Abstract
The viral protein Nef contributes to the optimal infectivity of human and simian immunodeficiency viruses. The requirement for Nef during viral biogenesis particles suggests that Nef might play a role in this process. Alternatively, because Nef is incorporated into viruses, it might play a role when progeny virions reach target cells. We challenged these hypotheses by manipulating the amounts of Nef incorporated in viruses while keeping its expression level constant in producer cells. This was achieved by forcing the incorporation of Nef into viral particles by fusing a Vpr sequence to the C-terminal end of Nef. A cleavage site for the viral protease was introduced between Nef and Vpr to allow the release of Nef fragments from the fusion protein during virus maturation. We show that the resulting Nef-CS-Vpr fusion partially retains the ability of Nef to downregulate cell surface CD4 and that high amounts of Nef-CS-Vpr are incorporated into viral particles compared with what is seen for wild-type Nef. The fusion protein is processed during virion maturation and releases Nef fragments similar to those found in viruses produced in the presence of wild-type Nef. Unlike viruses produced in the presence of wild-type Nef, viruses produced in the presence of Nef-CS-Vpr do not have an increase in infectivity and are as poorly infectious as viruses produced in the absence of Nef. These findings demonstrate that the presence of Nef in viral particles is not sufficient to increase human immunodeficiency virus type 1 infectivity and suggest that Nef plays a role during the biogenesis of viral particles.
In addition to the structural proteins common to all retroviruses, the genome of primate lentiviruses encode auxiliary proteins that regulate viral fitness in hosts. Among these proteins, Nef has proven to play a major role in the progression of infections with simian (SIV) and human (HIV) immunodeficiency viruses toward AIDS in primates (18, 28, 39, 40, 46, 47, 71). Single-round infection assays based on cell-free recombinant viruses also revealed that wild-type (WT) HIV type 1 (HIV-1) and SIV are more infectious than mutants lacking Nef expression (Δnef viruses) (4, 9, 37). The infectivity of Δnef viruses can be rescued by providing Nef in trans in virus-producing cells but not in target cells (4). This Nef-dependent increase of infectivity observed in vitro might explain the high pathogenicity of WT HIV and SIV in vivo compared with their Δnef counterparts.
Little is known about the mechanisms underlying the gain of infectivity conferred by Nef to HIV-1. One of the best-characterized functions of Nef concerns its ability to affect the trafficking of proteins expressed at the cell surface. Nef was initially shown to downregulate the cell surface expression levels of proteins such as CD4 and a subset of major histocompatibility complex class I molecules (22, 38, 76), but additional studies revealed that the expression of HIV but also SIV Nef promotes the downregulation of other cell surface proteins, such as mature major histocompatibility complex class II molecules, CD28, CD8, CXCR4, and CCR5, and the transferrin receptor (43, 55, 58, 59, 83, 84, 87; for a review, see reference 48). The Nef-dependent downregulation of CD4 in virus-producing cells was shown to favor efficient HIV-1 envelope glycoprotein incorporation into virions by preventing the formation of CD4/gp120 complexes at the cell surface (6, 26, 73). An effect of Nef on T-cell activation leading to optimal conditions for HIV-1 and SIV replication was also suggested (75, 81, 92); however, it has been shown that Nef-dependent T-cell protein kinase activation and infectivity increase are two independent properties of Nef (52, 74). The fact that infectivity enhancement can be seen when viruses are produced in non-T cells, such as 293T, HeLa, or COS-7 cells, which do not express CD4, further suggests that mechanisms other than CD4 downregulation contribute to the Nef-dependent increase of viral infectivity.
The route of viral entry was proposed to dictate the Nef dependence for optimal infectivity. Indeed, Nef increases the infectivity of HIV-1-based recombinant viruses pseudotyped with envelope glycoproteins from HIV-1 or amphotropic murine leukemia virus that promote membrane fusion and entry at the cell surface. On the contrary, Nef does not increase the infectivity of viruses pseudotyped with the G protein of the vesicular stomatitis virus (VSV-G) for which fusion and entry take place after endocytosis upon the acidification of endocytic vesicles (4, 5, 19, 53, 60). A specific role for Nef in HIV-1 entry into target cells at the fusion step has been suggested (72), but other studies reported that similar amounts of viral cores are released in target cells upon membrane fusion, regardless of the presence of Nef during the assembly of viral particles (16, 60, 86). Given that the Nef dependence for optimal infectivity of viral particles is relieved when target cells are treated with drugs that disrupt the cytoskeleton, it has been suggested that Nef might facilitate postfusion events such as the trafficking of viral cores through the cortical actin network (15), although this hypothesis has been recently challenged (65).
The incorporation of cellular proteins and accessory viral proteins into virions can have a positive or negative impact on HIV infectivity. ICAM-1 (intracellular adhesion molecule 1) expressed at the surface of virion-producing cells was shown to be incorporated into the viral membrane and to increase infectivity of progeny virions by facilitating HIV-1 attachment to target cells (10, 34, 70). On the contrary, the Vif-dependent increase of HIV-1 infectivity relies on the specific exclusion of APOBEC3G (the cellular apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3G) from virions during assembly (56, 79, 80; for a review, see reference 21). HIV and SIV Vpr proteins are incorporated into virions through an interaction with the p6 domain of the Gag precursor during assembly (1, 49, 50, 64, 78). Although Vpr is dispensable for optimal infection of dividing cells, the association of Vpr with viral cores of incoming virions and with the preintegration complex was shown to play an important role in facilitating the infection of macrophages (25, 42, 66, 67, 88).
Like the HIV and SIV Vpr accessory protein, Nef is incorporated into HIV and SIV virions (13, 33, 51, 89). The analysis of WT and Δnef viruses failed to identify significant differences in the protein content of virions other than the presence of Nef itself in WT viruses. This suggested that Nef molecules associated with the cores of incoming viruses might increase the efficiency of postentry events, resulting in an overall increase of viral infectivity. In support of this hypothesis, Nef was shown to increase the retrotranscription and expression of the integrated viral genome (4, 77, 92). However, since the infectivity of Δnef viruses cannot be rescued by providing Nef in target cells (4), the increase of infectivity might also rely on a Nef-dependent mechanism that takes place during viral biogenesis.
Here, we tried to determine whether the CD4-independent effect of Nef on HIV-1 infectivity depends on a specific function of Nef molecules incorporated into viral particles or on a function of Nef during the assembly of the virus. To this aim, we manipulated the proportionality between the amounts of Nef expressed during the assembly of the virus and the amounts of Nef incorporated into viral particles. Our results show that the presence of intact Nef in viral particles is not sufficient to increase HIV-1 infectivity.
MATERIALS AND METHODS
Cell culture.
293T and HeLa cells were obtained through the ATCC and grown in complete medium (Dulbecco's minimal essential medium supplemented with 10% fetal calf serum [FCS], 100 IU of penicillin/ml, and 100 μg of streptomycin/ml [Invitrogen, Cergy-Pontoise, France]). HeLa CD4 cells were obtained through the AIDS Research and Reference Reagents Program, Division of AIDS, NIAID, NIH, and grown in complete medium supplemented with G418, 0.2 mg/ml (PAA, Austria). All cells were grown at 37°C with 5% CO2.
Plasmids.
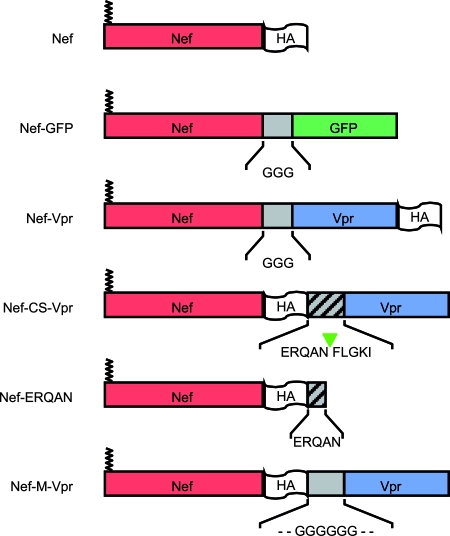
The construct encoding hemagglutinin (HA)-tagged HIV-1 VprLAI and green fluorescent protein-tagged Nef (Nef-GFP) were described elsewhere (29, 55). The pSRαNefLAI plasmids encoding HA-tagged WT HIV-1 NefLAI and the LL165/AA mutant were a kind gift from H. Gottlinger (University of Massachusetts Medical School, Worcester, MA). The Nef Xho construct used in mock transfections was generated by digesting pSRαNefLAI with XhoI, followed by 3′ end filling and blunt-end ligation as described elsewhere (30). The Nef-Vpr fusion was generated by standard overlapping PCR techniques. Briefly, the DNA sequences of NefLAI and VprLAI were amplified separately with specific primers containing appropriate sequences to allow 3′-to-5′-end annealing of the two PCR products in a second PCR and the generation of a Nef-Vpr fragment. The primers were also designed to allow the addition of three glycine residues between Nef and Vpr, the HA tag at the C-terminal end of Vpr, and a BamHI restriction site 3′ to the sequence of the HA tag. The final fragment was digested with XhoI and BamHI and subcloned into the pSRαNefLAI plasmid digested with the same restriction enzymes. Nef-M-Vpr and Nef-CS-Vpr were generated using the same strategy and appropriate primers to allow the addition of the HA tag between Nef and Vpr. A glycine stretch or the ERQANFLGKI sequence corresponding to the NC-p1 junction in the Gag polyprotein was inserted between the sequence of the HA tag and that of Vpr to generate Nef-M-Vpr and Nef-CS-Vpr, respectively. The amino acids are numbered according to the sequence of HIV-1 NefLAI.
Viral production.
Single-round-infection HIV-1 virions carrying the GFP gene were made in 293T cells as follows. 293T cells were seeded in T75 flasks at a density of ∼2.5 × 106 cells/T75 flask and transfected after 16 h by the calcium phosphate precipitation technique with a DNA mix containing the HIV-1-packaging plasmid (pCMVΔP1ΔenvpA), the HIV-1 vector encoding GFP (pHIvec2.GFP), the plasmids encoding the HIV-1 envelope glycoprotein HXBc2 (pSVIIIenv), and pSRαNefLAI at concentrations of 3, 3, 1, and 1 μg/T75 flask, respectively. In the case where viruses were pseudotyped with VSV-G glycoprotein, the pHCMV-G plasmid and a Rev-encoding plasmid (each at 1 μg/T75 flask) were used instead of pSVIIIenv HXBc2. The amounts of plasmids encoding HA-tagged Vpr and Nef-Vpr constructs were adjusted to achieve expression levels similar to that of WT Nef in producing cells. Elsewhere (see Fig. 5 below), 1 μg of the construct encoding Nef-GFP was used. Cells were washed 6 and 24 h posttransfection and then cultured in 10 ml of complete medium for 24 h, after which the supernatant was collected, spun to remove cell debris, filtered through a 0.45-μm-pore-size filter, and either ultracentrifuged to pellet viruses or stored at −80°C until use in infectivity experiments.
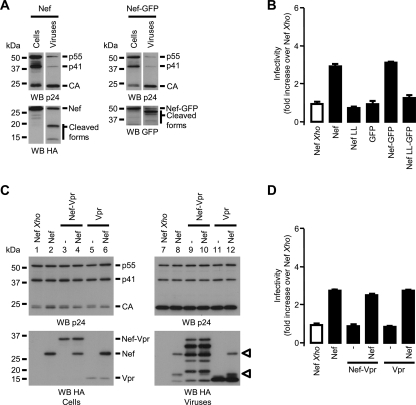
FIG. 5.
Differential consequences of the addition of GFP or Vpr to the C-terminal end of Nef. (A) Viruses were produced in the presence of WT Nef (left) or Nef-GFP (right). Cells and viruses were analyzed as described in the Fig. 2A legend. Nef-GFP was detected with GFP-specific antibodies. (B) Viral infectivities of viruses produced in the presence of the indicated variants of Nef were analyzed as described in the Fig. 2C legend. (C) Viruses were produced in the presence of the indicated combination of Nef, Nef-Vpr, or Vpr. Cells and viruses were analyzed as described in the Fig. 2A legend. Arrowheads point out the 27- and the 18-kDa forms of Nef. (D) Viral infectivities of viruses produced in the presence of the indicated combinations of Nef, Nef-Vpr, and/or Vpr were measured as indicated in the Fig. 2C legend and normalized to that of viruses produced in the presence of Nef Xho.
Ultracentrifugation.
Whole-virus pelleting was performed as follows. Supernatants obtained from 2.5 T75 flasks (25 ml/sample) were layered on 4 ml of phosphate-buffered saline (PBS) supplemented with 20% (wt/vol) sucrose in centrifuge tubes (Beckman Instruments, Inc., California). Samples were placed in swinging buckets on a Surespin 630 rotor and centrifuged at 4°C for 1.5 h at 27,000 rpm in a Sorvall centrifuge. Viral cores were pelleted as described previously with minor modifications (2). Briefly, supernatants (25 ml/sample) were centrifuged through a sucrose step gradient composed of 1.5 ml of PBS-sucrose 7.5%, 3.5 ml of PBS-sucrose 15%, and 3.5 ml of PBS-sucrose 30%. Igepal CA-630 (Sigma-Aldrich, Inc., MO) was added to the 15% sucrose fraction (0.03%) to allow shedding of the viral membrane. Tubes were centrifuged as described above. Pelleted material was resuspended in 80 μl of Laemmli sample buffer containing 5% of 2-mercaptoethanol (Sigma-Aldrich). The p24 contents of viral particles were analyzed by resolving 2 μl of the samples by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The incorporation of Nef, Vpr, and fusion protein into viral particles was analyzed by loading 20 μl of the samples on the gels.
Electrophoresis and Western blot analysis.
Virus-producing cells were solubilized and protein quantification was performed as described elsewhere (7), except that Igepal CA-630 was used instead of 3-[(-cholamidopropyl)dimethylammonio]-2-hydroxyl-1-propanesulfonic acid (CHAPS) in the lysis buffer. Cleared lysates containing ∼20 μg of total proteins were resolved by SDS-PAGE with 12% acrylamide (Sigma-Aldrich) to analyze the expression levels of the molecular species described in the figure legends. Proteins were then visualized by Western blotting followed by immunodetection with the following reagents. Rabbit polyclonal antibodies directed against HIV-1 p24 and p17 were obtained through the AIDS Research and Reference Reagents Program, Division of AIDS, NIAID, NIH and used at dilutions of 1:10,000 and 1:1,000, respectively. The rat polyclonal antibody directed against the HA tag (clone 3-F10; Roche Diagnostics GmbH, Germany) was used at a 1:500 dilution. GFP was detected with the rabbit polyclonal antibody (sc-8334; Santa Cruz Biotechnology, Inc., California) used at a 1:500 dilution. Secondary reagents were peroxidase-coupled goat antibodies directed against mouse or rat immunoglobulin G and used at a 1:2,000 dilution (Sigma-Aldrich).
Infectivity assay.
Virus concentration in cell culture supernatant was measured by reverse transcriptase (RT) assay as described elsewhere (69). HeLa CD4 cells were seeded in 24-well plates at a density of 30,000 cells/well. After an overnight incubation, viruses were added to the cells. The same RT counts were used for all the viruses used in the same experiment and varied from 200,000 to 400,000 RT cpm/ml between different experiments depending on the viral production. Sixteen hours postinfection, cells were washed once and cultured for 48 h. Cells were then harvested with trypsin (Invitrogen), fixed in 500 μl of PBS supplemented with formaldehyde at 3.7% (Sigma-Aldrich), and analyzed on a Cytomix FC500 cytometer (Beckman-Coulter, California). The percentage of GFP-positive cells was determined by analyzing the data with the RXP analysis software. Depending on the viral input, Nef-negative viruses infected between 5 and 10% of cells. Viral infectivity was calculated by normalizing the percentage of GFP-positive cells of each sample to that obtained in cells infected with viruses produced in the absence of Nef.
CD4 downregulation.
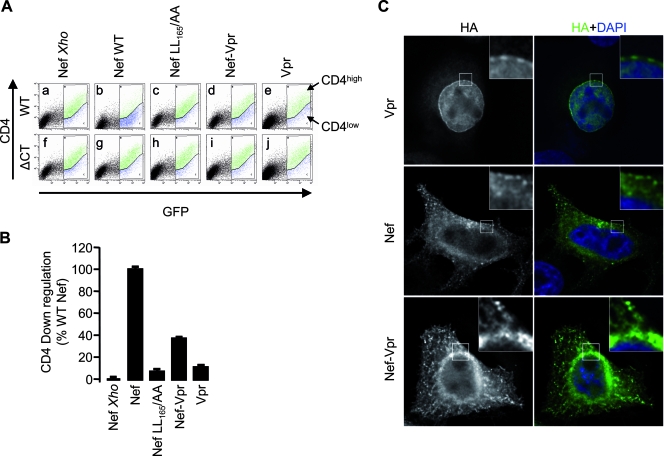
CD4 downregulation was analyzed in 293T cells coexpressing CD4 and various forms of Nef. 293T cells were seeded in T75 flasks at a density of ∼2.5 × 106 cells/T75 and transfected after 16 h by the calcium phosphate precipitation technique with a DNA mix containing a GFP-coding plasmid, plasmids coding WT CD4 or CD4 ΔCT, and the different Nef constructs at concentrations of 2, 4, and 7 μg/T75 flask, respectively. Cells were washed 6 hours posttransfection and harvested after an additional 36-h incubation time. Cells were detached with PBS and incubated in PBS supplemented with FCS at 2% (PBS-FCS). Cells (∼1 × 106) were pelleted and incubated for 45 min on ice with 20 μl of a 1:2 dilution of the phycoerythrin-Cy5-coupled anti-CD4 antibody (BD Biosciences, Belgium) in PBS-FCS. Cells were then washed twice in PBS-FCS and once in PBS, fixed in 500 μl of PBS supplemented with formaldehyde at 3.7%, and analyzed on a Cytomix FC500 cytometer. Data were analyzed with the RXP analysis software. The efficiency of CD4 downregulation was calculated for each construct as follows: (pCD4ΔCT construct/pCD4ΔCT Nef Xho) − (pCD4WT construct/pCD4WT Nef Xho), where p represents the percentage of cells coexpressing the indicated form of CD4 and Nef in the CD4high gate. The number obtained was normalized to that obtained in the presence of WT Nef and expressed as a percentage.
Immunofluorescence.
HeLa cells were seeded in 24-well plates on coverslips at a density of ∼125,000 cells/well and transfected after 16 h by Lipofectamine 2000 (Invitrogen) with the indicated constructs at a concentration of 0.25 μg/well. Cells were washed 6 h posttransfection, and coverslips were processed after a 24-h incubation time. Cells were washed twice in PBS and fixed for 30 min at 4°C in PBS supplemented with paraformaldehyde at 4% (Sigma-Aldrich). The reaction was quenched for 10 min at room temperature with PBS supplemented with 50 mM NH4Cl, and the mixture was washed twice in PBS supplemented with bovine serum albumin (BSA) at 0.1% (Sigma-Aldrich). Coverslips were then incubated with PBS-BSA supplemented with Triton X-100 at 0.1% (Sigma-Aldrich) and the anti-HA 3F10 antibody at a 1:250 dilution. Cells were incubated 45 min on ice, washed twice in PBS-BSA, and incubated 45 min on ice in PBS-BSA supplemented with Alexa488-coupled goat antibodies directed against rat immunoglobulin G (Invitrogen) at a 1:250 dilution. Coverslips were then washed twice in PBS-BSA and once in PBS and inverted on glass slides in mounting medium containing 4′,6′-diamidino-2-phenylindole (DAPI) (SlowFade; Invitrogen). Samples were examined under an epifluorescence microscope (Zeiss) with a cooled charge-coupled device camera (Micromax; Roper Scientific, Evry, France) by use of a plan apochromatic 100× objective coupled to a piezo positioning system allowing the acquisition of images in the z plane. The acquisition of images and the deconvolution of z stacks were done with MetaMorph (Universal Imaging, Downingtown, PA).
RESULTS
Viral infectivity correlates with cellular and viral levels of Nef.
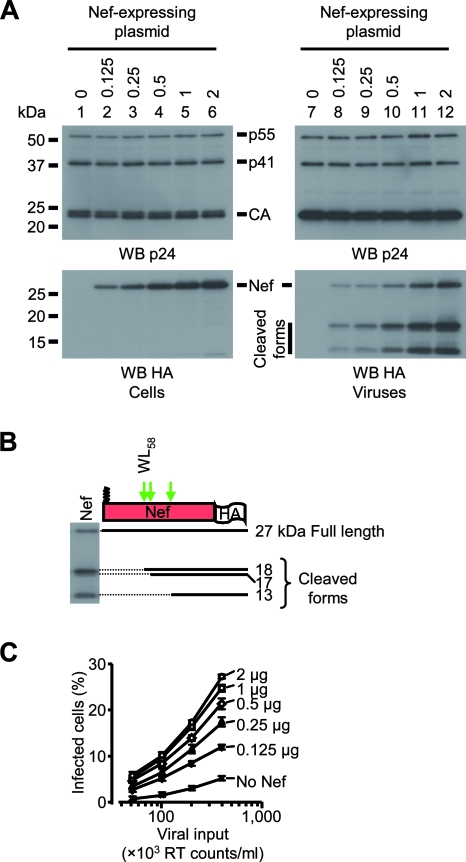
In order to determine whether HIV-1 infectivity enhancement requires the presence of Nef in virion-producing cells or in the virus, we analyzed the correlation between the cellular amounts of Nef, the amounts of Nef incorporated into viral particles, and viral infectivity. 293T cells were cotransfected with a fixed amount of plasmids required for the production of infectious viral particles and increasing amounts of a plasmid encoding WT Nef fused to the HA tag at its C-terminal end (Fig. 1). As shown in Fig. 2A, left, this resulted in increasing amounts of Nef expressed in virus-producing cells (bottom) without affecting the expression of the Gag polyprotein (top). The presence of Nef in virions pelleted from the cell culture supernatant was analyzed (Fig. 2A, right). The expression of increasing amounts of Nef in virus-producing cells did not affect the amount of viral particles released (Fig. 2A, right, top); however, it resulted in the cosedimentation of increasing amounts of Nef with virions (bottom). The presence of several additional anti-HA reactive molecular species migrating faster than Nef in the pelleted material reflects the processing of Nef by the viral protease (13) and confirms the presence of Nef in virions (Fig. 2B). The 18-kDa band likely derives from the processing of Nef at the WL58 motif previously identified, while the 13-kDa band corresponds to the cleavage of Nef in its C-terminal domain (35, 36). This fragment has been previously described (61), but the exact cleavage site has not been characterized yet. A minor band with an apparent molecular mass of 17 kDa was also detected, suggesting the presence of an additional cleavage site C terminal to the WL58 motif. We then analyzed the infectivity of viruses produced in the absence or in the presence of increasing amounts of Nef. As shown in Fig. 2C, Nef enhanced HIV-1 infectivity up to fivefold. Moreover, viral infectivity was strictly correlated to the amount of Nef expressed in the cells and incorporated into virions. From this correlation, one cannot conclude whether the increase of HIV-1 infectivity requires the presence of Nef in producer cells or in virions, and the necessity for uncoupling cellular and viral levels of Nef is also highlighted.
FIG. 1.
Nef-based constructs used in this study. The various domains of the chimeric proteins are illustrated. Except for Nef-GFP, all constructs are HA tagged to facilitate the immunodetection in Western blotting experiments. The HA tag was added to the C-terminal ends of the proteins in order to preserve the myristoylation site of Nef. In the case of Nef-CS-Vpr, an HIV-1 protease recognition motif corresponding to the NC-p1 junction in the Gag-Pol precursor was inserted between Nef and Vpr. The HA tag was moved between Nef and this motif in order to visualize Nef fragments generated from the cleavage of the fusion protein in the virus. A similar construct was also generated that contains a glycine stretch in place of the protease recognition motif. Because the processing of Nef-CS-Vpr is supposed to release Nef fragments with the ERQAN sequence C terminal to the HA tag, a Nef-ERQAN was also generated and analyzed in incorporation and infectivity experiments.
FIG. 2.
Expression of Nef during HIV-1 production: impact on viral infectivity. GFP reporter viruses were produced in 293T cells in the presence of increasing amounts of a Nef-coding plasmid (0.125 to 2 μg/T75 flasks). (A) Producer cells were solubilized (left) and viruses were pelleted from the cell culture supernatant by ultracentrifugation (right). Samples were resolved by SDS-PAGE and visualized by Western blotting (WB) followed by immunodetection with p24 (top)- and HA (bottom)-specific antibodies. (B) Molecular species derived from the processing of Nef in mature viruses. The lane showing the incorporation of Nef into viral particles (panel A, lane 10) is shown next to a schematic representation of Nef processed by HIV-1 protease. The position of the processing sites (green arrows) was determined according to published data and the measurement of the mobility of the anti-HA reactive proteins in the gel. (C) Viral infectivity was assayed with HeLa CD4 cells. HeLa CD4 cells were incubated with increasing amounts of viruses (50 × 103 to 400 × 103 RT cpm/ml) produced in the presence of the indicated amounts of Nef-coding plasmid, and the percentages of GFP-positive cells were measured by fluorescence-activated cell sorting 60 h postinfection.
A Nef-Vpr fusion protein is efficiently incorporated into HIV-1 particles.
In order to uncouple the concentrations of Nef in producer cells and in virions, we generated a construct encoding a fusion between Nef and Vpr. Previous work showed that more Vpr than Nef molecules are incorporated into virions (62, 90), suggesting that a Nef-Vpr fusion would be more efficiently incorporated into virions than WT Nef. In addition, various proteins fused to Vpr were shown to be efficiently incorporated into HIV-1 particles while retaining their original properties (17, 32, 57, 93, 94). Since the myristoylation of Nef is important for most of the functions of Nef investigated so far (8, 82, 91), we decided to fuse Vpr to the C-terminal end of Nef as depicted in Fig. 1.
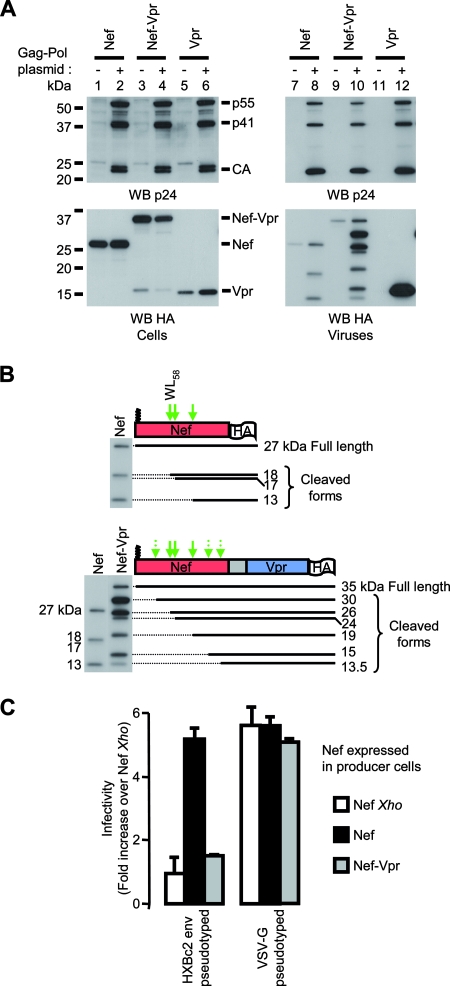
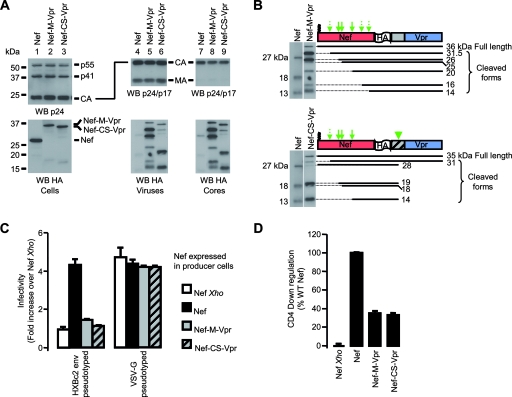
Viruses were produced in 293T cells expressing Nef, Nef-Vpr, or Vpr. Cell lysates and viruses were analyzed by Western blotting as described for Fig. 2. Figure 3A, top, shows that the presence of Nef, Nef-Vpr, or Vpr affected neither the expression levels of Gag in producer cells (lanes 2, 4, and 6) nor the release of viral particles in the supernatant (lanes 8, 10, and 12). Traces of Nef and Nef-Vpr but no Vpr was detected in the material pelleted from the supernatant of cells that did not express Gag-Pol (Fig. 3A, bottom, lanes 7 and 9). In the presence of Gag-Pol, however, significant amounts of Nef, Nef-Vpr, and Vpr cosedimented with virions. The incorporation of these proteins into viral particles was further confirmed by the presence of cleaved forms of Nef and Nef-Vpr (Fig. 3A, bottom, lanes 8 and 10). In agreement with published results (62, 90), higher amounts of Vpr than of Nef were found to be incorporated into viral particles in spite of similar cellular expression levels (Fig. 3A, bottom). Fusion of a Vpr sequence to the C-terminal end of Nef increased the amounts of Nef associated with viral particles (Fig. 3A, bottom, compare lanes 8 and 10). Analysis of the molecular species derived from the Nef-Vpr fusion protein showed that the processing of Nef-Vpr by HIV-1 protease resembles that of WT Nef (Fig. 3B, bottom). The fragments with apparent molecular masses of 26, 24, and 19 kDa probably reflect the cleavage of Nef-Vpr at sites identified in Nef, but the fusion protein also appears to be susceptible to the viral protease at three additional sites, generating 30-, 15-, and 13.5-kDa fragments.
FIG. 3.
Characterization of viruses produced in the presence of Nef, Nef-Vpr, and Vpr. 293T cells were cotransfected with Gag-Pol in combination with either Nef, Nef-Vpr, or Vpr expression plasmids as indicated, along with the plasmids required for the production of infectious particles. (A) Cells and viruses were analyzed as described in the Fig. 2A legend. (B) The processing of the Nef-Vpr fusion protein (bottom) was analyzed as described in the Fig. 2B legend and compared to that of WT Nef (top). Thick arrows indicate Nef-WT cleavage sites; thin arrows indicate three additional sites where Nef-Vpr also appears to be susceptible to the viral protease. (C) HXBc2 Env- or VSV-G-pseudotyped viruses were produced in the absence or in the presence of WT Nef or the Nef-Vpr fusion protein. Viral infectivity was measured as described in the Fig. 2C legend and normalized to that of viruses produced in the presence of Nef Xho.
We then compared the infectivities of viruses produced in the presence of Nef or Nef-Vpr. Figure 3C shows that viruses pseudotyped with the HIV-1 HXBc2 Env and produced in the presence of Nef were fivefold as infectious as viruses produced in the absence of Nef (Nef Xho). On the contrary, no increase of infectivity was detected when viruses were produced in the presence of Nef-Vpr. Consistent with previous results (5), VSV-G-pseudotyped viruses were equally infectious regardless of the presence of Nef. Of note is that the infectivity of VSV-G-pseudotyped viruses produced in the presence of Nef-Vpr was identical to that of viruses produced in the absence or in the presence of Nef. This means that the lack of effect of Nef-Vpr on the viral infectivity of HXBc2 Env-pseudotyped viruses cannot be explained by a deleterious effect of Nef-Vpr on the overall viral fitness.
Altogether, the results reported in Fig. 3 show that the expression of the Nef-Vpr fusion protein in virus-producing cells is not able to restore the infectivity of Δnef viruses, even if the fusion protein is efficiently incorporated and processed in viral particles.
CD4 downregulation activity and subcellular localization of Nef-Vpr.
In order to gain insight into the functional differences between Nef and Nef-Vpr, we analyzed the ability of Nef-Vpr to downregulate cell surface CD4 in 293T cells. Figure 4A shows that WT Nef was able to downregulate cell surface levels of WT CD4 but had no effect on CD4ΔCT (compare dot plots a and b to f and g). The quantification of CD4 downregulation activity revealed that Nef-Vpr retained 40% of the activity of WT Nef (Fig. 4B). As expected, Vpr and the well-characterized Nef LL165/AA mutant that fails to downregulate CD4 (11, 23, 41) displayed similar residual activities in this assay. The subcellular localization of these proteins was analyzed by immunofluorescence microscopy. As shown in Fig. 4C, Vpr mostly localized to the nucleus (top) and an evident Vpr staining was detected around the nucleus in a DAPI-negative area (top, insets), confirming the accumulation of Vpr at the nuclear envelope (29, 88). The staining of Nef-expressing cells revealed a punctated pattern in the cytoplasm and at the plasma membrane (Fig. 4C, middle) compatible with endocytic vesicles and clathrin-coated pits as previously described (14). As opposed to Vpr, Nef was detected neither in the nucleus nor at the nuclear envelope (Fig. 4C, middle, insets). The staining of cells expressing Nef-Vpr revealed punctated structures in the cytoplasm and at the plasma membrane that were reminiscent of Nef; however, Nef-Vpr was also detected in a perinuclear region (Fig. 4C, bottom, insets) distinct from the nuclear envelope. These subtle differences in the localizations of Nef and Nef-Vpr might explain why Nef-Vpr conserved a partial activity for CD4 downregulation but failed to increase viral infectivity.
FIG. 4.
CD4 downregulation activity and subcellular localization of Nef-Vpr. (A) Cell surface CD4 expression was analyzed with cells coexpressing CD4 or CD4ΔCT along with Nef variants or Vpr as indicated. Cells were also cotransfected with a plasmid encoding GFP to gate the analysis of cell surface CD4 expression on transfected cells. Dot plots of cells stained with a CD4-specific antibody are shown. (B) Quantification of the CD4 downregulation activity of Nef variants measured as described for panel A. (C) Cellular distribution of Nef, Nef-Vpr, and Vpr. HeLa cells were grown on coverslips and transfected with the indicated HA-tagged constructs. Twenty-four hours posttransfection, cells were permeabilized and stained with an HA-specific antibody and an Alexa488-coupled secondary antibody. Coverslips were transferred to slides in mounting medium supplemented with DAPI and samples were analyzed by immunofluorescence microscopy followed by deconvolution as described in Materials and Methods.
Vpr has a cis-inhibiting effect on the functions of Nef required for infectivity enhancement.
Figure 4 demonstrates that Nef-Vpr retains 40% of the CD4 downregulation activity mediated by Nef but lacks the specific functions that are important for viral infectivity enhancement. In order to determine whether this lack of function is specific to the addition of the Vpr sequence to the C-terminal end of Nef, the activity of another Nef-based fusion protein containing the GFP sequence (Fig. 1) was analyzed. It has been shown that the Nef-GFP fusion protein downregulates CD4 as efficiently as the native Nef protein and that the same motifs of Nef are required for CD4 downregulation both for WT Nef and in the context of the Nef-GFP fusion (11, 23, 41). We thus characterized the Nef-GFP fusion protein in incorporation and infectivity assays. Figure 5A shows that Nef-GFP was efficiently incorporated and processed in virions and was also able to complement Δnef viruses in the infectivity assay (Fig. 5B). As described previously (27), the introduction of the LL165/AA mutation in Nef severely altered the ability of Nef to increase viral infectivity. Therefore, these results indicate that the Vpr sequence specifically inhibits the functions of Nef that are important for infectivity enhancement in the context of the Nef-Vpr fusion protein.
We then investigated the inhibitory effect of Vpr on these functions. To address this question, viruses were produced in cells expressing Nef alone or in combination with Nef-Vpr or Vpr. The incorporation of these proteins and the infectivities of the corresponding virions were analyzed. Consistent with previous results (Fig. 3A), Nef-Vpr and Vpr were incorporated into viral particles better than WT Nef with respect to their cellular expression levels (Fig. 5C, bottom). The coexpression of Nef with either Nef-Vpr or Vpr resulted in the incorporation of both proteins in amounts comparable to those of proteins expressed alone (Fig. 5C, bottom, compare lanes 8, 9, and 11 to lanes 8, 10, and 12). Regardless of the proteins coexpressed with Nef in producer cells and coincorporated into viral particles, viruses produced in these conditions were threefold more infectious than viruses produced in the absence of Nef (Fig. 5D). This indicates that in the context of the Nef-Vpr fusion, Vpr has a cis- but not a trans-inhibitory effect on the functions of Nef required for infectivity enhancement.
Intravirion release of Nef from a Nef-CS-Vpr fusion protein does not rescue Nef functions essential for viral infectivity enhancement.
The use of Vpr as a carrier results in a more efficient incorporation of the Nef-Vpr fusion protein into virions than WT Nef; however, the Vpr sequence has a cis-inhibitory effect on Nef-mediated increase of viral infectivity. In order to release native Nef into virions after virus maturation, we generated a Nef-Vpr fusion protein containing an HIV-1 protease-specific cleavage site between Nef and Vpr (Nef-CS-Vpr). Another construct that included a glycine linker in place of the protease cleavage site (Nef-M-Vpr) was also generated and used as a control (Fig. 1).
Similar to what was observed with Nef-Vpr, high amounts of Nef-M-Vpr and Nef-CS-Vpr fusion protein were incorporated into viral particles relative to amounts of WT Nef (Fig. 6A, bottom, lanes 4 to 6). Figure 6B also shows that the molecular species generated from the processing of the Nef-M-Vpr fusion protein were similar to those generated from Nef-Vpr (Fig. 3B). The presence of a cleavage motif between Nef and Vpr modified the pattern of Nef-associated bands detected in virions (Fig. 6A, bottom, compare lanes 5 and 6), indicating that Nef-CS-Vpr is efficiently processed at the protease cleavage site. The comparison of the molecular species generated from the Nef-M-Vpr and Nef-CS-Vpr fusion proteins also revealed that most of the Nef-CS-Vpr molecules incorporated into virions were cleaved between Nef and Vpr (Fig. 6B). Nef released from Nef-CS-Vpr was itself processed at sites previously identified in Nef. In addition, Fig. 6A shows that the molecular species generated from the processing of the Nef-CS-Vpr fusion protein cosedimented with viral cores (bottom, compare lanes 4 to 6 and 7 to 9).
FIG. 6.
Characterization of a Nef-Vpr fusion protein containing a protease specific cleavage site between Nef and Vpr. (A) Viruses were produced in the presence of WT Nef, Nef-M-Vpr, or Nef-CS-Vpr as indicated. Cells (left) and viruses (middle) were analyzed as described in the Fig. 2A legend. Viral core purification (right) was performed by pelleting cell culture supernatant through a thin detergent layer as indicated in Materials and Methods. Viral matrix and capsid were detected with p17- and p24-specific antibodies, respectively. Film obtained with a short exposure time (30 s) is shown in order to better distinguish Nef-Vpr-derived fragments in whole viruses and cores. This explains the weak signal obtained with Nef, since Nef-Vpr fusion proteins are better incorporated than Nef. (B) The processing of the Nef-M-Vpr (top) and Nef-CS-Vpr (bottom) fusion proteins was analyzed as described in the Fig. 2B legend. For Nef lanes, film obtained with a longer exposure time was used (5 min). The arrowhead indicates cleavage of the Nef-CS-Vpr fusion protein at the HIV-1 protease-specific cleavage site (CS) introduced between Nef and VPR. The ability of the indicated constructs to increase HIV-1 infectivity (C) and to downregulate cell surface CD4 (D) was analyzed as described in the Fig. 2 and 4 legends, respectively.
The ability of Nef-CS-Vpr to increase HIV-1 infectivity was analyzed. As shown in Fig. 6C, there was no significant infectivity enhancement when viruses were produced in the presence of either Nef-M-Vpr or Nef-CS-Vpr compared with what was seen for viruses produced in the absence of Nef. The fact that both fusion proteins were 40% as efficient as WT Nef in the CD4 downregulation assay (Fig. 6D) confirms that Nef-CS-Vpr selectively lacks functions important for HIV-1 infectivity enhancement.
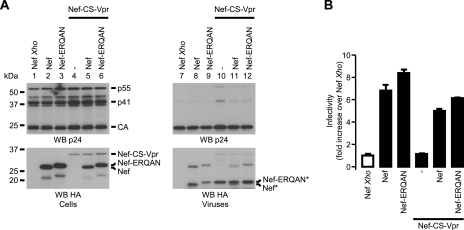
The ERQAN amino acids that remain fused to Nef following Nef-CS-Vpr processing likely account for the slight decrease in mobility of the molecular species derived from Nef-CS-Vpr compared with what was seen for fragments generated from the processing of WT Nef (Fig. 6B). In order to test this hypothesis and to rule out an inhibitory effect of these residues on the functions of Nef, an additional fusion protein was generated by fusing the ERQAN stretch C terminal to the HA tag (Nef-ERQAN [Fig. 1]). Viruses were produced in the presence of Nef, Nef-ERQAN, and Nef-CS-Vpr and analyzed as described previously. The amounts of Nef and Nef-CS-Vpr plasmids were adjusted to achieve similar incorporation levels of Nef into viruses, as opposed to what was the case for Fig. 3, 5, and 6, where similar expression levels were achieved in producing cells. Figure 7A shows that Nef and Nef-ERQAN display similar expression and incorporation levels in producing cells and viruses, respectively (bottom, lanes 2, 3, 8, and 9). As expected, the mobility of the Nef-ERQAN fusion was slightly lower than that of Nef both in cell lysates (Fig. 7A, lanes 2 and 3) and after cleavage by the viral protease (Nef* and Nef-ERQAN*; lanes 8 and 9). Of note is that the ∼19-kDa Nef fragment derived from the cleavage of the Nef-CS-Vpr by HIV-1 protease has an apparent molecular mass similar to that of Nef-ERQAN (Fig. 7A, compare lanes 9 and 10), which further confirms that the cleavage of Nef-CS-Vpr releases a Nef-ERQAN fragment in the virus. Figure 7B shows that viruses produced in the presence of Nef or Nef-ERQAN are more infectious than viruses produced in the absence of Nef. Similar to what was previously observed, Nef-CS-Vpr failed to increase viral infectivity. In addition, this fusion had a significant trans-inhibitory effect neither on Nef nor on Nef-ERQAN. Together, these results indicate that the presence of full-length Nef as well as Nef fragments in viruses is not sufficient to increase HIV-1 infectivity.
FIG. 7.
Characterization of the Nef-ERQAN fusion protein. (A) Viruses were produced in the presence of the indicated combination of Nef, Nef-ERQAN, and Nef-CS-Vpr. Cells and viruses were analyzed as described in the Fig. 2A legend. The amounts of Nef-CS-Vpr-coding plasmid cotransfected with Nef and Nef-ERQAN were adjusted to achieve incorporation of similar amounts of Nef-CS-Vpr and Nef in viral particles. (B) Viral infectivities of viruses produced in the presence of the indicated combinations of Nef, Nef-ERQAN, and Nef-CS-Vpr were measured as indicated in the Fig. 2C legend and normalized to that of viruses produced in the presence of Nef Xho. * indicates a product cleaved by the viral protease.
DISCUSSION
Soon after its initial description as a negative factor of HIV-1 replication (3, 54, 63), Nef was shown to be required for optimal HIV and SIV infectivity in vitro and pathogenicity in vivo (4, 9, 18, 28, 37, 39, 40, 46, 47, 71). Several mechanisms have been put forward to explain the gain of infectivity conferred by Nef to HIV-1; however, these mechanisms fail to fit all the experimental systems in which the gain of infectivity can be observed. Our work questioned the relationship between Nef incorporation into viral particles and viral infectivity. We characterized the ability of various Nef-based fusion proteins to increase viral infectivity. We found that the incorporation of the Nef-CS-Vpr fusion protein and the efficient release of full-length Nef and Nef fragments during virion maturation were not accompanied by an increase of viral infectivity. These data demonstrate that the presence of Nef in viral particles is not sufficient to promote the increase of viral infectivity.
The mechanism that drives Nef to the assembly platform of viral particles is not fully understood. Our results show that in the absence of Gag-Pol, significant amounts of Nef, Nef-Vpr, and Nef-GFP (not shown), but not of Vpr, can be pelleted from cell culture supernatants. This suggests that Nef can be found in vesicles secreted from cells independently of any other viral component. Thus, the incorporation of Nef into viral particles might only reflect the propensity of Nef to associate to membranes.
The single-amino-acid substitution (G2/A) that prevents Nef myristoylation abolishes the incorporation of Nef into viral particles and severely affects its ability to increase viral infectivity (8, 13, 20, 61, 91). Since myristoylation is also required for most of the other functions of Nef investigated so far, a direct link between the lack of incorporation and the lack of effect on viral infectivity cannot be drawn. Extensive site-directed mutagenesis has been performed to map the determinants on Nef responsible for its incorporation into virions. Mutation of the six lysine and arginine residues located in the amino terminus of Nef prevents its incorporation (8, 91). Despite retaining a WT-like myristoylation pattern, this mutant fails to increase viral infectivity and to downregulate cell surface CD4 (8, 91). Therefore, drawing definitive conclusions as to a particular phenotype is hazardous.
On the contrary, several Nef mutants that are efficiently incorporated into viral particles but fail to increase viral infectivity are reported in the literature. Such mutants do not allow one to discriminate whether Nef is required in producer cells or in the virus. A mutation in Nef affecting incorporation without compromising viral infectivity enhancement would support the hypothesis stating that the presence of Nef is required during viral production but not when progeny virions reach target cells. One study shows that a mutant of Nef, with alanine substitution for the N-terminal acidic cluster (EEEE69/AAAA), is not detected in pelleted viruses yet potently increases viral infectivity (31); however, these results have been challenged in a recent study mentioning that this mutant is efficiently incorporated into viral particles (68). These conflicting results leave the relevance of the incorporation of Nef into viral particles for infectivity increase unanswered.
Our results demonstrate that the presence of a functional form of Nef is important during the biogenesis of viral particles and that the presence of WT Nef in viral particles does not suffice to increase viral infectivity. In a recent work published by Qi and Aiken (68), Nef was fused to cyclophilin A (CypA) in order to control the incorporation of the resulting CypA-Nef fusion protein into viral particles. The authors showed that the CypA-Nef fusion protein retains the Nef-specific ability to increase HIV-1 infectivity. Of note is that when an alanine substitution was made for arginine 55 in CypA to prevent the CypA/Gag interaction, viruses produced in the presence of the corresponding mutant (CypA.R55A-Nef) were as poorly infectious as viruses produced in the absence of Nef. This indicates that Nef is not functional in the CypA.R55A-Nef construct and implies that it lacks the functions required to increase HIV-1 infectivity. In light of our results, this indicates that the Nef-dependent increase of HIV-1 infectivity requires a functional interaction between Nef and Gag. It also suggests that additional mechanisms are involved in virus-producing cells in order to achieve optimal infectivity, since the presence of Nef in viral particles does not suffice to increase HIV-1 infectivity.
If the Nef-dependent increase of HIV-1 infectivity reflects a specific function of Nef carried by incoming viruses to target cells, the loss of function of the Nef-CS-Vpr fusion protein suggests that Nef fragments released from WT Nef and Nef-CS-Vpr in mature virions might differ. It is well established that posttranslational modifications of Nef are required for Nef to exert specific functions such as CD4 downregulation (31, 45). Since Nef and Nef-Vpr do not have the same subcellular distribution, it is conceivable that Nef-Vpr does not undergo the same posttranslational modifications as WT Nef. Differences in maturation patterns could be responsible for the specific loss of infectivity enhancement of the Nef-Vpr fusion protein.
Alternatively, Nef could increase viral infectivity by modifying viral particles in the course of their biogenesis. In agreement with this hypothesis, Nef was found to alter the lipid composition of viral membranes (12, 95, 96) and to increase the incorporation of HIV-1 envelope glycoproteins into the membrane of the virus (6, 26, 73). Nef-associated kinases were also shown to phosphorylate HIV-1 matrix during viral assembly (85). Although these modifications might contribute to an overall increase of viral infectivity, additional mechanisms are likely involved. Indeed, it has been shown that the efficiency of the fusion process is not affected by Nef (16, 86) and that viruses harboring major deletions in matrix are as sensitive as WT viruses to the presence of Nef for optimal infectivity (30).
The LL165 motif of Nef is involved in the interaction between Nef and clathrin-associated AP complexes and its integrity is required for functions of Nef such as CD4 downregulation (11, 24, 27, 41, 44). The fact that the LL165/AA mutant no longer enhances viral infectivity suggests that an interaction between Nef and AP complexes is directly involved in this phenotype. It is possible that Nef affects the trafficking of cellular proteins and promotes their incorporation into viral particles. Nef might also promote the exclusion of cellular factor from viral particles by redirecting these factors away from HIV assembly platforms. A thorough proteomic analysis of WT and Δnef viruses should help identify differences that could explain the gain of infectivity conferred by Nef to WT viruses.
Our study suggests that Nef might promote structural modifications of the virus or might itself be subjected to structural modifications in order to increase HIV-1 infectivity. In either case, the Nef-dependent increase of HIV-1 infectivity should be investigated in virion-producing cells. The determination of the binding partners of Nef and Nef-Vpr might reveal factors that interact with Nef but fail to interact with Nef-Vpr. This might allow the identification of cellular factors specifically hijacked by Nef in the virus-producing cells and target cells to optimize postfusion events.
Acknowledgments
We thank Bastien Laumain for expert technical assistance in molecular biology.
This work was supported by grants from Agence Nationale de la Recherche sur le SIDA et les Hepatites Virales (ANRS) and Sidaction to S. Benichou and from Sidaction to S. Basmaciogullari. S. Basmaciogullari was supported successively by ANRS and INSERM fellowships. N. Laguette is supported by a Ph.D. fellowship from the French Ministry of Research.
Footnotes
Published ahead of print on 5 November 2008.
REFERENCES
- 1.Accola, M. A., A. A. Bukovsky, M. S. Jones, and H. G. Gottlinger. 1999. A conserved dileucine-containing motif in p6gag governs the particle association of Vpx and Vpr of simian immunodeficiency viruses SIVmac and SIVagm. J. Virol. 739992-9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Accola, M. A., A. Ohagen, and H. G. Gottlinger. 2000. Isolation of human immunodeficiency virus type 1 cores: retention of Vpr in the absence of p6gag. J. Virol. 746198-6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad, N., and S. Venkatesan. 1988. Nef protein of HIV-1 is a transcriptional repressor of HIV-1 LTR. Science 2411481-1485. [DOI] [PubMed] [Google Scholar]
- 4.Aiken, C., and D. Trono. 1995. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J. Virol. 695048-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 715871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arganaraz, E. R., M. Schindler, F. Kirchhoff, M. J. Cortes, and J. Lama. 2003. Enhanced CD4 down-modulation by late stage HIV-1 nef alleles is associated with increased Env incorporation and viral replication. J. Biol. Chem. 27833912-33919. [DOI] [PubMed] [Google Scholar]
- 7.Basmaciogullari, S., B. Pacheco, S. Bour, and J. Sodroski. 2006. Specific interaction of CXCR4 with CD4 and CD8alpha: functional analysis of the CD4/CXCR4 interaction in the context of HIV-1 envelope glycoprotein-mediated membrane fusion. Virology 35352-67. [DOI] [PubMed] [Google Scholar]
- 8.Bentham, M., S. Mazaleyrat, and M. Harris. 2006. Role of myristoylation and N-terminal basic residues in membrane association of the human immunodeficiency virus type 1 Nef protein. J. Gen. Virol. 87563-571. [DOI] [PubMed] [Google Scholar]
- 9.Bertsch, C., D. Cluet, C. Beyer, L. Gloeckler, A. Cecile, J. P. Gut, and A. M. Aubertin. 2002. Properties of a chimeric simian-human immunodeficiency virus expressing an hybrid HIV-1 Nef/SIVmac Nef protein. Arch. Virol. 1471963-1975. [DOI] [PubMed] [Google Scholar]
- 10.Bounou, S., J. F. Giguere, R. Cantin, C. Gilbert, M. Imbeault, G. Martin, and M. J. Tremblay. 2004. The importance of virus-associated host ICAM-1 in human immunodeficiency virus type 1 dissemination depends on the cellular context. FASEB J. 181294-1296. [DOI] [PubMed] [Google Scholar]
- 11.Bresnahan, P. A., W. Yonemoto, S. Ferrell, D. Williams-Herman, R. Geleziunas, and W. C. Greene. 1998. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol. 81235-1238. [DOI] [PubMed] [Google Scholar]
- 12.Brugger, B., E. Krautkramer, N. Tibroni, C. E. Munte, S. Rauch, I. Leibrecht, B. Glass, S. Breuer, M. Geyer, H. G. Krausslich, H. R. Kalbitzer, F. T. Wieland, and O. T. Fackler. 2007. Human immunodeficiency virus type 1 Nef protein modulates the lipid composition of virions and host cell membrane microdomains. Retrovirology 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bukovsky, A. A., T. Dorfman, A. Weimann, and H. G. Gottlinger. 1997. Nef association with human immunodeficiency virus type 1 virions and cleavage by the viral protease. J. Virol. 711013-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burtey, A., J. Z. Rappoport, J. Bouchet, S. Basmaciogullari, J. Guatelli, S. M. Simon, S. Benichou, and A. Benmerah. 2007. Dynamic interaction of HIV-1 Nef with the clathrin-mediated endocytic pathway at the plasma membrane. Traffic 861-76. [DOI] [PubMed] [Google Scholar]
- 15.Campbell, E. M., R. Nunez, and T. J. Hope. 2004. Disruption of the actin cytoskeleton can complement the ability of Nef to enhance human immunodeficiency virus type 1 infectivity. J. Virol. 785745-5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavrois, M., J. Neidleman, W. Yonemoto, D. Fenard, and W. C. Greene. 2004. HIV-1 virion fusion assay: uncoating not required and no effect of Nef on fusion. Virology 32836-44. [DOI] [PubMed] [Google Scholar]
- 17.Cavrois, M., C. De Noronha, and W. C. Greene. 2002. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 201151-1154. [DOI] [PubMed] [Google Scholar]
- 18.Chakrabarti, L. A., K. J. Metzner, T. Ivanovic, H. Cheng, J. Louis-Virelizier, R. I. Connor, and C. Cheng-Mayer. 2003. A truncated form of Nef selected during pathogenic reversion of simian immunodeficiency virus SIVmac239Δnef increases viral replication. J. Virol. 771245-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chazal, N., G. Singer, C. Aiken, M. L. Hammarskjold, and D. Rekosh. 2001. Human immunodeficiency virus type 1 particles pseudotyped with envelope proteins that fuse at low pH no longer require Nef for optimal infectivity. J. Virol. 754014-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen, Y. L., D. Trono, and D. Camaur. 1998. The proteolytic cleavage of human immunodeficiency virus type 1 Nef does not correlate with its ability to stimulate virion infectivity. J. Virol. 723178-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiu, Y. L., and W. C. Greene. 2008. The APOBEC3 cytidine deaminases: an innate defensive network opposing exogenous retroviruses and endogenous retroelements. Annu. Rev. Immunol. 26317-353. [DOI] [PubMed] [Google Scholar]
- 22.Cohen, G. B., R. T. Gandhi, D. M. Davis, O. Mandelboim, B. K. Chen, J. L. Strominger, and D. Baltimore. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10661-671. [DOI] [PubMed] [Google Scholar]
- 23.Coleman, S. H., R. Madrid, N. Van Damme, R. S. Mitchell, J. Bouchet, C. Servant, S. Pillai, S. Benichou, and J. C. Guatelli. 2006. Modulation of cellular protein trafficking by human immunodeficiency virus type 1 Nef: role of the acidic residue in the ExxxLL motif. J. Virol. 801837-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman, S. H., N. Van Damme, J. R. Day, C. M. Noviello, D. Hitchin, R. Madrid, S. Benichou, and J. C. Guatelli. 2005. Leucine-specific, functional interactions between human immunodeficiency virus type 1 Nef and adaptor protein complexes. J. Virol. 792066-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206935-944. [DOI] [PubMed] [Google Scholar]
- 26.Cortes, M. J., F. Wong-Staal, and J. Lama. 2002. Cell surface CD4 interferes with the infectivity of HIV-1 particles released from T cells. J. Biol. Chem. 2771770-1779. [DOI] [PubMed] [Google Scholar]
- 27.Craig, H. M., M. W. Pandori, and J. C. Guatelli. 1998. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc. Natl. Acad. Sci. USA 9511229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, V. A. Lawson, S. Crowe, A. Maerz, S. Sonza, J. Learmont, J. S. Sullivan, A. Cunningham, D. Dwyer, D. Dowton, and J. Mills. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270988-991. [DOI] [PubMed] [Google Scholar]
- 29.Depienne, C., P. Roques, C. Creminon, L. Fritsch, R. Casseron, D. Dormont, C. Dargemont, and S. Benichou. 2000. Cellular distribution and karyophilic properties of matrix, integrase, and Vpr proteins from the human and simian immunodeficiency viruses. Exp. Cell Res. 260387-395. [DOI] [PubMed] [Google Scholar]
- 30.Dorfman, T., E. Popova, M. Pizzato, and H. G. Gottlinger. 2002. Nef enhances human immunodeficiency virus type 1 infectivity in the absence of matrix. J. Virol. 766857-6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fackler, O. T., A. Moris, N. Tibroni, S. I. Giese, B. Glass, O. Schwartz, and H. G. Krausslich. 2006. Functional characterization of HIV-1 Nef mutants in the context of viral infection. Virology 351322-339. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher, T. M., III, M. A. Soares, S. McPhearson, H. Hui, M. Wiskerchen, M. A. Muesing, G. M. Shaw, A. D. Leavitt, J. D. Boeke, and B. H. Hahn. 1997. Complementation of integrase function in HIV-1 virions. EMBO J. 165123-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forshey, B. M., and C. Aiken. 2003. Disassembly of human immunodeficiency virus type 1 cores in vitro reveals association of Nef with the subviral ribonucleoprotein complex. J. Virol. 774409-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fortin, J. F., R. Cantin, G. Lamontagne, and M. Tremblay. 1997. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J. Virol. 713588-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freund, J., R. Kellner, J. Konvalinka, V. Wolber, H. G. Krausslich, and H. R. Kalbitzer. 1994. A possible regulation of negative factor (Nef) activity of human immunodeficiency virus type 1 by the viral protease. Eur. J. Biochem. 223589-593. [DOI] [PubMed] [Google Scholar]
- 36.Gaedigk-Nitschko, K., A. Schon, G. Wachinger, V. Erfle, and B. Kohleisen. 1995. Cleavage of recombinant and cell derived human immunodeficiency virus 1 (HIV-1) Nef protein by HIV-1 protease. FEBS Lett. 357275-278. [DOI] [PubMed] [Google Scholar]
- 37.Garcia, J. V., and J. L. Foster. 1996. Structural and functional correlates between HIV-1 and SIV Nef isolates. Virology 226161-166. [DOI] [PubMed] [Google Scholar]
- 38.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350508-511. [DOI] [PubMed] [Google Scholar]
- 39.Gorry, P. R., M. Churchill, J. Learmont, C. Cherry, W. B. Dyer, S. L. Wesselingh, and J. S. Sullivan. 2007. Replication-dependent pathogenicity of attenuated nef-deleted HIV-1 in vivo. J. Acquir. Immune Defic. Syndr. 46390-394. [DOI] [PubMed] [Google Scholar]
- 40.Gorry, P. R., D. A. McPhee, E. Verity, W. B. Dyer, S. L. Wesselingh, J. Learmont, J. S. Sullivan, M. Roche, J. J. Zaunders, D. Gabuzda, S. M. Crowe, J. Mills, S. R. Lewin, B. J. Brew, A. L. Cunningham, and M. J. Churchill. 2007. Pathogenicity and immunogenicity of attenuated, nef-deleted HIV-1 strains in vivo. Retrovirology 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenberg, M., L. DeTulleo, I. Rapoport, J. Skowronski, and T. Kirchhausen. 1998. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr. Biol. 81239-1242. [DOI] [PubMed] [Google Scholar]
- 42.Heinzinger, N. K., M. I. Bukinsky, S. A. Haggerty, A. M. Ragland, V. Kewalramani, M. A. Lee, H. E. Gendelman, L. Ratner, M. Stevenson, and M. Emerman. 1994. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA 917311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hrecka, K., T. Swigut, M. Schindler, F. Kirchhoff, and J. Skowronski. 2005. Nef proteins from diverse groups of primate lentiviruses downmodulate CXCR4 to inhibit migration to the chemokine stromal derived factor 1. J. Virol. 7910650-10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janvier, K., Y. Kato, M. Boehm, J. R. Rose, J. A. Martina, B. Y. Kim, S. Venkatesan, and J. S. Bonifacino. 2003. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J. Cell Biol. 1631281-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin, Y. J., C. Y. Cai, X. Zhang, and S. J. Burakoff. 2008. Lysine 144, a ubiquitin attachment site in HIV-1 Nef, is required for Nef-mediated CD4 down-regulation. J. Immunol. 1807878-7886.18523251 [Google Scholar]
- 46.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65651-662. [DOI] [PubMed] [Google Scholar]
- 47.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332228-232. [DOI] [PubMed] [Google Scholar]
- 48.Kirchhoff, F., M. Schindler, A. Specht, N. Arhel, and J. Munch. 2008. Role of Nef in primate lentiviral immunopathogenesis. Cell. Mol. Life Sci. 652621-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kondo, E., and H. G. Gottlinger. 1996. A conserved LXXLF sequence is the major determinant in p6gag required for the incorporation of human immunodeficiency virus type 1 Vpr. J. Virol. 70159-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kondo, E., F. Mammano, E. A. Cohen, and H. G. Gottlinger. 1995. The p6gag domain of human immunodeficiency virus type 1 is sufficient for the incorporation of Vpr into heterologous viral particles. J. Virol. 692759-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kotov, A., J. Zhou, P. Flicker, and C. Aiken. 1999. Association of Nef with the human immunodeficiency virus type 1 core. J. Virol. 738824-8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo, T., R. A. Livingston, and J. V. Garcia. 1997. Infectivity enhancement by human immunodeficiency virus type 1 Nef is independent of its association with a cellular serine/threonine kinase. J. Virol. 719524-9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo, T., J. L. Douglas, R. L. Livingston, and J. V. Garcia. 1998. Infectivity enhancement by HIV-1 Nef is dependent on the pathway of virus entry: implications for HIV-based gene transfer systems. Virology 241224-233. [DOI] [PubMed] [Google Scholar]
- 54.Luciw, P. A., C. Cheng-Mayer, and J. A. Levy. 1987. Mutational analysis of the human immunodeficiency virus: the orf-B region down-regulates virus replication. Proc. Natl. Acad. Sci. USA 841434-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madrid, R., K. Janvier, D. Hitchin, J. Day, S. Coleman, C. Noviello, J. Bouchet, A. Benmerah, J. Guatelli, and S. Benichou. 2005. Nef-induced alteration of the early/recycling endosomal compartment correlates with enhancement of HIV-1 infectivity. J. Biol. Chem. 2805032-5044. [DOI] [PubMed] [Google Scholar]
- 56.Mariani, R., D. Chen, B. Schrofelbauer, F. Navarro, R. Konig, B. Bollman, C. Munk, H. Nymark-McMahon, and N. R. Landau. 2003. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell 11421-31. [DOI] [PubMed] [Google Scholar]
- 57.McDonald, D., M. A. Vodicka, G. Lucero, T. M. Svitkina, G. G. Borisy, M. Emerman, and T. J. Hope. 2002. Visualization of the intracellular behavior of HIV in living cells. J. Cell Biol. 159441-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michel, N., K. Ganter, S. Venzke, J. Bitzegeio, O. T. Fackler, and O. T. Keppler. 2006. The Nef protein of human immunodeficiency virus is a broad-spectrum modulator of chemokine receptor cell surface levels that acts independently of classical motifs for receptor endocytosis and Galphai signaling. Mol. Biol. Cell 173578-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Michel, N., I. Allespach, S. Venzke, O. T. Fackler, and O. T. Keppler. 2005. The Nef protein of human immunodeficiency virus establishes superinfection immunity by a dual strategy to downregulate cell-surface CCR5 and CD4. Curr. Biol. 15714-723. [DOI] [PubMed] [Google Scholar]
- 60.Miller, M. D., M. T. Warmerdam, K. A. Page, M. B. Feinberg, and W. C. Greene. 1995. Expression of the human immunodeficiency virus type 1 (HIV-1) nef gene during HIV-1 production increases progeny particle infectivity independently of gp160 or viral entry. J. Virol. 69579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller, M. D., M. T. Warmerdam, S. S. Ferrell, R. Benitez, and W. C. Greene. 1997. Intravirion generation of the C-terminal core domain of HIV-1 Nef by the HIV-1 protease is insufficient to enhance viral infectivity. Virology 234215-225. [DOI] [PubMed] [Google Scholar]
- 62.Muller, B., U. Tessmer, U. Schubert, and H. G. Krausslich. 2000. Human immunodeficiency virus type 1 Vpr protein is incorporated into the virion in significantly smaller amounts than Gag and is phosphorylated in infected cells. J. Virol. 749727-9731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niederman, T. M., B. J. Thielan, and L. Ratner. 1989. Human immunodeficiency virus type 1 negative factor is a transcriptional silencer. Proc. Natl. Acad. Sci. USA 861128-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paxton, W., R. I. Connor, and N. R. Landau. 1993. Incorporation of Vpr into human immunodeficiency virus type 1 virions: requirement for the p6 region of Gag and mutational analysis. J. Virol. 677229-7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pizzato, M., E. Popova, and H. G. Gottlinger. 2008. Nef can enhance the infectivity of receptor-pseudotyped human immunodeficiency virus type 1 particles. J. Virol. doi: 10.1128/JVI.01150-08. [DOI] [PMC free article] [PubMed]
- 66.Popov, S., M. Rexach, L. Ratner, G. Blobel, and M. Bukrinsky. 1998. Viral protein R regulates docking of the HIV-1 preintegration complex to the nuclear pore complex. J. Biol. Chem. 27313347-13352. [DOI] [PubMed] [Google Scholar]
- 67.Popov, S., M. Rexach, G. Zybarth, N. Reiling, M. A. Lee, L. Ratner, C. M. Lane, M. S. Moore, G. Blobel, and M. Bukrinsky. 1998. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 17909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qi, M., and C. Aiken. 2008. Nef enhances HIV-1 infectivity via association with the virus assembly complex. Virology 373287-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rho, H. M., B. Poiesz, F. W. Ruscetti, and R. C. Gallo. 1981. Characterization of the reverse transcriptase from a new retrovirus (HTLV) produced by a human cutaneous T-cell lymphoma cell line. Virology 112355-360. [DOI] [PubMed] [Google Scholar]
- 70.Rizzuto, C. D., and J. G. Sodroski. 1997. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J. Virol. 714847-4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sawai, E. T., M. S. Hamza, M. Ye, K. E. Shaw, and P. A. Luciw. 2000. Pathogenic conversion of live attenuated simian immunodeficiency virus vaccines is associated with expression of truncated Nef. J. Virol. 742038-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schaeffer, E., R. Geleziunas, and W. C. Greene. 2001. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J. Virol. 752993-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schiavoni, I., S. Trapp, A. C. Santarcangelo, V. Piacentini, K. Pugliese, A. Baur, and M. Federico. 2004. HIV-1 Nef enhances both membrane expression and virion incorporation of Env products. A model for the Nef-dependent increase of HIV-1 infectivity. J. Biol. Chem. 27922996-23006. [DOI] [PubMed] [Google Scholar]
- 74.Schindler, M., D. Rajan, A. Specht, C. Ritter, K. Pulkkinen, K. Saksela, and F. Kirchhoff. 2007. Association of Nef with p21-activated kinase 2 is dispensable for efficient human immunodeficiency virus type 1 replication and cytopathicity in ex vivo-infected human lymphoid tissue. J. Virol. 8113005-13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schrager, J. A., V. Der Minassian, and J. W. Marsh. 2002. HIV Nef increases T cell ERK MAP kinase activity. J. Biol. Chem. 2776137-6142. [DOI] [PubMed] [Google Scholar]
- 76.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2338-342. [DOI] [PubMed] [Google Scholar]
- 77.Schwartz, O., V. Marechal, O. Danos, and J. M. Heard. 1995. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J. Virol. 694053-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Selig, L., J. C. Pages, V. Tanchou, S. Preveral, C. Berlioz-Torrent, L. X. Liu, L. Erdtmann, J. Darlix, R. Benarous, and S. Benichou. 1999. Interaction with the p6 domain of the Gag precursor mediates incorporation into virions of Vpr and Vpx proteins from primate lentiviruses. J. Virol. 73592-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sheehy, A. M., N. C. Gaddis, and M. H. Malim. 2003. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat. Med. 91404-1407. [DOI] [PubMed] [Google Scholar]
- 80.Sheehy, A. M., N. C. Gaddis, J. D. Choi, and M. H. Malim. 2002. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418646-650. [DOI] [PubMed] [Google Scholar]
- 81.Simmons, A., V. Aluvihare, and A. McMichael. 2001. Nef triggers a transcriptional program in T cells imitating single-signal T cell activation and inducing HIV virulence mediators. Immunity 14763-777. [DOI] [PubMed] [Google Scholar]
- 82.Sol-Foulon, N., C. Esnault, Y. Percherancier, F. Porrot, P. Metais-Cunha, F. Bachelerie, and O. Schwartz. 2004. The effects of HIV-1 Nef on CD4 surface expression and viral infectivity in lymphoid cells are independent of rafts. J. Biol. Chem. 27931398-31408. [DOI] [PubMed] [Google Scholar]
- 83.Stove, V., I. Van de Walle, E. Naessens, E. Coene, C. Stove, J. Plum, and B. Verhasselt. 2005. Human immunodeficiency virus Nef induces rapid internalization of the T-cell coreceptor CD8αβ. J. Virol. 7911422-11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swigut, T., N. Shohdy, and J. Skowronski. 2001. Mechanism for down-regulation of CD28 by Nef. EMBO J. 201593-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Swingler, S., P. Gallay, D. Camaur, J. Song, A. Abo, and D. Trono. 1997. The Nef protein of human immunodeficiency virus type 1 enhances serine phosphorylation of the viral matrix. J. Virol. 714372-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tobiume, M., J. E. Lineberger, C. A. Lundquist, M. D. Miller, and C. Aiken. 2003. Nef does not affect the efficiency of human immunodeficiency virus type 1 fusion with target cells. J. Virol. 7710645-10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Venzke, S., N. Michel, I. Allespach, O. T. Fackler, and O. T. Keppler. 2006. Expression of Nef downregulates CXCR4, the major coreceptor of human immunodeficiency virus, from the surfaces of target cells and thereby enhances resistance to superinfection. J. Virol. 8011141-11152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vodicka, M. A., D. M. Koepp, P. A. Silver, and M. Emerman. 1998. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 12175-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Welker, R., H. Hohenberg, U. Tessmer, C. Huckhagel, and H. G. Krausslich. 2000. Biochemical and structural analysis of isolated mature cores of human immunodeficiency virus type 1. J. Virol. 741168-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Welker, R., H. Kottler, H. R. Kalbitzer, and H. G. Krausslich. 1996. Human immunodeficiency virus type 1 Nef protein is incorporated into virus particles and specifically cleaved by the viral proteinase. Virology 219228-236. [DOI] [PubMed] [Google Scholar]
- 91.Welker, R., M. Harris, B. Cardel, and H. G. Krausslich. 1998. Virion incorporation of human immunodeficiency virus type 1 Nef is mediated by a bipartite membrane-targeting signal: analysis of its role in enhancement of viral infectivity. J. Virol. 728833-8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wolf, D., S. I. Giese, V. Witte, E. Krautkramer, S. Trapp, G. Sass, C. Haller, K. Blume, O. T. Fackler, and A. S. Baur. 2008. Novel (n) PKC kinases phosphorylate Nef for increased HIV transcription, replication and perinuclear targeting. Virology 37045-54. [DOI] [PubMed] [Google Scholar]
- 93.Wu, X., H. Liu, H. Xiao, J. Kim, P. Seshaiah, G. Natsoulis, J. D. Boeke, B. H. Hahn, and J. C. Kappes. 1995. Targeting foreign proteins to human immunodeficiency virus particles via fusion with Vpr and Vpx. J. Virol. 693389-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yao, X. J., G. Kobinger, S. Dandache, N. Rougeau, and E. Cohen. 1999. HIV-1 Vpr-chloramphenicol acetyltransferase fusion proteins: sequence requirement for virion incorporation and analysis of antiviral effect. Gene Ther. 61590-1599. [DOI] [PubMed] [Google Scholar]
- 95.Zheng, Y. H., A. Plemenitas, C. J. Fielding, and B. M. Peterlin. 2003. Nef increases the synthesis of and transports cholesterol to lipid rafts and HIV-1 progeny virions. Proc. Natl. Acad. Sci. USA 1008460-8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zheng, Y. H., A. Plemenitas, T. Linnemann, O. T. Fackler, and B. M. Peterlin. 2001. Nef increases infectivity of HIV via lipid rafts. Curr. Biol. 11875-879. [DOI] [PubMed] [Google Scholar]