Abstract
Induction of broadly cross-reactive neutralizing antibodies (NAb) is an important goal for a prophylactic human immunodeficiency virus type 1 (HIV-1) vaccine. Some HIV-infected patients make a NAb response that reacts with diverse strains of HIV-1, but most candidate vaccines have induced NAb only against a subset of highly sensitive isolates. To better understand the nature of broad NAb responses that arise during natural infection, we screened patients for sera able to neutralize diverse HIV strains and explored the frequency and phenotype of their peripheral Envelope-specific B cells. We screened 113 HIV-infected patients of various clinical statuses for the prevalence of broad NAb. Sera able to neutralize at least four of five viral isolates were found in over one-third of progressors and slow progressors, but much less frequently in aviremic long-term nonprogressors. Most Env-specific antibody-secreting B cells were CD27hi CD38hi plasmablasts, and the total plasmablast frequency was higher in HIV-infected patients than in uninfected donors. We found that 0.0031% of B cells and 0.047% of plasmablasts secreted Env-specific immunoglobulin G (IgG) in an enzyme-linked immunospot (ELISPOT) assay. We developed a novel staining protocol to label HIV-specific B cells with Env gp140 protein. A total of 0.09% of B cells were found to be Env-specific by this method, a frequency far higher than that indicated by ELISPOT assay. gp140-labeled B cells were predominantly CD27+ and surface IgG+. These data describe the breadth and titer of serum NAb and the frequency and phenotype of HIV-specific B cells in a cohort of patients with broad cross-neutralizing antibody responses that are potential goals for vaccines for HIV.
There is a growing consensus that eliciting neutralizing antibodies (NAb) will be necessary for an effective vaccine for human immunodeficiency virus (HIV). Historically, many licensed vaccines have relied on the induction of pathogen-neutralizing antibodies (reviewed in references 58 and 66). In most experimental animal model systems of viral infection, vaccine-elicited antiviral memory T cells alone have been unable to prevent infection or provide sterilizing immunity (reviewed in reference 1). Similarly, in the simian immunodeficiency virus and SHIV-nonhuman primate models of HIV, T-cell based vaccines can lower peak or setpoint viremia after challenge but have not prevented infection (19, 76, 85). In contrast, passive antibody transfer studies in nonhuman primate models of HIV infection have demonstrated that neutralizing antibodies, when present at the time of challenge, can protect from infection (43, 74, 89). Furthermore, the risk of mother-to-child transmission of HIV may be reduced by maternal neutralizing antibodies (9, 73). For these reasons, it is currently believed that the generation of HIV-specific NAb, possibly in combination with a cellular immune response, should be a major goal for candidate vaccines for HIV.
Eliciting NAb to HIV, however, has been a challenging goal. One of the major obstacles to the development of an efficacious antibody response is the extraordinary diversity of HIV Envelope (Env) (45, 46), the surface glycoprotein that is the target of neutralizing antibodies (70, 84). The structural features of HIV Env, such as flexible loops and extensive glycosylation, also provide resistance to neutralization (reviewed in reference 62). To date, HIV vaccines in clinical trials have elicited narrowly directed HIV-binding antibody with no or weak neutralizing activity against primary isolates (30, 44, 81). In contrast to vaccinees, most HIV-infected patients develop neutralizing antibodies. During the early stage of infection, the NAb response is often narrowly directed, neutralizing only autologous isolates from earlier in the infection but not those contemporaneous with the serum sample. It is believed that this phenomenon is caused by progressive immunologic escape from the neutralizing antibody response (2, 4, 53, 69, 83). In addition, most sera from infected patients neutralize laboratory isolates but typically not the majority of the more difficult to neutralize heterologous primary isolates. However, over time some patients make broadly cross-reactive antibodies that are able to neutralize viruses of diverse lineages, even across clades (10, 55). Understanding the mechanism of cross-neutralization and the generation of these antibodies may provide information that is crucial for the development of effective immunogens.
Several aspects of broadly cross-neutralizing HIV-specific antibody responses in infected patients remain poorly understood. First, the clinical characteristics of patients with a broad NAb response remain unclear. Few studies have screened large numbers of patients for a broad NAb response or compared patients with various clinical courses. In addition, studies have yielded variable results, using diverse assay platforms, viral isolates, definitions of breadth, and classifications of patient clinical status (10, 17, 18, 20, 55, 63, 64). Efforts to standardize neutralization assays across laboratories have led to the wide use of luciferase-based assays with standard panels of pseudovirus isolates (42, 68). Several studies using this format have examined the cross-reactivity of monoclonal antibodies (14) and pooled sera of different clades (15), but the level of breadth in individual patient sera has not been well described with the current, standardized assays.
Second, the frequency and phenotype of HIV-specific B cells in patients with broadly cross-neutralizing antibodies is unknown. HIV+ patients exhibit many altered B-cell characteristics compared to uninfected donors, including increased spontaneous immunoglobulin G (IgG) secretion (37), increased expression of markers of activation and terminal differentiation, and a decreased proportion of circulating memory cells (reviewed in reference 50). However, the frequency, phenotype, specificity, and immunoglobulin class of HIV-specific B cells have not been well defined. Enzyme-linked immunospot (ELISPOT) assays allow enumeration of HIV-specific antibody-secreting cells (ASC) (27, 57, 75); however, the assay does not allow recovery of the HIV-specific cells for later characterization or cloning. Furthermore, to date no reagent has been available to allow marking of individual HIV-specific cells; thus, the phenotypic characteristics of Env-specific B cells have remained unknown.
In the present study, we evaluated NAb breadth in a set of 113 patients and assembled cohorts of patients with or without broadly cross-reactive NAb. Cells from these patients were used to analyze the phenotype and frequency of Env-specific B cells. The plasmablast subset (CD3− CD19+ CD20−/lo CD27hi CD38hi) (88) was found to be increased in HIV-infected donors compared to uninfected controls. In addition, the vast majority of HIV Env-specific ASC were found within this plasmablast subset. Labeling Env-specific B cells permitted an examination of their frequency, immunoglobulin class, and phenotype. Importantly, the frequency of gp140-labeled B cells was an order of magnitude greater than the frequency measured on the basis of ASC in ELISPOT assays. These results describe the neutralizing antibody response in a cohort of patients screened for broadly cross-neutralizing antibodies and provide a characterization of peripheral blood B cells and HIV-specific B cells of these patients. The frequency of the B cells of these patients and the breadth of neutralization demonstrate what is possible for the humoral response to HIV to achieve and are potentially important goals for the induction of neutralizing antibodies in vaccinees.
MATERIALS AND METHODS
Study participants.
Patients in the present study signed informed consent and participated in NIAID protocols at the National Institutes of Health, Bethesda, MD. HIV infection was documented by HIV1/2 immunoassay. The study included 113 HIV-infected patients who were classified as long-term nonprogressors (LTNP), slow progressors, or progressors. LTNP were defined as having set point plasma viral RNA levels of <50 copies/ml (or below the available limit of detection at the time of sampling), stable CD4 T-cell counts, and no opportunistic infections in the absence of antiretroviral (ARV) treatment. Slow progressors were defined as having a detectable viral load, a stable CD4 T-cell count above 400 cells/μl, being diagnosed with HIV for at least 7 years, and off ARV treatment for at least 5 years. All other patients were classified as progressors (those off of ARV treatment for <5 years, diagnosed with HIV-1 <7 years prior to sampling, and/or with CD4+ T-cell counts declining and/or <400 cells/μl). All had been infected for at least 1 year. Some of the patients have been previously described (40, 48). All patients were presumed to be infected with clade B virus based on the locations of current and former residences. Phlebotomy and leukapheresis were performed at time points during which patients were not using ARVs. An additional 35 HIV-1-seronegative donors were recruited from the National Institutes of Health donor apheresis clinic.
Storage of samples.
Serum was stored at −80°C. Peripheral blood mononuclear cells (PBMC) were purified from leukapheresis packs using Ficoll density centrifugation with lymphocyte separation medium (MP Biomedicals, Solon, Ohio). PBMC were frozen in Recovery cell culture freezing medium (Gibco, Carlsbad, CA) using a CryoMed controlled-rate freezer (ThermoForma, Waltham, MA) and stored at −140°C. PBMC were thawed in media containing complete RPMI with 10% fetal calf serum (FCS; Gibco) and 50 U/ml of benzonase (Novagen, Darmstadt, Germany) and rested overnight in RPMI with 10% FCS at 106 cells/ml.
Flow cytometry.
Multicolor flow cytometry was performed by using standard protocols (33) using the antibodies CD3 (fluorescein isothiocyanate or Pacific Blue), CD14 (Pacific Blue), CD19 (phycoerythrin [PE]-Cy7), CD20 (allophycocyanin [APC]-Cy7), CD27 (PE), CD38 (APC), and IgG (PE-Cy5) (BD Biosciences, San Jose, CA); IgA (APC) and IgM (PE) (Jackson Immunoresearch, West Grove, PA); and Qdot605-streptavidin (Invitrogen, Carlsbad, CA). All stainings were done at 4°C for 30 min. Plasmablasts were identified with a panel including CD3, CD19, CD20, CD27, and CD38, with the addition of CD14 when gp140 labeling was performed (see below). The data was collected on a FACSAria or an LSR II flow cytometer using FACSDiva software (BD Biosciences), and cell sorting was conducted on a FACSAria. Color compensation was performed using single-stained samples for each fluorochrome used in addition to an unstained control. The data were further analyzed by using FlowJo software (TreeStar, Cupertino, CA). A minimum of 2,000,000 events was collected for each patient sample.
Virus neutralization assays.
Thawed patient serum was heat-inactivated at 56°C for 30 min prior to assay. The TZM-bl assay was used as previously described (77). Briefly, pseudovirus stocks were prepared by transfection of 293T cells with an env-deficient backbone (pSG3ΔEnv) (82) and an expression plasmid for the env gene of interest. The env genes, all of which were cloned from CCR5-using primary isolates, were previously described (40). Serum was diluted in Dulbecco's modified Eagle medium-10% FCS (Gibco) and mixed with pseudovirus. After 30 min, 10,000 TZM-bl cells were added, and the plates were incubated for 48 h. Assays were developed with a luciferase assay system (Promega, Madison, WI), and the relative light units (RLU) were read on a luminometer (Perkin-Elmer, Waltham, MA). The percent neutralization was calculated as follows: % neutralization = 100 × (Vo − Vn)/Vo, where Vn is the RLU in the virus and antibody wells and V0 is the RLU in the virus-only wells. A neutralization dose-response curve was fit by nonlinear regression using a four-parameter hill slope equation programmed into JMP statistical software (SAS Institute, Inc., Cary, NC). The reciprocal dilution at which 50% of the virus is neutralized (ID50) is reported for all virus-serum pairs. Based on background levels in sera from uninfected donors and titers against a control pseudovirus using murine leukemia virus Env, the cutoff for neutralization was set at an ID50 of 100.
B-cell ELISPOT assay.
MultiScreen-IP plates (Millipore, Billerica, MA) were washed twice with 30% ethanol, rinsed three times with phosphate-buffered saline (PBS), and then coated with 100 μl of antigen in PBS at 4°C overnight. The antigens included 2.5 μg/ml anti-kappa light chain plus 2.5 μg/ml anti-lambda light chain (Rockland, Gilbertsville, PA) (anti-Ig), 10 μg/ml HIV-1 YU2 Env gp120 (produced in S2 cells and purified over a 17b affinity column), and 5 μg/ml keyhole limpet hemocyanin (Sigma, St. Louis, MO). Plates were washed six times with PBS and blocked with RPMI with 10% FCS for 2 h at room temperature. Rested PBMC or sorted B cells were plated in at least 100 μl of medium and incubated overnight at 37°C. The following morning, plates were washed six times in PBS with 0.25% Tween 20 (Sigma) (PBS-T) and incubated with 100 μl of 1:10,000 biotin-IgG (Jackson Immunoresearch) for 1 h at room temperature. Plates were then washed and incubated with 100 μl of 1:60 streptavidin-AP (R&D Research Systems, Minneapolis, MN) for 1 h at room temperature. The plates were then washed with PBS-T, PBS, and dI water and developed with 100 μl of BCIP/NBT (R&D Research Systems) for 20 to 30 min at room temperature, and the reaction was stopped with dI water. Spot quantitation was performed by using a CTL ELISPOT reader (Cellular Technology, Ltd., Cleveland, OH) with manual correction as needed. The average number of spots in keyhole limpet hemocyanin wells was subtracted from the average number of spots in anti-immunoglobulin or gp120 wells when the spots per well was calculated.
gp140 stain.
Biotinylated, trimeric, cleavage-defective gp140 (biotinylated gp140-F trimer) was used for staining patient B cells. It was derived from gp140Δ683(−/FT), which consists of HIV-1 YU2 Env amino acids 1 to 683 fused to the T4 phage fibritin trimerization domain (as described in reference 91). The gp140Δ683(−/FT) sequence was modified by the addition of the sequence encoding the Avitag signal for biotinylation (LNDIFEAQKIEWHE) at the 3′ end of the gene and subcloned into pCDNA3.1(−). The resulting plasmid was used to transfect 293 freestyle cells at a density of 1.2 × 106/ml using 293 fectin (Invitrogen) according to the manufacturer's protocol. After 4 days in culture in shake flasks at 37°C, the supernatant containing secreted proteins was collected by centrifugation at 3,500 rpm for 30 min. The gp140-F trimers were purified by lentil lectin affinity chromatography, followed by chelation chromatography over a Ni-charged column (GE Healthcare, Piscataway, NJ). Biotin ligase Bir A (Avidity, Denver, CO) was used to biotinylate the protein at the Avitag sequence only, distal to the relevant epitopes. Biotinylation of the gp140-F trimers was confirmed by enzyme-linked immunosorbent assay using streptavidin-horseradish peroxidase (Sigma). The antigenic structure was not affected by biotinylation, as demonstrated by the binding of monoclonal antibodies b12, F105, and 17b with or without soluble CD4 (unpublished data).
For staining with biotinylated gp140-F trimers, cells were thawed and rested overnight as described above and washed in PBS with 0.5% bovine serum albumin (BSA; Sigma) and 0.5 mM EDTA (Quality Biologicals, Gaithersburg, MD). A total of 50 × 106 to 150 × 106 cells in 50 μl were blocked using unlabeled anti-CD4 antibody (clone SK3; BD Biosciences) at 0.5 mg/ml in PBS-0.5% BSA-0.5 mM EDTA (5) for 15 min at 4°C. Then, 8 μg of biotinylated gp140 was added, and the cells were incubated for 30 min at 4°C. The cells were washed twice in PBS-0.5% BSA-0.5 mM EDTA, stained with Qdot605-streptavidin (Invitrogen) for 30 min in the presence of cell surface antibodies at 4°C, and then washed again. The data were collected on a FACSAria or an LSR II flow cytometer (BD) and analyzed with FlowJo software. The Qdot605-streptavidin+ gate was positioned such that the positive population in cells stained with Qdot605-streptavidin only (no protein) was 0.01% of the CD19+ B cells. The same gate was used for the corresponding gp140-labeled sample from the same patient in order to exclude background staining.
Statistical analysis.
The Wilcoxon two-sample test was used to compare independent groups. Paired data were compared by using the Wilcoxon signed rank test. Correlations were determined by using the Spearman rank method. The frequencies of not-broad neutralizing antibodies in LTNP versus all viremic patients were compared by using the Fisher exact test. The Bonferroni method was used to adjust P values for multiple testing.
RESULTS
Initial screen for broad cross-neutralizing sera.
In order to better understand the spectrum of breadth of neutralization of HIV-1 in infected patients using current standardized assays, we evaluated sera from a large cohort of HIV-infected patients (n = 113) with a variety of clinical characteristics. Table 1 shows the median and range for each patient group for CD4+ T-cell count, viral load, and number of years since HIV diagnosis at the time of screening. Twenty-four patients were LTNP, also called elite controllers, from the cohort described previously (47, 48); they control viremia without the use of ARVs to <50 copies/ml of plasma, with a median CD4+ T-cell count of 964 cells/μl. Thirty-seven patients were classified as slow progressors (defined as having a stable CD4+ T-cell count of ≥400 cells/μl, no ARV within 5 years, and infected ≥7 years). The remaining 52 patients were classified as progressors (those patients with <400 CD4+ T cells or declining, and/or having taken ARVs within 5 years of screening, and/or diagnosed 1 to 7 years prior to screening). All patients were off of ARV at the time of screening.
TABLE 1.
Clinical characteristics of patient groups
| Patient group | No. of patients | Median (range)
|
||
|---|---|---|---|---|
| Viral RNA copies/ml plasma | No. of CD4+ T cells/μl | No. of yr since diagnosis | ||
| LTNP | 24 | <50 (<50-1,000) | 967 (371-1,813) | 10 (3-22) |
| Slow progressor | 37 | 1,665 (76-54,723) | 663 (402-1,069) | 15 (6-23) |
| Progressor | 52 | 18,531 (101-304,718) | 424 (185-948) | 7 (1-21) |
For the purpose of screening for breadth of neutralization, we used a TZM-bl cell line-based HIV neutralization assay (42, 77) with panels of isolates from clades A, B, and C. All assays used pseudoviruses made with primary isolate env genes. The initial screen of 113 sera was performed with a five-isolate minipanel, and the findings were validated for a subset of sera (n = 42) with an extended panel of 20 isolates from clades A, B, and C (see below). The number of isolates neutralized in the set of five correlated well with the number neutralized in the set of 20 (r = 0.84, P < 0.001). The minipanel consisted of JRFL (clade B), CAAN.A2 (clade B), THRO.18 (clade B), Q168.a2 (clade A), and ZM106.9 (clade C). In a previous analysis with a subset of these sera, JRFL and Q168.a2 were moderately sensitive, CAAN.A2 and THRO.18 were moderately resistant, and ZM106.9 was highly resistant to neutralization by patient sera (40).
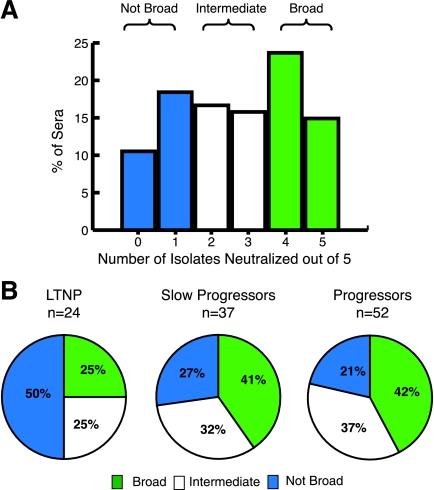
Using the minipanel, we found a range of neutralization breadth among the 113 sera. As shown in Fig. 1A, 38% of sera were able to neutralize four or five of five isolates and were classified as broad; 33% of sera were able to neutralize two or three of five isolates and were classified as intermediate; and 29% of sera neutralized zero or one of five isolates and were classified as not broad. Broad sera were found in all patient groups (Fig. 1B). There was less breadth in the LTNP group, with 50% of the LTNP sera classified as not broad compared to 27 and 21% in slow progressors and progressors, respectively (P = 0.04, Fisher exact test). Conversely, only 25% of the LTNP sera were classified as broad compared to >40% in each of the viremic groups.
FIG. 1.
Distribution of broadly cross-reactive sera. (A) Percentages of 113 sera that neutralize the indicated number of isolates. Sera that neutralize none or one of five are classified as not broad (blue), two or three of five as intermediate (white), and four or five of five as broad (green). (B) Percentages of broad, intermediate, and not-broad sera in LTNP, slow progressor, and progressor patient groups (colors are as defined in panel A).
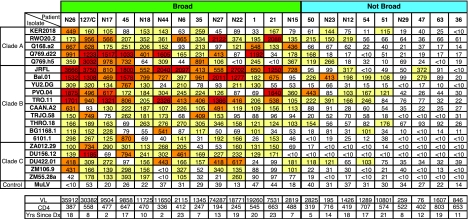
We then sought to further extend the characterization of the neutralizing antibody response. Using the expanded panel of 20 isolates, we determined the serum titers from a subset of patients that had been characterized as broad or not broad in the initial screen. Isolates from clades A, B, and C with a range of neutralization sensitivities (38-40) were included. Figure 2 shows the ID50 neutralization titers for the expanded panel, along with the clinical characteristics of 22 patients. Nine of the sera were remarkably broad, neutralizing fifteen or more isolates, including most of the clade A and C viruses. Patients in the broad group had higher plasma viral RNA compared to those in the not-broad group (median, 11,650 versus 1,289 RNA copies/ml, P = 0.003), while the CD4 count and years since diagnosis with HIV did not correlate with breadth (P = 0.2 and P > 0.5, respectively).
FIG. 2.
Clinical characteristics and neutralization data for patients in B-cell analysis. Each column shows data from a single patient. In the top panel, each row shows the ID50 of serum against the indicated virus isolate. ID50 values: white, <100; yellow, 100 to 400; orange, 401 to 1,000; red, >1,000. In the bottom panel, abbreviations are defined as follows: VL, viral load in copies/ml; CD4, CD4+ T cells/μl; Yrs Since Dx, years since HIV diagnosis. Clinical data are from the same time point or within 3 months of the serum sample. Broad and not-broad categories are shown as classified for Fig. 1.
We next characterized the frequency and phenotype of B cells in the 13 patients with broad sera, 9 with not-broad sera, 8 additional HIV-infected patients, and 18 uninfected donors. Patient sera were classified as broad or not broad based on the five-virus minipanel. When the cells of all 30 patients were examined, the frequency of B cells in lymphocytes was lower in patients than in uninfected controls, with median frequencies of 6.0 and 15.8%, respectively (P < 0.001). No significant differences emerged between patients with or without broad NAb in the frequency of B cells or IgG+ B cells (data not shown). The levels of CD19+ CD27+ memory B cells were somewhat lower in the patients with broad sera relative to not broad sera (median 20% of B cells versus 35%), although this trend did not reach statistical significance. CD19+ CD27+ frequency showed a weak but significant negative correlation with viral load (r = −0.49, P = 0.02) when all patients were analyzed.
HIV-specific ASC.
In order to examine the phenotype of HIV-specific B cells, we first searched for B-cell subsets that are enriched for cells actively secreting Env-specific IgG. We hypothesized that plasmablasts would be such a population, since they have been shown to represent most of the ASC in the peripheral blood of uninfected donors and are increased in frequency during the acute response to vaccination (60, 88). We therefore examined whether the plasmablast subset is also increased in chronic HIV infection. Figure 3A shows typical staining for plasmablasts, defined as: CD3− CD19+ CD20−/lo CD27hi CD38hi (60, 88). We found that HIV-infected patients had a significant increase in plasmablast frequency compared to uninfected donors, with medians of 1.6 and 0.46% of B cells, respectively (P = 0.001) (Fig. 3B). The fraction of plasmablasts that were surface IgG+ was also higher in HIV-infected patients (median of 32.3% versus 21.3%, P = 0.04). The plasmablast frequency was not different between the broad and not-broad groups and did not correlate with viral load or CD4+ T-cell count (data not shown).
FIG. 3.
The plasmablast frequency is increased in HIV-infected patients. (A) Flow cytometric analysis of plasmablasts. A representative sample is shown. Plasmablasts are CD3− CD19+ CD20−/lo CD27hi CD38hi. The large gate in the forward-scatter/side-scatter plot was set to include plasmablasts based on backgating. (B) Plasmablasts as a percentage of CD19+ lymphocytes in HIV-positive patients and uninfected donors. Horizontal bar, median value.
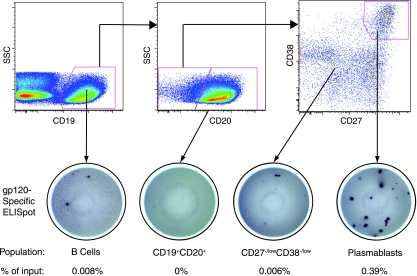
Using a B-cell ELISPOT assay, we measured the cells that actively secrete IgG or HIV Env-specific IgG. HIV gp120 was used as the target antigen on ELISPOT plates; trimeric gp140 gave equivalent results to gp120 (data not shown). One might expect to find differences in the responses to these two proteins since a gp120 ELISPOT assay does not detect cells specific for gp41 and an ELISPOT assay using intact gp140 trimer is unable to detect gp120 epitopes that are buried in the trimer. It is possible that increases in detection of gp41-specific cells with the gp140 protein are offset by the loss of inner binding sites on gp120. Alternatively, the fraction of the total envelope response comprised by these specificities is below the limit of detection in our assay. In this assay, 0.07% of unstimulated PBMC secreted IgG (Table 2). The overall frequency of gp120-specific IgG ASC varied considerably between patients, with a median of 2.5 per 106 PBMC (range, 0 to 17 per 106, n = 15), a finding similar to the range reported by other groups (57, 75). We next purified B cells and B-cell subpopulations by fluorescence-activated cell sorting and evaluated the sorted cells by ELISPOT assay. A total of 0.0031% of B cells secreted anti-gp120 IgG. Cells secreting anti-gp120 IgG were largely limited to the plasmablast population (Fig. 4): a median of 58% of IgG ASC and up to 92% of the gp120-specific ASC were plasmablasts. The median frequency of anti-gp120 IgG ASC in 11 patients was 0.047% of total plasmablasts and 0.49% of IgG-secreting plasmablasts (Table 2). The frequency of gp120-specific IgG ASC in PBMC or in plasmablasts did not correlate with viral load, CD4 count, or years since diagnosis at the time of sampling. Likewise, no differences were noted between broad and not-broad cohorts for the frequencies of ASC.
TABLE 2.
Frequencies of ASC in different cell populations
| Cell type | Median (range)
|
||
|---|---|---|---|
| % IgG secretinga | % gp120 specificb | gp120-specific as % of IgG secretingc | |
| PBMC | 0.066 (0.021-0.18) | 0.00025 (0-0.0017) | 0.47 (0-2.4) |
| B cells | 0.71 (0.22-1.2) | 0.0031 (0.0013-0.0088) | 0.22 (0.58-1.1) |
| Plasmablasts | 20 (3.0-32) | 0.047 (0-0.47) | 0.49 (0-2.2) |
Percentage of input cells secreting IgG in an ELISPOT assay.
Percentage of input cells secreting anti-gp120 IgG.
Frequency of gp120-specific/frequency of IgG secreting × 100.
FIG. 4.
gp120-specific ASC are plasmablasts. The top panels present representative flow cytometry data showing gating strategy for plasmablasts. The bottom panels show representative anti-gp120 IgG ELISPOT wells from the indicated populations. Input cell numbers: CD19+ well, 30,000; CD19+ CD20+ well, 200,000; CD27−/lo CD38−/lo, 25,000; plasmablasts, 4,000.
We were unable to immortalize sorted plasmablasts by infection with Epstein-Barr virus (EBV). Seven attempts using a high-titer EBV stock to infect plasmablasts yielded no transformants, whereas nonplasmablast, CD20−/lo B cells from the same samples were consistently transformed (data not shown). This was not due to cell death during sorting, since the sorted plasmablasts survived to secrete IgG in the ELISPOT assay. Nor was it a problem with receptor expression, since most plasmablasts expressed CD21, the main receptor for EBV (data not shown).
Frequency and phenotype of envelope-specific B cells.
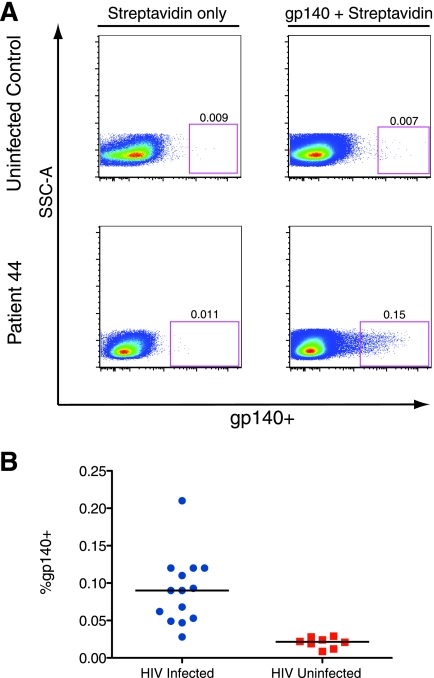
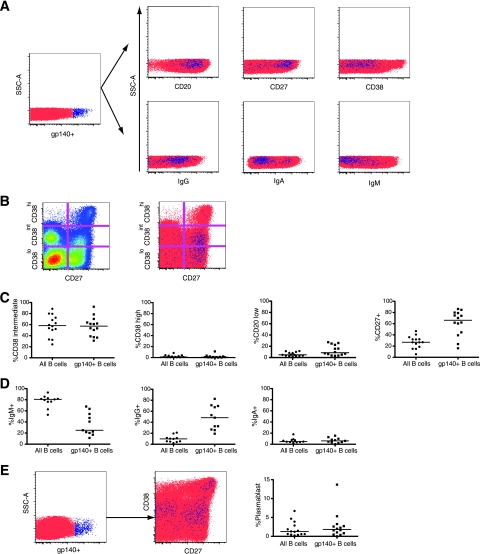
In order to directly analyze the frequency and phenotype of HIV-specific B cells by flow cytometry, we developed a protocol for staining B cells with a recombinant HIV Env protein, biotinylated gp140-F trimer (hereafter referred to as gp140). This method allows the measurement of total Env-specific cells. Trimeric gp140 was chosen because it better reflects the conformation and epitopes of the native Env spike than monomeric gp120 (90, 91). Although the ELISPOT assay, described above, measures the cells that are actively secreting anti-Env IgG (mainly plasmablasts), it does not account for the large fraction of peripheral antigen-experienced B cells that are not actively secreting antibody. The ELISPOT assay therefore was likely to underestimate the frequency of total Env-specific B cells. Cells were stained with biotinylated gp140 and detected with streptavidin-Qdot605. Figure 5 shows representative examples of staining CD19+ cells with gp140. To validate the specificity of staining, we compared gp140-labeled B cells in HIV-infected patients to labeled cells from uninfected donors. Background levels were assessed on cells stained with streptavidin-Qdot605 only. The Qdot605-Streptavidin+ gate was positioned such that the positive population in cells stained with Qdot605-streptavidin only (no protein) was 0.01% of the CD19+ B cells. The same gate was used for the corresponding gp140-labeled sample from the same patient. The level of binding to B cells of HIV-uninfected controls was similar to the background due to streptavidin-Qdot605 alone (median of 0.021% [range, 0.008 to 0.029%] in eight donors). B cells labeled with gp140 were clearly visible in HIV-infected patients (Fig. 5A). The median frequency of gp140 binding to B cells of 14 HIV-infected patients was 0.09% (range, 0.028 to 0.21%) (Fig. 5B). The frequency of gp140-labeled B cells in individual patients did not correlate with breadth of NAb, viral load, CD4 count, or years since HIV diagnosis at the time of sampling. Since the recombinant Env proteins used in both the ELISPOT assay and B-cell staining were derived from HIV-1 strain YU2, we compared neutralization titers against HIV-1 YU2 to data from both assays and did not find significant correlations (data not shown).
FIG. 5.
gp140 specifically stains Env-specific B cells. (A) PBMC were labeled with biotinylated gp140-F trimer (right panels) or buffer only (left panels) and later stained with Qdot605-streptavidin, along with antibodies for lymphocyte markers. Panels show CD3− CD14− CD19+ cells. The gate shows gp140-labeled cells and is set so that ∼0.01% of cells are positive in the streptavidin-only sample for each patient. Representative plots of an uninfected control (top) and an HIV-infected patient (bottom) are shown. (B) Percent of B cells that are labeled with gp140+ in HIV-infected patients and uninfected controls.
We then examined the phenotype of Env gp140-specific peripheral B cells and compared the results to total B cells (Fig. 6A to C). Surface expression of the activation marker CD38 was highly variable between patients and, overall, CD38intermediate and CD38hi cells were present among gp140-labeled cells at frequencies close to those of total B cells in each patient. A total of 64% of gp140-labeled B cells expressed the memory marker CD27 compared to 25% of all CD19+ B cells. Conversely, the frequency of gp140-labeled cells was 0.17% (range, 0.08 to 0.41%) in the CD19+CD27+ cells, higher than in total B cells (0.09%). Although it has been shown in humans that all CD19+ CD27+ cells are memory cells, not all memory B cells are CD27+ (25, 26). We therefore used surface immunoglobulin class as an indicator of switched memory phenotype (Fig. 6D). gp140-labeled B cells were highly enriched for surface IgG compared to total B cells, with a median 9.7% of total B cells but 48% of gp140-labeled B cells expressing surface IgG, and a concomitant reduction of surface IgM+ cells (medians of 81% versus 25%, respectively). The surface IgA frequency was 6.0% in the gp140-labeled cells, a level similar to that in total B cells. Together, these data indicate that the majority of gp140-labeled B cells were class-switched memory cells. Total gp140-labeled B cells were 50-fold more frequent and IgG+gp140+ B cells were 15-fold more frequent than gp120-specific ASC within B cells of the same patients, indicating that the ELISPOT assay underestimates the true frequency of Env-specific B cells. The frequency of gp140-specific plasmablasts was also examined (Fig. 6E). A median of 1.8% of gp140-labeled B cells were plasmablasts, similar to total B cells. A total of 0.05% of plasmablasts stained positive for gp140, while 0.07% of the plasmablasts from the same patients secreted anti-Env gp120 antibody in the ELISPOT assay. Thus, although the ELISPOT assay underestimated the frequency of total HIV-specific B cells, the numbers of Env-specific cells within the plasmablasts were concordant when measured by gp140 staining or by ELISPOT assay.
FIG. 6.
Frequencies of B cells and gp140-labeled B cells expressing surface markers. (A) Left panel, CD3− CD19+ cells from a representative patient. Right panels, CD3− CD19+ cells of the same sample stained for B-cell markers. gp140-labeled cells (blue) are shown on the background of all B cells (red). Totals of 1.8 × 105 to 3.4 × 105 gated events are shown. (B) Gating strategy for CD27 and CD38. Left panel, CD3− CD19+ cells from a representative patient. Right panel, gp140-labeled cells (blue) on the background of total B cells (red). (C and D) Percent of cells of the indicated phenotype in total B cells or gp140-labeled B cells. Horizontal bars, medians. (E) Left panel, CD3− CD19+ cells from a representative patient. Center panel, CD3− CD14− CD19+ CD20−/lo cells of the same sample. The CD27hi CD38hi cells are plasmablasts. gp140-labeled cells (blue) are shown on the background of other B cells (red). A total of 4.7 × 105 gated events are shown. Right panel, percentage of B cells or gp140 labeled B cells that are plasmablasts.
DISCUSSION
The study of broadly cross-reactive NAb and the cells that make them is a critical line of research for HIV vaccine development. In the present study, we assembled cohorts of patients with or without broadly cross-reactive neutralizing antibodies and explored the frequency and phenotype of Env-specific B cells in these patients. The prevalence of patients with broadly cross-neutralizing sera was 38%. In addition, several sera showed extraordinary breadth, neutralizing most primary isolates studied from clades A, B, and C. Investigations of the B cells from these patients showed that the HIV Env-specific ASC frequency was not different between patients with or without broad NAb and that the plasmablast B-cell subset contained the majority of active Env-specific ASC. We have also developed a staining technique that, for the first time, allows flow cytometric analysis of individual Env-specific B cells. Measurements of the total Env-specific B-cell population showed that their frequency was 50-fold larger than estimates based upon ASC in ELISPOT assays.
The prevalence of patients with broadly cross-neutralizing antibodies was greater than that observed in prior studies. A total of 42 of the 113 patient sera in the present study could neutralize four or five out of five isolates, and >70% could neutralize two or more. An extended analysis (Fig. 2) showed that nine extremely broad sera were able to neutralize at least 15 of 20 isolates, including most of the neutralization-resistant and non-clade B viruses. In the present study, a broadly cross-neutralizing antibody response was much less common among LTNP (elite controllers) than viremic patients, a finding that is consistent with other recent observations (8, 63). We also found that the progressor and slow progressor groups did not differ in the proportion of patients with breadth (Fig. 1B), which is similar to recent findings in a different cohort (63). Earlier studies, using different assays and patient group definitions, found more broad NAb in the sera of slow progressors (16, 18, 20, 52, 64, 93), although these comparisons were made to patients with severe CD4+ T-cell depletion and a global decline in immunity. Some previous studies using a PBMC-based assay with multiple rounds of viral replication have found at least some cross-neutralization of primary isolates within clade B in more than half of chronically infected patients (18, 53), but others found a much more limited prevalence of breadth when isolates of non-B clades were included (11, 55). The high proportion of sera able to neutralize multiple heterologous and non-B primary isolates in the present study implies that breadth is more common than previously thought. It remains possible that this finding is due to differences between the PBMC-based assay and the cell-line based TZM-bl assay. Indeed, discordant results have been reported using the same virus-serum pairs in both assays (14), although the TZM-bl assay does not always give higher titers (68). Ultimately, the relevance of the neutralization breadth and titer measured in this assay can only be confirmed by in vivo studies of passive transfer and vaccine efficacy trials. However, if the same assay platform is used in vaccine clinical trials, then the data presented here set a standard to which vaccine samples can be compared.
These results confirm and extend prior work that indicated that it is possible for the human B-cell response to generate cross-neutralizing antibodies and potentially set a goal for immunization. Early candidate vaccines focused on eliciting antibodies using recombinant Env proteins, but the antibodies made by vaccinees had neutralizing activity only against laboratory strains, not primary isolates (29, 44), and the vaccines had no protective efficacy (65). A recent phase I study of DNA prime, recombinant Env gp120 boost regimens found weak neutralizing antibody with modest breadth (81); the titers were an improvement over other previous studies, but much less than the levels we found in most patients using the same assays. By measuring the levels and the breadth of NAb in patients, we describe what is possible for the immune system to achieve, and these results may provide some reference for the level of breadth and titer that is potentially achievable in vaccinees.
The development of broadly cross-neutralizing antibodies may be partially dependent on ongoing exposure of B cells to HIV antigen. Half of the aviremic LTNP had sera classified as not broad, neutralizing only one or none of the five test isolates. In other cohorts of LTNP, autologous neutralizing activity was found to be very low (7) and heterologous neutralization was rare (63). Among the viremic patients studied here, viral load had a weak, positive association with breadth (P = 0.003), similar to some previous observations (23). ARV treatment can lead to declines in both neutralizing activity (7) and HIV-specific ASC (57). Collectively, these findings support a link between prolonged exposure to viral antigen and the development of cross-reactive NAb. However, antigen exposure is not always predictive of breadth, since some patients failed to develop broad NAb even after 20 years of untreated viremia (Fig. 2), while some aviremic LTNP did develop broad NAb (Fig. 1B). Thus, viremia is likely an important but not exclusive driving force in the development of broad NAb.
We also explored the frequency and phenotype of ASC in patients with or without broadly cross-neutralizing antibodies. A population of particular interest was CD19+ CD20−/lo CD27hi CD38hi plasmablasts, which are derived from memory B cells, as shown by their extensively mutated immunoglobulin genes (88). Upon activation by antigen or within days of vaccination, memory B cells proliferate and differentiate into plasmablasts, which appear at high frequencies and are the major ASC in the peripheral blood (60, 88). Most of these cells die by apoptosis, while some traffic to bone marrow or inflamed tissue and become long-lived plasma cells that maintain continuous production of antibodies (reviewed in references 32 and 87). Plasmablasts are also increased during episodes of active systemic lupus erythematosus (59), infectious mononucleosis (49), and acute rotavirus infection (34) and appear at a higher frequency in the cerebrospinal fluid of HIV-infected patients compared to controls (21). We show here that in the setting of chronic HIV infection, plasmablast frequency in the blood is significantly higher in patients than in uninfected donors (1.6% versus 0.46%, P = 0.001). In addition, plasmablasts accounted for most of the ASC in HIV+ patient PBMC. Although Env-specific ASC were found to be almost entirely contained within this population, there was no relationship between the frequency of ASC or Env-specific plasmablasts and the development of a broadly cross-neutralizing antibody response. Although viral load had a positive association with NAb breadth, CD4+ T-cell counts, years since diagnosis, and the other B-cell subsets examined did not correlate with broad NAb in these patients.
The observation that the majority of cells actively secreting anti-Env antibodies are plasmablasts has implications for the generation of Env-specific monoclonal antibodies. Since the cells that actively secrete anti-Env gp120 IgG are plasmablasts, we attempted to generate immortalized cell lines from sorted plasmablasts by EBV transformation but were unable to do so in seven attempts. Prior work has suggested that this difficulty is caused by lytic infection of highly activated B cells. Immortalization of B cells by EBV requires latency, as opposed to lytic replication. The EBV gene BZLF-1 switches viral replication to the lytic phase, and there is both direct and indirect evidence for its upregulation in plasmablasts. Plasmablasts express XBP-1 (6), a B-cell transcription factor that transactivates BZLF-1 (12, 79), and it has been directly shown that BZLF-1 is upregulated in CD38hi B cells (36). EBV transformation alone will not capture the ASC, such as plasmablasts, that may contain the specificities contributing to neutralization. This may explain difficulties encountered by many laboratories in cloning neutralizing antibodies against HIV via EBV immortalization. Single-cell PCR and cloning of B-cell antigen receptors of sorted plasmablasts (88) or gp140-stained cells allows the immortalization step to be bypassed and thus may be a viable alternative method for obtaining monoclonal antibodies from these cells.
Use of a technique that permits direct staining of Env-specific B cells permitted an investigation of their frequency and phenotype. Cells actively secreting antibody make up only a subset of all antigen-experienced B cells, and therefore ELISPOT assays are likely to underestimate the frequency of total Env-specific B cells. Furthermore, since cells are destroyed during the assay, we could not recover the HIV-specific cells from the ELISPOT analyses for later characterization or cloning. For these reasons, we developed techniques to measure the total frequency of Env-specific B cells using direct staining by a labeled Env protein, biotinylated gp140-F trimer. We observed that gp140-labeled B cells are mainly but not entirely CD27+ and half express surface IgG. The proportion that express IgM was low but variable. The majority of these cells are most likely antigen-experienced cells, given that they were not found above the limit of detection in uninfected controls and are consistent with the large fraction of human memory cells that are IgM+ (3, 80). Unexpectedly, the frequencies of CD38intermediate and CD38hi B cells and of plasmablasts were not different in gp140-labeled cells compared to total B cells, suggesting that Env-specific cells are not more activated than those of other specificities during chronic viremia. In addition, no differences were found in these surface phenotypes between patients with or without broad NAb. However, it should be noted that these surface markers may not reliably discriminate functional memory or activated B cells. CD27− memory B cells have been described in normal donors (25, 26, 86), and a population of CD27− memory B cells that are specific for HIV in an ELISPOT assay was recently described (51). Likewise, the expression of the activation marker CD38 can be highly heterogeneous (71). The use of these markers to distinguish B-cell activation and memory subsets is still being refined (71, 80).
The frequency of B cells staining with Env gp140 was considerably higher than the frequency of Env-specific ASC observed. The ELISPOT assay, which did not include an in vitro activation step, only measured cells that were actively secreting antibody, and not all HIV-specific cells would be expected to be ASC at any given time. Thus, the ELISPOT data greatly underestimated the number of Env-specific B cells. This was not simply due to differences in the techniques of measurement. Previous studies using an ELISPOT assay to measure HIV-specific B cells in unstimulated PBMC found frequencies similar to those reported here (57, 75). In vitro stimulation of B cells might be expected to allow measurement of more of the HIV-specific cells, but in one study using this approach the frequency within B cells of Env-specific ASC was similar to our findings for unstimulated cells (27). In addition, although monomeric gp120 was used for ELISPOT assay while trimeric gp140 was used for staining, this difference did not influence the results as the two proteins gave equivalent data when used side-by-side in ELISPOT assays. Therefore, we infer that technical issues do not account for this difference. However, within the plasmablast subset, the frequencies of Env-specific stained cells and ASC were very similar (medians of 0.05% versus 0.07%). Thus, for the subpopulation that is most likely to secrete antibody, the ELISPOT and staining assays did give similar results. Therefore, these results suggest that the vast majority of HIV-specific B cells are not ASC and not measured in the ELISPOT assay and that the true frequency of HIV-specific B cells is considerably higher than previously appreciated.
Several studies that compare the antigenic structures of recombinant Env proteins suggest that trimeric gp140 is a better mimic of the conformation of native gp160 spike than gp120 monomer (13, 61, 78, 90, 91). Investigations of the trimeric gp140-F construct used here (91) showed better binding of neutralizing MAb, including b12, to trimeric gp140 than to gp120. Conversely, the trimeric gp140 was bound less well by non-neutralizing MAb that recognize epitopes thought to be buried in the trimeric spike structure, such as C11 (54), implying exposure of relevant epitopes and occlusion of non-neutralizing epitopes (91). In addition, there is some evidence that trimeric gp140 elicits more and/or broader neutralizing antibodies than gp120 when used as an immunogen (24, 31, 35, 41, 92). However, the gp140-F trimers are not perfect mimics of the gp160 spike: they contain non-HIV protein domains, and the cleavage site between gp120 and gp41 is mutated in order to stabilize the quaternary structure. These factors, in addition to the reduced binding to non-neutralizing antibodies, suggest that the frequencies of Env-specific B cells reported here may still be an underestimate.
It remains unknown whether patients with broadly cross-reactive neutralizing activity have an oligoclonal response, targeting one or a few highly conserved epitopes via antibodies akin to the known monoclonal antibodies or whether they produce a variety of antibody species, each targeting epitopes found on a subset of isolates. The latter possibility seems less likely, given that neutralization in many broad patients extended to viruses in clades A and C that represent envelopes to which they were unlikely to be exposed. Rather, it is more likely that this breadth is mediated by a limited number of clones directed against conserved epitopes. An early analysis of two of the broad-serum patients within this cohort supports this hypothesis. The majority of neutralization was attributable to anti-gp120 antibodies with limited specificities. In one patient this was almost exclusively mediated by antibodies directed to the CD4 binding site, while for the second patient there was substantial anti-CD4 binding site activity, as well as activity directed to additional non-V3 epitopes on gp120 (40). Phage display and panning technology have been used to generate monoclonal antibodies from patients with broadly cross-neutralizing antibodies; studies of one patient have yielded three broadly cross-neutralizing monoclonal antibodies against gp120 and gp41 (22). Thus far, the full range of specificities that mediate the breadth of polyclonal sera in a single individual have not been determined at the monoclonal antibody level, in part because of the extremely low frequencies of neutralizing antibodies in vivo. Enrichment of Env-specific B cells using the techniques described here and sorting for plasmablasts or for Env-specific B cells may be helpful in achieving this goal.
In summary, the results of the present study provide a context for the induction of humoral immunity by HIV vaccines. The frequency and phenotype of HIV-specific B cells reported here, although not correlated with breadth, are examples of what is potentially achievable by the human immune response. In addition, the number of isolates neutralized and the titer of neutralizing antibodies are goals that are theoretically achievable in prophylactic vaccines. Further work, using the direct staining techniques described here for isolation of HIV-specific B cells, could potentially uncover the specificities and mechanism by which some patients mediate broad cross-neutralization.
Acknowledgments
We thank James Arthos, Jeffrey Cohen, Jason Ho, Laurie Lamoreaux, and Mark Louder for technical assistance and invaluable advice and Nancy Cogliano-Schutta, Catherine Rehm, Gregg Roby, and Sara Stallings for coordinating samples and patient visits.
This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and a grant from the NIH Intramural AIDS Targeted Antiviral Program.
Footnotes
Published ahead of print on 15 October 2008.
REFERENCES
- 1.Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding their relation. Science 27254-60. [DOI] [PubMed] [Google Scholar]
- 2.Albert, J., B. Abrahamsson, K. Nagy, E. Aurelius, H. Gaines, G. Nystrom, and E. M. Fenyo. 1990. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS 4107-112. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, S. M., M. M. Tomayko, and M. J. Shlomchik. 2006. Intrinsic properties of human and murine memory B cells. Immunol. Rev. 211280-294. [DOI] [PubMed] [Google Scholar]
- 4.Arendrup, M., C. Nielsen, J. E. Hansen, C. Pedersen, L. Mathiesen, and J. O. Nielsen. 1992. Autologous HIV-1 neutralizing antibodies: emergence of neutralization-resistant escape virus and subsequent development of escape virus neutralizing antibodies. J. Acquir. Immune Defic. Syndr. 5303-307. [PubMed] [Google Scholar]
- 5.Arthos, J., C. Cicala, E. Martinelli, K. Macleod, D. Van Ryk, D. Wei, Z. Xiao, T. D. Veenstra, T. P. Conrad, R. A. Lempicki, S. McLaughlin, M. Pascuccio, R. Gopaul, J. McNally, C. C. Cruz, N. Censoplano, E. Chung, K. N. Reitano, S. Kottilil, D. J. Goode, and A. S. Fauci. 2008. HIV-1 envelope protein binds to and signals through integrin α4β7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 9301-309. [DOI] [PubMed] [Google Scholar]
- 6.Avery, D. T., J. I. Ellyard, F. Mackay, L. M. Corcoran, P. D. Hodgkin, and S. G. Tangye. 2005. Increased expression of CD27 on activated human memory B cells correlates with their commitment to the plasma cell lineage. J. Immunol. 1744034-4042. [DOI] [PubMed] [Google Scholar]
- 7.Bailey, J. R., K. G. Lassen, H. C. Yang, T. C. Quinn, S. C. Ray, J. N. Blankson, and R. F. Siliciano. 2006. Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active antiretroviral therapy. J. Virol. 804758-4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey, J. R., T. M. Williams, R. F. Siliciano, and J. N. Blankson. 2006. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. J. Exp. Med. 2031357-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barin, F., G. Jourdain, S. Brunet, N. Ngo-Giang-Huong, S. Weerawatgoompa, W. Karnchanamayul, S. Ariyadej, R. Hansudewechakul, J. Achalapong, P. Yuthavisuthi, C. Ngampiyaskul, S. Bhakeecheep, C. Hemwutthiphan, and M. Lallemant. 2006. Revisiting the role of neutralizing antibodies in mother-to-child transmission of HIV-1. J. Infect. Dis. 1931504-1511. [DOI] [PubMed] [Google Scholar]
- 10.Beirnaert, E., S. De Zutter, W. Janssens, and G. van der Groen. 2001. Potent broad cross-neutralizing sera inhibit attachment of primary HIV-1 isolates (groups M and O) to peripheral blood mononuclear cells. Virology 281305-314. [DOI] [PubMed] [Google Scholar]
- 11.Beirnaert, E., P. Nyambi, B. Willems, L. Heyndrickx, R. Colebunders, W. Janssens, and G. van Der Groen. 2000. Identification and characterization of sera from HIV-infected individuals with broad cross-neutralizing activity against group M (env clade A-H) and group O primary HIV-1 isolates. J. Med. Virol. 6214-24. [PubMed] [Google Scholar]
- 12.Bhende, P. M., S. J. Dickerson, X. Sun, W. H. Feng, and S. C. Kenney. 2007. X-box-binding protein 1 activates lytic Epstein-Barr virus gene expression in combination with protein kinase D. J. Virol. 817363-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74627-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 7813232-13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown, B. K., L. Wieczorek, E. Sanders-Buell, A. R. Borges, M. L. Robb, D. L. Birx, N. L. Michael, F. E. McCutchan, and V. R. Polonis. 2008. Cross-clade neutralization patterns among HIV-1 strains from the six major clades of the pandemic evaluated and compared in two different models. Virology 375529-538. [DOI] [PubMed] [Google Scholar]
- 16.Cao, J., J. McNevin, S. Holte, L. Fink, L. Corey, and M. J. McElrath. 2003. Comprehensive analysis of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-secreting CD8+ T cells in primary HIV-1 infection. J. Virol. 776867-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao, Y., L. Qin, L. Zhang, J. Safrit, and D. D. Ho. 1995. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N. Engl. J. Med. 332201-208. [DOI] [PubMed] [Google Scholar]
- 18.Carotenuto, P., D. Looij, L. Keldermans, F. de Wolf, and J. Goudsmit. 1998. Neutralizing antibodies are positively associated with CD4+ T-cell counts and T-cell function in long-term AIDS-free infection. AIDS 121591-1600. [DOI] [PubMed] [Google Scholar]
- 19.Casimiro, D. R., F. Wang, W. A. Schleif, X. Liang, Z. Q. Zhang, T. W. Tobery, M. E. Davies, A. B. McDermott, D. H. O'Connor, A. Fridman, A. Bagchi, L. G. Tussey, A. J. Bett, A. C. Finnefrock, T. M. Fu, A. Tang, K. A. Wilson, M. Chen, H. C. Perry, G. J. Heidecker, D. C. Freed, A. Carella, K. S. Punt, K. J. Sykes, L. Huang, V. I. Ausensi, M. Bachinsky, U. Sadasivan-Nair, D. I. Watkins, E. A. Emini, and J. W. Shiver. 2005. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with dna and recombinant adenoviral vaccine vectors expressing Gag. J. Virol. 7915547-15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cecilia, D., C. Kleeberger, A. Munoz, J. V. Giorgi, and S. Zolla-Pazner. 1999. A longitudinal study of neutralizing antibodies and disease progression in HIV-1-infected subjects. J. Infect. Dis. 1791365-1374. [DOI] [PubMed] [Google Scholar]
- 21.Cepok, S., G. von Geldern, T. Nolting, V. Grummel, R. Srivastava, D. Zhou, H. P. Hartung, O. Adams, G. Arendt, and B. Hemmer. 2007. Viral load determines the B-cell response in the cerebrospinal fluid during human immunodeficiency virus infection. Ann. Neurol. 62458-467. [DOI] [PubMed] [Google Scholar]
- 22.Choudhry, V., M. Y. Zhang, I. A. Sidorov, J. M. Louis, I. Harris, A. S. Dimitrov, P. Bouma, F. Cham, A. Choudhary, S. M. Rybak, T. Fouts, D. C. Montefiori, C. C. Broder, G. V. Quinnan, Jr., and D. S. Dimitrov. 2007. Cross-reactive HIV-1 neutralizing monoclonal antibodies selected by screening of an immune human phage library against an envelope glycoprotein (gp140) isolated from a patient (R2) with broadly HIV-1 neutralizing antibodies. Virology 36379-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deeks, S. G., B. Schweighardt, T. Wrin, J. Galovich, R. Hoh, E. Sinclair, P. Hunt, J. M. McCune, J. N. Martin, C. J. Petropoulos, and F. M. Hecht. 2006. Neutralizing antibody responses against autologous and heterologous viruses in acute versus chronic human immunodeficiency virus (HIV) infection: evidence for a constraint on the ability of HIV to completely evade neutralizing antibody responses. J. Virol. 806155-6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Earl, P. L., W. Sugiura, D. C. Montefiori, C. C. Broder, S. A. Lee, C. Wild, J. Lifson, and B. Moss. 2001. Immunogenicity and protective efficacy of oligomeric human immunodeficiency virus type 1 gp140. J. Virol. 75645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehrhardt, G. R., J. T. Hsu, L. Gartland, C. M. Leu, S. Zhang, R. S. Davis, and M. D. Cooper. 2005. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J. Exp. Med. 202783-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fecteau, J. F., G. Cote, and S. Neron. 2006. A new memory CD27-IgG+ B-cell population in peripheral blood expressing VH genes with low frequency of somatic mutation. J. Immunol. 1773728-3736. [DOI] [PubMed] [Google Scholar]
- 27.Fondere, J. M., M. F. Huguet, A. Macura-Biegun, V. Baillat, V. Ohayon, J. Reynes, and J. P. Vendrell. 2004. Detection and enumeration of circulating HIV-1-specific memory B cells in HIV-1-infected patients. J. Acquir. Immune Defic. Syndr. 35114-119. [DOI] [PubMed] [Google Scholar]
- 28.Reference deleted.
- 29.Graham, B. S. 2002. Clinical trials of HIV vaccines. Annu. Rev. Med. 53207-221. [DOI] [PubMed] [Google Scholar]
- 30.Graham, B. S., and J. R. Mascola. 2005. Lessons from failure-preparing for future HIV-1 vaccine efficacy trials. J. Infect. Dis. 191647-649. [DOI] [PubMed] [Google Scholar]
- 31.Grundner, C., Y. Li, M. Louder, J. Mascola, X. Yang, J. Sodroski, and R. Wyatt. 2005. Analysis of the neutralizing antibody response elicited in rabbits by repeated inoculation with trimeric HIV-1 envelope glycoproteins. Virology 33133-46. [DOI] [PubMed] [Google Scholar]
- 32.Hofer, T., G. Muehlinghaus, K. Moser, T. Yoshida, E. M. H., K. Hebel, A. Hauser, B. Hoyer, E. O. Luger, T. Dorner, R. A. Manz, F. Hiepe, and A. Radbruch. 2006. Adaptation of humoral memory. Immunol. Rev. 211295-302. [DOI] [PubMed] [Google Scholar]
- 33.Holmes, K., B. Fowlkes, I. Schmid, and J. Giorgi. 1995. Preparation of cells and reagents for flow cytometry, p. 5.3.1-5.3.23. In J. Coligan, A. Kruisbeek, D. Margulies, E. Sheevac, and W. Strober (ed.), Current protocols in immunology, vol. 1. Green Publishing Associates, New York, NY. [DOI] [PubMed] [Google Scholar]
- 34.Jaimes, M. C., O. L. Rojas, E. J. Kunkel, N. H. Lazarus, D. Soler, E. C. Butcher, D. Bass, J. Angel, M. A. Franco, and H. B. Greenberg. 2004. Maturation and trafficking markers on rotavirus-specific B cells during acute infection and convalescence in children. J. Virol. 7810967-10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim, M., Z. S. Qiao, D. C. Montefiori, B. F. Haynes, E. L. Reinherz, and H. X. Liao. 2005. Comparison of HIV type 1 ADA gp120 monomers versus gp140 trimers as immunogens for the induction of neutralizing antibodies. AIDS Res. Hum. Retrovir. 2158-67. [DOI] [PubMed] [Google Scholar]
- 36.Laichalk, L. L., and D. A. Thorley-Lawson. 2005. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J. Virol. 791296-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lane, H. C., H. Masur, L. C. Edgard, G. Whalen, A. H. Rook, and A. S. Fauci. 1983. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 309453. [DOI] [PubMed] [Google Scholar]
- 38.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 7910108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li, M., J. F. Salazar-Gonzalez, C. A. Derdeyn, L. Morris, C. Williamson, J. E. Robinson, J. M. Decker, Y. Li, M. G. Salazar, V. R. Polonis, K. Mlisana, S. A. Karim, K. Hong, K. M. Greene, M. Bilska, J. Zhou, S. Allen, E. Chomba, J. Mulenga, C. Vwalika, F. Gao, M. Zhang, B. T. Korber, E. Hunter, B. H. Hahn, and D. C. Montefiori. 2006. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J. Virol. 8011776-11790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li, Y., S. A. Migueles, B. Welcher, K. Svehla, A. Phogat, M. K. Louder, X. Wu, G. M. Shaw, M. Connors, R. T. Wyatt, and J. R. Mascola. 2007. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat. Med. 131032-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, Y., K. Svehla, N. L. Mathy, G. Voss, J. R. Mascola, and R. Wyatt. 2006. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J. Virol. 801414-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mascola, J. R., P. D'Souza, P. Gilbert, B. H. Hahn, N. L. Haigwood, L. Morris, C. J. Petropoulos, V. R. Polonis, M. Sarzotti, and D. C. Montefiori. 2005. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J. Virol. 7910103-10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 734009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mascola, J. R., S. W. Snyder, O. S. Weislow, S. M. Belay, R. B. Belshe, D. H. Schwartz, M. L. Clements, R. Dolin, B. S. Graham, G. J. Gorse, M. C. Keefer, M. J. McElrath, M. C. Walker, K. F. Wagner, J. G. McNeil, F. E. McCutchan, D. S. Burke, et al. 1996. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J. Infect. Dis. 173340-348. [DOI] [PubMed] [Google Scholar]
- 45.McCutchan, F. E. 2000. Understanding the genetic diversity of HIV-1. AIDS 14(Suppl. 3)S31-S44. [PubMed] [Google Scholar]
- 46.McKnight, A., and M. M. Aasa-Chapman. 2007. Clade specific neutralizing vaccines for HIV: an appropriate target? Curr. HIV Res. 5554-560. [DOI] [PubMed] [Google Scholar]
- 47.Migueles, S. A., A. C. Laborico, H. Imamichi, W. L. Shupert, C. Royce, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, C. W. Hallahan, and M. Connors. 2003. The differential ability of HLA B*5701+ long-term nonprogressors and progressors to restrict human immunodeficiency virus replication is not caused by loss of recognition of autologous viral gag sequences. J. Virol. 776889-6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T-cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 31061-1068. [DOI] [PubMed] [Google Scholar]
- 49.Mockridge, C. I., A. Rahman, S. Buchan, T. Hamblin, D. A. Isenberg, F. K. Stevenson, and K. N. Potter. 2004. Common patterns of B-cell perturbation and expanded V4-34 immunoglobulin gene usage in autoimmunity and infection. Autoimmunity 379-15. [DOI] [PubMed] [Google Scholar]
- 50.Moir, S., and A. S. Fauci. 2008. Pathogenic mechanisms of B-lymphocyte dysfunction in HIV disease. J. Allergy Clin. Immunol. 12212-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moir, S., J. Ho, A. Malaspina, W. Wang, A. C. Dipoto, M. A. O'Shea, G. Roby, S. Kottilil, J. Arthos, M. A. Proschan, T. W. Chun, and A. S. Fauci. 2008. Evidence for HIV-associated B-cell exhaustion in a dysfunctional memory B-cell compartment in HIV-infected viremic individuals. J. Exp. Med. 2051797-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montefiori, D. C., G. Pantaleo, L. M. Fink, J. T. Zhou, J. Y. Zhou, M. Bilska, G. D. Miralles, and A. S. Fauci. 1996. Neutralizing and infection-enhancing antibody responses to human immunodeficiency virus type 1 in long-term nonprogressors. J. Infect. Dis. 17360-67. [DOI] [PubMed] [Google Scholar]
- 53.Moog, C., H. J. Fleury, I. Pellegrin, A. Kirn, and A. M. Aubertin. 1997. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J. Virol. 713734-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moore, J. P., Y. Cao, D. D. Ho, and R. A. Koup. 1994. Development of the ant-gp120 antibody response during seroconversion to human immunodeficiency virus type 1. J. Virol. 685142-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore, J. P., Y. Cao, J. Leu, L. Qin, B. Korber, and D. D. Ho. 1996. Inter- and intraclade neutralization of human immunodeficiency virus type 1: genetic clades do not correspond to neutralization serotypes but partially correspond to gp120 antigenic serotypes. J. Virol. 70427-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reference deleted.
- 57.Morris, L., J. M. Binley, B. A. Clas, S. Bonhoeffer, T. P. Astill, R. Kost, A. Hurley, Y. Cao, M. Markowitz, D. D. Ho, and J. P. Moore. 1998. HIV-1 antigen-specific and -nonspecific B-cell responses are sensitive to combination antiretroviral therapy. J. Exp. Med. 188233-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murphy, B. R., and R. M. Chanock. 2001. Immunization against viral diseases, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, PA.
- 59.Odendahl, M., A. Jacobi, A. Hansen, E. Feist, F. Hiepe, G. R. Burmester, P. E. Lipsky, A. Radbruch, and T. Dorner. 2000. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J. Immunol. 1655970-5979. [DOI] [PubMed] [Google Scholar]
- 60.Odendahl, M., H. Mei, B. F. Hoyer, A. M. Jacobi, A. Hansen, G. Muehlinghaus, C. Berek, F. Hiepe, R. Manz, A. Radbruch, and T. Dorner. 2005. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood 1051614-1621. [DOI] [PubMed] [Google Scholar]
- 61.Pancera, M., J. Lebowitz, A. Schon, P. Zhu, E. Freire, P. D. Kwong, K. H. Roux, J. Sodroski, and R. Wyatt. 2005. Soluble mimetics of human immunodeficiency virus type 1 viral spikes produced by replacement of the native trimerization domain with a heterologous trimerization motif: characterization and ligand binding analysis. J. Virol. 799954-9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pantophlet, R., and D. R. Burton. 2006. GP120: target for neutralizing HIV-1 antibodies. Annu. Rev. Immunol. 24739-769. [DOI] [PubMed] [Google Scholar]
- 63.Pereyra, F., M. M. Addo, D. E. Kaufmann, Y. Liu, T. Miura, A. Rathod, B. Baker, A. Trocha, R. Rosenberg, E. Mackey, P. Ueda, Z. Lu, D. Cohen, T. Wrin, C. J. Petropoulos, E. S. Rosenberg, and B. D. Walker. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 197563-571. [DOI] [PubMed] [Google Scholar]
- 64.Pilgrim, A. K., G. Pantaleo, O. J. Cohen, L. M. Fink, J. Y. Zhou, J. T. Zhou, D. P. Bolognesi, A. S. Fauci, and D. C. Montefiori. 1997. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J. Infect. Dis. 176924-932. [DOI] [PubMed] [Google Scholar]
- 65.Pitisuttithum, P., P. Gilbert, M. Gurwith, W. Heyward, M. Martin, F. van Griensven, D. Hu, J. W. Tappero, and K. Choopanya. 2006. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 1941661-1671. [DOI] [PubMed] [Google Scholar]
- 66.Plotkin, S. A. 2008. Vaccines: correlates of vaccine-induced immunity. Clin. Infect. Dis. 47401-409. [DOI] [PubMed] [Google Scholar]
- 67.Reference deleted.
- 68.Polonis, V. R., B. K. Brown, A. R. Borges, S. Zolla-Pazner, D. S. Dimitrov, M. Y. Zhang, S. W. Barnett, R. M. Ruprecht, G. Scarlatti, E. M. Fenyo, D. C. Montefiori, F. E. McCutchan, and N. L. Michael. 2008. Recent advances in the characterization of HIV-1 neutralization assays for standardized evaluation of the antibody response to infection and vaccination. Virology 375315-320. [DOI] [PubMed] [Google Scholar]
- 69.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 1004144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robey, W. G., L. O. Arthur, T. J. Matthews, A. Langlois, T. D. Copeland, N. W. Lerche, S. Oroszlan, D. P. Bolognesi, R. V. Gilden, and P. J. Fischinger. 1986. Prospect for prevention of human immunodeficiency virus infection: purified 120-kDa envelope glycoprotein induces neutralizing antibody. Proc. Natl. Acad. Sci. USA 837023-7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sanz, I., C. Wei, F. E. Lee, and J. Anolik. 2008. Phenotypic and functional heterogeneity of human memory B cells. Semin. Immunol. 2067-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reference deleted.
- 73.Scarlatti, G., T. Leitner, V. Hodara, E. Halapi, P. Rossi, J. Albert, and E. M. Fenyo. 1993. Neutralizing antibodies and viral characteristics in mother-to-child transmission of HIV-1. AIDS 7(Suppl. 2)S45-S48. [DOI] [PubMed] [Google Scholar]
- 74.Shibata, R., T. Igarashi, N. Haigwood, A. Buckler-White, R. Ogert, W. Ross, R. Willey, M. W. Cho, and M. A. Martin. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5204-210. [DOI] [PubMed] [Google Scholar]
- 75.Shirai, A., M. Cosentino, S. F. Leitman-Klinman, and D. M. Klinman. 1992. Human immunodeficiency virus infection induces both polyclonal and virus-specific B-cell activation. J. Clin. Investig. 89561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415331-335. [DOI] [PubMed] [Google Scholar]
- 77.Shu, Y., S. Winfrey, Z. Y. Yang, L. Xu, S. S. Rao, I. Srivastava, S. W. Barnett, G. J. Nabel, and J. R. Mascola. 2007. Efficient protein boosting after plasmid DNA or recombinant adenovirus immunization with HIV-1 vaccine constructs. Vaccine 251398-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Srivastava, I. K., L. Stamatatos, H. Legg, E. Kan, A. Fong, S. R. Coates, L. Leung, M. Wininger, J. J. Donnelly, J. B. Ulmer, and S. W. Barnett. 2002. Purification and characterization of oligomeric envelope glycoprotein from a primary R5 subtype B human immunodeficiency virus. J. Virol. 762835-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun, C. C., and D. A. Thorley-Lawson. 2007. Plasma cell-specific transcription factor XBP-1s binds to and transactivates the Epstein-Barr virus BZLF1 promoter. J. Virol. 8113566-13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tangye, S. G., and K. L. Good. 2007. Human IgM+ CD27+ B cells: memory B cells or “memory” B cells? J. Immunol. 17913-19. [DOI] [PubMed] [Google Scholar]
- 81.Wang, S., J. S. Kennedy, K. West, D. C. Montefiori, S. Coley, J. Lawrence, S. Shen, S. Green, A. L. Rothman, F. A. Ennis, J. Arthos, R. Pal, P. Markham, and S. Lu. 2008. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine 261098-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei, X., J. M. Decker, H. Liu, Z. Zhang, R. B. Arani, J. M. Kilby, M. S. Saag, X. Wu, G. M. Shaw, and J. C. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 461896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422307-312. [DOI] [PubMed] [Google Scholar]
- 84.Weiss, R. A., R. P. Clapham, and R. Cheingsong-Popov. 1985. Neutralization of human T-lymphotropic virus type III by sera of AIDS and AIDS-risk patients. Nature 31669-72. [DOI] [PubMed] [Google Scholar]
- 85.Wilson, N. A., J. Reed, G. S. Napoe, S. Piaskowski, A. Szymanski, J. Furlott, E. J. Gonzalez, L. J. Yant, N. J. Maness, G. E. May, T. Soma, M. R. Reynolds, E. Rakasz, R. Rudersdorf, A. B. McDermott, D. H. O'Connor, T. C. Friedrich, D. B. Allison, A. Patki, L. J. Picker, D. R. Burton, J. Lin, L. Huang, D. Patel, G. Heindecker, J. Fan, M. Citron, M. Horton, F. Wang, X. Liang, J. W. Shiver, D. R. Casimiro, and D. I. Watkins. 2006. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J. Virol. 805875-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wirths, S., and A. Lanzavecchia. 2005. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. Eur. J. Immunol. 353433-3441. [DOI] [PubMed] [Google Scholar]
- 87.Wrammert, J., and R. Ahmed. 2008. Maintenance of serological memory. Biol. Chem. 389537-539. [DOI] [PubMed] [Google Scholar]
- 88.Wrammert, J., K. Smith, J. Miller, W. A. Langley, K. Kokko, C. Larsen, N. Y. Zheng, I. Mays, L. Garman, C. Helms, J. James, G. M. Air, J. D. Capra, R. Ahmed, and P. C. Wilson. 2008. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453667-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu, W., R. Hofmann-Lehmann, H. M. McClure, and R. M. Ruprecht. 2002. Passive immunization with human neutralizing monoclonal antibodies: correlates of protective immunity against HIV. Vaccine 201956-1960. [DOI] [PubMed] [Google Scholar]
- 90.Yang, X., M. Farzan, R. Wyatt, and J. Sodroski. 2000. Characterization of stable, soluble trimers containing complete ectodomains of human immunodeficiency virus type 1 envelope glycoproteins. J. Virol. 745716-5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang, X., J. Lee, E. M. Mahony, P. D. Kwong, R. Wyatt, and J. Sodroski. 2002. Highly stable trimers formed by human immunodeficiency virus type 1 envelope glycoproteins fused with the trimeric motif of T4 bacteriophage fibritin. J. Virol. 764634-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang, X., R. Wyatt, and J. Sodroski. 2001. Improved elicitation of neutralizing antibodies against primary human immunodeficiency viruses by soluble stabilized envelope glycoprotein trimers. J. Virol. 751165-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang, Y. J., C. Fracasso, J. R. Fiore, A. Bjorndal, G. Angarano, A. Gringeri, and E. M. Fenyo. 1997. Augmented serum neutralizing activity against primary human immunodeficiency virus type 1 (HIV-1) isolates in two groups of HIV-1-infected long-term nonprogressors. J. Infect. Dis. 1761180-1187. [DOI] [PubMed] [Google Scholar]