Abstract
Hepatitis E virus (HEV) is an important human pathogen, although little is known about its biology and replication. Comparative sequence analysis revealed a hypervariable region (HVR) with extensive sequence variations in open reading frame 1 of HEV. To elucidate the role of the HVR in HEV replication, we first constructed two HVR deletion mutants, hHVRd1 and hHVRd2, with in-frame deletion of amino acids (aa) 711 to 777 and 747 to 761 in the HVR of a genotype 1 human HEV replicon. Evidence of HEV replication was detected in Huh7 cells transfected with RNA transcripts from mutant hHVRd2, as evidenced by expression of enhanced green fluorescent protein. To confirm the in vitro results, we constructed three avian HEV mutants with various HVR deletions: mutants aHVRd1, with deletion of aa 557 to 585 (Δ557-585); aHVRd2 (Δ612-641); and aHVRd3 (Δ557-641). Chickens intrahepatically inoculated with capped RNA transcripts from mutants aHVRd1 and aHVRd2 developed active viral infection, as evidenced by seroconversion, viremia, and fecal virus shedding, although mutant aHVRd3, with complete HVR deletion, was apparently attenuated in chickens. To further verify the results, we constructed four additional HVR deletion mutants using the genotype 3 swine HEV as the backbone. Mutants sHVRd2 (Δ722-781), sHVRd3 (Δ735-765), and sHVRd4 (Δ712-765) were shown to tolerate deletions and were infectious in pigs intrahepatically inoculated with capped RNA transcripts from the mutants, whereas mutant sHVRd1 (Δ712-790), with a nearly complete HVR deletion, exhibited an attenuation phenotype in infected pigs. The data from these studies indicate that deletions in HVR do not abolish HEV infectivity in vitro or in vivo, although evidence for attenuation was observed for HEV mutants with a larger or nearly complete HVR deletion.
Hepatitis E virus (HEV), the causative agent of human hepatitis E, is a nonenveloped, single-stranded, positive-sense RNA virus in the genus Hepevirus of the family Hepeviridae (9). Hepatitis E is an important public health disease in many developing countries and is also endemic in some industrialized countries (1, 2, 4, 8, 19, 41). HEV transmission occurs primarily by the fecal-oral route through contaminated drinking water or water supplies in areas with poor sanitation (35). The disease mainly affects young adults, and the mortality rate is generally less than 1%, but it can reach up to 28% among infected pregnant women (17, 28). A relatively high prevalence of anti-HEV antibodies in healthy individuals has been reported in the United States and other industrialized countries where HEV infections are only sporadic (33, 34, 51). HEV antibodies have also been detected in several other animal species, including rodents, pigs, and chickens (12, 30, 35, 54). In 1997, the first animal strain of HEV, swine HEV, was discovered and characterized from pigs in the United States (38). More recently, another strain of HEV, avian HEV, from chickens with hepatitis-splenomegaly syndrome was discovered and characterized in the United States (18). The discovery of animal strains of HEV and the existence of a population of individuals in industrialized countries who are seropositive for HEV have led to a hypothesis that animal reservoirs exist for HEV (34). Increasing evidence indicates that hepatitis E is indeed a zoonotic disease (35, 39) and that pigs (and perhaps other species) are animal reservoirs for HEV (34).
There are at least four major genotypes of HEV: genotype 1 (primarily Burmese-like Asian strains) (3, 50, 52); genotype 2 (a single Mexican strain) (21); genotype 3 (strains from rare endemic cases in industrialized countries, including the United States, Europe, and Japan, and swine HEV strains from pigs worldwide) (11, 38, 45-48); and genotype 4 (variant strains from endemic cases in Asia and swine HEV strains from pigs in Asia) (49, 57). All swine HEV strains identified thus far worldwide belong to either genotype 3 or 4 (20, 22, 40, 47). The avian HEV from chickens likely represents a new genus in the family Hepeviridae (25).
The genome of HEV is approximately 7.2 kb in length and consists of three open reading frames (ORFs) (14, 31, 43, 50, 55) and short 5′ and 3′ noncoding regions (NCR), followed by a poly(A) tail (8, 15, 29, 50). The ORF1 encodes a nonstructural polyprotein, which contains putative functional motifs characteristic of methyltransferase, protease, helicase, and RNA-dependent RNA polymerase (31, 42). ORF2 encodes the capsid protein of about 660 amino acids (aa). The small ORF3 encodes a phosphoprotein of about 123 aa, the biological function of which has yet to be fully defined (14, 15, 31, 50, 55, 58, 60). Though ORF1 is essential for HEV replication, it contains a highly heterogeneous and hypervariable region (HVR) among HEV strains (25, 32, 44, 53). The size differences among different HEV genomes are confined mainly to the HVR of ORF1 (25). The observed extensive inter- and intragenotypic sequence variations in the HVRs of HEV genomes suggest that the HVR may not be necessary for virus replication. However, sequences not required for virus infectivity or spread are normally rapidly lost in vivo, especially in small RNA viruses like HEV. Therefore, the fact that HEV does retain such a hypervariable sequence in its genome suggested a potential biological role for the HVR in HEV replication and/or pathogenesis, which warranted further investigation.
Reverse genetic systems for HEV have been recently established, permitting the manipulation of the HEV genome to explore the potential functions of viral genes (10, 24, 26). To elucidate the potential role of the HVR in HEV replication and/or pathogenesis, in this study we constructed various HVR deletion mutants using a genotype 1 human HEV replicon, an avian HEV infectious clone, and genotype 3 swine HEV infectious clones. The mutants were tested for infectivity in Huh7 liver cells, as well as in chickens and pigs. The results from this study indicate that deletions of HVR from the HEV genome do not affect virus viability in vitro or in vivo, although virus mutants with a larger or nearly complete HVR deletion were apparently attenuated in infected animals.
MATERIALS AND METHODS
Cells and infectious cDNA clones.
The genotype 1 human HEV (Sar55 strain) infectious clone (10) and a subclone of the Huh7 liver cell line (7, 15) were gifts from Suzanne Emerson and Robert Purcell at the Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, NIH. The infectious cDNA clones of the genotype 3 swine HEV (26) and avian HEV (24) were reported previously.
Sequence analysis of the HVR among known HEV strains.
In order to identify the length of the HVR and to determine the intragenotypic and intergenotypic sequence identities in the HVR among different HEV strains of the four major genotypes, the amino acid sequences flanking the HVR among known mammalian strains of HEV (Fig. 1) and the corresponding region of avian HEV were aligned and analyzed using the Clustal W method of the MegAlign program (DNAStar, Inc.). The putative HVR for each genotype of mammalian HEV and the corresponding HVR in avian HEV were predicted on the basis of the sequence alignment.
FIG. 1.
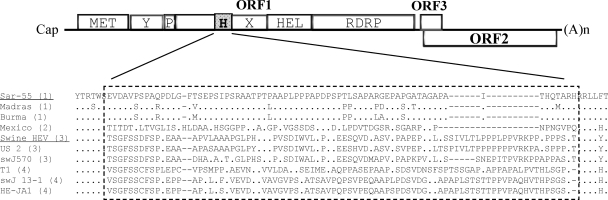
Schematic diagram showing the relative positions of the HVR of ORF1 from representative isolates in four major genotypes of mammalian HEV (genotypes 1 to 4, in parentheses), along with putative functional domains: MET, methyltransferase; P, papain-like cysteine protease; Y, Y domain; H, HVR; X, X domain; HEL, helicase; RDRP, RNA-dependent RNA polymerase. The amino acid sequences of different HEV strains were aligned using the Clustal W method of the MegAlign program (DNAStar, Inc.). The amino acid sequence in the HVR of the genotype 1 Sar55 human HEV strain is shown at the top, and only differences are indicated for the other strains. Amino acid sequences identical to the genotype 1 HEV Sar55 sequence are indicated by dots, and deletions are indicated by dashes. Representative strains from each genotype used in this study are underlined.
Construction of genotype 1 human HEV HVR deletion mutant replicons.
The enhanced green fluorescent protein (EGFP)-expressing HEV replicon was constructed in our laboratory (Y. W. Huang and X. J. Meng, unpublished data) using the infectious cDNA clone of genotype 1 human HEV (strain Sar55), pSK-HEV-2 (10), as the backbone. Briefly, the 5′ NCR, ORF1, and 3′ NCR in this EGFP replicon are intact, with part of the carboxy terminus of ORF2 fused to the EGFP gene. The amino terminus of the ORF2 gene (nt 5148 to 5816) downstream of the first methionine was removed, and the EGFP gene was inserted in frame with the ORF2 initiation codon. The EGFP replicon was shown to express the EGFP protein when transfected into Huh7 liver cells (Huang and Meng, unpublished).
Two HVR deletion mutants of the genotype 1 HEV replicon were constructed using fusion PCR (Fig. 2). Amino acid residues 711 to 777 and 747 to 761, corresponding to nucleotides (nt) 2131 to 2331 and 2239 to 2283, were deleted to construct the HVR deletion mutants hHVRd1 and hHVR2, respectively. The two fragments used for fusion PCR were first amplified with the primer sets Hu F/Hu r1 and Hu f1/Hu R for the mutant hHVRd1 and Hu F/Hu r2 and Hu f2/Hu R for the mutant hHVRd2 (Table 1). The PCR products amplified from each mutant were then used in the fusion PCR with primer set Hu F/Hu R (Table 1). To produce the two HVR deletion mutants, the fusion product was purified, digested with SphI and NsiI, and ligated into the backbone of the genotype 1 HEV EGFP replicon from which the SphI-NsiI region had been deleted.
FIG. 2.
(A) Schematic diagram showing the HVR (aa 707 to 777) in ORF1 of the genotype 1 human HEV (strain Sar55) replicon expressing EGFP. MET, methyltransferase; P, papain-like cysteine protease; Y, Y domain; H, HVR; X, X domain; HEL, helicase; RDRP, RNA-dependent RNA polymerase. The amino acid sequence of each HVR deletion mutant is aligned with that of the wild-type Sar55 HEV replicon to show the relative positions of the in-frame amino acid deletions: mutants hHVRd1 (aa 711 to 777) and hHVRd2 (aa 747 to 761). (B) Fluorescence microscopy of Huh7 liver cells at 6 days posttransfection with similar amounts of capped RNA transcripts from the wild-type Sar55 replicon with the EGFP gene (A), HVR deletion mutants hHVRd1 (B) and hHVRd2 (C), and mock-transfected cells (D).
TABLE 1.
Oligonucleotide primers used for construction of HVR deletion mutants, as well as for PCR and sequencing, in this study
| Primer ID | Primer sequence (5′ → 3′) |
|---|---|
| For construction of avian HEV HVR deletion mutants | |
| AvF | CCCGTTAACTGCGCACCACCGGCCG |
| Avf | CCCGTTAACGGCGGACCTGAGGTCA |
| AvR | CGGGTTAACAAGCCAGTCGGCGGCA |
| Avr | CGGGTTAACCTGTGGCGGCAAGGGC |
| For construction of swine HEV HVR-deletion mutants | |
| SwF | GACATCGCCGCTCGAGCCTCCCGCCTAA |
| Swf1 | ACATCTGGCTTTTCTCGCACTCGTCGTCTC |
| Swf2 | CCTGAGGCGGCCGCCCGTAAGCCACCAACA |
| Swf3 | CTGCCCCACCCTACCTTACCCAGCTCCATT |
| Swf4 | ACATCTGGCTTTTCTTTACCCAGCTCCATT |
| SwR | GGGACCTGGTATATACCCGAGCCTAGAA |
| Swr1 | AGAAAAGCCAGATGTTGACCAAGTCCGGGT |
| Swr2 | GGCGGCCGCCTCAGGGGGGGAGAAATCGCT |
| Swr3 | GGTAGGGTGGGGCAGCCCCGGGGCAGCGGC |
| For construction of genotype 1 human HEV replicon HVR-deletion mutants | |
| HuF | GGGAGCATGCTCAGAAGTTTATAACACGCC |
| Huf1 | TCGGAGGTTGATGCTCGCCGCCTGCTCTTT |
| Huf2 | CCCCCTGCACCGGATGGCGCTACCGCCGGG |
| HuR | GTACCTCTGGTAAAATGCATGACAGAGCCC |
| Hur1 | AGCATCAACCTCCGACCAAGTGCGGGTGTA |
| Hur2 | ATCCGGTGCAGGGGGGGGTAGAGGGGCCGC |
| For sequencing of the HVR region from viruses recovered from pigs and chickens | |
| Av N1 | TTACCATTGACTTTGAACGGCG |
| Av N3 | GCTTGTGCATTGACGATTTCCC |
| Sw N1 | CAGGTCAGGATTTCATCTAATGG |
| Av N2 | CCGGGCTGATGGTCTCGATTAG |
| Av N4 | CAATAGGTTACCCACGATGACG |
| Sw N2 | GCCCTCACGCATAATGAACTCAG |
Construction of avian HEV HVR deletion mutants using the avian HEV infectious cDNA clone as the backbone.
To further elucidate the role of the HVR in HEV replication, we utilized the genetically distinct avian HEV to produce three avian HEV HVR deletion mutants. Avian HEV shares only approximately 50% nucleotide sequence identity with the mammalian HEV strains (23-25), although avian HEV and mammalian HEV have similar genomic organizations. Since the complete sequences of avian HEV are available for only two strains, the putative HVR in avian HEV is derived from the corresponding HVR in mammalian HEV.
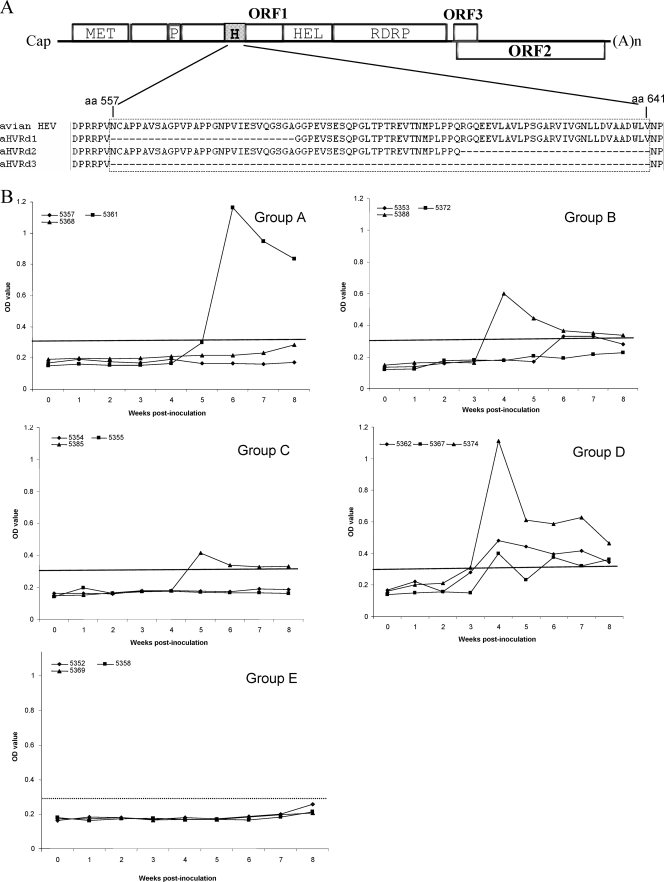
To construct the three avian HEV HVR deletion mutants with various lengths, the avian infectious cDNA clone (24), pT7-aHEV, was used as the backbone (Fig. 3). Mutant aHVRd1 was created by PCR to delete amino acid residues 557 to 585 (nt 1693 to 1779) using the primers Avf and AvR (Table 1). Similarly, to construct mutant aHVRd2, primers AvF and Avr were used to delete amino acid residues 612 to 641 (nt 1858 to 1947). All four primers contain a unique HpaI restriction site. The PCR products were purified with a GeneClean II kit, digested with HpaI, and ligated into the backbone of the avian HEV infectious clone pT7-aHEV from which the HpaI region had been deleted. For the construction of mutant aHVRd3, aa 557 to 641 (nt 1693 to 1947) were deleted by direct digestion of the avian HEV infectious cDNA clone with HpaI and religation of the ends after purification.
FIG. 3.
(A) Schematic diagram showing the HVR (aa 557 to 641) in ORF1 of avian HEV, along with putative functional domains: MET, methyltransferase; P, papain-like cysteine protease; H, HVR; HEL, helicase; and RDRP, RNA-dependent RNA polymerase. The amino acid sequence of each HVR deletion mutant is aligned with that of the wild-type strain of avian HEV to show the relative positions of the in-frame amino acid deletions: mutants aHVRd1 (aa 557 to 585), aHVRd2 (aa 612 to 641), and aHVRd3 (aa 557 to 641). (B) Seroconversion to IgG anti-HEV in chickens inoculated with capped RNA transcripts from the wild-type avian HEV infectious clone and its derived HVR deletion mutants. IgG anti-HEV was plotted as the ELISA optical density (OD) (A405), and the ELISA cutoff value was 0.30. Chickens 5357, 5361, and 5368 were each inoculated with RNA transcripts from HVR deletion mutant aHVRd1 (group A); chickens 5353, 5372, and 5388 with RNA transcripts from mutant aHVRd2 (group B); and chickens 5354, 5355, and 5385 with RNA transcripts from mutant sHVRd3 (group C). Chickens 5362, 5367, and 5374 (group D) were each inoculated with RNA transcripts from the wild-type avian HEV infectious cDNA clone as positive controls, and chickens 5352, 5358, and 5369 (group E) were intrahepatically inoculated with PBS buffer as negative controls.
Construction of swine HEV HVR deletion mutants using the genotype 3 swine HEV infectious cDNA clone as the backbone.
To more definitively verify the role of the HVR in HEV replication, we subsequently constructed four additional HVR deletion mutants with a different genotype, the genotype 3 swine HEV. Briefly, the infectious cDNA clone pSHEV-3 of the prototype genotype 3 swine HEV (26) was used as the backbone for the construction of four HVR deletion mutants using fusion PCR. Amino acid residues 712 to 790, 722 to 781, 735 to 765, and 712 to 765 (corresponding to nt 2160 to 2396, 2190 to 2369, 2229 to 2321, and 2160 to 2321, respectively) were deleted from the infectious cDNA clone pSHEV-3 to produce HVR deletion mutants sHVRd1, sHVRd2, sHVRd3, and sHVRd4, respectively (Fig. 4). The two fragments used for fusion PCR were first amplified with the primer sets Sw F/Sw r1 and Sw f1/Sw R for the mutant sHVRd1, Sw F/Sw r2 and Sw f2/Sw R for the mutant sHVRd2, Sw F/Sw r3 and Sw f3/Sw R for the mutant sHVRd3, and Sw F/Sw r1 and Sw f4/Sw R for the mutant sHVRd4 (Table 1). PCR products amplified from each mutant were then used in the fusion PCR with primer set Sw F/Sw R (Table 1). The fusion PCR products were purified, digested with XhoI and SexAI, and ligated into the backbone of the genotype 3 swine HEV infectious cDNA clone pSHEV-3 from which the XhoI-SexAI region had been deleted.
FIG. 4.
(A) Schematic diagram showing the HVR (aa 707 to 790) in ORF1 of the genotype 3 swine HEV. MET, methyltransferase; P, papain-like cysteine protease; Y, Y domain; H, HVR; X, X domain; HEL, helicase; and RDRP, RNA-dependent RNA polymerase. The amino acid sequence of each HVR deletion mutant is aligned with that of the wild-type strain of the genotype 3 swine HEV to show the relative positions of the in-frame amino acid deletions: mutants sHVRd1 (aa 712 to 790), sHVRd2 (aa 722 to 781), sHVRd3 (aa 735 to 765), and sHVRd4 (aa 712 to 765). (B) Seroconversion to IgG anti-HEV in pigs inoculated with capped RNA transcripts from the wild-type swine HEV infectious clone and its derived HVR deletion mutants. IgG anti-HEV was plotted as the ELISA optical density (OD) (A405), and the ELISA cutoff value was 0.30. Pigs 286, 296, and 608 were each inoculated with capped RNA transcripts from HVR deletion mutant sHVRd1 (group A); pigs 291, 295, and 613 with RNA transcripts from mutant sHVRd2 (group B); pigs 292, 604, and 606 with RNA transcripts from mutant sHVRd3 (group C); pigs 288, 293, and 300 with RNA transcripts from mutant sHVRd4 (group D); and pigs 289, 294, and 612 with RNA transcripts from wild-type genotype 3 swine HEV infectious clone pSHEV-3 (group E). Pigs 603, 609, and 611 were intrahepatically inoculated with PBS buffer as negative controls (group F).
In vitro transcription.
For demonstration of the viability of the HVR deletion mutants in Huh7 liver cells, the genotype 1 human HEV EGFP replicon and its derived HVR deletion mutants (hHVRd1 and hHVRd2) were first linearized with BglII and purified by phenol-chloroform extraction and ethanol precipi tation. Capped RNA transcripts were synthesized with the mMESSAGE mMACHINE T7 kit (Ambion) from the mutant and wild-type replicons (24). Briefly, each transcription reaction was performed in a 20-μl reaction mixture containing 1 μg linearized cDNA template, 2 μl 10× reaction buffer, 10 μl 2× nucleoside triphosphate/Cap, 2 μl enzyme mixture, and an additional 1 μl 30 mM GTP stock for capping. The mixtures were incubated at 37°C for 1.5 h, and 0.5 μl of each reaction mixture was run on a 0.8% agarose gel to check the quality of the RNA transcripts. Each transcription mixture was cooled on ice and used for the transfection of Huh7 liver cells as previously described (7).
For avian HEV mutants, the full-length cDNA clone of avian HEV and the three avian HEV HVR deletion mutants were linearized by digestion with XhoI and purified by phenol-chloroform extraction. Each in vitro transcription reaction was performed in a 300-μl reaction as described previously (24) to generate capped full-length RNA transcripts. RNA transcripts from each cDNA clone were diluted 1:4 with cold RNase-, DNase-, and proteinase-free phosphate-buffered saline (PBS) buffer, frozen on dry ice, and used for intrahepatic inoculation of chickens on the same day.
For the genotype 3 swine HEV mutants, the full-length cDNA clone of swine HEV and the four HVR deletion mutants were linearized with XbaI and purified. Capped RNA transcripts were synthesized from each cDNA clone in a 600-μl reaction as described previously (27). Once the RNA transcripts were examined for quality on an agarose gel, the transcription reaction mixture from each clone was diluted with 4 volumes of cold RNase-, DNase-, and proteinase-free PBS buffer; aliquoted into 1-ml vials; and immediately frozen on dry ice until it was used for intrahepatic inoculation of pigs the next day.
In vitro transfection of Huh7 liver cells with genotype 1 human HEV HVR deletion mutant replicons.
The Huh7 cells grown in a six-well plate were washed once with serum-free medium prior to transfection. Five microliters of the capped RNA transcripts generated from the wild type or HVR deletion mutants of the genotype 1 HEV EGFP replicon were mixed with l ml of Opti-MEM (Invitrogen) containing 10 μl of DMRIE-C (Invitrogen), and the mixture was overlaid on cells in a drained well. After 4 h of incubation at 34.5°C, the mixture was aspirated, fresh Dulbecco's modified Eagle's medium containing 10% fetal bovine serum was added, and the cultures were continuously incubated at 34.5°C. Expression of EGFP in transfected cells was examined on days 4, 5, and 6 posttransfection with a fluorescence microscope (Nikon).
Evaluation of the infectivity of avian HEV HVR deletion mutants in specific-pathogen-free (SPF) chickens.
The lack of an efficient cell culture system for HEV propagation limited our ability to test the viability and infectivity of mutant or wild-type viruses in cell cultures. We had previously developed a unique procedure (intrahepatic inoculation of chickens with capped RNA transcripts from avian HEV infectious clones via percutaneous injection) to successfully determine the infectivity of avian HEV cDNA clones (24). Therefore, to test the infectivity and replication competency of the avian HEV HVR deletion mutants, we utilized the percutaneous-injection procedure for intrahepatic inoculation of chickens with capped RNA transcripts from the wild-type avian HEV infectious clone and HVR deletion mutants as described previously (24).
Briefly, 15 11-week-old SPF chickens that were negative for avian HEV RNA and antibodies were divided into five groups (groups A through E), with 3 chickens in each group (Fig. 3). The RNA transcripts were injected immediately, after a quick thaw, into four different sites in each liver, with approximately 100 μl per injection site. Three chickens in group A (5357, 5361, and 5368) were each injected with 400 μl of RNA transcripts from mutant aHVRd1; chickens 5353, 5372, and 5388 in group B with RNA transcripts from mutant clone aHVRd2; and chickens 5354, 5355, and 5385 in group C with RNA transcripts from mutant aHVRd3. Chickens 5362, 5367, and 5374 in group D were each injected with RNA transcripts from the wild-type avian HEV infectious clone and served as positive controls. The three chickens in group E (5352, 5358, and 5369) were injected similarly with PBS buffer as negative controls (Fig. 3). Fecal swabs and sera were collected from each inoculated chicken at weekly intervals and tested by RT-PCR for avian HEV RNA. Weekly serum samples were also tested by enzyme-linked immunosorbent assay (ELISA) for seroconversion to avian HEV antibodies as previously described (23, 25). All inoculated chickens were necropsied at 8 weeks postinoculation (p.i.).
Determination of the infectivities of the genotype 3 swine HEV HVR deletion mutants in SPF pigs.
The in vivo transfection system developed in our previous studies for testing the infectivities of swine HEV infectious cDNA clones and mutants (26) was used to determine the infectivities of HVR deletion mutants in pigs. Briefly, 18 6-week-old SPF pigs that were seronegative for HEV were assigned to six groups of three each (groups A, B, C, D, E, and F). An ultrasound-guided technique was used to inoculate the pigs intrahepatically with capped RNA transcripts from each of the mutants, as described previously (26). The RNA transcripts were thawed and immediately injected into five different sites in each liver with 200 μl per injection site. The three pigs (no. 286, 296, and 608) in group A were each injected with 1 ml of the RNA transcripts from mutant sHVRd1. Similarly, pigs 291, 295, and 613 in group B were each injected with RNA transcripts from mutant sHVRd2; pigs 292, 604, and 606 (group C) with RNA transcripts from sHVRd3; and pigs 288, 293, and 300 (group D) with the RNA transcripts from mutant sHVRd4. The three pigs 603, 609, and 611 in group F were each injected similarly with 1 ml of PBS buffer and served as negative controls, and the three pigs 289, 294, and 612 in group E were intrahepatically injected with 1 ml of the RNA transcripts from the wild-type pSHEV-3 infectious clone and served as positive controls (Fig. 4). Fecal samples and sera were collected from all inoculated pigs at weekly intervals until they were necropsied at 10 weeks p.i. Fecal and serum samples were tested by reverse transcription (RT)-PCR (16, 36, 37, 59) for swine HEV RNA, and weekly serum samples were also tested by ELISA for immunoglobulin G (IgG) antibodies to swine HEV (16, 36).
Detection and sequencing of viruses recovered from experimentally infected chickens and pigs.
For the chicken study, fecal materials collected from inoculated chickens at 3 weeks p.i. were tested by RT-PCR using the primers specific for the avian HEV HVR. A nested PCR with external primers Av N1 and Av N2 and internal primers Av N3 and Av N4 (Table 1) were used to amplify the region flanking the avian HEV HVR. Similarly, for the pig study, fecal materials collected from the inoculated pigs at 4 weeks p.i. were tested by RT-PCR using the primers specific for the genotype 3 swine HEV HVR. A One-step RT-PCR kit (Invitrogen) was used to amplify the region flanking the swine HEV HVR using the Sw N1 and Sw N2 primers (Table 1). The amplified PCR products from pigs and chickens were purified with a GeneClean II kit and sequenced at the Virginia Bioinformatic Institute. The sequences obtained from viruses recovered from the infected chickens and pigs were compared with the sequences of the original viruses used as the inocula.
RESULTS
The HVR is highly variable among HEV strains.
Sequence analyses confirmed the existence of an HVR in ORF1 of HEV strains (Fig. 1). The intergenotypic amino acid sequence identity in the HVR among HEV isolates in different genotypes differed by as much as 71%, whereas the intragenotypic amino acid sequence identities among isolates within the same genotype differed by 31% among genotype 1 isolates, 41% among genotype 3 isolates, 46% among genotype 4 isolates, and 30% between the only two available avian HEV isolates (data not shown). The variability of the HVR in genotype 2 is unknown, since only one strain of genotype 2 HEV has been sequenced to date. The predicted HVR for the HEV strains used in the present study includes ORF1 aa 707 to 777 in genotype 1 human HEV (Sar55 strain) (Fig. 2), aa 707 to 790 in genotype 3 swine HEV (pSHEV-3 infectious clone) (Fig. 4), and aa 557 to 641 in avian HEV (Fig. 3). It was previously predicted, based on sequence comparison of an apparently avirulent strain and the prototype pathogenic strain of avian HEV, that the region spanning aa 554 to 614 in ORF1 of avian HEV is hypervariable (5). However, further sequence comparisons with mammalian HEV strains revealed that the avian HEV genome downstream of the originally predicted HVR at aa 615 to 641 also displayed significant sequence variations. Therefore, we considered aa 557 to 641 the HVR of avian HEV for the purpose of constructing avian HEV HVR deletion mutants in this study (Fig. 3).
The HVR of genotype 1 human HEV is not required for virus replication in vitro.
We constructed two genotype 1 human HEV HVR deletion mutants using a strain Sar55 HEV replicon expressing EGFP (Fig. 2). Huh7 cells were transfected with capped RNA transcripts from a wild-type replicon and the two mutants, hHVRd1 and hHVRd2. The transfected cells were examined by fluorescence microscopy on days 4, 5, and 6 posttransfection for evidence of EGFP expression. EGFP fluorescence signal was detected in Huh7 cells transfected with the wild-type Sar55 replicon, as well as in those transfected with the mutant hHVRd2 replicon (Fig. 2), but not in the cells transfected with the mutant hHVRd1 replicon. Fluorescence was first detected on day 4, and the EGFP signal intensity increased on days 5 and 6 posttransfection. Expression of EGFP by the HVR deletion mutant hHVRd2 indicated that the mutant is replication competent in Huh7 liver cells.
The HVR (aa 557 to 585 and aa 612 to 641) of avian HEV tolerated deletions.
To validate the dispensability of the HVR for HEV replication observed in our in vitro study with the genotype 1 human HEV replicon, we selected the genetically distinct avian HEV for an in vivo study. Three avian HEV HVR deletion mutants were generated using the avian HEV infectious cDNA clone as the backbone (Fig. 3). The abilities of the three avian HEV HVR deletion mutants to infect chickens were tested by direct intrahepatic inoculation of SPF chickens with capped RNA transcripts from each mutant. Seroconversion to IgG anti-avian HEV was observed in all HVR deletion mutant groups (A, B, and C), as well as in the positive control group (D). In each group, however, only one or two chickens out of the three that were inoculated seroconverted (Fig. 3): only one chicken (no. 5361) in group A (aHVRd1) seroconverted at 6 weeks p.i., chickens 5388 and 5353 of group B (aHVRd2) seroconverted at 4 and 6 weeks p.i., and chicken 5354 of group C (aHVRd3) seroconverted at 5 weeks p.i. All the chickens in the positive control group D seroconverted at 4 weeks p.i. The three negative control chickens (5352, 5358, and 5369) remained seronegative through the experiment.
Avian HEV-specific RNA in feces was detected variably in inoculated chickens (Table 2). In group A chickens (aHVRd1), fecal virus shedding began at 3 weeks p.i. for chicken 5361. Fecal virus shedding was delayed until 6 weeks p.i. in chicken 5357 and was not detected in chicken 5368. In group B chickens (aHVRd2), fecal virus shedding began at 2 and 3 weeks p.i. for chickens 5388 and 5353 but was undetectable in chicken 5372. None of the chickens in group C (aHVRd3) had detectable avian HEV RNA in the feces. Viremia could not be detected in group A or C chickens (Table 1) and was transient in chicken 5353 but lasted for 4 weeks in chicken 5388 of group B. Transient viremia was detected in all chickens of positive control group D.
TABLE 2.
Detection of avian HEV RNAa
| Group | Chicken no. | Result (fecal/serum) at week p.i.:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| A (aHVRd1) | 5357 | −/− | −/− | −/− | −/− | −/− | −/− | +/− | −/− | −/− |
| 5361 | −/− | −/− | −/− | +/− | −/− | −/− | +/− | −/− | −/− | |
| 5368 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | |
| B (aHVRd2) | 5353 | −/− | −/− | −/− | +/+ | −/− | −/− | −/− | −/− | +/− |
| 5372 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | |
| 5388 | −/− | −/− | +/− | +/+ | −/+ | −/+ | −/+ | −/− | −/− | |
| C (aHVRd3) | 5354 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| 5355 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | |
| 5385 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | |
| D (wild-type clone) (positive control group) | 5362 | −/− | −/− | +/− | +/+ | −/− | −/− | −/− | −/− | −/− |
| 5367 | −/− | −/− | − | −/− | +/− | −/− | −/− | −/− | −/− | |
| 5374 | −/− | −/− | −/+ | +/+ | −/− | −/− | −/− | −/− | −/− | |
| E (negative control group) | 5352 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| 5358 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | |
| 5369 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | |
Positive (+) or negative (−) samples at the indicated week p.i. in SPF chickens intrahepatically inoculated with capped RNA transcripts of avian HEV HVR deletion mutants, as well as the wild-type avian HEV infectious clone.
The HVR of genotype 3 swine HEV is not required for in vivo infectivity.
To further verify the results from the avian HEV chicken study and the in vitro genotype 1 HEV replicon study, we subsequently constructed four swine HEV mutants using the genotype 3 swine HEV infectious cDNA clone as the backbone (Fig. 4). The abilities of the four genotype 3 swine HEV mutants to infect pigs were tested by direct intrahepatic inoculation of SPF pigs with capped RNA transcripts from each mutant. Since the intrahepatic inoculation of RNA transcripts of avian HEV HVR mutants in chickens was a blind percutaneous procedure (24), some chickens may not have received, or received much less, inocula in the livers. To ensure that all animals received equal amounts of RNA inocula, in the pig study with the genotype 3 HEV HVR deletion mutants, we used an ultrasound-guided technique for the intrahepatic injection to make sure that the RNA inocula were injected directly into the liver. All the pigs in groups A, B, C, and D, which were injected with capped RNA transcripts from respective HVR deletion mutants, seroconverted to IgG anti-HEV, indicating that active swine HEV infections had occurred in the inoculated pigs (Fig. 4). All the pigs in group B (sHVRd 2), group C (sHVRd 3), and group D (sHVRd 4) seroconverted at about 3 to 5 weeks p.i. The three pigs in group A (sHVRd 1) had a delayed seroconversion at 6 to 7 weeks p.i. The positive control pigs in group E (pSHEV-3) seroconverted at 3 to 4 weeks p.i. The three negative control pigs in group F remained seronegative throughout the course of study (Fig. 4).
Fecal virus shedding occurred variably in pigs of groups B, C, and D (Table 3). There was no fecal virus shedding in group A pigs. Delayed fecal virus shedding occurred in pigs 291 and 295 of group B at 9 to 10 weeks p.i., while there was no fecal virus shedding in pig 613. Fecal virus shedding occurred as early as 1 week p.i. in group C pigs and at 2 weeks p.i. in group D pigs and lasted for 5 to 8 weeks. Viremia was not detected in group A or B pigs (Table 3). Viremia was detected only in pig 604 of group C at 3 and 6 weeks p.i. and only in pig 288 of group D at 5 weeks p.i. (Table 3).
TABLE 3.
Detection of HEV RNAa
| Group | Pig no. | Result (fecal/serum) at week p.i.:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| A (sHVRd1) | 286 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| 296 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | |
| 608 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | |
| B (sHVRd2) | 291 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | +/− | −/− |
| 295 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | +/− | +/− | |
| 613 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | |
| C (sHVRd3) | 292 | −/− | −/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | ++/−/− |
| 604 | −/− | −/− | −/− | +/+ | −/− | +/− | +/+ | +/− | +/− | −/− | +/− | |
| 606 | −/− | −/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | |
| D (sHVRd4) | 288 | −/− | −/− | +/− | +/− | +/− | +/+ | +/− | +/− | +/− | +/− | −/− |
| 293 | −/− | −/− | +/− | +/− | +/− | −/− | −/− | −/− | +/− | −/− | +/− | |
| 300 | −/− | −/− | −/− | +/− | +/− | +/− | +/− | −/− | +/− | −/− | +/− | |
| E (wild-type clone) | 289 | −/− | −/− | −/− | −/− | −/− | +/− | +/− | −/− | −/− | −/− | −/− |
| (Positive control | 294 | −/− | −/− | +/− | +/+ | +/− | +/− | −/− | −/− | −/− | −/− | −/− |
| group) | 612 | −/− | −/− | −/− | −/− | +/− | +/− | −/− | +/− | −/− | +/− | −/− |
| F (negative control | 603 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| group) | 609 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| 611 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | |
In samples that were positive (+) or negative (−) at the indicated week p.i. in SPF chickens intrahepatically inoculated with capped RNA transcripts of swine HEV HVR deletion mutants, as well as the wild-type infectious clone.
Viruses recovered from infected chickens and pigs retained their respective deletions in the HVR.
Viruses recovered from the feces of chicken no. 5361 from group A and chicken no. 5388 from group B at 3 weeks p.i. were sequenced to confirm the presence of deletions in the HVR. Sequence analyses revealed that the recovered virus from chicken no. 5361 retained its nt 1693 to 1779 deletion and the virus recovered from chicken no. 5388 also retained its nt 1858 to 1947 deletion.
Similarly, we also amplified and sequenced the HVRs of the rescued viruses from selected pigs inoculated with the genotype 3 HVR deletion mutants. The introduced deletions nt 2190 to 2369 for group B pigs, nt 2229 to 2321 for group C pigs, and nt 2160 to 2321 for group D pigs were retained intact in the viruses recovered from the fecal samples collected at 4 weeks p.i.
DISCUSSION
The objective of this study was to assess the role of the HVR in ORF1 of HEV in virus replication and/or pathogenesis. Sequence analysis of known HEV strains revealed an HVR with a high degree of variability at both amino acid and nucleotide sequence levels. This region overlaps the proline-rich hinge region of ORF1 (25, 32, 44, 53). It is known that inherent structural constraints can influence the vulnerability of genomic segments to replication errors during virus infection, resulting in the accumulation of mutations for genetic diversity (13). The size differences in HEV genomes from different genotypes are confined mainly to the HVR of ORF1, which spanned 105 aa as originally proposed (31, 53). As the sequences of additional HEV isolates were published, it became clear that the first 35 aa in the originally described HVR among HEV strains (53) is not hypervariable. Thus, the true HVR is 70 to 72 aa for genotype 1 HEV, 68 aa for genotype 2 HEV, 80 to 86 aa for genotype 3 HEV, 84 aa for all genotype 4 HEVs, and 84 aa for avian HEV (based on the corresponding region in mammalian HEVs). HEV genomes exhibited increased divergence in the HVR encompassing aa 707 to 777 for genotype 1 human HEV, aa 707 to 790 for genotype 3 swine HEV, and aa 557 to 641 for avian HEV. Extensive sequence variations observed among isolates in the four major genotypes of mammalian HEV and avian HEV, as well as within each genotype, suggested that the HVR may not be necessary for virus replication. It has been shown that a 507-nt deletion in a variable nonstructural region of rubella virus, a virus distantly related to HEV, is not required for virus replication (56). Therefore, we hypothesize that the HVR of HEV is not required for virus infectivity.
To test our hypothesis, we first constructed two genotype 1 human HEV HVR deletion mutants using the EGFP-expressing Sar55 HEV replicon as the backbone: mutants hHVRd1 (aa 711 to 777 deleted) and hHVRd2 (aa 747 to 761 deleted). The wild-type Sar55 EGFP replicon, which was constructed in our laboratory (Huang and Meng, unpublished), was shown to be replication competent and expressed EGFP when transfected into Huh7 liver cells. The two HVR deletion mutants (hHVRd1 and hHVRd2) were tested for viability and replication competency in Huh7 cells. EGFP fluorescence signal was detected in Huh7 cells transfected with the mutant hHVRd2, as well as with the wild-type Sar55 replicon, but not in the cells transfected with the HVR deletion mutant hHVRd1. The results from this experiment showed that the mutant hHVRd2 with partial HVR deletion is viable, and thus, the HVR is dispensable for virus replication in vitro. The absence of EGFP expression for mutant hHVRd1, which contains a deletion of the nearly complete HVR, suggested that this mutant is not replication competent. Therefore, it is likely that the end sequences of the HVR for genotype 1 human HEV may be important for virus viability. Nevertheless, partial deletion of HVR sequence in the middle region, as revealed by mutant hHVRd2 (Fig. 2), apparently does not affect the replication ability of the genotype 1 human HEV in vitro.
To further confirm our results from the in vitro study with genotype 1 human HEV replicon mutants, we utilized a genetically distinct chicken strain of HEV (avian HEV); we constructed three avian HEV mutants with various deletions in the HVR and tested the mutants for the ability to infect chickens. Based on the amino acid sequence alignment of avian HEV with other mammalian HEV strains, we found that the region spanning aa 557 to 641 in avian HEV is highly divergent and thus termed it the HVR for avian HEV. A total of three avian HEV HVR deletion mutants with various lengths were constructed: aHVRd1, with a partial deletion in the 5′ end of the HVR; aHVRd2, with a partial deletion in the 3′ end of the HVR; and aHVRd3, with the deletion of the nearly complete HVR (Fig. 3). The infectivities of the three avian HEV mutants were tested in chickens by intrahepatically inoculating the RNA transcripts from each mutant into the livers of live chickens. The kinetics of virus replication appears to be different in chickens infected with different mutants and wild-type avian HEV (Fig. 3). Although seroconversion was observed in chickens inoculated with all three mutants, only one or two out of the three inoculated chickens had seroconverted (Fig. 3). Since the percutaneous intrahepatic-injection procedure used in this study to inoculate RNA transcripts into chicken livers is a blind procedure (24), it was quite possible that the RNA transcripts were not injected into the livers of some chickens or that only a small amount was injected (24). This may explain why not all inoculated chickens seroconverted to avian HEV antibodies. Fecal virus shedding and viremia were detected only in mutants aHVRd1 and aHVRd2. Deletions in the HVR may influence the replicative competence of the virus and thus may attenuate avian HEV. Therefore, attenuation of HVR deletion mutants to replicate at lower levels could explain why viral RNA was not detected in sera from group A chickens (aHVRd1) or in feces and sera of group C chickens (aHVRd3). Clearly, future studies are warranted to explore any potential role of the HVR in virus attenuation, which is beyond the scope of this study. The results from this avian HEV and chicken study indicated that the HVR of avian HEV is not essential for virus infectivity in vivo, although the avian HEV mutant with complete HVR deletion displayed an apparent attenuation phenotype.
In order to definitively verify our results from the avian HEV and chicken study, as well as from the in vitro genotype 1 HEV replicon mutant study, we subsequently constructed four genotype 3 swine HEV mutants with various HVR deletions: sHVRd1, with the deletion of the nearly complete HVR sequence; sHVRd2 and sHVRd3, with partial deletions of HVR sequences in the middle region; and sHVRd4, with a deletion of partial HVR sequence at the 5′ end (Fig. 4). The infectivities of these four mutants were tested in pigs by intrahepatic inoculation of capped RNA transcripts from each mutant via an ultrasound-guided inoculation procedure. Similar to our observations in the chicken study, we found that mutants sHVRd2, sHVRd3, and sHVRd4, with partial deletions of the HVR sequences at the 5′ end and in the middle region, are viable and infectious in pigs. Seroconversion was observed for all HVR deletion mutants; however, there was a delayed seroconversion with no detectable viral RNA in feces or sera for pigs inoculated with mutant sHVRd1, which contains the nearly complete HVR deletion, an indication of attenuation for the sHVRd1 virus. Viral RNA was detected much later during infection, at 9 weeks p.i., in pigs (no. 291 and no. 295) infected with mutant sHVRd2, which contains a larger sequence deletion of the HVR than mutants sHVRd3 and sHVRd4. Fecal virus shedding was detected at 1 and 2 weeks p.i. in pigs inoculated with mutants sHVRd3 and sHVRd4 and lasted for 5 to 8 weeks. These results suggest that mutants sHVRd1 and sHVRd2, with larger sequence deletions of the HVR, may be attenuated to replicate at lower levels, and it appears that the lengths of HVR deletions may affect the level of virus replication and attenuation. Again, additional studies to explore the role of the HVR in HEV attenuation, which is not within the scope of this study, will provide more insights into the role of the HVR in the biology and pathogenesis of HEV.
The patterns of viremia and fecal virus shedding in experimentally infected pigs and chickens (Tables 2 and 3) are somewhat different from that observed in HEV-infected humans. In humans, viremia usually precedes fecal virus shedding, whereas in the pig and chicken studies fecal virus shedding was detected prior to viremia, which is consistent with our previous animal studies (5, 16). It is believed that tissues in the gastrointestinal tract are the initial sites of swine HEV and avian HEV replication, and thus, the virus is excreted to the feces before entering the bloodstream. In fact, it has recently been demonstrated that after oral injection or intravenous injection, swine HEV and avian HEV first replicate in various gastrointestinal tissues in chickens and pigs before reaching the target organ, the liver, via the bloodstream (6, 59), and this may explain why fecal virus shedding precedes viremia in HEV-infected pigs and chickens.
Since ORF1 contains domains essential for HEV replication, proper folding of the encoded polyprotein is essential for its role in virus replication either individually or by interacting with host alleles. The results from our in vitro, as well as in vivo, animal studies showed that the deletions in the HVR did not influence the viability of the virus, and thus, the polyprotein encoded by ORF1 appears to be properly folded in viable mutants. The HVR, aa 747 to 761 of genotype 1 human HEV, aa 557 to 585 and 612 to 641 of avian HEV, and aa 712 to 765 of genotype 3 swine HEV, apparently has no major effect on the host-mediated processing of the polyprotein, as the mutant viruses are viable and infectious in animals. Since unneeded sequences in virus genomes normally are lost rapidly during in vivo replication, it is possible that the HVR, although not essential for virus infectivity, may play a biological role in HEV pathogenesis. In fact, the results from the animal studies with limited numbers of pigs and chickens suggested that deletions of a larger or nearly complete HVR from the HEV genome apparently attenuated the virus. Therefore, additional studies with larger numbers of animals are warranted to fully evaluate the biological role of HVR in HEV replication and pathogenesis.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AI050611 and AI065546).
We thank Suzanne Emerson and Robert Purcell at the Laboratory of Infectious Diseases, National Institutes of Health, Bethesda, MD, for generously providing us the genotype 1 human HEV (Sar55 strain) infectious clone and a subclone of the Huh7 liver cell line. We also thank Padma Billam for her technical assistance.
Footnotes
Published ahead of print on 22 October 2008.
REFERENCES
- 1.Aikawa, T., M. Kojima, M. Takahashi, T. Nishizawa, and H. Okamoto. 2002. Identification of indigenous hepatitis E virus from a Japanese patient who contracted sporadic acute hepatitis in 1982. J. Infect. Dis. 1861535-1537. [DOI] [PubMed] [Google Scholar]
- 2.Arankalle, V. A., M. S. Chadha, S. A. Tsarev, S. U. Emerson, A. R. Risbud, K. Banerjee, and R. H. Purcell. 1994. Seroepidemiology of water-borne hepatitis in India and evidence for a third enterically-transmitted hepatitis agent. Proc. Natl. Acad. Sci. USA 913428-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arankalle, V. A., L. P. Chobe, M. V. Joshi, M. S. Chadha, B. Kundu, and A. M. Walimbe. 2002. Human and swine hepatitis E viruses from Western India belong to different genotypes. J. Hepatol. 36417-425. [DOI] [PubMed] [Google Scholar]
- 4.Billam, P., F. F. Huang, Z. F. Sun, F. W. Pierson, R. B. Duncan, F. Elvinger, D. K. Guenette, T. E. Toth, and X. J. Meng. 2005. Systematic pathogenesis and replication of avian hepatitis E virus in specific-pathogen-free adult chickens. J. Virol. 793429-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billam, P., Z. F. Sun, and X. J. Meng. 2007. Analysis of the complete genomic sequence of an apparently avirulent strain of avian hepatitis E virus (avian HEV) identified major genetic differences compared with the prototype pathogenic strain of avian HEV. J. Gen. Virol. 881538-1544. [DOI] [PubMed] [Google Scholar]
- 6.Billam, P., F. W. Pierson, W. Li, T. LeRoith, R. B. Duncan, and X. J. Meng. 2008. Development and validation of a negative-strand-specific reverse transcription-PCR assay for detection of a chicken strain of hepatitis E virus: identification of nonliver replication sites. J. Clin. Microbiol. 462630-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emerson, S. U., H. Nguyen, U. Torian, and R. H. Purcell. 2006. ORF3 protein of hepatitis E virus is not required for replication, virion assembly, or infection of hepatoma cells in vitro. J. Virol. 8010457-10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emerson, S. U., and R. H. Purcell. 2003. Hepatitis E virus. Rev. Med. Virol. 13145-154. [DOI] [PubMed] [Google Scholar]
- 9.Emerson, S. U., and R. H. Purcell. 2004. Running like water—the omnipresence of hepatitis E. N. Engl. J. Med. 3512367-2368. [DOI] [PubMed] [Google Scholar]
- 10.Emerson, S. U., M. Zhang, X. J. Meng, H. Nguyen, M. St Claire, S. Govindarajan, Y. K. Huang, and R. H. Purcell. 2001. Recombinant hepatitis E virus genomes infectious for primates: importance of capping and discovery of a cis-reactive element. Proc. Natl. Acad. Sci. USA 9815270-15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erker, J. C., S. M. Desai, G. G. Schlauder, G. J. Dawson, and I. K. Mushahwar. 1999. A hepatitis E virus variant from the United States: molecular characterization and transmission in cynomolgus macaques. J. Gen. Virol. 80681-690. [DOI] [PubMed] [Google Scholar]
- 12.Favorov, M. O., M. Y. Kosoy, S. A. Tsarev, J. E. Childs, and H. S. Margolis. 2000. Prevalence of antibody to hepatitis E virus among rodents in the United States. J. Infect. Dis. 181449-455. [DOI] [PubMed] [Google Scholar]
- 13.Gouvea, V., N. Snellings, M. J. Popek, C. F. Longer, and B. L. Innis. 1998. Hepatitis E virus: complete genome sequence and phylogenetic analysis of a Nepali isolate. Virus Res. 5721-26. [DOI] [PubMed] [Google Scholar]
- 14.Graff, J., H. Nguyen, C. Kasorndorkbua, P. G. Halbur, M. St Claire, R. H. Purcell, and S. U. Emerson. 2005. In vitro and in vivo mutational analysis of the 3′-terminal regions of hepatitis E virus genomes and replicons. J. Virol. 791017-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graff, J., U. Torian, H. Nguyen, and S. U. Emerson. 2006. A bicistronic subgenomic mRNA encodes both the ORF2 and ORF3 proteins of hepatitis E virus. J. Virol. 805919-5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halbur, P. G., C. Kasorndorkbua, C. Gilbert, D. Guenette, M. B. Potters, R. H. Purcell, S. U. Emerson, T. E. Toth, and X. J. Meng. 2001. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J. Clin. Microbiol. 39918-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamid, S. S., S. M. Jafri, H. Khan, H. Shah, Z. Abbas, and H. Fields. 1996. Fulminant hepatic failure in pregnant women: acute fatty liver or acute viral hepatitis? J. Hepatol 2520-27. [DOI] [PubMed] [Google Scholar]
- 18.Haqshenas, G., H. L. Shivaprasad, P. R. Woolcock, D. H. Read, and X. J. Meng. 2001. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J. Gen. Virol. 822449-2462. [DOI] [PubMed] [Google Scholar]
- 19.Harrison, T. J. 1999. Hepatitis E virus—an update. Liver 19171-176. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh, S. Y., X. J. Meng, Y. H. Wu, S. T. Liu, A. W. Tam, D. Y. Lin, and Y. F. Liaw. 1999. Identity of a novel swine hepatitis E virus in Taiwan forming a monophyletic group with Taiwan isolates of human hepatitis E virus. J. Clin. Microbiol. 373828-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, C. C., D. Nguyen, J. Fernandez, K. Y. Yun, K. E. Fry, D. W. Bradley, A. W. Tam, and G. R. Reyes. 1992. Molecular cloning and sequencing of the Mexico isolate of hepatitis E virus (HEV). Virology 191550-558. [DOI] [PubMed] [Google Scholar]
- 22.Huang, F. F., G. Haqshenas, D. K. Guenette, P. G. Halbur, S. K. Schommer, F. W. Pierson, T. E. Toth, and X. J. Meng. 2002. Detection by reverse transcription-PCR and genetic characterization of field isolates of swine hepatitis E virus from pigs in different geographic regions of the United States. J. Clin. Microbiol. 401326-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang, F. F., G. Haqshenas, H. L. Shivaprasad, D. K. Guenette, P. R. Woolcock, C. T. Larsen, F. W. Pierson, F. Elvinger, T. E. Toth, and X. J. Meng. 2002. Heterogeneity and seroprevalence of a newly identified avian hepatitis E virus from chickens in the United States. J. Clin. Microbiol. 404197-4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, F. F., F. W. Pierson, T. E. Toth, and X. J. Meng. 2005. Construction and characterization of infectious cDNA clones of a chicken strain of hepatitis E virus (HEV), avian HEV. J. Gen. Virol. 862585-2593. [DOI] [PubMed] [Google Scholar]
- 25.Huang, F. F., Z. F. Sun, S. U. Emerson, R. H. Purcell, H. L. Shivaprasad, F. W. Pierson, T. E. Toth, and X. J. Meng. 2004. Determination and analysis of the complete genomic sequence of avian hepatitis E virus (avian HEV) and attempts to infect rhesus monkeys with avian HEV. J. Gen. Virol. 851609-1618. [DOI] [PubMed] [Google Scholar]
- 26.Huang, Y. W., G. Haqshenas, C. Kasorndorkbua, P. G. Halbur, S. U. Emerson, and X. J. Meng. 2005. Capped RNA transcripts of full-length cDNA clones of swine hepatitis E virus are replication competent when transfected into Huh7 cells and infectious when intrahepatically inoculated into pigs. J. Virol. 791552-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang, Y. W., T. Opriessnig, P. G. Halbur, and X. J. Meng. 2007. Initiation at the third in-frame AUG codon of open reading frame 3 of the hepatitis E virus is essential for viral infectivity in vivo. J. Virol. 813018-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hussaini, S. H., S. J. Skidmore, P. Richardson, L. M. Sherratt, B. T. Cooper, and J. G. O'Grady. 1997. Severe hepatitis E infection during pregnancy. J. Viral Hepat. 451-54. [DOI] [PubMed] [Google Scholar]
- 29.Jameel, S. 1999. Molecular biology and pathogenesis of hepatitis E virus. Exp. Rev. Mol. Med. 19991-16. [DOI] [PubMed] [Google Scholar]
- 30.Kabrane-Lazizi, Y., J. B. Fine, J. Elm, G. E. Glass, H. Higa, A. Diwan, C. J. Gibbs, Jr., X. J. Meng, S. U. Emerson, and R. H. Purcell. 1999. Evidence for widespread infection of wild rats with hepatitis E virus in the United States. Am. J. Trop. Med. Hyg. 61331-335. [DOI] [PubMed] [Google Scholar]
- 31.Koonin, E. V., A. E. Gorbalenya, M. A. Purdy, M. N. Rozanov, G. R. Reyes, and D. W. Bradley. 1992. Computer-assisted assignment of functional domains in the nonstructural polyprotein of hepatitis E virus: delineation of an additional group of positive-strand RNA plant and animal viruses. Proc. Natl. Acad. Sci. USA 898259-8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krawczynski, K. 1993. Hepatitis E. Hepatology 17932-941. [PubMed] [Google Scholar]
- 33.Mast, E. E., I. K. Kuramoto, M. O. Favorov, V. R. Schoening, B. T. Burkholder, C. N. Shapiro, and P. V. Holland. 1997. Prevalence of and risk factors for antibody to hepatitis E virus seroreactivity among blood donors in Northern California. J. Infect. Dis. 17634-40. [DOI] [PubMed] [Google Scholar]
- 34.Meng, X. J. 2000. Novel strains of hepatitis E virus identified from humans and other animal species: is hepatitis E a zoonosis? J. Hepatol. 33842-845. [DOI] [PubMed] [Google Scholar]
- 35.Meng, X. J. 2003. Swine hepatitis E virus: cross-species infection and risk in xenotransplantation. Curr. Top. Microbiol. Immunol. 278185-216. [DOI] [PubMed] [Google Scholar]
- 36.Meng, X. J., P. G. Halbur, J. S. Haynes, T. S. Tsareva, J. D. Bruna, R. L. Royer, R. H. Purcell, and S. U. Emerson. 1998. Experimental infection of pigs with the newly identified swine hepatitis E virus (swine HEV), but not with human strains of HEV. Arch. Virol. 1431405-1415. [DOI] [PubMed] [Google Scholar]
- 37.Meng, X. J., P. G. Halbur, M. S. Shapiro, S. Govindarajan, J. D. Bruna, I. K. Mushahwar, R. H. Purcell, and S. U. Emerson. 1998. Genetic and experimental evidence for cross-species infection by swine hepatitis E virus. J. Virol. 729714-9721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng, X. J., R. H. Purcell, P. G. Halbur, J. R. Lehman, D. M. Webb, T. S. Tsareva, J. S. Haynes, B. J. Thacker, and S. U. Emerson. 1997. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. USA 949860-9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meng, X. J., B. Wiseman, F. Elvinger, D. K. Guenette, T. E. Toth, R. E. Engle, S. U. Emerson, and R. H. Purcell. 2002. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J. Clin. Microbiol. 40117-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okamoto, H., M. Takahashi, T. Nishizawa, K. Fukai, U. Muramatsu, and A. Yoshikawa. 2001. Analysis of the complete genome of indigenous swine hepatitis E virus isolated in Japan. Biochem. Biophys. Res. Commun. 289929-936. [DOI] [PubMed] [Google Scholar]
- 41.Panda, S. K., and S. Jameel. 1997. Hepatitis E virus: from epidemiology to molecular biology. Viral Hepat. Rev. 3227-251. [Google Scholar]
- 42.Panda, S. K., I. H. Ansari, H. Durgapal, S. Agrawal, and S. Jameel. 2000. The in vitro-synthesized RNA from a cDNA clone of hepatitis E virus is infectious. J. Virol. 742430-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reyes, G. R., C. C. Huang, A. W. Tam, and M. A. Purdy. 1993. Molecular organization and replication of hepatitis E virus (HEV). Arch. Virol. Suppl. 715-25. [DOI] [PubMed] [Google Scholar]
- 44.Reyes, G. R., C. C. Huang, P. O. Yarbough, and A. W. Tam. 1991. Hepatitis E virus. Comparison of ‘New and Old World’ isolates. J. Hepatol 13(Suppl. 4)S155-S161. [DOI] [PubMed] [Google Scholar]
- 45.Schlauder, G. G., G. J. Dawson, J. C. Erker, P. Y. Kwo, M. F. Knigge, D. L. Smalley, J. E. Rosenblatt, S. M. Desai, and I. K. Mushahwar. 1998. The sequence and phylogenetic analysis of a novel hepatitis E virus isolated from a patient with acute hepatitis reported in the United States. J. Gen. Virol. 79447-456. [DOI] [PubMed] [Google Scholar]
- 46.Schlauder, G. G., S. M. Desai, A. R. Zanetti, N. C. Tassopoulos, and I. K. Mushahwar. 1999. Novel hepatitis E virus (HEV) isolates from Europe: evidence for additional genotypes of HEV. J. Med. Virol. 57243-251. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi, M., T. Nishizawa, H. Miyajima, Y. Gotanda, T. Iita, F. Tsuda, and H. Okamoto. 2003. Swine hepatitis E virus strains in Japan form four phylogenetic clusters comparable with those of Japanese isolates of human hepatitis E virus. J. Gen. Virol. 84851-862. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi, M., T. Nishizawa, and H. Okamoto. 2003. Identification of a genotype III swine hepatitis E virus that was isolated from a Japanese pig born in 1990 and that is most closely related to Japanese isolates of human hepatitis E virus. J. Clin. Microbiol. 411342-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi, M., T. Nishizawa, A. Yoshikawa, S. Sato, N. Isoda, K. Ido, K. Sugano, and H. Okamoto. 2002. Identification of two distinct genotypes of hepatitis E virus in a Japanese patient with acute hepatitis who had not travelled abroad. J. Gen. Virol. 831931-1940. [DOI] [PubMed] [Google Scholar]
- 50.Tam, A. W., M. M. Smith, M. E. Guerra, C. C. Huang, D. W. Bradley, K. E. Fry, and G. R. Reyes. 1991. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology 185120-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas, D. L., P. O. Yarbough, D. Vlahov, S. A. Tsarev, K. E. Nelson, A. J. Saah, and R. H. Purcell. 1997. Seroreactivity to hepatitis E virus in areas where the disease is not endemic. J. Clin. Microbiol. 351244-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsarev, S. A., L. N. Binn, P. J. Gomatos, R. R. Arthur, M. K. Monier, H. van Cuyck-Gandre, C. F. Longer, and B. L. Innis. 1999. Phylogenetic analysis of hepatitis E virus isolates from Egypt. J. Med. Virol. 5768-74. [DOI] [PubMed] [Google Scholar]
- 53.Tsarev, S. A., S. U. Emerson, G. R. Reyes, T. S. Tsareva, L. J. Legters, I. A. Malik, M. Iqbal, and R. H. Purcell. 1992. Characterization of a prototype strain of hepatitis E virus. Proc. Natl. Acad. Sci. USA 89559-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsarev, S. A., T. S. Tsareva, S. U. Emerson, M. K. Rippy, P. Zack, M. Shapiro, and R. H. Purcell. 1995. Experimental hepatitis E in pregnant rhesus monkeys: failure to transmit hepatitis E virus (HEV) to offspring and evidence of naturally acquired antibodies to HEV. J. Infect. Dis. 17231-37. [DOI] [PubMed] [Google Scholar]
- 55.Tyagi, S., H. Korkaya, M. Zafrullah, S. Jameel, and S. K. Lal. 2002. The phosphorylated form of the ORF3 protein of hepatitis E virus interacts with its non-glycosylated form of the major capsid protein, ORF2. J. Biol. Chem. 27722759-22767. [DOI] [PubMed] [Google Scholar]
- 56.Tzeng, W. P., and T. K. Frey. 2003. Complementation of a deletion in the rubella virus p150 nonstructural protein by the viral capsid protein. J. Virol. 779502-9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, Y., R. Ling, J. C. Erker, H. Zhang, H. Li, S. Desai, I. K. Mushahwar, and T. J. Harrison. 1999. A divergent genotype of hepatitis E virus in Chinese patients with acute hepatitis. J. Gen. Virol. 80169-177. [DOI] [PubMed] [Google Scholar]
- 58.Wang, Y., H. Zhang, Z. Li, W. Gu, H. Lan, W. Hao, R. Ling, H. Li, and T. J. Harrison. 2001. Detection of sporadic cases of hepatitis E virus (HEV) infection in China using immunoassays based on recombinant open reading frame 2 and 3 polypeptides from HEV genotype 4. J. Clin. Microbiol. 394370-4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams, T. P., C. Kasorndorkbua, P. G. Halbur, G. Haqshenas, D. K. Guenette, T. E. Toth, and X. J. Meng. 2001. Evidence of extrahepatic sites of replication of the hepatitis E virus in a swine model. J. Clin. Microbiol. 393040-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zafrullah, M., M. H. Ozdener, S. K. Panda, and S. Jameel. 1997. The ORF3 protein of hepatitis E virus is a phosphoprotein that associates with the cytoskeleton. J. Virol. 719045-9053. [DOI] [PMC free article] [PubMed] [Google Scholar]