Abstract
We have discovered two metal ion binding compounds, pyrithione (PT) and hinokitiol (HK), that efficiently inhibit human rhinovirus, coxsackievirus, and mengovirus multiplication. Early stages of virus infection are unaffected by these compounds. However, the cleavage of the cellular eukaryotic translation initiation factor eIF4GI by the rhinoviral 2A protease was abolished in the presence of PT and HK. We further show that these compounds inhibit picornavirus replication by interfering with proper processing of the viral polyprotein. In addition, we provide evidence that these structurally unrelated compounds lead to a rapid import of extracellular zinc ions into cells. Imported Zn2+ was found to be localized in punctate structures, as well as in mitochondria. The observed elevated level of zinc ions was reversible when the compounds were removed. As the antiviral activity of these compounds requires the continuous presence of the zinc ionophore PT, HK, or pyrrolidine-dithiocarbamate, the requirement for zinc ions for the antiviral activity is further substantiated. Therefore, an increase in intracellular zinc levels provides the basis for a new antipicornavirus mechanism.
Curing virus infections harbors an enormous economic potential, and the search for new antiviral substances is of great interest for worldwide health. We have previously described the commonly used NF-κB inhibitor and metal ion chelator pyrrolidine-dithiocarbamate (PDTC) to significantly inhibit the replication of several picornaviruses such as human rhinovirus (HRV), poliovirus, coxsackievirus, and mengovirus (9, 22). These examples suggest that a common step in the life cycle of these picornaviruses is the target for the antiviral drug. In particular, we have demonstrated that PDTC has negative effects on picornavirus replication by influencing the processing of the viral polyprotein (21, 22).
The antiviral activity of PDTC is not restricted to the family Picornaviridae, since PDTC was shown to prevent the multiplication of human influenza virus, a member of the Orthomyxoviridae (33, 34). However, due to strong differences in the life cycle and host-cell interaction between human influenza virus and picornaviruses, it is likely that entirely different mechanisms might be relevant for the antiviral action of PDTC against these viruses.
Currently, the precise mode of the antiviral action of PDTC is unknown, although several theories have been substantiated with experimental evidence. Antioxidative properties of PDTC are postulated to be the reason for antiviral effects against influenza virus infections (33), which is not the case for human rhinovirus multiplication (9).
We have demonstrated that the antiviral effects of PDTC are metal ion dependent, and, in particular, Zn2+ ions play a pivotal role. To underline the hypothesis that influx of zinc into the cells has antiviral capacity, pyrithione (PT) and hinokitiol (HK) were examined. PT is known to be a zinc ionophore that leads to a rapid increase in intracellular zinc levels (27), and HK is a chelator of divalent metal ions (2).
We provide evidence that both PT and HK inhibit replication of picornaviruses by impairing viral polyprotein processing. The basis of the antiviral activity is dependent upon the availability of zinc ions. We show that the import of extracellular zinc ions is a key feature of the common antiviral property of these compounds.
MATERIALS AND METHODS
Cell, media, and reagents.
HeLa cells (strain Ohio; European Collection of Cell Cultures, Salisbury, United Kingdom) were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (all obtained from Gibco, Invitrogen, Austria). PDTC (pyrrolidine-dithiocarbamate ammonium salt), pyrithione (2-mercaptopyridine N-oxide sodium salt), Zn-EDTA, Ca-EDTA, Mg-EDTA, and N,N,N′,N′-tetrakis (2-pyridylmethyl) ethylenediamine (TPEN) were purchased from Sigma-Aldrich, Austria. HK (β-thujaplicin) from Calbiochem was dissolved in dimethyl sulfoxide and stored at −20°C.
Virus preparation and titration.
Strain HRV2 was obtained from the American Type Culture Collection and routinely grown in suspension cultures of HeLa cells (strain Ohio; Flow Laboratories, McLean, VA) as described previously (31). CVB3 (Nancy strain) was derived from transcription of the p53CVB3/T7 plasmid (36). The mengovirus strain EMCV was obtained upon transfection of in vitro-transcribed RNA from cDNA clone pM16.1 (5).
Virus titers in 50% tissue culture infectious doses (TCID50) were determined according to Reed and Muench (26). From our experience, typical interassay variation is less than ±0.5 log TCID50/ml.
Infection of cells.
HeLa cells were grown in six-well plates and infected at 70% confluence with HRV2 with indicated multiplicity of infection (MOI) in RPMI medium supplemented with 2% fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. After 2 h of infection, input virus was removed, and cells were washed three times with acidic HEPES buffer and once with phosphate-buffered saline (PBS). For further incubation, infection medium was applied for the indicated amounts of times. If indicated, chemicals were present during parts or the whole period of infection. Whenever HK was used, application of 30 mM MgCl2 was essential to avoid cytotoxicity. Virus production was determined in the supernatants of infected cells by TCID50 determination.
Confluent monolayers of HeLa cells were infected with CVB3 or mengovirus for 30 min. Cells were then washed twice with PBS and cultured in medium at 37°C in the presence or absence of indicated chemicals. At indicated time points, the cells were disrupted by three cycles of freezing and thawing, and virus titers were determined by end point titration.
Cell viability assay.
HeLa cells were infected with HRV2 (MOI = 50) or mock infected in the presence or absence of different concentrations of PT or HK-MgCl2. At 24 h postinfection (p.i.), a CellTiter 96 AQueous nonradioactive cell proliferation assay (Promega, WI) was performed according to the manufacturer's protocol. Extinction at 492 nm reflecting cell viability was measured in a Labsystems Multiscan RC plate reader.
Western blot analysis.
HeLa cells were infected with HRV2 as described for virus infection. At 0, 2, 4, 6, 8, and 24 h p.i., the medium was removed, and the cells were lysed by the addition of protein sample buffer. Protein extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), electroblotted onto polyvinylidene difluoride membranes, and analyzed using a rabbit polyclonal antibody against eIF4GI (37), as described previously (9).
Pulse-labeling and immunoprecipitation.
HeLa cells were preincubated with 10 μM PT or 125 μM HK-30 mM MgCl2 for 30 min and infected with HRV2 (MOI = 50) in the presence of the substances. At 6 h p.i., newly synthesized proteins were labeled with [32S]Met/Cys (Hartmann Analytic, Germany) for 1 hour. At 7, 8, and 9 h p.i., VP2 or VP2-comprising precursor proteins were isolated by immunoprecipitation using the monoclonal VP2-specific 8F5 antibodies (30) and analyzed by SDS-PAGE and autoradiography as described previously (21).
HeLa cells infected with CVB3 (MOI = 50) were incubated with 125 μM PDTC, 75 μM HK-30 mM MgCl2, or 10 μM PT in the presence or absence of 10 μM TPEN. Five hours p.i., cells were starved half an hour prior to 1 h of labeling with [35S]Met/Cys. After labeling, cells were lysed, and cellular extracts were subjected to immunoprecipitation using either antibodies specific for 3D or 3C (kindly provided by C. Cameron, Pennsylvania State University) as described previously (22). Then, the protein pattern was analyzed by SDS-PAGE and autoradiography.
Radioactive zinc uptake studies.
HeLa cells in serum-free culture medium (zinc concentration of 0.6 μM as measured by atomic absorption analysis) were incubated with 5 μM 65Zn2+ (0.82 mCi/mg Zn2+; Hartmann Analytic, Germany) in the presence of indicated concentrations of PDTC, PT, or HK-MgCl2. At the indicated time points, the cells were harvested by filtration on GF/C glass fiber filters (Whatman, Maidstone, United Kingdom). To remove extracellular 65Zn2+, filters were washed with a buffer containing EDTA (15 mM HEPES, 100 mM glucose, 150 mM KCl, 1 mM EDTA [pH 7]), and the amount of intracellular 65Zn2+ was determined in a Packard Cobra II γ-counter.
Fluorescence microscopy.
HeLa cells grown in μ-slide VI slides (Ibidi, Germany) were loaded with 5 μM FluoZin-3 acetoxymethyl ester (Invitrogen) or 1 μM RhodZin-3 acetoxymethyl ester (Invitrogen) in PBS for 15 min at 37°C. Subsequently, cells were incubated for 1 h in growth medium to allow de-esterification. Then, PDTC, PT, or HK was added to the medium for the indicated incubation times. In some experiments, chemicals were removed after 30 min, and fresh medium was applied for 1 h. Fluorescence was monitored in a live-cell microscope system (Olympus cellR) using fluorescein isothiocyanate settings for FluoZin-3 and Cy3 settings for RhodZin-3. A Hamamatsu Photonics Orca-ER camera was used.
RESULTS AND DISCUSSION
PT and HK inhibit picornavirus multiplication and increase cell viability of infected cells.
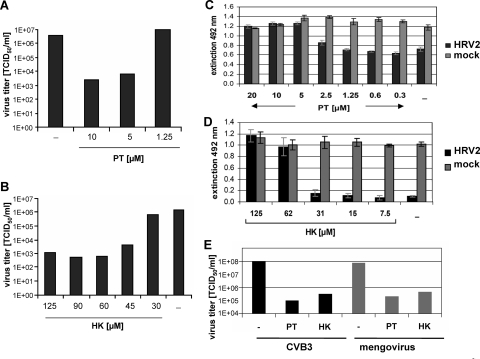
We have previously discovered that the drug PDTC substantially interferes with the multiplication of several picornaviruses (9, 22). These antiviral effects of PDTC are dependent on metal ions, in particular zinc ions. To further substantiate the hypothesis that zinc ions play a major role in virus inhibition, two structurally different metal ion binding compounds, PT (4, 12, 17, 35) and HK (2, 23, 32), were analyzed regarding their effects on HRV production (Fig. 1). HeLa cells were infected with HRV serotype 2 with an MOI of 20 in the presence or absence of PT or HK. At 24 h p.i., supernatants were collected, and virus titers were determined. The presence of 5 μM and 10 μM PT decreased viral titers by around 3 orders of magnitude (Fig. 1A). Likewise, HK significantly diminished viral multiplication in a dose-dependent manner (Fig. 1B). Similar results were obtained using a different HRV serotype (HRV14) or an alveolar carcinoma cell line (A549) (data not shown).
FIG. 1.
PT and HK dramatically reduce picornavirus multiplication and increase cell viability. Viral titers of HRV2-infected HeLa cells (MOI = 20) in the presence or absence of indicated concentrations of PT (A) or HK plus 30 mM MgCl2 (B) were determined 24 h p.i. Results for one representative experiment of three experiments are shown. HeLa cells were infected with HRV2 (MOI = 50) or mock infected, and different concentrations of PT (C) or HK-MgCl2 (D) were added. At 24 h p.i., a CellTiter 96 AQueous nonradioactive cell proliferation assay was performed according to the manufacturer's protocol. Extinction at 492 nm reflects cell viability. The means ± standard deviations of quintuples obtained in one representative experiment of three performed are shown. (E) HeLa cells were infected with CVB3 or mengovirus (MOI = 10) and incubated with 10 μM PT or 75 μM HK-30 mM MgCl2, respectively. Virus production of infected cells was determined by TCID50 assay at 24 h p.i. The results for one of two representative experiments are shown. −, control.
To quantify cell viability a colorimetric viability assay was performed. HeLa cells infected with HRV2 showed a significant loss of viability 24 h p.i. (Fig. 1C and D). In contrast, PT (Fig. 1C) and HK (Fig. 1D) retained viability to normal levels at concentrations between 5 μM and 20 μM PT and 62 μM and 125 μM HK, respectively. Based on additional experiments, 50% inhibitory concentrations of 3 μM for PT and 42 μM for HK were determined. Importantly, no cytotoxicity of PT and HK was found in mock-infected HeLa cells (Fig. 1C and D, gray bars) and A549 cells (data not shown) under these conditions. The absence of cytotoxicity is in contrast to reports about proapoptotic activities of PT and HK in other cell lines such as HL-60 cells (15, 19, 20).
It is of great interest to investigate whether PT and HK act specifically for HRV or whether these drugs target a common step in the picornavirus life cycle. Thus, the role of PT and HK during infection with coxsackievirus strain B3 (CVB3) and the mengovirus strain EMCV (which will hereafter be referred to as mengovirus) was examined (Fig. 1E). In a single-round infection experiment with CVB3 or mengovirus, viral titers of around 108 TCID50/ml were obtained. In the presence of PT or HK, the titers were significantly reduced by around 3 and 2 orders of magnitude, respectively. These data are in agreement with the antiviral activities of PDTC against these viruses that were reported previously (22).
PT and HK abolish eIF4GI cleavage and affect viral polyprotein processing.
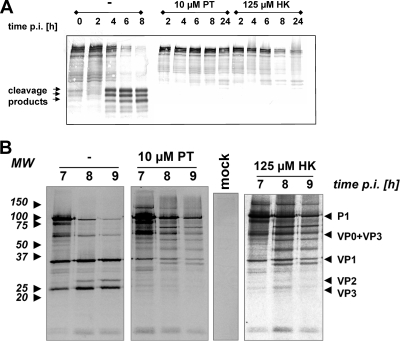
Different steps of the HRV infection in the presence of PT or HK were studied to analyze whether PT and HK act mechanistically similar to PDTC. Early events in the viral life cycle, such as virus entry and uncoating, were not affected by PT and HK (data not shown). A hallmark of rhinovirus infection is the cleavage of the eukaryotic translation initiation factors eIF4GI and eIF4GII by the viral 2Apro (7, 11), leading to the shutoff of host-cell protein synthesis (8). To test whether PT and HK affect eIF4GI cleavage, protein extracts of HRV2-infected cells were analyzed by Western blotting (Fig. 2A). In the absence of PT or HK, around 50% cleavage of eIF4GI was obtained at 4 h p.i. At 6 h and 8 h p.i., eIF4GI was nearly completely processed into its typical cleavage products. In the presence of PT or HK, no significant amounts of eIF4GI cleavage products were found within 24 h of infection. This is in agreement with data obtained with PDTC (9).
FIG. 2.
PT and HK abolish HRV2-triggered eIF4GI cleavage and affect viral polyprotein processing. (A) HRV2-infected HeLa cells (MOI = 20) were either mock treated or treated with 10 μM PT or 125 μM HK-30 mM MgCl2. After the indicated time points, protein extracts were collected and analyzed by Western blotting using an antibody specific for eIF4GI and its cleavage products. (B) HeLa cells were preincubated with 10 μM PT or 125 μM HK-30 mM MgCl2 for 30 min and infected with HRV2 (MOI = 50) in the presence of the substances. At 6 h p.i., newly synthesized proteins were labeled with [32S]Met/Cys for 1 hour. At the indicated time points, VP2 or VP2-containing precursor proteins were isolated by immunoprecipitation and analyzed by SDS-PAGE and autoradiography. Immunoprecipitation of mock-infected labeled cells did not show any signal. MW, molecular weight in thousands.
As PDTC shows a clear inhibition of viral polyprotein processing (21), P1 processing was also examined in the presence of PT or HK (Fig. 2B) by a pulse-chase labeling experiment, followed by immunoprecipitation with a VP2-specific antibody, 8F5, which pulls down VP2, VP2-containing precursors, and viral proteins bound to VP2 (30). Labeling of viral proteins started at 6 h p.i. for 60 min, and subsequently, cell lysates were prepared every hour. In untreated cells, the processing of the P1 precursor protein was clearly visible between 7 h and 9 h p.i. as a reduction of the amount of the corresponding polyprotein band. At the same time, smaller bands comprising VP2 and VP3 appeared. In the presence of either PT or HK, the processing of the P1 protein was blocked, and no mature VP2 or VP3 products could be detected. Moreover, various intermediate processing products were obtained in the presence of PT or HK, giving an identical picture to the processing defects observed using PDTC (21).
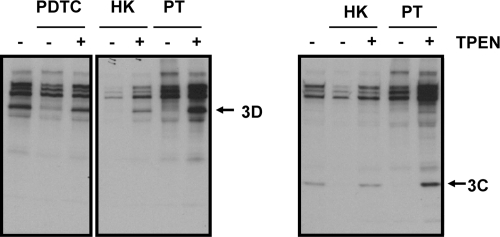
To specifically monitor the activity of 3CDpro, which is responsible for the processing of the P1 region and which autocleaves itself into 3Cpro and 3Dpol, the polyprotein-processing pattern of coxsackievirus infection in the presence of PDTC, PT, and HK was investigated (Fig. 3). CVB3-infected HeLa cells incubated with PDTC, HK, or PT were starved half an hour prior to 1 h of labeling at 5.5 h p.i. Subsequently, immunoprecipitation experiments using polyclonal antibodies raised against CVB3 3Cpro and 3Dpol were performed and analyzed as described above. PDTC, PT, and HK abolished coxsackievirus polyprotein processing into the mature viral proteins 3D and 3C in a similar manner indicating a common antiviral mechanism of all three compounds. The presence of 10 μM of the strong metal ion chelator TPEN restored viral polyprotein processing, suggesting that the observed inhibition is based on the availability of metal ions.
FIG. 3.
PT and HK abrogate 3D and 3C processing of CVB3. HeLa cells infected with coxsackievirus B3 (MOI = 50) were incubated with 125 μM PDTC, 75 μM HK-30 mM MgCl2, or 10 μM PT in the presence or absence of 10 μM TPEN. At 5 h p.i., cells were starved half an hour prior to 1 h of labeling with [35S]Met/Cys. Harvested cell lysates were subjected to immunoprecipitation using either antibodies specific for 3D (left panel) or 3C (right panel) and then analyzed by SDS-PAGE and autoradiography. Results for a representative experiment are shown. −, TPEN absent; +, TPEN present.
These data indicate that polyprotein processing of picornaviruses is a sensitive step for treatment with zinc ionophores. We have evidence that viruses such as respiratory syncytial virus (belonging to the family of Paramyxoviridae) which do not depend on polyprotein processing during their life cycle are not inhibited by PDTC treatment (data not shown). An unspecific adverse effect of PDTC on general cellular functions that lead to virus inhibition is therefore unlikely.
EDTA abolishes the antipicornavirus effects of PT and HK.
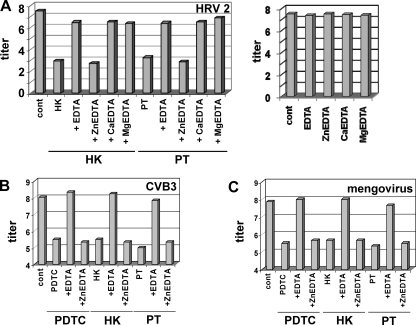
We and others have shown that treatment with PDTC or PT leads to a rapid elevation of the concentration of free cytoplasmic Zn2+ ions (6, 22, 16-18). The importance of metal ions was further substantiated by experiments using EDTA as a general extracellular metal ion chelator (Fig. 4). In the presence of 10 μM EDTA, the antiviral properties of PT and HK against HRV2 (Fig. 4A), CVB3 (Fig. 4B), and mengovirus (Fig. 4C) are lost, indicating the involvement of metal ions. By using Zn2+-, Ca2+-, or Mg2+-saturated EDTA (25), we demonstrate that only Zn-EDTA retains the antiviral property of PT or HK. This indicates that the availability of Zn2+ ions is a prerequisite for the antiviral activity of these compounds. None of the EDTA-metal ion complexes alone had any antiviral effect at the concentrations tested.
FIG. 4.
Zinc ions are responsible for the antiviral property of PT and HK. HeLa cells were infected with HRV2 (MOI = 20) (A), CVB3 (MOI = 10) (B), or mengovirus (MOI = 10) (C) and incubated with 10 μM EDTA or 10 μM of Zn-EDTA, Ca-EDTA, and Mg-EDTA, respectively. If indicated, 10 μM PT (A, B, and C), 125 μM HK-30 mM MgCl2 (A), or 75 μM HK-MgCl2 (B and C) was added at the time of infection. The virus titers in the supernatants of the infected cells were determined by a TCID50 assay at 24 h p.i. and shown as log TCID50/ml. Viral infection without addition of compounds is labeled as “cont.” The results for one representative experiment of three are shown.
PDTC, PT, and HK treatments cause a rapid and efficient influx of 65Zn2+ into cells.
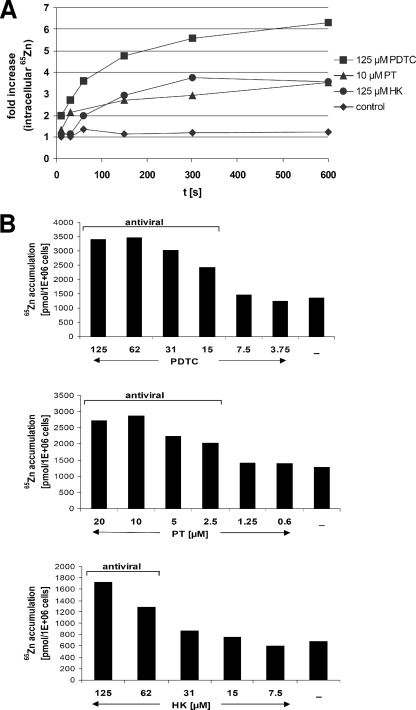
It is unclear whether PDTC, PT, and HK mobilize zinc ions localized in intracellular storage vesicles or allow extracellular ions to enter the cytoplasm. To directly monitor import of extracellular zinc ions into HeLa cells, we employed radioactive 65Zn2+ to determine the effects of PT, HK, and PDTC on zinc import (Fig. 5A). HeLa cells were incubated for different time periods with 5 μM 65Zn2+ and PDTC, PT, or HK and then harvested by filtration on GF/C glass fiber filters. The amount of intracellular 65Zn2+ was measured by γ-counting of the filter membranes. In the presence of 65Zn2+ alone, no significant increase in radioactivity of the collected cells was measured, whereas the presence of either PT or HK facilitated a threefold increase of intracellular 65Zn2+ within a few minutes. PDTC elevated the intracellular 65Zn2+ level sixfold. These results demonstrate that all three substances facilitate import of extracellular zinc ions via a rapid uptake mechanism.
FIG. 5.
PDTC, PT, and HK cause import of extracellular 65Zn2+ levels in a dose-dependent manner. (A) HeLa cells in serum-free culture medium were incubated with 5 μM 65Zn2+ and 125 μM PDTC, 10 μM PT, or 125 μM HK-30 mM MgCl2. At the indicated time points, the cells were harvested and washed with a buffer containing EDTA, and the amount of intracellular 65Zn2+ was determined in a Packard Cobra II γ-counter. Statistical variations between different samples are determined to be a maximal ±10% (data not shown). The results for one of three experiments are shown. (B) HeLa cells in serum-free culture medium were incubated with 5 μM 65Zn2+ and various concentrations of PDTC (top panel), PT (middle panel), or HK-MgCl2 (lower panel) for 15 min. Then, the cells were collected and washed, and the amount of intracellular 65Zn2+ was determined as described above. The results for one of five experiments are shown.
As shown in Fig. 5B, this zinc uptake is dose dependent. The presence of more than 15 μM PDTC, 2.5 μM PT, or 62 μM HK caused significant 65Zn2+ uptake after 15 min. It is noteworthy that this concentration range was found to be antiviral (Fig. 1) (9), suggesting that the influx of zinc ions is essential for mediating the common antiviral property of these compounds.
Intracellular localization of zinc ions imported via PT, HK, and PDTC.
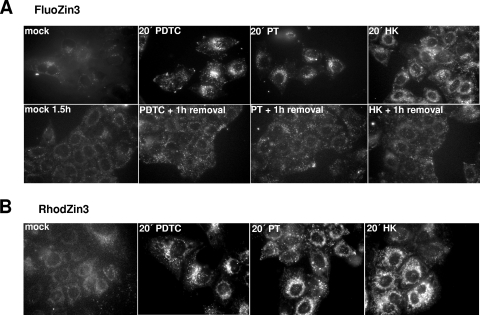
To directly visualize the ability of PDTC, PT, and HK to increase the intracellular pool of labile Zn2+, specific zinc indicators were used (Fig. 6). FluoZin-3 is a Zn2+-sensitive and -specific fluorescent probe that has no specific cellular localization (14). In cells loaded with FluoZin-3, intracellular fluorescence was minimal (Fig. 6A, top panel) due to a very low level of free zinc ions (24). After induction with 10 μM PT, 125 μM PDTC, or 125 μM HK, a rapid and significant increase in fluorescence was found reflecting an elevation of the intracellular labile “chelatable” zinc level. Noteworthy, these experiments were carried out in normal growth medium and apparently the Zn2+ concentration present in the medium is sufficient to lead to ionophore-mediated zinc ion transport.
FIG. 6.
Zinc ionophores influence the labile Zn2+ pool. HeLa cells were loaded with 5 μM FluoZin-3 (A) or 1 μM RhodZin-3 (B) in PBS for 15 min at 37°C. After extensive washing and 1 h of de-esterification in medium, zinc uptake was induced by treatment with 125 μM PDTC, 10 μM PT, and 125 μM HK-30 mM MgCl2 in growth medium. If indicated, after 30 min of chemical treatment, ionophores were removed for 1 hour (PDTC + 1 h removal, PT + 1 h removal, HK + 1 h removal). Pictures were taken at the indicated time points by live fluorescence microscopy with 60× optics. Examples of images of three independent experiments were chosen.
The observed punctate pattern of FluoZin-3 fluorescence is in agreement with recent data from rainbow trout cells incubated with high levels of extracellular zinc (24). In a large variety of mammalian cell types, vesicular storage sites for zinc which have been designated “zincosomes” can be detected (1). Zincosomes play a role in detoxifying excess zinc and are suggested to colocalize with endosomal compartments and lysosomes (13).
The uptake of zinc ions is reversible. When the zinc ionophores PDTC, PT, and HK were removed, the fluorescence declined close to background within 1 h (Fig. 6A, bottom panel).
To specifically monitor the free mitochondrial zinc pool, RhodZin-3 was used (28). HeLa cells loaded with RhodZin-3 exhibited a very weak fluorescence, indicating low levels of labile Zn2+ inside the mitochondrial compartment (Fig. 6B). In contrast, upon 20 min of treatment with PDTC, PT, or HK, the signal of RhodZin-3 fluorescence was strongly enhanced. This mitochondrial localized signal was found to be constant over at least 80 min of PT incubation (data not shown). The role of a labile Zn2+ fraction in mitochondria during viral infection is currently unclear and has to be the subject of further studies.
Concluding remarks.
Conclusively, all three antiviral substances are potent zinc ionophores increasing the intracellular pool of labile zinc. This strong elevation is constant as long as the zinc ionophores are present but is reversible after removal of PDTC, PT, or HK.
The reversibility of the elevation of free-zinc concentrations is in agreement with data that we had obtained when virus multiplication was monitored in a setup when PT, HK, and PDTC were present for short intervals only. In contrast to the significant viral titer reduction evoked by permanently present PDTC, PT, or HK, a short (1 h) pulse of zinc ionophore treatment between 2 h and 5 h p.i. was not sufficient to significantly reduce viral titers (data not shown). These data indicate that a prolonged presence of ionophores during viral replication is needed to exert antiviral effects. In previous data, we have shown that a clear reduction of virus titer was obtained when PDTC treatment started up to 4 h after infection, continuing to 24 h (9).
In conclusion, we have identified a set of three antiviral compounds against HRV, coxsackievirus, and mengovirus infections, which differ in their chemical structures but appear to use import of zinc ions as a common trigger for exerting their antiviral effect. It was reported that PDTC reduces coxsackievirus B3 replication through inhibition of the ubiquitin-proteasome pathway (29). We tested the proteasome inhibitor MG-132 at nontoxic concentrations for antiviral properties against HRV2 and HRV14. However, functional MG-132 concentrations that cause a massive accumulation of ubiquitinylated proteins within 8 h of incubation were not antiviral (data not shown). Thus, proteasomal inhibition is not likely to be the major cause of the antiviral activity of PDTC, PT, or HK.
Importantly, PDTC, PT, and HK lead to efficient import of zinc ions that are naturally present in the cell culture medium. Uptake of Zn2+ into cells interferes with the viral life cycle. Already in 1974, Butterworth and colleagues have shown that adding high concentrations of zinc ions to cells impairs picornavirus polyprotein processing (3); however, the mechanistic basis still remains incompletely understood. On the one hand, viral proteases might be affected in their functions directly; on the other hand, zinc ions could contribute to folding problems of the viral polyprotein that lead to inseparable precursors. Folding inhibition was recently suggested as a target for antiviral strategies (10).
Several attempts have been employed to create mutants resistant against PDTC during human rhinovirus or influenza virus infections. However, no resistant viruses were obtained, hinting at a cellular mechanism of action which is expected to be similar for HK and PT. On account of these findings, PDTC, PT, and HK may provide an interesting basis for the development of new classes of antipicornaviral therapeutics.
Acknowledgments
This work was supported by grant LS05-039 of the Vienna Science and Technology Fund to J.S. and by grant NWO-VIDI-6 917.46.305 of The Netherlands Organization for Scientific Research and the M.W. Beijerink Virology Fund from the Royal Netherlands Academy of Sciences to F.J.M.V.K.
We thank C. Cameron (Pennsylvania State University) for the kind gift of antibodies, Andrea Trienoll for technical help, and Hans Goldenberg for helpful discussions.
Footnotes
Published ahead of print on 15 October 2008.
REFERENCES
- 1.Beyersmann, D., and H. Haase. 2001. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals 14331-341. [DOI] [PubMed] [Google Scholar]
- 2.Bryant, F., and B. T. Overell. 1953. Quantitative chromatographic analysis of organic acids in plant tissue extracts. Biochim. Biophys. Acta 10471-476. [DOI] [PubMed] [Google Scholar]
- 3.Butterworth, B. E., and B. D. Korant. 1974. Characterization of the large picornaviral polypeptides produced in the presence of zinc ion. J. Virol. 14282-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crutchfield, C. E., III, E. J. Lewis, and B. D. Zelickson. 1997. The highly effective use of topical zinc pyrithione in the treatment of psoriasis: a case report. Dermatol. Online J. 33. [PubMed] [Google Scholar]
- 5.Duke, G. M., and A. C. Palmenberg. 1989. Cloning and synthesis of infectious cardiovirus RNAs containing short, discrete poly(C) tracts. J. Virol. 631822-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erl, W., C. Weber, and G. K. Hansson. 2000. Pyrrolidine dithiocarbamate-induced apoptosis depends on cell type, density, and the presence of Cu(2+) and Zn(2+). Am. J. Physiol. Cell Physiol. 278C1116-C1125. [DOI] [PubMed] [Google Scholar]
- 7.Etchison, D., and S. Fout. 1985. Human rhinovirus 14 infection of HeLa cells results in the proteolytic cleavage of the p220 cap-binding complex subunit and inactivates globin mRNA translation in vitro. J. Virol. 54634-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etchison, D., S. C. Milburn, I. Edery, N. Sonenberg, and J. W. Hershey. 1982. Inhibition of HeLa cell protein synthesis following poliovirus infection correlates with the proteolysis of a 220,000-dalton polypeptide associated with eucaryotic initiation factor 3 and a cap binding protein complex. J. Biol. Chem. 25714806-14810. [PubMed] [Google Scholar]
- 9.Gaudernak, E., J. Seipelt, A. Triendl, A. Grassauer, and E. Kuechler. 2002. Antiviral effects of pyrrolidine dithiocarbamate on human rhinoviruses. J. Virol. 766004-6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geller, R., M. Vignuzzi, R. Andino, and J. Frydman. 2007. Evolutionary constraints on chaperone-mediated folding provide an antiviral approach refractory to development of drug resistance. Genes Dev. 21195-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gradi, A., H. Imataka, Y. V. Svitkin, E. Rom, B. Raught, S. Morino, and N. Sonenberg. 1998. A novel functional human eukaryotic translation initiation factor 4G. Mol. Cell. Biol. 18334-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guthery, E., L. A. Seal, and E. L. Anderson. 2005. Zinc pyrithione in alcohol-based products for skin antisepsis: persistence of antimicrobial effects. Am. J. Infect. Control 3315-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haase, H., and D. Beyersmann. 2002. Intracellular zinc distribution and transport in C6 rat glioma cells. Biochem. Biophys. Res. Commun. 296923-928. [DOI] [PubMed] [Google Scholar]
- 14.Haase, H., and W. Maret. 2003. Intracellular zinc fluctuations modulate protein tyrosine phosphatase activity in insulin/insulin-like growth factor-1 signaling. Exp. Cell Res. 291289-298. [DOI] [PubMed] [Google Scholar]
- 15.Inamori, Y., H. Tsujibo, H. Ohishi, F. Ishii, M. Mizugaki, H. Aso, and N. Ishida. 1993. Cytotoxic effect of hinokitiol and tropolone on the growth of mammalian cells and on blastogenesis of mouse splenic T cells. Biol. Pharm. Bull. 16521-523. [DOI] [PubMed] [Google Scholar]
- 16.Kim, C. H., J. H. Kim, C. Y. Hsu, and Y. S. Ahn. 1999. Zinc is required in pyrrolidine dithiocarbamate inhibition of NF-kappaB activation. FEBS Lett. 44928-32. [DOI] [PubMed] [Google Scholar]
- 17.Kim, C. H., J. H. Kim, S. J. Moon, K. C. Chung, C. Y. Hsu, J. T. Seo, and Y. S. Ahn. 1999. Pyrithione, a zinc ionophore, inhibits NF-kappaB activation. Biochem. Biophys. Res. Commun. 259505-509. [DOI] [PubMed] [Google Scholar]
- 18.Kim, C. H., J. H. Kim, J. Xu, C. Y. Hsu, and Y. S. Ahn. 1999. Pyrrolidine dithiocarbamate induces bovine cerebral endothelial cell death by increasing the intracellular zinc level. J. Neurochem. 721586-1592. [DOI] [PubMed] [Google Scholar]
- 19.Kondoh, M., E. Tasaki, S. Araragi, M. Takiguchi, M. Higashimoto, Y. Watanabe, and M. Sato. 2002. Requirement of caspase and p38MAPK activation in zinc-induced apoptosis in human leukemia HL-60 cells. Eur. J. Biochem. 2696204-6211. [DOI] [PubMed] [Google Scholar]
- 20.Kondoh, M., E. Tasaki, M. Takiguchi, M. Higashimoto, Y. Watanabe, and M. Sato. 2005. Activation of caspase-3 in HL-60 cells treated with pyrithione and zinc. Biol. Pharm. Bull. 28757-759. [DOI] [PubMed] [Google Scholar]
- 21.Krenn, B. M., B. Holzer, E. Gaudernak, A. Triendl, F. J. van Kuppeveld, and J. Seipelt. 2005. Inhibition of polyprotein processing and RNA replication of human rhinovirus by pyrrolidine dithiocarbamate involves metal ions. J. Virol. 7913892-13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanke, K., B. M. Krenn, W. J. Melchers, J. Seipelt, and F. J. van Kuppeveld. 2007. PDTC inhibits picornavirus polyprotein processing and RNA replication by transporting zinc ions into cells. J. Gen. Virol. 881206-1217. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto, D., Y. Kusagaya, N. Endo, A. Sometani, S. Takeo, T. Suzuki, Y. Arima, K. Nakajima, and Y. Suzuki. 1998. Thujaplicin-copper chelates inhibit replication of human influenza viruses. Antiviral Res. 3989-100. [DOI] [PubMed] [Google Scholar]
- 24.Muylle, F. A., D. Adriaensen, W. De Coen, J. P. Timmermans, and R. Blust. 2006. Tracing of labile zinc in live fish hepatocytes using FluoZin-3. Biometals 19437-450. [DOI] [PubMed] [Google Scholar]
- 25.Perrin, D. D. 1979. Stability constants of metal-ion complexes: organic ligands. Pergamon, Oxford, United Kingdom.
- 26.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27493-497. [Google Scholar]
- 27.Sensi, S. L., L. M. Canzoniero, S. P. Yu, H. S. Ying, J. Y. Koh, G. A. Kerchner, and D. W. Choi. 1997. Measurement of intracellular free zinc in living cortical neurons: routes of entry. J. Neurosci. 179554-9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sensi, S. L., D. Ton-That, J. H. Weiss, A. Rothe, and K. R. Gee. 2003. A new mitochondrial fluorescent zinc sensor. Cell Calcium 34281-284. [DOI] [PubMed] [Google Scholar]
- 29.Si, X., B. M. McManus, J. Zhang, J. Yuan, C. Cheung, M. Esfandiarei, A. Suarez, A. Morgan, and H. Luo. 2005. Pyrrolidine dithiocarbamate reduces coxsackievirus B3 replication through inhibition of the ubiquitin-proteasome pathway. J. Virol. 798014-8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skern, T., C. Neubauer, L. Frasel, P. Grundler, W. Sommergruber, M. Zorn, E. Kuechler, and D. Blaas. 1987. A neutralizing epitope on human rhinovirus type 2 includes amino acid residues between 153 and 164 of virus capsid protein VP2. J. Gen. Virol. 68315-323. [DOI] [PubMed] [Google Scholar]
- 31.Skern, T., W. Sommergruber, D. Blaas, C. Pieler, and E. Kuechler. 1984. Relationship of human rhinovirus strain 2 and poliovirus as indicated by comparison of the polymerase gene regions. Virology 136125-132. [DOI] [PubMed] [Google Scholar]
- 32.Trust, T. J., and R. W. Coombs. 1973. Antibacterial activity of beta-thujaplicin. Can. J. Microbiol. 191341-1346. [DOI] [PubMed] [Google Scholar]
- 33.Uchide, N., and K. Ohyama. 2003. Antiviral function of pyrrolidine dithiocarbamate against influenza virus: the inhibition of viral gene replication and transcription. J. Antimicrob. Chemother. 528-10. [DOI] [PubMed] [Google Scholar]
- 34.Uchide, N., K. Ohyama, T. Bessho, B. Yuan, and T. Yamakawa. 2002. Effect of antioxidants on apoptosis induced by influenza virus infection: inhibition of viral gene replication and transcription with pyrrolidine dithiocarbamate. Antiviral Res. 56207-217. [DOI] [PubMed] [Google Scholar]
- 35.Warner, R. R., J. R. Schwartz, Y. Boissy, and T. L. Dawson, Jr. 2001. Dandruff has an altered stratum corneum ultrastructure that is improved with zinc pyrithione shampoo. J. Am. Acad. Dermatol. 45897-903. [DOI] [PubMed] [Google Scholar]
- 36.Wessels, E., D. Duijsings, R. A. Notebaart, W. J. Melchers, and F. J. van Kuppeveld. 2005. A proline-rich region in the coxsackievirus 3A protein is required for the protein to inhibit endoplasmic reticulum-to-Golgi transport. J. Virol. 795163-5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan, R., W. Rychlik, D. Etchison, and R. E. Rhoads. 1992. Amino acid sequence of the human protein synthesis initiation factor eIF-4 gamma. J. Biol. Chem. 26723226-23231. [PubMed] [Google Scholar]