Abstract
Avian influenza A virus A/teal/HK/W312/97 (H6N1) possesses seven gene segments that are highly homologous to those of highly pathogenic human influenza H5N1 viruses, suggesting that a W312-like H6N1 virus might have been involved in the generation of the A/HK/97 H5N1 viruses. The continuous circulation and reassortment of influenza H6 subtype viruses in birds highlight the need to develop an H6 vaccine to prevent potential influenza pandemics caused by the H6 viruses. Based on the serum antibody cross-reactivity data obtained from 14 different H6 viruses from Eurasian and North American lineages, A/duck/HK/182/77, A/teal/HK/W312/97, and A/mallard/Alberta/89/85 were selected to produce live attenuated H6 candidate vaccines. Each of the H6 vaccine strains is a 6:2 reassortant ca virus containing HA and NA gene segments from an H6 virus and the six internal gene segments from cold-adapted A/Ann Arbor/6/60 (AA ca), the master donor virus that is used to make live attenuated influenza virus FluMist (intranasal) vaccine. All three H6 vaccine candidates exhibited phenotypic properties of temperature sensitivity (ts), ca, and attenuation (att) conferred by the internal gene segments from AA ca. Intranasal administration of a single dose of the three H6 ca vaccine viruses induced neutralizing antibodies in mice and ferrets and fully protected mice and ferrets from homologous wild-type (wt) virus challenge. Among the three H6 vaccine candidates, the A/teal/HK/W312/97 ca virus provided the broadest cross-protection against challenge with three antigenically distinct H6 wt viruses. These data support the rationale for further evaluating the A/teal/HK/W312/97 ca vaccine in humans.
Influenza type A viruses cause seasonal epidemics and occasional pandemics in humans. There are a total of 16 hemagglutinin (HA) subtypes (H1 to H16) and 9 neuraminidase (NA) subtypes (N1 to N9) of influenza virus A identified (10, 42). While only a limited number of HA and NA subtypes are circulating in humans and other mammalian species, all the HA and NA subtypes are found in avian species. Avian influenza (AI) viruses are the source of novel subtypes that infect humans. In the past century, AI viruses caused three influenza pandemics when a pandemic strain with a novel HA from an avian virus infected immunologically naïve humans and spread efficiently from person to person. The H1N1 virus that caused the 1918 catastrophic pandemic was possibly derived from an avian-like virus that had been adapted in a mammalian host (31, 39). The 1957 Asian H2N2 and 1968 Hong Kong H3N2 pandemic strains were reassortants generated by reassortment between an avian and a human influenza virus (18).
Thus, pandemic influenza virus strains could emerge from direct transmission of AI viruses or from reassortment between an AI and a currently circulating human strain. Direct transmission of AI viruses to humans has occurred for H5N1 viruses since 1997. During the 1997 Hong Kong outbreak of the highly pathogenic AI (HPAI) H5N1 virus in chickens, there were at least 18 confirmed human cases of infection by an avian H5N1 virus (9, 37). Additional human cases of avian H5N1 infections have been reported since 2003; there have been 376 confirmed cases and 238 fatalities reported in many countries in Asia, Europe, and Africa as of April 2008 (http://www.who.int/csr/disease/avian_influenza/en/). In addition to the HPAI H5N1 viruses, avian viruses from other subtypes, including H9N2, H5N1, H7N7, H7N3, and H10N7, have been implicated in human infections. An HPAI H7N7 virus caused 89 human infections including one fatal case during a severe disease outbreak in domestic poultry in Netherlands in 2003 (11, 22). Thus, avian viruses could constitute a potential pandemic threat to public health.
The H6 subtype is one of the most commonly recognized subtypes in domestic ducks in southern China (7, 34) and in migratory birds in North America and Europe (27, 35). H6 viruses have caused several outbreaks in commercial poultry worldwide that resulted in decreased egg production and increased mortality (1, 40, 41). Although natural human infection with this virus subtype has not yet been reported, H6N1 viruses can replicate in the upper respiratory tract and cause mild clinical symptoms in experimental infection (3). A recent study showed significantly elevated antibody titers against H5, H6, and H7 AI viruses in United States veterinarians who have been exposed to birds, suggesting that human infections with H6 viruses could have occurred (29). The continuing prevalence of H6 viruses and frequent reassortment in avian populations highlight the potential for H6 viruses and H6 reassortants to cross the species barrier to infect humans and cause human-to-human transmission. In addition, studies of HPAI H5N1 viruses showed important contributions of internal protein genes such as PB2 and NS1 to virus replication and virulence in hosts (14, 17, 33). The high level of homology of the internal protein genes of H6N1 A/teal/Hong Kong/W312/97 (tl/HK/97)-like viruses to those of the 1997 human H5N1 viruses raises concerns about W312-like H6 viruses. The continued cocirculation of H5N1, H6N1, and H9N2 influenza viruses in southern China could lead to frequent reassortment, which would greatly increase the genetic diversity of influenza viruses in this region (7, 8).
Vaccination is one of the most effective preventive measures for the control of influenza (36). FluMist, a live attenuated influenza virus (LAIV) vaccine, was licensed in the United States in 2003 and elicits both systemic and local mucosal immunity (4, 28). This live attenuated vaccine approach has been used to generate live attenuated H5N1, H7N3, and H9N2 vaccine viruses. These candidate vaccines were found to be safe and efficacious in conferring protection against wild-type (wt) virus challenge in mice and ferrets (5, 21, 24, 38) and are being evaluated in phase 1 clinical studies.
In this study, we describe the selection and generation of three live attenuated H6 vaccine candidates that contain the HA and NA gene segments from wt H6 viruses and the six internal protein gene segments from the cold-adapted A/Ann Arbor/6/60 (AA ca) vaccine donor virus, which is used to produce seasonal LAIV. We analyzed the H6 vaccine candidates for their temperature sensitivity (ts), ca, and attenuation (att) phenotypes and for their immunogenicity and protective efficacy in ferrets and mice. We conclude that tl/HK/97 ca is a promising H6 vaccine candidate that provides broad protection against diverse influenza H6 viruses.
MATERIALS AND METHODS
Viruses and cells.
wt H6 AI viruses used in this study were obtained from the influenza virus repository at St. Jude Children's Research Hospital, Memphis, TN (Table 1). Virus stocks were propagated in the allantoic cavities of 9- to 11-day-old embryonated specific-pathogen-free hen's eggs (Charles River Laboratories, North Franklin, CT) at 37°C. The allantoic fluids were harvested at 72 h postinoculation and tested for hemagglutinating activity using 0.5% turkey red blood cells (Lampire Biological Laboratories, Pipersville, PA). Allantoic fluids were pooled, aliquoted, and stored at −80°C until use. Fifty-percent tissue culture infectious dose (TCID50) titers were determined in Madin-Darby canine kidney (MDCK) cells (ATCC, Manassas, VA) as described previously (12).
TABLE 1.
AI virus isolates of the H6 subtype included in this study
| Virus name | Abbreviation | Subtype | Phylogenetic lineagea |
|---|---|---|---|
| A/duck/HK/182/77 | dk/HK/77 | H6N9 | Eurasian |
| A/teal/HK/W312/97 | tl/HK/97 | H6N1 | Eurasian |
| A/quail/HK/1721-30/99 | qu/HK/99 | H6N1 | Eurasian |
| A/mallard/Alberta/89/85 | ma/Alb/85 | H6N2 | North American |
Phylogenetic analysis based on HA amino acid sequence was generated using the neighbor joining method rooted on the AA H2 HA.
Chicken embryo kidney (CEK) cells were obtained from Charles River Laboratories (North Franklin, CT) and maintained in M199 medium (HyClone, Logan, UT). Vero (African green monkey kidney) cells were maintained in OptiPRO serum-free medium (Invitrogen, Carlsbad, CA), and MDCK cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (Invitrogen).
Generation of reassortant H6 ca viruses by plasmid rescue.
Reassortant H6 ca viruses were generated using the eight-plasmid reverse genetics system (16). Viral RNA was extracted from H6 wt viruses by use of the QIAamp viral RNA extraction kit (Qiagen, Valencia, CA). The HA and NA cDNAs of H6 wt strains were amplified by one-step reverse transcriptase-PCR using viral RNA as a template and HA- or NA-specific oligonucleotide primers (primer sequences are available upon request). The HA and NA cDNAs were cloned into the BsmBI site of the pAD3000 vector, and the sequences of the resulting clones were confirmed. The six internal gene segments of AA ca were also cloned into the pAD3000 vector (20). To produce the 6:2 reassortant H6 ca viruses, Vero cells were transfected with the six AA ca internal gene plasmids and the HA and NA plasmids derived from selected wt H6 viruses by electroporation. Briefly, 3 μg of each of the eight plasmids was added to a suspension of approximately 5.0 × 106 viable Vero cells in Opti-MEM I in a volume of 300 μl and electroporated at a setting of 220 V, 950 microfarads by use of a Bio-Rad gene pulser Xcell system (Bio-Rad, Hercules, CA). The electroporated cells were resuspended in 2 ml of Opti-MEM I and overlaid onto a 50%-confluent CEK cell monolayer that had been washed once with Opti-MEM I. The electroporated Vero cells were cocultured with CEK cells at 33°C for approximately 20 h and replenished with fresh Opti-MEM I containing 0.5 μg/ml N-p-tosyl-l-phenylalanine chloromethyl ketone trypsin (Sigma-Aldrich, St. Louis, MO). After 3 to 7 days of incubation at 33°C, the culture supernatants were harvested, filtered with a 0.8-μm Minisart syringe filter (Sartorius, Edgewood, NY), and inoculated into embryonated hen's eggs followed by incubation at 33°C for 3 days. The egg-amplified viruses were titered by plaque assay in CEK cells or by a TCID50 assay in CEK cells or MDCK cells.
Plaque assay in CEK cells.
Confluent CEK cells in six-well plates were infected with 10-fold serially diluted virus and absorbed for 1 h with constant rotation. The inoculum was removed and cells were overlaid with 0.8% low-melting-point agarose in 1× minimal essential medium (MEM) and incubated at 33°C for 4 days. Plaques were immunostained with a chicken polyclonal serum against AA.
Phenotypic assays.
The ts and ca phenotypes of H6 wt and ca viruses were assessed by virus titration at 39, 33, and 25°C in CEK cells by the TCID50 assay. In brief, serial 10-fold dilutions of virus samples in MEM were added to the washed CEK cell monolayers in 96-well plates and incubated at 39, 33, and 25°C. Replicates of eight wells were used for each dilution. Cells incubated at 33 or 39°C were examined for cytopathic effect (CPE) at 6 days postinfection (dpi), and cells incubated at 25°C were examined for CPE at 10 dpi. A virus is defined as ts if its titer at 39°C is at least 100-fold lower than its titer at 33°C. A virus is defined as ca if the difference in titers at 33 and 25°C is less than 100-fold (20).
Ferret studies.
Male ferrets of 7 to 8 weeks of age (Triple F Farm, Sayre, PA) were used in the study. Ferret study protocols were approved by MedImmune's Animal Care and Use Committee. To examine the replication of H6 wt and ca viruses in the respiratory tracts of ferrets, groups of two ferrets were inoculated intranasally (i.n.) with 107 PFU of H6 wt or ca viruses. At 3 and 5 dpi, ferrets were euthanized, and their lungs and nasal turbinates (NT) were harvested. Ten-percent (wt/vol) tissue suspensions of lung homogenate were prepared and virus titers were determined by infection in eggs and calculated as the 50% egg infectious dose (EID50) per gram of tissue as described previously (15). Virus titers in the NT tissue homogenate were determined by plaque assay in CEK cells and expressed as log10 PFU per gram of tissue.
To examine H6 ca viruses for their immunogenicity and protective efficacy, groups of three or four ferrets were inoculated i.n. with 107 PFU of H6 ca virus, namely, A/duck/Hong Kong/182/77 (dk/HK/77 ca), A/mallard/Alberta/89/85 (ma/Alb/85 ca), tl/HK/97 ca, or H1N1 A/New Caledonia/20/99 ca or with MEM (mock immunized) in a volume of 0.25 ml/nostril. Serum was collected prior to virus inoculation and at 32 dpi the animals were challenged with homologous and heterologous H6 wt viruses at a dose of 107 PFU administered i.n. The body weights and temperatures of these animals were measured prior to challenge infection and daily after challenge infection. On day 3 postchallenge, the NT and lungs were collected and virus titers were determined as log10 PFU per gram of NT or log10 EID50 per gram of lung tissue as described above. Ferret serum antibody levels against homologous or heterologous H6 wt or ca viruses were measured by hemagglutination inhibition (HAI) and microneutralization assays as described previously (12).
Mouse studies.
Six- to 8-week-old female BALB/c mice (Taconic Farms, Inc., Germantown, NY) were used in all mouse experiments. Mouse experiments were approved by the National Institutes of Health Animal Care and Use Committee and were conducted at the NIH.
One-dose study.
Groups of 44 lightly anesthetized mice were inoculated i.n. with 106 TCID50 of H6 ca viruses, namely, tl/HK/97 ca, dk/HK/77 ca, and ma/Alb/85 ca. Each virus was diluted in Leibovitz (L15) medium (Invitrogen, Carlsbad, CA) to a final volume of 50 μl/mouse. Mock-inoculated controls were administered L15 medium alone. Neutralizing antibody (NtAb) responses to H6 wt viruses (dk/HK/77, ma/Alb/85, tl/HK/97, and A/quail/HK/1721-30/99 [qu/HK/99]) were determined for sera collected prior to inoculation (prebleed) and at 4 weeks postinoculation by the method described previously (12). The NtAb titer was defined as the reciprocal of the highest dilution of serum that completely neutralized the infectivity of 100 TCID50 of virus in MDCK cells, identified by the absence of CPE from the neutralized samples on day 4. A NtAb titer that was fourfold lower than the homologous NtAb titer was considered significantly different and indicative of a lack of cross-reactivity as previously described (2). At 4 weeks postinoculation, groups of eight mice were challenged i.n. with 105 TCID50 of the H6 wt viruses dk/HK/77 and ma/Alb/85. Groups of 14 mice were challenged with 105 TCID50 of wt tl/HK/97 or wt qu/HK/99. Four mice per H6 challenge virus were sacrificed on days 2 and 4 postchallenge, and lungs and NT were harvested and stored at −80°C. Organs were weighed and homogenized in L15 medium containing 1× antibiotic-antimycotic (Invitrogen) to make 10% and 5% (wt/vol) tissue homogenates of lung and NT, respectively. Tissue homogenates clarified by centrifugation at 1,500 rpm for 5 min were titered in MDCK cells as described previously (38). Virus titers in TCID50/g were calculated by the method of Reed and Muench (30).
Two-dose study.
Groups of 44 lightly anesthetized mice were inoculated i.n. with 106 TCID50 of H6 ca viruses tl/HK/97 ca, dk/HK/77 ca, and ma/Alb/85 ca on day 0 and administered a second dose of each virus 4 weeks after the first dose. Mock-inoculated controls were administered L15 medium alone. At 4 weeks after the second dose of H6 ca virus, groups of eight mice were challenged i.n. with H6 wt viruses as follows: 105 TCID50 of dk/HK/77 and 105 TCID50 of ma/Alb/85. Groups of 14 mice were challenged with wt viruses as follows: 105 TCID50 and tl/HK/97 or 105 TCID50 of qu/HK/99. As described for the one-dose study, immunogenicity and viral replication were determined following virus challenge. NtAb responses to H6 wt viruses (dk/HK/77, ma/Alb/85, tl/HK/97, and qu/HK/99) were determined for sera collected prior to inoculation with the H6 ca viruses, 4 weeks after the first dose, and 2 weeks after the second dose.
RESULTS
Generation of H6 ca viruses.
Based on genetic analysis and the antigenic cross-reactivity of postinfection sera of mice and ferrets infected with 14 H6 viruses (including H6N1, H6N2, H6N5, H6N8, and H6N9 viruses) isolated from different continents from 1973 to 2001 (12), three H6 viruses (Table 1), namely, dk/HK/77 (H6N9), ma/Alb/85 (H6N2), and tl/HK/97 (H6N1), were selected for vaccine development. These three H6 strains represent diverse strains in the H6 HA phylogenetic tree (8, 13).
The six internal protein gene segments of AA ca, the master donor virus, confer the characteristic ts, ca, and att phenotypes to the seasonal influenza virus FluMist (i.n.) vaccines (20). This AA ca donor virus was used to generate live attenuated H6 vaccine candidates. The 6:2 H6 AA ca reassortant (H6 ca) viruses contained the HA and NA gene segments from an H6 wt virus and the six internal gene segments from the AA ca virus, which were generated using the eight-plasmid reverse genetics system (16). Viruses rescued from transfected Vero/CEK cocultures were amplified once in embryonated hen's eggs, and virus titers were determined by plaque assay in CEK cells at 33°C. Each of the H6 ca virus had a plaque morphology similar to that of the corresponding wt virus (data not shown). tl/HK/97 ca formed the largest plaques and ma/Alb/85 ca formed the smallest plaques among the three ca viruses. All three H6 ca viruses replicated efficiently in eggs to titers greater than 108 PFU/ml.
H6 ca viruses are ca and ts.
The six internal gene segments from AA ca were expected to confer the ts, ca, and att phenotypes to the reassortant ca viruses. The H6 wt viruses replicated equally well at 39 and 33°C, but all three ca viruses were restricted in replication at 39°C, with a 1,000-fold reduction in titer compared to what was seen at 33°C, confirming the ts phenotype (Table 2). The three ca viruses replicated better at 25°C than did the H6 wt virus counterparts. The wt H6 viruses did not exhibit the ca phenotype; in each case, the replication of the wt virus at 25°C was reduced by more than 1,000-fold compared to the replication of the same virus at 33°C. As expected, the H6 ca viruses expressed the ca phenotype; the titer of each virus at 25°C was within 100-fold of its titer at 33°C (6, 26). Thus, the three H6 ca viruses displayed the expected ts and ca phenotypes.
TABLE 2.
H6 ca viruses exhibit ts and ca phenotypesa
| Virus | Virus titer (log10 TCID50/ml) at:
|
Phenotype
|
|||||
|---|---|---|---|---|---|---|---|
| 39°C | 33°C | 25°C | Δ33/39°C | Δ33/25°C | tsb | cac | |
| dk/HK/77 | |||||||
| wt | 8.5 | 8.3 | 4.0 | −0.2 | 4.3 | − | − |
| ca | 4.2 | 8.1 | 6.3 | 3.9 | 1.8 | + | + |
| tl/HK/97 | |||||||
| wt | 9.0 | 8.7 | 4.2 | −0.3 | 4.5 | − | − |
| ca | 5.2 | 8.7 | 8.2 | 3.5 | 0.5 | + | + |
| ma/Alb/85 | |||||||
| wt | 8.5 | 8.0 | 4.2 | −0.5 | 3.8 | − | − |
| ca | 5.0 | 8.2 | 7.3 | 3.2 | 0.9 | + | + |
| AA ca | 5.2 | 9.3 | 8.2 | 4.1 | 1.1 | + | + |
CEK cells were inoculated with H6 wt and ca viruses or AA ca and were incubated at 39, 33, and 25°C. After incubation for 6 days at 39°C and 33°C or 10 days at 25°C to reach peak titers, cells were examined for CPE, and virus titers (TCID50) at each temperature were determined.
ts, difference between the mean TCID50 at 33°C and 39°C of ≥100-fold.
ca, difference between the mean TCID50 at 33°C and 25°C of ≤100-fold.
H6 ca viruses are attenuated in ferrets.
To determine whether the H6 ca viruses were attenuated in vivo, the levels of replication in the NT and lungs 3 and 5 days following i.n. administration were compared with those of H6 wt viruses (Table 3). The three H6 wt viruses replicated efficiently in the upper and lower respiratory tracts of ferrets and caused fever and weight loss. The titers of the H6 ca viruses in the NT were significantly lower than the corresponding wt viruses and were not detected in the lungs of ferrets at 3 or 5 dpi, confirming the att phenotype of the H6 ca viruses. No signs of disease were observed in ferrets infected with H6 ca viruses.
TABLE 3.
Replication of wt and ca H6 viruses in ferretsa
| Virus | dpi | Virus titer in:
|
|||
|---|---|---|---|---|---|
| Lungs (log10 EID50/g ± SE)
|
NT (log10 PFU/g ± SE)
|
||||
| wt | ca | wt | ca | ||
| dk/HK/77 | 3 | 7.0 ± 0.5 | ≤1.5b | 5.4 ± 0.1 | 2.5 ± 0.2 |
| 5 | 6.0 ± 0.5 | ≤1.5 | 6.1 ± 0.0 | 2.6 ± 0.6 | |
| tl/HK/97 | 3 | 6.3 ± 0.2 | ≤1.5 | 6.0 ± 0.3 | 2.1 ± 0.0 |
| 5 | 7.2 ± 0.3 | ≤1.5 | 5.4 ± 0.0 | 2.4 ± 0.3 | |
| ma/Alb/85 | 3 | 6.5 ± 0.0 | ≤1.5 | 3.9 ± 0.0 | 2.0 ± 0.0 |
| 5 | 5.8c | NTd | 2.1c | NTd | |
Groups of four ferrets were inoculated i.n. with 107.0 PFU (0.25 ml per nostril) of the indicated H6 wt and ca viruses. At 3 and 5 dpi, NT and lungs of two ferrets were collected and virus titers were determined. Each data set represents the mean for two ferrets.
Below the limit of detection.
Data from one ferret.
NT, not tested.
Immunogenicity of the H6 ca viruses in ferrets.
The immunogenicity of the three H6 ca viruses was evaluated in ferrets. A single dose of each H6 ca virus elicited neutralizing and HAI antibodies to the homologous wt viruses at titers that ranged from 160 to 269 in NtAb and from 128 to 861 in HAI assays (Table 4). However, both the HAI and NtAb titers against heterologous viruses were significantly lower than the titers against the homologous virus. dk/HK/77 ca and tl/HK/97 ca postinfection sera cross-reacted with each other at a low level but were poorly reactive with ma/Alb/85. ma/Alb/85 ca postinfection antiserum did not cross-react well with tl/HK/97 and dk/HK/77 viruses. These data indicate that a single dose of the three H6 ca viruses is immunogenic in ferrets; however, the serum antibodies had limited cross-reactivity against heterologous H6 viruses.
TABLE 4.
Immunogenicity of H6 ca viruses after a single dose of vaccine in ferretsa
| Test antigen used in serological assay | Geometric mean titer of serum antibody in ferrets immunized withb:
|
|||||
|---|---|---|---|---|---|---|
| dk/HK/77 ca
|
tl/HK/97 ca
|
ma/Alb/85 ca
|
||||
| NtAb | HAI | NtAb | HAI | NtAb | HAI | |
| dk/HK/77 ca | 160 | 215 | 48 | 27 | 25 | 4 |
| tl/HK/97 ca | 57 | 16 | 269 | 128 | 20 | 4 |
| ma/Alb/85 ca | 24 | 4 | 34 | 4 | 190 | 861 |
Groups of four ferrets were inoculated i.n. with 107.0 PFU of the indicated H6 ca viruses. Serum was collected 14 days after immunization.
NtAb and HAI titers were determined by microneutralization assay and HAI assay using horse erythrocytes. Antibody titers against homologous viruses are underlined. An undetectable serum NtAb titer was assigned a value of 20 and an undetectable HAI antibody titer was assigned a value of 4.
Efficacy of H6 ca viruses in ferrets.
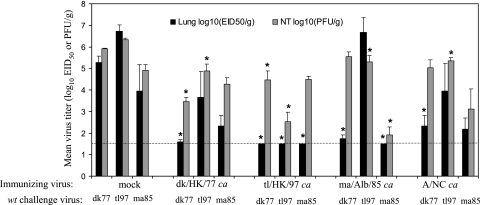
The ability of the three H6 ca vaccine candidates to provide protection against H6 wt virus challenge infection was examined in ferrets (Fig. 1). H6 wt viruses, namely, dk/HK/77, tl/HK/97, and ma/Alb/85, replicated well in the upper and lower respiratory tracts of mock-vaccinated ferrets, with NT titers of 105.9, 106.3, and 104.9 PFU/g, respectively, and lung titers of 105.3, 106.7, and 103.9 EID50/g, respectively. Vaccination with the H6 ca viruses fully protected ferrets from the replication of homologous H6 wt viruses in the lungs, except for a single animal vaccinated with dk/HK/77 ca, for which a low titer of challenge virus was detected. Homologous challenge virus titers in the NT were significantly reduced to 103.5, 102.5, and 101.9 PFU/g in ferrets immunized with dk/HK/77 ca, tl/HK/97 ca, and ma/Alb/85 ca, respectively.
FIG. 1.
Efficacy of H6 ca viruses in ferrets. Groups of three or four ferrets were inoculated i.n. with phosphate-buffered saline (mock), dk/HK/77 ca, tl/HK/97 ca, ma/Alb/85 ca, or H1N1 A/New Caledonia/20/99 (A/NC ca), and 32 days later, the animals were challenged with H6 wt viruses dk/HK/77, tl/HK/97, and ma/Alb/85. Three days postchallenge, virus titers in the lungs and NT tissues were determined by PFU (NT) or EID50 (lungs) assay. Bars represent the means and standard errors of titers. The lower limit of detection is represented by the dotted line. *, P value of <0.05 compared to that for mock-immunized ferrets.
The three H6 ca viruses differed in their abilities to protect ferrets from heterologous wt virus challenge. Vaccination with dk/HK/77 ca offered partial protection against replication of the heterologous tl/HK/97 and ma/Alb/85 wt viruses in the lungs. tl/HK/97 ca vaccine provided complete protection from replication of the three diverse wt H6 viruses in the lungs and a low level of protection from heterologous challenge virus replication in the NT. ma/Alb/85 ca offered significant protection against replication of dk/HK/77 wt virus in the lungs but no protection in the NT, and it did not offer significant protection against the replication of tl/HK/97 wt virus in the lungs. A/New Caledonia/20/99 (H1N1) ca vaccine offered partial protection from replication of the dk/HK/77 wt virus in the lungs and from replication of the tl/HK/97 wt virus in the NT. Thus, among the three H6 ca vaccine candidates, tl/HK/97 ca offered the broadest protection against heterologous H6 wt virus challenge infection.
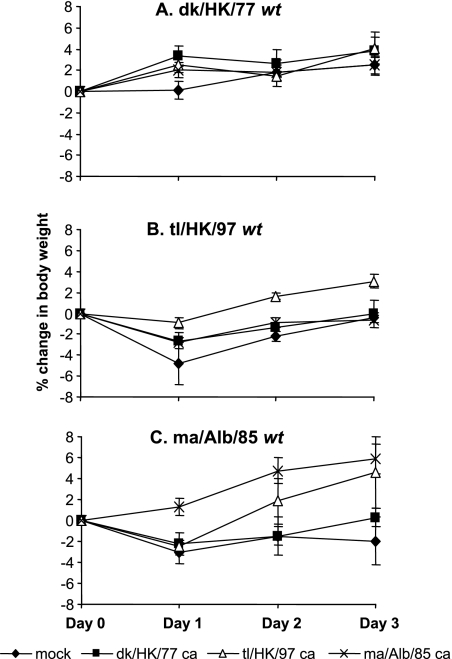
The effect of vaccination on the morbidity of ferrets was assessed by reference to weight loss after H6 wt virus challenge infection (Fig. 2). Although the body weight changes varied among different ferret studies, it was consistently observed that among the three wt H6 viruses, the weight loss was greatest for tl/HK/97- and the least for dk/HK/77-infected ferrets. The greatest reductions in body weight in tl/HK/97 wt virus- and ma/Alb/85 wt virus-infected ferrets were observed 1 day after infection and were −4.77% and −3.10%, respectively. The ferrets started to recover from weight loss from the second day after challenge. dk/HK/77 wt was less virulent and caused minimal weight loss. Vaccination with H6 ca vaccines prevented weight loss in the ferrets that received homologous H6 wt virus challenge infection and offered varied degrees of protection against heterologous wt virus challenge. dk/HK/77 ca did not prevent weight loss resulting from tl/HK/97 and ma/Alb/85 infection. tl/HK/97 ca reduced weight loss caused by ma/Alb/85 and tl/HK97 on day 1 postchallenge, and the ferrets recovered on day 2 postchallenge. ma/Alb/85 ca prevented weight loss from homologous wt virus infection only and provided limited protection against tl/HK/97 wt virus infection. Thus, consistent with the protective effect observed for the H6 ca viruses against the replication of H6 wt challenge viruses in the respiratory tract, tl/HK/97 also offered great protection against weight loss from H6 wt virus infection compared to what was seen for dk/HK/77 ca and ma/Alb/85 ca.
FIG. 2.
Ferret body weight change following H6 wt virus challenge. Groups of ferrets were mock vaccinated or vaccinated with dk/HK/77 ca, tl/HK/97 ca, or ma/Alb/85 ca. After 32 days, ferrets were challenged with homologous and heterologous H6 wt viruses dk/HK/77 (A), tl/HK/97 (B), and ma/Alb/85 (C). Body weight was measured each day from day 0 to day 3 after challenge and is represented as mean body weight loss or gain from the body weights of four ferrets at the time of challenge.
Immunogenicity of H6 ca viruses in mice.
To determine the magnitude and breadth of the immune response induced by infection with the three H6 ca viruses, mice were inoculated i.n. with one or two doses of dk/HK/77 ca, ma/Alb/85 ca, or tl/HK/97 ca. Inoculation with each of the H6 ca viruses induced significant NtAb titers against homologous H6 wt viruses, with a further increase in titer observed following a second dose of vaccine (Table 5). The breadth of the NtAb responses induced by a single dose or two doses of dk/HK/77 ca and ma/Alb/85 ca vaccines was narrow, as demonstrated by the lack of NtAb titers against heterologous H6 wt viruses. In contrast, one or two doses of tl/HK/97 ca induced a more broadly cross-reactive NtAb response, as demonstrated by NtAb titers against heterologous wt viruses dk/HK/77 and qu/HK/99.
TABLE 5.
Immunogenicity of a single dose and of two doses of H6 ca viruses in micea
| Test antigen used in NtAb assay | Geometric mean titer of serum NtAb in mice immunized withb:
|
|||||
|---|---|---|---|---|---|---|
| dk/HK/77 ca
|
ma/Alb/85 ca
|
tl/HK/97 ca
|
||||
| 1 Dose | 2 Doses | 1 Dose | 2 Doses | 1 Dose | 2 Doses | |
| dk/HK/77 wt | 214 | 528 | 44 | 29 | 27 | 85 |
| ma/Alb/85 wt | 10c | 10 | 314 | 3,044 | 10 | 15 |
| tl/HK/97 wt | 10 | 10 | 10 | 10 | 94 | 543 |
| qu/HK/99 wt | 10 | 10 | 10 | 10 | 86 | 388 |
Groups of eight mice were inoculated i.n. with 106 TCID50 of H6 ca viruses. NtAb titers were determined for sera collected 4 weeks after the first dose and 2 weeks after the second dose of vaccination by microneutralization assay in MDCK cells.
Antibody titers against homologous viruses are underlined.
Undetectable titer was assigned as 10.
Efficacy of H6 ca viruses in mice.
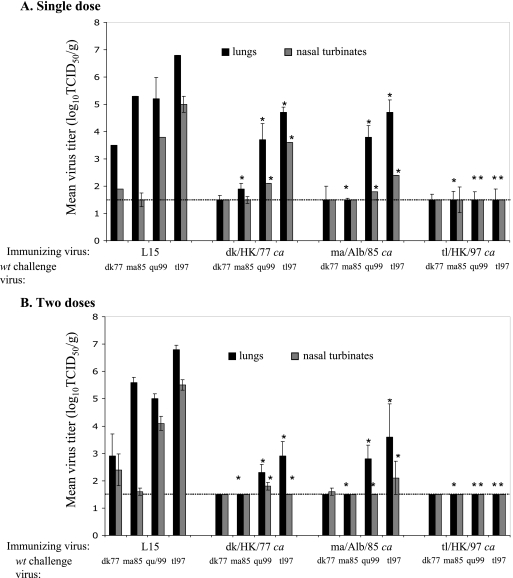
To determine whether infection with the H6 ca viruses induced protection, we inoculated mice with either a single dose or two doses of the H6 ca viruses and subsequently challenged them with both homologous and heterologous H6 wt viruses. Four weeks after a single dose, or 2 weeks after a second dose of H6 ca virus, the mice were challenged with the homologous virus or heterologous H6 wt virus dk/HK/77, tl/HK/97, ma/Alb/85, or qu/Hk/99, an H6N1 virus that is antigenically related to tl/HK/97 (12). Virus titers in the lungs and NT of mock-immunized mice 4 days postchallenge with each of the H6 wt viruses were similar to titers observed in previous studies (12) (Fig. 3). A single dose of each of the H6 ca viruses provided complete protection against challenge with homologous wt virus in the upper and lower respiratory tracts (Fig. 3A.). Vaccination with a single dose of dk/HK/77 ca and ma/Alb/85 ca resulted in a significant decrease in virus titers in the upper and lower respiratory tracts of mice challenged with lethal doses of heterologous tl/HK/97 and qu/HK/99. A single dose of tl/HK/97 ca completely protected mice against challenge with the three heterologous dk/HK/77, ma/Alb/85, and qu/HK/99 viruses. A similar pattern of results was observed for mice administered two doses of H6 ca virus prior to challenge with H6 wt viruses (Fig. 3B). A significant reduction in the virus titers was observed in the upper and lower respiratory tracts of mice challenged with a lethal dose of tl/HK/97 wt and qu/HK/99 wt following two doses of either dk/HK/77 ca or ma/Alb/85 ca compared to what was seen for a single dose of ca virus. Thus, the mice data also demonstrated that tl/HK/97 offered protection against H6 wt virus challenge.
FIG. 3.
Efficacy of H6 ca viruses in mice. Groups of four mice were inoculated i.n. with either one dose (A) or two doses (B) of 106 TCID50 of H6 ca viruses. Mice were challenged i.n. with homologous and heterologous H6 wt viruses 4 weeks after one dose or 4 weeks after two doses of H6 ca virus. Virus titers were determined for lungs and NT on day 4 postchallenge. Each bar represents the mean and standard error of titers for four mice. Statistical significance was determined using the nonparametric Mann-Whitney U test, and * indicates a P value of <0.05. The lower limit of detection is represented by the dotted lines.
DISCUSSION
H6 subtype viruses are phylogenetically diverse. To select H6 strains that can provide a broader protection against H6 wt viruses, we previously evaluated 14 different H6 wt viruses for their replication, immunogenicity, and cross-reactivity in mice and ferrets (12). The tl/HK/97 (H6N1) wt virus elicited the most broadly cross-reactive antibodies and provided complete protection against homologous and heterologous virus challenge in mice. dk/HK/77 (H6N9) and ma/Alb/85 (H6N2) wt viruses also protected mice from homologous virus challenge and provided significant protection against heterologous H6 wt virus challenge. Thus, these three viruses, belonging to different clades in the phylogenetic tree, were selected for further vaccine development.
The three live attenuated H6 ca vaccines were generated by plasmid-based reverse genetics using the same AA ca donor virus that is used to produce seasonal live attenuated influenza virus FluMist (i.n.) vaccines. The vaccine viruses were rescued from the transfection of Vero cells suitable for vaccine manufacture with eight plasmids. The three H6 ca vaccine viruses displayed ts and ca phenotypes in cell culture and the att phenotype in ferrets, which are conferred by the internal protein gene segments of the AA ca virus.
The H6 ca vaccine viruses induced similar levels of neutralizing antibodies in mice and ferrets. A single dose of H6 ca vaccine viruses produced a robust NtAb response, with a homologous titer of more than 100 after a single dose, and provided complete protection from replication of the homologous wt virus in the lower respiratory tract. The vaccine induced antibodies that cross-reacted with heterologous viruses less well than with the homologous virus. The protective efficacy of the H6 ca vaccines was consistent with the data obtained from immunization of mice with the H6 wt virus (12). Although tl/HK/97 ca produced very modest titers of NtAb to dk/HK/77 wt and ma/Alb/85 wt, a single dose of tl/HK/97 ca completely protected mice and ferrets from pulmonary replication of these three wt H6 viruses, indicating that the level of NtAb may not predict the breadth of the cross-protective immune response in these two animal models. In addition to the HA-mediated cross-protective immunity, cellular immune responses against the conserved internal proteins might also contribute to the protection against viral replication. The three H6 ca viruses contain different NA subtypes, and it is not expected that the NA-induced immunity would offer any protection against a different NA subtype. It was noted that the A/New Caledonia/20/99 (H1N1)-infected ferrets were partially protected from dk/HK/77 (H6N9) wt virus replication in the upper respiratory tract (∼1,000-fold) and from tl/HK/97 (H6N1) wt replication in the lungs (∼10-fold). However, the magnitude of reduction in challenge virus replication is much less than what was seen for H6 ca virus-immunized animals. Heterosubtypic immunity against seasonal influenza virus infection (32) and against H5N1 wt virus infection has been reported for mice (19, 23, 25). The mechanistic basis of this type of immunity remains undetermined; however, this type of immunity has been speculated to be mediated either by the NA or by the internal proteins of the virus. Live attenuated vaccines have also demonstrated induction of mucosal and cellular immune responses that contribute to cross-protection from heterologous viruses. Our data are consistent with our previous study on the H6 wt viruses (12) in that tl/HK/97 ca offered a broader protective efficacy in mice and ferrets.
Influenza pandemic preparedness has focused mostly on influenza virus H5 and H7 subtypes. However, it is not possible to predict which influenza virus subtype will cause an influenza pandemic. It is important to prepare influenza virus vaccines against different influenza virus subtypes and carefully evaluate the safety and immunogenicity of these candidate vaccines in clinical studies prior to a pandemic. The preclinical evaluation of the three H6 vaccine candidates described in this study strongly indicated that H6N1 tl/HK97 ca is a promising vaccine candidate and that further clinical evaluation of this vaccine is justified.
Acknowledgments
This work was performed under a Cooperative Research and Development Agreement (CRADA no. AI-0155) between the Laboratory of Infectious Diseases, NIAID, and MedImmune and was supported in part by the Intramural Research Program of the NIAID, NIH.
We thank Chin-fen Yang's group at MedImmune for providing wt H6 sequence analysis. We thank Scott Jacobson, Kim Ngo, Stephanie Gee, and other staff members at MedImmune's Animal Care Facility for helping with the ferret studies along with members of H.J.'s group for discussions and assistance. We thank Jadon Jackson, the staff of SoBran Inc., and the Comparative Medicine Branch, NIAID, for excellent technical support for the mouse studies. We also thank Robert G. Webster for providing H6 wt virus isolates used in this study.
Footnotes
Published ahead of print on 22 October 2008.
REFERENCES
- 1.Abolnik, C., S. Bisschop, T. Gerdes, A. Olivier, and R. Horner. 2007. Outbreaks of avian influenza H6N2 viruses in chickens arose by a reassortment of H6N8 and H9N2 ostrich viruses. Virus Genes 3437-45. [DOI] [PubMed] [Google Scholar]
- 2.Archetti, I., and F. L. J. Horsfall. 1950. Persistent antigenic variation of influenza A viruses after incomplete neutralization in ovo with heterologous immune serum. J. Exp. Med. 92441-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beare, A. S., and R. G. Webster. 1991. Replication of avian influenza viruses in humans. Arch. Virol. 11937-42. [DOI] [PubMed] [Google Scholar]
- 4.Belshe, R., M. S. Lee, R. E. Walker, J. Stoddard, and P. M. Mendelman. 2004. Safety, immunogenicity and efficacy of intranasal, live attenuated influenza vaccine. Expert Rev. Vaccines 3643-654. [DOI] [PubMed] [Google Scholar]
- 5.Chen, H., Y. Matsuoka, D. Swayne, Q. Chen, N. J. Cox, B. R. Murphy, and K. Subbarao. 2003. Generation and characterization of a cold-adapted influenza A H9N2 reassortant as a live pandemic influenza virus vaccine candidate. Vaccine 214430-4436. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Z., A. Aspelund, G. Kemble, and H. Jin. 2006. Genetic mapping of the cold-adapted phenotype of B/Ann Arbor/1/66, the master donor virus for live attenuated influenza vaccines (FluMist). Virology 345416-423. [DOI] [PubMed] [Google Scholar]
- 7.Cheung, C. L., D. Vijaykrishna, G. J. Smith, X. H. Fan, J. X. Zhang, J. Bahl, L. Duan, K. Huang, H. Tai, J. Wang, L. L. Poon, J. S. Peiris, H. Chen, and Y. Guan. 2007. Establishment of influenza A virus (H6N1) in minor poultry species in southern China. J. Virol. 8110402-10412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin, P. S., E. Hoffmann, R. Webby, R. G. Webster, Y. Guan, M. Peiris, and K. F. Shortridge. 2002. Molecular evolution of H6 influenza viruses from poultry in Southeastern China: prevalence of H6N1 influenza viruses possessing seven A/Hong Kong/156/97 (H5N1)-like genes in poultry. J. Virol. 76507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claas, E. C., A. D. Osterhaus, R. van Beek, J. C. De Jong, G. F. Rimmelzwaan, D. A. Senne, S. Krauss, K. F. Shortridge, and R. G. Webster. 1998. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351472-477. [DOI] [PubMed] [Google Scholar]
- 10.Fouchier, R. A., V. Munster, A. Wallensten, T. M. Bestebroer, S. Herfst, D. Smith, G. F. Rimmelzwaan, B. Olsen, and A. D. Osterhaus. 2005. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 792814-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fouchier, R. A., P. M. Schneeberger, F. W. Rozendaal, J. M. Broekman, S. A. Kemink, V. Munster, T. Kuiken, G. F. Rimmelzwaan, M. Schutten, G. J. Van Doornum, G. Koch, A. Bosman, M. Koopmans, and A. D. Osterhaus. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl. Acad. Sci. USA 1011356-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillim-Ross, L., C. Santos, Z. Chen, A. Aspelund, C.-F. Yang, D. Ye, H. Jin, G. Kemble, and K. Subbarao. 2008. Avian influenza H6 viruses productively infect and cause illness in mice and ferrets. J. Virol. 8210854-10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatchette, T. F., D. Walker, C. Johnson, A. Baker, S. P. Pryor, and R. G. Webster. 2004. Influenza A viruses in feral Canadian ducks: extensive reassortment in nature. J. Gen. Virol. 852327-2337. [DOI] [PubMed] [Google Scholar]
- 14.Hatta, M., P. Gao, P. Halfmann, and Y. Kawaoka. 2001. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 2931840-1842. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann, E., K. Mahmood, Z. Chen, C. Yang, J. Spaete, H. B. Greenberg, M. L. Herlocher, H. Jin, and G. Kemble. 2005. Multiple gene segments control the temperature sensitivity and attenuation phenotypes of ca B/Ann Arbor/1/66. J. Virol. 7911014-11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann, E., G. Neumann, Y. Kawaoka, G. Hobom, and R. G. Webster. 2000. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc. Natl. Acad. Sci. USA 976108-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horimoto, T., and Y. Kawaoka. 2005. Influenza: lessons from past pandemics, warnings from current incidents. Nat. Rev. Microbiol. 3591-600. [DOI] [PubMed] [Google Scholar]
- 18.Horimoto, T., and Y. Kawaoka. 2001. Pandemic threat posed by avian influenza A viruses. Clin. Microbiol. Rev. 14129-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichinohe, T., S. Tamura, A. Kawaguchi, A. Ninomiya, M. Imai, S. Itamura, T. Odagiri, M. Tashiro, H. Takahashi, H. Sawa, W. M. Mitchell, D. R. Strayer, W. A. Carter, J. Chiba, T. Kurata, T. Sata, and H. Hasegawa. 2007. Cross-protection against H5N1 influenza virus infection is afforded by intranasal inoculation with seasonal trivalent inactivated influenza vaccine. J. Infect. Dis. 1961313-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin, H., B. Lu, H. Zhou, C. Ma, J. Zhao, C. F. Yang, G. Kemble, and H. Greenberg. 2003. Multiple amino acid residues confer temperature sensitivity to human influenza virus vaccine strains (FluMist) derived from cold-adapted A/Ann Arbor/6/60. Virology 30618-24. [DOI] [PubMed] [Google Scholar]
- 21.Joseph, T., J. McAuliffe, B. Lu, L. Vogel, D. Swayne, H. Jin, G. Kemble, and K. Subbarao. 2008. A live attenuated cold adapted influenza A H7N3 virus vaccine provides protection against homologous and heterologous H7 viruses in mice and ferrets. Virology 378123-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koopmans, M., B. Wilbrink, M. Conyn, G. Natrop, H. van der Nat, H. Vennema, A. Meijer, J. van Steenbergen, R. Fouchier, A. D. Osterhaus, and A. Bosman. 2004. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet 363587-593. [DOI] [PubMed] [Google Scholar]
- 23.Kreijtz, J. H., R. Bodewes, G. van Amerongen, T. Kuiken, R. A. Fouchier, A. D. Osterhaus, and G. F. Rimmelzwaan. 2007. Primary influenza A virus infection induces cross-protective immunity against a lethal infection with a heterosubtypic virus strain in mice. Vaccine 25612-620. [DOI] [PubMed] [Google Scholar]
- 24.Li, S., C. Liu, A. Klimov, K. Subbarao, M. L. Perdue, D. Mo, Y. Ji, L. Woods, S. Hietala, and M. Bryant. 1999. Recombinant influenza A virus vaccines for the pathogenic human A/Hong Kong/97 (H5N1) viruses. J. Infect. Dis. 1791132-1138. [DOI] [PubMed] [Google Scholar]
- 25.Lu, X., L. E. Edwards, J. A. Desheva, D. C. Nguyen, A. Rekstin, I. Stephenson, K. Szretter, N. J. Cox, L. G. Rudenko, A. Klimov, and J. M. Katz. 2006. Cross-protective immunity in mice induced by live-attenuated or inactivated vaccines against highly pathogenic influenza A (H5N1) viruses. Vaccine 246588-6593. [DOI] [PubMed] [Google Scholar]
- 26.Maassab, H. F., and D. C. DeBorde. 1985. Development and characterization of cold-adapted viruses for use as live virus vaccines. Vaccine 3355-369. [DOI] [PubMed] [Google Scholar]
- 27.Munster, V. J., C. Baas, P. Lexmond, J. Waldenström, A. Wallensten, T. Fransson, G. F. Rimmelzwaan, W. E. Beyer, M. Schutten, B. Olsen, A. D. Osterhaus, and R. A. Fouchier. 2007. Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathogens 3e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy, B. R., and K. Coelingh. 2002. Principles underlying the development and use of live attenuated cold-adapted influenza A and B virus vaccines. Viral Immunol. 15295-323. [DOI] [PubMed] [Google Scholar]
- 29.Myers, K. P., S. F. Setterquist, A. W. Capuano, and G. C. Gray. 2007. Infection due to 3 avian influenza subtypes in United States veterinarians. Clin. Infect. Dis. 454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27493-497. [Google Scholar]
- 31.Reid, A., J. K. Taubenberger, and T. G. Fanning. 2004. Evidence of an absence: the genetic origins of the 1918 pandemic influenza virus. Nat. Rev. Microbiol. 2909-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulman, J. L., and E. D. Kilbourne. 1965. Induction of partial specific heterotypic immunity in mice by a single infection with influenza A virus. J. Bacteriol. 89170-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo, S. H., E. Hoffmann, and R. G. Webster. 2004. The NS1 gene of H5N1 influenza viruses circumvents the host anti-viral cytokine responses. Virus Res. 103107-113. [DOI] [PubMed] [Google Scholar]
- 34.Shortridge, K. F. 1992. Pandemic influenza: a zoonosis? Semin. Respir. Infect. 711-25. [PubMed] [Google Scholar]
- 35.Spackman, E., D. E. Stallknecht, R. D. Slemons, K. Winker, D. L. Suarez, M. Scott, and D. E. Swayne. 2005. Phylogenetic analyses of type A influenza genes in natural reservoir species in North America reveals genetic variation. Virus Res. 11489-100. [DOI] [PubMed] [Google Scholar]
- 36.Subbarao, K., and T. Joseph. 2007. Scientific barriers to developing vaccines against avian influenza viruses. Nat. Rev. Immunol. 7267-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subbarao, K., A. Klimov, J. Katz, H. Regnery, W. Lim, H. Hall, M. Perdue, D. Swayne, C. Bender, J. Huang, M. Hemphill, T. Rowe, M. Shaw, X. Xu, K. Fukuda, and N. Cox. 1998. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 279393-396. [DOI] [PubMed] [Google Scholar]
- 38.Suguitan, A. L. J., J. McAuliffe, K. L. Mills, H. Jin, G. Duke, B. Lu, C. J. Luke, B. Murphy, D. E. Swayne, G. Kemble, and K. Subbarao. 2006. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 3e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taubenberger, J. K., A. H. Reid, R. M. Lourens, R. Wang, G. Jin, and T. G. Fanning. 2005. Characterization of the 1918 influenza virus polymerase genes. Nature 437889-893. [DOI] [PubMed] [Google Scholar]
- 40.Wang, C. W., and C. H. Wang. 2003. Experimental selection of virus derivatives with variations in virulence from a single low-pathogenicity H6N1 avian influenza virus field isolate. Avian Dis. 471416-1422. [DOI] [PubMed] [Google Scholar]
- 41.Woolcock, P. R., D. L. Suarez, and D. Kuney. 2003. Low-pathogenicity avian influenza virus (H6N2) in chickens in California, 2000-02. Avian Dis. 47872-881. [DOI] [PubMed] [Google Scholar]
- 42.Wright, P. F., and R. G. Webster. 2001. Orthomyxoviruses, p. 1533-1579. In D. M. Knipe, P. M. Howley, et al. (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.