Abstract
Proper folding of newly synthesized viral proteins in the cytoplasm is a prerequisite for the formation of infectious virions. The major capsid protein Vp1 of simian virus 40 forms a series of disulfide-linked intermediates during folding and capsid formation. In addition, we report here that Vp1 is associated with cellular chaperones (HSP70) and a cochaperone (Hsp40) which can be coimmunoprecipitated with Vp1. Studies in vitro demonstrated the ATP-dependent interaction of Vp1 and cellular chaperones. Interestingly, viral cochaperones LT and ST were essential for stable interaction of HSP70 with the core Vp1 pentamer Vp1 (22-303). LT and ST also coimmunoprecipitated with Vp1 in vivo. In addition to these identified (co)chaperones, stable, covalently modified forms of Vp1 were identified for a folding-defective double mutant, C49A-C87A, and may represent a “trapped” assembly intermediate. By a truncation of the carboxyl arm of Vp1 to prevent the Vp1 folding from proceeding beyond pentamers, we detected several apparently modified Vp1 species, some of which were absent in cells transfected with the folding-defective mutant DNA. These results suggest that transient covalent interactions with known or unknown cellular and viral proteins are important in the assembly process.
Simian virus 40 (SV40), a polyomavirus, has an icosahedral capsid whose structure is known at atomic resolution (34, 48). The capsid is built from a major capsid protein (Vp1) and two minor, internally embedded capsid proteins (Vp2 and Vp3). One Vp1 monomer folds into a core β-barrel domain of jelly roll topology along with the N- and C-terminal arms. Five Vp1 monomers interdigitate their secondary structures to form pentamers that are tied together via interactions of their C-terminal arms (34). Little is known about how Vp1 folds into the icosahedral structure. Vp1 folds in the cytoplasm of infected host cells first through monomeric intermediates and then through oligomeric intermediates that contain transitory disulfide bonds (29). Two nonviable viral mutants harboring double cysteine mutations in Vp1 (i.e., C49A-C87A and C87A-C254A) fail to traffic to the nucleus but accumulate, at reduced levels, as punctuate speckles in the cytoplasm (30). This finding suggests that the disulfide redox, Vp1 folding, and cytoplasmic-nuclear trafficking of Vp1 are all tightly linked (30).
Protein folding is generally assisted by molecular chaperones involving transiently and/or partially unfolded proteins (reviewed in reference 37). In particular, Hsc70 and Hsp70, members of the HSP70 family, have been implicated in the life cycle of polyomaviruses (37). Infection by SV40 or murine polyomavirus induces higher levels of production of stress-inducible Hsp70 and/or its homologs (26, 27, 53). The in vivo association of the constitutively expressed protein Hsc70 with Vp1 proteins of SV40 and murine polyomavirus has also been reported (8, 43). The HSP70 chaperones use ATP hydrolysis cycles to toggle between two states: binding to (ADP-bound state) and release from (ATP-bound state) protein substrates. The ATPase activity and substrate selection of these chaperones are regulated by the Hsp40 cochaperone via their J domains (20, 40, 56). SV40 oncoproteins LT and ST are viral cochaperones and contain an N-terminal J domain (5, 15, 17, 24, 46) that interacts with Hsc70 (44, 45, 47, 50, 55). The J domain is necessary for viral DNA replication, transformation, transcriptional activation, and virion assembly (14, 51). The DnaK and DnaJ proteins, HSP70-HSP40 homologs in bacteria, have been shown to copurify with full-length murine polyomavirus Vp1, and their binding to chaperone and cochaperone is dependent on the presence of the Vp1 N and C termini (6). Furthermore, an Hsc70-SV40 LT pair can catalyze the formation of capsid-like structures from SV40 Vp1 pentamer-Vp3 complexes (6). We hypothesize that specific chaperone/cochaperone sets assist the proper folding of the first Vp1 monomer as well as the assembly of monomers into pentamers in the cytoplasm. Thus, we have begun to study how Vp1 folds via the recruitment of cellular HSP70 chaperones and J-domain cochaperones. We have confirmed the association of Vp1 with (co)chaperones in vivo but extend these findings to suggest a role for the viral cochaperones LT/ST. To our knowledge, the current finding that SV40 LT and ST may play the critical roles in Vp1 folding as viral (co)chaperones has not previously been reported.
MATERIALS AND METHODS
Preparation of plasmids and recombinant proteins.
The construction and preparation of SV40 genomes, nonoverlapping SV40 (NO-SV40) (22), and its mutant counterparts NO-SV40 C49A-C87A and NO-SV40 C87A-C207S (30), have been reported previously. The codons encoding the 69 carboxy-terminal amino acids of Vp1 were amplified from pSV-Vp1 by using an XbaI- and a SacI-tagged primer (5′-CAG GTC CAT GGT CTA GAC GGT CTG TGA AAA ACC CCT ACC CAA TTT CC-3′ and 5′-CAA GAA TTC GAG CTC GCC CAA CTT G-3′, respectively) and cloned in pGEX-3XS (9), resulting in a plasmid encoding a glutathione S-transferase (GST) fusion protein (pGEX-Vp1C69). The constitutive mammalian expression vector for six-histidine-tagged (His-tagged), carboxy-58-residue-truncated Vp1 (pCI-Vp1ΔC58-H6) was constructed in pBlueScript II KS(+) with a series of fragments as follows: the cytomegalovirus/T7 promoter of pcDNA 3.1 (Invitrogen); the synthetic intron of pFlp (11); the His-tagged, Vp1-coding sequence of pBS-Vp1ΔC58-flexible linker-H6 lacking the region encoding the 63 C-terminal amino acids (30); and the SV40 polyadenylation signal (11). The Vp1ΔC58 construct is in pCI and contains Vp1 (1A-298P), with an additional three amino acids, MKM, at the N terminus and four amino acids, GPAS, at the C-terminus, followed by a flexible linker (three repeats of GGGGS), EFESGR and the six-histidine tag (His tag) (expected mass, 35.6 kDa). The C49A-C87A and C87A-C207S mutant counterparts of pCI-Vp1ΔC58-H6 were made by substituting suitable fragments from respective NO-SV40 mutants (30).
The recombinant proteins Vp1ΔC58-His6 (amino acids [aa] 1 to 303 of Vp1, referred to as ΔC) and Vp1ΔN20ΔC58-His6 (aa 22 to 303 of Vp1) were prepared from Escherichia coli containing pQE-Vp1-2CA-ΔC58 and pQE-Vp1-2CA-ΔN (2-21)ΔC58, respectively, as described before (31). Vp1ΔC58 contains Vp1 (1A-303F), with MKM at the N terminus and SGRGITPLGTDPRS at the C-terminus, followed by the His tag (expected mass, 35.6 kDa). For pentamer preparation, cysteines at positions 104 and 254 were replaced with alanines to avoid formation of Vp1 aggregates in E. coli. His-tagged Vp1ΔC58 proteins in the soluble fraction of the E. coli lysate were first enriched via Ni-nitrilotriacetic acid resin and eluted from the resin with imidazole, and the pentamers were further purified by sedimentation through a sucrose gradient as described previously (31). GST fusion proteins, GST-Vp1 (aa 1 to 361, whole Vp1 protein) and GST-Vp1C69 (aa 292 to 361, referred to as C69), were prepared from E. coli containing pGEX-Vp1 and pGEX-Vp1C69, respectively, as described previously (9).
Cell culture, heat shock treatment, virus infection, DNA transfection, metabolic radiolabeling, and immunofluorescence.
The culture conditions for the TC7 subline of African green monkey kidney cells (23) and the SV40-transformed COS-7 cell line (18) have previously been described. The 293TT cell line, a gift from John T. Schiller and Christopher B. Buck, is a derivative of the human embryonic kidney cell line 293T and expresses both SV40 LT and ST (3). For heat shock treatment, TC7 cells were incubated at 43°C for 1 h under standard culture conditions before subcellular fractionation of the cell lysate (see below). SV40 infection (23) and NO-SV40 DNA transfection (32) in TC7 cells were previously described. COS-7 cells were transfected with pCI-Vp1ΔC58-H6 DNAs by using FuGENE 6 transfection reagent (Roche) according to the manufacturer's instructions. The transfected cells were labeled with [35S]methionine for 40 min as described before (36). Immunofluorescence analysis of transfected cells was performed as described previously (7).
Cell lysis and subcellular fractionation.
Whole-cell lysates were prepared in radioimmunoprecipitation assay (RIPA) buffer containing NP-40, sodium deoxycholate, sodium dodecyl sulfate (SDS), and DNase I, as described previously (8), except that Tris-Cl was replaced with HEPES (pH 7.6). The NP-40-soluble, cytosolic fraction (Sol) and the double-detergent (Tween 20 and sodium deoxycholate)-soluble, cytoskeletal fraction (Csk) were fractionated as described previously (35). The hypotonic cytosolic fraction was prepared according to published reports (1, 57), with slight modifications. Briefly, the cells were collected and washed with buffered saline. The cells were resuspended in 9 volumes of 20 mM HEPES (pH 7.5) containing protease inhibitors (32) and incubated on ice for 30 min with occasional agitation. The cells were homogenized using a Dounce homogenizer for 12 strokes and supplemented with sucrose to give a final concentration of 250 mM. After centrifugation at 6,000 × g at 4°C for 10 min and at 100,000 × g for 60 min, the supernatant was collected as the hypotonic cytosolic fraction.
Cellular and viral proteins in the cytoplasmic fractions (1.2 × 105 cells) were separated by SDS-polyacrylamide gel electrophoresis (PAGE), and lamin B was detected by Western blot analysis using anti-lamin B antibody (Calbiochem). We note that lamin B protein, which is one of the major components of the nuclear lamina underlying the nuclear envelope of eukaryotic cells, was absent in all cytoplasmic fractions tested and was found as expected in the detergent-solubilized post-Csk fraction (data not shown).
In vitro chaperone binding.
To assess (co)chaperone-Vp1 binding, extracts were incubated with recombinant Vp1 proteins with/without ATP (5 mM) for 30 min at 37°C, followed by treatment with 50 μg/ml hexokinase for 30 min at 37°C. The (co)chaperone binding to Vp1 was determined by immunoprecipitation (IP) and immunoblotting as described below. The extracts used included the NP-40-soluble Sol fraction prepared from 4 × 107 293TT cells, both before and after depletion of LT/ST (see below). Also used was an extract from 5 × 106 heat-shocked TC7 (hs TC7) cells. The levels of Hsc70 in the Sol fraction were comparable among these extracts, but the levels of Hsp70 were not. The level of Hsp70 in 293TT Sol was five times higher than that in hs TC7 Sol (data not shown). The level of Hsp70 in the hs TC7 lysate was two to three times higher than that in the TC7 cell lysate. To ensure that the levels of Hsp70 among the input lysates were the same for Hsp70-Vp1 binding, the lysates were adjusted to contain equivalent amounts of Hsp70 by addition of suitable amounts of TC7 lysate. The 293TT Sol devoid of SV40 LT/ST was prepared by first incubating 640 μl of the lysate with 80 μl of mouse pAb419 monoclonal antibody which recognizes the epitope in the J domain common to both LT and ST and then by removing antibody-LT/ST complexes by using protein G-agarose.
Antibodies and affinity resins.
Various primary antibodies were used in IP, Western blot analysis, immunoadsorption, and immunofluorescence analysis. Rabbit polyclonal anti-Vp1 serum (23) and affinity-purified rabbit anti-Vp1 immunoglobulin Gs (IgGs) (39) were described previously. Rat anti-Hsc70 (SPA-815), mouse anti-Hsp70 (SPA-810), and mouse anti-TCP-1α (CTA-122) monoclonal IgGs and rabbit polyclonal anti-Hsp40 serum (SPA-400) were from StressGen. Mouse monoclonal IgGs against a common epitope on SV40 LT and ST antigens (SV40 Ab-1), a unique LT epitope (SV40 Ab-2), and a unique ST epitope (SV40 Ab-3) were from Oncogene Research Products. Mouse monoclonal anti-SV40 LT/ST antibody (pAb419, a gift from Kathleen Rundell) was used to deplete SV40 LT/ST from the 293TT lysate. Mouse monoclonal IgGs against β-galactosidase (β-Gal), protein disulfide isomerase (PDI), and tetra-histidine (His) were from Promega, Affinity BioReagent, and Qiagen, respectively.
For IPs and resin pulldown assays, protein G-agarose, glutathione-Sepharose, TrueBlot anti-mouse Ig beads, and Talon metal affinity resin were bought from Roche, GE Healthcare, eBioscience, and Clontech, respectively. For secondary antibodies, horseradish peroxidase-conjugated anti-rabbit, anti-mouse, and anti-rat IgG antibodies were bought from MP Biomedicals, and TrueBlot horseradish peroxidase-conjugated anti-rabbit antibody was bought from eBioscience. Secondary antibodies for immunofluorescence (fluorescein isothiocyanate- or rhodamine-conjugated anti-rabbit, anti-mouse, and anti-rat antibodies) were bought from MP Biomedicals.
IP.
IP and elution under nonreducing or reducing conditions were performed as described before (29, 36). The lysate was prepared in 0.4 to 0.8 ml of 20 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 4 mM glutathione, and protease inhibitors (32), and the cell debris was removed by centrifugation at 16,000 × g at 4°C for 20 min. The supernatant was incubated for 1 h at 4°C with 25 μl of a suitable resin (50% slurry)—protein G-agarose (in most cases) or anti-mouse Ig beads (where indicated)—to preadsorb nonspecifically binding proteins. After removal of the resins, the supernatant was mixed with a primary antibody and incubated at 4°C for 2 h, followed by addition of another 25 μl of fresh resin, followed by further incubation for 1 h. The resin-bound immunoprecipitated proteins were eluted under the reducing conditions in the Laemmli sample buffer (28) or under nonreducing conditions by incubating the resins in 50 mM HEPES, pH 6.8, 2.5% SDS, 10% glycerol, 0.005% bromophenol blue, and 4 mM NEM at 80 to 90°C for 15 min.
GST pulldown assay.
Lysates were brought to a volume of 0.5 ml in 50 mM HEPES, pH 7.5, 150 mM NaCl, 2 mM dithiothreitol, 0.5% NP-40, and 1 mM phenylmethylsulfonyl fluoride, then mixed with a 20-μl bed volume of glutathione-Sepharose beads (to which 35 pmol of GST or GST-Vp1 had been immobilized), and incubated with rotation at room temperature for 2 h. The beads were collected, washed twice with 50 mM HEPES (pH 7.5), 150 mM NaCl, and 0.5% NP-40, washed once with 50 mM HEPES (pH 7.5), 300 mM NaCl, and 0.5% NP-40, and finally, the bound proteins were eluted from the resin under the reducing conditions in the Laemmli sample buffer.
Metal affinity resin pulldown assay.
Each hypotonic cytosolic lysate was brought to a final volume of 0.8 ml and final concentrations of 40 mM Tris-Cl (pH 8.0), 150 mM NaCl, 0.5% NP-40, 0.1% sodium deoxycholate, 4 mM glutathione, and protease inhibitors. Each lysate was then incubated at 4°C for 1 h with 30 μl of Talon resin (50% slurry) to preadsorb nonspecifically binding proteins, and the resin was removed by centrifugation. Fresh and pretreated Talon resin (30 μl) was added to the preadsorbed supernatant, incubated for 1 h, and then washed with the buffer. The bound proteins were eluted under either reducing or nonreducing conditions. In some experiments, lysates were first denatured before the pulldown assay was performed. In this case, each lysate was brought to 0.5 ml in RIPA buffer and then denatured by addition of 1 ml of a denaturing buffer containing 9 M urea, 60 mM Tris (pH 7.6), 150 mM NaCl, and 15% glycerol. This mixture was then sonicated, cleared of debris by centrifugation at 6,800 × g for 10 min at 4°C, mixed with 30 μl of Ni-nitrilotriacetic acid resin (50% slurry) that had been equilibrated with 6 M urea, 40 mM Tris-Cl (pH 8.0), 150 mM NaCl, and 10% glycerol, and then incubated at 4°C for 1 h. The resins were collected and washed twice each with the buffer, which contained 6 M urea, 40 mM Tris-Cl (pH 8.0), and 10% glycerol, in addition to 150 mM NaCl for a first wash, 1 M NaCl for the second, and 150 mM NaCl and 30 mM imidazole (pH 8.0) for the third. The resin-bound proteins were eluted by boiling them in the Laemmli sample buffer for 10 min, the resins were removed by centrifugation, and the resin-free proteins were analyzed by SDS-PAGE and Western blotting.
Fluorography and Western blot analysis.
For fluorography, [35S]methionine-labeled samples were separated by SDS-PAGE and fixed, and the gel was treated with Amplify fluorographic reagent (Amersham) according to the manufacturer's instructions. The gel was vacuum dried and exposed to X-ray film at −70°C. Western blot analysis was performed essentially according to the protocol recommended for the TrueBlot system (eBioscience) and detected using either SuperSignal West Pico or West Femto enhanced chemiluminescent substrate system (Pierce).
RESULTS
Vp1 is associated with Hsc70, Hsp70, Hsp40, LT, and ST during the late phase of SV40 infection.
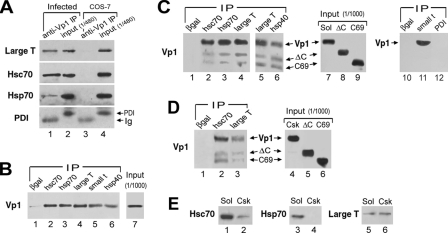
The interactions of SV40 Vp1 with the cellular chaperones Hsc70 and Hsp70, the cellular cochaperone Hsp40, and the SV40 viral cochaperone LT/ST were examined by co-IP. Whole-cell lysate from TC7 cells at 50 h postinfection was solubilized in RIPA buffer containing multiple detergents and DNase I. This lysate, which contained both cytoplasmic and nuclear contents, was immunoreacted with an anti-Vp1 antibody. Proteins in immune complexes were then separated by SDS gel electrophoresis before Western blot analysis to detect the tumor antigens and the (co)chaperones. LT, Hsc70, and Hsp70 were found to have been associated with Vp1 (Fig. 1A, lane 1, upper three rows). These proteins were not detected in IPs of the control cell line COS-7, which does not express Vp1, although COS-7 extracts had comparable amounts of Hsc70, Hsp70, and LT. PDI, an endoplasmic reticulum-resident chaperone that was abundantly present in the lysate of COS-7 and infected TC7 cells (lanes 4 and 2, respectively), was not found in the Vp1-IP (Fig. 1A, lanes 1 and 3 of the PDI row), consistent with our previous observation (29). In reciprocal assays, Vp1 was detected in the IPs of Hsc70, Hsp70, a unique LT epitope, a unique ST epitope, and Hsp40 (Fig. 1B, lanes 2 through 6) but not in the IP of β-Gal (lane 1). These results suggest that a fraction of Vp1 in the SV40-infected whole-cell lysate associates with Hsc70, Hsp70, Hsp40, and unique segment of either SV40 LT or SV40 ST.
FIG. 1.
Association of SV40 Vp1 with cellular/viral (co)chaperones during SV40 infection. (A) The RIPA lysate prepared from 107 SV40-infected TC7 cells at 50 h postinfection was immunoprecipitated with affinity-purified anti-Vp1 IgG and analyzed for the presence of LT, Hsc70, Hsp70, or PDI by Western blotting. As a control, the same number of uninfected COS-7 cells was also analyzed. For input, 1/480 of the lysate used for immunoreactions was loaded. PDI and an immunoglobulin heavy chain (Ig) were detected with anti-PDI antibody (arrow). (B) The RIPA lysate from 106 infected cells was immunoprecipitated with antibodies against β-Gal, Hsc70, Hsp70, a unique LT epitope, a unique ST epitope, and Hsp40, and the IPs were probed for Vp1 by Western blotting. One-thousandth of the input lysate was analyzed for Vp1 in the same blot. (C) The Sol fraction from 106 infected cells, which contains endogenous and infection-based Vp1, was mixed with two recombinant Vp1 proteins, Vp1ΔC58-His6 pentamer (ΔC) and GST-Vp1C69 (C69). After IP with antibodies against β-Gal, Hsc70, Hsp70, Hsp40, a unique LT epitope, a unique ST epitope, and PDI, the IPs and 1/1,000 of the inputs were analyzed by anti-Vp1 Western blotting. The reactions shown in lanes 4, 5, and 10, to 12 were performed using TrueBlot anti-mouse beads. (D) The Csk fraction prepared from 2.5 × 106 infected cells was immunoprecipitated with antibodies against β-Gal, Hsc70, or a unique LT epitope, and Vp1 in the IPs was analyzed by anti-Vp1 Western blotting. Vp1 in 1/1,000 of the input was also analyzed. (E) The Sol fraction from 104 infected cells or the Csk fraction from 2.5 × 104 infected cells was analyzed for Hsc70, Hsp70, and LT by Western blotting.
Vp1 undergoes folding and oligomerization in the cytosol of infected cells (29), so it was of interest to determine where the various (co)chaperones were found. Two cytoplasmic subfractions, a “Sol” fraction and “Csk” fraction, were prepared (29, 35, 36), and the existence of (co)chaperones was tested by Western blot analysis. The Sol fraction contained Hsc70, Hsp70, and LT, whereas the Csk fraction contained Hsc70 and LT (Fig. 1E). Although LT is primarily found in the nucleus, we found some LT in the cytosolic fraction as well. This is not surprising. Others have reported that LT is also found at the cytoplasmic membrane (4), and LT would be present at least transiently in the cytoplasm after synthesis. We ruled out the possibility of cross-contamination of the cytosolic fraction with nuclear proteins by monitoring lamin B, a marker of nuclear resident proteins. Lamin B was not detected in the Sol or Csk fraction, implying that little cross-contamination of the nuclear proteins occurred during the preparation of the cytoplasmic fractions (data not shown).
It was also important to determine whether Vp1 genuinely associates with cellular and viral (co)chaperones in the cytoplasm or whether the association occurs after cell lysis. Accordingly, IPs were performed in the presence of exogenously added recombinant Vp1 proteins to help gauge the level of Vp1 interactions that might have occurred in the test tube rather than in the cell prior to lysis. We used a His-tagged pentamer of Vp1 lacking the last 58 residues (Vp1ΔC58-His6; ΔC) and the C-terminal 69 residues of Vp1 fused to GST (GST-Vp1C69; C69) (Fig. 2A), because these could be distinguished from infection-derived full-length Vp1 on SDS gels. The amount of infection-derived Vp1 in the Sol or Csk fraction was determined by Western blotting (Fig. 1C, lane 7, and D, lane 4), and an amount of the recombinant protein (ΔC or C69) equal to that of infection-derived Vp1 was added to the respective fraction (Fig. 1C, lanes 8 and 9, and D, lanes 5 and 6). Infection-derived Vp1 coimmunoprecipitated with Hsc70, Hsp70, Hsp40, and LT from the Sol fraction (Fig. 1C, lanes 2 through 6) and with Hsc70 and LT from the Csk fraction (Fig. 1D, lanes 2 and 3) at much greater levels than did exogenously added ΔC or C69. Vp1 was found in the Sol fraction in the unique-ST IP (Fig. 1C, lane 11) but not in the IP of either β-Gal or PDI (lane 10 or 12, respectively), confirming the result described above for the whole-cell lysate (Fig. 1B). These results show that Vp1 associates with cellular and viral (co)chaperones in the infected cell cytoplasm.
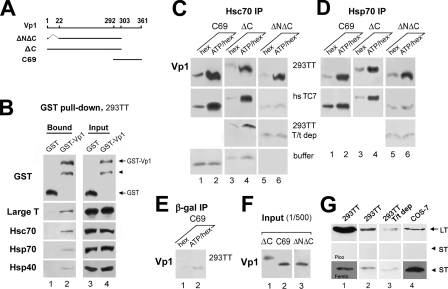
FIG. 2.
In vitro binding of recombinant Vp1 proteins with (co)chaperone proteins. (A) Schematic diagram of Vp1 amino acid residues of recombinant Vp1 proteins. Diagrams are shown for the Vp1ΔN20ΔC58-His6 pentamer (ΔNΔC; aa 22 to 303), the Vp1ΔC58-His6 pentamer (ΔC; aa 1 to 303), and GST-Vp1C69 (C69; aa 292 to 361). GST-Vp1 is full-length Vp1 (aa 1 to 361). (B) Vp1 interacts with (co)chaperones. GST or GST-Vp1 was immobilized onto glutathione-Sepharose resin and reacted with the Sol fraction of 4 × 107 293TT cells. The resin-bound proteins were eluted and probed for GST proteins, a unique region of LT, Hsc70, Hsp70, and Hsp40 by Western blotting. To detect GST in input, a 1/3 volume of input GST and GST-Vp1 was loaded (lanes 3 and 4, top row), and for LT, Hsc70, Hsp70, and Hsp40, a 1/160 dilution of input Sol was used (lanes 3 and 4, bottom four rows). An unlabeled arrowhead points to a degraded product of GST-Vp1. (C, D) Binding of recombinant Vp1 proteins to Hsc70 (C) or to Hsp70 (D). GST-Vp1C69 (C69), the Vp1ΔC58-His6 pentamer (ΔC), or the Vp1ΔN20ΔC58-His6 core pentamer (ΔNΔC) was mixed with the Sol fraction from 4 × 106 of 293TT cells or hs TC7 cells, with the 293TT Sol fraction that had been depleted of LT/ST (T/t), or with the lysis buffer in the presence of hexokinase (hex) or ATP, followed by hexokinase addition (ATP/hex). After incubation, chaperone-Vp1 binding was assessed by IP with an anti-Hsc70 antibody (C) or with an anti-Hsp70 antibody (D), followed by anti-Vp1 Western blotting. (E) GST-Vp1C69 was mixed with the 293TT Sol fraction and treated with hexokinase or ATP/hexokinase as described for panels C and D. After IP with an anti-β-Gal antibody, Vp1 was visualized by anti-Vp1 Western blots. (F) The Vp1 in 1/500 of input Vp1ΔC58-His6, GST-Vp1C69, or Vp1ΔN20ΔC58-His6 used for panels B, C, and D was visualized by anti-Vp1 Western blotting. (G) The Sol fraction from 2.4 × 106 293TT cells (lane 1), 6.0 × 105 293TT cells (lane 2), 6.0 × 105 LT/ST-depleted 293TT cells (lane 3), or 3.6 × 105 COS-7 cells (lane 4) was analyzed for LT and ST by using antibody to a common epitope of both LT and ST by Western blotting. SuperSignal West Pico (Pico) or West Femto (Femto) was used to visualize LT or ST. An arrow or arrowhead indicates LT or ST, respectively.
ATP stimulates in vitro binding of Hsc70 and Hsp70 with Vp1.
A small but detectable level of exogenous ΔC or C69 was coimmunoprecipitated with Hsc70, Hsp70, and Hsp40 (Fig. 1C and D). This is consistent with reports by others (8) indicating that these (co)chaperones can associate with Vp1 in vivo and in vitro. These observations led us to ask whether Vp1 interacts, as a substrate, with Hsc70 or Hsp70 through ATP hydrolysis cycles and which parts of Vp1 mediate the chaperone-substrate interaction. These questions were first addressed using full-length Vp1 (GST-Vp1) or the protein's carboxy-terminal 69 residues tagged with GST (GST-Vp1C69; C69) (Fig. 2A). GST-Vp1 (Fig. 2B, lane 2), but not GST alone (lane 1), pulled down all four (co)chaperones from the Sol extract of 293TT cells (lanes 3 and 4). In order to further assess the Vp1-chaperone interaction, we prepared two His-tagged Vp1 pentamers and used them for the binding study. One of these, Vp1ΔC58-His6, lacks the last 58 aa (ΔC), and the other, Vp1ΔN20ΔC58-His6, lacks both carboxy-terminal sequences and the first 21 aa (Vp1ΔNΔC). Vp1ΔNΔC is also referred to as the core pentamer, because it is the minimum portion of Vp1 that can assemble into a pentamer.
For the chaperones, Sol fractions were prepared from either 293TT or hs TC7 cells. The heat shock treatment of mammalian cells induces Hsp70 (33, 54). The recombinant Vp1 proteins and Sol fractions were mixed and incubated with ATP to allow new rounds of substrate binding by the chaperones, followed by further incubation with the ATP depletion system to lock the chaperone complexes in the ADP-bound form. The samples were then immunoprecipitated with an anti-Hsc70 or anti-Hsp70 antibody, and Vp1 in the IPs was detected by Western blot analysis with an anti-Vp1 antibody. When either 293TT or hs TC7 Sol was used (top or second row in Fig. 2C or D), substantial increases in coprecipitated ΔC (lane 4) and C69 (lane 2) were observed in the presence of ATP compared to the levels in the absence of ATP (lanes 1 and 3). This stimulation effect of ATP was not obtained for the low background level of Vp1 found in IPs with the lysis buffer (Fig. 2C, bottom row) or in anti-β-Gal antibody IP (Fig. 2E). Thus, the present results demonstrate that Vp1 can interact with either Hsc70 or Hsp70 as a substrate via ATPase cycles.
Role of LT/ST in Vp1-chaperone interaction.
A strikingly different picture in heat shock protein binding was obtained with the Vp1ΔNΔC core pentamer, which lacked both the N and the C termini of Vp1. The association of the core pentamer with Hsc70 or Hsp70 was strongly augmented by ATP only with the 293TT Sol fraction (upper rows in Fig. 2C and D, compare lane 6 with lane 5) but not with the hs TC7 Sol fraction, which does not express LT or ST (middle rows in Fig. 2C and D, lanes 5 and 6). The 293TT cell line is derived from 293T cells, which contain SV40 LT at a very low level due to a splicing bias in favor of the SV40 ST-mRNA (16), by transfection with a plasmid that is designed to express SV40 LT constitutively (3). Therefore, the 293TT lysate is expected to have both LT/ST, with LT at a high level. This has been confirmed (Fig. 2G, lane 1). The finding described above implies that SV40 LT and ST are required for the Vp1ΔNΔC core pentamer-chaperone interaction. To further test this possibility, we performed a binding study with a 293TT lysate depleted of both LT and ST (293TT T/t dep), prepared by incubating the lysate with mouse pAb419 monoclonal antibody, which recognizes a common epitope within the N-terminal J domain of LT and ST (12, 19). We confirmed greatly reduced levels of both LT and ST (Fig. 2G, lanes 2 and 3), but the levels of Hsp70 and Hsc70 were not altered by the antibody depletion step (data not shown). We found that the 293TT T/t dep lysate did not support the binding of either of the two chaperones to the core Vp1ΔNΔC pentamer, affirming the important role of LT and ST in the pentamer-chaperone interaction (Fig. 2C and D, third rows from the top, lane 6, compared with the top rows).
Taken together with the previous section, our results show that three regions of Vp1 are involved in the ATP-dependent interaction with cellular chaperones. These regions are the N terminus (aa 1 to 21), core (aa 22 to 303), and C terminus (aa 292 to 361). When either the N or the C terminus of Vp1 is present, endogenous chaperones in 293TT or hs TC7 (perhaps together with the Hsp40 cochaperone) are sufficient for Vp1 binding. However, for binding of the chaperone to the Vp1ΔNΔC core pentamer, an additional LT/ST protein is required and may be providing a (co)chaperone function via the DnaJ domain.
Hsp70, Hsc70, and LT partly colocalize with wild-type and C49A-C87A mutant Vp1 proteins.
We previously identified three nonviable Vp1 double cysteine mutants that are defective in cytoplasmic Vp1 folding (C49A-C87A and C87A-C254A) and never move to the cell nucleus. A third mutant undergoes nuclear translocation but has subsequent defects in nuclear virion assembly (C87A-C207A) (30). These are studied by transfection of full-length mutant and wild-type viral genomes found within the NO-SV40 plasmid. In the wild-type transfection, Hsp70, LT, and Hsc70 localized in the nucleus along with wild-type Vp1 (Fig. 3A to C, panels a and e). A similar nuclear colocalization was observed in the C87A-C207S mutant, whose defect occurs after nuclear entry (panels d and h). In C49A-C87A or C87A-C254A transfection, the majority of mutant Vp1 was found in cytoplasmic regions (panels b and c). Likewise, the localization of Hsp70 and Hsc70 changed from primarily nuclear to predominantly cytoplasmic. The majority of LT remained in the nuclei of cells, although in a small population of C87A-C254A mutant-transfected cells, LT was partly localized in the cytoplasm. We interpret that there is a small amount of cytoplasmic LT in all cells but that this may be below the level of detection for fluorescence microscopy. These results suggest the aberrant accumulation of cellular and viral (co)chaperones when folding-defective Vp1 proteins fail to move to subsequent steps in assembly. This is consistent with a transient association of these (co)chaperones with Vp1 during normal Vp1 biogenesis. Whether the cytoplasmic LT localization is an exception or reflects a physiological state of certain cells is not known. Yet, the observation quantitatively corroborates the biochemical results described above (Fig. 1).
FIG. 3.
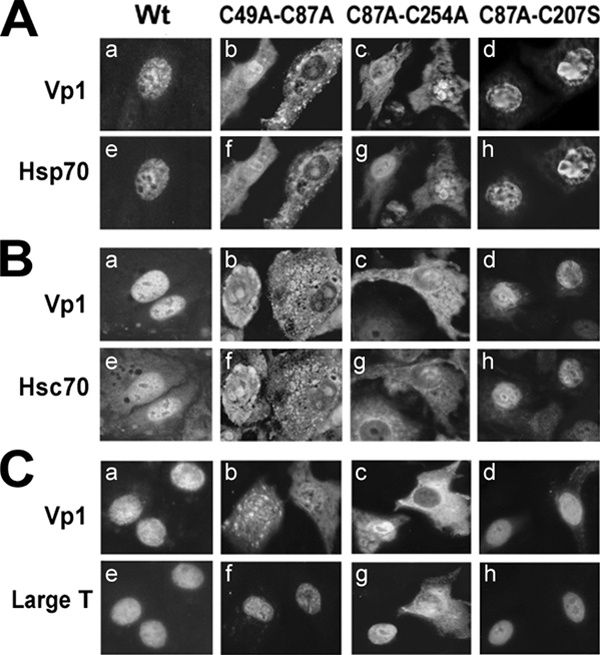
Subcellular localization of Vp1 and Hsp70, Hsc70, and large T antigen in cells transfected with wild-type and double-cysteine-mutant NO-SV40. TC7 cells were transfected with wild-type (Wt), C49A-C87A, C87A-C254A, or C87A-C207S NO-SV40 DNA; fixed at 40 to 48 h posttransfection; and double stained with anti-Vp1 serum and either anti-Hsp70 (A), anti-Hsc70 (B), or anti-LT unique (C) antibodies followed by rhodamine-labeled (a to d) or fluorescein-labeled (e to h) secondary antibodies.
Newly synthesized wild-type and C49A-C87A mutant Vp1 proteins form different folding intermediates.
In the SV40-infected cytoplasm, newly synthesized Vp1 forms first a series of disulfide-bonded monomers and then oligomers (29). We tested whether the cytoplasmic-mutant Vp1 proteins fold through intermediates different from those for wild-type Vp1. We focused on analysis of C49A-C87A mutant Vp1 in comparison with wild-type and nuclear-assembly-defective C87A-C207S mutant Vp1 proteins by DNA transfection. Any difference in the intermediates formed with these mutants may result from the altered interaction(s) with (co)chaperones.
To visualize transiently formed assembly intermediates, transfected TC7 cells were pulse-labeled with [35S]methionine for 5 min at 72 h posttransfection and were either immediately harvested or chased for 10 or 40 min prior to harvesting. The Vp1 IPs prepared from the Sol or Csk fraction were separated by SDS-PAGE under nonreducing (Fig. 4A and C) or reducing (Fig. 4B and D) conditions, and the labeled proteins were detected by fluorography. Most of the Vp1 synthesized within the 5-min pulse period resided in the Sol fraction (Fig. 4A and B, lanes 1). As a function of chase time, the location of Vp1 shifted into the Csk fraction (Fig. 4C and D), and the proportions of disulfide-linked oligomers, including dimers (labeled 2), trimers (3), tetramers, and higher molecules (4+), increased in this cellular compartment as well. Vp1 of the nuclear-assembly-defective C87A-C207S mutant produced a profile of intermediates similar to that for the wild type (Fig. 4A to D, lanes 4 to 6).
FIG. 4.
Formation of newly synthesized Vp1 folding intermediates in cells transfected with mutant DNAs. (A to D) Profiles of Vp1 intermediates in cytoplasmic fractions. The Sol (A and B) or Csk (C and D) fraction was prepared from TC7 cells 72 h posttransfection of wild-type (Wt), C87A-C207S mutant (87/207), or C49A-C87A mutant (49/87) NO-SV40 DNA, following 5 min of [35S]methionine pulse labeling with no chase (lanes 1, 4, and 7), with 10 min of chase (lanes 2, 5, and 8), or with 40 min of chase (lanes 3, 6, and 9). Aliquots of the Sol or Csk fraction (equivalent to 6 × 105 cells) were immunoprecipitated with affinity-purified anti-Vp1 IgG, and the IPs were denatured under nonreducing (A and C) or reducing (B and D) conditions, resolved by SDS-PAGE, and processed for fluorography. Bands corresponding to monomeric, dimeric, trimeric, and higher oligomeric forms of Vp1 are denoted 1, 2, 3, and 4+, respectively. A single asterisk marks a 73- to 75-kDa doublet present prominently in C49A-C87A samples. (E) Steady-state intracellular Vp1 species. Samples of 4 × 105 whole cells transfected with wild-type, C87A-C208S, or C49A-C87A NO-SV40 DNA were separated by nonreducing or reducing SDS-PAGE and processed for anti-Vp1 Western blots. Lane 4 represents a longer exposure of the image in lane 3, and lanes 8 to 10 represent longer exposures of the images in lanes 5 to 7, respectively.
The profile of the cytoplasmic, folding-defective C49A-C87A mutant Vp1 was different from that of wild-type Vp1. The monomers and dimers were present throughout the pulse-chase experiment, but trimers and possibly higher oligomers were observed only immediately after pulse-labeling and did not accumulate during the chase period (Fig. 4A and C, lanes 8 and 9). This was confirmed by Western blot analysis, in which lower proportions of disulfide-linked Vp1 oligomers (Fig. 4E, lanes 3 and 4) were detected in the cells transfected with C49A-C87A mutant DNA than in those transfected with the wild-type or the C87A-C207S mutant counterpart (lanes 1 and 2). Furthermore, we observed the presence of a 73- to 75-kDa doublet (Fig. 4A to D) that was particularly prominent in the profile of the C49A-C87A mutant Vp1 protein. This doublet, smaller than a Vp1 dimer, was resistant to reduction and accumulated during the chase period in both the Sol and Csk fractions (Fig. 4A to D, lanes 7 to 9). A doublet of comparable size was also detected by anti-Vp1 Western blot analysis after long film exposure in unlabeled cells transfected with the mutant DNA (Fig. 4E, lane 10). Since the doublet also existed at very low levels in wild-type and C87A-C207S samples (Fig. 4E, lanes 8 and 9), it may represent a transient intermediate of Vp1 biogenesis or degradation which accumulates as a result of stalled Vp1 biosynthesis. These results suggest that the lack of the Cys49-Cys87 pair impairs the normal folding progress of Vp1, perhaps due to altered modes of interaction with chaperones, and that the stalled folding intermediates may be subjected to covalent modification or cleavage and fated for degradation, leading to C49A-C87A Vp1 at a lower yet steady level.
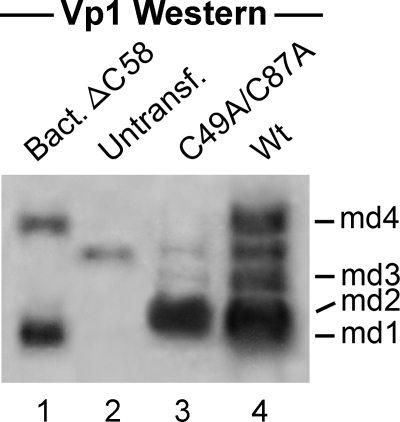
We hypothesized that transfection with Vp1ΔC58 DNA, in which the truncated Vp1 is prevented from proceeding to assembly beyond pentamers, might reveal intermediates that would otherwise be difficult to observe. Furthermore, the nonreducible Vp1-related proteins present in transfected cells may be enriched by denaturation and by affinity chromatography via the His tag. The presence of nonreducible Vp1 species in COS-7 cells that express both LT and ST was tested (Fig. 2G, lane 3) (12, 19). Wild-type Vp1ΔC58 and C49A-C87A Vp1ΔC58 were constructed in a mammalian expression plasmid under the control of a constitutive cytomegalovirus promoter, pCI-Vp1ΔC58-H6. COS-7 cells transfected with wild-type DNA (Fig. 5, lane 4) or its mutant counterpart (Fig. 5, lane 3) were harvested at 40 h posttransfection. Extracts were prepared using RIPA buffer and then denatured in urea before application to metal affinity columns. The proteins were freed of resins by boiling in Laemmli sample buffer, and proteins retained on the resin were separated by SDS-PAGE and were subjected to Western blotting using an anti-Vp1 (Fig. 5) or anti-His (not shown) antibody. For wild-type Vp1ΔC58, several nonreducible, higher-molecular-weight forms were detected by Western blotting for either Vp1 (Fig. 5, lane 4) or the His tag (not shown). In addition to the monomer-length protein (Fig. 5, md1), protein bands that were larger than the monomer form of Vp1 but smaller than a dimer form which would comigrate with md4 were found (md2 and md3). The protein with mobility at 58 to 60 kDa was host derived and present in the control untransfected cells (lane 2). Because proteins md2, md3, and md4 are nonreducible forms larger than the monomer but smaller than a dimer, they appear to have an unidentified covalent protein modification(s). The C49A-C87A mutant Vp1 showed accumulation of md2, which is likely to be a conformationally altered monomer form of md1, but accumulated far less of the higher-molecular-weight forms, especially md4. The profiles of both anti-Vp1 and anti-His (not shown) Western blots for the wild-type and C49A-C87A samples are mirror images, indicating the preservation of carboxy-terminal amino acids, including the His tag. These results indicate that transient forms of covalently modified wild-type Vp1ΔC58 species md2, md3, and md4 are present in the cell cytoplasm, in addition to unmodified md1. The absence of the more slowly migrating mutant md3 and md4 species as well as md1 suggests that the mutation affected conversions from md2 to md3 and to md4. Although we do not know at present which modified species represent the 73- to 75-kDa intermediate observed in infection (29) or in the transfection experiment described above, these results are consistent with the interpretation that SV40 Vp1 folds via discrete pathways and that certain intermediates of folding-defective mutant Vp1 accumulate in the cytoplasm.
FIG. 5.
Covalent and nonreducing Vp1 modifications observed in the cytoplasm of cells transfected with Vp1ΔC58-H6. COS-7 cells were transfected with DNA of the pCI-Vp1ΔC58-H6 wild type (Wt; lane 4) and DNA of its C49A-C87A mutant (lane 3) and harvested at 40 h posttransfection in RIPA buffer. Following urea denaturation, each lysate equivalent to 1.8 × 107 cells was subjected to a metal affinity resin pulldown assay. The resin-bound proteins were analyzed by anti-His (not shown) or anti-Vp1 Western blotting. Bands corresponding to monomeric (40-kDa) and dimeric (80-kDa) forms are denoted md1 and md4, respectively. Two other bands, md2 (45-kDa) and md3 (50-kDa), are also marked. Lane 1 contains marker Vp1ΔC58 produced in bacteria, and lane 2 contains untransfected lysate.
The C49A-C87A mutant Vp1 pentamer is more extensively associated with Hsp70 and Hsc70 than the wild-type Vp1 pentamer.
To study the effect of the C49A-C87A mutation on the interaction of Vp1 with Hsp70 and Hsc70 in vivo, we introduced these mutations into the His-tagged, C terminus-truncated construct, pCI-Vp1ΔC58-H6. The C-terminally truncated Vp1 protein can mediate effective Hsc70 and Hsp70 binding in vitro (Fig. 2C and D). Furthermore, although pentamers can form, the truncation prevents later steps in assembly, and this may allow chaperone-associated intermediates to accumulate. Following transfection, the intracellular localizations of the mutant and wild-type Vp1ΔC58 were the same as those of the full-length molecules, with the wild-type form being predominantly nuclear while the C49A-C87A mutant was found in the cytoplasm (Fig. 6A).
FIG. 6.
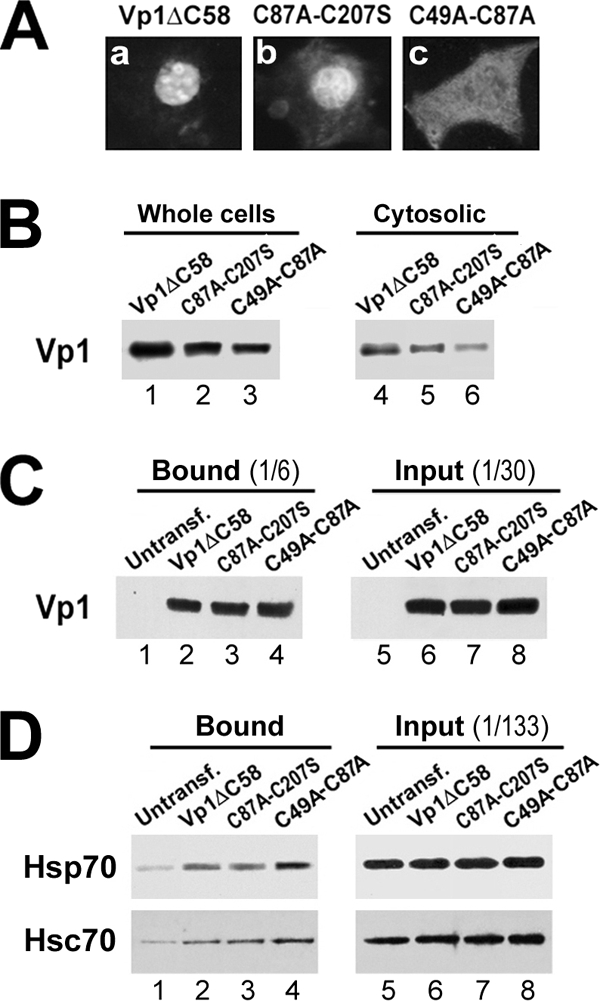
Association of Vp1ΔC58-H6 and double cysteine mutants with Hsc70 and Hsp70 in COS-7 cells. (A) COS-7 cells were transfected with pCI-Vp1ΔC58-H6 with the wild-type Vp1 sequence, Vp1ΔC58 (a), or C87A-C207S (b) or C49A-C87A (c) mutant counterpart for 38 h, fixed, and reacted with an anti-Vp1 serum followed by a rhodamine-labeled secondary antibody. (B) The whole-cell lysates (105 cells) or the hypotonic cytosolic fractions were analyzed for Vp1 content by anti-Vp1 Western blotting. (C and D) The Vp1ΔC58 concentrations of the three cytosolic fractions for panel B were adjusted to be equal between samples by adding the suitable amounts of the untransfected COS-7 cytosolic fraction. The untransfected and adjusted transfected lysates equivalent to 107 cells were subjected to a metal affinity resin pulldown assay. The resin-bound proteins were analyzed by anti-Vp1 (C) and anti-Hsp70/anti-Hsc70 (D) Western blotting along with the input lysates.
Cytoplasmic extracts of transfected cells were used to isolate wild-type and mutant Vp1 proteins by using metal affinity columns. Because the C49A-C87A mutation leads to lower accumulated levels of Vp1 (Fig. 6B), the wild-type extract was adjusted with COS-7 cell extract so that the starting Vp1 levels were the same (Fig. 6C). When the resin-bound proteins were probed by an anti-Hsp70 or anti-Hsc70 antibody, we found that Hsp70 (Fig. 6D, upper row) and Hsc70 (Fig. 6D, lower row) associated with wild-type, C87A-C207S, or C49A-C87A Vp1ΔC58. The amounts of Hsp70 and Hsc70 bound to metal affinity columns along with His-tagged Vp1 constructs were noticeably higher for C49A-C87A than for the wild-type or C87A-C207S protein (Fig. 6D, compare lane 4 with lanes 2 and 3 in both rows). The data suggest that the misfolding-prone C49A-C87A Vp1 mutant has increased association with Hsp70 and Hsc70.
DISCUSSION
SV40 Vp1 goes through folding and oligomerization in the cytoplasm of infected cells, and this involves cellular and viral (co)chaperones. The proper folding of newly synthesized SV40 Vp1 in the cytoplasm is a prerequisite for the formation of infectious virions in the nucleus. The present results show that Hsc70/Hsp70 and virally encoded T antigens play critical roles in the normal folding of wild-type SV40 Vp1 and perhaps in the downstream fate of abnormally folded mutant Vp1.
Association of cellular HSP70 with SV40 Vp1.
Viruses have developed strategies to efficiently produce their own infectious particles by either encoding chaperone genes in their genomes or recruiting cellular chaperones (2, 8, 37, 42, 43). Our findings on the in vivo and in vitro association of SV40 Vp1 with the stress-inducible Hsp70 protein as well as the constitutively expressed Hsc70 protein indicate the requirement of cellular chaperones in SV40 Vp1 biogenesis, largely corroborating early reports on the recruitment of cellular chaperones for virion assembly (8, 42, 43).
Our results suggest the presence of two distinct HSP70 binding patterns for three regions of SV40 Vp1. Vp1 molecules with either the C-terminal 69 aa or the N-terminal 21 aa bind both Hsc70 and Hsp70 in an energy-dependent manner. When both the N and the C termini are missing, as in the core pentamer, HSP70 recruitment is still dependent on ATP and further requires additional virally encoded J-domain cochaperone SV40 LT/ST. Despite this new insight, the present findings raise the question of whether the two Vp1-interacting HSP70 family chaperones play redundant or distinct roles in SV40 Vp1 folding. Monkey Hsc70 and Hsp70 amino acid sequences have 84% homology, although their C-terminal substrate-binding domains are more divergent than their N-terminal ATPase domains (42). Though each HSP70 chaperone has a broad affinity to unfolded polypeptide regions containing exposed hydrophobic side chains and an accessible polypeptide backbone, the preferred pattern of amino acid residues in the substrates varies between different HSP70 members (20). Thus, Hsc70 and Hsp70 may have preferences for differently exposed, unstructured regions of Vp1 or different conformations of Vp1 that occur during its folding and trafficking. Further interaction experiments in vitro using various segments of Vp1 are needed to identify binding preferences for Hsc70 and Hsp70.
SV40 tumor antigens are (co)chaperones for Vp1 folding.
HSP70 proteins with a low basal rate of ATP hydrolysis require the cooperation of ATPase-stimulating J-domain proteins in performing chaperoning functions (20, 21, 38). Our finding that binding of HSP70 to the core Vp1 protein requires prior binding of LT/ST implies that the SV40 J-domain proteins orchestrate and chaperone the core Vp1 folding in the cytoplasm. SV40 ST, sharing the same J domain as LT, can functionally substitute for bacterial DnaJ in promoting the growth of E. coli (17), consistent with the idea of ST also being a candidate cochaperone. It is not clear whether LT/ST binding to the Vp1 core pentamer is via the unique segments or the conserved J domain located on both LT/ST. However, it is clear that the chaperone activity is lost once the LT/ST is depleted in the 293TT lysate (Fig. 2C and D).
There are at least three advantages to LT/ST being a cochaperone for Vp1 folding during SV40 infection. First, LT/ST may be a more efficient recruiter and stimulator of either Hsc70 or Hsp70 than Hsp40 is. Although J domains of different proteins share similar overall structures (reviewed in reference 21), the primary sequence of LT/ST J domains has no more than 40% homology to that of DnaJ and the human Hsp40 homolog Hdj-2 (and presumably the monkey counterpart) (25), and the remaining parts and Hsp40 are unrelated. Coupling between the Hsp40 homolog and the Hsp70 homolog appears to be universal (21). For example, the ability of LT to functionally cooperate with Hsc70 depends on specific regions of LT (not only in the N-terminal J domain but also in the C terminus), and a chimeric LT containing the J domain of DnaJ does not show this function (49, 50, 52). Second, LT/ST may be more effective at presenting Vp1 as a substrate to the Hsc70 or Hsp70 chaperone than to the Hsp40 cochaperone. J-domain proteins are known to vary in their spectra of polypeptide substrates (13, 37, 51). The mutually nonhomologous regions of LT/ST and Hsp40 can conceivably mediate different substrate specificities. Thus, LT/ST may contain determinants for binding to the certain parts of the Vp1 core before delivering it to Hsc70 or Hsp70. Third, LT/ST may supplement the cochaperone activity of Hsp40. Hsp40 is induced by heat stress (41) as well as by SV40 infection (P. Liu and P. P. Li, unpublished data). Yet, the efficient folding of all the capsid proteins being produced during the late phase of infection may require additional contributions of LT/ST as a cochaperone, even if LT is no more effective than Hsp40 in cochaperoning Vp1 folding. The essential issue would be to figure out a mechanism(s) for the two tumor antigens, separately or simultaneously, to chaperone Vp1 core folding in cooperation with the HSP70 protein in the cytoplasm. Further in vitro experiments using various regions of Vp1 as well as LT and ST are expected to identify binding preferences of the J domain common to the SV40's two T antigens (the LT-unique segment and the ST-unique segment). In short, in addition to their known roles in viral DNA replication, transcriptional regulation, transformation, and virion maturation (10, 14, 51), our present data strongly support the idea that the two SV40 T antigens play a critical role in Vp1 folding in the cytoplasm.
The 73- to 75-kDa Vp1 folding intermediate and its abnormal accumulation and covalent and nonreducible Vp1 modifications.
The presence of the 73- to 75-kDa doublet, found during SV40 infection among protein species that sediment as a large protein complex (29), is difficult to relate to Vp1 folding, since the doublet's amounts in both the whole-cell lysate and the cytoplasmic fraction are quite small. The pulse-chase experiments have shown accumulation of the 73- to 75-kDa Vp1 folding intermediate in a larger amount with C49A-C87A Vp1 than with the wild type. The aberrant 73- to 75-kDa intermediate, smaller than a Vp1 dimer, is resistant to reduction, is presumed to be posttranslationally modified, and is kept behind from normal folding and oligomerization processes. Using recombinant DNAs encoding the truncated Vp1 protein, in which the last 58 aa are replaced with a His tag to prevent the intermediates from proceeding beyond pentamers, we were able to enrich and detect, in addition to monomeric md1, three Vp1 species (md2, md3, and md4), all of which appeared to have an unidentified covalent protein modification(s). Some of them were absent in cells transfected with the folding-defective mutant DNA. We suggest that these new species represent folding intermediates of SV40 Vp1ΔC58: perhaps monomer md1 to modified md2 and then to md3 and md4. That the md1 is absent in the cells transfected with the C49A-C87A mutant DNA might suggest that the mutant Vp1 protein is stalled at the md2 stage. Alternatively, some of them may also represent dead-end products that are being routed for degradation. Nonetheless, these new findings support the view that the 73- to 75-kDa doublet is a transient intermediate in normal Vp1 folding during infection. Further experiments, however, are necessary for identifying the nature of chemical modifications, the amino acid(s) of Vp1 at which modifications occur, and the consequence of each modification(s) to Vp1 folding in cytoplasm.
In conclusion, we have identified candidate cellular and viral chaperones which may mediate efficient intracellular folding of SV40 Vp1 in the cytoplasm. These proteins are likely to work together with additional enzymatic and/or regulatory proteins in the cell. Pertinent studies that dissect the regulatory network and the interactive or functional domains involved are expected to provide further insight in the mechanism of Vp1 folding during SV40 infection.
Acknowledgments
We are grateful to Akira Nakanishi for immunofluorescence analysis and helpful discussions, to Kathleen Rundell for the gift of monoclonal antibody pAb419, and to John T. Schiller and Christopher B. Buck for sharing the 293TT cell line. We are also grateful to Akiko Nakamura, Jennifer Jung-Hwa Lee, Doris Li, and Julie Garchow for technical assistance; to Ann Roman and Lisa Barrow-Lating for technical information; to Yuichiro Itoh for editorial assistance; to Mark Arbing and the UCLA-DOE Protein Expression Technology Center for assistance in protein expression and insightful discussions; to James Pipas for helpful discussions; and to Kathleen Rundell for professional editing. Critically reading the manuscript, Kathleen Rundell gave us helpful insight and discussions.
This work was supported by R01 CA50574 from the National Institutes of Health.
Footnotes
Published ahead of print on 22 October 2008.
REFERENCES
- 1.Abdul, K. M., K. Terada, T. Gotoh, R. M. Hafizur, and M. Mori. 2002. Characterization and functional analysis of a heart-enriched DnaJ/ Hsp40 homolog dj4/DjA4. Cell Stress Chaperones 7156-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodsky, J. L., and J. M. Pipas. 1998. Polyomavirus T antigens: molecular chaperones for multiprotein complexes. J. Virol. 725329-5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buck, C. B., D. V. Pastrana, D. R. Lowy, and J. T. Schiller. 2004. Efficient intracellular assembly of papillomaviral vectors. J. Virol. 78751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butel, J. S., S. S. Tevethia, and J. L. Melnick. 1972. Oncogenicity and cell transformation by papovavirus SV40: the role of the viral genome. Adv. Cancer Res. 151-55. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, K. S., K. P. Mullane, I. A. Aksoy, H. Stubdal, J. Zalvide, J. M. Pipas, P. A. Silver, T. M. Roberts, B. S. Schaffhausen, and J. A. DeCaprio. 1997. DnaJ/hsp40 chaperone domain of SV40 large T antigen promotes efficient viral DNA replication. Genes Dev. 111098-1110. [DOI] [PubMed] [Google Scholar]
- 6.Chromy, L. R., J. M. Pipas, and R. L. Garcea. 2003. Chaperone-mediated in vitro assembly of polyomavirus capsids. Proc. Natl. Acad. Sci. USA 10010477-10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clever, J., and H. Kasamatsu. 1991. Simian virus 40 Vp2/3 small structural proteins harbor their own nuclear transport signal. Virology 18178-90. [DOI] [PubMed] [Google Scholar]
- 8.Cripe, T. P., S. E. Delos, P. A. Estes, and R. L. Garcea. 1995. In vivo and in vitro association of hsc70 with polyomavirus capsid proteins. J. Virol. 697807-7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean, D. A., P. P. Li, L. M. Lee, and H. Kasamatsu. 1995. Essential role of the Vp2 and Vp3 DNA-binding domain in simian virus 40 morphogenesis. J. Virol. 691115-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeCaprio, J. A. 1999. The role of the J domain of SV40 large T in cellular transformation. Biologicals 2723-28. [DOI] [PubMed] [Google Scholar]
- 11.Dymecki, S. M. 1996. A modular set of Flp, FRT and lacZ fusion vectors for manipulating genes by site-specific recombination. Gene 171197-201. [DOI] [PubMed] [Google Scholar]
- 12.Fahrbach, K. M., R. B. Katzman, and K. Rundell. 2008. Role of SV40 ST antigen in the persistent infection of mesothelial cells. Virology 370255-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan, C. Y., S. Lee, and D. M. Cyr. 2003. Mechanisms for regulation of Hsp70 function by Hsp40. Cell Stress Chaperones 8309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fanning, E., X. Zhao, and X. Jiang. Polyomavirus life cycle. In B. Damania and J. Pipas (ed.), DNA tumor viruses, in press. Springer-Verlag, Berlin, Germany.
- 15.Fewell, S. W., J. M. Pipas, and J. L. Brodsky. 2002. Mutagenesis of a functional chimeric gene in yeast identifies mutations in the simian virus 40 large T antigen J domain. Proc. Natl. Acad. Sci. USA 992002-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu, X. Y., and J. L. Manley. 1987. Factors influencing alternative splice site utilization in vivo. Mol. Cell. Biol. 7738-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genevaux, P., F. Lang, F. Schwager, J. V. Vartikar, K. Rundell, J. M. Pipas, C. Georgopoulos, and W. L. Kelley. 2003. Simian virus 40 T antigens and J domains: analysis of Hsp40 cochaperone functions in Escherichia coli. J. Virol. 7710706-10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gluzman, Y. 1981. SV40-transformed simian cells support the replication of early SV40 mutants. Cell 23175-182. [DOI] [PubMed] [Google Scholar]
- 19.Harlow, E., L. V. Crawford, D. C. Pim, and N. M. Williamson. 1981. Monoclonal antibodies specific for simian virus 40 tumor antigens. J. Virol. 39861-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartl, F. U., and M. Hayer-Hartl. 2002. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 2951852-1858. [DOI] [PubMed] [Google Scholar]
- 21.Hennessy, F., W. S. Nicoll, R. Zimmermann, M. E. Cheetham, and G. L. Blatch. 2005. Not all J domains are created equal: implications for the specificity of Hsp40-Hsp70 interactions. Protein Sci. 141697-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishii, N., A. Nakanishi, M. Yamada, M. H. Macalalad, and H. Kasamatsu. 1994. Functional complementation of nuclear targeting-defective mutants of simian virus 40 structural proteins. J. Virol. 688209-8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasamatsu, H., and A. Nehorayan. 1979. Intracellular localization of viral polypeptides during simian virus 40 infection. J. Virol. 32648-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelley, W. L., and C. Georgopoulos. 1997. The T/t common exon of simian virus 40, JC, and BK polyomavirus T antigens can functionally replace the J-domain of the Escherichia coli DnaJ molecular chaperone. Proc. Natl. Acad. Sci. USA 943679-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelley, W. L., and S. J. Landry. 1994. Chaperone power in a virus? Trends Biochem. Sci. 19277-278. [DOI] [PubMed] [Google Scholar]
- 26.Khandjian, E. W., and H. Turler. 1983. Simian virus 40 and polyoma virus induce synthesis of heat shock proteins in permissive cells. Mol. Cell. Biol. 31-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kingston, R. E., A. Cowie, R. I. Morimoto, and K. A. Gwinn. 1986. Binding of polyomavirus large T antigen to the human hsp70 promoter is not required for trans activation. Mol. Cell. Biol. 63180-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 29.Li, P. P., A. Nakanishi, S. W. Clark, and H. Kasamatsu. 2002. Formation of transitory intrachain and interchain disulfide bonds accompanies the folding and oligomerization of simian virus 40 Vp1 in the cytoplasm. Proc. Natl. Acad. Sci. USA 991353-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, P. P., A. Nakanishi, V. Fontanes, and H. Kasamatsu. 2005. Pairs of Vp1 cysteine residues essential for simian virus 40 infection. J. Virol. 793859-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, P. P., A. Nakanishi, D. Shum, P. C. Sun, A. M. Salazar, C. F. Fernandez, S. W. Chan, and H. Kasamatsu. 2001. Simian virus 40 Vp1 DNA-binding domain is functionally separable from the overlapping nuclear localization signal and is required for effective virion formation and full viability. J. Virol. 757321-7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, P. P., A. Nakanishi, M. A. Tran, A. M. Salazar, R. C. Liddington, and H. Kasamatsu. 2000. Role of simian virus 40 Vp1 cysteines in virion infectivity. J. Virol. 7411388-11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, S., S. Chien, and P. I. Branemark. 1999. Heat shock-induced necrosis and apoptosis in osteoblasts. J. Orthop. Res. 17891-899. [DOI] [PubMed] [Google Scholar]
- 34.Liddington, R. C., Y. Yan, J. Moulai, R. Sahli, T. L. Benjamin, and S. C. Harrison. 1991. Structure of simian virus 40 at 3.8-A resolution. Nature 354278-284. [DOI] [PubMed] [Google Scholar]
- 35.Lin, W., T. Hata, and H. Kasamatsu. 1984. Subcellular distribution of viral structural proteins during simian virus 40 infection. J. Virol. 50363-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin, W., J. L. Shurgot, and H. Kasamatsu. 1986. The synthesis and transport of SV40 structural proteins. Virology 154108-120. [DOI] [PubMed] [Google Scholar]
- 37.Mayer, M. P. 2005. Recruitment of Hsp70 chaperones: a crucial part of viral survival strategies. Rev. Physiol. Biochem. Pharmacol. 1531-46. [DOI] [PubMed] [Google Scholar]
- 38.Michels, A. A., B. Kanon, A. W. Konings, K. Ohtsuka, O. Bensaude, and H. H. Kampinga. 1997. Hsp70 and Hsp40 chaperone activities in the cytoplasm and the nucleus of mammalian cells. J. Biol. Chem. 27233283-33289. [DOI] [PubMed] [Google Scholar]
- 39.Nakanishi, A., J. Clever, M. Yamada, P. P. Li, and H. Kasamatsu. 1996. Association with capsid proteins promotes nuclear targeting of simian virus 40 DNA. Proc. Natl. Acad. Sci. USA 9396-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohtsuka, K., and M. Hata. 2000. Mammalian HSP40/DNAJ homologs: cloning of novel cDNAs and a proposal for their classification and nomenclature. Cell Stress Chaperones 598-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohtsuka, K., A. Masuda, A. Nakai, and K. Nagata. 1990. A novel 40-kDa protein induced by heat shock and other stresses in mammalian and avian cells. Biochem. Biophys. Res. Commun. 166642-647. [DOI] [PubMed] [Google Scholar]
- 42.Sainis, I., C. Angelidis, G. Pagoulatos, and I. Lazaridis. 1994. The hsc70 gene which is slightly induced by heat is the main virus inducible member of the hsp70 gene family. FEBS Lett. 355282-286. [DOI] [PubMed] [Google Scholar]
- 43.Sainis, L., C. Angelidis, G. N. Pagoulatos, and L. Lazaridis. 2000. HSC70 interactions with SV40 viral proteins differ between permissive and nonpermissive mammalian cells. Cell Stress Chaperones 5132-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawai, E. T., and J. S. Butel. 1989. Association of a cellular heat shock protein with simian virus 40 large T antigen in transformed cells. J. Virol. 633961-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sawai, E. T., G. Rasmussen, and J. S. Butel. 1994. Construction of SV40 deletion mutants and delimitation of the binding domain for heat shock protein to the amino terminus of large T-antigen. Virus Res. 31367-378. [DOI] [PubMed] [Google Scholar]
- 46.Sock, E., J. Enderich, and M. Wegner. 1999. The J domain of papovaviral large tumor antigen is required for synergistic interaction with the POU-domain protein Tst-1/Oct6/SCIP. Mol. Cell. Biol. 192455-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srinivasan, A., A. J. McClellan, J. Vartikar, I. Marks, P. Cantalupo, Y. Li, P. Whyte, K. Rundell, J. L. Brodsky, and J. M. Pipas. 1997. The amino-terminal transforming region of simian virus 40 large T and small t antigens functions as a J domain. Mol. Cell. Biol. 174761-4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stehle, T., S. J. Gamblin, Y. W. Yan, and S. C. Harrison. 1996. The structure of simian virus 40 refined at 3.1 A resolution. Structure 4165-182. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan, C. S., P. Cantalupo, and J. M. Pipas. 2000. The molecular chaperone activity of simian virus 40 large T antigen is required to disrupt Rb-E2F family complexes by an ATP-dependent mechanism. Mol. Cell. Biol. 206233-6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan, C. S., S. P. Gilbert, and J. M. Pipas. 2001. ATP-dependent simian virus 40 T-antigen-Hsc70 complex formation. J. Virol. 751601-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sullivan, C. S., and J. M. Pipas. 2002. T antigens of simian virus 40: molecular chaperones for viral replication and tumorigenesis. Microbiol. Mol. Biol. Rev. 66179-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sullivan, C. S., J. D. Tremblay, S. W. Fewell, J. A. Lewis, J. L. Brodsky, and J. M. Pipas. 2000. Species-specific elements in the large T-antigen J domain are required for cellular transformation and DNA replication by simian virus 40. Mol. Cell. Biol. 205749-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor, I. C., W. Solomon, B. M. Weiner, E. Paucha, M. Bradley, and R. E. Kingston. 1989. Stimulation of the human heat shock protein 70 promoter in vitro by simian virus 40 large T antigen. J. Biol. Chem. 26416160-16164. [PubMed] [Google Scholar]
- 54.Uney, J. B., B. H. Anderton, and S. M. Thomas. 1993. Changes in heat shock protein 70 and ubiquitin mRNA levels in C1300 N2A mouse neuroblastoma cells following treatment with iron. J. Neurochem. 60659-665. [DOI] [PubMed] [Google Scholar]
- 55.Whalen, K. A., R. de Jesus, J. A. Kean, and B. S. Schaffhausen. 2005. Genetic analysis of the polyomavirus DnaJ domain. J. Virol. 799982-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young, J. C., V. R. Agashe, K. Siegers, and F. U. Hartl. 2004. Pathways of chaperone-mediated protein folding in the cytosol. Nat. Rev. Mol. Cell Biol. 5781-791. [DOI] [PubMed] [Google Scholar]
- 57.Zhang, J., and H. Herscovitz. 2003. Nascent lipidated apolipoprotein B is transported to the Golgi as an incompletely folded intermediate as probed by its association with network of endoplasmic reticulum molecular chaperones, GRP94, ERp72, BiP, calreticulin, and cyclophilin B. J. Biol. Chem. 2787459-7468. [DOI] [PubMed] [Google Scholar]