Abstract
The infection of humans with the rodent-borne lymphocytic choriomeningitis virus (LCMV) can lead to central nervous system disease in adults or severe neurological disease with hydrocephalus and chorioretinitis in children infected congenitally. Although LCMV-induced meningitis and encephalitis have been studied extensively, the immunopathological mechanisms underlying LCMV infection-associated ocular disease remain elusive. We report here that the intraocular administration of the neurotropic LCMV strain Armstrong (Arm) elicited pronounced chorioretinitis and keratitis and that infection with the more viscerotropic strains WE and Docile precipitated less severe immunopathological ocular disease. Time course analyses revealed that LCMV Arm infection of the uvea and neuroretina led to monophasic chorioretinitis which peaked between days 7 and 12 after infection. Analyses of T-cell-deficient mouse strains showed that LCMV-mediated ocular disease was strictly dependent on the presence of virus-specific CD8+ T cells and that the contribution of CD4+ T cells was negligible. Whereas the topical application of immunosuppressive agents did not prevent the development of chorioretinitis, passive immunization with hyperimmune sera partially prevented retinal and corneal damage. Likewise, mice displaying preexisting LCMV-specific T-cell responses were protected against LCMV-induced ocular disease. Thus, antibody- and/or T-cell-based vaccination protocols could be employed as preventive strategies against LCMV-mediated chorioretinitis.
The eye is highly vulnerable to virus infection, and intraocular (i.o.) infection can precipitate blindness. Viral retinitis in humans is usually caused by herpesviruses, including cytomegalovirus (CMV) and herpes simplex virus. However, the prevalence of chorioretinitis caused by less common viral pathogens such as West Nile virus has increased over recent years (19). Likewise, congenital exposure to lymphocytic choriomeningitis virus (LCMV) can lead to severe chorioretinitis (5, 29, 30). Considering the high prevalence of LCMV in the human population, with up to 5% of adults being seropositive for LCMV (11, 23, 27, 38), it is important to investigate the virological and immunopathological mechanisms underlying LCMV-induced ocular disease and to evaluate potential prevention and treatment options.
LCMV was initially isolated by Armstrong and Lillie in 1933 from the cerebrospinal fluid of a woman who was suspected to suffer from St. Louis encephalitis (2). Since then, numerous cases of congenital LCMV infection in humans have been reported (4, 5). In recent years, several cases of acquired LCMV infection in adults, including lethal infection of transplant recipients (17), have been described. Commonly, human infection occurs through the ingestion or inhalation of infected murine urine, feces, or saliva. Approximately one-third of individuals with acquired LCMV infection remain asymptomatic or present with only mild symptoms. About half of the remaining individuals develop central nervous system (CNS) disease, predominantly aseptic meningitis or meningoencephalitis and, less frequently, chorioretinitis. However, in view of the fact that LCMV is not one of the infectious agents routinely evaluated when patients present with uveitis, it has been assumed that the role of LCMV as a causative agent of chorioretinitis is underestimated (9). As a consequence, information regarding the phenotype and incidence of the virus is limited, and the mechanisms by which LCMV causes chorioretinitis remain elusive.
The primary host and reservoir of LCMV is the common mouse, Mus musculus. Neonatal mice, which lack a fully developed immune system, remain asymptomatic because the overwhelming LCMV infection leads to immunological tolerance. Immunocompetent adult mice usually clear infection with LCMV strains that exhibit slow or intermediate replication kinetics, such as Armstrong (Arm) and WE, via a potent cytotoxic T-lymphocyte (CTL) response (7). Particular LCMV strains, such as the rapidly replicating strain Docile, have been shown previously to exhaust the CD8+ T-cell response and therefore establish persistence in multiple tissues (18, 31). Despite various replication kinetics, the different LCMV strains exhibit distinct patterns of tissue tropism; e.g., Arm causes mainly CNS disease (1), whereas WE elicits severe liver pathology (45).
LCMV-induced pathology of the CNS in mice has been well-studied and represents a reliable model of experimental immunopathology (28). However, only very few studies, including those by Ticho and colleagues more than 30 years ago on LCMV infection of the eye in the virus's natural host (39, 40) and a recent study on LCMV-mediated experimental ocular disease in rats (8), have addressed LCMV-mediated neuroretinal immunopathology. The importance of this disease in humans motivated us to readdress the issue and to thoroughly investigate the immunopathological mechanisms underlying LCMV-induced chorioretinitis. Our study revealed pronounced differences between different LCMV strains, with the neurotropic strain Arm eliciting the most severe chorioretinitis and keratitis. Virus-specific effector CTLs, but not CD4+ T cells, were mandatory for the development of immunopathological eye disease. Topical immunosuppressive treatment of ongoing i.o. LCMV infection could only improve keratitis but not the potentially more vision-damaging inflammation of the retina. Whereas pretreatment with hyperimmune sera mitigated the severity of the disease, LCMV-induced ocular pathology was inhibited in mice immune to LCMV. Hence, our findings suggest that appropriate vaccines may provide protection against this virus-mediated immunopathological disease of the eye.
MATERIALS AND METHODS
Mice and viruses.
C57BL/6 mice were obtained from Charles River (Sulzfeld, Germany). β2-microglobulin-deficient (β2m−/−) mice (24), CD4+ T-cell-deficient major histocompatibility complex class II (MHC-II) knockout (IAb−/−) mice (12), type II interferon (IFN) receptor-deficient (ifngr−/−) mice, type I and II IFN receptor-deficient (ifnagr−/−) mice (41), and P14 T-cell receptor (TCR) transgenic mice expressing a TCR recognizing the LCMV gp33 epitope in the context of H-2Db (35) were obtained from the Institut für Labortierkunde (University of Zurich, Switzerland). P14 TCR transgenic mice were further crossed with C57BL/6 mice expressing the congenic marker Thy 1.1. Mice were kept in individually ventilated cages under conventional conditions at the Research Department of the Kantonal Hospital of St. Gallen, St. Gallen, Switzerland. LCMV Arm, WE, and Docile strains, obtained from Rolf M. Zinkernagel (University of Zürich, Switzerland), were propagated on L929 cells at a low multiplicity of infection and quantified as described previously (26). All animals were treated according to the Statement for the Use of Animals in Ophthalmic and Vision Research promulgated by the Association for Research in Vision and Ophthalmology and to the Federal Swiss Regulations on Animal Welfare.
Virus infection.
i.o. injection was performed with the aid of a surgical microscope. A 35-gauge needle was introduced through the temporal corner of the limbus, and 1.0 μl of a virus suspension containing 103 PFU of the LCMV strain indicated in the figure legends in minimal essential medium-2% fetal calf serum was applied intravitreally. In order to control for potential injection-associated local inflammatory responses, 1.0 μl of phosphate-buffered saline was injected i.o. into intravenously (i.v.) LCMV Arm (103 PFU)-infected mice.
Immunization and topical treatment.
For passive immunization, polyclonal immune sera were generated in C57BL/6 mice by repeated i.v. infection with 106 PFU of LCMV Arm. Mice were treated intraperitoneally with 200 μg/ml of a depleting rat anti-CD8 monoclonal antibody (YTS169.4.2) on day 3 and 1 day before LCMV infection. Pooled sera from days 40, 50, and 60 postinfection exhibited significant neutralizing titers as determined by a focus reduction assay (6). Pooled sera from naïve C57BL/6 mice served as a control. Active, T-cell-based immunity against LCMV was induced by i.v. infection with 200 PFU of LCMV WE.
Eye drops of 1% cyclosporine A (obtained from the pharmacy of the Kantonal Hospital of St. Gallen, St. Gallen, Switzerland) and 1% prednisolone acetate eye drops (PredForte; Allergan, Irvine, California) were applied twice daily, with one drop placed onto each cornea of mice i.o. infected with LVM Arm.
Adoptive transfer and immunohistology.
For the visualization of LCMV-specific CD8+ T cells, single-cell suspensions from spleens of P14 mice were subjected to hypotonic red blood cell lysis and CD8+ T cells were sorted using a magnetic separation system according to the protocols of the manufacturer (Miltenyi Biotec, Bergisch-Gladbach, Germany). Recipient C57BL/6 mice were injected i.v. with 106 P14 Thy 1.1+ splenocytes in 500 μl of balanced salt solution 12 h prior to LCMV infection. At different time points after LCMV infection, mice were sacrificed and eyes were enucleated, snap-frozen in liquid nitrogen, and stored at −80°C. Frozen tissue sections were cut in a cryostat and fixed in acetone for 10 min. Sections were incubated with antibodies against CD8 (clone YTS169.4.2), CD4 (clone YTS191.1.2), LCMV nucleoprotein (clone VL4), Thy 1.1 (clone HIS51; eBioscience), or F4/80 (clone BM8; Biomedicals AG), followed by goat anti-rat immunoglobulin (Caltag Labs) and alkaline phosphatase-labeled donkey anti-goat immunoglobulin (Jackson ImmunoResearch Labs). Alkaline phosphatase was visualized by using 7-bromo-3-hydroxy-2-naphthoic-o-anisidide (AS-BI) phosphate/new fuchsine, and sections were counterstained with hemalum. For the quantitative evaluation of the swelling of the cornea, three to five serial cross-sections through the eye were measured by using a Leica DM R microscope, a Leica DC300 FX camera, and Leica IM1000 (version 1.20) computer-aided morphometry software. For the semiquantitative assessment of inflammatory alterations in retinal lesions, sections were evaluated by two observers in a blinded fashion by using the following criteria: grade 0, no infiltration; grade 1, confined minor infiltration (foci of <10 cells) in any retinal layer; grade 2, confined major infiltration (foci of >10 cells) within the retina; grade 3, multiple clusters of inflammatory cells involving at least 10% of the retina; and grade 4, multiple clusters of inflammatory cells covering more than 30% of the retina.
Intracellular cytokine staining.
Spleens were removed at the times after infection indicated below. Single-cell suspensions of 106 splenocytes were incubated for 5 h at 37°C in 200 ml of culture medium containing 25 U/ml interleukin-2 and 5 mg/ml brefeldin A (Sigma-Aldrich) in 96-well round-bottom plates. The cells were stimulated with phorbol myristate acetate (50 ng/ml) and ionomycin (500 ng/ml) as a positive control or left untreated as a negative control. Peptide-specific responses were analyzed after cells were stimulated with 10−6 M gp33 (KAVYNFATC) or np396 (FQPGNGQFI) peptide (both from Neosystems, France) and surface stained, as described elsewhere (22). The percentage of CD8+ T cells producing IFN-γ was determined using a FACSCalibur flow cytometer and CellQuest software (BD Biosciences).
Construction of tetrameric class I molecule-peptide complexes and flow cytometry.
MHC-I (H-2Db) monomers in complexes with LCMV gp33-41 were produced as described previously (22) and tetramerized by the addition of streptavidin-phycoerythrin (Molecular Probes). At the times after immunization indicated below, single-cell suspensions were prepared from spleens or ocular tissue. For the isolation of retina-infiltrating cells, dissected ocular tissue was incubated with collagenase A (Sigma, Buchs, Switzerland) for 60 min at 37°C and the mixture was then filtered through a 70-μm-pore-size cell strainer. Aliquots of retina-infiltrating cells, splenocytes, or peripheral blood mononuclear cells (from 300 μl of blood) were stained using 50 μl of a solution containing tetrameric class I molecule-peptide complexes at 37°C for 10 min, followed by anti-CD8-fluorescein isothiocyanate (BD Pharmingen) at 4°C for 20 min. Absolute cell counts were determined by counting leukocytes in an improved Neubauer chamber.
Statistical analysis.
To determine statistically significant differences, the unpaired two-tailed Student t test was used. P values smaller than 0.05 were considered statistically significant. Statistical data analysis was performed using GraphPad Prism version 5 for Windows (GraphPad Software Inc.).
RESULTS
LCMV-induced chorioretinopathy.
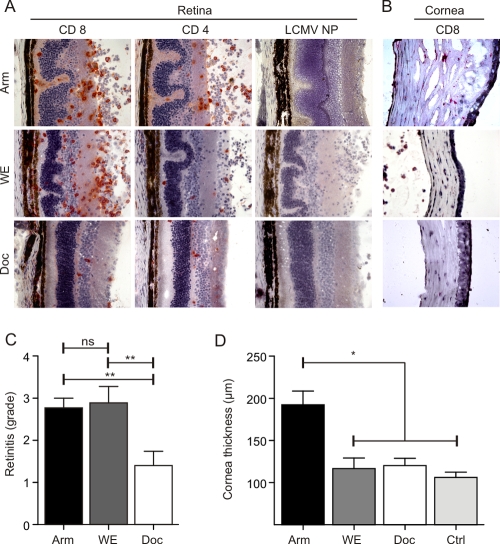
Different LCMV strains exhibit distinct replication patterns (7) and various target organ tropism patterns (1, 45). In order to assess how strain differences affect LCMV-mediated ocular disease, adult C57BL/6 mice were infected i.o. with 103 PFU of LCMV Arm, WE, or Docile. In situ analysis on day 12 postinfection revealed that the outcomes of ocular infection with the different strains varied to a high degree (Fig. 1). i.o. infection with LCMV Arm and WE elicited severe chorioretinitis, with the infiltration of the retina by CD8+ and CD4+ T cells and pronounced folding of the photoreceptor layer, whereas infection with Docile precipitated only very mild inflammatory reactions (Fig. 1A). The thickening of the cornea and infiltration by CD8+ T cells was noted only following i.o. infection with LCMV Arm (Fig. 1B). A semiquantitative analysis of retinal lesions (Fig. 1C) and morphometric determination of cornea swelling (Fig. 1D) confirmed that i.o. infection with LCMV Arm elicited significant ocular disease.
FIG. 1.
In situ analysis of LCMV-induced chorioretinitis and keratitis. C57BL/6 mice were infected i.o. with 103 PFU of LCMV strain Arm, WE, or Docile (Doc). (A and B) Eyes were analyzed by immunohistology on day 12 postinfection for the presence of CD8+ and CD4+ T cells and LCMV nucleoprotein (NP). Representative images of retinas (A) and corneas (B) from three independent experiments are shown. (C) Semiquantitative analysis of immunopathological retinitis following infection with the indicated LCMV strains. Data are mean retinitis scores ± standard errors of the means (SEM; Arm, n = 16 mice; WE, n = 5 mice; Docile, n = 6 mice). (D) Measurement of corneal thickness using digital morphometry. Values are mean corneal thicknesses ± SEM (Arm, n = 13 mice; WE, n = 5 mice; Docile, n = 6 mice; control [Ctrl], n = 10 naïve C57BL/6 mice). **, P < 0.001; *, P < 0.05; ns, not significant.
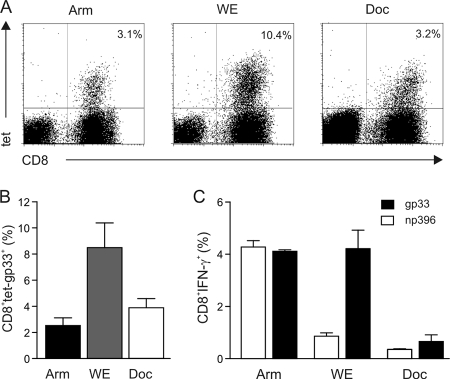
Since LCMV-mediated immunopathology is mediated almost exclusively by virus-specific CD8+ T cells (44), we next determined CTL responses induced by the three different strains following i.o. infection. Tetramer analysis on day 12 postinfection revealed that LCMV WE had induced the most pronounced expansion of gp33-specific CD8+ T cells (Fig. 2A and B). However, an assessment of the functionality of both gp33- and np396-specific CD8+ T cells showed that only Arm infection had induced fully functional, i.e., IFN-γ-producing, CD8+ T cells (Fig. 2C). The lack of efficient CD8+ T-cell responses in the case of Docile infection was due most likely to the rapid spread of Docile virus throughout secondary lymphoid organs, leading to the exhaustion of LCMV-specific CTL responses (7, 31). Indeed, i.o. infection with the three different LCMV strains led to rapid spread to spleens and cervical lymph nodes, with high viral titers on day 4 postinfection. Whereas Arm and WE titers declined until day 8 postinfection, the replication of Docile—as in the i.v. infection—could not be halted by the immune system (data not shown). Thus, the different strains used in this study can spread systemically following i.o. inoculation and establish productive infection of secondary lymphoid organs.
FIG. 2.
LCMV-induced CD8+ T-cell responses after i.o. infection. C57BL/6 mice were infected i.o. with 103 PFU of LCMV Arm, WE, and Docile (Doc), and CD8+ T-cell responses were analyzed on day 12 postinfection. (A) Representative dot blots from MHC-I tetramer analysis for the indicated viral strain. Values in the upper right quadrants indicate percentages of H-2Db/gp33-binding cells within the CD8+ T-cell compartment. (B) Mean percentages ± SEM of gp33 tetramer (tet)-positive cells in the CD8+ T-cell compartment (Arm, n = 3 mice; WE, n = 3 mice; Docile, n = 3 mice). (C) CTL reactivity determined by intracellular IFN-γ secretion in response to restimulation with LCMV gp33 and np396 peptides. Values are mean percentages ± SEM of IFN-γ-secreting cells within the CD8+ T-cell compartment (Arm, n = 3 mice; WE, n = 3 mice; Docile, n = 3 mice).
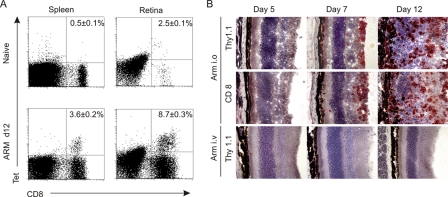
Next, we assessed whether LCMV-specific CD8+ T cells can home to ocular tissues following the initial replication of the virus within the eye. Tetramer analysis on day 12 post-i.o. infection with Arm revealed that high numbers of LCMV gp33-specific CD8+ T cells accumulated in the retina (Fig. 3A). To visualize LCMV-specific CTLs in situ, Thy 1.1+ P14 TCR transgenic CD8+ T cells were adoptively transferred into Thy 1.2+ C57BL/6 recipients prior to i.o. infection with LCMV Arm. Following LCMV infection, P14 TCR transgenic CD8+ T cells expanded vigorously (43) and homed, as shown in Fig. 3B, to virus-infected tissues. It is noteworthy that also under conditions of elevated CTL precursor frequencies, LCMV-specific CTLs did not home to ocular tissues if the initial site of replication had not been the eye (Fig. 3B, bottom row). Overall, the results of these initial experiments revealed that i.o. infection with Arm is best-suited for the study of LCMV-mediated immunopathological ocular disease and that LCMV infection of ocular tissue is a crucial prerequisite for the observed ocular disease.
FIG. 3.
Recruitment of LCMV-specific CD8+ T cells to the retina after i.o. infection. (A) C57BL/6 mice were infected i.o. with 103 PFU of LCMV Arm, and CD8+ T-cell responses were evaluated on day 12 (d12) postinfection by tetramer (Tet) analysis. Representative dot blots from MHC-I tetramer analysis are shown, with values in the upper right quadrants indicating mean percentages ± SEM (n = 3 mice) of H-2Db/gp33-binding cells within the CD8+ T-cell compartment. (B) In situ analysis for the presence of P14 TCR transgenic CD8+ Thy 1.1+ T cells in the retina. Thy 1.2+ C57BL/6 mice received 106 purified P14 TCR transgenic cells 12 h before i.o. (top and middle rows) or i.v. (bottom row) infection with LCMV Arm. Representative images of the retina are shown.
Immunopathological mechanisms of LCMV-induced chorioretinitis and keratitis.
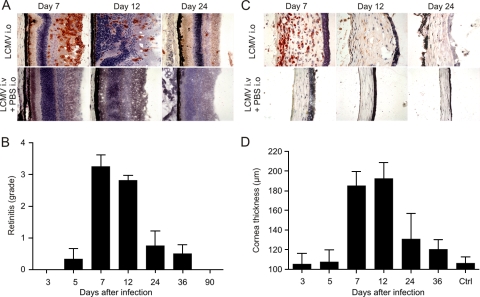
Viral infections can precipitate genuine autoimmune reactions which may contribute to the maintenance of the disease process through the activation of self-reactive lymphocytes (epitope spreading) and/or the cytokine-driven amplification of self-reactive T and B cells (bystander activation) (42). In coxsackievirus B3 infection, for example, self-limiting virus-mediated inflammation is followed by a prolonged autoimmune phase with immune reactivity against cardiac antigens (16, 25). Likewise, the infection of the eye with a neurotropic coronavirus precipitates autoimmune reactivity against retinal antigen (21). Since LCMV-induced inflammation leads to the damage of the uvea, various antigens that can induce uveitis may be released (e.g., S antigen, rhodopsin, or phosducin). To assess whether self-maintaining chronic autoimmune uveitis develops following acute LCMV infection in the eye, we performed a detailed time course analysis of LCMV-induced chorioretinitis and keratitis following i.o. inoculation with LCMV Arm. The anterior chamber of LCMV Arm-infected eyes showed progressive inflammatory reactions starting from day 5 on, with decreasing depth of the anterior chamber, the production of fibrinous exudates, and the formation of anterior synechiae (data not shown). Immunohistochemical analysis revealed that the infiltration with CD8+ T cells started around the ciliary body by day 5, with the loss of the integrity of the iris and the ciliary body by day 12. Infiltration was dominated by CD8+ and CD4+ cells. In addition, CD11c+ dendritic cells and F4/80+ macrophages were found in clusters within the inflammatory lesions (data not shown). The evaluation of the retinal alterations revealed that the level of disease activity was highest between days 7 and 12 after infection and that a slow healing process resulted in a complete loss of inflammatory foci in the retina by 2 to 3 months postinfection (Fig. 4A and B).
FIG. 4.
Time course analysis of immunopathological alteration in the retina and cornea following i.o. infection with 103 PFU of LCMV Arm. Eyes were analyzed at the indicated time points postinfection. (A) Immunohistological analysis for the presence of CD8+ T cells. Representative images of the retina are shown. PBS, phosphate-buffered saline. (B) Semiquantitative analysis of immunopathological retinitis. Data are mean retinitis scores ± SEM (day 3, n = 7; day 5, n = 4; day 7, n = 12; day 12, n = 16; day 24, n = 4; day 36, n = 4; day 90, n = 3). (C) Immunohistological staining for CD8. Representative images of the cornea are shown. (D) Measurement of corneal thickness using digital morphometry. Values are mean corneal thicknesses ± SEM (day 3, n = 5; day 5, n = 4; day 7, n = 12; day 12, n = 13; day 24, n = 3; day 36, n = 3; control [Ctrl], n = 10 naïve C57BL/6 mice).
After i.o. infection with LCMV Arm, the cornea remained clear until day 5. On day 7, clinical signs of ocular disease, which started with the mild opacity of the cornea, were externally visible. This development was followed by a progressive loss of corneal clarity peaking on day 12 and persisting until the end of the experiment. Infiltrations of the corneal stroma were detected and were accompanied by the loss of endothelial cells and the disruption of the lamellar array (Fig. 4C). Corneal thickness measurements showed the progressive edema of the cornea, with a maximum between days 7 and 12 postinfection (Fig. 4D). The resolution of the corneal tissue damage was comparable to that of the retinal inflammation, i.e., the swelling of the cornea was completely resolved 2 to 3 months postinfection (Fig. 4D). It is noteworthy that i.v. infection with LCMV ARM, even following the i.o. application of carrier phosphate-buffered saline, did not result in ocular inflammation (Fig. 4A and C), indicating that replicating virus had to be present within the eye to cause the disease. In summary, the monophasic development and synchronous resolution of chorioretinitis and keratitis suggest that autoimmune reactions against uveal proteins may not play a dominant role in the prolongation of the ocular disease following LCMV infection.
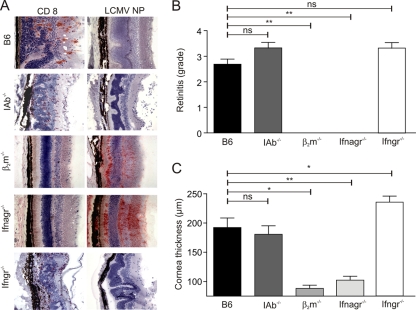
Next, we determined the relative contributions of CD8+ and CD4+ T cells to the disease process by using i.o. infection of IAb−/− and β2m−/− mice. The lack of CD4+ T cells in MHC-II-deficient mice (12) mitigated the chorioretinits only mildly (Fig. 5A and B) and did not affect corneal swelling (Fig. 5C). The lack of LCMV antigen in the retinas of IAb−/− mice on day 12 postinfection (Fig. 5A) suggests that CD8+ T cells had completely cleared the viral infection even without support from Th cells. Indeed, the lack of CD8+ T cells in β2m−/− mice completely prevented chorioretinits and keratitis (Fig. 5) but resulted in the persistence of LCMV antigen in the retina (Fig. 5A). Overwhelming LCMV infection, which leads to the widespread distribution of the virus and the exhaustion of virus-specific CD8+ T cells, can be observed in mice lacking the type I IFN receptor (32). Since the lack of the type II IFN receptor ameliorates autoimmune diseases (15), we assessed whether the lack of both the type I and II IFN receptors or the lack of the type II IFN receptor alone had an impact on chorioretinitis and keratitis following i.o. LCMV Arm inoculation. As shown in Fig. 5, ifnagr−/− mice did not develop chorioretinits or keratitis. The persistence of viral antigen in the retinas of ifnagr−/− mice (Fig. 5A) indicates that the disease developed only in the presence of a functional virus-specific CD8+ T-cell response. In the absence of the type II IFN receptor, immunopathological ocular disease developed comparably to that in C57BL/6 controls (Fig. 5), indicating that the type II IFN receptor most likely affected neither the development of the antiviral T-cell response nor the disease process.
FIG. 5.
Impact of CD8+ and CD4+ T cells and of the IFN system on LCMV-induced ocular disease. (A) The indicated mouse strains were infected i.o. with 103 PFU of LCMV Arm, and eyes were analyzed on day 12 postinfection by immunohistology for the presence of CD8+ T cells and LCMV nucleoprotein (NP). Representative images of the retina are shown. (B and C) Semiquantitative analysis of immunopathological retinitis (B) and measurements of corneal thickness using digital morphometry (C) following the infection of the indicated mouse strains. Data are mean retinitis scores ± SEM (B) and mean corneal thicknesses ± SEM (C) (B6, n = 16; IAb−/−, n = 3; β2m−/−, n = 4; ifnagr−/−, n = 6; ifngr−/−, n = 4). **, P < 0.001; *, P < 0.05; ns, not significant.
Prevention and treatment of LCMV-induced chorioretinitis and keratitis.
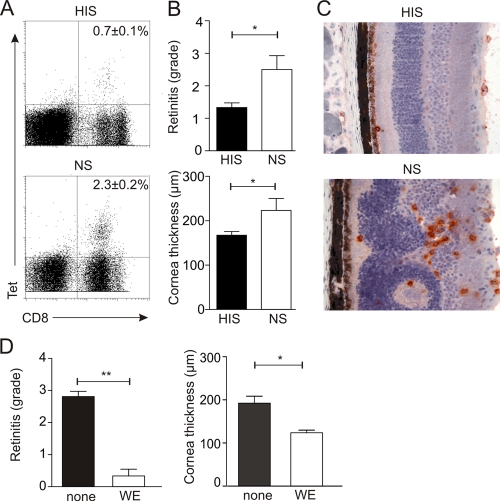
Antibodies play only a minor role in the resolution of acute LCMV infection (10). However, preexisting neutralizing antibodies are able to lower viral titers and protect against lethal LCMV choriomeningitis (3, 37). In order to assess whether neutralizing antibodies could prevent LCMV-induced chorioretinitis and keratitis, we passively immunized C57BL/6 mice with anti-LCMV hyperimmune sera before i.o. challenge. The presence of neutralizing antibodies efficiently attenuated LCMV-specific CD8+ T-cell responses (Fig. 6A). The reduced vigor of the CTL response was associated with significantly diminished chorioretinitis and keratitis (Fig. 6B and C). However, LCMV Arm-induced ocular disease was nearly completely abolished in mice immune to LCMV WE (Fig. 6D). It is noteworthy that preimmunization with WE did not affect the clearance of Arm following i.o. infection. Thus, the results of these vaccination experiments indicate that appropriate T-cell vaccination and passive immunization with neutralizing antibodies represent potential means for the prevention of LCMV-induced ocular disease.
FIG. 6.
Prevention of LCMV-induced chorioretinitis and keratitis. (A to C) C57BL/6 mice were pretreated with hyperimmune sera (HIS) or normal mouse sera (NS) before i.o. challenge with 103 PFU of LCMV Arm. (A) Representative dot blots from MHC-I tetramer (Tet) analysis on day 12 postinfection. Values in the upper right quadrants are mean percentages ± SEM of H-2Db/gp33-binding cells within the splenic CD8+ T-cell compartment. (HIS, n = 12 mice; NS, n = 7 mice.) (B) Semiquantitative analysis of immunopathological retinitis (upper panel) and measurements of corneal thickness using digital morphometry (lower panel). (HIS, n = 12 mice; NS, n = 7 mice.) (C) In situ analysis of anti-CD8-stained cryosections of retinas from C57BL/6 mice treated with hyperimmune sera against LCMV or with naïve sera as a control. (D) Analysis of immunopathological retinitis (left panel) and digital morphometry measurements of corneal thicknesses (right panel) in C57BL/6 mice receiving LCMV WE 8 days before i.o. infection with LCMV Arm. Values are mean retinitis grades ± SEM (left panel) and mean corneal thicknesses ± SEM (right panel) on day 12 after LCMV Arm infection (n = 6 mice). *, P < 0.05; **, P < 0.001.
Inflammatory processes of unclear origin in the uvea (so-called autoimmune uveitis) are commonly treated with an immunosuppressive regimen including steroids or cyclosporine. We thus evaluated the effectiveness of such topical treatment options for the LCMV-induced immunopathology of the cornea and retina. The daily topical application of prednisolone eye drops led to a significant reduction of corneal swelling but did not influence the retinitis severity (Fig. 7). Likewise, the local application of cyclosporine, which inhibits T-cell activation, led to a small improvement in corneal immunopathology but exerted no significant effect on the inflammation in the retina (Fig. 7). In summary, these results indicate that appropriate vaccination against LCMV represents the most promising approach to avoid potentially debilitating LCMV-induced ocular disease.
FIG. 7.
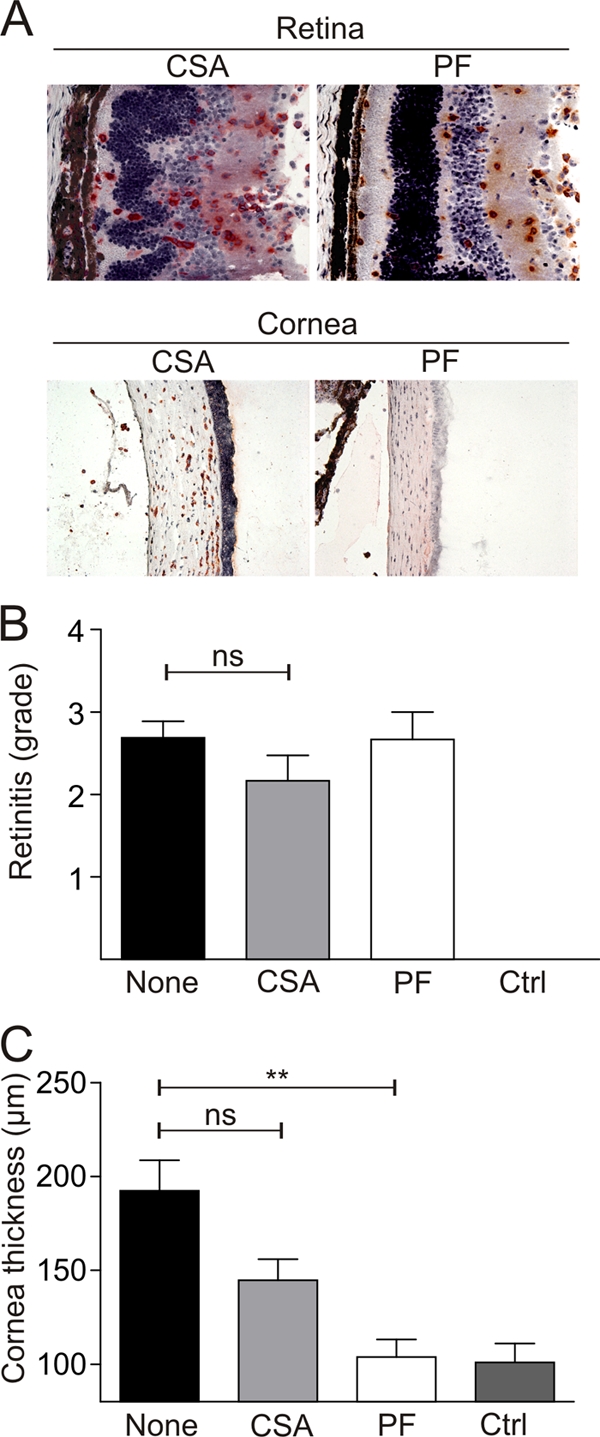
Analysis of LCMV Arm-infected eyes treated with 1% prednisolone acetate eye drops (PredForte; PF) or 1% cyclosporine A eye drops (CSA). (A) In situ analysis (by CD8 staining) of retinas and corneas of C57BL/6 mice treated topically with PF or CSA (day 12 post-i.o. infection). Representative images of the retinas and corneas from two independent experiments are shown. (B and C) Semiquantitative analysis of immunopathological retinitis (B) and measurements of corneal thickness using digital morphometry (C). Values are mean retinitis grades ± SEM (B) and mean corneal thicknesses ± SEM (C) on day 12 after LCMV Arm infection. (Untreated [none], n = 16 mice; CSA, n = 6 mice; PF, n = 6 mice; control [Ctrl], n = 10 naïve C57BL/6 mice.) **, P < 0.001; ns, not significant.
DISCUSSION
LCMV infection in humans can lead to severe and potentially lethal disease. It is mainly the CNS that is affected in humans, with aseptic meningitis in immunocompetent adults or chorioretinitis and structural brain anomalies following congenital infection. Studies of LCMV-induced choriomeningitis in mice during the last decades have provided great insight into the delicate virus-host balance and the particular immunopathological mechanisms underlying the disease (28). However, a major drawback in studying LCMV-induced CNS disease following intracranial injection is the uniformly lethal course of the disease. Once LCMV has established an infection in the meninges and effector CTLs have gained access to the CNS, the ensuing immunopathological damage within the CNS does not permit studies beyond the acute phase of the viral infection. Thus, detailed studies of later stages of the disease, such as the healing process, or potential autoimmune complications are impractical with the intracranial infection model. The present study established that the retina, as a readily accessible part of the CNS, is well-suited for investigations of the long-term effects of LCMV-mediated immunopathological disease in this organ system.
Two readout systems were found to be robust and reliable in the assessment of LCMV-induced ocular disease. The assessment of the accumulation of mononuclear inflammatory cells (predominantly CD8+ T cells) in the retinal layers provided a direct measure for the damage in this particular part of the CNS. It appears that, during infection with LCMV and primarily with the Arm strain, cells in the cornea become infected with the virus, leading to CTL infiltration and pronounced corneal thickening. Morphometric evaluation of the cornea-swelling reaction provided an exact second measure for the vigor of the immunopathological reaction. Using the i.o. infection with LCMV Arm and the carefully validated readout systems, this study revealed that LCMV-mediated ocular disease depends critically on the delicate balance between functional antiviral CTLs and the presence of viral antigen in the eye. In the absence of CD8+ T cells or under conditions of impaired CTL function (i.e., CTL exhaustion during LCMV Docile infection or the absence of the type I IFN receptor), viral antigen persisted in the eye but disease did not develop. Correspondingly, the rapid reduction of the viral load through the administration of neutralizing antibodies or the generation of efficient antiviral T cells effectively reduced or even prevented chorioretinitis and keratitis.
The activation of CD8+ T cells during viral infection critically depends on professional antigen-presenting cells (mainly dendritic cells and macrophages) that present antigenic peptides in the context of host MHC-I molecules to naïve T cells within the secondary lymphoid organs (20, 34, 36). However, the details of how and where viral antigen from the i.o. space is presented to CTLs remain unclear. A recent study examined the route by which antigen from the anterior segment of the eye migrates to draining lymph nodes (14). Previous attempts to trace the route of antigen-presenting cell migration from the anterior uveal tissues showed that dendritic cells or macrophages do not migrate to draining lymph nodes. Therefore, immune responses in the draining lymph nodes were assumed to be initiated by antigens escaping from the eye extracellularly via the canal of Schlemm into the blood (14). This view is compatible with our results which suggest that i.o. injection with LCMV leads to a generalized LCMV infection with a systemic specific T-cell response similar to that in i.v. infected animals.
Immunopathological destruction of the retina may also be caused by viruses which are considered to be mainly cytolytic. Indeed, CMV, an important ocular pathogen in immunosuppressed patients, causes the destruction of retinal cells mainly through direct cytopathicity. This finding in AIDS patients was well-documented before the era of highly active antiretroviral therapy (13). However, with the introduction of highly active antiretroviral therapy, a new form of CMV retinitis has evolved, termed immune recovery uveitis, which is associated with an improvement in T-cell numbers and enhanced CD8+ T-cell reactivity against CMV (33). Although the exact nature of this syndrome in humans is currently unknown, the findings presented in this study suggest that immunopathological damage by activated T cells recognizing persisting viral antigen in the eye may be the underlying pathological mechanism.
An enhanced understanding of the factors that trigger ocular immune responses and ocular immunopathology may facilitate therapeutic interventions. The results presented herein suggest that treatment strategies against immunopathological uveitis should aim at reducing excessive local i.o. immune reactivity without impairing general immune defense mechanisms for the elimination of extraocular virus. Lessons learned from the LCMV-induced chorioretinitis model may also shed light on the pathogenesis of other retinal diseases.
Acknowledgments
We thank Rolf Zinkernagel for helpful discussions and careful reading of the manuscript. We thank Rita de Giuli for generating MHC-I tetramers, Harindra Hewage for excellent animal husbandry, and Andre Fitsche for help with immunohistochemistry.
This study was supported by a grant from the OPOS Foundation, St. Gallen, Switzerland. We declare that we do not have competing commercial or financial interests.
Footnotes
Published ahead of print on 22 October 2008.
REFERENCES
- 1.Ahmed, R., and M. B. Oldstone. 1988. Organ-specific selection of viral variants during chronic infection. J. Exp. Med. 1671719-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong, C., and R. D. Lillie. 1934. Experimental lymphocytic chriomeningitis of monkeys and mice produced by a virus encountered in studies of the 1993 St. Louis encephalitis epidemic. Public Health Rep. 491019-1027. [Google Scholar]
- 3.Baldridge, J. R., and M. J. Buchmeier. 1992. Mechanisms of antibody-mediated protection against lymphocytic choriomeningitis virus infection: mother-to-baby transfer of humoral protection. J. Virol. 664252-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton, L. L., and M. B. Mets. 2001. Congenital lymphocytic choriomeningitis virus infection: decade of rediscovery. Clin. Infect. Dis. 33370-374. [DOI] [PubMed] [Google Scholar]
- 5.Barton, L. L., M. B. Mets, and C. L. Beauchamp. 2002. Lymphocytic choriomeningitis virus: emerging fetal teratogen. Am. J. Obstet. Gynecol. 1871715-1716. [DOI] [PubMed] [Google Scholar]
- 6.Battegay, M., D. Moskophidis, H. Waldner, M. A. Brundler, W. P. Fung Leung, T. W. Mak, H. Hengartner, and R. M. Zinkernagel. 1993. Impairment and delay of neutralizing antiviral antibody responses by virus-specific cytotoxic T cells. J. Immunol. 1515408-5415. [PubMed] [Google Scholar]
- 7.Bocharov, G., B. Ludewig, A. Bertoletti, P. Klenerman, T. Junt, P. Krebs, T. Luzyanina, C. Fraser, and R. M. Anderson. 2004. Underwhelming the immune response: effect of slow virus growth on CD8+-T-lymphocyte responses. J. Virol. 782247-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonthius, D. J., B. Nichols, H. Harb, J. Mahoney, and B. Karacay. 2007. Lymphocytic choriomeningitis virus infection of the developing brain: critical role of host age. Ann. Neurol. 62356-374. [DOI] [PubMed] [Google Scholar]
- 9.Bonthius, D. J., and S. Perlman. 2007. Congenital viral infections of the brain: lessons learned from lymphocytic choriomeningitis virus in the neonatal rat. PLoS Pathog. 3e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brundler, M. A., P. Aichele, M. Bachmann, D. Kitamura, K. Rajewsky, and R. M. Zinkernagel. 1996. Immunity to viruses in B cell-deficient mice: influence of antibodies on virus persistence and on T cell memory. Eur. J. Immunol. 262257-2262. [DOI] [PubMed] [Google Scholar]
- 11.Childs, J. E., G. E. Glass, T. G. Ksiazek, C. A. Rossi, J. G. Oro, and J. W. Leduc. 1991. Human-rodent contact and infection with lymphocytic choriomeningitis and Seoul viruses in an inner-city population. Am. J. Trop. Med. Hyg. 44117-121. [DOI] [PubMed] [Google Scholar]
- 12.Cosgrove, D., D. Gray, A. Dierich, J. Kaufman, M. Lemeur, C. Benoist, and D. Mathis. 1991. Mice lacking MHC class II molecules. Cell 661051-1066. [DOI] [PubMed] [Google Scholar]
- 13.Deayton, J. R., P. Wilson, C. A. Sabin, C. C. Davey, M. A. Johnson, V. C. Emery, and P. D. Griffiths. 2000. Changes in the natural history of cytomegalovirus retinitis following the introduction of highly active antiretroviral therapy. AIDS 141163-1170. [DOI] [PubMed] [Google Scholar]
- 14.Dullforce, P. A., K. L. Garman, G. W. Seitz, R. J. Fleischmann, S. M. Crespo, S. R. Planck, D. C. Parker, and J. T. Rosenbaum. 2004. APCs in the anterior uveal tract do not migrate to draining lymph nodes. J. Immunol. 1726701-6708. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson, U., M. O. Kurrer, R. Bingisser, H. P. Eugster, P. Saremaslani, F. Follath, S. Marsch, and U. Widmer. 2001. Lethal autoimmune myocarditis in interferon-gamma receptor-deficient mice: enhanced disease severity by impaired inducible nitric oxide synthase induction. Circulation 10318-21. [DOI] [PubMed] [Google Scholar]
- 16.Fairweather, D., Z. Kaya, G. R. Shellam, C. M. Lawson, and N. R. Rose. 2001. From infection to autoimmunity. J. Autoimmun. 16175-186. [DOI] [PubMed] [Google Scholar]
- 17.Fischer, S. A., M. B. Graham, M. J. Kuehnert, C. N. Kotton, A. Srinivasan, F. M. Marty, J. A. Comer, J. Guarner, C. D. Paddock, D. L. DeMeo, W. J. Shieh, B. R. Erickson, U. Bandy, A. DeMaria, Jr., J. P. Davis, F. L. Delmonico, B. Pavlin, A. Likos, M. J. Vincent, T. K. Sealy, C. S. Goldsmith, D. B. Jernigan, P. E. Rollin, M. M. Packard, M. Patel, C. Rowland, R. F. Helfand, S. T. Nichol, J. A. Fishman, T. Ksiazek, and S. R. Zaki. 2006. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N. Engl. J. Med. 3542235-2249. [DOI] [PubMed] [Google Scholar]
- 18.Gallimore, A., A. Glithero, A. Godkin, A. C. Tissot, A. Pluckthun, T. Elliott, H. Hengartner, and R. M. Zinkernagel. 1998. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 1871383-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg, S., and L. M. Jampol. 2005. Systemic and intraocular manifestations of West Nile virus infection. Surv. Ophthalmol. 503-13. [DOI] [PubMed] [Google Scholar]
- 20.Hickman, H. D., K. Takeda, C. N. Skon, F. R. Murray, S. E. Hensley, J. Loomis, G. N. Barber, J. R. Bennink, and J. W. Yewdell. 2008. Direct priming of antiviral CD8+ T cells in the peripheral interfollicular region of lymph nodes. Nat. Immunol. 9155-165. [DOI] [PubMed] [Google Scholar]
- 21.Hooks, J. J., C. Percopo, Y. Wang, and B. Detrick. 1993. Retina and retinal pigment epithelial cell autoantibodies are produced during murine coronavirus retinopathy. J. Immunol. 1513381-3389. [PubMed] [Google Scholar]
- 22.Junt, T., H. Nakano, T. Dumrese, T. Kakiuchi, B. Odermatt, R. M. Zinkernagel, H. Hengartner, and B. Ludewig. 2002. Antiviral immune responses in the absence of organized lymphoid T cell zones in plt/plt mice. J. Immunol. 1686032-6040. [DOI] [PubMed] [Google Scholar]
- 23.Kallio-Kokko, H., J. Laakkonen, A. Rizzoli, V. Tagliapietra, I. Cattadori, S. E. Perkins, P. J. Hudson, A. Cristofolini, W. Versini, O. Vapalahti, A. Vaheri, and H. Henttonen. 2006. Hantavirus and arenavirus antibody prevalence in rodents and humans in Trentino, Northern Italy. Epidemiol. Infect. 134830-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koller, B. H., P. Marrack, J. W. Kappler, and O. Smithies. 1990. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science 2481227-1230. [DOI] [PubMed] [Google Scholar]
- 25.Krebs, P., M. O. Kurrer, M. Kremer, R. De Giuli, I. Sonderegger, A. Henke, R. Maier, and B. Ludewig. 2007. Molecular mapping of autoimmune B cell responses in experimental myocarditis. J. Autoimmun. 28224-233. [DOI] [PubMed] [Google Scholar]
- 26.Ludewig, B., S. Ehl, U. Karrer, B. Odermatt, H. Hengartner, and R. M. Zinkernagel. 1998. Dendritic cells efficiently induce protective antiviral immunity. J. Virol. 723812-3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marrie, T. J., and M. F. Saron. 1998. Seroprevalence of lymphocytic choriomeningitis virus in Nova Scotia. Am. J. Trop. Med. Hyg. 5847-49. [DOI] [PubMed] [Google Scholar]
- 28.McGavern, D. B., D. Homann, and M. B. Oldstone. 2002. T cells in the central nervous system: the delicate balance between viral clearance and disease. J. Infect. Dis. 186(Suppl. 2)S145-S151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mets, M. B. 1999. Childhood blindness and visual loss: an assessment at two institutions including a “new” cause. Trans. Am. Ophthalmol. Soc. 97653-696. [PMC free article] [PubMed] [Google Scholar]
- 30.Mets, M. B., L. L. Barton, A. S. Khan, and T. G. Ksiazek. 2000. Lymphocytic choriomeningitis virus: an underdiagnosed cause of congenital chorioretinitis. Am. J. Ophthalmol. 130209-215. [DOI] [PubMed] [Google Scholar]
- 31.Moskophidis, D., F. Lechner, H. Pircher, and R. M. Zinkernagel. 1993. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362758-761. [DOI] [PubMed] [Google Scholar]
- 32.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 2641918-1921. [DOI] [PubMed] [Google Scholar]
- 33.Mutimer, H. P., Y. Akatsuka, T. Manley, E. L. Chuang, M. Boeckh, R. Harrington, T. Jones, and S. R. Riddell. 2002. Association between immune recovery uveitis and a diverse intraocular cytomegalovirus-specific cytotoxic T cell response. J. Infect. Dis. 186701-705. [DOI] [PubMed] [Google Scholar]
- 34.Oehen, S., B. Odermatt, U. Karrer, H. Hengartner, R. Zinkernagel, and C. Lopez-Macias. 2002. Marginal zone macrophages and immune responses against viruses. J. Immunol. 1691453-1458. [DOI] [PubMed] [Google Scholar]
- 35.Pircher, H., K. Burki, R. Lang, H. Hengartner, and R. M. Zinkernagel. 1989. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature 342559-561. [DOI] [PubMed] [Google Scholar]
- 36.Probst, H. C., and M. van den Broek. 2005. Priming of CTLs by lymphocytic choriomeningitis virus depends on dendritic cells. J. Immunol. 1743920-3924. [DOI] [PubMed] [Google Scholar]
- 37.Seiler, P., M. A. Brundler, C. Zimmermann, D. Weibel, M. Bruns, H. Hengartner, and R. M. Zinkernagel. 1998. Induction of protective cytotoxic T cell responses in the presence of high titers of virus-neutralizing antibodies: implications for passive and active immunization. J. Exp. Med. 187649-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stephensen, C. B., S. R. Blount, R. E. Lanford, K. V. Holmes, R. J. Montali, M. E. Fleenor, and J. F. Shaw. 1992. Prevalence of serum antibodies against lymphocytic choriomeningitis virus in selected populations from two U.S. cities. J. Med. Virol. 3827-31. [DOI] [PubMed] [Google Scholar]
- 39.Ticho, U., G. A. Cole, and A. M. Silverstein. 1974. Immunopathologic uveitis in the mouse due to lymphocytic choriomeningitis virus. Investig. Ophthalmol. 1333-38. [PubMed] [Google Scholar]
- 40.Ticho, U., A. M. Silverstein, and G. A. Cole. 1974. Immunopathogenesis of LCM virus-induced uveitis: the role of T lymphocytes. Investig. Ophthalmol. 13229-231. [PubMed] [Google Scholar]
- 41.van den Broek, M. F., U. Muller, S. Huang, M. Aguet, and R. M. Zinkernagel. 1995. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 694792-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanderlugt, C. L., and S. D. Miller. 2002. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat. Rev. Immunol. 285-95. [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann, C., K. Brduscha-Riem, C. Blaser, R. M. Zinkernagel, and H. Pircher. 1996. Visualization, characterization, and turnover of CD8+ memory T cells in virus-infected hosts. J. Exp. Med. 1831367-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zinkernagel, R. M. 1996. Immunology taught by viruses. Science 271173-178. [DOI] [PubMed] [Google Scholar]
- 45.Zinkernagel, R. M., E. Haenseler, T. Leist, A. Cerny, H. Hengartner, and A. Althage. 1986. T cell-mediated hepatitis in mice infected with lymphocytic choriomeningitis virus. Liver cell destruction by H-2 class I-restricted virus-specific cytotoxic T cells as a physiological correlate of the 51Cr-release assay? J. Exp. Med. 1641075-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]