with resting conidia at day 28. Further, IL-13 acts with TNF-α and histamines to increase mucus production and promote bronchoconstriction (4, 5, 22, 27). CCL2 neutralization in the lung at the day 14 and 28 time points after conidium challenge decreases airway inflammation and airway hyper-responsiveness in a model of fungal asthma (6). Our observation that CCL2 levels significantly decreased in TLR9−/− mice challenged with resting conidia could explain the decreased airway hyper-responsiveness we observed in challenges with resting conidia. However, we observed that airway hyper-responsiveness in the TLR9+/+ and TLR9−/− groups challenged with swollen conidia were similar, which was unexpected since the TLR9−/− mice contained markedly greater amounts of fungus and/or fungal material. Two potential explanations might reconcile this observation. First, Th2 factors that drive airway hyper-responsiveness, namely, IL-5, IL-13, CCL11, and CCL21 were significantly lower in the TLR9−/− group than in the TLR9+/+ group. Second, containment of the fungus in the lungs of the TLR9−/− mice might have prevented disseminated infection and thus limited fungal antigen exposure in the lung. We noted a disconnect between serum IgE levels and the airway response in TLR9−/− mice that was in keeping with other previous findings regarding IgE and airway hyper-responsiveness (26). Serum IgE alone is not an accurate indicator of airway hyper-responsiveness in mice. The containment of fungus in the lungs of TLR9−/− mice was remarkable and similar to what is described clinically as fungal granulomas (44). The factors precipitating the marked fibrotic response in TLR9−/− mice are the subject of ongoing studies in the laboratory, but we postulate that soluble factors such as CCL2 and CCL5 might contribute to this phenotype. Both chemokines have well-described profibrotic roles in the lung. Thus, the lack of TLR9 resulted in a pulmonary environment characterized by less allergic airway disease due to resting conidia but increased remodeling due to the challenge with swollen conidia.
Our data suggest that sustained dectin-1 levels mediated through TLR9 are important in antifungal responses against swollen conidia in the immunocompetent host. The immune response to resting conidia is mediated by MyD88-dependent TLR activation, including signaling through TLR2, TLR4, and TLR9 (2, 3). The lack of TLR9 is compensated for by other TLRs in the case of resting conidia. However, TLR9 is required for full dectin-1 expression, and its absence renders mice susceptible to swollen conidia. IL-17 has been demonstrated to play a role in inflammation during allergic asthma since it negatively regulates allergic asthma disease (51). From the present study it was apparent that TLR9−/− mice displayed lower dectin-1 expression at various times during the course of IPA (data not shown) and chronic fungal asthma. One consequence of the decreased dectin-1 expression appeared to be a concomitant trend toward decreased IL-17 levels in asthmatic TLR9−/− mice exposed to swollen conidia. As mentioned above, the absence of IL-17 has been shown previously to augment features of allergic airway inflammation, and our present data appeared to confirm these previous studies. Thus, the presence of TLR9 appears to be required for appropriate dectin-1 expression during fungal asthma responses evoked by swollen conidia.
Dendritic cells mediate the immune response against Aspergillus (7). During IPA infection, dendritic cell recruitment and activation are key to survival. Dendritic cell recruitment occurs initially through recognition of Aspergillus conidia by alveolar macrophages in the lung and secretion of chemokine and cytokine signals (55). Further, dendritic cells themselves recognize and engulf Aspergillus conidia and aid in the development of Th and neutrophil responses to the fungus (7, 21). Dectin-1 receptor expression on dendritic cells mediates inflammatory responses to A. fumigatus conidia. Silencing of dectin-1 results in reduced expression of proinflammatory cytokines (TNF-α and IL-12) (37). Further, dectin-1 Fc targeting of Aspergillus β-glucans has proven to be an effective means of improving the immune response to Aspergillus conidia (35). In the present study we observed a transient decrease in dectin-1 transcript expression in TLR9−/− dendritic cells. It is possible that other cell types, including macrophages, also show impaired dectin-1 expression, and these studies are ongoing.
In summary, TLR9 is relevant for pulmonary fungal responses against swollen conidia and is important for regulating inflammation that aids in the containment and clearance of Aspergillus (Fig. 10). This role appears to be related to altered dectin-1 expression. Dectin-1 plays an important role in the recognition of swollen Aspergillus conidia during IPA and fungal asthma, and the levels of dectin-1 are decreased in the absence of TLR9. Further studies are warranted to elucidate the direct role of dectin-1 in the fungal asthma response to swollen A. fumigatus conidia.
FIG. 10.
Model for conidium recognition and innate immune responses in TLR9+/+ and TLR9−/− mice. The recognition of resting conidia through TLR9 and swollen conidia through TLR9 and dectin-1 promotes cytokine responses leading to fungal clearance. The absence of TLR9, however, produces an impaired cytokine response and fungal growth in TLR9−/− mice challenged with resting conidia. Decreased levels of dectin-1 expression are observed in TLR9−/− mice. This decrease also produces a defective cytokine response in TLR9−/− mice challenged with swollen conidia and fungal growth.
Acknowledgments
This study was supported, in part, by the NIH (grant HL069865 to C.M.H.).
We thank Erica O'Connor and Priya Kulasekaran for technical contributions to this study. We thank Robin Kunkel for providing graphic artist expertise.
Editor: A. Casadevall
Footnotes
Published ahead of print on 20 October 2008.
REFERENCES
- 1.Ahmad-Nejad, P., S. Mrabet-Dahbi, K. Breuer, M. Klotz, T. Werfel, U. Herz, K. Heeg, M. Neumaier, and H. Renz. 2004. The Toll-like receptor 2 R753Q polymorphism defines a subgroup of patients with atopic dermatitis having severe phenotype. J. Allergy Clin. Immunol. 113565-567. [DOI] [PubMed] [Google Scholar]
- 2.Bellocchio, S., C. Montagnoli, S. Bozza, R. Gaziano, G. Rossi, S. S. Mambula, A. Vecchi, A. Mantovani, S. M. Levitz, and L. Romani. 2004. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J. Immunol. 1723059-3069. [DOI] [PubMed] [Google Scholar]
- 3.Bellocchio, S., S. Moretti, K. Perruccio, F. Fallarino, S. Bozza, C. Montagnoli, P. Mosci, G. B. Lipford, L. Pitzurra, and L. Romani. 2004. TLRs govern neutrophil activity in aspergillosis. J. Immunol. 1737406-7415. [DOI] [PubMed] [Google Scholar]
- 4.Blease, K., C. Jakubzick, J. M. Schuh, B. H. Joshi, R. K. Puri, and C. M. Hogaboam. 2001. IL-13 fusion cytotoxin ameliorates chronic fungal-induced allergic airway disease in mice. J. Immunol. 1676583-6592. [DOI] [PubMed] [Google Scholar]
- 5.Blease, K., C. Jakubzick, J. Westwick, N. Lukacs, S. L. Kunkel, and C. M. Hogaboam. 2001. Therapeutic effect of IL-13 immunoneutralization during chronic experimental fungal asthma. J. Immunol. 1665219-5224. [DOI] [PubMed] [Google Scholar]
- 6.Blease, K., B. Mehrad, N. W. Lukacs, S. L. Kunkel, T. J. Standiford, and C. M. Hogaboam. 2001. Antifungal and airway remodeling roles for murine monocyte chemoattractant protein-1/CCL2 during pulmonary exposure to Aspergillus fumigatus conidia. J. Immunol. 1661832-1842. [DOI] [PubMed] [Google Scholar]
- 7.Bozza, S., R. Gaziano, A. Spreca, A. Bacci, C. Montagnoli, P. di Francesco, and L. Romani. 2002. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J. Immunol. 1681362-1371. [DOI] [PubMed] [Google Scholar]
- 8.Bozza, S., T. Zelante, S. Moretti, P. Bonifazi, A. DeLuca, C. D'Angelo, G. Giovannini, C. Garlanda, L. Boon, F. Bistoni, P. Puccetti, A. Mantovani, and L. Romani. 2008. Lack of Toll IL-1R8 exacerbates Th17 cell responses in fungal infection. J. Immunol. 1804022-4031. [DOI] [PubMed] [Google Scholar]
- 9.Bretz, C., G. Gersuk, S. Knoblaugh, N. Chaudhary, J. Randolph-Habecker, R. C. Hackman, J. Staab, and K. A. Marr. 2008. MyD88 signaling contributes to early pulmonary responses to Aspergillus fumigatus. Infect. Immun. 76952-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown, G. D. 2006. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 633-43. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter, K. J., J. L. Ewing, J. M. Schuh, T. L. Ness, S. L. Kunkel, M. Aparici, M. Miralpeix, and C. M. Hogaboam. 2005. Therapeutic targeting of CCR1 attenuates established chronic fungal asthma in mice. Br. J. Pharmacol. 1451160-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carpenter, K. J., and C. M. Hogaboam. 2005. Immunosuppressive effects of CCL17 on pulmonary antifungal responses during pulmonary invasive aspergillosis. Infect. Immun. 737198-7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carvalho, A., A. C. Pasqualotto, L. Pitzurra, L. Romani, D. W. Denning, and F. Rodrigues. 2008. Polymorphisms in Toll-like receptor genes and susceptibility to pulmonary aspergillosis. J. Infect. Dis. 197618-621. [DOI] [PubMed] [Google Scholar]
- 14.Chignard, M., V. Balloy, J. M. Sallenave, and M. Si-Tahar. 2007. Role of Toll-like receptors in lung innate defense against invasive aspergillosis: distinct impact in immunocompetent and immunocompromised hosts. Clin. Immunol. 124238-243. [DOI] [PubMed] [Google Scholar]
- 15.Da Silva, C. A., D. Hartl, W. Liu, C. G. Lee, and J. A. Elias. 2008. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J. Immunol. 1814279-4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dennehy, K. M., and G. D. Brown. 2007. The role of the beta-glucan receptor Dectin-1 in control of fungal infection. J. Leukoc. Biol. 82253-258. [DOI] [PubMed] [Google Scholar]
- 17.Dennehy, K. M., G. Ferwerda, I. Faro-Trindade, E. Pyz, J. A. Willment, P. R. Taylor, A. Kerrigan, S. V. Tsoni, S. Gordon, F. Meyer-Wentrup, G. J. Adema, B. J. Kullberg, E. Schweighoffer, V. Tybulewicz, H. M. Mora-Montes, N. A. Gow, D. L. Williams, M. G. Netea, and G. D. Brown. 2008. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur. J. Immunol. 38500-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denning, D. W., B. R. O'Driscoll, C. M. Hogaboam, P. Bowyer, and R. M. Niven. 2006. The link between fungi and severe asthma: a summary of the evidence. Eur. Respir. J. 27615-626. [DOI] [PubMed] [Google Scholar]
- 19.Eder, W., W. Klimecki, L. Yu, E. von Mutius, J. Riedler, C. Braun-Fahrlander, D. Nowak, and F. D. Martinez. 2004. Toll-like receptor 2 as a major gene for asthma in children of European farmers. J. Allergy Clin. Immunol. 113482-488. [DOI] [PubMed] [Google Scholar]
- 20.Ferwerda, G., F. Meyer-Wentrup, B. J. Kullberg, M. G. Netea, and G. J. Adema. 2008. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell. Microbiol. 102058-2066. [DOI] [PubMed] [Google Scholar]
- 21.Gafa, V., M. E. Remoli, E. Giacomini, M. C. Gagliardi, R. Lande, M. Severa, R. Grillot, and E. M. Coccia. 2007. In vitro infection of human dendritic cells by Aspergillus fumigatus conidia triggers the secretion of chemokines for neutrophil and Th1 lymphocyte recruitment. Microbes Infect. 9971-980. [DOI] [PubMed] [Google Scholar]
- 22.Galli, S. J., M. Tsai, and A. M. Piliponsky. 2008. The development of allergic inflammation. Nature 454445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gersuk, G. M., D. M. Underhill, L. Zhu, and K. A. Marr. 2006. Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J. Immunol. 1763717-3724. [DOI] [PubMed] [Google Scholar]
- 24.Goodridge, H. S., and D. M. Underhill. 2008. Fungal recognition by TLR2 and dectin-1. Handb. Exp. Pharmacol. 200887-109. [DOI] [PubMed] [Google Scholar]
- 25.Green, B. J., E. R. Tovey, J. K. Sercombe, F. M. Blachere, D. H. Beezhold, and D. Schmechel. 2006. Airborne fungal fragments and allergenicity. Med. Mycol. 44(Suppl. 1)S245-255. [DOI] [PubMed] [Google Scholar]
- 26.Hamelmann, E., K. Takeda, A. Haczku, G. Cieslewicz, L. Shultz, Q. Hamid, Z. Xing, J. Gauldie, and E. W. Gelfand. 2000. Interleukin (IL)-5 but not immunoglobulin E reconstitutes airway inflammation and airway hyper-responsiveness in IL-4-deficient mice. Am. J. Respir. Cell Mol. Biol. 23327-334. [DOI] [PubMed] [Google Scholar]
- 27.Hogaboam, C. M., K. Blease, B. Mehrad, M. L. Steinhauser, T. J. Standiford, S. L. Kunkel, and N. W. Lukacs. 2000. Chronic airway hyperreactivity, goblet cell hyperplasia, and peribronchial fibrosis during allergic airway disease induced by Aspergillus fumigatus. Am. J. Pathol. 156723-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hogaboam, C. M., K. J. Carpenter, J. M. Schuh, and K. F. Buckland. 2005. Aspergillus and asthma: any link? Med. Mycol. 43(Suppl. 1)S197-S202. [DOI] [PubMed] [Google Scholar]
- 29.Hohl, T. M., H. L. Van Epps, A. Rivera, L. A. Morgan, P. L. Chen, M. Feldmesser, and E. G. Pamer. 2005. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog. 1e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knutsen, A. 2006. Genetic and respiratory tract risk factors for aspergillosis. Med. Mycol. 4461-70. [DOI] [PubMed] [Google Scholar]
- 31.Kurup, V. P., J. Q. Xia, R. Crameri, D. A. Rickaby, H. Y. Choi, S. Fluckiger, K. Blaser, C. A. Dawson, and K. J. Kelly. 2001. Purified recombinant Aspergillus fumigatus allergens induce different responses in mice. Clin. Immunol. 98327-336. [DOI] [PubMed] [Google Scholar]
- 32.Lauener, R. P., T. Birchler, J. Adamski, C. Braun-Fahrlander, A. Bufe, U. Herz, E. von Mutius, D. Nowak, J. Riedler, M. Waser, and F. H. Sennhauser. 2002. Expression of CD14 and Toll-like receptor 2 in farmers' and non-farmers' children. Lancet 360465-466. [DOI] [PubMed] [Google Scholar]
- 33.LeibundGut-Landmann, S., O. Gross, M. J. Robinson, F. Osorio, E. C. Slack, S. V. Tsoni, E. Schweighoffer, V. Tybulewicz, G. D. Brown, J. Ruland, and C. Reis e Sousa. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8630-638. [DOI] [PubMed] [Google Scholar]
- 34.Luther, K., A. Torosantucci, A. A. Brakhage, J. Heesemann, and F. Ebel. 2007. Phagocytosis of Aspergillus fumigatus conidia by murine macrophages involves recognition by the dectin-1 beta-glucan receptor and Toll-like receptor 2. Cell Microbiol. 9368-381. [DOI] [PubMed] [Google Scholar]
- 35.Mattila, P. E., A. E. Metz, R. R. Rapaka, L. D. Bauer, and C. Steele. 2008. Dectin-1 Fc targeting of Aspergillus fumigatus β-glucans augments innate defense against invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 521171-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehrad, B., R. M. Strieter, and T. J. Standiford. 1999. Role of TNF-alpha in pulmonary host defense in murine invasive aspergillosis. J. Immunol. 1621633-1640. [PubMed] [Google Scholar]
- 37.Mezger, M., S. Kneitz, I. Wozniok, O. Kurzai, H. Einsele, and J. Loeffler. 2008. Proinflammatory response of immature human dendritic cells is mediated by dectin-1 after exposure to Aspergillus fumigatus germ tubes. J. Infect. Dis. 197924-931. [DOI] [PubMed] [Google Scholar]
- 38.Mezger, M., M. Steffens, M. Beyer, C. Manger, J. Eberle, M. R. Toliat, T. F. Wienker, P. Ljungman, H. Hebart, H. J. Dornbusch, H. Einsele, and J. Loeffler. 2008. Polymorphisms in the chemokine (C-X-C motif) ligand 10 are associated with invasive aspergillosis after allogeneic stem-cell transplantation and influence CXCL10 expression in monocyte-derived dendritic cells. Blood 111534-536. [DOI] [PubMed] [Google Scholar]
- 39.Moss, R. B. 2005. Pathophysiology and immunology of allergic bronchopulmonary aspergillosis. Med. Mycol. 43(Suppl. 1)S203-S206. [DOI] [PubMed] [Google Scholar]
- 40.Nagai, H., J. Guo, H. Choi, and V. Kurup. 1995. Interferon-gamma and tumor necrosis factor-alpha protect mice from invasive aspergillosis. J. Infect. Dis. 1721554-1560. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura, K., A. Miyazato, G. Xiao, M. Hatta, K. Inden, T. Aoyagi, K. Shiratori, K. Takeda, S. Akira, S. Saijo, Y. Iwakura, Y. Adachi, N. Ohno, K. Suzuki, J. Fujita, M. Kaku, and K. Kawakami. 2008. Deoxynucleic acids from Cryptococcus neoformans activate myeloid dendritic cells via a TLR9-dependent pathway. J. Immunol. 1804067-4074. [DOI] [PubMed] [Google Scholar]
- 42.Nolles, G., M. O. Hoekstra, J. P. Schouten, J. Gerritsen, and H. F. Kauffman. 2001. Prevalence of immunoglobulin E for fungi in atopic children. Clin. Exp. Allergy 311564-1570. [DOI] [PubMed] [Google Scholar]
- 43.O'Driscoll, B. R., L. C. Hopkinson, and D. W. Denning. 2005. Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions. BMC Pulmon. Med. 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinckard, J. K., D. B. Rosenbluth, K. Patel, L. P. Dehner, and J. D. Pfeifer. 2003. Pulmonary hyalinizing granuloma associated with Aspergillus infection. Int. J. Surg. Pathol. 1139-42. [DOI] [PubMed] [Google Scholar]
- 45.Ramirez-Ortiz, Z. G., C. A. Specht, J. P. Wang, C. K. Lee, D. C. Bartholomeu, R. T. Gazzinelli, and S. M. Levitz. 2008. Toll-like receptor 9-dependent immune activation by unmethylated CpG motifs in Aspergillus fumigatus DNA. Infect. Immun. 762123-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roilides, E., A. Dimitriadou-Georgiadou, T. Sein, I. Kadiltsoglou, and T. J. Walsh. 1998. Tumor necrosis factor alpha enhances antifungal activities of polymorphonuclear and mononuclear phagocytes against Aspergillus fumigatus. Infect. Immun. 665999-6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romani, L. 2004. Immunity to fungal infections. Nat. Rev. Immunol. 41-23. [DOI] [PubMed] [Google Scholar]
- 48.Romani, L., and P. Puccetti. 2007. Controlling pathogenic inflammation to fungi. Expert Rev. Anti-Infect. Ther. 51007-1017. [DOI] [PubMed] [Google Scholar]
- 49.Romani, L., T. Zelante, A. De Luca, F. Fallarino, and P. Puccetti. 2008. IL-17 and therapeutic kynurenines in pathogenic inflammation to fungi. J. Immunol. 1805157-5162. [DOI] [PubMed] [Google Scholar]
- 50.Schelenz, S., D. A. Smith, and G. J. Bancroft. 1999. Cytokine and chemokine responses following pulmonary challenge with Aspergillus fumigatus: obligatory role of TNF-alpha and GM-CSF in neutrophil recruitment. Med. Mycol. 37183-194. [DOI] [PubMed] [Google Scholar]
- 51.Schnyder-Candrian, S., D. Togbe, I. Couillin, I. Mercier, F. Brombacher, V. Quesniaux, F. Fossiez, B. Ryffel, and B. Schnyder. 2006. Interleukin-17 is a negative regulator of established allergic asthma. J. Exp. Med. 2032715-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schuh, J. M., K. Blease, S. L. Kunkel, and C. M. Hogaboam. 2003. Chemokines and cytokines: axis and allies in asthma and allergy. Cytokine Growth Factor Rev. 14503-510. [DOI] [PubMed] [Google Scholar]
- 53.Steele, C., R. R. Rapaka, A. Metz, S. M. Pop, D. L. Williams, S. Gordon, J. K. Kolls, and G. D. Brown. 2005. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 1e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toyotome, T., Y. Adachi, A. Watanabe, E. Ochiai, N. Ohno, and K. Kamei. 2008. Activator protein 1 is triggered by Aspergillus fumigatus beta-glucans surface-exposed during specific growth stages. Microb. Pathog. 44141-150. [DOI] [PubMed] [Google Scholar]
- 55.Walsh, T. J., E. Roilides, K. Cortez, S. Kottilil, J. Bailey, and C. A. Lyman. 2005. Control, immunoregulation, and expression of innate pulmonary host defenses against Aspergillus fumigatus. Med. Mycol. 43(Suppl. 1)S165-S172. [DOI] [PubMed] [Google Scholar]
- 56.Willment, J. A., A. S. Marshall, D. M. Reid, D. L. Williams, S. Y. Wong, S. Gordon, and G. D. Brown. 2005. The human beta-glucan receptor is widely expressed and functionally equivalent to murine dectin-1 on primary cells. Eur. J. Immunol. 351539-1547. [DOI] [PubMed] [Google Scholar]
- 57.Yao, Z., and W. Liao. 2006. Fungal respiratory disease. Curr. Opin. Pulmon. Med. 12222-227. [DOI] [PubMed] [Google Scholar]
- 58.Zelante, T., A. De Luca, P. Bonifazi, C. Montagnoli, S. Bozza, S. Moretti, M. L. Belladonna, C. Vacca, C. Conte, P. Mosci, F. Bistoni, P. Puccetti, R. A. Kastelein, M. Kopf, and L. Romani. 2007. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur. J. Immunol. 372695-2706. [DOI] [PubMed] [Google Scholar]