Abstract
The measles virus (MV) accessory proteins V and C play important roles in MV replication and pathogenesis. Infection with recombinant MV lacking either V or C causes more cell death than infection with the parental vaccine-equivalent virus (MVvac), and C-deficient virus grows poorly relative to the parental virus. Here, we show that a major effector of the C phenotype is the RNA-dependent protein kinase PKR. Using human HeLa cells stably deficient in PKR as a result of RNA interference-mediated knockdown (PKRkd cells), we demonstrated that a reduction in PKR partially rescued the growth defect of C knockout (Cko) virus but had no effect on the growth of either wild-type (WT) or V knockout (Vko) virus. Increased growth of the Cko virus in PKRkd cells correlated with increased viral protein expression, while defective growth and decreased protein expression in PKR-sufficient cells correlated with increased phosphorylation of PKR and the α subunit of eukaryotic initiation factor 2. Furthermore, infection with WT, Vko, or especially Cko virus caused significantly less apoptosis in PKRkd cells than in PKR-sufficient cells. Although apoptosis induced by Cko virus infection in PKR-sufficient cells was blocked by a caspase antagonist, the growth of Cko virus was not restored to the WT level by treatment with this pharmacologic inhibitor. Taken together, these results indicate that PKR plays an important antiviral role during MV infection but that the virus growth restriction by PKR is not dependent upon the induction of apoptosis. Furthermore, the results establish that a principal function of the MV C protein is to antagonize the proapoptotic and antiviral activities of PKR.
Measles virus (MV), a member of the genus Morbillivirus of the family Paramyxoviridae, causes a highly contagious acute febrile disease. Despite the availability of an effective live vaccine, MV remains a major pathogen of global concern, with more than 500,000 measles-related deaths annually (27, 59). In addition, measles disease occasionally reemerges in industrialized nations, due in part to a lack of adherence to vaccine recommendations (10, 29). As measles continues to cause significant morbidity and mortality worldwide, understanding interactions between MV and the host at the molecular level that affect virulence is imperative for optimizing immunization and treatment strategies.
The 15.9-kb genome of MV consists of six genes (the N, P/V/C, M, F, H, and L genes), all of which are monocistronic with the exception of the P gene, which encodes three proteins: the phosphoprotein P and the accessory proteins V and C. The synthesis of V protein begins at the same translation initiation codon as that of P protein; however, a frameshift occurs in the V mRNA due to a cotranscriptional RNA-editing process that involves the insertion of a single nontemplated guanosine by the viral polymerase. Thus, V protein shares its first N-terminal 231 amino acids with the P protein but has a unique 68-amino-acid cysteine-rich C terminus that has the ability to bind zinc (9, 32, 57). C protein of MV is a small (186-amino-acid) basic protein synthesized from an alternative translation start site located 22 nucleotides downstream from the P and V translation start site (4).
The MV V and C proteins are major virulence factors, and viruses deficient in V and C replicate poorly in some cell lines and animal models (16-18, 21, 35, 41, 50, 53, 55). V proteins of many paramyxoviruses including MV have been shown to inhibit interferon (IFN) induction through interaction with the mda-5 cytoplasmic RNA sensor (1, 13, 36). MV C protein also suppresses IFN induction (17, 35, 36), most likely through the downregulation of viral RNA synthesis (36). In addition to inhibiting IFN production, the V protein of MV suppresses IFN signal transduction by preventing the phosphorylation (18, 51, 60) and nuclear accumulation (18, 39, 43) of STAT1 and STAT2. MV C protein may also inhibit IFN signaling (23, 50), although the available evidence suggests that C protein is a less potent antagonist than V (23, 51). V and C proteins are also important for viral RNA production and genome replication (3, 55), and C is important for virus assembly (16). Finally, MV V and C play a role in preventing MV-induced cell death (14, 53).
The protein kinase regulated by RNA (PKR) is an IFN-regulated protein kinase that is activated through binding to RNA (34, 46). The activation of the kinase leads to autophosphorylation, dimerization, and the subsequent phosphorylation of substrate proteins, the best-characterized of which is the α subunit of eukaryotic translation initiation factor 2 (eIF-2α) (48). PKR phosphorylates eIF-2α on serine 51 (40, 46), leading to the inhibition of translation (47). PKR, present at significant constitutive levels in most cells, is transcriptionally induced by IFN treatment and virus infection (48, 54). PKR inhibits the multiplication of many viruses, and the importance of PKR as an antiviral factor is further illustrated by the number of viral gene products that antagonize PKR function (56). In addition to changing the translation pattern in cells, a major biological function of PKR is to mediate apoptosis caused by double-stranded RNA (dsRNA) stress or virus infection (2, 15, 56, 61, 62). Furthermore, PKR is also involved in cell signaling pathways, including the NF-κB (31) and mitogen-activated protein kinase (25) pathways.
Here, we present evidence that PKR plays a pivotal role during MV infection. Using human HeLa cells either stably deficient in PKR expression or expressing normal levels of PKR, as well as wild-type (WT) and derived isogenic mutant MVs lacking the expression of either the V or C protein (V knockout [Vko] or Cko virus, respectively), we examined the role of PKR in MV multiplication and MV-induced cell death. We observed that PKR contributed to the growth defect of the Cko virus but that the expression of the kinase had no effect on the growth of the WT or Vko virus. Moreover, we found that PKR is a mediator of MV-induced apoptosis but that the antiviral activity of PKR toward MV is not dependent upon apoptosis induction.
MATERIALS AND METHODS
Cells and viruses.
Parental HeLa (PKR+) and Vero cells were maintained in Dulbecco's modified Eagle's medium supplemented with 5% (vol/vol) fetal bovine serum (HyClone), 100 μg/ml of penicillin, and 100 U/ml of streptomycin (GIBCO/Invitrogen) as described previously (61, 62). HeLa cells with the stable knockdown of PKR by RNA interference (PKRkd cells) (see references 61 and 62) were maintained in the above-named medium containing 1 μg/ml puromycin (Sigma). HeLa cells transfected with the pSUPER.retro.puro vector with the H1 promoter, which was used for the construction of short hairpin expression constructs to silence human PKR (62), were selected and maintained in the puromycin-containing medium. These PKR-expressing drug-resistant knockdown control cells, called PKRkd-con cells, were used as an additional PKR-positive control. Treatment with the pan-caspase inhibitor z-VAD-fmk (benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone; EMD/Calbiochem), where indicated, was with a concentration of 100 μM beginning after the virus inoculum was removed.
The recombinant parental virus MVvac GFP(H), herein designated WT, as well as V-deficient (Vko) and C-deficient (Cko) versions of this virus, was constructed based on the Moraten vaccine strain as described previously (18), except that a gene encoding green fluorescent protein (GFP) was inserted downstream of the H gene. For the Vko virus, the V protein was selectively abolished by the mutation of the V gene editing site and the introduction of a stop codon. The mutations introduced into the WT MVvac strain to inactivate V or C protein expression were the same as those introduced previously into the WT IC-B strain (17). For the Cko virus, C was silenced by mutating the start codon for C and introducing a stop codon. The mutations in V did not affect the amino acid sequence of P or C; likewise, the mutations in C did not affect the amino acid sequence of P or V.
An E3L deletion mutant of the Copenhagen strain (VC-2) of vaccinia virus (VVΔE3L) was generously provided by B. Jacobs (11, 12).
Virus infections and growth assays.
PKR+, PKRkd, and/or PKRkd-con cells were seeded into 12- or 6-well plates. Twenty-four hours later, cell monolayers were washed once with OptiMEM and then infected with WT, Vko, or Cko MV at a multiplicity of infection (MOI) of 0.1 50% tissue culture infective doses (TCID50)/cell or 5 TCID50/cell as indicated. The monolayers were allowed to adsorb the inoculum for 2 h at 37°C. After adsorption, the monolayers were washed twice with OptiMEM, and Dulbecco's modified Eagle's medium containing 5% (vol/vol) serum was added to each well. For vaccinia virus infections, the cells were similarly seeded into plates and then infected with VVΔE3L at an MOI of 5 PFU/cell. For superinfection experiments, cells were infected first with WT MV at an MOI of 5 TCID50/cell for 38 h and then with VVΔE3L at an MOI of 5 PFU/cell. Virus adsorption for VVΔE3L infections was for 1 h at 37°C. For MV growth assays, the infected cells were harvested by being scraped into the medium at either 48 h (MOI of 0.1) or 36 h (MOI of 5) after infection. Virus was released from the cells by three freeze-thaw cycles, followed by centrifugation to remove cellular debris. Virus yields were determined by 50% TCID50 titration on Vero cells according to the Spearman-Kärber method (17, 30).
Western immunoblot analysis.
Whole-cell extracts were prepared at the times postinfection indicated in the figures and legends in the presence of 1 mM phenylmethylsulfonyl fluoride and 1% (vol/vol) protease inhibitor cocktail (Sigma) as described previously (62), except that 1% (vol/vol) phosphatase inhibitor cocktail (Sigma) was also added. Protein concentrations of the extracts were determined by the Bradford method. Proteins were fractionated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-10% PAGE) or SDS-12% PAGE and transferred onto nitrocellulose, and the membranes were blocked in either 3% (wt/vol) bovine serum albumin (for the detection of phosphoproteins) or 5% (wt/vol) skim milk (for the detection of all other proteins). For N and P protein detection, rabbit antipeptide antisera recognizing the respective carboxyl-terminal amino acids were generated. For this endeavor, the peptide (C)SEEQGSDTDTPTVYNDRNLLD (the amino-terminal cysteine was added for coupling purposes), corresponding to MV N amino acids 505 to 525, and the peptide (C)ARKSPSEPSGPGAPAGNVP, corresponding to MV P amino acids 254 to 273, were synthesized and coupled to keyhole limpet hemocyanin. These conjugates were used to produce a rabbit antiserum. Rabbit polyclonal antibodies against MV V, C, and H were described previously (8, 16). Antibody against vaccinia virus I3 was generously provided by P. Tracktman (Medical College of Wisconsin, Milwaukee). Rabbit polyclonal antibodies from the indicated sources were used to detect GFP (Santa Cruz), human PKR (Santa Cruz), eIF-2α (Cell Signaling), PKR phosphorylated at Thr446 (Santa Cruz), and eIF-2α phosphorylated at Ser51 (Cell Signaling). Mouse monoclonal antibodies were used to detect β-actin (Sigma) and human poly(ADP-ribose) polymerase (PARP; BD Biosciences).
Cell viability assays.
Cells were seeded into 24-well dishes and 24 h later infected at an MOI of 5 as described above. At 48 h postinfection, 100 μl of a 5-mg/ml solution of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT reagent; Invitrogen) was added to each well, and the cells were incubated at 37°C for 30 min. The medium was then removed, the MTT dye was released from the cells with 1 ml of dimethyl sulfoxide, and the absorbance at 540 nm was measured with a Beckman DU-65 spectrophotometer.
RESULTS
The growth defect of C-deficient MV is partially rescued in PKR-deficient HeLa cells.
Prior studies showed that MV V and C proteins are dispensable for replication in cultivated cells (42, 49), although C-deficient virus replicates poorly relative to WT virus (16-18, 21, 35, 41, 53, 55). Furthermore, several studies have demonstrated that PKR is activated (19), eIF-2α is phosphorylated (20, 35), and translation is inhibited (35) in response to MV, but a definitive role for PKR in MV infection has yet to be established. To test whether PKR plays an obligatory role in limiting the growth of mutant MV deficient in either C or V protein expression, a HeLa clonal line in which PKR expression is stably knocked down by RNA interference (62) was examined. These PKRkd cells have about 2 to 5% of the PKR protein found in either parental cells (PKR+) or drug-treated control cells (PKRkd-con) (61, 62). We therefore studied the growth of WT and mutant recombinant MVs in PKR-deficient (PKRkd) and PKR-sufficient (PKR+ and PKRkd-con) human cells. The recombinant MVvac GFP(H) strain, as well as the isogenic V- and C-deficient (Vko and Cko, respectively) mutants of this virus, was derived from the Moraten vaccine and carries a GFP gene downstream of the H gene. The vaccine backbone was chosen for these experiments because only vaccine-lineage strains can enter cells through CD46 (37), a receptor expressed on PKR-deficient and -sufficient cells.
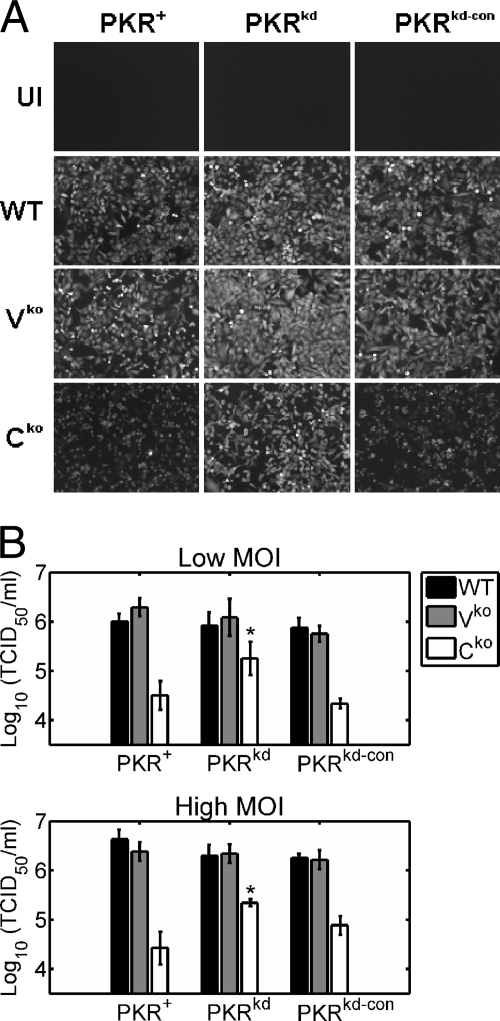
Among infected cell cultures expressing PKR, the intensity of the GFP reporter signal in Cko virus-infected cells was reduced compared to that in WT- and Vko virus-infected cells as viewed by fluorescence microscopy (Fig. 1A). Viral growth assays revealed that the Cko virus yield was ∼30- to 50-fold lower than the yield of WT or Vko virus in cells with sufficient PKR (Fig. 1B). These results are in agreement with the findings of previous studies which have reported attenuated MV growth in the absence of C protein (16-18, 21, 35, 53) but relatively normal growth in the absence of V protein (18, 21).
FIG. 1.
Growth of V and C mutant viruses compared to WT MV in PKR+, PKRkd, and PKRkd-con HeLa cells. PKR+, PKRkd, and PKRkd-con cells were infected with WT, Vko, or Cko recombinant virus. (A) Fluorescence images of cells infected at an MOI of 5 were taken at 36 h after infection. UI, uninfected cells. (B) Virus yields from PKR-sufficient and PKR-deficient cells at 48 h after infection at an MOI of 0.1 (low MOI) or 36 h after infection at an MOI of 5 (high MOI) were determined by 50% TCID50 titration on Vero cells. The results shown are the means with standard deviations (n = 3). * , P of <0.05 by Student's t test for comparison of the Cko virus yields in PKRkd cells and those in PKR+ or PKRkd-con cells.
In contrast, in PKRkd cells, the GFP signal for the Cko virus was similar to that for the WT virus (Fig. 1A). Moreover, Cko virus yields were only ∼5- to 10-fold lower than those of the WT and Vko viruses in these PKR-deficient cells (Fig. 1B). These results suggest that PKR plays a role in limiting MV growth and that the accessory C protein counterbalances the PKR antiviral pathway.
The C-deficient MV exhibits impaired protein expression in PKR-sufficient cells but not in PKR-deficient cells.
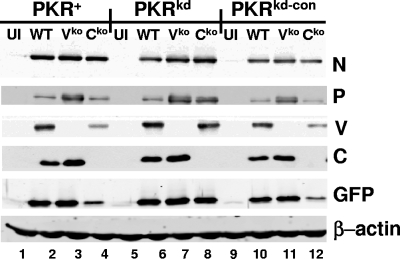
Next, we performed Western immunoblot analyses with antibodies against several viral proteins to determine if increased viral protein expression levels correlated with the increased yield of the Cko virus seen in PKR-deficient cells. Compared to PKR-sufficient cells, PKRkd cells infected with the Cko virus expressed slightly increased levels of N protein but greatly enhanced levels of P, V, and GFP (Fig. 2), indicating that the protein expression defect of the Cko virus was relieved in the absence of PKR. In PKR-sufficient cells, the level of P protein was increased upon infection with the Vko virus compared to the level observed upon infection with WT or Cko virus. The Vko virus is an editing mutant, and all P transcripts of the Vko virus express P, while 50% of the P transcripts of the WT or Cko virus express V. This increase in the P protein level for the Vko virus was reported previously (17). Similar results for protein expression were observed when infections were performed at an MOI of 5 (data not shown).
FIG. 2.
Viral protein expression levels in WT, Vko, and Cko virus-infected PKR+, PKRkd, and PKRkd-con cells. Cells were infected at an MOI of 0.l, and whole-cell extracts were prepared at 48 h after infection and analyzed by Western immunoblotting by using monospecific antibodies against the indicated MV proteins or β-actin. UI, uninfected cells.
Infection with Cko MV leads to increased PKR activation and eIF-2α phosphorylation.
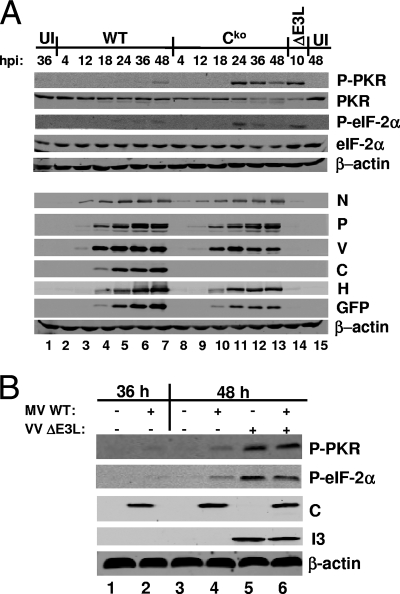
Upon RNA binding, PKR dimerizes and undergoes autophosphorylation, thereby leading to an active kinase that can then phosphorylate the translation initiation factor eIF-2α, causing a block in translation initiation. To determine whether the viral protein expression defect observed in Cko virus-infected cells was accompanied by increased PKR activation and subsequent eIF-2α phosphorylation, we analyzed PKR and eIF-2α phosphorylation levels upon infection with WT and Cko viruses. In PKR-sufficient cells, PKR phosphorylation at threonine 446 was enhanced only at late times after infection with the WT virus. This phosphorylation of PKR correlated with a modest increase in eIF-2α phosphorylation (Fig. 3A, lanes 6 and 7). Infection with the Cko virus, in contrast, led to high levels of PKR and eIF-2α phosphorylation by 24 h postinfection, which then decreased at later times postinfection (Fig. 3A, lanes 11 to 13). As a positive control, the phosphorylation of eIF-2α in parallel cultures of cells infected with VVΔE3L was also measured; infection with this mutant virus led to highly increased levels of PKR-dependent eIF-2α phosphorylation (Fig. 3A, lane 14), as well-established previously (48, 61). No increase in eIF-2α phosphorylation in PKRkd cells was observed upon infection with any of the MVs (data not shown).
FIG. 3.
PKR and eIF-2α phosphorylation in WT and Cko virus-infected PKR+ cells. (A) Cells were left uninfected (UI) or were infected with WT or Cko MV at an MOI of 5 TCID50/cell or VVΔE3L at an MOI of 5 PFU/cell. Whole-cell extracts were prepared at the indicated times postinfection. Western blot analyses were performed using antibodies against phospho-PKR (P-PKR), PKR, phospho-eIF-2α (P-eIF-2α), eIF-2α, N, P, V, C, H, GFP, and β-actin. hpi, hours postinfection. (B) Cells were left uninfected or were infected with WT MV at an MOI of 5 TCID50/cell. At 38 h after infection with MV, the cells were superinfected with VVΔE3L at an MOI of 5 PFU/cell where indicated (+). Whole-cell extracts were prepared at the indicated times and analyzed by Western blotting with antibodies against phospho-PKR, phospho-eIF-2α, β-actin, MV C, and VV I3. +, present; −, absent.
The observed decrease in protein expression by the Cko virus correlated with the timing of increased eIF-2α phosphorylation (Fig. 3A). At 18 h postinfection, before PKR activation in response to either WT or Cko virus was detected, the levels of protein expression by the two viruses were similar (Fig. 3A, lanes 4 and 10). At 36 and 48 h postinfection, the levels of Cko virus N and P were slightly reduced and those of other virus proteins (V, H, and GFP) were more reduced relative to the levels of WT proteins (Fig. 3A, lanes 6, 7, 12, and 13). Taken together, these results indicate that the Cko MV is a more potent activator of PKR than the WT virus and that PKR is a major contributor to the protein expression defect of the Cko virus.
We next tested whether the effect of MV C protein on PKR activation was direct or indirect. To this end, PKR-sufficient parental HeLa cells were infected with WT MV for 38 h to allow the ample expression of C protein and then superinfected with VVΔE3L for 10 h. As expected, infection with WT MV alone caused only slight PKR activation (Fig. 3B, lanes 2 and 4) whereas infection with VVΔE3L alone caused greatly enhanced PKR activation (Fig. 3B, lane 5). Preinfection with WT MV had little effect on either PKR or eIF-2α phosphorylation induced by VVΔE3L (Fig. 3B, lane 6), indicating that the expression of C protein cannot inhibit PKR activation in trans and that C protein is an indirect antagonist of PKR activation.
PKR is a mediator of MV-induced apoptosis.
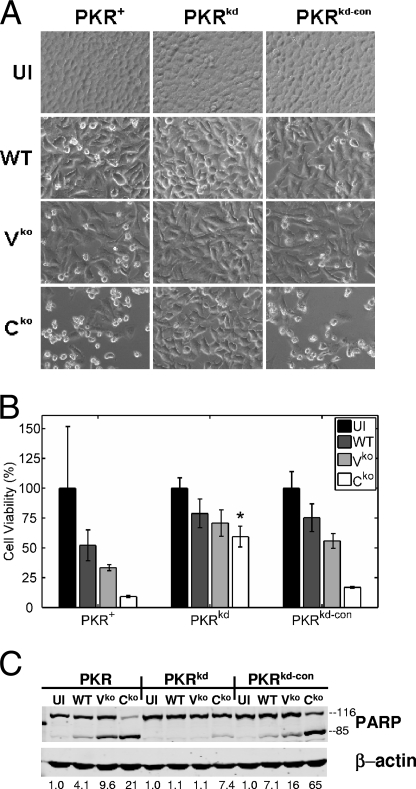
The PKR kinase has been shown previously to mediate apoptosis induced by a variety of stimuli, including dsRNA and virus infection (48, 56). During our studies of MV growth in PKR-sufficient and PKR-deficient cells, we consistently observed a reduced cytopathic effect after the infection of PKRkd cells compared to that observed after the infection of PKR+ or PKRkd-con cells, as illustrated by the phase-contrast images shown in Fig. 4A. To quantify the effect, the colorimetric MTT assay was employed. The MTT assay revealed that the number of viable PKRkd cells was indeed increased compared to the numbers of viable PKR+ or PKRkd-con cells following infection, especially with the Cko virus (Fig. 4B). As MV is known to induce apoptosis (22), we further considered whether the PKR-mediated cell death was apoptotic. Western immunoblot analyses using antibodies against the caspase substrate PARP revealed much less PARP cleavage in the PKRkd cells deficient in PKR than in either of the PKR-sufficient control cells (Fig. 4C), consistent with the notion that the PKR-mediated cell death was apoptotic. The degree of PARP cleavage seen in cells with sufficient PKR was least in WT-infected cells, intermediate in Vko mutant-infected cells, and most pronounced in Cko mutant-infected cells (Fig. 4C), which is consistent with the data in previous reports implicating C and V proteins in protecting against MV-induced cell death (14, 53). Taken together, these results suggest that PKR plays an important role in the apoptosis induced by MV.
FIG. 4.
MV-induced apoptosis is impaired in PKR-deficient cells. PKR+, PKRkd, and PKRkd-con cells were infected with WT, Vko, or Cko virus or left uninfected (UI). (A) Phase-contrast images taken at 36 h postinfection. (B) Results of the colorimetric MTT assay to measure cell viability 48 h postinfection, displayed as percentages of the number of viable uninfected cells (n = 4). * , P of <0.05 by Student's t test for comparison of the Cko virus yields in PKRkd cells and those in PKR+ or PKRkd-con cells. (C) Western immunoblot analyses performed on whole-cell extracts prepared at 36 h postinfection, using antibodies against human PARP and β-actin. The quantity of PARP cleavage based on the immunoblots, expressed as a ratio of PARP85 to total PARP [PARP85 ÷ (PARP85 + PARP116)], is shown below each lane.
The inhibition of apoptosis is not sufficient to eliminate the growth defect of C-deficient MV.
In the experiments described above, the observed PKR-mediated inhibition of Cko mutant virus multiplication and viral protein production correlated with PKR-mediated apoptosis. We therefore considered whether PKR prevented the growth of the Cko virus by enhancing cell death induced by the virus.
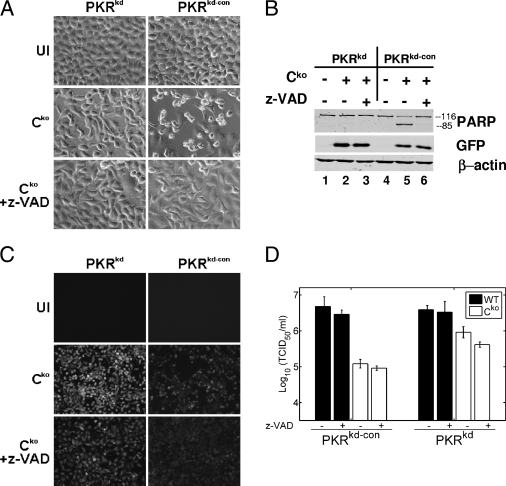
To test this hypothesis, we asked whether the inhibition of apoptosis alone could increase the yield of Cko virus in cells with sufficient PKR to the levels obtained in PKRkd cells. Accordingly, we examined the growth of the WT and Cko mutant viruses in PKRkd and PKRkd-con cells either treated with the pancaspase inhibitor z-VAD-fmk or left untreated. The treatment of PKR-sufficient cells with z-VAD-fmk prevented the cells from undergoing apoptosis induced by infection with the Cko virus, as revealed by a lack of cell blebbing and rounding (Fig. 5A) and also a lack of PARP cleavage (Fig. 5B, compare lanes 5 and 6). As expected, very little apoptosis in the PKRkd cell cultures was observed, either in the presence or in the absence of the caspase antagonist (Fig. 5A and B, lanes 2 and 3). Surprisingly, treatment with z-VAD-fmk did not increase Cko virus growth in PKR-sufficient cells (Fig. 5C and D). If anything, titers of both the WT and Cko viruses were slightly decreased by treatment with z-VAD-fmk, which may reflect the drug's nonspecific inhibition (33) of an essential protease (i.e., furin) (5) needed for virus replication. Virus protein production was unaffected by treatment with the drug (Fig. 5B and data not shown). These results suggest that the inhibition of apoptosis alone is not enough to restore the growth of the Cko virus and suggest that PKR's antiviral pathway is not dependent on the induction of apoptosis.
FIG. 5.
Inhibition of apoptosis does not restore the WT host range phenotype in Cko MV. PKRkd and PKRkd-con cells infected with Cko or WT virus (MOI of 5) or left uninfected (UI) were treated with medium alone or medium containing the caspase inhibitor z-VAD-fmk (100 μM). Cells were analyzed at 36 h postinfection. (A) Phase-contrast images. (B) Western immunoblot analyses using antibodies against human PARP, GFP, and β-actin. (C) Fluorescence images. (D) Virus yields determined by 50% TCID50 titration on Vero cells. The results shown are means ± standard deviations (n = 4).
DISCUSSION
As host cells evolve mechanisms to interfere with virus infection, viruses counteract by evolving strategies to suppress host defenses. In the case of paramyxoviruses, the virus-host interplay is modulated by virulence factors encoded by the P gene (27, 44, 48). The P gene products, including the P, V, and C proteins, evade the host's innate immune system by using a variety of mechanisms that include not only the inhibition of IFN signal transduction, but also the suppression of IFN production (26, 27, 44, 48). The MV V, C, and P proteins are implicated in the inhibition of STAT1 and STAT2 phosphorylation and nuclear accumulation and thus impair the expression of IFN-regulated genes (7, 18, 23, 38, 39, 50, 51, 60). V protein inhibits IFN induction by interfering with the mda-5 pathway (1, 13), while C protein may also suppress IFN production (17, 35, 36). Moreover, both the V and C proteins of MV function to prevent apoptosis (14, 53), which may be an additional host mechanism to limit virus multiplication and spread.
Here, we present evidence that the RNA-dependent protein kinase PKR plays a key role in restricting MV multiplication and inhibiting MV-induced apoptosis and that the MV C protein counteracts these PKR-mediated activities. Using HeLa cells with sufficient PKR expression, we showed that the Cko MV mutant produced ∼30- to 50-fold-lower infectious titers than WT or V-deficient virus. The growth defect of this Cko virus correlated with reduced levels of expression of several virus proteins. These findings are consistent with the results of previous studies that described growth and viral protein synthesis defects of C-deficient MV in a variety of cell lines and animal models (16-18, 21, 35, 53). We have now shown that, in HeLa cells deficient in PKR expression, the Cko virus growth defect was largely reversed—producing only ∼5-fold-lower titers of Cko virus than of WT virus—and that viral protein levels were almost entirely if not completely restored to WT levels. These results establish that PKR is a major contributor to the growth defect of the Cko virus.
What is the mechanism by which PKR inhibits the multiplication of the Cko mutant virus? Our results suggest that the induction of apoptosis is not a requirement for the PKR-mediated antiviral effect. Although increased Cko virus yields correlated with the decreased apoptosis of PKR-deficient cells, the inhibition of the apoptotic process of PKR-sufficient cells by the pharmacological caspase inhibitor z-VAD-fmk was not sufficient to complement the growth defect of the Cko virus.
The enhancement of Cko virus yields in PKR-deficient cells correlated with an increase in viral protein expression, suggesting that PKR limits Cko virus multiplication by interfering with the translation of viral proteins. The best-characterized substrate of PKR is translation initiation factor eIF-2α (48), which when phosphorylated leads to the inhibition of protein synthesis (47). Indeed, we observed increased PKR and eIF-2α phosphorylation by 24 h postinfection with the Cko virus relative to that observed after infection with the WT virus. The results of our studies carried out with a vaccine (Moraten-equivalent) strain of recombinant WT virus and an engineered isogenic Cko mutant of this strain in HeLa cells extend the recent findings of Nakatsu et al. (35), where viral translation inhibition was seen in A549 cells infected with a recombinant C-deficient MV derived from the virulent IC-B strain, in that we have definitively shown that PKR is an upstream cellular factor responsible for the protein expression defect. Although eIF-2α phosphorylation appears to be the major mechanism leading to the decreased protein expression of the Cko virus, it is conceivable that other PKR-mediated effects, including PKR action on PP2A phosphatase activity (58) or NF-κB-dependent signal transduction responses (6), also play a role.
The WT and Vko viruses that we utilized expressed comparable levels of C protein and replicated equally well in PKR-sufficient and -deficient cells, whereas the Cko virus that did not express detectable C protein grew poorly in PKR-sufficient cells but well in PKR-deficient cells. We considered a mechanism of C antagonism of PKR in which C inhibits PKR activation through a physical interaction. We were unable to detect such an interaction through coimmunoprecipitation analysis (data not shown). Another possibility is that the effect of MV C protein on PKR activity is indirect; for example, C protein may inhibit the production of aberrant RNAs which have the ability to activate PKR. C proteins of several paramyxoviruses have been shown previously to regulate viral RNA synthesis (3, 36, 45, 52). Our data for MVvac, like those of Takeuchi et al. for Sendai virus (52), favor an indirect model of C protein inhibition of PKR activity; WT MV was unable to inhibit PKR activation following infection with a mutant vaccinia virus (VVΔE3L) that was shown previously to induce high levels of PKR-dependent eIF-2α phosphorylation (61). Likewise, Newcastle disease virus-induced phosphorylation of PKR was not impaired by prior infection with Sendai virus (52).
Although protein expression by the Cko virus was nearly or completely restored in PKR-deficient cells, the virus yield was only partially rescued, suggesting that the decreased growth of the Cko virus may not be due entirely to impaired viral protein expression. The C protein, therefore, likely affects additional steps of virus multiplication, such as virus assembly (16), and/or host factors in addition to PKR are important for limiting the replication of the Cko virus. As C protein has been shown previously to be an effector of IFN synthesis (17, 35, 36), IFN-regulated proteins other than PKR may be involved in restricting MV replication.
The P/V/C gene products of paramyxoviruses are known virulence factors, determinants of host range and viral pathogenesis, and modulators of cell apoptotic and signal transduction pathways (26-28, 44, 48). The V and C proteins play important roles in antagonizing STAT-dependent IFN-α/β signal transduction (26, 44). Our results, together with those of the recent studies of simian virus 5 (SV5) by Gainey et al. (24) and of Sendai virus by Takeuchi et al. (52), suggest that the V and C gene products are also important for antagonizing the PKR-mediated antiviral response. While we found that the C protein product, but not the V protein product, of the MV P gene is a PKR-dependent virulence factor and modulator of viral protein expression, Gainey et al. (24) reported that the P/V proteins of SV5 (which does not encode a C protein) are important viral factors for antagonizing PKR-mediated translation inhibition. More recently, Takeuchi et al. (52) showed that the C protein of Sendai virus inhibits PKR activation by limiting the generation of dsRNAs. Some notable differences exist between our experiments and those of Gainey et al. and Takeuchi et al., however. Upon infection of PKR-deficient cells compared to PKR-sufficient cells with the Cko MV, we observed the complementation of the apoptosis induction phenotype and the partial restoration of infectious virus yield, biological parameters that were not considered by the other studies with SV5 and Sendai virus (24, 52). Moreover, our results which establish PKR as a mediator of MV-induced apoptosis are to our knowledge the first example of a role for PKR in paramyxovirus-induced cell death.
Acknowledgments
This work was supported in part by research grants AI-12520, AI-20611, and AI-63476 from the National Institute of Allergy and Infectious Diseases, NIH, Public Health Service.
We thank P. Zhang of our laboratory for helpful discussions.
Footnotes
Published ahead of print on 12 November 2008.
REFERENCES
- 1.Andrejeva, J., K. S. Childs, D. F. Young, T. S. Carlos, N. Stock, S. Goodbourn, and R. E. Randall. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-β promoter. Proc. Natl. Acad. Sci. USA 10117264-17269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balachandran, S., P. C. Roberts, T. Kipperman, K. N. Bhalla, R. W. Compans, D. R. Archer, and G. N. Barber. 2000. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/caspase-8 death signaling pathway. J. Virol. 741513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bankamp, B., J. Wilson, W. J. Bellini, and P. A. Rota. 2005. Identification of naturally occurring amino acid variations that affect the ability of the measles virus C protein to regulate genome replication and transcription. Virology 336120-129. [DOI] [PubMed] [Google Scholar]
- 4.Bellini, W. J., G. Englund, S. Rozenblatt, H. Arnheiter, and C. D. Richardson. 1985. Measles virus P gene codes for two proteins. J. Virol. 53908-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolt, G., and I. R. Pedersen. 1998. The role of subtilisin-like proprotein convertases for cleavage of the measles virus fusion glycoprotein in different cell types. Virology 252387-398. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet, M. C., R. Weil, E. Dam, A. G. Hovanessian, and E. F. Meurs. 2000. PKR stimulates NF-κB irrespective of its kinase function by interacting with the IκB kinase complex. Mol. Cell. Biol. 204532-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caignard, G., M. Guerbois, J.-L. Labernardière, Y. Jacob, L. M. Jones, F. Wild, F. Tangy, and P.-O. Vidalain. 2007. Measles virus V protein blocks Jak1-mediated phosphorylation of STAT1 to escape IFN-α/β signaling. Virology 368351-362. [DOI] [PubMed] [Google Scholar]
- 8.Cathomen, T., H. Y. Naim, and R. Cattaneo. 1998. Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J. Virol. 721224-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cattaneo, R., K. Kaelin, K. Baczko, and M. A. Billeter. 1989. Measles virus editing provides an additional cysteine-rich protein. Cell 56759-764. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control. 2008. Measles—United States, January 1-April 25, 2008. MMWR Morb. Mort. Wkly. Rep. 57494-498. [PubMed] [Google Scholar]
- 11.Chang, H. W., and B. L. Jacobs. 1993. Identification of a conserved motif that is necessary for binding of the vaccinia virus E3L gene-products to double-stranded-RNA. Virology 194537-547. [DOI] [PubMed] [Google Scholar]
- 12.Chang, H. W., L. H. Uribe, and B. L. Jacobs. 1995. Rescue of vaccinia virus lacking the E3L gene by mutants of E3L. J. Virol. 696605-6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Childs, K., N. Stock, C. Ross, J. Andrejeva, L. Hilton, M. Skinner, R. Randall, and S. Goodbourn. 2007. Mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology 359190-200. [DOI] [PubMed] [Google Scholar]
- 14.Cruz, C. D., H. Palosaari, J.-P. Parisien, P. Devaux, R. Cattaneo, T. Ouchi, and C. M. Horvath. 2006. Measles virus V protein inhibits p53 family member p73. J. Virol. 805644-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Der, S. D., Y.-L. Yang, C. Weissmann, and B. R. G. Williams. 1997. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc. Natl. Acad. Sci. USA 943279-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devaux, P., and R. Cattaneo. 2004. Measles virus phosphoprotein gene products: conformational flexibility of the P/V protein amino-terminal domain and C protein infectivity factor function. J. Virol. 7811632-11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devaux, P., G. Hodge, M. B. McChesney, and R. Cattaneo. 2008. Attenuation of V- or C-defective measles viruses: infection control by the inflammatory and interferon responses of rhesus monkeys. J. Virol. 825359-5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devaux, P., V. von Messling, W. Songsungthong, C. Springfeld, and R. Cattaneo. 2007. Tyrosine 110 in the measles virus phosphoprotein is required to block STAT1 phosphorylation. Virology 36072-83. [DOI] [PubMed] [Google Scholar]
- 19.Dhib-Jalbut, S., J. Xia, H. Rangaviggula, Y.-Y. Fang, and T. Lee. 1999. Failure of measles virus to activate nuclear factor-κB in neuronal cells: implications on the immune response to viral infections in the central nervous system. J. Immunol. 1624024-4029. [PubMed] [Google Scholar]
- 20.Donze, O., J. Deng, J. Curran, R. Sladek, D. Picard, and N. Sonenberg. 2004. The protein kinase PKR: a molecular clock that sequentially activates survival and death programs. EMBO J. 23564-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Escoffier, C., S. Manie, S. Vincent, C. P. Muller, M. Billeter, and D. Gerlier. 1999. Nonstructural C protein is required for efficient measles virus replication in human peripheral blood cells. J. Virol. 731695-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esolen, L., S. Park, J. Hardwick, and D. Griffin. 1995. Apoptosis as a cause of death in measles virus-infected cells. J. Virol. 693955-3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fontana, J. M., B. Bankamp, W. J. Bellini, and P. A. Rota. 2008. Regulation of interferon signaling by the C and V proteins from attenuated and wild-type strains of measles virus. Virology 37471-81. [DOI] [PubMed] [Google Scholar]
- 24.Gainey, M. D., P. J. Dillon, K. M. Clark, M. J. Manuse, and G. D. Parks. 2008. Paramyxovirus-induced shutoff of host and viral protein synthesis: role of the P and V proteins in limiting PKR activation. J. Virol. 82828-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goh, K. C., M. J. deVeer, and B. R. G. Williams. 2000. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 194292-4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gotoh, B., T. Komatsu, K. Takeuchi, and J. Yokoo. 2001. Paramyxovirus accessory proteins as interferon antagonists. Microbiol. Immunol. 45787-800. [DOI] [PubMed] [Google Scholar]
- 27.Griffin, D. 2007. Measles virus, p. 1551-1585. In P. Howley, D. Griffin, R. Lamb, M. Martin, B. Roizman, and S. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 28.Haller, O., G. Kochs, and F. Weber. 2006. The interferon response circuit: induction and suppression by pathogenic viruses. Virology 344119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansen, V. A. A., N. Stollenwerk, H. J. Jensen, M. E. Ramsay, W. J. Edmunds, and C. J. Rhodes. 2003. Measles outbreaks in a population with declining vaccine uptake. Science 301804. [DOI] [PubMed] [Google Scholar]
- 30.Karber, G. 1931. Beitrag zur kollektiven behandlung pharmakologischer reihenversuche. Naunyn-Schmiedeberg's Arch. Pharmacol. 162480-483. [Google Scholar]
- 31.Kumar, A., Y. L. Yang, V. Flati, S. Der, S. Kadereit, A. Deb, J. Haque, L. Reis, C. Weissmann, and B. R. G. Williams. 1997. Deficient cytokine signaling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-κB. EMBO J. 16406-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liston, P., and D. J. Briedis. 1994. Measles virus V protein binds zinc. Virology 198399-404. [DOI] [PubMed] [Google Scholar]
- 33.Martin, U., N. Jarasch, M. Nestler, A. Rassmann, T. Munder, S. Seitz, R. Zell, P. Wutzler, and A. Henke. 2007. Antiviral effects of pan-caspase inhibitors on the replication of coxsackievirus B3. Apoptosis 12525-533. [DOI] [PubMed] [Google Scholar]
- 34.McCormack, S. J., D. C. Thomis, and C. E. Samuel. 1992. Mechanism of interferon action: identification of a RNA binding domain within the N-terminal region of the human RNA-dependent p1/eIF-2α protein kinase. Virology 18847-56. [DOI] [PubMed] [Google Scholar]
- 35.Nakatsu, Y., M. Takeda, S. Ohno, R. Koga, and Y. Yanagi. 2006. Translational inhibition and increased interferon induction in cells infected with C protein-deficient measles virus. J. Virol. 8011861-11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakatsu, Y., M. Takeda, S. Ohno, Y. Shirogane, M. Iwasaki, and Y. Yanagi. 2008. Measles virus circumvents the host interferon response by different actions of the C and V proteins. J. Virol. 828296-8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navaratnarajah, C. K., V. H. L. Leonard, and R. Cattaneo. 2009. Measles virus: glycoprotein complex assembly, receptor attachment, and cell entry, p. 59-76. In D. E. Griffin and M. B. A. Oldstone (ed.), Measles history and basic biology, vol. 329. Springer-Verlag, New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohno, S., N. Ono, M. Takeda, K. Takeuchi, and Y. Yanagi. 2004. Dissection of measles virus V protein in relation to its ability to block alpha/beta interferon signal transduction. J. Gen. Virol. 852991-2999. [DOI] [PubMed] [Google Scholar]
- 39.Palosaari, H., J.-P. Parisien, J. J. Rodriguez, C. M. Ulane, and C. M. Horvath. 2003. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J. Virol. 777635-7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pathak, V. K., D. Schindler, and J. W. Hershey. 1988. Generation of a mutant form of protein synthesis initiation factor eIF-2 lacking the site of phosphorylation by eIF-2 kinases. Mol. Cell. Biol. 8993-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patterson, J. B., D. Thomas, H. Lewicki, M. A. Billeter, and M. B. A. Oldstone. 2000. V and C proteins of measles virus function as virulence factors in vivo. Virology 26780-89. [DOI] [PubMed] [Google Scholar]
- 42.Radecke, F., and M. A. Billeter. 1996. The nonstructural C protein is not essential for multiplication of Edmonston B strain measles virus in cultured cells. Virology 217418-421. [DOI] [PubMed] [Google Scholar]
- 43.Ramachandran, A., J.-P. Parisien, and C. M. Horvath. 2008. STAT2 is a primary target for measles virus V protein-mediated IFN-α/β signaling inhibition. J. Virol. 828330-8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Randall, R. E., and S. Goodbourn. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 891-47. [DOI] [PubMed] [Google Scholar]
- 45.Reutter, G. L., C. Cortese-Grogan, J. Wilson, and S. A. Moyer. 2001. Mutations in the measles virus C protein that up regulate viral RNA synthesis. Virology 285100-109. [DOI] [PubMed] [Google Scholar]
- 46.Samuel, C. E. 1979. Mechanism of interferon action: phosphorylation of protein synthesis initiation factor eIF-2 in interferon-treated human cells by a ribosome-associated kinase processing site specificity similar to hemin-regulated rabbit reticulocyte kinase. Proc. Natl. Acad. Sci. USA 76600-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samuel, C. E. 1993. The eIF-2 alpha protein kinases, regulators of translation in eukaryotes from yeasts to humans. J. Biol. Chem. 2687603-7606. [PubMed] [Google Scholar]
- 48.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider, H., K. Kaelin, and M. A. Billeter. 1997. Recombinant measles viruses defective for RNA editing and V protein synthesis are viable in cultured cells. Virology 227314-322. [DOI] [PubMed] [Google Scholar]
- 50.Shaffer, J. A., W. J. Bellini, and P. A. Rota. 2003. The C protein of measles virus inhibits the type I interferon response. Virology 315389-397. [DOI] [PubMed] [Google Scholar]
- 51.Takeuchi, K., S.-I. Kadota, M. Takeda, N. Miyajima, and K. Nagata. 2003. Measles virus V protein blocks interferon IFN-α/β but not IFN-γ signaling by inhibiting STAT1 and STAT2 phosphorylation. FEBS Lett. 545177-182. [DOI] [PubMed] [Google Scholar]
- 52.Takeuchi, K., T. Komatsu, Y. Kitagawa, K. Sada, and B. Gotoh. 2008. Sendai virus C protein plays a role in restricting PKR activation by limiting the generation of intracellular double-stranded RNA. J. Virol. 8210102-10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeuchi, K., M. Takeda, N. Miyajima, Y. Ami, N. Nagata, Y. Suzaki, J. Shahnewaz, S.-I. Kadota, and K. Nagata. 2005. Stringent requirement for the C protein of wild-type measles virus for growth both in vitro and in macaques. J. Virol. 797838-7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka, H., and C. Samuel. 1994. Mechanism of interferon action: structure of the mouse PKR gene encoding the interferon-inducible RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 917995-7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tober, C., M. Seufert, H. Schneider, M. A. Billeter, I. C. D. Johnston, S. Niewiesk, V. ter Meulen, and S. Schneider-Schaulies. 1998. Expression of measles virus V protein is associated with pathogenicity and control of viral RNA synthesis. J. Virol. 728124-8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toth, A. M., P. Zhang, S. Das, C. X. George, and C. E. Samuel. 2006. Interferon action and the double-stranded RNA-dependent enzymes ADAR1 adenosine deaminase and PKR protein kinase. Prog. Nucleic Acid Res. Mol. Biol. 81369-434. [DOI] [PubMed] [Google Scholar]
- 57.Wardrop, E. A., and D. J. Briedis. 1991. Characterization of V protein in measles virus-infected cells. J. Virol. 653421-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams, B. R. G. 2001. Signal integration via PKR. Sci. STKE 2001re2. [DOI] [PubMed] [Google Scholar]
- 59.World Health Organization. 2008. Measles. World Health Organization, Geneva, Switzerland.
- 60.Yokota, S.-I., H. Saito, T. Kubota, N. Yokosawa, K.-I. Amano, and N. Fujii. 2003. Measles virus suppresses interferon-α signaling pathway: suppression of Jak1 phosphorylation and association of viral accessory proteins, C and V, with interferon-α receptor complex. Virology 306135-146. [DOI] [PubMed] [Google Scholar]
- 61.Zhang, P., B. L. Jacobs, and C. E. Samuel. 2008. Loss of protein kinase PKR expression in human HeLa cells complements the vaccinia virus E3L deletion mutant phenotype by restoration of viral protein synthesis. J. Virol. 82840-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, P., and C. E. Samuel. 2007. Protein kinase PKR plays a stimulus- and virus-dependent role in apoptotic death and virus multiplication in human cells. J. Virol. 818192-8200. [DOI] [PMC free article] [PubMed] [Google Scholar]