Abstract
Control of bacterial colonization at mucosal surfaces depends on rapid activation of the innate immune system. Interleukin-15 (IL-15) directs the development, maturation, and function of a population of cells positive for NK1.1, such as natural killer (NK) cells, which are critical components of the innate immune defense against several viral and bacterial pathogens. Using IL-15-deficient mice, in vivo depletion of NK1.1+ cells from wild-type mice, and in vivo overexpression of IL-15 from a recombinant adenovirus, we tested the role of IL-15 and NK1.1+ cells in innate protection of the murine gut and reticuloendothelial system from Salmonella enterica serovar Typhimurium infection. IL-15 and the NK1.1+ cell population provided innate protection from serovar Typhimurium in mice at the enteric mucosae and in the reticuloendothelial system during murine typhoid. Interestingly, serovar Typhimurium extensively colonized the gut of IL-15−/− mice and wild-type C57BL/6 mice depleted of NK1.1+ cells prior to infection, even though the animals were not pretreated with antibiotics to reduce colonization resistance and there was an absence of overt inflammation in the colon and cecum. Enhanced dissemination of Salmonella from the gut of mice depleted of NK1.1+ cells correlated with a localized disruption of IL-17 in the colon. These data suggest a relationship between the gut ecosystem and the innate mucosal immune system, which may be linked via IL-15 and NK1.1+ cells.
Drug resistance continues to erode the efficacy of conventional antibiotics against many serious bacterial pathogens, contributing to a doubling of the death rate due to infectious disease in the past two decades (33). This problem is particularly germane to Salmonella enterica serovar Typhimurium infections, where multidrug resistance has been on the rise globally for almost a decade (10). These gram-negative bacteria infect humans using virulence factors that promote invasiveness and immune system avoidance (8). A notable concern is the multidrug-resistant strain of serovar Typhimurium DT104, which has emerged in North America (and globally) as a serious threat to public health due to its association with increased morbidity (31) and higher rates of death (18, 19).
One approach to bridge the widening innovation gap in anti-infectives involves harnessing innate immune defenses of the susceptible host. Although this strategy is in its infancy, the potential revolutionary impact for the treatment of infectious diseases and for improving human and animal health has called for a critical exploration of its potential (35). The thinking behind this paradigm is that immune enhancement could reduce reliance on traditional antibiotic therapies, thus preserving their useful life span and engendering a more resilient host environment for pathogenic microbes.
The vast majority of pathogens, including Salmonella, enter hosts and initiate infection at mucosal surfaces. Besides physical barriers limiting the ability of microbial pathogens to attach and transit through epithelial cells, the inducible innate immune response is a central first line of defense, comprising many cell types and cytokines, of which NK cells and interleukin-15 (IL-15) are of particular interest. NK cells are well known for their early innate contribution to antiviral defenses and their ability to kill tumor cells without prior exposure. The development, maturation, and function of these cells are dependent on IL-15, identified for its ability to stimulate proliferation of the IL-2-dependent CTLL-2 T-cell line in the presence of neutralizing anti-IL-2 antibodies (4). IL-15 is produced by a variety of myeloid and stromal cell types (2, 5, 9, 22) and is required for proper NK and NKT cell development and function. Mice lacking IL-15 or IL-15 receptor alpha subunit have no NK cells and few NKT cells (23, 29). Overexpression of IL-15 in IL-15-transgenic mice leads to an increase in NK cells and NK-cell-derived gamma interferon (IFN-γ), which is associated with resistance to tumors (47), Mycobacterium bovis (44), Listeria monocytogenes (46), and toxic shock induced by Escherichia coli (20). In humans, IL-15 is more prominently elevated in those suffering from systemic salmonellosis than in those suffering from infection localized strictly to the gastrointestinal tract (32). Previous work using a mouse model of infection showed that IL-15 was implicated in the host defense against the swine pathogen Salmonella enterica serovar Choleraesuis (21). However, an avirulent strain was used in these studies, and so it was not possible to assess the role of this cytokine in defense against virulent microbes that can actively subvert innate immunity (8). The murine model of Salmonella colitis employs an antibiotic pretreatment regimen prior to infection that produces a qualitative shift in the gut microbiota in favor of the Firmicutes and Cytophaga-Flavobacterium-Bacteroidetes phyla (41), the effect of which is reduced innate colonization resistance of the animal. The ability of Salmonella serovar Typhimurium to colonize the gut of antibiotic-pretreated animals is enhanced by the ensuing inflammatory process, leading to colitis and typhlitis (1). It is also thought that this underlying inflammation is necessary to allow serovar Typhimurium to outcompete the normal microbiota and colonize the gut to high levels (43). However, whether IL-15 and/or NK cells are important in innate protection of the nonperturbed gut (absence of antibiotic treatment) and systemic tissues following infection with virulent serovar Typhimurium has not been studied. We used a well-characterized model of murine typhoid to study the role of IL-15 and NK1.1+ cells in innate microbial defense against serovar Typhimurium. Three different experimental approaches, involving IL-15 knockout mice, in vivo depletion of NK1.1+ cells, and in vivo overexpression of IL-15, provide evidence that IL-15 and NK1.1+ cells are required for innate control of Salmonella infection in the gut and at systemic sites of infection targeted by this pathogen.
MATERIALS AND METHODS
Bacterial strains and manipulations.
Wild-type Salmonella enterica serovar Typhimurium strain SL1344 was used throughout this study. Bacteria were routinely cultured in Luria broth (LB) supplemented with streptomycin at 50 μg/ml. Prior to infection, SL1344 was cultured overnight in LB with shaking at 225 rpm. Overnight cultures were washed in 100 mM HEPES (pH 8.0)-0.9% NaCl and then resuspended in the same buffered solution.
Animal experiments.
Experiments were performed in accordance with protocols approved by the Animal Ethics Committee at McMaster University and by the Canadian Council on Animal Care. Mice were housed under specific-pathogen-free conditions in a level II barrier facility. Female C57BL/6 (B6) mice or IL-15−/− mice (Taconic) were infected orally with 0.5 × 107 to 1 × 107 CFU of serovar Typhimurium suspended in 0.1 ml of 100 mM HEPES (pH 8.0)-0.9% NaCl. At 48 to 72 h after infection, the colon, cecum, spleen, and liver were collected separately into 1 ml of sterile phosphate-buffered saline (PBS) on ice and homogenized (MixerMill 400; Retsch). Spleen and liver tissue homogenates were diluted in PBS and plated on LB agar containing streptomycin for determination of total Salmonella CFU. Tissue homogenates from the cecum and the colon were plated on bismuth sulfite agar. Plates were incubated at 37°C, and CFU were enumerated after 16 h.
NK1.1+-cell depletion experiments.
Seven- to 9-week old female C57BL/6 mice were injected intraperitoneally with 200 μg of anti-mouse NK1.1 antibody (PK136 mouse immunoglobulin G2a hybridoma HB191; ATCC) daily for 2 days prior to challenge. On day 3 after the first injection, mice were infected by oral gavage with 5 × 106 CFU serovar Typhimurium and sacrificed on day 2 postinfection. To verify NK1.1+-cell depletion, half of the spleen was used for fluorescence-activated cell sorting (FACS) analysis of splenocytes (see below) and the other half was used for bacterial load determinations. Cecum and colon tissues were also harvested for bacterial enumeration. For cytokine determination experiments, two groups of four B6 mice were depleted of NK1.1+ cells as described above and were inoculated with serovar Typhimurium or sterile buffer. As a control, two groups of B6 mice were treated identically but were left NK1.1+ cell replete. The small intestine and colon were collected at 48 h after infection, and the feces were removed. Tissues were flash frozen in liquid nitrogen at the site of surgery and then homogenized in 1 ml PBS containing protease inhibitor cocktail. Tissue homogenates were centrifuged, and murine IL-17 and IFN-γ were measured in the supernatant using a DuoSet enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems) according to the manufacturer's instructions.
In vivo expression of IL-15 using recombinant adenovirus.
A recombinant adenovirus expressing human IL-15 was generated in the parental virus, Ad-d170.3, obtained from the Robert E. Fitzhenry Vector Laboratory (Centre for Gene Therapeutics, Hamilton, Canada). Seven- to nine-week-old female C57BL/6 mice were administered 5 × 108 PFU of recombinant adenovirus (Ad-IL-15) or wild-type virus (Ad) by intravenous tail injection on days 1 and 4. Mice were challenged on day 5 with 5 × 106 CFU of serovar Typhimurium and sacrificed on day 7. For IL-15 cytokine analysis, blood samples were collected from mice at the time of sacrifice and serum was isolated after 24 h at 4°C. IL-15 levels were quantified using a hIL-15 DuoSet ELISA kit (R&D Systems) according to the manufacturer's instructions.
FACS.
Spleens were isolated from mice, and single-cell suspensions were prepared. Splenocytes were enumerated using a hemacytometer and resuspended in 0.2% bovine serum albumin-PBS for FACS analysis. Cells were plated at 1 × 106 per well in 96-well round-bottom plates. Each well was washed and blocked using CD16/CD32 antibody (eBioscience) for 15 min on ice. Cells were then washed and surface stained for 30 min on ice with phycoerythrin-conjugated anti-mouse NK1.1 antibody (clone PK136; BD Pharmingen). Stained cells (100,000 gated events) were analyzed on a FACSCanto flow cytometer. All FACS data was analyzed using the FlowJo flow cytometry analysis software program (Tree Star Inc., Ashland, OR).
Murine histopathology.
Colons and ceca from experimental animals were fixed in paraformaldehyde and transferred to 70% ethanol until processed for paraffin embedding. The samples were sectioned at 5 μm and stained with hematoxylin and eosin. For pathological scoring, six fields per sample were examined and scored as described previously to facilitate comparisons (6). This scoring system was as follows: for lumen tissue, sum of empty, score = 0; necrotic epithelial cells, scant = 1, moderate = 2, and dense = 3; and polymorphonuclear cells (PMNs), scant = 2, moderate = 3, and dense = 4; for surface epithelium, sum of no pathological change, score = 0; regenerative change, mild = 1, moderate = 2, and severe = 3; desquamation, patchy = 1, diffuse = 2; for PMNs in epithelium, score = 1; ulceration, score = 1; for mucosa, sum of no pathological change, score = 0; crypt abscesses, rare (<15%) = 1, moderate (15% to 50%) = 2, and abundant (>50%) = 3; presence of mucinous plugs = 1; and presence of granulation tissue = 1; for submucosa, sum of no pathological change = 0; mononuclear cell infiltrate, 1 small aggregate = 0, more than one aggregate = 1, and large aggregates plus increased single cells = 2; PMN infiltrate, none = 0, single = 1, and aggregates = 2; and edema, mild = 0, moderate = 1, and severe = 2.
Statistical tests.
Survival plots were analyzed using the log-rank (Mantel-Cox) test. Bacterial loads were expressed as the means with standard errors and compared between groups using a two-tailed Mann-Whitney nonparametric test. P values of <0.5 were considered significant.
RESULTS
IL-15−/− mice are more susceptible to Salmonella infection than wild-type animals.
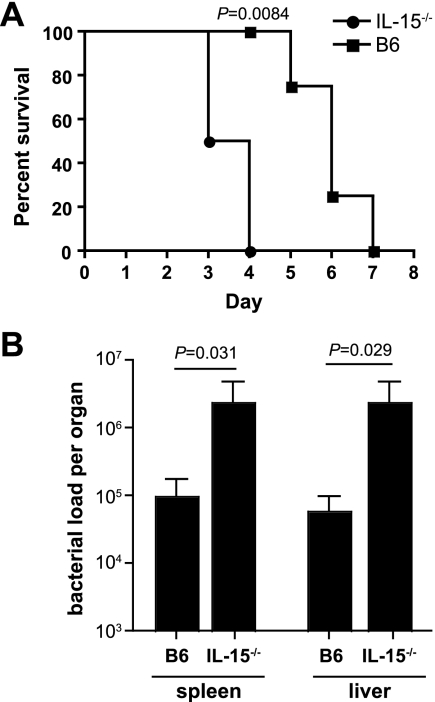
IL-15 is a pleiotropic cytokine involved in innate immune protection against some bacterial and viral infections. However, the role of IL-15 in innate protection against serovar Typhimurium infection is not known. We began our work using the well-characterized model of murine salmonellosis, which offers the ability to examine infection outcomes at both enteric and systemic sites. IL-15−/− mice and congenic wild-type mice (B6) were infected with wild-type serovar Typhimurium by oral gavage and monitored following infection. All IL-15−/− mice infected with serovar Typhimurium died by day 4 after infection (Fig. 1A), with a median survival of 3.5 days, whereas wild-type mice had a prolonged survival time after infection compared to IL-15−/− mice (P = 0.0084, log rank, Mantel-Cox). The median survival time of 6.5 days for infected B6 mice was characteristic of the infection dynamic for wild-type serovar Typhimurium, suggesting that mice deficient in IL-15 had a defect in innate control of early pathogen colonization. To test this, the bacterial load in the spleen and liver was measured 2 days after infection and was found to be ∼50 times higher in IL-15−/− mice than in B6 mice (Fig. 1B).
FIG. 1.
IL-15−/− mice are more susceptible to Salmonella infection. (A) Survival plots of wild-type mice (B6) and IL-15 knockout mice (IL-15−/−) (n = 8 per group) infected with serovar Typhimurium by the oral route (for mean survival time for IL-15−/− mice versus that for B6 mice, P = 0.0084. (B) Bacterial loads in the spleen and liver of B6 mice and IL-15−/− mice (n = 8 per group). Data are the means with standard errors from two separate experiments (B6 versus IL-15−/− mice: spleen, P = 0.031; liver, P = 0.029.
Serovar Typhimurium reaches high bacterial load in gut of IL-15-deficient mice.
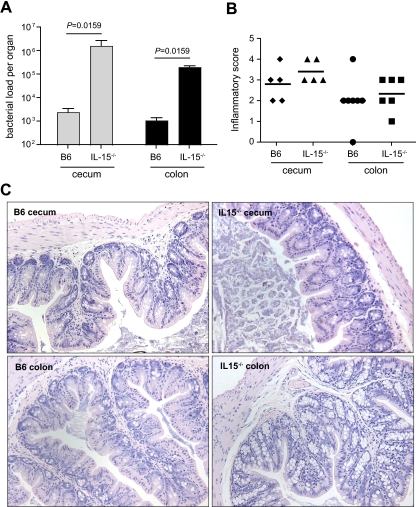
In the absence of antibiotic pretreatment, serovar Typhimurium reaches a low bacterial density in the murine cecum and colon due to innate colonization resistance and infection leads to scant inflammatory response in gut tissues (1). The increased systemic bacterial load in IL-15−/− mice prompted us to examine the role of IL-15 in innate protection at the gut mucosae. For these experiments, we orally infected specific-pathogen-free non-antibiotic-treated IL-15−/− mice and B6 mice and determined bacterial loads in the cecum and colon 48 h postinfection. In accord with previous data (1, 7), wild-type mice that were not pretreated with antibiotics prior to infection had low-level colonization of enteric tissues (Fig. 2A). In contrast, IL-15−/− mice had a ∼1,000-fold increase in the bacterial load in the cecum and colon (P = 0.0159) (Fig. 2A). This greater bacterial colonization of the gut was not accompanied by overt inflammation in either the colon or the cecum (Fig. 2B). The sum inflammatory scores were similar for both B6 mice and IL-15−/− mice in the cecum (2.0 ± 1.2 and 2.3 ± 0.8, respectively) and colon (2.8 ± 0.8 and 3.4 ± 0.5, respectively). Using a similar scoring matrix, these values were comparable to those reported previously for Salmonella-infected B6 mice not treated with antibiotic prior to infection (1). For comparison, inflammatory scores following Salmonella infection of antibiotic-pretreated animals are ∼20 using similar metrics (1, 6, 7). The overall structure of gut tissues from infected mice with extensive bacterial loads was preserved, with little to no infiltration of polymorphonuclear cells and mononuclear cells in the lumen, epithelium, and submucosa (Fig. 2C). These data indicated that IL-15 participates in innate control of Salmonella colonization of the gut and that in this genetic background, inflammation is not required for serovar Typhimurium to overcome colonization resistance as reported for wild-type animals (30, 43).
FIG. 2.
Serovar Typhimurium colonizes the gut of IL-15-deficient mice in the absence of inflammation. (A) Bacterial load in the cecum and colon of wild-type B6 mice and IL-15−/− mice at day 3 after oral infection (n = 8 per group). Data are the means with standard errors from two separate experiments (B6 versus IL-15−/− mice: cecum, P = 0.0159; liver, P = 0.0159). (B) Immunological scores in the cecum and colon from mice colonized with Salmonella serovar Typhimurium. Ceca and colons were harvested from infected mice at day 3 after infection and processed for histopathological examination as outlined in Methods. Data are aggregate scores of pathology from five tissue sections from five to seven individual mice per group. Each data point represents an individual animal and shows the scatter of data, with horizontal lines representing the means. (C) Sections of cecum and colon from infected mice stained with hematoxylin and eosin. Representative images are shown from those animals scored in panel B.
NK1.1+ cells are a major effector-cell population controlling acute salmonellosis.
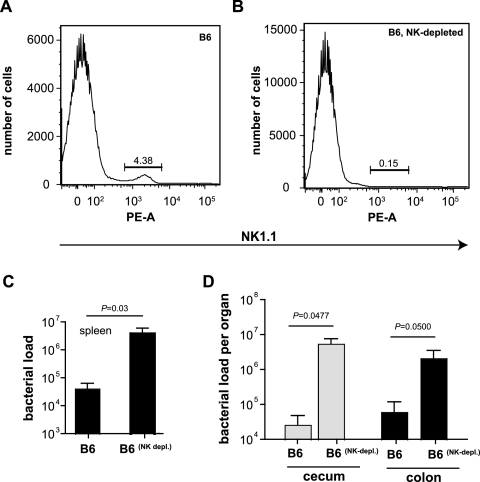
IL-15 is a key mediator of NK1.1+-cell development and function (11). NK cells are a source of IFN-γ during Salmonella infection of streptomycin-pretreated mice (17), but the role of NK1.1+ cells in protecting the murine gut without prior antibiotic treatment has not been studied. To examine the role of NK1.1+ cells in controlling early stages of serovar Typhimurium infection, we depleted NK1.1+ cells from wild-type C57BL/6 mice using a depleting monoclonal antibody and measured cecal pathology, splenic bacterial load, and colonization of the cecum and colon following oral infection. We first verified NK1.1+-cell depletion in our experimental animals by quantifying NK1.1-positive splenocytes at necropsy. FACS analysis of the splenocyte population showed an expected number of NK1.1+ cells (4.38%) from B6 mice (Fig. 3A) and a reduction of this proportion to 0.15% in NK1.1+-cell-depleted animals (Fig. 3B). To examine the role of the NK1.1+ cell population in early colonization dynamics, we quantified the bacterial load in the spleen and in the gut 48 h postinfection. Similar to what we observed for IL-15-deficient mice, depletion of NK1.1+ cells resulted in a ∼100-fold increase in numbers of Salmonella serovar Typhimurium bacteria in the spleen (Fig. 3C) and a ∼500-fold increase in numbers in both the cecum and the colon (Fig. 3D).
FIG. 3.
NK1.1+ cells are a major effector-cell group during acute salmonellosis. Analysis of NK1.1+ cells from B6 mice (A) or NK-cell-depleted mice (B). The percentage of NK1.1+ cells from the analyzed population is shown in each panel. (C) Bacterial loads in the spleens of wild-type mice (B6) and NK1.1+-cell-depleted mice infected with serovar Typhimurium for 2 days (n = 5 per group). Data are the means with standard errors from two separate experiments (P = 0.03). (D) Bacterial loads in the cecum and colon of wild-type mice (B6) or mice depleted of NK cells and then infected with Salmonella for 2 days (n = 5 per group). Data are the means with standard errors from two separate experiments (cecum, B6 versus B6NK-depleted, P = 0.0477; colon, B6 versus B6NK-depleted, P = 0.05).
Delivery of a recombinant adenovirus expressing IL-15 enhances innate resistance and delays systemic colonization by Salmonella serovar Typhimurium.
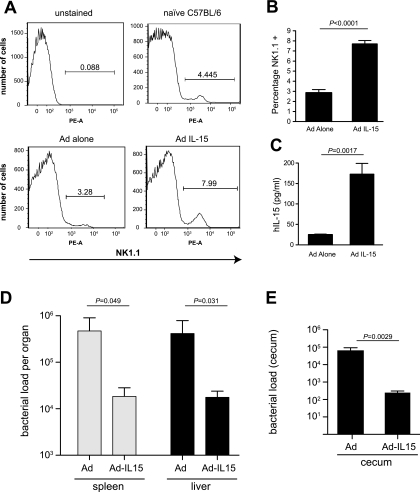
The previous experiments with IL-15−/− animals and NK1.1+-cell-depleted B6 mice provided evidence for IL-15 and NK cells in providing innate protection from Salmonella infection both in the gut and in systemic tissues. Based on this, we reasoned that overexpression of IL-15 above endogenous levels in wild-type mice should provide additional protection from early colonization by Salmonella, which it did. To do this, we delivered to mice a recombinant nonreplicating adenovirus expressing human IL-15 (Ad-IL-15) or a control virus not expressing IL-15 (Ad) and then infected the animals with Salmonella by oral gavage. As expected, delivery to mice of Ad-IL-15 but not of Ad alone increased the proportion of NK1.1-positive cells in the spleen (Fig. 4A and B) and led to enhanced serum IL-15 levels (Fig. 4C). To test whether this increased IL-15 and NK1.1-positive cell population affected bacterial colonization, we quantified the bacterial load in systemic tissues and in the gut 2 days after infection, a time point corresponding to maximal NK cell numbers and circulating IL-15. Mice treated with Ad-IL-15 had a significantly lower bacterial load in the spleen and liver at 2 days postinfection (Fig. 4D) than mice treated with the Ad control. Treatment with Ad-IL-15 prior to Salmonella infection also reduced the bacterial load in the cecum by approximately 1,000-fold, to levels that were near the limit of detection of our experimental setup (Fig. 4E). These data corroborate a role for IL-15 and NK1.1+ cells in providing innate protection from serovar Typhimurium infection at systemic and enteric sites targeted by this pathogen.
FIG. 4.
Delivery of a recombinant adenovirus expressing IL-15 enhances mucosal and systemic innate defense against colonization by serovar Typhimurium. C57BL/6 mice were administered 5 × 108 PFU of adenovirus (Ad) alone,or recombinant adenovirus expressing human IL-15 (Ad IL-15) via intravenous tail vein injection as described in Materials and Methods. Mice were challenged with serovar Typhimurium and then sacrificed on day 2 postinfection. (A) FACS analysis of splenocytes isolated from naive mice or mice receiving injections of either Ad or Ad IL-15 and stained for the NK-cell marker NK1.1. (B) Average percentages of NK1.1+ populations in Ad alone-treated and Ad-IL-15-treated mice (five mice per group) were calculated from FACS data (P < 0.0001 for NK1.1+ cells from Ad-alone-treated mice versus those from mice receiving Ad-IL-15). (C) Serum from mice treated with Ad alone or with Ad -L-15 was analyzed by ELISA for human IL-15 (hIL-15). Data shown are the means with standard errors from five mice per group (P = 0.0017). (D) Bacterial load in the spleen and liver from mice pretreated with either Ad or Ad-IL-15 prior to infection with serovar Typhimurium (n = 5 per group). Data are mean with standard errors from five mice per group (Ad alone versus Ad-IL-15: spleen, P = 0.049; liver, P = 0.031). (E) Bacterial load in the cecum from mice pretreated with either Ad or Ad-IL-15 prior to infection with serovar Typhimurium (n = 5 per group). Data are means with standard errors from five mice per group (Ad alone versus Ad-IL-15: P = 0.0029).
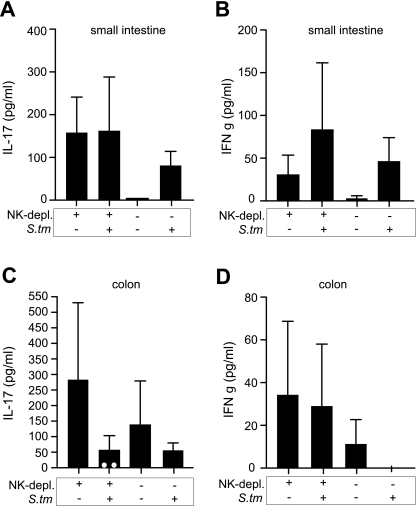
Enhanced dissemination of Salmonella in NK1.1+-cell-depleted mice correlates with blunted IL-17 response in the colon.
Previous work with rhesus macaques demonstrated that an IL-17 deficiency induced by chronic viral infection promotes dissemination of Salmonella from the gut. To test whether the enhanced dissemination of Salmonella from the gut that we observed in NK1.1+-cell-depleted B6 mice was related to IL-17, we depleted two groups of B6 mice of NK1.1+ cells and inoculated them with serovar Typhimurium or left them uninfected. Nondepleted B6 mice were used as controls and treated identically. In the absence of NK1.1+-cell depletion, Salmonella infection resulted in an increase in IL-17 and IFN-γ in the small intestine, consistent with previous data (Fig. 5A and B) (38). In mice uninfected but depleted of NK1.1+ cells, we noted a marked increase in basal IL-17 levels compared to results for control mice that were not depleted of NK1.1+ cells, suggesting that NK1.1+ cells may serve to negatively regulate IL-17 production in the gut. IL-17 protein levels in the small intestine did not change appreciably at 48 h following Salmonella infection in mice depleted of NK1.1+ cells. However, in the colon of mice depleted of NK1.1+ cells, this increase in IL-17 was normalized following infection with Salmonella (Fig. 5C) and reached undetectable levels in two infected mice. IFN-γ levels in the colon or small intestine did not correlate with bacterial loads, consistent with previous data showing that regional IFN-γ production in the gut is insufficient for control of Salmonella infection (13). Together, these data suggest that the enhanced dissemination of Salmonella from the gut of NK1.1-depleted mice may be related to a localized IL-17 deficiency in the colon following Salmonella infection.
FIG. 5.
A marked decrease in colon IL-17 following infection of NK1.1-depleted mice correlates with enhanced bacterial dissemination from the gut. Two groups of B6 mice were depleted of NK1.1 cells and then infected orally with Salmonella or left uninfected. Two groups of control mice were not depleted of NK1.1 cells but otherwise were treated identically. At 48 h following infection, IL-17 levels (A and C) or IFN-γ levels (B and D) were measured in the small intestine and colon using an ELISA. Data are the means with standard errors. White circles in panel C denote two animals with undetectable levels of IL-17.
DISCUSSION
With the steady increase in bacterial resistance to current antibiotics, modulation of innate immunity is viewed as a promising approach to the development of antimicrobial therapies (35, 12). Toll-like-receptor agonists and cationic host defense peptides have already shown promise as agents of protection in animal models of human infectious diseases (14, 15, 40). Alternatively, direct expansion of the effector-cell population responsible for innate protection stands to offer similar advantages (26). Tackling these issues with bacterial pathogens is challenging, because these microbes have evolved strategies to subvert innate host defenses (8). Animal models capable of capturing the nuances of both the microbial and host elements of this dynamic interaction are therefore essential. Our interest in this area relates to the potential role of infectious agents in the pathobiology of inflammatory bowel disease, particularly the contribution of enteric bacterial pathogens. By using knockout animals, in vivo depletion of immune cells, and targeted overexpression of IL-15 from a recombinant adenovirus, we have shown that IL-15 and the population of NK1.1+ cells are key mediators of innate defense against serovar Typhimurium at mucosal surfaces and systemic sites targeted by this pathogen.
IL-15 is implicated in the pathogenesis of human inflammatory bowel diseases (IBDs), such as Crohn's disease, ulcerative colitis, and celiac disease. A greater percentage of peripheral blood mononuclear cells from patients with Crohn's disease or ulcerative colitis produced IL-15 than was the case with normal donors, which was further augmented in vitro following lipopolysaccharide stimulation (25). Similarly, IL-15 was detected in inflamed mucosal biopsies from IBD patients but not from controls (27, 39), supporting the hypothesis that IL-15-mediated processes may underlie the chronic inflammatory conditions of IBD. The finding that IL-15 contributed to murine intestinal inflammation induced by the chemical stimulant dextran sodium sulfate was consistent with this hypothesis (48), leading to the suggestion that antagonizing IL-15 may improve IBD outcomes. However, our data may moderate this idea, because although removal of IL-15 from the system can ameliorate the symptoms of chronic colitis by reducing inflammation (48), it may in fact bring about an increased susceptibility to infectious disease at the enteric mucosal surface. This work supports a model wherein IL-15 and NK1.1+-cell defense systems participate in innate resistance to serovar Typhimurium in the gut. While it is likely that NK cells are the subset of NK1.1+ cells participating in this innate pathway due to their increased numbers during Salmonella infection relative to NKT cells (3, 24), we cannot exclude at this point the involvement of NKT cells or NK1.1+ αβ T cells, which may participate in an IL-4-IL-12 axis via macrophages (34).
The mechanism of enhanced dissemination of Salmonella serovar Typhimurium from the gut to systemic tissues is an area of interest and appears to involve both bacterial (45) and host (38) factors. In antibiotic-pretreated Il17ra−/− mice, Salmonella dissemination from the gut to systemic tissues is enhanced (38). In mice with a nonperturbed commensal microbiota used in our experiments, a local IL-17 deficiency in the colon was noted following Salmonella infection of NK1.1+-cell-depleted animals and reached undetectable levels in two animals in the group. Although the group mean IL-17 level in the colon was similar to that of infected, nondepleted animals, the overall change in colon IL-17 levels following infection was more pronounced in mice depleted of NK1.1+ cells, which may account for the enhanced dissemination of Salmonella from the gut in these animals. In the gut, IFN-γ levels were generally low and did not correlate with bacterial load or enhanced dissemination of infection, which is consistent with data that systemic IFN-γ (36) but not local IFN-γ in the gut (13) correlated with enhanced control of Salmonella infection. The source of this IFN-γ in animals depleted of NK1.1+ cells is likely the population of intraepithelial lymphocytes, although additional work is required to establish this and to understand the interplay between NK1.1+ cells and IL-17 production in the gut following Salmonella infection. Interestingly, we saw a marked increase in IL-17 in the colon and small intestine of mice depleted of NK1.1+ cells. A recent report noted that NK cell depletion exacerbated experimental collagen-induced arthritis in mice and that NK cell depletion increased the frequency of IL-17-secreting cells in draining lymph nodes (28). These data suggest a negative regulatory function for NK cells in modulating IL-17 levels during arthritis, and a similar relationship between NK cells and IL-17 may also exist in the gut based on our depletion experiments.
Innate colonization resistance is thought to result from the dense microbial community residing in the gut, providing competing conditions for invading pathogens (42). However, the molecular basis of colonization resistance is still poorly understood. Previous work revealed that manipulating the ecology of the gut with antibiotics prior to infection leads to a loss of colonization resistance, high bacterial loads of Salmonella in the cecum and colon, and colitis (1, 7, 16). More recently it has been suggested that inflammation is necessary and sufficient for enteropathogenic bacteria to successfully compete with the microbiota and overcome colonization resistance (43). However, Salmonella serovar Typhimurium colonized the gut of IL-15−/− mice and wild-type mice depleted of NK1.1+ cells just prior to infection in the absence of inflammation. Although evidence from the literature supports a link between the microbiota and innate defenses in maintaining intestinal homeostasis and ergo innate resistance mechanisms, the details of this cross talk are not fully understood. Of relevance, the probiotic Lactobacillus casei elicited murine and human NK cell activities and augmented IL-15 gene expression in mucosal epithelial cells (37), suggesting that the microbiota can influence NK cell and IL-15 activities. The relationship between the microbiota and the IL-15-directed innate defenses reported here is the subject of follow-up studies ongoing in our laboratory. In light of the protective role we have identified for IL-15 and NK1.1+ cells following acute serovar Typhimurium infection, further studies to quantify the cytokine networks and cellular components of the inflammatory response to infectious triggers following chemical or genetic perturbation in the gut are warranted. Our work here has identified two new candidates, IL-15 and NK1.1-positive cells, that would be expected to provide innate protection in these models.
Acknowledgments
We thank members of the Coombes and Ashkar laboratories for helpful discussions on this work.
This research was funded by operating grants to B.K.C. from the Canadian Institutes of Health Research (CIHR; MOP-82704) and to A.A.A. from the CIHR. A.A.A. is a recipient of a Career Award in Health Sciences from Rx&D/CIHR. B.K.C. is the recipient of a New Investigator Award from the CIHR (MSH-83721), a Young Investigator Award from the American Society for Microbiology, and an Early Researcher Award from the Ontario Ministry of Research and Innovation.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 17 November 2008.
REFERENCES
- 1.Barthel, M., S. Hapfelmeier, L. Quintanilla-Martinez, M. Kremer, M. Rohde, M. Hogardt, K. Pfeffer, H. Russmann, and W. D. Hardt. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 712839-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blauvelt, A., H. Asada, V. Klaus-Kovtun, D. J. Altman, D. R. Lucey, and S. I. Katz. 1996. Interleukin-15 mRNA is expressed by human keratinocytes, Langerhans cells, and blood-derived dendritic cells and is downregulated by ultraviolet B radiation. J. Investig. Dermatol. 1061047-1052. [DOI] [PubMed] [Google Scholar]
- 3.Brigl, M., L. Bry, S. C. Kent, J. E. Gumperz, and M. B. Brenner. 2003. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat. Immunol. 41230-1237. [DOI] [PubMed] [Google Scholar]
- 4.Carson, W. E., J. G. Giri, M. J. Lindemann, M. L. Linett, M. Ahdieh, R. Paxton, D. Anderson, J. Eisenmann, K. Grabstein, and M. A. Caligiuri. 1994. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 1801395-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carson, W. E., M. E. Ross, R. A. Baiocchi, M. J. Marien, N. Boiani, K. Grabstein, and M. A. Caligiuri. 1995. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J. Clin. Investig. 962578-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coburn, B., Y. Li, D. Owen, B. A. Vallance, and B. B. Finlay. 2005. Salmonella enterica serovar Typhimurium pathogenicity island 2 is necessary for complete virulence in a mouse model of infectious enterocolitis. Infect. Immun. 733219-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coombes, B. K., B. A. Coburn, A. A. Potter, S. Gomis, K. Mirakhur, Y. Li, and B. B. Finlay. 2005. Analysis of the contribution of Salmonella pathogenicity islands 1 and 2 to enteric disease progression using a novel bovine ileal loop model and a murine model of infectious enterocolitis. Infect. Immun. 737161-7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coombes, B. K., Y. Valdez, and B. B. Finlay. 2004. Evasive maneuvers by secreted bacterial proteins to avoid innate immune responses. Curr. Biol. 14R856-R867. [DOI] [PubMed] [Google Scholar]
- 9.Doherty, T. M., R. A. Seder, and A. Sher. 1996. Induction and regulation of IL-15 expression in murine macrophages. J. Immunol. 156735-741. [PubMed] [Google Scholar]
- 10.European Commission. 2007. Surveillance of enteric pathogens in Europe and beyond: international surveillance network for the enteric infections—Salmonella, VTEC O157 and Campylobacter. 1786/2002/EC. European Commission, Brussels, Belgium. http://ecdc.europa.eu/en/files/pdf/Activities/ENTER_NET/annual_report2005.pdf.
- 11.Fehniger, T. A., and M. A. Caligiuri. 2001. Interleukin 15: biology and relevance to human disease. Blood 9714-32. [DOI] [PubMed] [Google Scholar]
- 12.Finlay, B. B., and R. E. Hancock. 2004. Can innate immunity be enhanced to treat microbial infections? Nat. Rev. Microbiol. 2497-504. [DOI] [PubMed] [Google Scholar]
- 13.Gajendran, N., H. W. Mittrucker, K. Bordasch, E. Heinemann, M. Koch, and S. H. Kaufmann. 2007. Regional IFN-gamma expression is insufficient for efficacious control of food-borne bacterial pathogens at the gut epithelial barrier. Int. Immunol. 191075-1081. [DOI] [PubMed] [Google Scholar]
- 14.Gill, N., P. M. Deacon, B. Lichty, K. L. Mossman, and A. A. Ashkar. 2006. Induction of innate immunity against herpes simplex virus type 2 infection via local delivery of Toll-like receptor ligands correlates with beta interferon production. J. Virol. 809943-9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hancock, R. E., and H. G. Sahl. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 241551-1557. [DOI] [PubMed] [Google Scholar]
- 16.Hapfelmeier, S., K. Ehrbar, B. Stecher, M. Barthel, M. Kremer, and W. D. Hardt. 2004. Role of the Salmonella pathogenicity island 1 effector proteins SipA, SopB, SopE, and SopE2 in Salmonella enterica subspecies 1 serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun. 72795-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrington, L., C. V. Srikanth, R. Antony, H. N. Shi, and B. J. Cherayil. 2007. A role for natural killer cells in intestinal inflammation caused by infection with Salmonella enterica serovar Typhimurium. FEMS Immunol. Med. Microbiol. 51372-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helms, M., S. Ethelberg, and K. Molbak. 2005. International Salmonella Typhimurium DT104 infections, 1992-2001. Emerg. Infect. Dis. 11859-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helms, M., P. Vastrup, P. Gerner-Smidt, and K. Molbak. 2002. Excess mortality associated with antimicrobial drug-resistant Salmonella typhimurium. Emerg. Infect. Dis. 8490-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiromatsu, T., T. Yajima, T. Matsuguchi, H. Nishimura, W. Wajjwalku, T. Arai, Y. Nimura, and Y. Yoshikai. 2003. Overexpression of interleukin-15 protects against Escherichia coli-induced shock accompanied by inhibition of tumor necrosis factor-alpha-induced apoptosis. J. Infect. Dis. 1871442-1451. [DOI] [PubMed] [Google Scholar]
- 21.Hirose, K., H. Nishimura, T. Matsuguchi, and Y. Yoshikai. 1999. Endogenous IL-15 might be responsible for early protection by natural killer cells against infection with an avirulent strain of Salmonella choleraesuis in mice. J. Leukoc. Biol. 66382-390. [DOI] [PubMed] [Google Scholar]
- 22.Jonuleit, H., K. Wiedemann, G. Muller, J. Degwert, U. Hoppe, J. Knop, and A. H. Enk. 1997. Induction of IL-15 messenger RNA and protein in human blood-derived dendritic cells: a role for IL-15 in attraction of T cells. J. Immunol. 1582610-2615. [PubMed] [Google Scholar]
- 23.Kennedy, M. K., M. Glaccum, S. N. Brown, E. A. Butz, J. L. Viney, M. Embers, N. Matsuki, K. Charrier, L. Sedger, C. R. Willis, K. Brasel, P. J. Morrissey, K. Stocking, J. C. Schuh, S. Joyce, and J. J. Peschon. 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191771-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirby, A. C., U. Yrlid, and M. J. Wick. 2002. The innate immune response differs in primary and secondary Salmonella infection. J. Immunol. 1694450-4459. [DOI] [PubMed] [Google Scholar]
- 25.Kirman, I., and O. H. Nielsen. 1996. Increased numbers of interleukin-15-expressing cells in active ulcerative colitis. Am. J. Gastroenterol. 911789-1794. [PubMed] [Google Scholar]
- 26.Lauzon, N. M., F. Mian, R. MacKenzie, and A. A. Ashkar. 2006. The direct effects of Toll-like receptor ligands on human NK cell cytokine production and cytotoxicity. Cell. Immunol. 241102-112. [DOI] [PubMed] [Google Scholar]
- 27.Liu, Z., K. Geboes, S. Colpaert, G. R. D'Haens, P. Rutgeerts, and J. L. Ceuppens. 2000. IL-15 is highly expressed in inflammatory bowel disease and regulates local T cell-dependent cytokine production. J. Immunol. 1643608-3615. [DOI] [PubMed] [Google Scholar]
- 28.Lo, C. K., Q. L. Lam, L. Sun, S. Wang, K. H. Ko, H. Xu, C. Y. Wu, B. J. Zheng, and L. Lu. 2008. Natural killer cell degeneration exacerbates experimental arthritis in mice via enhanced interleukin-17 production. Arthritis Rheum. 582700-2711. [DOI] [PubMed] [Google Scholar]
- 29.Lodolce, J. P., D. L. Boone, S. Chai, R. E. Swain, T. Dassopoulos, S. Trettin, and A. Ma. 1998. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 9669-676. [DOI] [PubMed] [Google Scholar]
- 30.Lupp, C., M. L. Robertson, M. E. Wickham, I. Sekirov, O. L. Champion, E. C. Gaynor, and B. B. Finlay. 2007. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2119-129. [DOI] [PubMed] [Google Scholar]
- 31.Martin, L. J., M. Fyfe, K. Dore, J. A. Buxton, F. Pollari, B. Henry, D. Middleton, R. Ahmed, F. Jamieson, B. Ciebin, S. A. McEwen, and J. B. Wilson. 2004. Increased burden of illness associated with antimicrobial-resistant Salmonella enterica serotype typhimurium infections. J. Infect. Dis. 189377-384. [DOI] [PubMed] [Google Scholar]
- 32.Mizuno, Y., H. Takada, A. Nomura, C. H. Jin, H. Hattori, K. Ihara, T. Aoki, K. Eguchi, and T. Hara. 2003. Th1 and Th1-inducing cytokines in Salmonella infection. Clin. Exp. Immunol. 131111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morens, D. M., G. K. Folkers, and A. S. Fauci. 2004. The challenge of emerging and re-emerging infectious diseases. Nature 430242-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naiki, Y., H. Nishimura, T. Kawano, Y. Tanaka, S. Itohara, M. Taniguchi, and Y. Yoshikai. 1999. Regulatory role of peritoneal NK1.1+ alpha beta T cells in IL-12 production during Salmonella infection. J. Immunol. 1632057-2063. [PubMed] [Google Scholar]
- 35.National Academy of Sciences. 2006. Treating infectious diseases in a microbial world: report on two workshops on novel antimicrobial therapeutics. National Academy of Sciences 0-309-65490-4. National Academies Press, Washington, DC. [PubMed]
- 36.Nishimura, H., M. Tagaya, H. Tsunobuchi, H. Suzuki, I. Nakashima, and Y. Yoshikai. 2001. Mice lacking interleukin-2 (IL-2)/IL-15 receptor beta chain are susceptible to infection with avirulent Salmonella enterica subsp. enterica serovar Choleraesuis, but mice lacking IL-2 are resistant. Infect. Immun. 691226-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogawa, T., Y. Asai, R. Tamai, Y. Makimura, H. Sakamoto, S. Hashikawa, and K. Yasuda. 2006. Natural killer cell activities of synbiotic Lactobacillus casei ssp. casei in conjunction with dextran. Clin. Exp. Immunol. 143103-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raffatellu, M., R. L. Santos, D. E. Verhoeven, M. D. George, R. P. Wilson, S. E. Winter, I. Godinez, S. Sankaran, T. A. Paixao, M. A. Gordon, J. K. Kolls, S. Dandekar, and A. J. Baumler. 2008. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat. Med. 14421-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakai, T., K. Kusugami, H. Nishimura, T. Ando, T. Yamaguchi, M. Ohsuga, K. Ina, A. Enomoto, Y. Kimura, and Y. Yoshikai. 1998. Interleukin 15 activity in the rectal mucosa of inflammatory bowel disease. Gastroenterology 1141237-1243. [DOI] [PubMed] [Google Scholar]
- 40.Scott, M. G., E. Dullaghan, N. Mookherjee, N. Glavas, M. Waldbrook, A. Thompson, A. Wang, K. Lee, S. Doria, P. Hamill, J. J. Yu, Y. Li, O. Donini, M. M. Guarna, B. B. Finlay, J. R. North, and R. E. Hancock. 2007. An anti-infective peptide that selectively modulates the innate immune response. Nat. Biotechnol. 25465-472. [DOI] [PubMed] [Google Scholar]
- 41.Sekirov, I., N. M. Tam, M. Jogova, M. L. Robertson, Y. Li, C. Lupp, and B. B. Finlay. 2008. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect. Immun. 764726-4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stecher, B., and W. D. Hardt. 2008. The role of microbiota in infectious disease. Trends Microbiol. 16107-114. [DOI] [PubMed] [Google Scholar]
- 43.Stecher, B., R. Robbiani, A. W. Walker, A. M. Westendorf, M. Barthel, M. Kremer, S. Chaffron, A. J. Macpherson, J. Buer, J. Parkhill, G. Dougan, C. von Mering, and W. D. Hardt. 2007. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 52177-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Umemura, M., H. Nishimura, K. Hirose, T. Matsuguchi, and Y. Yoshikai. 2001. Overexpression of IL-15 in vivo enhances protection against Mycobacterium bovis bacillus Calmette-Guerin infection via augmentation of NK and T cytotoxic 1 responses. J. Immunol. 167946-956. [DOI] [PubMed] [Google Scholar]
- 45.Worley, M. J., G. S. Nieman, K. Geddes, and F. Heffron. 2006. Salmonella typhimurium disseminates within its host by manipulating the motility of infected cells. Proc. Natl. Acad. Sci. USA USA 10317915-17920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yajima, T., H. Nishimura, R. Ishimitsu, K. Yamamura, T. Watase, D. H. Busch, E. G. Pamer, H. Kuwano, and Y. Yoshikai. 2001. Memory phenotype CD8(+) T cells in IL-15 transgenic mice are involved in early protection against a primary infection with Listeria monocytogenes. Eur. J. Immunol. 31757-766. [DOI] [PubMed] [Google Scholar]
- 47.Yajima, T., H. Nishimura, W. Wajjwalku, M. Harada, H. Kuwano, and Y. Yoshikai. 2002. Overexpression of interleukin-15 in vivo enhances antitumor activity against MHC class I-negative and -positive malignant melanoma through augmented NK activity and cytotoxic T-cell response. Int. J. Cancer 99573-578. [DOI] [PubMed] [Google Scholar]
- 48.Yoshihara, K., T. Yajima, C. Kubo, and Y. Yoshikai. 2006. Role of interleukin 15 in colitis induced by dextran sulphate sodium in mice. Gut 55334-341. [DOI] [PMC free article] [PubMed] [Google Scholar]