Abstract
Mannheimia haemolytica, a commensal organism of the upper respiratory tract in cattle, is the principal bacterial pathogen associated with the bovine respiratory disease complex. Adherence to the respiratory mucosa is a crucial event in its pathogenesis. However, the bacterial components that contribute to this process are not fully characterized. In this study, we demonstrated that M. haemolytica adhered to bovine bronchial epithelial cells (BBEC) in vitro and that adherence was inhibited by anti-M. haemolytica antibody. Western blot analysis of M. haemolytica proteins that bind to BBEC showed a dominant protein band with an apparent molecular mass of ∼30 kDa. Peptide sequences for the 30-kDa BBEC-binding proteins, as determined by liquid chromatography-tandem mass spectrometry, matched two M. haemolytica surface proteins: heat-modifiable outer membrane protein A (OmpA) and lipoprotein 1 (Lpp1). Western blotting showed that the 30-kDa protein band is recognized by both anti-M. haemolytica OmpA and anti-Lpp1 antibodies. Furthermore, incubation with anti-OmpA and anti-Lpp1 antibodies significantly inhibited M. haemolytica binding to BBEC monolayers. In summary, these results suggest that OmpA and Lpp1 contribute to adherence of M. haemolytica to bovine respiratory epithelial cells.
Mannheimia haemolytica is the principal bacterial pathogen of the bovine respiratory disease complex (40, 54). The bacteria colonize the nasal cavity and tonsillar crypts in healthy cattle (18, 54). However, following stress or viral infection, M. haemolytica bacteria rapidly increase in number and descend into the lungs, leading to acute pneumonia (18, 19). It is still not clear what mechanisms allow M. haemolytica to transform from a commensal organism to a pathogen that escapes clearance from the respiratory tract and invades the lung.
The adherence of respiratory pathogens to the mucosal epithelium is a critical step in host colonization and infection (1). Previous studies demonstrated the ability of M. haemolytica to adhere to epithelial cells in vitro (7, 20, 52) and the mucosal surface of respiratory tissue explants (8, 34). M. haemolytica produces several surface components that potentially can contribute to adherence. Fimbriae and glycocalyx were identified on M. haemolytica cells grown in culture and on bacteria associated with tracheal tissues isolated from experimentally infected cows (33, 34). However, the role of these structures in M. haemolytica adhesion has never been investigated. Similar to other gram-negative bacteria, M. haemolytica produces major outer membrane protein A (OmpA) (31, 55). This protein is also referred to as heat-modifiable outer membrane protein (OMP) or, in M. haemolytica, PomA (2, 31, 55). OmpA participates in specific binding to cell receptors and mediates adherence of several pathogenic bacteria to host cells (4, 12, 45, 46, 51). Based on sequence homology, it has been suggested that the OmpA of M. haemolytica might play a role in colonization of the respiratory tracts of cattle and sheep (13). M. haemolytica cells also express a high-molecular-weight protein, similar to the high-molecular-weight and Hia adhesin proteins of Haemophilus influenzae, but its functional activity and interaction with target cells have not been reported (30). A 68-kDa adhesin with hemagglutinating activity for rabbit erythrocytes specifically recognizes glycoproteins containing N-acetyloglucosamine (GlcNAc) on bovine neutrophils and tracheal epithelial cells (14, 23). Binding of this 68-kDa adhesin to bovine neutrophils induces an oxidative burst (14).
Genome sequence analysis of M. haemolytica revealed a wide repertoire of putative adhesins similar to those characterized in other respiratory pathogens (21). These include filamentous hemagglutinin FhaB, as described in Bordetella pertussis, two trimeric autotransporter adhesins resembling those of H. influenzae and Neisseria meningitidis, and proteins with dual immunoglobulin protease/adhesin function that have orthologues in H. influenzae and N. meningitidis. It is not known whether these proteins are expressed by M. haemolytica cells or play any role in its adhesion to bovine respiratory epithelial cells.
In the present study, we sought to identify M. haemolytica proteins that interact with bovine bronchial epithelial cells (BBEC) and are involved in bacterial adherence. For this purpose, biotinylated M. haemolytica surface proteins were analyzed for binding to BBEC monolayers. We identified at least two 30-kDa proteins, heat-modifiable OmpA and lipoprotein 1 (Lpp1), which associate with BBEC and are candidate adhesins. We also demonstrate for the first time the utility of using fixed monolayers of epithelial cells for affinity purification of bacterial adhesins.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
M. haemolytica A1 (isolated from a pneumonic bovine lung) was kindly provided by R. E. Briggs (Ames, IA). Bacteria were incubated without shaking in brain heart infusion (BHI) broth (Difco Laboratories) at 37°C. Escherichia coli DH5α (Invitrogen) was used for both plasmid generation and recombinant protein expression. For cloning experiments, E. coli cultures were grown in Luria-Bertani medium (43) supplemented with 100 μg/ml of ampicillin. For recombinant protein expression, the bacteria were grown in BHI broth supplemented with 100 μg/ml of ampicillin.
Primary BBEC.
Primary BBEC were kindly provided by D. S. Allen-Gipson (Omaha, NE). BBEC were maintained at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium/F-12 medium (Mediatech, Inc.) supplemented with 10% heat-inactivated fetal bovine serum, 2 mM glutamine, and Pen/Strep (Sigma), for up to 12 to 14 passages.
FITC labeling of bacteria.
Overnight cultures of M. haemolytica were centrifuged and washed with phosphate-buffered saline (PBS). The bacteria (∼2 × 109) were added to 10 ml of 0.01% fluorescein isothiocyanate (FITC; Sigma) in 0.2 M Na2CO3/NaHCO3 buffer, pH 9.6, and incubated on ice for 15 min. Bacterial cells were then washed and resuspended in RPMI medium at a final concentration of 1 × 108 CFU/ml.
Fluorescence microscopy.
Confluent BBEC monolayers in 24-well tissue culture plates were washed with RPMI medium and incubated with FITC-labeled M. haemolytica at a multiplicity of infection (MOI) of 100 bacterial cells per epithelial cell (MOI of 100:1) for 2 h at 37°C in 5% CO2. Unbound bacteria were removed by five washes with RPMI medium. Cell-associated bacteria were visualized by fluorescence microscopy using an Olympus IX70 microscope (Olympus). In some experiments, FITC-labeled M. haemolytica (2.5 ×107) was preincubated for 45 min at room temperature with a 1:100 dilution of rabbit polyclonal anti-M. haemolytica antibody (a generous gift from R. Y. C Lo, Guelph, Canada) or normal rabbit serum (Cappel Laboratories) before bacteria were added to BBEC.
Adhesion and invasion assay.
Confluent BBEC monolayers in 24-well tissue culture plates (approximately 2.5 ×105 cells per well) were washed with RPMI medium and incubated with M. haemolytica (MOI of 100:1) for 2 h at 37°C in 5% CO2 or at 4°C without 5% CO2. The epithelial cells were then washed five times with RPMI medium and lysed with 1% saponin (Sigma) in RPMI medium for 20 min at room temperature to release adherent bacteria. The number of CFU in each well was quantified by plating serial dilutions of cell lysates on blood agar plates. In some experiments, binding of E. coli DH5α to BBEC was analyzed as a control in a similar manner. To analyze whether internalization of M. haemolytica occurred, we performed a gentamicin protection assay. Briefly, BBEC monolayers were incubated with M. haemolytica (MOI of 100:1) for 1 and 3 h at 37°C in 5% CO2. After five washes with RPMI medium, epithelial cells were incubated for an additional 1 h with 100 μg/ml gentamicin. BBEC were then washed three times with RPMI medium to remove antibiotic, lysed as described above, and plated on blood agar plates to enumerate the CFU of bacteria.
Biotinylation of bacterial surface proteins.
An overnight culture of M. haemolytica (200 ml) was centrifuged and washed with PBS. The bacteria were mixed with 2 ml of biotinamidocaproate-N-hydroxysuccinimide ester (Sigma) in PBS (0.025%, wt/vol, solution) and incubated at room temperature for 1 h with gentle shaking. The bacterial cells were collected by centrifugation, washed three times, and resuspended in 1 ml of PBS. The bacterial cells were sonicated three time (45 s each) on ice, using a Vibra Cell sonicator (Sonics and Materials, Inc.), and centrifuged for 10 min at 10,000 × g. The protein concentration in the resulting supernatants was determined using a bicinchoninic acid assay (Pierce).
Isolation of OMPs.
M. haemolytica OMPs were isolated as described by Shin et al. (46) with minor modifications. Briefly, a bacterial culture (1 liter) was harvested by centrifugation and exposed to two freeze-thaw cycles on dry ice. The bacterial pellet was resuspended in 20 ml of 10 mM HEPES, pH 7.4, containing lysozyme (5 mg/ml; Pierce), DNase I (40 μg/ml; Roche), and RNase A (100 μg/ml; Sigma) and then incubated at 4°C for 30 min. Cells were disrupted by sonication (three times for 1 min each time) on ice, using a Vibra Cell sonicator, in the presence of a protease inhibitor cocktail (1:20; Sigma). After centrifugation (6,500 × g, 5 min), the bacterial cell lysate was subjected to ultracentrifugation at 25,000 rpm in an SW28 rotor (Beckman Coulter) at 15°C for 1 h. The pellet containing the crude cell envelope was washed with cold PBS and extracted with 5 ml of 2% Sarkosyl (N-lauroylsarcosine; in PBS) for 30 min at room temperature. Sarkosyl-insoluble outer membrane fractions were pelleted at 30,000 rpm in an SW41 rotor for 1 h and solubilized in 10 mM HEPES (pH 7.4), containing 0.7% n-octyl-glucoside (Research Products International Corp.). In some experiments, isolated OMPs were biotinylated according to the method described by Duk et al. (17), with minor modifications. Briefly, 200 μg of OMP resuspended in 250 μl of PBS containing 0.7% n-octyl-glucoside was mixed with 400 of μl 0.025% solution of biotinamidocaproate-N-hydroxysuccinimide ester (Sigma) and incubated for 1 h at room temperature. The proteins were dialysed for several hours against PBS at 4°C.
Binding of biotinylated proteins to BBEC monolayers.
BBEC growing in six-well plates were washed with PBS and incubated with bacterial lysates containing biotinylated surface proteins (100 to 200 μg per well) or with biotinylated OMPs (30 μg per well) for 1.5 h at room temperature. The cell monolayers were washed five time with PBS and then lysed with 150 μl of mammalian protein extraction reagent (M-PER; Pierce). Total proteins were mixed with Laemmli loading buffer and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 4 to 20% gradient gel. Proteins were blotted onto nitrocellulose (Bio-Rad) and probed with a 1:10,000 dilution of horseradish peroxidase (HRP)-conjugated anti-biotin antibody (Sigma) or a 1:250 dilution of rabbit polyclonal anti-M. haemolytica antibody, followed by 1:3,000 dilution of HRP-conjugated anti-rabbit antibody (Santa Cruz Biotechnology, Inc.). The ESL Western Chemiluminescence Reagent Plus (Pierce) was used to visualize proteins. In separate experiments, binding of biotinylated bacterial proteins to fixed BBEC monolayers was evaluated. BBEC were treated with Karnovsky's fixative (mixture 2, containing 2% paraformaldehyde, 2.5% glutaraldehyde, and 0.1 M sodium phosphate buffer; Electron Microscopy Sciences) for 10 min at room temperature and then washed two times with PBS before they were incubated with biotinylated M. haemolytica proteins as described above.
Isolation and identification of BBEC-binding proteins.
BBEC monolayers in T75 flasks were washed with PBS and fixed with Karnovsky's fixative (mixture 2; Electron Microscopy Sciences) for 10 min at room temperature. The fixed cells were washed two times with PBS and incubated overnight with the M. haemolytica OMP fraction (200 μg/ml) at 4°C. Unbound proteins were removed by washing five times with PBS. Bound proteins were eluted from cell monolayers using M-PER (Pierce) and concentrated using a Centricon YM-10 column (Millipore). The proteins (20 μg) were mixed with SDS boiling buffer (5% SDS, 10% glycerol, 60 mM Tris, pH 6.8, 5% mercaptoethanol) and subjected to two-dimensional electrophoresis (2-DE; Kendrick Labs, Inc., Madison, WI). The gel was stained with Coomassie blue, dried between sheets of cellophane, and digitally scanned. The proteins of interest (30 kDa) were excised from the Coomassie blue-stained gels and submitted for liquid chromatography (LC)-electrospray ionization tandem mass spectrometry (MS/MS) analysis (Protein Core Facility of the Columbia University Medical Center, New York, NY). Analysis of MS/MS spectral data was performed using Mascot software (http://www.matrixscience.com).
Alternatively, fixed BBEC monolayers were incubated with M. haemolytica whole-cell lysates in PBS (1 mg/ml). Bacterial proteins bound to BBEC were eluted using 1× Laemmli buffer and concentrated as described above. The proteins were separated by SDS-PAGE in 4 to 20% gradient gels and stained with Coomassie blue or transferred into nitrocellulose and probed with bovine polyclonal anti-M. haemolytica OmpA (a generous gift from A. W. Confer, Stillwater, OK) or chicken anti-M. haemolytica rLpp1 antibodies.
Plasmid construction.
The M. haemolytica lpp1 gene was amplified by PCR from total M. haemolytica genomic DNA using the following pair of primers: lpp5′, 5′-GGCGCATGCCATGAGTTTCAAGAAAATTTTAG-3′; and lpp3′, 5′-GTTCCGCGGCCAACCTTTTACTACAC-3′. The PCR product was cloned into the dsRed vector (Clontech), in which the dsRed gene was replaced with a DNA segment encoding antigen V5 and a His tag (amplified previously from the pcDNA3.1 vector [Invitrogen]) (unpublished data). Standard recombinant DNA methods (43) or procedures specified by the manufacturer were used. The resulting plasmid, designated lpp1/V5/His-dsRed, was verified by DNA sequencing with appropriate vector-specific primers.
Expression and purification of recombinant Lpp1.
E. coli DH5α harboring the expression vector lpp1/V5/His-dsRed was grown at 37°C in 100 ml of BHI medium with 100 μg/ml ampicillin until an optical density at 600 nm of 0.2 was obtained. Protein expression was induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside and incubated for 12 h at 37°C. The bacteria were harvested by centrifugation (10 min at 6,500 × g), and the bacterial pellets were resuspended in 10 ml of 50 mM NaH2PO4, 300 mM NaCl buffer (pH 8.0). The bacterial cells were disrupted by sonication (two times for 45 s each time), and the lysates were centrifuged at 10,000 × g for 10 min. The supernatants were loaded onto Ni-nitrilotriacetic acid-agarose columns equilibrated with the same buffer. The columns were washed with 50 mM NaH2PO4, 300 mM NaCl buffer (pH 8.0) containing 20 mM imidazole. Bound recombinant proteins were eluted from the columns using an imidazole gradient (100 mM, 200 mM, and 400 mM). Eluted proteins were dialyzed for 1 h against water and then overnight against PBS. The proteins were concentrated using Centricon YM-10 columns (Millipore). Protein concentrations in the samples were determined by bicinchoninic acid assay (Pierce).
Anti-Lpp1 antibody preparation.
Purified recombinant Lpp1 (rLpp1) was used to raise antibodies in chickens by the method of Schwarzkopf et al. (44). Briefly, 100 μg of purified rLpp1 resuspended in 500 μl of PBS was mixed with an equal volume of Freund complete adjuvant, emulsified, and injected intramuscularly in four separate locations in single-combed White Leghorn laying hens (1 ml per bird). A booster injection prepared with incomplete adjuvant was given to the hen 1 week after the initial injection. The eggs from days 19 to 21 were collected, and antibodies were purified from egg yolks by the polyethylene glycol (PEG 8000; Fisher) precipitation method (37).
Enzyme-linked immunosorbent assay (ELISA).
BBEC monolayers in 96-well plates were washed with Hank's balanced salt solution (HBSS) and blocked with 1% bovine serum albumin (BSA; Pierce) for 45 min. Increasing amounts of purified recombinant Lpp1 in HBSS were added to the wells and incubated for 1 h with gentle shaking. The monolayers were washed three times with HBSS and incubated with a 1:5,000 dilution of HRP-conjugated anti-V5 antibody (Invitrogen) for another hour. After plates were washed, the reaction was developed with TMB (3,3′,5,5′-tetramethylbenzidine; Pierce), and the absorbance was read at 450 nm. For inhibition studies, rLpp1 (1 μM) was preincubated with increasing amounts of chicken anti-rLpp1 antibody for 45 min at room temperature in a total volume of 100 μl.
Flow cytometry.
Overnight cultures of M. haemolytica were centrifuged, washed three times with PBS, and resuspended in PBS. The bacterial cells (5 × 108 CFU in 0.3 ml of PBS) were incubated with an equal volume of 4% paraformaldehyde (Sigma) at room temperature for 20 min. The fixed bacterial cells were then washed two times with PBS and incubated with 2% BSA (Pierce) for 45 min to block nonspecific binding. To detect Lpp1, bacterial cells were incubated for 1.5 h with chicken anti-rLpp1 antibody (1 μg/ml in PBS in a final volume 0.3 ml). As a control, bacterial cells were similarly incubated with irrelevant chicken immunoglobulin Y (IgY) antibody. The bacterial cells were then washed two times with PBS and incubated at room temperature for 1 h in the dark with FITC-conjugated goat anti-chicken IgY (diluted 1:250; Promega) and propidium iodide (5 μg/ml). The bacterial cells were washed three times with PBS and resuspended in PBS at a final concentration of 5 × 106 cells per ml. Flow cytometry analysis of 100,000 events was performed using a FACScan (Becton Dickinson).
Confocal microscopy.
M. haemolytica cells (∼108) were washed with PBS, incubated at room temperature on glass coverslips for 10 min, and fixed on the coverslips with 4% paraformaldehyde (Sigma) for 20 min. After three washes with PBS, nonspecific binding was blocked by incubation of slides with 2% BSA (Pierce) in PBS for 45 min. To detect Lpp1, bacteria were incubated for 1.5 h with chicken anti-rLpp1 antibody or irrelevant chicken IgY antibody as control, followed by FITC-conjugated goat anti-chicken IgY (diluted 1:250; Promega). Fluorescence was visualized by laser scanning confocal microscopy using an Eclipse TE200-U microscope (Nikon).
Inhibition assay.
M. haemolytica suspensions (107 CFU per 100 μl of RPMI medium) were preincubated with different dilutions (1:300, 1:70, and 1:35) of bovine anti-OmpA or chicken anti-rLpp1 antibodies for 45 min at room temperature. Bacteria were added to BBEC monolayers in 96-well tissue culture plates and incubated for 1 h at 37°C. Normal bovine and rabbit sera were used as controls. After five washes with RPMI, BBEC were lysed with 1% saponin (Sigma) for 20 min. Bacteria in the cell lysates were suspended by vigorous pipetting, and the numbers of CFU were determined by plating of serial dilutions on blood agar. Control experiments demonstrated that saponin treatment did not have a deleterious effect on M. haemolytica viability as determined by CFU counts.
Statistical analysis.
Data are presented as the means ± standard errors of the means (SEM). Analysis of variance followed by a Tukey posttest for significance was performed using the Prism 5 (GraphPad) statistical software package.
RESULTS
M. haemolytica binds to BBEC.
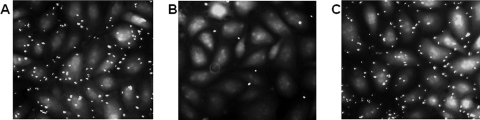
Adherence of M. haemolytica to primary cultures of BBEC was visualized by fluorescence microscopy using FITC-labeled bacteria (Fig. 1A). The number of FITC-labeled bacteria adhered per epithelial cell was 4.2 ± 0.6 (estimated to be 4.1% of the inoculum). Bacterial binding was significantly inhibited (94% ± 7.2%; P < 0.05) by preincubation of bacterial cells with rabbit anti-M. haemolytica antibody (gift from R. Y. C. Lo, University of Guelph, Canada) raised against whole bacterial cells (Fig. 1B). As a control, incubation of M. haemolytica with normal rabbit serum had no inhibitory effect on bacterial adherence to epithelial cells (109% ± 6.8% binding compared to a control with no serum) (Fig. 1C). We also performed experiments in which we compared M. haemolytica binding to BBEC at 37°C and 4°C using unlabeled bacteria. Significantly fewer (33% ± 6.9% less, mean ± SEM of three separate experiments; P < 0.05) M. haemolytica cells bound to BBEC monolayers following a 2-h incubation at 4°C than at 37°C (data not shown). As an additional control, we performed a binding experiment at 37°C with E. coli DH5α. Following a 2-h incubation, the relative number of adherent E. coli DH5α cells was 51% ± 7.6% (mean ± SEM of three separate experiments; P < 0.05) of the number of M. haemolytica cells (data not shown). We also investigated whether M. haemolytica was internalized by BBEC using a gentamicin protection assay. We did not recover any CFU of M. haemolytica after gentamicin treatment of BBEC monolayers incubated with bacteria for 1 h and 3 h at 37°C (data not shown). These results suggest that M. haemolytica did not invade BBEC during these times of incubation.
FIG. 1.
Adherence of M. haemolytica to BBEC. Primary cultures of BBEC were incubated with FITC-labeled M. haemolytica (A), FITC-labeled M. haemolytica preincubated with a 1:100 dilution of rabbit anti-M. haemolytica antibody (B), or FITC-labeled M. haemolytica preincubated with a 1:100 dilution of normal rabbit serum (C). BBEC were incubated with bacteria at an MOI of 100:1 for 2 h at 37°C. After several washes to remove unbound bacteria, cell-associated bacteria were visualized by fluorescence microscopy using an Olympus IX70 microscope (Olympus). Photos are representative of three independent experiments.
M. haemolytica proteins bind to BBEC monolayers.
To identify putative adhesins of M. haemolytica, bacterial cells were biotinylated and disrupted by sonication, and the resulting lysates were incubated with BBEC monolayers. BBEC with bound bacterial proteins were lysed with M-PER reagent and analyzed by Western blotting using an anti-biotin antibody. This analysis showed the presence of a major protein band with a molecular mass of ∼30 kDa and a minor protein band with a molecular mass of ∼90 kDa (Fig. 2B). Similar protein bands were observed when the blot was probed with anti-M. haemolytica antibody although an additional minor band with a molecular mass of ∼60 kDa was also detected (Fig. 2C). In contrast, no protein bands were identified when BBEC were incubated with biotin-labeled proteins from E. coli DH5α as a control (data not shown). This observation is consistent with data showing relatively less adherence of E. coli DH5α to BBEC. To exclude the possibility that inefficient biotinylation of surface proteins affected the observed profiles of adhesive proteins, M. haemolytica OMPs were isolated, labeled with biotin, and tested for binding to BBEC monolayers. As shown in Fig. 3C (lane 1), staining with anti-biotin antibody consistently detected a dominant protein band of ∼30 kDa. In some experiments additional minor bands were observed with molecular mass of 18 kDa, 37 kDa, and 45 kDa (data not shown). As described in Materials and Methods, BBEC monolayers with bound bacterial proteins were lysed with M-PER. Interestingly, when BBEC monolayers were solubilized with Laemmli buffer, Western blotting revealed additional ∼37-kDa protein bands (Fig. 3C, lane 2). Similar heterogenic migration of bacterial proteins in SDS-PAGE has been described for the OmpA protein family (2, 49, 31, 53). OmpA can undergo conformational changes when heated in the presence of SDS or 2-mercaptoethanol and migrates in the gel in two forms (2, 49). The apparent molecular mass reported for M. haemolytica OmpA is 38 kDa for the denatured form and 30 kDa for the incompletely denatured form (55). Because the molecular masses of the protein bands we detected by Western blotting with anti-biotin antibody were similar to the molecular mass of OmpA, we hypothesized that the 30-kDa BBEC-binding proteins might represent OmpA of M. haemolytica.
FIG. 2.
Binding of biotinylated M. haemolytica surface proteins to BBEC monolayers. Panel A illustrates Western blot analysis of a total cell lysate (5 μg) of biotinylated M. haemolytica cells probed with HRP-conjugated anti-biotin antibody. In panels B to D, BBEC monolayers were incubated with lysates of biotinylated M. haemolytica cells for 2 h at room temperature. After a washing step, cell monolayers were lysed with M-PER, and total lysates were separated in 4 to 20% gradient gels. The proteins were transferred to nitrocellulose and probed with HRP-conjugated anti-biotin antibody (B and D) or anti-M. haemolytica antibody (C). Lanes in panels B and C are as follows: lane 1, BBEC control; lane 2, BBEC incubated with 100 μg of bacterial proteins; lane 3, BBEC incubated with 200 μg of bacterial proteins. In panel D, native (lane 1) and fixed (lane 2) BBEC were incubated with 200 μg of bacterial proteins. Data are representative of three independent experiments. Molecular mass markers (in kDa) are shown on the right.
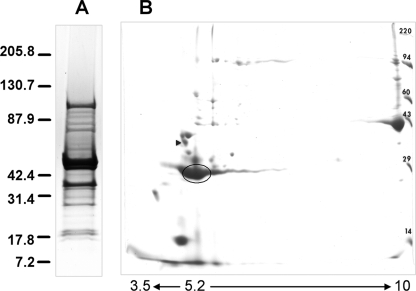
FIG. 3.

Binding of biotinylated M. haemolytica OMPs to BBEC monolayers. (A) Biotinylated M. haemolytica OMPs (10 μg) stained with Coomassie blue. (B) Biotinylated M. haemolytica OMPs (1 μg) probed with HRP-conjugated anti-biotin antibody. (C) BBEC monolayers incubated with 30 μg of biotinylated M. haemolytica OMPs for 2 h at room temperature. After a washing step, cell monolayers were lysed with M-PER (lane 1) or Laemmli buffer (lane 2), and total lysates were separated in 4 to 20% gradient gels. The proteins were transferred to nitrocellulose and probed with HRP-conjugated anti-biotin antibody. Data are representative of three independent experiments. Molecular mass markers (in kDa) are shown on the right.
Molecular characterization of 30-kDa BBEC-binding proteins.
To determine the identity of the 30-kDa proteins, BBEC-binding proteins were affinity purified from the M. haemolytica OMP fraction using fixed monolayers of BBEC. To demonstrate that the same M. haemolytica proteins bind to fixed and unfixed BBEC, a control experiment with biotinylated bacterial proteins was performed. As shown in Fig. 2D, the same dominant 30-kDa band was detected for native and fixed bronchial epithelial cells.
M. haemolytica OMPs that bound to the fixed BBEC were eluted with M-PER, concentrated, and then separated by 2-DE. Examination of a Coomassie blue-stained gel revealed the presence of a major 30-kDa spot and additional spots with molecular masses of 14 and 43 kDa (Fig. 4B). These additional proteins (14 and 43 kDa) were not observed by SDS-PAGE when whole M. haemolytica lysate, instead of the OMP fraction, was used for binding to fixed BBEC (Fig. 5A). The 30-kDa protein spot was excised from the 2-DE gel and digested with trypsin, and the peptide masses were determined by LC-MS/MS. The MS/MS peptide ions were analyzed using the Mascot protein database, resulting in identification of the proteins listed in Table 1. These results showed that the greatest number of peptides matched with two M. haemolytica proteins: a heat-modifiable OMP (homologue of OmpA) and Lpp1. As a control, BBEC-binding proteins were separated by SDS-PAGE, and the 30-kDa protein band was excised from the gel and analyzed by LC-MS/MS. Using this approach, the same proteins, OmpA and Lpp1, were identified as dominant proteins. However, we also found additional peptide homologues of the OMP P2 of Actinobacillus pleuropneumoniae (data not shown). We also assessed the identity of the ∼43-kDa protein spot shown in Fig. 4 by LC-MS/MS. A homology search of the protein database identified this protein as OMP44 of M. haemolytica, with high homology of this protein to bacterial porins identified in other Pasteurellaceae family members (OmpH of Pasteurella multocida and OMP P2 of H. influenzae). To confirm the results obtained by LC-MS/MS for a 30-kDa spot, bacterial BBEC-binding proteins were blotted and probed with bovine polyclonal anti-M. haemolytica OmpA and chicken anti-M. haemolytica rLpp1 antibodies. This analysis showed that the 30-kDa protein band was recognized by both antibodies (Fig. 5B and C).
FIG. 4.
2-DE of M. haemolytica OMPs eluted from fixed BBEC. (A) SDS-PAGE analysis of M. haemolytica OMPs stained with Coomassie blue prior to incubation with BBEC. (B) M. haemolytica OMPs eluted from fixed BBEC were separated by 2-DE using pH 3.5 to 10 immobilized pH gradient strips and 12.5% SDS-PAGE. The proteins were stained with Coomassie blue. The circled 30-kDa protein spot was excised and analyzed by LC-MS/MS. The proteins identified in this spot are listed in Table 1. The positions of molecular mass markers (in kDa) are indicated on the right. The black arrowhead indicates the tropomyosin standard with pI 5.2 and molecular mass of 32.7 kDa.
FIG. 5.
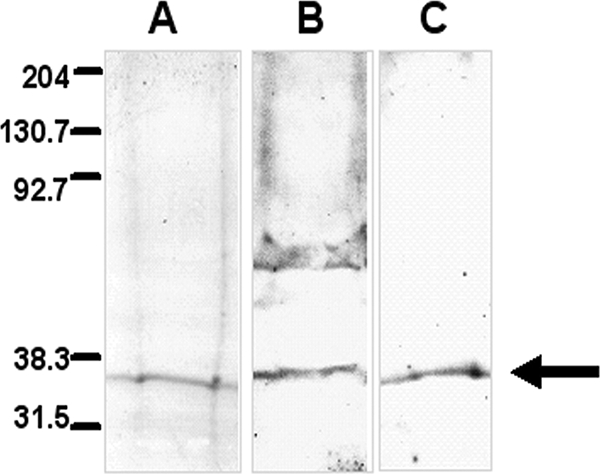
SDS-PAGE and Western blot analysis of M. haemolytica proteins that bind to BBEC. Fixed monolayers of BBEC were incubated with unlabeled M. haemolytica proteins (1 mg/ml) overnight at 4°C. After a washing step, bacterial proteins were eluted using Laemmli buffer, concentrated, and separated in 4 to 20% gradient gels. The proteins were stained with Coomassie blue (A) or transferred to nitrocellulose. The blotted proteins were probed with bovine anti-M. haemolytica OmpA (B) or chicken anti-rLpp1 (C) antibodies followed by appropriate HRP-conjugated secondary antibodies. The arrow indicates the M. haemolytica proteins eluted from the fixed BBEC monolayers. Data are representative of three independent experiments. Molecular mass markers (in kDa) are shown on the left.
TABLE 1.
BBEC bound proteins identified by LC-MS/MS for the 30-kDa 2-DE protein spot
| Protein name | NCBI accession no. | Theoretical mass (kDa) | Organism | No. of peptides matched |
|---|---|---|---|---|
| Heat-modifiable OMP | Q6XAY3 | 40.5 | M. haemolytica | 16 |
| Outer membrane Lpp1 | AAA25538 | 30 | M. haemolytica | 16 |
| Serotype-specific antigen 1 | AAA25549 | 103 | M. haemolytica | 4 |
| Outer membrane Lpp2 | Q2VRC4 | 30.2 | M. haemolytica | 2 |
| Outer membrane Lpp3 | Q2VRC9 | 29.1 | M. haemolytica | 1 |
| OmpA homolog | A60336 | 37.9 | A. actinomycetemcomitans | 1 |
rLpp1 binds to BBEC.
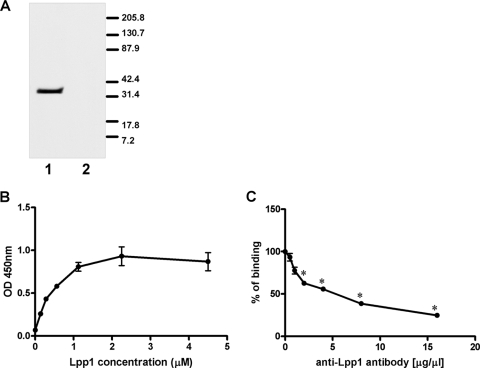
The heat-modifiable OMP of M. haemolytica is a homologue of OmpA, which functions as an adhesin for several gram-negative pathogens (22, 38, 45). In contrast, little is known about the adhesive properties of Lpp1. A BLAST search did not reveal similarity of Lpp1 with any known adhesin. To test whether M. haemolytica Lpp1 itself has the ability to bind to BBEC, recombinant M. haemolytica Lpp1 was prepared. For this purpose, the M. haemolytica lpp1 gene was amplified by PCR, tagged with V5 antigen and six His residues, and expressed as a fusion protein in E. coli DH5α. As shown in Fig. 6, after rLpp1 was purified on Ni-nitrilotriacetic acid agarose, the recombinant protein was recognized by V5-specific and rLpp1-specific antibodies. Binding of rLpp1 to BBEC was assessed by Western blotting and a cell ELISA method. As shown in Fig. 7A and B, rLpp1 bound to BBEC monolayers. The cell ELISA method showed that rLpp1 binding to BBEC was dose dependent, saturable at approximately 1 μM, and significantly inhibited by chicken anti-rLpp1 antibodies (Fig. 7B and C).
FIG. 6.

SDS-PAGE and Western blot analysis of rLpp1. Purified rLpp1 (2 μg) was separated in 4 to 20% gradient gels. The proteins were stained with Coomassie blue (A) or transferred to nitrocellulose and probed with HRP-conjugated anti-V5 (B) or chicken anti-Lpp1 (C) antibodies. Data are representative of three independent experiments. Molecular mass markers (in kDa) are shown on the left.
FIG. 7.
Binding of rLpp1 to BBEC. (A) BBEC monolayers were incubated with 50 μg purified rLpp1 (lane 1) or with HBSS (lane 2) for 2 h at room temperature. BBEC were lysed with M-PER, and total lysates were separated in 4 to 20% gradient gels. The proteins were transferred to nitrocellulose and probed with HRP-conjugated anti-V5 antibody. Molecular mass markers (in kDa) are shown on the left. (B) BBEC cell monolayers in 96-well plates were incubated with increasing concentrations of purified rLpp1 for 1 h at room temperature. Cells then were incubated with HRP-conjugated anti-V5 antibody. The reaction was developed using TMB, and absorbance was read at 450 nm. (C) rLpp1 (1 μM) was preincubated with increasing amounts of anti-Lpp1 antibody before being incubated with BBEC for 1 h at room temperature. Panel A is a photograph of a representative blot from one of three independent experiments. In panels B and C, data are the mean ± SEM of triplicate wells from one representative experiment of three independent experiments that were performed. *, P < 0.05 compared to control (no antibody). OD, optical density.
Detection of Lpp1 on the surface of M. haemolytica.
The expression of Lpp1 on the surface of M. haemolytica was analyzed by flow cytometry and confocal microscopy using a chicken IgY antibody against recombinant Lpp1. Flow cytometry analysis of propidium iodide-stained M. haemolytica showed that a majority of bacterial cells (approximately 75% as quantified in three independent experiments) were recognized by anti-rLpp1 antibody (Fig. 8A). Positive staining of the surface of M. haemolytica cells with anti-rLpp1 antibody was also observed using confocal microscopy (Fig. 8B and C).
FIG. 8.
Detection of Lpp1 on the surface of M. haemolytica cells. (A) Flow cytometry analysis of M. haemolytica cells stained with chicken anti-rLpp1 antibody and FITC-conjugated anti-chicken IgY. (B to D) The expression of Lpp1 on the surface of M. haemolytica was analyzed by laser scanning confocal microscopy at an emission wavelength of 488 nm. M. haemolytica cells were stained with chicken anti-rLpp1 antibody (B and C) or an irrelevant chicken IgY (D) as a control and then incubated with FITC-conjugated anti-chicken IgY antibody. Photos are representative of three independent experiments. Magnifications, ×200 (B and D) and ×1,500 (C). Ab, antibody.
M. haemolytica binding to BBEC is inhibited by anti-OmpA and anti-rLpp1 antibodies.
Preincubation of M. haemolytica cells with bovine anti-M. haemolytica OmpA or chicken anti-rLpp1 antibodies significantly inhibited bacterial binding to BBEC monolayers (Fig. 9). We did not observe an additive effect when anti-OmpA and anti-rLpp1 antibodies were used together (data not shown). Control experiments with normal rabbit serum or normal bovine serum showed no inhibitory effect on M. haemolytica binding to BBEC (data not shown). These results suggest that OmpA and Lpp1 are involved in adherence of M. haemolytica to bovine respiratory epithelial cells. Because neither of these antibodies blocked M. haemolytica adherence completely, we cannot exclude the possibility that other bacterial proteins also contribute to M. haemolytica binding to epithelial cells.
FIG. 9.
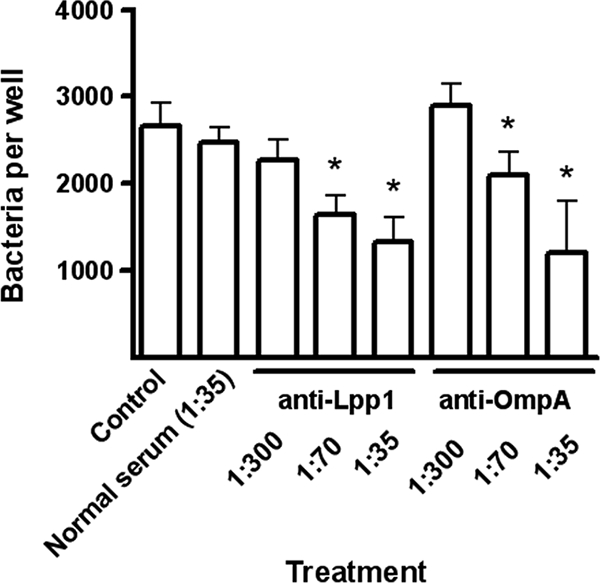
Inhibition of M. haemolytica adherence to BBEC by anti-OmpA or anti-Lpp1 antibodies. M. haemolytica cells were preincubated with different dilutions (1: 300, 1:70, and 1: 35) of bovine anti-OmpA or chicken anti-rLpp1 antibodies for 45 min at room temperature. BBEC incubated with normal rabbit serum or RPMI medium were included as controls. Bacteria were added to BBEC monolayers in 96-well tissue culture plates and incubated for 1 h at 37°C. After plates were washed with RPMI medium, BBEC were lysed with 1% saponin followed by serial dilution and plating on blood agar. Data are the mean ± SEM of triplicate wells from one representative experiment of three experiments that were performed. *, P < 0.05 compared to control.
DISCUSSION
Previous studies showed that M. haemolytica adheres to different types of cultured epithelial cells (7, 20, 52). However, adhesins that allow M. haemolytica to interact with receptors on the epithelial cell surface are poorly described. In the present study, we demonstrate adherence of M. haemolytica to bovine bronchial epithelial cells and show that M. haemolytica surface proteins with apparent molecular masses of 30 kDa bound selectively to BBEC.
LC-MS/MS analysis of the 30-kDa BBEC-binding proteins identified at least two M. haemolytica surface proteins: a heat-modifiable OMP (homologue of OmpA) and Lpp1. OmpA is one of the major OMPs in gram-negative bacteria. OmpA has been reported to be involved in bacterial adherence and invasion and also activation of host defense mechanisms for many gram-negative pathogens (4, 16, 36, 38, 45). For example, OmpA of meningitis-causing E. coli contributes to invasion of human brain microvascular endothelial cells in vitro (27, 38, 39). Klebsiella pneumoniae OmpA binds to and activates human bronchial epithelial cells, macrophages, and dendritic cells (24, 36, 48). Intratracheal injection of purified K. pneumoniae OmpA into mice results in lung neutrophilia (36). The recent reports of Choi et al. (5, 6) showed that OmpA can act as a potential virulence factor during infection caused by Acinetobacter baumanni. The direct binding and subsequent subcellular targeting of A. baumanni OmpA induced apoptosis of host cells in vitro and in vivo. Adhesive properties of OmpA have also been identified in members of the Pasteurellaceae family. For example, the OmpA of nontypeable H. influenzae (P5 protein) binds to human nasopharyngeal mucin and carcinoembryonic antigen-related cell adhesion molecules (4, 22, 41). Recognition of host receptors by P5 appears to be critical for adherence of nontypeable H. influenzae to A549 lung epithelial cells and nasopharynx colonization of chinchillas (4, 25, 47). Dabo et al. (12) demonstrated interaction of the recombinant OmpA of P. multocida with Madin-Darby bovine kidney cells and extracellular matrix molecules such as heparin and fibronectin. Fibronectin-binding activity has also been reported for OmpA from M. haemolytica (29). In our study, we show that M. haemolytica OmpA binds to BBEC. Preincubation of M. haemolytica cells with anti-OmpA antibody significantly reduced bacterial binding to BBEC, suggesting that OmpA interaction with BBEC is important for M. haemolytica adherence.
Interestingly, we identified another M. haemolytica protein, Lpp1, which binds to BBEC and migrates in SDS-PAGE together with OmpA. M. haemolytica Lpp1 (also known as PlpA or Plp1) represents one of three very similar membrane lipoproteins with molecular masses of 28 to 30 kDa (9). Each of these three lipoproteins was immunogenic and recognized by serum from calves naturally exposed to M. haemolytica or vaccinated with live or killed M. haemolytica (11). Furthermore, the serum antibody response against recombinant Lpp1 or Lpp3 correlated with resistance of calves to M. haemolytica-induced pneumonic pasteurellosis (10, 11). In vitro investigation demonstrated that an M. haemolytica mutant that was unable to produce these three lipoproteins was more susceptible to bovine complement-mediated killing (35). The strong reaction of antiserum raised against whole M. haemolytica cells with these lipoproteins suggested that they are exposed on the surface of the bacterial cells. However, the surface localization and adhesive properties of these lipoproteins were not specifically investigated. Our work demonstrates that purified rLpp1 binds to BBEC monolayers in a dose-dependent manner and that binding of rLpp1 and M. haemolytica cells to BBEC was inhibited by anti-rLpp1 antibody. Furthermore, we show by flow cytometry and confocal microscopy that Lpp1 was localized on the surface of M. haemolytica cells. Although the amino acid sequence of Lpp1 shows no homology with any known adhesin, our results suggest that Lpp1 can contribute to M. haemolytica adherence to BBEC. Comparison of the Lpp1 sequence with the NCBI protein database indicates that Lpp1 is strongly related to the ATP-binding cassette (ABC) transporters. A previous report described a 68-kDa adhesin of M. haemolytica that binds to bovine tracheal epithelial cells and neutrophils (23). Interestingly, BLAST analysis of this protein's N-terminal sequence (ANEVNVYIYKQPYLI) identified it as an iron binding protein with homology to iron (III) ABC transporters. Several other ABC transporters involved in nutrient or metal ion uptake have been reported to contribute to adherence of pathogenic bacteria to their host cells. For example, adherence of Streptococcus pneumoniae to nasopharyngeal cells and type II pneumocytes was mediated by PsaA lipoprotein, which is a member of the ABC transporter family implicated in manganese transport (3, 32, 42). Similarly, the glutamine ABC transporter (product of glnQ) of group B streptococcus contributed to binding and invasion of respiratory epithelial cells in vitro and virulence in an experimental animal model (50). Although the ABC transporter function of M. haemolytica Lpp1 remains unknown, we infer that, like other ABC transporters, Lpp1 may participate in bacterial adhesion.
Homologues of both M. haemolytica OmpA and Lpp1 are reported to be major protein components of leukotoxin-enriched outer membrane vesicles produced by Aggregatibacter actinomycetemcomitans, another member of the Pasteurellaceae family (26). These vesicles are thought to represent a vehicle for the in vivo delivery of virulence factors to susceptible host cells (28). Although the mechanism involved in the interaction of bacterial vesicles with target cells is not fully understood, it has been shown that association of A. actinomycetemcomitans vesicles with HL60 cells is independent of leukotoxin (15). Therefore, it is tempting to speculate that the adhesive properties of vesicle-associated OmpA or Lpp1 of A. actinomycetemcomitans are responsible for attachment of leukotoxin-containing particles to target cell membranes. Similarly, perhaps M. haemolytica OmpA or Lpp1 is involved in delivery of leukotoxin to bovine leukocytes.
In our experiments we consistently identified 30-kDa M. haemolytica proteins as dominant adhesive proteins. In some experiments we also detected other M. haemolytica proteins associated with BBEC. For example, Western blot analyses with biotinylated M. haemolytica surface proteins showed the presence of a minor 90-kDa protein band that was recognized by anti-M. haemolytica antibody. However, this protein band was not detected when unbiotinylated total bacterial lysates or isolated OMP fractions were used in binding assays. One potential explanation for these results is that the 90-kDa band represents a trimer of OmpA. However, we cannot exclude the possibility that the 90-kDa band represents some other adhesive protein of M. haemolytica. Additional protein spots (∼43 kDa and 14 kDa) were also observed in our 2D analysis of M. haemolytica OMP associated with BBEC monolayers. When the 43-kDa spot was analyzed by LC-MS/MS, a homology search of the NCBI protein database identified this protein as OMP44 of M. haemolytica that shows high homology to bacterial porins (OmpH and OMP P2) identified in other Pasteurellaceae family members. These results could explain the observation that anti-OmpA and anti-Lpp1 antibodies only partially inhibited M. haemolytica binding to BBEC. Therefore, we cannot exclude the possibility that other adhesive proteins are involved in M. haemolytica adherence to bovine bronchial epithelial cells.
In summary, our results demonstrate that OmpA and Lpp1 contribute to adherence of M. haemolytica to BBEC in vitro. Additional studies are required to define the receptors on bovine respiratory epithelial cells that are involved in M. haemolytica binding. Defining the mechanisms by which M. haemolytica interacts with respiratory tract epithelial cells will provide new insight into the pathogenesis of the bovine respiratory disease complex.
Acknowledgments
We thank Mark W. Cook and David L. Trott of the University of Wisconsin-Madison for help in generating chicken anti-rLpp1 antibodies, Diane S. Allen-Gipson of the University of Nebraska Medical Center (Omaha, NE) for providing the primary bovine bronchial epithelial cells, Reggie Y. C. Lo of the University of Guelph (Guelph, Canada) for providing the rabbit anti-M. haemolytica antibody, and Anthony W. Confer of the Oklahoma State University (Stillwater, OK) for providing the bovine polyclonal anti-M. haemolytica OmpA antibody. We also thank Dhammika N. Atapattu and Raksha Tiwari (University of Wisconsin-Madison) for their advice and critical reading of the manuscript.
This work was supported by National Research Initiative Competitive Grants 2004-35204-14841 and 2006-35204-17522 from the USDA Cooperative State Research, Education, and Extension Service and by the Walter and Martha Renk Endowed Laboratory for Food Safety.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 3 November 2008.
REFERENCES
- 1.Beachey, E. H. 1981. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J. Infect. Dis. 143325-345. [DOI] [PubMed] [Google Scholar]
- 2.Beher, M. G., C. A. Schnaitman, and A. P. Pugsley. 1980. Major heat-modifiable outer membrane protein in gram-negative bacteria: comparison with the OmpA protein of Escherichia coli. J. Bacteriol. 143906-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry, A. M., and J. C. Paton. 1996. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect. Immun. 645255-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bookwalter, J. E., J. A. Jurcisek, S. D. Gray-Owen, S. Fernandez, G. McGillivary, and L. O. A. Bakaletz. 2008. A carcinoembryonic antigen-related cellular adhesion molecule 1 homologue plays a pivotal role in nontypeable Haemophilus influenzae colonization of the chinchilla nasopharynx via the outer membrane protein P5-homologous adhesin. Infect. Immun. 7648-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi, C. H., E. Y. Lee, Y. C. Lee, T. I. Park, H. J. Kim, S. H. Hyun, S. A. Kim, S. K. Lee, and J. C. Lee. 2005. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell Microbiol. 71127-1138. [DOI] [PubMed] [Google Scholar]
- 6.Choi, C. H., S. H. Hyun, J. Y. Lee, J. S. Lee, Y. S. Lee, S. A. Kim, J. P. Chae, S. M. Yoo, and J. C. Lee. 2008. Acinetobacter baumannii outer membrane protein A targets the nucleus and induces cytotoxicity. Cell Microbiol. 10309-319. [DOI] [PubMed] [Google Scholar]
- 7.Clarke, J. M., and R. J. Morton. 2000. Development of an in vitro fluorometric assay to study adherence of Pasteurella haemolytica to bovine cells. Am. J. Vet. Res. 61129-132. [DOI] [PubMed] [Google Scholar]
- 8.Clarke, J. M., R. J. Morton, C. R. Clarke, R. W. Fulton, and J. T. Saliki. 2001. Development of an ex vivo model to study adherence of Mannheimia haemolytica serovar 1 to mucosal tissues of the respiratory tract of cattle. Am. J. Vet. Res. 62805-811. [DOI] [PubMed] [Google Scholar]
- 9.Cooney, B. J., and R. Y. Lo. 1993. Three contiguous lipoprotein genes in Pasteurella haemolytica A1 which are homologous to a lipoprotein gene in Haemophilus influenzae type b. Infect. Immun. 614682-4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craven, R. C., A. W. Confer, and M. J. Gentry. 1991. Cloning and expression of a 30 kDa surface antigen of Pasteurella haemolytica. Vet. Microbiol. 2763-78. [DOI] [PubMed] [Google Scholar]
- 11.Dabo, S. M., A. W. Confer, D. Styre, and G. L. Murphy. 1994. Expression, purification and immunologic analysis of three Pasteurella haemolytica A1 28-30 kDa lipoproteins. Microb. Pathog. 17149-158. [DOI] [PubMed] [Google Scholar]
- 12.Dabo, S. M., A. W. Confer, and R. A. Quijano-Blas. 2003. Molecular and immunological characterization of Pasteurella multocida serotype A:3 OmpA: evidence of its role in P. multocida interaction with extracellular matrix molecules. Microb. Pathog. 35147-157. [DOI] [PubMed] [Google Scholar]
- 13.Davies, R. L., and I. Lee. 2004. Sequence diversity and molecular evolution of the heat-modifiable outer membrane protein gene (OmpA) of Mannheimia (Pasteurella) haemolytica, Mannheimia glucosida, and Pasteurella trehalosi. J. Bacteriol. 1865741-5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De la Mora, A., F. Trigo, L. Jaramillo, Y. Garfias, C. Solórzano, C. Agundis, A. Pereyra, R. Lascurain, E. Zenteno, and F. Suárez-Güemes. 2006. The N-acetyl-D-glucosamine specific adhesin from Mannheimia haemolytica activates bovine neutrophils oxidative burst. Vet. Immunol. Immunopathol. 113148-156. [DOI] [PubMed] [Google Scholar]
- 15.Demuth, D. R., D. James, Y. Kowashi, and S. Kato. 2003. Interaction of Actinobacillus actinomycetemcomitans outer membrane vesicles with HL60 cells does not require leukotoxin. Cell Microbiol. 5111-121. [DOI] [PubMed] [Google Scholar]
- 16.Duim, B., L. D. Bowler, P. P. Eijk, H. M. Jansen, J. Dankert, and L. van Alphen. 1997. Molecular variation in the major outer membrane protein P5 gene of nonencapsulated Haemophilus influenzae during chronic infections. Infect. Immun. 651351-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duk, M., E. Lisowska, J. H. Wu, and A. M. Wu. 1994. The biotin/avidin-mediated microtiter plate lectin assay with the use of chemically modified glycoprotein ligand. Anal. Biochem. 221266-272. [DOI] [PubMed] [Google Scholar]
- 18.Frank, G. H., and P. C. Smith. 1983. Prevalence of Pasteurella haemolytica in transported calves. Am. J. Vet. Res. 44981-985. [PubMed] [Google Scholar]
- 19.Frank, G. H. 1986. The role of Pasteurella haemolytica in the respiratory disease complex. Vet. Med. 81838-846. [Google Scholar]
- 20.Galdiero, M., M. G. Pisciotta, A. Marinelli, G. Petrillo, and E. Galdiero. 2002. Coinfection with BHV-1 modulates cell adhesion and invasion by P. multocida and Mannheimia (Pasteurella) haemolytica. New Microbiol. 25427-436. [PubMed] [Google Scholar]
- 21.Gioia, J., X. Qin, H. Jiang, K. Clinkenbeard, R. Lo, Y. Liu, G. E. Fox, S. Yerrapragada, M. P. McLeod, T. Z. McNeill, L. Hemphill, E. Sodergren, Q. Wang, D. M. Muzny, F. J. Homsi, G. M. Weinstock, and S. K. Highlander. 2006. The genome sequence of Mannheimia haemolytica A1: insights into virulence, natural competence, and Pasteurellaceae phylogeny. J. Bacteriol. 1887257-7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill, D. J., M. A. Toleman, D. J. Evans, S. Villullas, L. van Alphen, and M. Virji. 2001. The variable P5 proteins of typeable and non-typeable Haemophilus influenzae target human CEACAM1. Mol. Microbiol. 39850-862. [DOI] [PubMed] [Google Scholar]
- 23.Jaramillo, L., F. Díaz, P. Hernández, H. Debray, F. Trigo, G. Mendoza, and E. Zenteno. 2000. Purification and characterization of an adhesin from Pasteurella haemolytica. Glycobiology 1031-37. [DOI] [PubMed] [Google Scholar]
- 24.Jeannin, P., T. Renno, L. Goetsch, I. Miconnet, J. P. Aubry, Y. Delneste, N. Herbault, T. Baussant, G. Magistrelli, C. Soulas, P. Romero, J. C. Cerottini, and J. Y. Bonnefoy. 2000. OmpA targets dendritic cells, induces their maturation and delivers antigen into the MHC class I presentation pathway. Nat. Immunol. 1502-509. [DOI] [PubMed] [Google Scholar]
- 25.Jiang, Z., N. Nagata, E. Molina, L. O. Bakaletz, H. Hawkins, and J. A. Patel. 1999. Fimbria-mediated enhanced attachment of nontypeable Haemophilus influenzae to respiratory syncytial virus-infected respiratory epithelial cells. Infect. Immun. 67187-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato, S., Y. Kowashi, and D. R. Demuth. 2002. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb. Pathog. 321-13. [DOI] [PubMed] [Google Scholar]
- 27.Kim, K. S. 2001. Escherichia coli translocation at the blood-brain barrier. Infect. Immun. 695217-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuehn, M. J., and N. C. Kesty. 2005. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 192645-2655. [DOI] [PubMed] [Google Scholar]
- 29.Lo, R. Y., and L. S. Sorensen. 2007. Outer membrane protein OmpA of Mannheimia haemolytica A1 is involved in the binding of fibronectin. FEMS Microbiol. Lett. 274226-231. [DOI] [PubMed] [Google Scholar]
- 30.Lo, R. Y. C. 2001. Genetic analysis of virulence factors of Mannheimia (Pasteurella) haemolytica A1. Vet. Microbiol. 8323-35. [DOI] [PubMed] [Google Scholar]
- 31.Mahasreshti, P. J., G. L. Murphy, J. H. 3rd Wyckoff, S. Farmer, R. E. Hancock, and A. W. Confer. 1997. Purification and partial characterization of the OmpA family of proteins of Pasteurella haemolytica. Infect. Immun. 65211-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McAllister. L. J., H. J. Tseng, A. D. Ogunniyi, M. P. Jennings, A. G. McEwan, and J. C. Paton. 2004. Molecular analysis of the psa permease complex of Streptococcus pneumoniae. Mol. Microbiol. 53889-901. [DOI] [PubMed] [Google Scholar]
- 33.Morck, D. W., T. J. G. Raybould, S. D. Acres, L. A. Babiuk, J. Nelligan, and W. J. Costerton. 1987. Electron microscopic description of glycocalyx and fimbriae on the surface of Pasteurella haemolytica A1. Can. J. Vet. Res. 5183-88. [PMC free article] [PubMed] [Google Scholar]
- 34.Morck, D. W., M. E. Olson, S. D. Acres, P.-Y. Daoust, and J. W. Costerton. 1989. Presence of bacterial glycocalyx and fimbriae on Pasteurella haemolytica in feedlot cattle with pneumonic pasteurellosis. Can. J. Vet. Res. 53167-171. [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy, G. L., L. C. Whitworth, A. W. Confer, J. D. Gaskins, K. Pandher, and S. M. Dabo. 1998. Characterization of a Pasteurella haemolytica A1 mutant deficient in production of three membrane lipoproteins. Am. J. Vet. Res. 591275-1280. [PubMed] [Google Scholar]
- 36.Pichavant, M., Y. Delneste, P. Jeannin, C. Fourneau, A. Brichet, A. B. Tonnel, and P. Gosset. 2003. Outer membrane protein A from Klebsiella pneumoniae activates bronchial epithelial cells: implication in neutrophil recruitment J. Immunol. 1716697-6705. [DOI] [PubMed] [Google Scholar]
- 37.Polson, A., M. B. von Wechmar, and M. H. van Regenmortel. 1980. Isolation of viral IgY antibodies from yolks of immunized hens. Immunol. Commun. 9475-493. [DOI] [PubMed] [Google Scholar]
- 38.Prasadarao, N. V., C. A. Wass, J. N. Weiser, M. F. Stins, S. H. Huang, and K. S. Kim. 1996. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect. Immun. 64146-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prasadarao, N. V., C. A. Wass, M. F. Stins, H. Shimada, and K. S. Kim. 1999. Outer membrane protein A-promoted actin condensation of brain microvascular endothelial cells is required for Escherichia coli invasion. Infect. Immun. 675775-5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purdy, C. W., R. H. Raleigh, J. K. Collins, J. L. Watts, and D. C. Straus. 1997. Serotyping and enzyme characterization of Pasteurella haemolytica and Pasteurella multocida isolates recovered from pneumonic lungs of stressed feeder calves. Curr. Microbiol. 34244-249. [DOI] [PubMed] [Google Scholar]
- 41.Reddy, M. S., J. M. Bernstein, T. F. Murphy, and H. S. Faden. 1996. Binding between outer membrane proteins of nontypeable Haemophilus influenzae and human nasopharyngeal mucin. Infect. Immun. 641477-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romero-Steiner, S., T. Pilishvili, J. S. Sampson, S. E. Johnson, A. Stinson, G. M. Carlone, and E. W. Ades. 2003. Inhibition of pneumococcal adherence to human nasopharyngeal epithelial cells by anti-PsaA antibodies. Clin. Diagn. Lab. Immunol. 10246-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 44.Schwarzkopf, C., C. Staak, I. Behn, and M. Erhard. 2001. Immunisation, p. 25-64. In R. Schade, I. Behn, M. Erhard, A. Hlinak, and C. Staak (ed.), Chicken egg yolk antibodies, production and application: IgY-technology. Springer-Verlag, Berlin, Germany.
- 45.Serino, L., B. Nesta, R. Leuzzi, M. R. Fontana, E. Monaci, B. T. Mocca, E. Cartocci, V. Masignani, A. E. Jerse, R. Rappuoli, and M. Pizza. 2007. Identification of a new OmpA-like protein in Neisseria gonorrhoeae involved in the binding to human epithelial cells and in vivo colonization. Mol. Microbiol. 641391-1403. [DOI] [PubMed] [Google Scholar]
- 46.Shin, S., G. Lu, M. Cai, and K. S. Kim. 2005. Escherichia coli outer membrane protein A adheres to human brain microvascular endothelial cells. Biochem. Biophys. Res. Commun. 3301199-1204. [DOI] [PubMed] [Google Scholar]
- 47.Sirakova, T., P. E. Kolattukudy, D. Murwin, J. Billy, E. Leake, D. Lim, T. DeMaria, and L. Bakaletz. 1994. Role of fimbriae expressed by nontypeable Haemophilus influenzae in pathogenesis of and protection against otitis media and relatedness of the fimbrin subunit to outer membrane protein A. Infect. Immun. 622002-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soulas, C., T. Baussant, J. P. Aubry, Y. Delneste, N. Barillat, G. Caron, T. Renno, J. Y. Bonnefoy, and P. Jeannin. 2000. Outer membrane protein A (OmpA) binds to and activates human macrophages. J. Immunol. 1652335-2340. [DOI] [PubMed] [Google Scholar]
- 49.Spinola, S. M., G. E. Griffiths, K. L. Shanks, and M. S. Blake. 1993. The major outer membrane protein of Haemophilus ducreyi is a member of the OmpA family of proteins. Infect. Immun. 611346-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamura, G. S., A. Nittayajarn, and D. L. Schoentag. 2002. A glutamine transport gene, glnQ, is required for fibronectin adherence and virulence of group B streptococci. Infect. Immun. 702877-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres, A. G., and J. B. Kaper. 2003. Multiple elements controlling adherence of enterohemorrhagic Escherichia coli O157:H7 to HeLa cells. Infect. Immun. 714985-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vilela, C. L., J. Fitzpatrick, and K. L. Morgan. 2004. In vitro adherence and invasion of ovine mammary epithelium by Mannheimia (Pasteurella) haemolytica. Vet. J. 167211-213. [DOI] [PubMed] [Google Scholar]
- 53.Wilson, M. E. 1991. The heat-modifiable outer membrane protein of Actinobacillus actinomycetemcomitans: relationship to OmpA proteins. Infect. Immun. 592505-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zecchinon, L., T. Fett, and D. Desmecht. 2005. How Mannheimia haemolytica defeats host defence through a kiss of death mechanism. Vet. Res. 36133-156. [DOI] [PubMed] [Google Scholar]
- 55.Zeng, H., K. Pandher, and G. L. Murphy. 1999. Molecular cloning of the Pasteurella haemolytica pomA gene and identification of bovine antibodies against PomA surface domains. Infect. Immun. 674968-4973. [DOI] [PMC free article] [PubMed] [Google Scholar]