Abstract
The attachment, entry, and fusion of Kaposi's sarcoma-associated herpesvirus (KSHV) with target cells are mediated by complex machinery containing, among others, viral glycoprotein H (gH) and its alleged chaperone, gL. We observed that KSHV gH, in contrast to its homologues in several other herpesviruses, is transported to the cytoplasm membrane independently from gL, but not vice versa. Mutational analysis revealed that the N terminus of gH is sufficient for gL interaction. However, the entire extracellular part of gH is required for efficient gL secretion. The soluble ectodomain of gH was sufficient to interact with the surfaces of potential target cells in a heparin-dependent manner, and binding was further enhanced by coexpression of gL. Surface plasmon resonance revealed a remarkably high affinity of gH for glycosaminoglycans. Heparan sulfate (HS) proteoglycans of the syndecan family act as cellular receptors for the gH/gL complex. They promoted KSHV infection, and expression of gH/gL on target cells inhibited subsequent KSHV infection. Whereas gH alone was able to bind to HS, we observed that only the gH/gL complex adhered to heparan sulfate-negative cells at lamellipodium-like structures.
The entry of Kaposi's sarcoma-associated herpesvirus (KSHV), also termed human herpesvirus 8 (HHV-8), into target cells is only poorly understood. In general, herpesviruses enter the cell through at least two consecutive steps involving different viral glycoproteins and different cellular receptors. Attachment is the first step in this process. In KSHV, this step is mediated through engagement of heparan sulfate proteoglycans (HSPGs) on the cell surface (5, 9). Attachment via binding to cell surface HSPGs is widely used among different pathogens (15, 44), especially herpesviruses (26, 59). Of the KSHV envelope proteins, gB (3), K8.1 (5, 9), and the complement control protein (KCP) (52) are known to interact with heparan sulfates. Notably, heparin and other sulfated sugars are extremely potent at blocking KSHV infection and fusion (25). Receptors responsible for entry are engaged in the next step. In herpes simplex virus (HSV), the prototypic herpesvirus, gD binds to a spectrum of cellular proteins, including members of the tumor necrosis factor receptor family (37) and nectins (14, 19), which are at least in part responsible for the tropism of the virus (36, 63). A glycosaminoglycan molecule similar to those already involved in attachment, 3-O-heparan sulfate, is also specifically engaged by gD to induce HSV entry (51). Thus, glycosaminoglycan interaction does not generally seem to be confined exclusively to the early attachment steps in viral infection. Following binding to one or more entry receptors or coreceptors, herpesviruses enter the target cell via fusion at the plasma membrane or via endocytosis (2, 20). Fusion seems to be generally mediated by a multiprotein complex. In HSV, this complex comprises gD, gB, gH, and gL (12). Among the cellular receptors promoting KSHV entry are integrins (4, 18) and the recently described fusion receptor xCT (27). Given the HSV example, additional receptors are likely to be used for the infection of certain cells or by different routes.
Within the herpesvirus family, gH, together with gL, was shown to take part in the process of membrane fusion in HSV (23), human cytomegalovirus (35), and KSHV (45). At least in HSV, gH is not fully processed and functional in the absence of gL (24, 47). Not much is known about specific receptors targeted by gH, except the affinity for integrins via an RGD sequence in HSV (43) and the interaction with CD46 in HHV-6 (38). Recently, it has been shown for murine herpesvirus 68 (MHV-68) that cell fusion mediated by gH is independent of the presence of gL, whereas binding to cells seems to be dependent on gL (22). Recently, the same group reported interaction of MHV-68 gH/gL with glycosaminoglycans (21). We show here that KSHV gH is expressed on the cell surface in the absence of gL, whereas gL surface expression is dependent on gH coexpression. Furthermore, we observed that both gH alone and the gH/gL complex are able to bind heparin structures with high affinity. They target heparan sulfate proteoglycans of the syndecan family, which promote KSHV entry. Finally, we show that the gH/gL complex is also able to interact with the surfaces of heparan sulfate-negative cells.
MATERIALS AND METHODS
Expression plasmids.
KSHV glycoprotein open reading frames were amplified from a library of overlapping KSHV genome fragments obtained from a Kaposi's sarcoma biopsy specimen (GenBank accession no. U93872) using the Expand HiFi DNA polymerase according to the manufacturer's instructions (Roche, Basel, Switzerland). pAB34 contains the open reading frame for KSHV gH (GenBank accession no. AF448055) in a pcDNA3.1 (Invitrogen) backbone. pAB34Flag for expression of N-terminally tagged gH was constructed by inserting the nucleic acid sequence (GACTACAAGGACGACGATGACAAG) coding for a Flag epitope (DYKDDDDK) between codons 33 and 34 of the gH open reading frame and thus 12 amino acids (aa) after the most likely signal cleavage site (the signal peptide cleavage site after aa 21, according to SignalP). The amino acid sequence was not altered otherwise. A similar construct, pAB80, was used to express gH with a Flag epitope localized more proximal to the C terminus between positions 568 and 569 of gH. The transmembrane region of gH is most likely located between aa 704 and 726, according to TMHMM (32). The sequence coding for KSHV gL (GenBank accession no. U93872 and AAB62638) was cloned into pcDNA3.1-Myc-His with a C-terminal myc tag (pAB37). The expression plasmid pAB74 contains sequences coding for the extracellular domain of KSHV gH (aa 22 to 705) fused to the carboxy terminus of the 21-aa murine immunoglobulin κ [Igκ] signal peptide (Protein Information Resource locus, KVMS32) and the amino terminus of the Fc part from human IgG1 (GenBank accession no. S72664; aa 146 to 374) in a pcDNA3.1 backbone. This plasmid was used to express the soluble ectodomain of gH fused to the IgG1 Fc (gHΔTM-Fc), including a C-terminal myc epitope. Expression plasmid pAB67 contains the coding sequence for the extracellular domain of KSHV open reading frame K14 (aa 27 to 230) fused to the same signal peptide and Fc part (K14ΔTM-Fc). Plasmid pAB61 contained sequences coding for the signal peptide and Fc fragment only.
cDNA molecules encoding syndecans 1, 2, and 4 were synthesized by reverse transcription and amplification using the Titan One Tube reverse transcription-PCR system (Roche) and total cellular RNA from either 293T (syndecans 2 and 4) or JSC-1 (syndecan 1) cells. cDNAs were cloned into pcDNA4a myc/his. Syndecan 1/CD138 expression was verified by flow cytometry with phycoerythrin (PE)-coupled anti-CD138 antibody B-A38 (Serotec, Oxford, United Kingdom). Expression of all other syndecans was verified with anti-myc antibody 9E10 by immunofluorescence in 293T cells (data not shown).
Expression and purification of recombinant proteins.
Fc fusion proteins were prepared and purified as described previously (9). Briefly, 293T cells were transfected by the calcium phosphate method or with Lipofectamine and Plus Reagent (Invitrogen, Carlsbad, CA). The cells were kept in culture for up to 2 weeks, and supernatant was collected every 2 days. Fc fusion proteins were purified from the supernatant by affinity to protein A. The proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining (60). The protein concentration was determined using the Bio-Rad protein assay (Bio-Rad, Hercules, CA) according to the manufacturer's instructions.
Cell culture and transfection.
The cell lines sog-9, Akata, BCBL-1, BC-3, and JSC-1 were obtained from the ATCC. KSHV-positive B cells (BC-3, JSC-1, and BCBL-1) and KSHV-negative B cells (Akata and BJAB) were maintained in RPMI 1640 supplemented with 100 mg/ml gentamicin, 350 mg/ml l-glutamine, 1 mM sodium pyruvate (Sigma Chemicals, St. Louis, MO), 0.05 mM beta-mercaptoethanol (cell culture grade; Gibco BRL, Carlsbad, CA), and 10% (BCBL-1, Akata, and BJAB) or 20% (BC-3 and JSC) heat-inactivated fetal calf serum (FCS) (PAA Laboratories GmbH, Pasching, Austria). Primary human foreskin fibroblasts (HFF) were a kind gift from Klaus Korn (Erlangen, Germany). HEK293T, HeLa, and 9E10 cells were obtained from the ATCC. These cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FCS. Transfection for immunofluorescence and fluorescence-activated cell sorter (FACS) analysis with Lipofectamine and Plus Reagent (Invitrogen) was carried out in 12-well plates according to the manufacturer's instructions. Briefly, 2 μg DNA was transfected with 2 μl Plus Reagent and 2 μl Lipofectamine in a total volume of 400 μl Optimem. Cells were incubated with the transfection mixture overnight. Media were exchanged the following day, and the cells were harvested 1 or 2 days later.
Immunofluorescence.
For immunofluorescence assays, cells were cultured and transfected on coverslips. The cells were washed twice with phosphate-buffered saline (PBS) and fixed with 3% paraformaldehyde for 15 min. After fixation, the cells were washed once in PBS. Paraformaldehyde autofluorescence was quenched with 100 mM glycine in PBS, followed by washing with PBS. Where indicated, cells were permeabilized for 10 min in 0.1% NP-40 in PBS. Blocking was carried out at room temperature for 30 min with 1% bovine serum albumin (BSA) and 5% FCS in PBS. After three washing steps (PBS), the cells were incubated with the respective primary antibody diluted in blocking buffer, followed by three washing steps and 30 min of incubation with anti-mouse Cy3-labeled Fab fragment (Amersham Biosciences, Little Chalfont, United Kingdom) for detection. After three further washing steps, the coverslips were mounted with Vectashield antifading mounting medium (Vector Laboratories, Burlingame, CA) and sealed with nail hardener. Monoclonal antibody anti-Flag M2 (Sigma, St. Louis, MO) was used at a dilution of 1:1,000. Monoclonal antibody anti-myc 9E10 was purified from hybridoma supernatant by the same protocol as Fc fusion proteins.
Immunofluorescence binding assays were done as described previously (9). In binding assays with Fc fusion proteins, 1% BSA in PBS was used for blocking. BSA incubation was followed by incubation with 2 mg/ml CohnII IgG fraction from human serum to block Fc-binding sites. However, omission of this blocking step did not yield different results when protein concentrations that were not above 10 μg/ml were used. The cells were washed again and incubated with the respective Fc fusion protein in PBS with 1% BSA. After three washing steps, bound proteins were detected via their C-terminal myc epitopes with monoclonal antibody 9E10 as described above. Where indicated, the incubation with Cohn's fraction II was omitted and a Cy3-labeled antibody against human Fc fragments (Sigma) was used for direct detection of bound Fc fusion proteins. The endoplasmic reticulum (ER) marker calreticulin was detected by rabbit polyclonal anti-calreticulin antibody (Acris, Germany) and anti-rabbit-fluorescein isothiocyanate (FITC) secondary antibody (Dako, Glostrup, Denmark). Cells were visualized using a Zeiss Axioplan fluorescence microscope (Carl Zeiss Microimaging, Göttingen, Germany). Images were recorded with a Spot Diagnostic Imaging camera and software (Diagnostic Instruments, Burroughs, MI). Confocal images were acquired on a Leica (Leica Microsystems, Wetzlar, Germany) TCS SP5.
FACS analysis.
Cells were harvested 1 or 2 days after transfection with Lipofectamine and Plus Reagent (Invitrogen). The cells were washed once with PBS and once with FACS buffer (10% FCS and 0.05% sodium azide in PBS). The cells were then incubated with the respective primary antibody (anti-Flag, 1:1,000; anti-myc, 1:200) in FACS buffer for 1 h, followed by washing and incubation with anti-mouse-FITC, 1:50 in FACS buffer, for 30 min. For binding assays, cells were incubated with the respective proteins diluted in FACS buffer at the indicated concentrations for 1 h on ice. The cells were washed twice in FACS buffer and incubated for 30 min with FITC-coupled secondary anti-human IgG antibody (Dako, Glostrup, Denmark), 1:50 in FACS buffer. After two washing steps, the cells were analyzed on a FACSCalibur system (BD Biosciences, San Jose, CA). Syndecan 1 expression was detected with a PE-coupled anti-CD138 antibody (Serotec) according to the manufacturer's protocol. PE-coupled mouse IgG1 isotype antibody (Becton Dickinson, Franklin Lakes, NJ) served as a control.
SPR measurement.
Surface plasmon resonance (SPR) experiments were performed on a BIAcore biosensor system using a streptavidin-coated (SA) biosensor chip (BIAcore AB, Uppsala, Sweden) as described previously (9). Briefly, purified Fc fusion proteins were biotinylated and coupled to flow cell 2 of the SA sensor chip. Flow cell 1 was used as a reference to correct for changes in the buffer composition and nonspecific binding to the sensor chip surface. For SPR measurements, heparin diluted in PBS at various concentrations (see below) was injected at a flow rate of 4 μl/min. Following injection of the glycosaminoglycan solution, the biosensor was rinsed with running buffer at the same flow rate for 200 s. The flow rate was then increased to 20 μl/min, and 10 μl of a 0.1 M NaOH/0.1% sodium dodecyl sulfate solution was injected to regenerate the chip surface. The binding of various glycosaminoglycans (heparin, N-acetylheparin, N-acetyl-de-O-sulfated heparin, de-N-sulfated acetylated heparin, and chondroitin sulfates A, B, and C, all obtained from Sigma Chemicals) was measured using gHΔTM-Fc (flow cell 1) versus Fc (flow cell 2) coupled to SA sensor chips and glycosaminoglycan solutions (10 μg/ml in PBS). SPR data were analyzed with BIAevaluation 3.0 software (BIAcore AB). Briefly, for estimation of the association, or on-rate, constant, the middle portion of the association curves (5 s to 75 s [see Fig. 6A]) was used. For estimation of the dissociation, or off-rate, constant, the first part of the dissociation phase of the curve (135 s to 175 s [see Fig. 5A]) was used. These kinetics data were fitted most adequately by assuming a simple bimolecular reaction model for interaction between the soluble analyte and the immobilized ligand (Langmuir model). The goodness of fit was estimated by calculating χ2 values and inspecting residuals (the difference between observed and calculated values).
FIG. 6.
Binding of gHΔTM-Fc and gHΔTM-Fc/gL and KSHV infection are enhanced by overexpression of syndecans. (A) (I) Purified gHΔTM-Fc at 10 μg/ml or K14ΔTM-Fc as a control was incubated with 293T cells transfected with syndecan expression plasmids. Concentrated gHΔTM-Fc also binds vector-transfected 293T cells (presumably through endogenous HSPGs), but binding is strongly augmented by syndecan expression. Binding of gHΔTM-Fc could be blocked by the addition of heparin (100 U/ml) to the recombinant protein prior to binding. One of three representative experiments is shown. (II) Silver-stained gel displaying 1 μg of the respective Fc fusion proteins. (B) (I) KSHV infection is enhanced by syndecan expression. 293T cells were transfected with expression plasmids for syndecans 1, 2, and 4. After 2 days, the cells were inoculated overnight with recombinant rKSHV.219. On day 3 after infection, 100,000 cells per sample were analyzed by FACS for expression of the enhanced-GFP reporter gene of rKSHV.219. The experiment was carried out in triplicate; the error bars represent standard deviations. (II) Virus was preincubated with heparin (100 U/ml), and cells transfected with expression plasmid for syndecan 1 or with empty vector were infected and analyzed as described above. Heparin strongly inhibits KSHV infection, and the enhancing effect of syndecan overexpression can be reverted.
FIG. 5.
Analysis of gH binding to glycosaminoglycans by surface plasmon analysis. (A) SPR with gHΔTM-Fc immobilized on a biosensor chip and various concentrations of heparin. The KD of the gHΔTM-Fc-heparin complex was determined at 1.5 × 10−8 by using the Langmuir model. (B) The gHΔTM-Fc fusion protein was immobilized on a biosensor chip, and the binding of different glycosaminoglycans (10 μg/ml) in solution was measured by SPR. The strongest binding could be detected with heparin, a heparan sulfate-glycosaminoglycan. It consists of strongly sulfated N-acetylglucosamine and iduronic acid residues. (C) Structures of the predominant components of the glycosaminoglycan preparations used for affinity measurements. l-Iduronate (shown here) is more abundant than d-glucoronate in heparin. Heparin preparations contain a variety of variants of this basic disaccharide unit with variations in the sulfation and acetylation patterns. N-Acetylheparin is almost identical to de-N-sulfated acetylated heparin, with the latter only partially acetylated at amino residues.
Virus preparation.
Recombinant KSHV.219 (rKSHV.219) (55) was latently propagated in 293 cells. rKSHV.219-293 cells were grown to density in 75-cm2 culture vessels. Lytic replication was induced by treatment with 3 mM sodium butyrate for 24 h. The medium was then exchanged for 10 ml 293 medium without butyrate. The cell culture supernatant was collected after 4 days, and debris was removed by 5 min of centrifugation at 2,000 × g in 50-ml tubes. Virus was pelleted from the supernatants by centrifugation at 2,000 × g overnight in 50-ml tubes. After careful aspiration of the supernatant, the pellet was resuspended in “the last drop” overnight. The volume was then adjusted by the addition of 293 medium to achieve a 30-fold concentration. The virus was stored at 4°C. Before use, virus stocks were centrifuged for 5 min at 2,000 × g to remove the remaining debris.
Infection assay.
293T cells were transfected with Lipofectamine and Plus Reagent 2 days prior to infection. The cells were then incubated overnight with sixfold-concentrated infectious rKSHV.219 supernatant (30×-concentrated stock fivefold diluted in 293T medium). The medium was exchanged the next day, and the cells were analyzed by FACS 3 days after infection. The virus was diluted to yield ∼1% infected cells as determined by green fluorescent protein (GFP) fluorescence after 3 days, corresponding to a multiplicity of infection of ∼0.01. A total of 100,000 cells were scored by FACS analysis per infection.
RESULTS
gH expression on the cell surface is independent of gL.
KSHV gH is predicted to be a type I transmembrane protein of 730 aa. The N-terminal 21 aa likely constitute a secretory signal peptide (8). A transmembrane helix is predicted between aa 704 and 726 (28). In the case of HHV-8 gL, the first 20 aa are predicted to constitute the signal peptide (28, 39). To test whether gH and gL are expressed independently of each other, plasmids encoding Flag-tagged gH (pAB34Flag) and/or myc-tagged gL (pAB37) were transfected into HeLa or 293T cells. A Flag epitope was inserted into the extracellular part of gH at either position 33 (pAB34Flag) (Fig. 1A and B) or position 569 (pAB80) (data not shown) of the gH amino acid chain. Analysis of glycoprotein expression and localization was done by immunofluorescence using HeLa cells (Fig. 1A). As we achieved only low transfection efficiency (5 to 10%) with the HeLa cells in our immunofluorescence experiments, we also analyzed surface expression of gH and gL by FACS analysis using 293T cells (Fig. 1B shows one representative experiment out of three independent experiments). Interestingly, gH expression and membrane localization appeared to be essentially unaltered by the presence of gL. In contrast to gH, gL was not detectable on the cell surface in the absence of gH (Fig. 1B, II) in FACS analysis. gL alone was also not detectable by immunofluorescence assay without permeabilization. When cells were transfected with gL expression constructs only, the protein accumulated in a perinuclear area, mostly colocalizing with the ER marker calreticulin (Fig. 1A, II). Coexpression of gH clearly shifted gL expression away from the ER toward the membrane. The percentage of gH-positive cells in FACS analysis could not be conspicuously increased by cotransfection of gL (Fig. 1B, I). Thus, KSHV gH is transported to the plasma membrane independently of gL. In contrast, gL was detectable at the cell surface only when coexpressed with gH (Fig. 1A and B).
FIG. 1.
gH is expressed at the cell surface independently of gL. (A) HeLa cells were transfected with expression plasmids for gH-Flag, gL-myc, or both. The cells were fixed with 3% paraformaldehyde 2 days after transfection and either permeabilized with 0.1% NP-40 (II) for detection of intracellular and surface proteins or left unpermeabilized (I) for detection of surface expression only. gH-Flag and gL-myc were detected by the anti-Flag antibody M2 or the anti-myc antibody 9E10, respectively, followed by Cy3-labeled anti-mouse secondary antibody and immunofluorescence microscopy. (I, middle row) gH was readily detectable at the surfaces of nonpermeabilized cells in the absence of gL. gL surface expression was clearly dependent on gH coexpression. (I, top row) In the absence of gH, gL could not be detected on the surfaces of unpermeabilized cells. Identical camera settings were used for each row, and the pictures were taken at ×630 magnification. Confocal microscopy with permeabilized cells (II) revealed colocalization of gL with the ER marker calreticulin when expressed alone. Upon gH cotransfection, gL was relocalized away from the ER. gH was localized to both the ER and membranous structures with and without coexpression of gL. (B) 293T cells were transfected as in panel A and analyzed by FACS analysis for gH and gL surface expression. The cells were incubated with anti-Flag and anti-myc antibodies, followed by FITC-labeled anti-mouse antibody. The results were quantified by counting the proportion of cells exceeding a threshold adjusted to no more than 1% positive cells in the negative control (one representative experiment is shown).
gL requires gH for export and forms a soluble complex with the extracellular part of gH.
As we have demonstrated, gL translocation to the cell surface was dependent on gH. We also tried, but failed, to express soluble recombinant gL in eukaryotic cells without gH. This led us to ask whether gL is a type II transmembrane protein, as previously suggested (39), or rather secreted from the cell. We therefore designed a secretion assay cotransfecting expression constructs for gHΔTM-Fc, gHΔTM-Fc deletion mutants, and gL. gL was not detectable in the supernatant without gHΔTM-Fc coexpressed (Fig. 2, lane 7). When gHΔTM-Fc was coexpressed, gL was readily secreted (Fig. 2, lane 1). Deletion of the C-terminal membrane-proximal part of gH in gHΔTM-Fc resulted in abrogation of detectable gL secretion (Fig. 2, lanes 4 through 6). Immunoprecipitation from cellular lysates clearly showed that the N-terminal part of gH strongly interacts with gL but is not able to efficiently secrete gL (Fig. 2, lanes 4 through 6). This is surprising, as these gL binding mutants are very efficiently secreted themselves but are apparently unable to export bound gL in relevant amounts (Fig. 2, lanes 4 through 6). We therefore propose that the whole extracellular domain of gH is required for efficient secretion of gL, with the N terminus stably binding gL and the C-terminal parts of the gH exodomain transiently assisting in gL export. These findings could be confirmed by immunofluorescence analysis of transfected cells (see Fig. 1 posted at http://www.viro.med.uni-erlangen.de/supplement/jvi/JVI01170-08/suppl-fig.pdf); whereas gH variants containing the complete or N-terminal part of the protein colocalized with gL, a variant containing only aa 465 to 705 did not. Furthermore, KSHV gL is not a transmembrane protein but a secreted protein that forms a soluble complex with the extracellular domain of gH.
FIG. 2.
The overall integrity of gH is essential for efficient gL secretion. Several N- and C-terminal deletion mutants of gH (without transmembrane and intracellular domains) were created and C-terminally fused to Fc. The gH-Fc constructs were cotransfected with a gL expression construct, and expression, as well as secretion, of both proteins was assayed by Western blot analysis. Western blot analysis of gH-Fc (anti-Fc) of the cell culture supernatant and cell lysate showed expression and secretion of the gH mutants (lanes 1 through 6). gL was not efficiently secreted by itself (lane 7). Only full-length extracellular gH was able to efficiently secrete gL (lane 1). C-terminal truncation (lanes 4 through 6) and N-terminal truncation (lanes 2 and 3) of gH almost abrogated gL secretion. Very weak residual gL secretion could be observed after N-terminal truncation of the extracellular part of gH (lanes 2 and 3, asterisks). Precipitation of the gH mutants from cellular lysates with protein A was followed by Western blot analysis for gL. Full-length gHΔTM-Fc efficiently precipitates gL. The N-terminal part of gH strongly interacts with gL in immunoprecipitation but is not able to efficiently cosecrete gL (lanes 4 through 6).
Binding of the extracellular domain of gH and gH/gL to cell surface glycosaminoglycans.
As gH reached the cytoplasm membrane in the absence of gL, we speculated whether KSHV gH alone might have functions in infection, e.g., by binding to target cells. Recombinant gHΔTM-Fc was expressed for binding assays; Fc alone, as well as K14ΔTM-Fc (not shown), served as a control. All three proteins could be readily purified to near homogeneity from the supernatant of transfected HEK293T cells by protein A affinity chromatography (see Fig. 6A, II). HFF or Vero cells were incubated with purified Fc fusion proteins, and binding was detected by either antibodies against the myc epitope or anti-human Fc, respectively. As shown in Fig. 3, gHΔTM-Fc protein, but not the Fc protein, efficiently bound to both nonpermeabilized HFF and Vero cells (Fig. 3A and B, respectively, left and middle). A similar fine-grained binding pattern was observed with gHΔTM-Fc on HeLa, 293T, mouse L, and human microvascular endothelial cells (data not shown). Binding of KSHV gHΔTM-Fc was completely abolished when the proteins were preincubated with heparin (Fig. 3A, right) or cells were treated with heparinase III (Fig. 3B, right). A FACS binding assay with purified gHΔTM-Fc on Vero cells (Fig. 3C) also revealed strong binding of gHΔTM-Fc that could be completely abolished by preincubation of the protein with heparin. This indicates that HSPGs are the molecules predominantly responsible for gH binding to the cell surface.
FIG. 3.
Binding of the gH ectodomain to cell surfaces is heparin dependent. (A) After fixation and blocking with human Cohn fraction II, HFF were incubated with gHΔTM-Fc (middle), Fc (left), or gHΔTM-Fc that was preincubated for 10 min with heparin (right). The protein concentration was 2 μg/ml. Binding was detected with a monoclonal antibody directed to the C-terminal myc epitope and Cy3-labeled anti-mouse immunoglobulin G antibody. Nuclei were counterstained with DAPI. The pictures were taken at ×630 magnification with filters for either red (upper row; Cy3) or blue (lower row; DAPI) fluorescence. gHΔTM-Fc, but not Fc alone, binds in a speckled pattern to HFF surfaces. Binding can be abolished by preincubation of gHΔTM-Fc with heparin. (B) Vero cells were seeded on coverslips, fixed with paraformaldehyde, and treated either with 20 U/ml heparinase III in 0.1% BSA (right) in PBS or with 0.1% BSA alone (left and middle) for 4 hours at 37°C. The cells were then probed directly with gHΔTM-Fc or Fc at a concentration of 10 μg/ml, followed by detection with anti-human-Cy3 secondary antibody and DAPI counterstaining of nuclei. The pictures were taken at ×400 magnification (upper row, Cy3 fluorescence; lower row, DAPI). Again, gHΔTM-Fc (middle column) but not Fc protein alone (left) efficiently bound to the cell surface. Binding of gHΔTM-Fc was almost fully abrogated by pretreatment of the cells with heparinase III. (C) Vero cells were briefly trypsinized, detached, and then incubated with purified gHΔTM-Fc at a concentration of 10 μg/ml or with gHΔTM-Fc preincubated for 10 min with 100 U/ml heparin. Bound protein was detected by FACS analysis with anti-human-FITC secondary antibody.
To analyze the binding of the gH/gL complex, nonconcentrated supernatant from cells transfected with an expression plasmid for gHΔTM-Fc and gL was incubated with 293T target cells, followed by FACS analysis for binding. Bound protein was detected with anti-Fc-FITC (Fig. 4). Binding of gHΔTM-Fc/gL could be blocked by the addition of heparin (Fig. 4), with only some residual binding remaining.
FIG. 4.
Binding of soluble gH-Fc/gL is susceptible to heparin blocking. 293T cells were incubated with supernatants of transfected cells expressing gHΔTM-Fc/gL or a control protein. FACS analysis showed that gHΔTM-Fc binds to 293T cells (black line), whereas control protein (Fc) does not (gray filled histogram). Binding of gHΔTM-Fc/gL could be blocked by the addition of heparin to the binding reaction (gray line).
High affinity of gH for heparin.
We performed BIAcore SPR experiments with immobilized gHΔTM-Fc to determine the dissociation constant (KD) of the gHΔTM-Fc-heparin complex. A KD of 15 nM was calculated, fitting the data shown in Fig. 5A to the Langmuir model. To further elucidate the physicochemical requirements for gH binding to glycosaminoglycans, SPR was performed with immobilized gHΔTM-Fc versus Fc and seven different glycosaminoglycans (Fig. 5B).
This clearly showed that N-sulfation is important for the interaction of gH with glycosaminoglycans: two different preparations of heparin lacking an N-linked sulfate group (N-acetylheparin and de-N-sulfated acetylated heparin) (Fig. 5B) exhibited decreased affinity for gHΔTM-Fc, although binding was abolished only by complete desulfation of heparin residues in N-acetylated-de-O-sulfated heparin. In contrast to chondroitin-sulfate B/dermatan sulfate, chondroitin-sulfate A and C did not bind despite the presence of negatively charged sulfate groups. This suggests that the iduronic acid backbone common to chondroitin-sulfate B/dermatan sulfate and heparin is a structural requirement for recognition by gH, as well as the negative charges caused by both N- and O-linked sulfate groups.
gH and the gH/gL complex bind syndecans.
HSPGs are present on most cell types. As phospholipase C treatment did not alter gHΔTM-Fc binding visibly (data not shown), we focused our interest on HSPGs of the syndecan family. Although widespread, the spectrum of membrane-spanning proteoglycans in mammalian cells is essentially limited to syndecans 1 to 4, beta-glycan, and CD44v3 (10). In order to verify whether the high affinity of gH for glycosaminoglycans resulted in increased interaction of gH with syndecan-bearing KSHV target cells, we transfected HEK293T cells with expression constructs for human syndecans 1, 2, and 4. One day after transfection, the cells were incubated with recombinant purified gHΔTM-Fc at a concentration of 10 μg/ml (Fig. 6A). Recombinantly expressed and purified K14ΔTM-Fc at 10 μg/ml (Fig. 6A) served as a control. Binding of Fc fusion proteins was detected by FITC-labeled anti-human Fc antibodies and FACS analysis. Cells exceeding a fluorescence threshold were scored positive. One representative experiment is shown in Fig. 6. Compared to vector-transfected cells with intrinsic low-level expression of HSPGs, increased binding of gHΔTM-Fc, but not control protein, was seen with cells transfected with syndecans 1, 2, and 4. Similar results were obtained with supernatants from gHΔTM-Fc/gL-expressing cells. All syndecans clearly increased the binding of gHΔTM-Fc.
Syndecan expression enhances KSHV infection.
To assess the biological relevance of syndecan binding, we overexpressed syndecans 1, 2, and 4 in 293T cells and then inoculated these cells with rKSHV.219. Three days after transfection, cells expressing the GFP reporter gene from rKSHV.219 were counted by FACS analysis to measure viral entry. 293T cells expressing any of the syndecan proteoglycans showed a significant increase in KSHV infection (Fig. 6B, I), clearly indicating a role for these molecules as entry factors. Preincubation of the virus with heparin strongly inhibited infection and also reverted the enhancing effect of syndecan 1 expression (Fig. 6B, II).
The gH/gL complex also recognizes receptors other than HSPGs and binds to lamellipodium-like structures.
As can be seen in Fig. 4, binding of the gHΔTM-Fc/gL complex on 293T cells could be blocked almost completely by the addition of heparin. Nevertheless, a very small residual shift was still observed in FACS analysis, hinting at the potential presence of additional cellular receptors. To assess whether the gH/gL complex also binds receptors other than HSPGs, we chose to incubate heparan- and chondroitin sulfate-negative sog9 mouse fibroblasts (7) with soluble glycoproteins and checked for binding of the gHΔTM-Fc/gL complex via indirect immunofluorescence against the Fc part. The gHΔTM-Fc/gL complex clearly bound to the membranes of sog9 cells (Fig. 7 A, lower row), whereas control protein (Fc) and gHΔTM-Fc alone did not (Fig. 7A, upper left). Rather intense binding was observed in membrane areas resembling lamellipodium-like structures (Fig. 7A, enlargements) at the edges of cells, especially when these cells were apparently in the process of spreading out on the coverslip. FACS (Fig. 7C) and cell-linked immunosorbent assay (Fig. 7B) binding assays confirmed the binding of gHΔTM-Fc/gL to sog9 cells. Preincubation of gHΔTM-Fc/gL with heparin altered the affinity of gHΔTM-Fc/gL (Fig. 7C), presumably by complex formation with highly charged heparin molecules, but was not able to block interaction with the cell surface, even at high concentrations. Binding on sog9 cells was dependent on gL coexpression and could not be blocked by addition of heparin (Fig. 7A, lower left).
FIG. 7.
The gHΔTM-Fc/gL complex binds to lamellipodium-like structures on heparan-deficient sog9 cells. (A) sog9 cells were fixed with 3% paraformaldehyde for 15 min and incubated with the supernatants of 293T cells transfected with expression plasmid for gHΔTM-Fc, Fc, or gHΔTM-Fc, together with an expression plasmid for gL (gHΔTM-Fc/gL). Binding of the Fc fusion proteins was detected with anti-human Fc-Cy3 secondary antibody. Binding was dependent on gL coexpression, as gHΔTM-Fc alone did not bind. The addition of heparin did not inhibit binding. gHΔTM-Fc/gL staining was clearly con- centrated in lammellipodium-like regions, where cells were spreading on the coverslip (indicated by white arrowheads). (B) A binding cell-linked immunosorbent assay with soluble gHΔTM-Fc/gL. sog 9 cells were treated and incubated as described above in 12-well tissue culture dishes. Bound protein was detected with an anti-human-horseradish peroxidase secondary antibody, and luminescence was quantified densitometrically. The error bars indicate standard deviations. (C) FACS binding assay with gHΔTM-Fc/gL. sog9 cells were detached by brief treatment with trypsin/EDTA and incubated with glycoprotein-containing supernatants. The glycoproteins were preincubated with heparin at 100 U/ml for 10 min. Bound protein was detected with an anti-human-FITC secondary antibody.
Expression of gH/gL renders target cells more resistant to KSHV infection.
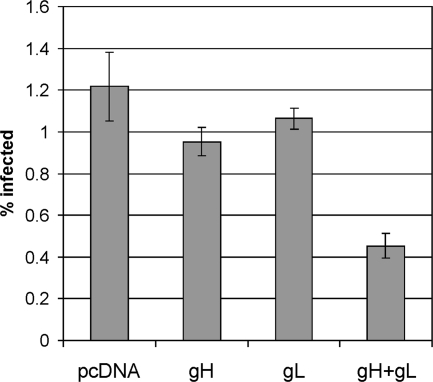
To assess whether binding of the gH/gL complex to cellular receptors is important for KSHV infection, we expressed these proteins in 293T cells before infection with recombinant rKSHV.219. Our hypothesis was that this should block the association of virion gH/gL with these receptors or lead to downregulation of the receptors. As can be seen in Fig. 8, expression of gH or gL alone led to slight reduction of KSHV infection. gH preexpression consistently gave a reduction in KSHV of around 20%. Preexpression of gL caused a still smaller reduction. However, preexpression of the gH/gL complex caused a drastic and highly significant drop in KSHV susceptibility of over 60%, which roughly corresponds to the transfection efficiency typically achieved.
FIG. 8.
Expression of gH/gL in target cells prior to infection reduces KSHV susceptibility. 293T cells were transfected with expression plasmids for gH, gL, or both. Transfection of gH alone and to a lesser degree gL alone led to a decrease in KSHV susceptibility. Preexpression of gH and gL together reduced KSHV susceptibility markedly. The error bars represent standard deviations of three independent experiments.
DISCUSSION
We report here that KSHV gH reaches the cell surface independently of gL, whereas gL is retained within the cell without coexpression of gH. These findings point to a fundamental difference between KSHV and HSV with respect to the export of gH and gL. KSHV gL is a soluble protein that is retained within the cell without gH being present. It has been shown for both HSV and varicella zoster virus, as well as the KSHV-related Epstein-Barr virus, that gH is retained within the cell when expressed without gL (24, 33). Formation of a gH-gL heterodimer is absolutely required for gH to reach the cell surface as a functional glycoprotein in the HSV model (24). However, there are also data from animal herpesviruses showing that gH is not entirely dependent on gL in all of its functions. Some reports suggest that even HSV gH is not entirely dependent on gL with respect to cell surface expression in CHO cells (49). Another example is pseudorabies virus, where gH seems to fulfill at least some of its functions in the absence of gL (30, 31). In addition, it was reported recently that a gL deletion mutant of MHV-68 retains infectivity (22) and that gL is not required for the fusion activity of MHV-68 gH. This is particularly noteworthy, as MHV-68 and KSHV are both members of the rhadinovirus subfamily. With respect to KSHV, our finding that gH was readily expressed on the cell surface in the absence of gL partially contrasts with previous publications. Naranatt and coworkers observed that gL, but not gH, of KSHV reached the cell surface (39), and a more recent paper using a C-terminally tagged gL supported this finding (42). Here, we report the opposite: gH by itself reached the cell surface, but gL did not. We also show that gL, at least when complexed with soluble gH, is a soluble protein, apparently lacking a transmembrane domain. An interesting paper by Cairns et al. (11) reported findings of HHV-2 gH- and gL-independent export. A region responsible for ER retention was characterized. The region critical for ER retention in HHV-2 gH was mapped through deletions and mutations from aa 29 to 48 (in HHV-2 gH). The region from aa 48 to 63 also proved to be important (Fig. 9). Several amino acids in the first region were shown to be critical for ER retention. Interestingly, the feature was not even conserved between HHV-1 and HHV-2, despite high sequence homology. This suggests that ER retention signals can easily be transferred to other positions during the course of evolution. KSHV and HHV-2 gHs are by far more divergent than those of the alphaherpesviruses. In fact, a whole block of around 25 aa in which the retention motif is located in HHV-2 is missing in KSHV gH. Figure 9 shows an alignment of the first 80 aa of HHV-2 gH and KSHV gH generated with the TCoffe Tool (46). In our opinion, it seems likely that the feature of ER retention, or “retention” more generally, has somehow migrated to gL during evolution. What is fascinating in our eyes is the fact that there is a conserved mechanism of “retention” or “impaired processing” that keeps one part of the gH/gL complex inside the cell as long as possible.
FIG. 9.
Alignment of the N termini of HHV-2 and KSHV gH. Amino acid sequences of HHV-2 (reference sequence, NP_044491.1 ) and KSHV gH (reference sequence, YP_001129375.1 ) were aligned using the tool TCoffee. Matching and similar amino acids are highlighted in blue, turquoise, and gray (with decreasing similarity in that order). The region critical for ER retention in HHV-2 is highlighted by a black bar. The greater part of this region is obviously deleted in the corresponding KSHV sequence.
Taken together, all our gH variants were transported to the plasma membrane or secreted. Furthermore, we mapped the region in gH that is mainly responsible for secretion of gL to the C terminus of the extracellular part of gH; deletions there clearly abrogated gL secretion. Without gH, gL was not efficiently exported. We speculate that the use of rodent or monkey cells might explain the results obtained by the other groups. If the hydrophobic signal peptide was not properly cleaved, gL might have been expressed similarly to a type II transmembrane protein. Coexpression of gL did not significantly alter gH transport or stability. What caused the discrepancy between two earlier reports and our data on KSHV gH surface transport remains to be solved. One possibility again is the use of different cell lines and expression systems.
Although gH of KSHV is transported to the plasma membrane without gL, this does not imply that gL is dispensable. However, gH without gL may well have additional functions, for example, blocking gH/gL binding sites on the host cell during egress in order to prevent premature triggering of the fusion machinery, or it could simply serve as a decoy for the immune system. The affinity of gH for certain HSPGs may not serve merely the purpose of attachment. Instead, it could represent a way to concentrate the virus at specific sites of entry. The gH binding we observed on many cells was clearly of a finely grained pattern. The selectivity of proteoglycan engagement by gH is implied by the SPR data, in which heparan sulfate is favored over chondroitin sulfate, with the exception of an affinity for chondroitin sulfate B/dermatan sulfate. This suggests that the epimer iduronic acid (common to heparan and dermatan sulfate), and not glucuronic acid, is important for the generation of the binding motif apart from negative charges provided by the sulfate residues. To date, not much is known about the compositions of glycosaminoglycans with respect to different cell types, but this might be a key to the understanding of the tropism of KSHV and other pathogens in vivo. At least for syndecan 1, the structures and ligand binding characteristics of the heparan sulfate side chains were shown to differ between cell types (29).
Syndecans compose a family of four closely related type I membrane proteins carrying heparan sulfate side chains (see references 6 and 13 for an overview). They play a major role in attachment to the extracellular matrix, in growth factor signaling, and in the formation of focal adhesions (34, 56, 57; see reference 54 for a review). We found that all three syndecans (1, 2, and 4) tested were able to increase the binding of gHΔTM-Fc, with and without gL, to target cells. Furthermore, all syndecans clearly enhanced KSHV infection. Our results suggest that the gH/gL complex is part of an intricate attachment machinery that engages HSPGs with different affinities. In our hands, gHΔTM-Fc exhibited higher affinity for cells and HSPGs than K8.1, another HSPG binding protein of KSHV. We suggest that the uncommonly high affinity of gH and gH/gL for cellular HSPGs may be the final stage in a multistep attachment sequence involving the glycoproteins K8.1, gB, KCP, and gH/gL and cellular HSPGs, as well as lectins. Syndecan engagement may be a turning point at which attachment ends and entry begins. Syndecans 1 and 4 were reported to trigger endocytosis (17, 53). Syndecan 4 is known to be a component of focal adhesions (56) and to induce protein kinase C signaling upon clustering (40, 41, 62), recruiting protein kinase C to focal contacts (34). It is noteworthy that KSHV enters at least some cells via endocytosis and that focal adhesions play a major role in KSHV entry (50). KSHV, like many other viruses, seems to mimic processes occurring during the attachment of cells to extracellular matrix (ECM) molecules in which integrins and syndecans or other HSPGs are cross-linked by fibronectin (48, 58). High-affinity binding of gH/gL to syndecans may not only promote attachment, but also foster internalization of the virus and thus ultimately enhance infection, as shown in our experiments.
Binding of the gH/gL complex on heparan sulfate-deficient cells also fits into this pattern of mimicking ECM interactions. Intense binding was clearly localized to structures resembling lammellipodia localized at the very edges of cells. Lamellipodia, in turn, are rich in integrins and other components of focal adhesions. A combination of HSPG and integrin interaction at focal adhesions seems to be a general feature of the entry processes of many pathogens and possibly mimics ECM molecules, like fibronectin, that also combine HSPG and integrin binding sites. Notably, this heparan sulfate-independent cell surface interaction required coexpression of gL.
Interestingly, syndecan 1 is present on PEL cells far more abundantly than on other cell lines tested here and was reported to disappear upon induction of lytic replication (1). It is not known from which type of B cell PEL is derived in the first place, at what stage of differentiation KSHV infection occurs, and which B-cell population is most susceptible to KSHV. Nevertheless, in light of our results, syndecan 1 expression, together with other entry factors, may play a role in the B-cell tropism of KSHV and thus ultimately in the development of PEL. It is surprising that shedding of syndecan 1 promotes the progression of tumors (61) and is a marker of PEL progression in a mouse xenograft model (16). The engagement of lammellipodium-like structures by the gH/gL complex, very similar to gB, combined with the affinity for syndecans may target the virus toward structures involved in very basal cellular functions, like migration and ECM attachment, functions that are deregulated in oncogenesis.
Acknowledgments
We thank Gaby Sander for providing rKSHV.219-positve 293 cells and Angela Holzer for excellent technical support.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 643 and GRK1071), the IZKF Erlangen, the European Community research project TargetHerpes, and the Mainzer Akademie der Wissenschaften und der Literatur.
Footnotes
Published ahead of print on 22 October 2008.
REFERENCES
- 1.Adang, L. A., C. H. Parsons, and D. H. Kedes. 2006. Asynchronous progression through the lytic cascade and variations in intracellular viral loads revealed by high-throughput single-cell analysis of Kaposi's sarcoma-associated herpesvirus infection. J. Virol. 8010073-10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akula, S. M., P. P. Naranatt, N. S. Walia, F. Z. Wang, B. Fegley, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J. Virol. 777978-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2001. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284235-249. [DOI] [PubMed] [Google Scholar]
- 4.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108407-419. [DOI] [PubMed] [Google Scholar]
- 5.Akula, S. M., F. Z. Wang, J. Vieira, and B. Chandran. 2001. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology 282245-255. [DOI] [PubMed] [Google Scholar]
- 6.Alexopoulou, A. N., H. A. Multhaupt, and J. R. Couchman. 2007. Syndecans in wound healing, inflammation and vascular biology. Int. J. Biochem. Cell. Biol. 39505-528. [DOI] [PubMed] [Google Scholar]
- 7.Banfield, B. W., Y. Leduc, L. Esford, K. Schubert, and F. Tufaro. 1995. Sequential isolation of proteoglycan synthesis mutants by using herpes simplex virus as a selective agent: evidence for a proteoglycan-independent virus entry pathway. J. Virol. 693290-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340783-795. [DOI] [PubMed] [Google Scholar]
- 9.Birkmann, A., K. Mahr, A. Ensser, F. Titgemeyer, B. Fleckenstein, and F. Neipel. 2001. Cell surface heparan sulfate is a receptor for human herpesvirus 8 and interacts with envelope glycoprotein K8.1. J. Virol. 7511583-11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bishop, J. R., M. Schuksz, and J. D. Esko. 2007. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 4461030-1037. [DOI] [PubMed] [Google Scholar]
- 11.Cairns, T. M., L. S. Friedman, H. Lou, J. C. Whitbeck, M. S. Shaner, G. H. Cohen, and R. J. Eisenberg. 2007. N-terminal mutants of herpes simplex virus type 2 gH are transported without gL but require gL for function. J. Virol. 815102-5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campadelli-Fiume, G., M. Amasio, E. Avitabile, A. Cerretani, C. Forghieri, T. Gianni, and L. Menotti. 2007. The multipartite system that mediates entry of herpes simplex virus into the cell. Rev. Med. Virol. 17313-326. [DOI] [PubMed] [Google Scholar]
- 13.Carey, D. J. 1997. Syndecans: multifunctional cell-surface co-receptors. Biochem. J. 3271-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cocchi, F., L. Menotti, P. Mirandola, M. Lopez, and G. Campadelli-Fiume. 1998. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 729992-10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dechecchi, M. C., A. Tamanini, A. Bonizzato, and G. Cabrini. 2000. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology 268382-390. [DOI] [PubMed] [Google Scholar]
- 16.Foussat, A., J. Wijdenes, L. Bouchet, G. Gaidano, F. Neipel, K. Balabanian, P. Galanaud, J. Couderc, and D. Emilie. 1999. Human interleukin-6 is in vivo an autocrine growth factor for human herpesvirus-8-infected malignant B lymphocytes. Eur. Cytokine Netw. 10501-508. [PubMed] [Google Scholar]
- 17.Fuki, I. V., M. E. Meyer, and K. J. Williams. 2000. Transmembrane and cytoplasmic domains of syndecan mediate a multi-step endocytic pathway involving detergent-insoluble membrane rafts. Biochem. J. 351607-612. [PMC free article] [PubMed] [Google Scholar]
- 18.Garrigues, H. J., Y. E. Rubinchikova, C. M. Dipersio, and T. M. Rose. 2008. Integrin αVβ3 binds to the RGD motif of glycoprotein B of Kaposi's sarcoma-associated herpesvirus and functions as an RGD-dependent entry receptor. J. Virol. 821570-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 2801618-1620. [DOI] [PubMed] [Google Scholar]
- 20.Gianni, T., G. Campadelli-Fiume, and L. Menotti. 2004. Entry of herpes simplex virus mediated by chimeric forms of nectin 1 retargeted to endosomes or to lipid rafts occurs through acidic endosomes. J. Virol. 7812268-12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillet, L., S. Colaco, and P. G. Stevenson. 2008. The murid herpesvirus-4 gH/gL binds to glycosaminoglycans. PLoS ONE 3e1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillet, L., J. S. May, S. Colaco, and P. G. Stevenson. 2006. Glycoprotein L disruption reveals two functional forms of the murine gammaherpesvirus-68 glycoprotein H. J. Virol. 81280-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harman, A., H. Browne, and T. Minson. 2002. The transmembrane domain and cytoplasmic tail of herpes simplex virus type 1 glycoprotein H play a role in membrane fusion. J. Virol. 7610708-10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutchinson, L., H. Browne, V. Wargent, N. Davis-Poynter, S. Primorac, K. Goldsmith, A. C. Minson, and D. C. Johnson. 1992. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J. Virol. 662240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue, N., J. Winter, R. B. Lal, M. K. Offermann, and S. Koyano. 2003. Characterization of entry mechanisms of human herpesvirus 8 by using an Rta-dependent reporter cell line. J. Virol. 778147-8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson, T., F. M. Ellard, R. A. Ghazaleh, S. M. Brookes, W. E. Blakemore, A. H. Corteyn, D. I. Stuart, J. W. Newman, and A. M. King. 1996. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J. Virol. 705282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaleeba, J. A. R., and E. A. Berger. 2006. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 3111921-1924. [DOI] [PubMed] [Google Scholar]
- 28.Kall, L., A. Krogh, and E. L. L. Sonnhammer. 2004. A combined transmembrane topology and signal peptide prediction method. J. Mol. Biol. 3381027-1036. [DOI] [PubMed] [Google Scholar]
- 29.Kato, M., H. Wang, M. Bernfield, J. T. Gallagher, and J. E. Turnbull. 1994. Cell surface syndecan-1 on distinct cell types differs in fine structure and ligand binding of its heparan sulfate chains. J. Biol. Chem. 26918881-18890. [PubMed] [Google Scholar]
- 30.Klupp, B. G., W. Fuchs, E. Weiland, and T. C. Mettenleiter. 1997. Pseudorabies virus glycoprotein L is necessary for virus infectivity but dispensable for virion localization of glycoprotein H. J. Virol. 717687-7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klupp, B. G., and T. C. Mettenleiter. 1999. Glycoprotein gL-independent infectivity of pseudorabies virus is mediated by a gD-gH fusion protein. J. Virol. 733014-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krogh, A., B. Larsson, G. von Heijne, and E. L. Sonnhammer. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305567-580. [DOI] [PubMed] [Google Scholar]
- 33.Li, Q., M. K. Spriggs, S. Kovats, S. M. Turk, M. R. Comeau, B. Nepom, and L. M. Hutt-Fletcher. 1997. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J. Virol. 714657-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim, S. T., R. L. Longley, J. R. Couchman, and A. Woods. 2003. Direct binding of syndecan-4 cytoplasmic domain to the catalytic domain of protein kinase C alpha (PKC alpha) increases focal adhesion localization of PKC alpha. J. Biol. Chem. 27813795-13802. [DOI] [PubMed] [Google Scholar]
- 35.Lopper, M., and T. Compton. 2004. Coiled-coil domains in glycoproteins B and H are involved in human cytomegalovirus membrane fusion. J. Virol. 788333-8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menotti, L., A. Cerretani, and G. Campadelli-Fiume. 2006. A herpes simplex virus recombinant that exhibits a single-chain antibody to HER2/neu enters cells through the mammary tumor receptor, independently of the gD receptors. J. Virol. 805531-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87427-436. [DOI] [PubMed] [Google Scholar]
- 38.Mori, Y., X. Yang, P. Akkapaiboon, T. Okuno, and K. Yamanishi. 2003. Human herpesvirus 6 variant A glycoprotein H-glycoprotein L-glycoprotein Q complex associates with human CD46. J. Virol. 774992-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naranatt, P. P., S. M. Akula, and B. Chandran. 2002. Characterization of gamma-2-human herpesvirus-8 glycoproteins gH and gL. Arch. Virol. 1471349-1370. [DOI] [PubMed] [Google Scholar]
- 40.Oh, E. S., A. Woods, and J. R. Couchman. 1997. Multimerization of the cytoplasmic domain of syndecan-4 is required for its ability to activate protein kinase C. J. Biol. Chem. 27211805-11811. [DOI] [PubMed] [Google Scholar]
- 41.Oh, E. S., A. Woods, and J. R. Couchman. 1997. Syndecan-4 proteoglycan regulates the distribution and activity of protein kinase C. J. Biol. Chem. 2728133-8136. [DOI] [PubMed] [Google Scholar]
- 42.Omerovic, J., and R. Longnecker. 2007. Functional homology of gHs and gLs from EBV-related γ-herpesviruses for EBV-induced membrane fusion. Virology 365157-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parry, C., S. Bell, T. Minson, and H. Browne. 2005. Herpes simplex virus type 1 glycoprotein H binds to αvβ3 integrins. J. Gen. Virol. 867-10. [DOI] [PubMed] [Google Scholar]
- 44.Patel, M., M. Yanagishita, G. Roderiquez, D. C. Bou-Habib, T. Oravecz, V. C. Hascall, and M. A. Norcross. 1993. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res. Hum Retrovir. 9167-174. [DOI] [PubMed] [Google Scholar]
- 45.Pertel, P. E. 2002. Human herpesvirus 8 glycoprotein B (gB), gH, and gL can mediate cell fusion. J. Virol. 764390-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poirot, O., E. O'Toole, and C. Notredame. 2003. Tcoffee@igs: a web server for computing, evaluating and combining multiple sequence alignments. Nucleic Acids Res. 313503-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts, S. R., M. Ponce de Leon, G. H. Cohen, and R. J. Eisenberg. 1991. Analysis of the intracellular maturation of the herpes simplex virus type 1 glycoprotein gH in infected and transfected cells. Virology 184609-624. [DOI] [PubMed] [Google Scholar]
- 48.Saoncella, S., F. Echtermeyer, F. Denhez, J. K. Nowlen, D. F. Mosher, S. D. Robinson, R. O. Hynes, and P. F. Goetinck. 1999. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc. Natl. Acad. Sci. USA 962805-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scanlan, P. M., V. Tiwari, S. Bommireddy, and D. Shukla. 2003. Cellular expression of gH confers resistance to herpes simplex virus type-1 entry. Virology 31214-24. [DOI] [PubMed] [Google Scholar]
- 50.Sharma-Walia, N., P. P. Naranatt, H. H. Krishnan, L. Zeng, and B. Chandran. 2004. Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements. J. Virol. 784207-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shukla, D., J. Liu, P. Blaiklock, N. W. Shworak, X. Bai, J. D. Esko, G. H. Cohen, R. J. Eisenberg, R. D. Rosenberg, and P. G. Spear. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 9913-22. [DOI] [PubMed] [Google Scholar]
- 52.Spiller, O. B., L. Mark, C. E. Blue, D. G. Proctor, J. A. Aitken, A. M. Blom, and D. J. Blackbourn. 2006. Dissecting the regions of virion-associated Kaposi's sarcoma-associated herpesvirus complement control protein required for complement regulation and cell binding. J. Virol. 804068-4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tkachenko, E., E. Lutgens, R. V. Stan, and M. Simons. 2004. Fibroblast growth factor 2 endocytosis in endothelial cells proceeds via syndecan-4-dependent activation of Rac1 and a Cdc42-dependent macropinocytic pathway. J. Cell Sci. 1173189-3199. [DOI] [PubMed] [Google Scholar]
- 54.Tumova, S., A. Woods, and J. R. Couchman. 2000. Heparan sulfate proteoglycans on the cell surface: versatile coordinators of cellular functions. Int. J. Biochem. Cell Biol. 32269-288. [DOI] [PubMed] [Google Scholar]
- 55.Vieira, J., and P. M. O'Hearn. 2004. Use of the red fluorescent protein as a marker of Kaposi's sarcoma-associated herpesvirus lytic gene expression. Virology 325225-240. [DOI] [PubMed] [Google Scholar]
- 56.Woods, A., and J. R. Couchman. 1994. Syndecan 4 heparan sulfate proteoglycan is a selectively enriched and widespread focal adhesion component. Mol. Biol. Cell 5183-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woods, A., and J. R. Couchman. 2001. Syndecan-4 and focal adhesion function. Curr. Opin. Cell Biol. 13578-583. [DOI] [PubMed] [Google Scholar]
- 58.Woods, A., J. R. Couchman, S. Johansson, and M. Hook. 1986. Adhesion and cytoskeletal organisation of fibroblasts in response to fibronectin fragments. EMBO J. 5665-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.WuDunn, D., and P. G. Spear. 1989. Initial interaction of herpes simplex virus with cells binding to heparan sulfate. J. Virol. 6352-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan, J. X., R. Wait, T. Berkelman, R. A. Harry, J. A. Westbrook, C. H. Wheeler, and M. J. Dunn. 2000. A modified silver staining protocol for visualization of proteins compatible with matrix-assisted laser desorption/ionization and electrospray ionization-mass spectrometry. Electrophoresis 213666-3672. [DOI] [PubMed] [Google Scholar]
- 61.Yang, Y., V. Macleod, H. Q. Miao, A. Theus, F. Zhan, J. D. Shaughnessy, Jr., J. Sawyer, J. P. Li, E. Zcharia, I. Vlodavsky, and R. D. Sanderson. 2007. Heparanase enhances syndecan-1 shedding: a novel mechanism for stimulation of tumor growth and metastasis. J. Biol. Chem. 28213326-13333. [DOI] [PubMed] [Google Scholar]
- 62.Yi, J. Y., I. Han, and E. S. Oh. 2006. Transmembrane domain-dependent functional oligomerization of syndecans. Sci. World J. 6457-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou, G., G. J. Ye, W. Debinski, and B. Roizman. 2002. Engineered herpes simplex virus 1 is dependent on IL13Rα 2 receptor for cell entry and independent of glycoprotein D receptor interaction. Proc. Natl. Acad. Sci. USA 9915124-15129. [DOI] [PMC free article] [PubMed] [Google Scholar]