Abstract
We have recently shown that human immunodeficiency virus type 1 (HIV-1) Pr55gag virus-like particles (HIV-VLPs), produced in a baculovirus expression system and presenting a gp120 molecule from a Ugandan HIV-1 isolate of clade A, induce maturation and activation of monocyte-derived dendritic cells (MDDCs) with a production of Th1- and Th2-specific cytokines. Furthermore, HIV-VLP-loaded MDDCs are able to induce a primary and secondary response in autologous human CD4+ T cells in an ex vivo immunization assay. In the present study, we show that similar data can be obtained directly with fresh peripheral blood mononuclear cells (PBMCs), and the HIV-1 seropositivity status, with either low or high viremia, does not significantly impair the immune activation status and the responsiveness of circulating monocyte CD14+ cell populations to an immunogenic stimulus. Some HIV-1-seropositive subjects, however, show a complete lack of maturation induced by HIV-VLPs in CD14+ circulating cells, which does not consistently correlate with an advanced status of HIV-1 infection. The established Th2 polarization in both HIV-seropositive groups is efficiently boosted by HIV-VLP induction and does not switch into a Th1 pattern, strongly suggesting that specific Th1 adjuvants would be required for therapeutic effectiveness in HIV-1-infected subjects. These results indicate the possibility of screening PBMCs for donor susceptibility to an immunogen treatment, which would greatly simplify the identification of “responsive” vaccinees as well as the understanding of eventual failures in individuals enrolled in clinical trials.
Virus-like particles (VLPs) represent a form of subunit vaccine based on viral capsid proteins which self-assemble into particulate structures closely resembling immature virus particles (18, 28, 33, 40). VLPs are replication and infection incompetent, lacking regulatory proteins and infectious genetic material, and can be employed to deliver antigenic structures. In particular, VLPs also efficiently reach the major histocompatibility complex class I pathway in antigen-presenting cells (APCs) in the absence of infection or intracellular replication (3, 49, 50). Considering these properties, VLPs represent a highly attractive vaccine approach and have been produced from a broad spectrum of enveloped and nonenveloped viruses, regardless of whether the particle structure is based on single or multiple capsid proteins (42).
The VLPs developed in our laboratory are based on the human immunodeficiency virus type 1 (HIV-1) Pr55gag precursor protein (HIV-VLPs) and display an entire gp120 molecule from a Ugandan HIV-1 isolate of the A clade (HIV-VLPAs) (6, 8), anchored through the transmembrane portion of Epstein-Barr virus gp220/350 (7). These HIV-VLPAs induce HIV-1-specific CD4+ and CD8+ T-cell responses and cross-clade neutralizing antibodies in immunized BALB/c mice (11). Moreover, the intraperitoneal and intranasal administration of HIV-VLPAs in mice induces antibody responses at systemic and mucosal (vaginal and intestinal) levels (9, 13).
Dendritic cells (DCs) are professional APCs specialized to capture and process antigens in vivo (30), converting proteins to peptides that are presented on major histocompatibility complex molecules and recognized by T cells. DCs also migrate to T-cell areas of lymphoid organs, where the two cell types interact to bring about clonal selection (32, 47, 55). In particular, two main DC types are present in human peripheral blood, known as myeloid DCs, the major subset, representing around 80% of blood DCs (23), and plasmacytoid DCs.
DCs derive from circulating monocytes which are characterized by expression of large amounts of CD14. However, it has been shown that monocytes in human peripheral blood are heterogeneous in terms of expression of antigenic markers (namely, CD14 and CD16), correlating to possible differential physiological activities of monocyte subsets: in particular, CD14hi CD16− cells, which are often called classic monocytes, and CD14+ CD16+ cells, which resemble mature tissue macrophages and appear more likely to be precursors of DCs (43, 48, 56). Nevertheless, both cellular subsets can differentiate ex vivo into DCs in the presence of granulocyte/macrophage colony-stimulating factor and interleukin-4 (IL-4) (51, 52).
An additional monocyte subset, defined by the expression of CD14, CD16, and CD64, has lately been reported, combining characteristics of monocytes and DCs, with high expression of CD86 and HLA-DR and high T-cell-stimulatory activity (29).
We have recently shown that baculovirus-expressed HIV-VLPs are able to induce maturation and activation of monocyte-derived DCs (MDDCs) and that this effect is partially mediated by the internal Toll-like receptors 3 and 9 (12). The HIV-VLP-activated MDDCs produce a pattern of cytokines indicative of both Th1 and Th2 pathways and induce primary and secondary responses in autologous human CD4+ T cells in an ex vivo immunization assay. The uptake of HIV-VLPs by DCs appears to be mainly mediated by a cytochalasin-D-sensitive pathway (12). Moreover, the HIV-VLP-activated MDDCs show specific transcriptional profiles of genes involved in the morphological and functional changes characterizing MDDC activation and maturation (1).
In addition, we have shown that the morphological and gene transcriptional maturation pattern induced by HIV-VLPs in ex vivo-generated MDDCs can also be observed in CD14+ uncultured peripheral blood mononuclear cells (PBMCs) (10). This experimental approach would greatly facilitate screening for responsiveness to vaccines and an understanding of eventual failures in individuals enrolled in clinical trials.
In an HIV-1 vaccine perspective, and in particular for therapeutic strategies, it is mandatory to evaluate the impact of HIV seropositivity status on responsiveness to immunization, considering a possible quantitative and/or qualitative impairment in circulating monocytes, DCs, and other APCs (4, 21, 22, 38).
In this study, the response to HIV-VLPs has been evaluated with CD14+ circulating monocytes, without distinction between different CD16 (+ or −) subsets, according to HIV-1 seropositivity status. An informative evaluation of PBMCs showing the individual vaccinees' immune responsiveness to a human papillomavirus-VLP vaccine has been reported (27, 45, 46). The results here reported indicate that the HIV-1 seropositivity status, with either low or high viremia, does not impair the immune activation status and the responsiveness of circulating monocyte CD14+-cell populations to an antigenic stimulus. Nevertheless, a complete lack of maturation induced by HIV-VLPs in CD14+ circulating cells is occasionally observed for a few HIV-1-seropositive subjects, without any consistent correlation to an advanced status of HIV-1 infection (high viremia levels and/or CD4+ counts). However, the established Th2 polarization in both HIV-seropositive groups is efficiently boosted by HIV-VLP induction but does not switch into a Th1 pattern.
MATERIALS AND METHODS
Cell culture medium.
PBMC culture medium consisted of RPMI 1640 medium (Life Technologies, Carlsbad, CA) supplemented with 2 mM l-glutamine (Sigma), 1% nonessential amino acids (Life Technologies), 1% sodium pyruvate (Life Technologies), 50 μM 2-mercaptoethanol (Sigma), 50 μg of gentamicin (Life Technologies) per ml, and 10% fetal calf serum (Life Technologies).
PBMC preparations and cell treatment.
All human specimens were obtained and processed at the Institute of Human Virology in Baltimore, MD, with informed consent, as approved by the University of Maryland—Baltimore Institutional Review Board. Fresh human PBMCs were isolated by Ficoll-Hypaque density gradient centrifugation and plated in six-well plates at a concentration of approximately 1 × 107/well in a maximum volume of 3 ml/well. PBMCs were pulsed with 6 μg/ml of HIV-VLPs. As a negative control, PBMCs were treated with phosphate-buffered saline (PBS). The residual endotoxin activity possibly present in the HIV-VLP preparation was inhibited by preincubation with polymyxin B sulfate (Sigma) at a concentration of 10 μg/ml. The absence of interference due to polymyxin B sulfate in the activation results was verified in parallel with PBMCs treated only with polymyxin. After 16 h, the cells were harvested, washed, and stained for phenotypic analysis by flow cytometry. The cellular supernatants were collected for quantification of cytokine production by enzyme-linked immunosorbent assay (ELISA) (University of Maryland Cytokine Core Lab, Baltimore, MD). During the development/optimization of the experimental assay, the absence of PBMC activation following treatment with either PBS, supernatant of SF9 cell culture transfected with a baculovirus expression vector (mock baculovirus supernatants), or heat-denatured VLP suspension in PBS (100°C for 10 min) has been repeatedly observed. Thus, the complete set of experiments was subsequently performed using only the PBS treatment as a negative control.
Flow cytometry.
Cells were incubated for 30 min at 4°C with the indicated murine monoclonal antibodies (BD Pharmingen, San Diego, CA), washed, and then fixed with 2% paraformaldehyde for analysis with a FACScalibur flow cytometer (BD Pharmingen). Data analysis was carried out using the FlowJo software program (Tree Star Inc., San Carlos, CA). All analyses were performed on freshly isolated PBMCs, and levels of activation markers were evaluated for cell populations gated for CD14 positivity (high or dim). The fraction of PBMCs that responded by upregulation of activation markers on the cell surface was calculated by overlaying the histograms of treated and untreated PBMCs and carrying out Overton subtraction of the curves.
Statistical analyses.
Intergroup comparisons were performed using the Mann-Whitney U test (for univariate nonparametric group analysis). All P values were two-tailed and were considered significant if less than 0.05.
RESULTS
Clinical parameters of subjects included in analysis.
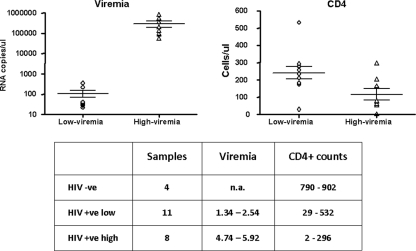
Twenty-three subjects were enrolled in the study. Nineteen were HIV-1 seropositive, of whom 11 showed a low HIV-1 viremia (<2.6 log RNA copies/ml) and 8 a high viremia (>4.69 log RNA copies/ml); the latter enrolled at their first visit and were naive for ART treatment. In particular, the log of viral RNA copies per milliliter ranged from 1.34 to 2.54 (mean, 107 copies/ml) for the low-viremia group and from 4.74 to 5.92 (mean, 295,120 copies/ml), for the high-viremia group (P < 0.0001). The CD4+ counts ranged from 29 to 532 cells/mm3 (mean, 240 cells/mm3) for the low-viremia group and from 2 to 296 cells/mm3 (mean, 117 cells/mm3) for the high-viremia group (P = 0.0298) (Fig. 1). Four healthy seronegative subjects were enrolled as controls.
FIG. 1.
Clinical parameters of subjects included in the analysis. The individual values of viremia and CD4+ cell counts observed for the enrolled subjects are reported. Means and standard errors for each group (low-viremia and high-viremia) are shown. −ve, negative; +ve, positive; n.a., not applicable.
Baculovirus HIV-VLPs induce maturation phenotype in CD14+ PBMCs.
Human PBMCs were isolated by Ficoll-Hypaque density gradient centrifugation, plated in six-well plates at a concentration of 1 × 107/well, and incubated with 6 μg/ml of HIV-VLPs. In parallel, PBMCs were incubated with PBS as a negative control. After 16 h, the expression of the surface maturation markers CD80, CD83, CD86, and HLA-DR was examined in the CD14+ monocyte population, whose distribution was comparable between seronegative and seropositive subjects, in both the CD14hi and CD14dim cellular subsets.
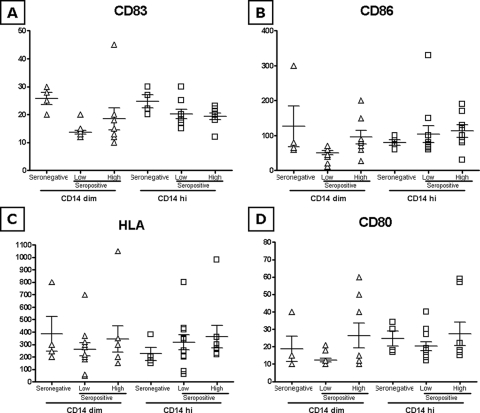
The basal expression of the four markers, in terms of mean fluorescence index (MFI), is largely comparable between seronegative and seropositive subjects, regardless of the HIV-1 viremia levels, in both CD14hi and CD14dim monocyte populations (Fig. 2A to D). The only exception is represented by basal CD83 expression, which is significantly lower in CD14dim monocyte populations of the low-viremia seropositive group (Fig. 2A).
FIG. 2.
Basal expression of activation markers and costimulatory molecules in CD14+ cells (A to D). The basal expression of CD80, CD83, CD86, and HLA-DR on PBMCs was analyzed on fixed cells by using a FACScalibur flow cytometer, and data analysis was carried out using the FlowJo software program. PBMCs were gated for CD14 positivity. The MFI for each sample in each group is represented in both CD14dim and CD14hi gating. Means and standard errors for each group are shown.
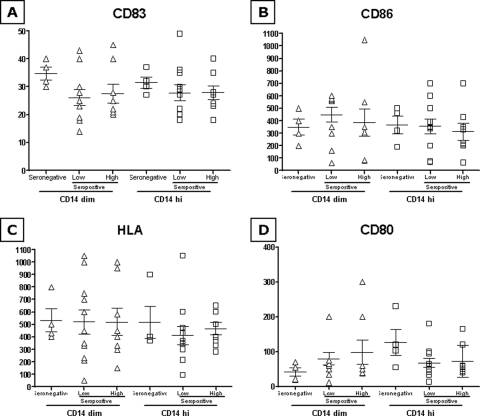
Similarly, the HIV-VLP-induced expression of the four markers is comparable between the seronegative and seropositive subjects, regardless of HIV-1 viremia levels, in both CD14hi and CD14dim monocyte populations (Fig. 3A to D). This observation is further confirmed by the MFI activation for each maturation marker (VLP versus basal), suggesting that overall the seropositivity status, with either low or high viremia, does not significantly affect the responsiveness to an immunogen stimulus (i.e., HIV-VLPs) of circulating monocyte CD14+ cell populations (Fig. 4A to D).
FIG. 3.
Expression of activation markers and costimulatory molecules induced by HIV-VLPs in CD14+ cells (A to D). PBMCs were incubated in the presence of HIV-VLPs for 16 h. The expression of CD80, CD83, CD86, and HLA-DR was analyzed on fixed cells by using a FACScalibur flow cytometer, and data analysis was carried out using the FlowJo software program. PBMCs were gated for CD14 positivity. The MFI for each sample in each group has been represented in both CD14dim and CD14hi gating. Means and standard errors for each group are shown.
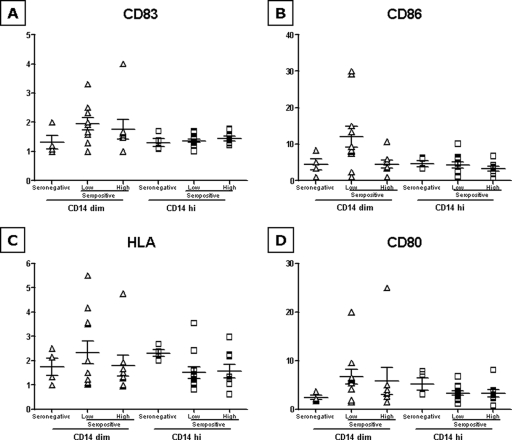
FIG. 4.
n-fold induction of activation markers and costimulatory molecules by HIV-VLPs in CD14+ cells (A to D). The n-fold increase in the MFI observed for each sample in each group after induction with HIV-VLPs, over the basal level (VLP versus PBS), is represented in both CD14dim and CD14hi gating. Means and standard errors for each group are shown.
In the panels for both basal and HIV-VLP-induced expression of the four markers, “hyperresponsive” subjects are present in low- as well as high-viremia groups (Fig. 2 to 4), although none of them is consistently an “outlier” for all four markers evaluated in this study.
Differential maturation phenotype HIV-1-seropositive subjects.
HIV-VLP-induced maturation phenotypes for individual HIV-1-seropositive subjects were evaluated in both low- and high-viremia groups. The results indicate that a clear maturation phenotype is observed for the majority of subjects, regardless of the levels of HIV-1 viremia; nevertheless, it is also evident that for a couple of subjects in both groups, a lack of maturation induced by HIV-VLPs in CD14+ circulating cells was observed when CD14hi as well as CD14dim monocyte populations were considered (Fig. 5 and 6).
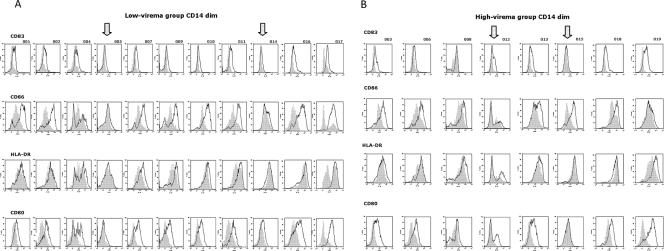
FIG. 5.
Individual pattern of activation in CD14dim cells. The expression of CD80, CD83, CD86, and HLA-DR in PBMCs was analyzed on fixed cells by using a FACScalibur flow cytometer, and data analysis was carried out using the FlowJo software program. The PBMCs were gated for CD14dim positivity. Results of a representative experiment are shown; the shaded curve represents the baseline data for untreated cells. (A) Expression of markers in low-viremia group. (B) Expression of markers in high-viremia group. In each panel, the arrow indicates the samples with no induction of expression.
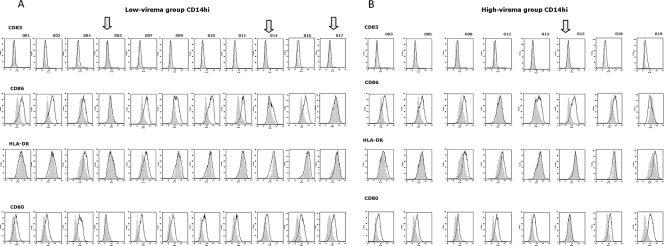
FIG. 6.
Individual pattern of activation in CD14hi cells. The expression of CD80, CD83, CD86, and HLA-DR in PBMCs was analyzed on fixed cells by using a FACScalibur flow cytometer, and data analysis was carried out using the FlowJo software program. PBMCs were gated for CD14dim positivity. The results of a representative experiment are shown; the shaded curve represents the baseline data for untreated cells. (A) Expression of markers in low-viremia group. (B) Expression of markers in high-viremia group. In each panel, the arrow indicates the samples with no induction of expression.
In particular, in the low-viremia group, subjects 5 and 14 showed a complete lack of activation for all four markers evaluated in both CD14hi and CD14dim monocyte populations, while subject 17 showed such an “anergic” phenotype only in the CD14hi monocyte population (Fig. 5A and 6A). Interestingly, the very low viremia (1.44 to 1.61 log RNA copies/ml) and the relatively normal CD4+ cell count (173 to 532 cells/μl) observed for these subjects would not predict any impairment in response to an antigen.
Similarly, in the high-viremia group, subjects 12 and 15 showed an incomplete activation of the four markers evaluated in both CD14hi and CD14dim monocyte populations (Fig. 5B and 6B). In particular, subject 15 showed activation only of the CD86 costimulatory molecule, which by itself has been shown to not be sufficient to drive T-cell immunity (26, 53, 55). Subject 12, instead, showed activation of costimulatory molecules (in particular, HLA-DR and CD80) only in a subpopulation of CD14dim monocytes (Fig. 5B).
These two subjects showed the highest viremia in this group (5.76 and 5.92 log RNA copies/ml), associated with a low CD4+ cell count (62 to 164 cells/μl), suggesting in the high-viremia group a possible correlation between higher viremia and an impaired response to an antigen, although further studies of a larger cohort of subjects will be needed to confirm this preliminary observation.
Cytokine production in HIV-VLP-loaded PBMCs.
In order to evaluate the impact of chronic HIV infection on the production of cytokines important for T-helper-cell activation, the levels of IL-12 p70, gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), IL-6, IL-4, and IL-10 were assessed in the supernatant of PBMCs loaded with HIV-VLPs.
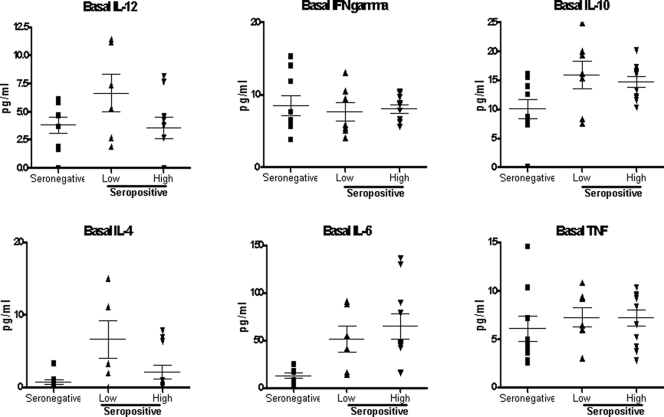
The average basal level of all evaluated cytokines was low (<20 pg/ml), with no significant difference between HIV-1-seronegative and -seropositive individuals; moreover, in the latter group, the level of viremia did not make a significant difference. The only exception was represented by IL-6, whose average basal levels in both the low- and high-viremia seropositive groups (51.06 and 64.66 pg/ml, respectively) were significantly higher than that in the seronegative group (12.37 pg/ml) (Fig. 7).
FIG. 7.
Basal expression of Th1 and Th2 cytokines in PBMCs. The basal expression of IL-12 p70, IFN-γ, TNF-α, IL-6, IL-4, and IL-10 by PBMCs was measured by ELISA in the culture supernatant. The value for each sample in each group is shown. Means and standard errors for each group are shown.
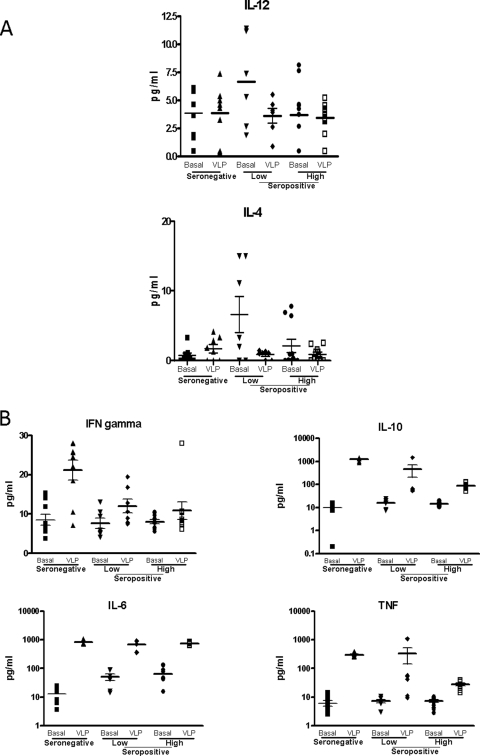
Treatment of PBMCs with HIV-VLPs did not induce any increase in the production of IL-12 p70 or IL-4, regardless of the HIV-1 serological status (Fig. 8A). On the contrary, HIV-VLPs induced a significantly increased production of the other Th2 cytokines in HIV-seronegative and -seropositive samples, with an average 1- to 2-logfold increase for IL-10, IL-6, and TNF-α. However, the increased production of IL-10 and TNF-α in the high-viremia seropositive group was significantly lower than those in the other groups (P < 0.05) (Fig. 8B). Moreover, the production of the Th1 IFN-γ was significantly increased of 2.5-fold by HIV-VLP treatment only in the healthy seronegative group, while in the seropositive groups the observed increased production was not statistically significant (P > 0.05) (Fig. 8B).
FIG. 8.
Expression of Th1 and Th2 cytokines induced by HIV-VLPs in PBMCs (A and B). PBMCs were incubated in the presence of HIV-VLPs for 16 h, and expression of IL-12 p70, IFN-γ, TNF-α, IL-6, IL-4, and IL-10 by PBMCs was measured in the culture supernatant by ELISA. For each group, the PBS (basal)- and VLP-induced (VLP) levels are reported. The value for each sample in each group is shown. The mean and SEM of the values in each group are shown.
DISCUSSION
We have previously reported that baculovirus-expressed HIV-VLPAs, which display gp120 derived from a Ugandan HIV-1 isolate of the subtype A, are strongly immunogenic in BALB/c mice, inducing HIV-1-specific CD4+ and CD8+ T-cell responses as well as cross-clade neutralizing antibodies at systemic and mucosal sites (9, 11, 13). We have also shown that these HIV-VLPs are able to induce maturation of ex vivo-generated immature human MDDCs, resulting in the expression of surface maturation markers and the increased production of Th1 and Th2 cytokines, and this observation can be confirmed on unselected PBMCs (1, 10).
Here we show that the basal and HIV-VLP-induced expression of CD80, CD83, CD86, and HLA-DR activation markers and costimulatory molecules, in terms of mean fluorescence, is largely comparable between seronegative and seropositive groups, regardless of HIV-1 viremia levels, in both CD14hi and CD14dim monocyte populations. The overall expression pattern suggests maturation/activation induced by VLPs, although for some specific markers and in some patients, the trend does not reach statistical significance. This observation suggests that the seropositivity status, with either low or high viremia, does not significantly impair the immune activation status and the responsiveness of circulating monocyte CD14+ cell populations to an immunogenic stimulus. Results obtained in parallel, with lipopolysaccharide used as a positive control, confirm the stimulation effects on PBMCs from the three groups analyzed in the present study (M. Monaco, personal communication).
Nevertheless, it is also evident that in both the low-viremia and high-viremia seropositive groups, some individuals show a complete lack of maturation induced by HIV-VLPs in CD14+ circulating cells, with both the CD14hi and CD14dim monocyte populations being considered. Such an “anergic” phenotype, at least in the low-viremia HIV-1-seropositive group, does not consistently correlate to an advanced status of HIV-1 infection (i.e., low CD4+ cell count and high viremia), indicating the need for individual evaluations to identify possible impairments in response to an immunogen.
The present results confirm and extend data from others showing a normal expression of surface molecules involved in antigen-specific T-cell activation on immature and mature DCs from HIV-1-infected and hepatitis C virus (HCV)-HIV-coinfected individuals (15, 25, 31). Furthermore, monocyte-derived DCs from either HCV-infected or HCV-HIV-coinfected persons have been previously shown to stimulate a mixed leukocyte reaction in purified, allogeneic CD4+ T cells comparable to that with DCs derived from healthy donors (36, 44, 54).
The average basal level of the IL-12 p70, IFN-γ, TNF-α, IL-6, IL-4, and IL-10 cytokines, relevant for T-helper cell activation and polarization, is low (<20 pg/ml) in HIV-1-seropositive individuals, as in healthy seronegative subjects. The only exception is represented by IL-6, whose average basal level in both low- and high-viremia seropositive groups is four to fivefold higher than that in the seronegative group. Given that IL-6 promotes Th2 differentiation (20) and inhibits IFN-γ production and Th1 differentiation (19), the higher basal levels of IL-6 in HIV-seropositive individuals suggest a Th2 polarization induced by an established HIV-1 infection, regardless of the viremia levels, as previously extensively reported (5, 16, 24).
Interestingly, in both HIV-seropositive groups, mainly Th2-polarizing cytokines (IL-6, IL-10, and TNF-α) were induced by HIV-VLPs, while the increased production of Th1 IFN-γ was not statistically significant (P > 0.05), suggesting the persistence of a prevalent Th2 status. However, the significantly lesser increase in production of IL-10 and TNF-α in the high-viremia seropositive group suggests a progressively functional impairment associated with an advanced status of HIV-1 infection. No significant difference in the secretion of the Th2 cytokine TNF-α or IL-10 was observed between PBMCs from healthy and low-viremia HIV-seropositive individuals, as previously reported by others in different experimental systems (14, 39).
The production of IL-4 and IL-12 p70 was not increased by HIV-VLPs in the seronegative or seropositive group. In particular, the production of IL-12 p70 by monocyte/macrophage cells and B lymphocytes is known to be inhibited by IL-10 and TNF-α (2, 17, 37), with a sequential detrimental effect on the IL-12-mediated induction of IFN-γ production by NK and T cells (34, 35, 41). Therefore, the high levels of IL-10 and TNF-α induced by HIV-VLPs could explain the lack of increased production of IL-12 p70 in all tested groups and the limited production of IFN-γ only in the seronegative group. The impairment of basal and antigen-induced production of Th1-polarizing cytokines for HIV-seropositive individuals is in concordance with previous observations of PBMCs from HIV-infected subjects exposed ex vivo to a panel of stimuli (i.e., Staphylococcus aureus, bacterial cell wall components) (25, 39). The correlation of the lymphokine response with the extent of stimulation of CD83, CD86, CD80, and HLA-DR markers for each subject of each group is currently under evaluation.
These results represent a proof-of-concept of the approach and have been generated using Env-presenting HIV-VLPs; additional VLPs and particulate structures (i.e., virosomes) (with or without presenting Env) are currently under evaluation. They show that in order to obtain a therapeutic effect in HIV-infected individuals, it would be mandatory to reduce the viremia and to use adjuvanting molecules able to shift the immune system toward a Th1-cell-mediated immune response to override the established Th2 status. Otherwise, the therapeutic vaccination would fail either for lack of effective activation of Th1/Th2 cells or because of detrimental further boosting of a Th2 response, useless for a therapeutic effect and inhibiting the possible ongoing Th1 response.
The overall results presented here show the possibility of screening donor susceptibility to an antigen treatment using PBMCs without the need of purification and ex vivo selection of DCs, simplifying the identification of “responsive” vaccinees and the understanding of eventual failures in individuals enrolled in clinical trials. When necessary, additional and more-detailed studies of fractionated cell types would allow identification and a better characterization of the individual cells involved in the in vivo response. This still-limited set of markers, which could be further broadened, has proven to be informative for identifying subjects who, in the genomic study described in a parallel work, are confirmed to be totally or partially anergic (Monaco, personal communication).
In conclusion, our results indicate that monocyte-derived DCs from HIV-1-seropositive patients retain a strong APC function and can be functional in active autologous immunotherapy strategies. However, specific Th1-driving adjuvant strategies might be necessary to obtain the efficient therapeutic effect sought.
Acknowledgments
We are indebted to Robin Flinko for invaluable technical support.
This study was supported by grants from the Ministero Italiano Università e Ricerca (MIUR, 2004), the Ministero Italiano della Sanità (Ricerca Corrente and Progetto Finalizzato AIDS 2006), the Institute of Human Virology, and the NIH (NIH grants to G.K.L.).
Footnotes
Published ahead of print on 22 October 2008.
REFERENCES
- 1.Aricò, E., E. Wang, M. L. Tornesello, M. Tagliamonte, G. K. Lewis, F. M. Marincola, F. M. Buonaguro, and L. Buonaguro. 2005. Immature monocyte derived dendritic cells gene expression profile in response to virus-like particles stimulation. J. Transl. Med. 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aste-Amezaga, M., X. Ma, A. Sartori, and G. Trinchieri. 1998. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J. Immunol. 1605936-5944. [PubMed] [Google Scholar]
- 3.Bachmann, M. F., M. B. Lutz, G. T. Layton, S. J. Harris, T. Fehr, M. Rescigno, and P. Ricciardi-Castagnoli. 1996. Dendritic cells process exogenous viral proteins and virus-like particles for class I presentation to CD8+ cytotoxic T lymphocytes. Eur. J. Immunol. 262595-2600. [DOI] [PubMed] [Google Scholar]
- 4.Barron, M. A., N. Blyveis, B. E. Palmer, S. MaWhinney, and C. C. Wilson. 2003. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J. Infect. Dis. 18726-37. [DOI] [PubMed] [Google Scholar]
- 5.Breen, E. C. 2002. Pro- and anti-inflammatory cytokines in human immunodeficiency virus infection and acquired immunodeficiency syndrome. Pharmacol. Ther. 95295-304. [DOI] [PubMed] [Google Scholar]
- 6.Buonaguro, L., F. M. Buonaguro, F. Russo, M. L. Tornesello, E. Beth-Giraldo, R. Wagner, H. Wolf, and G. Giraldo. 1998. A novel gp120 sequence from an HIV-1 isolate of the A clade identified in North Uganda. AIDS Res. Hum. Retrovir. 141287-1289. [DOI] [PubMed] [Google Scholar]
- 7.Buonaguro, L., F. M. Buonaguro, M. L. Tornesello, D. Mantas, E. Beth-Giraldo, R. Wagner, S. Michelson, M.-C. Prevost, H. Wolf, and G. Giraldo. 2001. High efficient production of Pr55gag virus-like particles expressing multiple HIV-1 epitopes, including a gp120 protein derived from an Ugandan HIV-1 isolate of subtype A. Antivir. Res. 4935-47. [DOI] [PubMed] [Google Scholar]
- 8.Buonaguro, L., E. Del Gaudio, M. Monaco, D. Greco, P. Corti, E. Beth-Giraldo, F. M. Buonaguro, and G. Giraldo. 1995. Heteroduplex mobility assay and phylogenetic analysis of V3 region sequences of human immunodeficiency virus type 1 isolates from Gulu, northern Uganda. J. Virol. 697971-7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buonaguro, L., C. Devito, M. L. Tornesello, U. Schroder, B. Wahren, J. Hinkula, and F. M. Buonaguro. 2007. DNA-VLP prime-boost intra-nasal immunization induces cellular and humoral anti-HIV-1 systemic and mucosal immunity with cross-clade neutralizing activity. Vaccine 255968-5977. [DOI] [PubMed] [Google Scholar]
- 10.Buonaguro, L., A. Monaco, E. Arico, E. Wang, M. L. Tornesello, G. K. Lewis, F. M. Marincola, and F. M. Buonaguro. 2008. Gene expression profile of peripheral blood mononuclear cells in response to HIV-VLPs stimulation. BMC Bioinform. 9(Suppl. 2)S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buonaguro, L., L. Racioppi, M. L. Tornesello, C. Arra, M. L. Visciano, B. Biryahwaho, S. D. K. Sempala, G. Giraldo, and F. M. Buonaguro. 2002. Induction of neutralizing antibodies and CTLs in Balb/c mice immunized with virus-like particles presenting a gp120 molecule from a HIV-1 isolate of clade A (HIV-VLPAs). Antivir. Res. 54189-201. [DOI] [PubMed] [Google Scholar]
- 12.Buonaguro, L., M. L. Tornesello, M. Tagliamonte, R. C. Gallo, L. X. Wang, R. Kamin-Lewis, S. Abdelwahab, G. K. Lewis, and F. M. Buonaguro. 2006. Baculovirus-derived human immunodeficiency virus type 1 virus-like particles activate dendritic cells and induce ex vivo T-cell responses. J. Virol. 809134-9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buonaguro, L., M. L. Visciano, M. L. Tornesello, M. Tagliamonte, B. Biryahwaho, and F. M. Buonaguro. 2005. Induction of systemic and mucosal cross-clade neutralizing antibodies in BALB/c mice immunized with human immunodeficiency virus type 1 clade A virus-like particles administered by different routes of inoculation. J. Virol. 797059-7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chehimi, J., S. E. Starr, I. Frank, A. D'Andrea, X. Ma, R. R. MacGregor, J. Sennelier, and G. Trinchieri. 1994. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J. Exp. Med. 1791361-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chougnet, C., S. S. Cohen, T. Kawamura, A. L. Landay, H. A. Kessler, E. Thomas, A. Blauvelt, and G. M. Shearer. 1999. Normal immune function of monocyte-derived dendritic cells from HIV-infected individuals: implications for immunotherapy. J. Immunol. 1631666-1673. [PubMed] [Google Scholar]
- 16.Clerici, M., and G. M. Shearer. 1993. A TH1→TH2 switch is a critical step in the etiology of HIV infection. Immunol. Today 14107-111. [DOI] [PubMed] [Google Scholar]
- 17.D'Andrea, A., M. Aste-Amezaga, N. M. Valiante, X. Ma, M. Kubin, and G. Trinchieri. 1993. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 1781041-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delchambre, M., D. Gheysen, D. Thines, C. Thiriart, E. Jacobs, E. Verdin, M. Horth, A. Burny, and F. Bex. 1989. The GAG precursor of simian immunodeficiency virus assembles into virus-like particles. EMBO J. 82653-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diehl, S., J. Anguita, A. Hoffmeyer, T. Zapton, J. N. Ihle, E. Fikrig, and M. Rincon. 2000. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity 13805-815. [DOI] [PubMed] [Google Scholar]
- 20.Diehl, S., C. W. Chow, L. Weiss, A. Palmetshofer, T. Twardzik, L. Rounds, E. Serfling, R. J. Davis, J. Anguita, and M. Rincon. 2002. Induction of NFATc2 expression by interleukin 6 promotes T helper type 2 differentiation. J. Exp. Med. 19639-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dieye, T. N., P. S. Sow, T. Simonart, A. Gueye-Ndiaye, S. J. Popper, M. L. Delforge, A. Dieye, A. D. Sarr, A. Crusiaux, J. P. Van Vooren, M. Devleeschouwer, P. Kanki, S. Mboup, L. Diakhate, and C. M. Farber. 2001. Immunologic and virologic response after tetanus toxoid booster among HIV-1- and HIV-2-infected Senegalese individuals. Vaccine 20905-913. [DOI] [PubMed] [Google Scholar]
- 22.Donaghy, H., B. Gazzard, F. Gotch, and S. Patterson. 2003. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood 1014505-4511. [DOI] [PubMed] [Google Scholar]
- 23.Dzionek, A., A. Fuchs, P. Schmidt, S. Cremer, M. Zysk, S. Miltenyi, D. W. Buck, and J. Schmitz. 2000. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 1656037-6046. [DOI] [PubMed] [Google Scholar]
- 24.Esser, R., H. von Briesen, M. Brugger, M. Ceska, W. Glienke, S. Muller, A. Rehm, H. Rubsamen-Waigmann, and R. Andreesen. 1991. Secretory repertoire of HIV-infected human monocytes/macrophages. Pathobiology 59219-222. [DOI] [PubMed] [Google Scholar]
- 25.Fan, Z., X. L. Huang, P. Kalinski, S. Young, and C. R. Rinaldo, Jr. 2007. Dendritic cell function during chronic hepatitis C virus and human immunodeficiency virus type 1 infection. Clin. Vaccine Immunol. 141127-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujii, S., K. Liu, C. Smith, A. J. Bonito, and R. M. Steinman. 2004. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J. Exp. Med. 1991607-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Pineres, A., A. Hildesheim, L. Dodd, T. J. Kemp, M. Williams, C. Harro, D. R. Lowy, J. T. Schiller, and L. A. Pinto. 2007. Cytokine and chemokine profiles following vaccination with human papillomavirus type 16 L1 virus-like particles 10. Clin. Vaccine Immunol. 14984-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gheysen, D., E. Jacobs, F. de Foresta, C. Thiriart, M. Francotte, D. Thines, and M. De Wilde. 1989. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell 59103-112. [DOI] [PubMed] [Google Scholar]
- 29.Grage-Griebenow, E., R. Zawatzky, H. Kahlert, L. Brade, H. Flad, and M. Ernst. 2001. Identification of a novel dendritic cell-like subset of CD64(+)/CD16(+) blood monocytes. Eur. J. Immunol. 3148-56. [DOI] [PubMed] [Google Scholar]
- 30.Guermonprez, P., J. Valladeau, L. Zitvogel, C. Thery, and S. Amigorena. 2002. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 20621-667. [DOI] [PubMed] [Google Scholar]
- 31.Huang, X. L., Z. Fan, B. A. Colleton, R. Buchli, H. Li, W. H. Hildebrand, and C. R. Rinaldo, Jr. 2005. Processing and presentation of exogenous HLA class I peptides by dendritic cells from human immunodeficiency virus type 1-infected persons. J. Virol. 793052-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itano, A. A., and M. K. Jenkins. 2003. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 4733-739. [DOI] [PubMed] [Google Scholar]
- 33.Kirnbauer, R., F. Booy, N. Cheng, D. R. Lowy, and J. T. Schiller. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 8912180-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi, M., L. Fitz, M. Ryan, R. M. Hewick, S. C. Clark, S. Chan, R. Loudon, F. Sherman, B. Perussia, and G. Trinchieri. 1989. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 170827-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kubin, M., M. Kamoun, and G. Trinchieri. 1994. Interleukin 12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J. Exp. Med. 180211-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Longman, R. S., A. H. Talal, I. M. Jacobson, C. M. Rice, and M. L. Albert. 2005. Normal functional capacity in circulating myeloid and plasmacytoid dendritic cells in patients with chronic hepatitis C. J. Infect. Dis. 192497-503. [DOI] [PubMed] [Google Scholar]
- 37.Ma, X., J. Sun, E. Papasavvas, H. Riemann, S. Robertson, J. Marshall, R. T. Bailer, A. Moore, R. P. Donnelly, G. Trinchieri, and L. J. Montaner. 2000. Inhibition of IL-12 production in human monocyte-derived macrophages by TNF. J. Immunol. 1641722-1729. [DOI] [PubMed] [Google Scholar]
- 38.Macatonia, S. E., R. Lau, S. Patterson, A. J. Pinching, and S. C. Knight. 1990. Dendritic cell infection, depletion and dysfunction in HIV-infected individuals. Immunology 7138-45. [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall, J. D., J. Chehimi, G. Gri, J. R. Kostman, L. J. Montaner, and G. Trinchieri. 1999. The interleukin-12-mediated pathway of immune events is dysfunctional in human immunodeficiency virus-infected individuals. Blood 941003-1011. [PubMed] [Google Scholar]
- 40.Miyanohara, A., T. Imamura, M. Araki, K. Sugawara, N. Ohtomo, and K. Matsubara. 1986. Expression of hepatitis B virus core antigen gene in Saccharomyces cerevisiae: synthesis of two polypeptides translated from different initiation codons. J. Virol. 59176-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy, E. E., G. Terres, S. E. Macatonia, C. S. Hsieh, J. Mattson, L. Lanier, M. Wysocka, G. Trinchieri, K. Murphy, and A. O'Garra. 1994. B7 and interleukin 12 cooperate for proliferation and interferon gamma production by mouse T helper clones that are unresponsive to B7 costimulation. J. Exp. Med. 180223-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noad, R., and P. Roy. 2003. Virus-like particles as immunogens. Trends Microbiol. 11438-444. [DOI] [PubMed] [Google Scholar]
- 43.Passlick, B., D. Flieger, and H. W. Ziegler-Heitbrock. 1989. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 742527-2534. [PubMed] [Google Scholar]
- 44.Piccioli, D., S. Tavarini, S. Nuti, P. Colombatto, M. Brunetto, F. Bonino, P. Ciccorossi, F. Zorat, G. Pozzato, C. Comar, S. Abrignani, and A. Wack. 2005. Comparable functions of plasmacytoid and monocyte-derived dendritic cells in chronic hepatitis C patients and healthy donors. J. Hepatol. 4261-67. [DOI] [PubMed] [Google Scholar]
- 45.Pinto, L. A., P. E. Castle, R. B. Roden, C. D. Harro, D. R. Lowy, J. T. Schiller, D. Wallace, M. Williams, W. Kopp, I. H. Frazer, J. A. Berzofsky, and A. Hildesheim. 2005. HPV-16 L1 VLP vaccine elicits a broad-spectrum of cytokine responses in whole blood. Vaccine 233555-3564. [DOI] [PubMed] [Google Scholar]
- 46.Pinto, L. A., J. Edwards, P. E. Castle, C. D. Harro, D. R. Lowy, J. T. Schiller, D. Wallace, W. Kopp, J. W. Adelsberger, M. W. Baseler, J. A. Berzofsky, and A. Hildesheim. 2003. Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles 28. J. Infect. Dis. 188327-338. [DOI] [PubMed] [Google Scholar]
- 47.Randolph, G. J., V. Angeli, and M. A. Swartz. 2005. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 5617-628. [DOI] [PubMed] [Google Scholar]
- 48.Randolph, G. J., G. Sanchez-Schmitz, R. M. Liebman, and K. Schakel. 2002. The CD16+ (FcγRIII+) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue setting. J. Exp. Med. 196517-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruedl, C., K. Schwarz, A. Jegerlehner, T. Storni, V. Manolova, and M. F. Bachmann. 2005. Virus-like particles as carriers for T-cell epitopes: limited inhibition of T-cell priming by carrier-specific antibodies. J. Virol. 79717-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruedl, C., T. Storni, F. Lechner, T. Bachi, and M. F. Bachmann. 2002. Cross-presentation of virus-like particles by skin-derived CD8(−) dendritic cells: a dispensable role for TAP. Eur. J. Immunol. 32818-825. [DOI] [PubMed] [Google Scholar]
- 51.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1791109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanchez-Torres, C., G. S. Garcia-Romo, M. A. Cornejo-Cortes, A. Rivas-Carvalho, and G. Sanchez-Schmitz. 2001. CD16+ and CD16− human blood monocyte subsets differentiate in vitro to dendritic cells with different abilities to stimulate CD4+ T cells. Int. Immunol. 131571-1581. [DOI] [PubMed] [Google Scholar]
- 53.Sporri, R., and C. Reis e Sousa. 2005. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat. Immunol. 6163-170. [DOI] [PubMed] [Google Scholar]
- 54.Stebbing, J., S. Patterson, S. Portsmouth, C. Thomas, R. Glassman, A. Wildfire, F. Gotch, M. Bower, M. Nelson, and B. Gazzard. 2004. Studies on the allostimulatory function of dendritic cells from HCV-HIV-1 co-infected patients. Cell Res. 14251-256. [DOI] [PubMed] [Google Scholar]
- 55.Steinman, R. M., and J. Banchereau. 2007. Taking dendritic cells into medicine. Nature 449419-426. [DOI] [PubMed] [Google Scholar]
- 56.Ziegler-Heitbrock, H. W., G. Fingerle, M. Strobel, W. Schraut, F. Stelter, C. Schutt, B. Passlick, and A. Pforte. 1993. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. Eur. J. Immunol. 232053-2058. [DOI] [PubMed] [Google Scholar]