Abstract
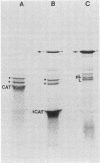
Streptococcus mutans LM7 (Bratthall serotype e) chromosomal DNA was partially digested with EcoRI and ligated into the positive-selection plasmid vector pOP203(A2+). The ligation mixture was transformed into Escherichia coli, and transformants were selected for tetracycline resistance. Recombinant-bearing clones were screened for their ability to ferment raffinose, using the procedure of Robeson et al. (J. Bacteriol. 153:211-221, 1983). One raffinose-fermenting clone was isolated and found to contain a plasmid with an insert consisting of four EcoRI fragments totalling approximately 10.3 kilobases (kb). This strain was capable of growth on defined medium plus raffinose or sucrose and generated reducing sugars from a sucrose substrate. Southern hybridization analysis of the four EcoRI fragments revealed homology not only to S. mutans LM7 chromosomal DNA but also to S. mutans serotypes b, c, and f. Subcloning of this fragment array into a streptococcal E. coli shuttle vector indicated that a 2.4-kb EcoRI fragment was essential for sucrase activity. E. coli minicell experiments revealed a gene product of 55 kilodaltons. These data along with restriction endonuclease analysis and Southern hybridizations suggested that the cloned S. mutans LM7 gene was closely related to the gtfA gene cloned by Robeson et al. from S. mutans PS13 (Bratthall serotype c). The shuttle plasmid containing the 2.4-kb fragment was transformed into Streptococcus sanguis, which subsequently displayed increased sucrase activity in both intracellular and extracellular fractions. Elevated levels of synthesis of alcohol-insoluble and water-insoluble glucans were observed with crude extracellular fractions of the S. sanguis strain bearing the 2.4-kb fragment. An isolate cured of the shuttle plasmid plus the 2.4-kb fragment displayed wild-type S. sanguis glucan synthesis. In S. sanguis, this gtfA allele may play a role in glucan synthesis by interacting with extant high-molecular-weight glucosyltransferases.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Bright J. S., Rosen S., Chorpenning F. W. Survey of the seven serological types of Streptococcus mutans in six-year-old children. J Dent Res. 1977 Nov;56(11):1421–1421. doi: 10.1177/00220345770560112501. [DOI] [PubMed] [Google Scholar]
- Chassy B. M., Beall J. R., Bielawski R. M., Porter E. V., Donkersloot J. A. Occurrence and distribution of sucrose-metabolizing enzymes in oral streptococci. Infect Immun. 1976 Aug;14(2):408–415. doi: 10.1128/iai.14.2.408-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. C., Modrich P. Positive-selection cloning vehicle useful for overproduction of hybrid proteins. J Bacteriol. 1983 May;154(2):1005–1008. doi: 10.1128/jb.154.2.1005-1008.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Evans R. P., Winter R. B., Macrina F. L. Molecular cloning of a pIP501 derivative yields a model replicon for the study of streptococcal conjugation. J Gen Microbiol. 1985 Jan;131(1):145–153. doi: 10.1099/00221287-131-1-145. [DOI] [PubMed] [Google Scholar]
- GUSTAFSSON B. E., QUENSEL C. E., LANKE L. S., LUNDQVIST C., GRAHNEN H., BONOW B. E., KRASSE B. The Vipeholm dental caries study; the effect of different levels of carbohydrate intake on caries activity in 436 individuals observed for five years. Acta Odontol Scand. 1954 Sep;11(3-4):232–264. doi: 10.3109/00016355308993925. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F., Chludzinski A. M. Rapid filter paper assay for the dextransucrase activity from Streptococcus mutans. J Dent Res. 1974 Nov-Dec;53(6):1355–1360. doi: 10.1177/00220345740530061101. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Dental caries. Annu Rev Med. 1975;26:121–136. doi: 10.1146/annurev.me.26.020175.001005. [DOI] [PubMed] [Google Scholar]
- Itakura K., Hirose T., Crea R., Riggs A. D., Heyneker H. L., Bolivar F., Boyer H. W. Expression in Escherichia coli of a chemically synthesized gene for the hormone somatostatin. Science. 1977 Dec 9;198(4321):1056–1063. doi: 10.1126/science.412251. [DOI] [PubMed] [Google Scholar]
- Kennedy N., Beutin L., Achtman M., Skurray R., Rahmsdorf U., Herrlich P. Conjugation proteins encoded by the F sex factor. Nature. 1977 Dec 15;270(5638):580–585. doi: 10.1038/270580a0. [DOI] [PubMed] [Google Scholar]
- Kral T. A., Daneo-Moore L. Glycerol incorporation in certain oral streptococci. Infect Immun. 1980 Dec;30(3):759–765. doi: 10.1128/iai.30.3.759-765.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of extracellular glucosyltransferase activity of Steptococcus mutans. Infect Immun. 1975 Oct;12(4):738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H., Ingersoll L. Immunological relationships between glucosyltransferases from Streptococcus mutans serotypes. Infect Immun. 1976 Sep;14(3):636–644. doi: 10.1128/iai.14.3.636-644.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lawson J. W., Gooder H. Growth and development of competence in the group H streptococci. J Bacteriol. 1970 Jun;102(3):820–825. doi: 10.1128/jb.102.3.820-825.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton N. W., Kakehashi S., Fitzgerald R. J. Recovery of specific "caries-inducing" streptococci from carious lesions in the teeth of children. Arch Oral Biol. 1970 May;15(5):461–463. doi: 10.1016/0003-9969(70)90073-7. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Evans R. P., Tobian J. A., Hartley D. L., Clewell D. B., Jones K. R. Novel shuttle plasmid vehicles for Escherichia-Streptococcus transgeneric cloning. Gene. 1983 Nov;25(1):145–150. doi: 10.1016/0378-1119(83)90176-2. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Jones K. R., Wood P. H. Chimeric streptococcal plasmids and their use as molecular cloning vehicles in Streptococcus sanguis (Challis). J Bacteriol. 1980 Sep;143(3):1425–1435. doi: 10.1128/jb.143.3.1425-1435.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Tobian J. A., Jones K. R., Evans R. P., Clewell D. B. A cloning vector able to replicate in Escherichia coli and Streptococcus sanguis. Gene. 1982 Oct;19(3):345–353. doi: 10.1016/0378-1119(82)90025-7. [DOI] [PubMed] [Google Scholar]
- Morrison D. A. Transformation in Escherichia coli: cryogenic preservation of competent cells. J Bacteriol. 1977 Oct;132(1):349–351. doi: 10.1128/jb.132.1.349-351.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perch B., Kjems E., Ravn T. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):357–370. doi: 10.1111/j.1699-0463.1974.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Roberts T. M., Swanberg S. L., Poteete A., Riedel G., Backman K. A plasmid cloning vehicle allowing a positive selection for inserted fragments. Gene. 1980 Dec;12(1-2):123–127. doi: 10.1016/0378-1119(80)90022-0. [DOI] [PubMed] [Google Scholar]
- Robeson J. P., Barletta R. G., Curtiss R., 3rd Expression of a Streptococcus mutans glucosyltransferase gene in Escherichia coli. J Bacteriol. 1983 Jan;153(1):211–221. doi: 10.1128/jb.153.1.211-221.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M. Essential dependence of smooth surface caries on, and augmentation of fissure caries by, sucrose and Streptococcus mutans infection. Infect Immun. 1979 Aug;25(2):526–531. doi: 10.1128/iai.25.2.526-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L., Fitzgerald R. J., Larson R. H. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):197–203. doi: 10.1128/iai.10.1.197-203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobian J. A., Cline M. L., Macrina F. L. Characterization and expression of a cloned tetracycline resistance determinant from the chromosome of Streptococcus mutans. J Bacteriol. 1984 Nov;160(2):556–563. doi: 10.1128/jb.160.2.556-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter R. B., Gold L. Overproduction of bacteriophage Q beta maturation (A2) protein leads to cell lysis. Cell. 1983 Jul;33(3):877–885. doi: 10.1016/0092-8674(83)90030-2. [DOI] [PubMed] [Google Scholar]