Abstract
Cell-mediated immunity and neutralizing antibodies contribute to control of human immunodeficiency virus/simian immunodeficiency virus (HIV/SIV) infection, but the role of nonneutralizing antibodies is not defined. Previously, we reported that sequential oral/oral or intranasal/oral (I/O) priming with replication-competent adenovirus type 5 host range mutant (Ad5hr)-SIV recombinants, followed by intramuscular envelope protein boosting, elicited systemic and mucosal cellular immunity and exhibited equivalent, significant reductions of chronic viremia after rectal SIVmac251 challenge. However, I/O priming gave significantly better control of acute viremia. Here, systemic and mucosal humoral immunity were investigated for potential correlates with the acute challenge outcome. Strong serum binding but nonneutralizing antibody responses against SIVmac251 were induced in both groups. Antibody responses appeared earlier and overall were higher in the I/O group. Reduced acute viremia was significantly correlated with higher serum binding titer, stronger antibody-dependent cellular cytotoxicity activity, and peak prechallenge and 2-week-postchallenge antibody-dependent cell-mediated viral inhibition (ADCVI). The I/O group consistently displayed greater anti-envelope immunoglobulin A (IgA) antibody responses in bronchoalveolar lavage and a stronger rectal anti-envelope IgA anamnestic response 2 weeks postchallenge. Pre- and postchallenge rectal secretions inhibited SIV transcytosis across epithelial cells. The inhibition was significantly higher in the I/O group, although a significant correlation with reduced acute viremia was not reached. Overall, the replicating Ad5hr-SIV priming/envelope boosting approach elicited strong systemic and mucosal antibodies with multiple functional activities. The pattern of elevated immune responses in the I/O group is consistent with its better control of acute viremia mediated, at least in part, by ADCVI activity and transcytosis inhibition.
Despite the successes of highly active antiretroviral therapy in slowing progression to AIDS after human immunodeficiency virus (HIV) infection, thus transforming a lethal disease into a manageable chronic infection (14), a vaccine able to prevent the transmission of HIV remains the ultimate goal. Antiretrovirals can only limit viral spread once HIV infection has been diagnosed and therapy has been initiated. Moreover, the availability of treatment is likely to be limited to countries that can afford the drugs (50). This can be a major hurdle in the developing world, where the majority of those newly infected live (26). Thus, the development of a safe, effective, easily administered HIV vaccine is urgently needed.
Historically, the best vaccine-mediated protection is achieved when administration of the vaccine mimics the natural route of infection, thereby establishing appropriate immunologic memory that can rapidly respond when an actual infection occurs. Most HIV infections occur via a mucosal route, including cervicovaginal and rectal tissues (26, 52). The prevention of mucosal transmission is a crucial consideration for the development of an effective HIV vaccine. Vaccinations with live attenuated simian immunodeficiency virus (SIV) have achieved 100% protection of vaccinated monkeys upon challenge (38, 56); however, this approach poses the potential risk that the vaccine virus might revert to a pathogenic form. Overtime, all macaques vaccinated as adults with SIVmac239Δ3 showed signs of immune dysregulation, more than half had T-cell depletion after 6.8 years of follow-up, and 18% developed AIDS (21). Further, a recent study reported evidence of virus recombinations between live-attenuated SIVmac239Δnef and a heterologous challenge virus (46). Safer yet effective mucosal vaccination strategies need to be explored, such as the use of benign viruses that naturally infect mucosa as vectors for live recombinant vaccines. We have pursued the use of E3-region deleted adenovirus (Ad) recombinant vaccines (18, 33, 44). This deletion removes genes encoding proteins involved in evading host immunity and also creates space for transgene insertion, while retaining the ability of recombinants to replicate in the host. Mucosal delivery of such Ad-HIV recombinants to chimpanzees, coupled with HIV envelope protein boosting, elicited humoral, cellular, and mucosal immune responses and protection against HIV challenge (29, 47). Further, in the same chimpanzee model, replication-competent Ad-HIV recombinants also exhibited better cellular immune responses and primed higher antibody titers after protein boosting compared to matched replication-defective Ad-HIV recombinants in similar regimens (45). In rhesus macaques, a series of studies utilizing a replicating Ad5 host range mutant (Ad5hr)-SIV recombinant priming/SIV envelope protein boosting regimen has demonstrated strong immunogenicity (31, 42, 58) and increasing protective efficacy (6, 59), culminating in potent, durable protection against intrarectal SIVmac251 challenge (32, 43). The contribution of a protein boost to protective efficacy was recently established by using the SHIV model (41).
Recently, we reported a comparative study of mucosal immunization routes. Rhesus macaques were primed sequentially by oral/oral (O/O) or intranasal/oral (I/O) administrations of replication-competent Ad5hr-SIV recombinants expressing env/rev, gag, and nef genes (60). Subsequently, both groups were boosted intramuscularly with native SIVmac251 envelope protein. Both the O/O and the I/O regimens elicited cellular immune responses in peripheral blood mononuclear cells (PBMC), as well as mucosal immunity, including memory cells in bronchial alveolar lavage (BAL), and gut-homing receptors on PMBC. After intrarectal challenge with the highly pathogenic SIVmac251, both groups exhibited significant protection and robust postchallenge cellular immunity. All immunized macaques exhibited reduced acute and chronic viremia. However, while the viral loads of both groups during the chronic phase were comparable, animals primed by the I/O route exhibited significantly lower peak acute viremia than the O/O group.
In the present study we explored the humoral immunity of these animals in depth, investigating both serum and mucosal samples and searching for potential immune responses that might be correlated with the lower peak viremia of the I/O group during acute infection. Specific antibodies are often considered as the most efficient defense against viral infections. However, in the case of HIV infection, despite generally vigorous and predominant antibody responses to the structural proteins, only a fraction of the elicited antibodies has been found to have neutralizing activity (40, 57). In the absence of effective neutralizing antibodies, nonneutralizing antibodies by actions of phagocytes, killer cells or the complement system can have substantial impact by clearing virus particles and infected cells (2, 10, 11). Hence, we focused on evaluation of systemic and mucosal functional antibody activities, including not only neutralization but also antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cell-mediated virus inhibition (ADCVI), and transcytosis inhibition.
MATERIALS AND METHODS
Vaccines, immunization, and challenge.
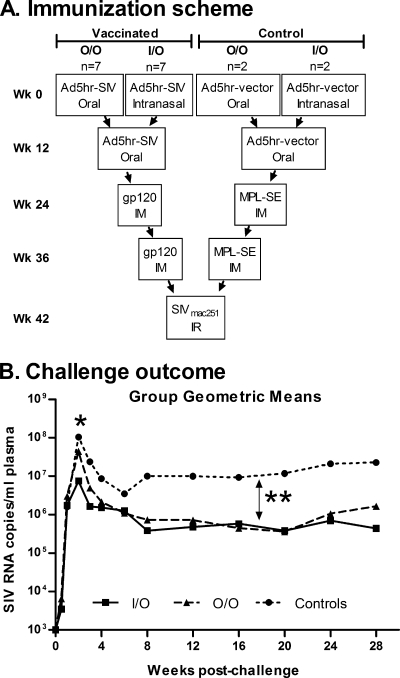
The vaccines and immunization regimen were previously described in detail (60) and are summarized in Fig. 1A. Briefly, rhesus macaques were primed at weeks 0 and 12 by the indicated routes with replication-competent Ad5hr-SIV recombinants carrying the SIVsmH4env/rev, SIV239gag (rev-independent), and SIVmac239nefΔ1-14 (nonmyristoylated) genes, boosted intramuscularly with native SIV gp120 protein in monophosphoryl lipid A-stable emulsion (MPL-SE) adjuvant at weeks 24 and 36, and challenged intrarectally with primary SIVmac251 at week 42. Control macaques received empty Ad5hr vector and adjuvant alone. The native SIV gp120 protein used for immunization and in vitro assays was purified from conditioned medium of HUT78 cells chronically infected with SIVmac251 by affinity chromatography using a mouse monoclonal antibody to SIV gp120. After reversed-phase high-pressure liquid chromatography using a C18 column, the protein was more than 95% pure and contained <20 U of endotoxin per mg of protein. All 18 colony-bred male Indian rhesus macaques (Macaca mulatta) were housed and maintained according to the standards of the American Association for Accreditation of Laboratory Animal Care. The animal protocol was reviewed and approved by the Animal Care and Use Committee prior to implementation.
FIG. 1.
Immunization of rhesus macaques and challenge outcome. Regimen summarized from reference (60). (A) Three Ad5hr recombinants containing SIVsmH4env/rev, SIV239gag, and SIV239nef were administered at 5 × 108 PFU/recombinant/dose either orally as enteric coated tablets or intranasally in PBS. SIV gp120 was administered at 100 μg/dose in MPL-SE. Controls received a total Ad5hr vector dose of 1.5 × 109 PFU and MPL-SE only. The SIVmac251 challenge dose was 10 50% monkey infectious doses. (B) Summary of challenge results (60). *, Both O/O and I/O groups exhibited reduced peak viremia (weeks 1 to 4) compared to controls (P = 0.042 for both). At week 2 postchallenge, viremia of the I/O group was significantly lower than that of the O/O group (P = 0.026). **, Both the O/O and the I/O groups exhibited reduced chronic viremia (weeks 12 to 28) compared to controls (P = 0.048 and 0.038, respectively).
Sample collection.
Serum samples were collected routinely over the course of immunization, divided into aliquots, and stored at −70°C until analyzed. BAL samples were obtained at time points indicated from both immunized and control macaques. Samples were collected by flushing one bronchus with 30 to 50 ml of phosphate-buffered saline (PBS). After centrifugation, the supernatants were stored at −70°C until analyzed. Nasal and rectal samples were collected by using cotton-tipped swabs, which were placed in 1 ml of PBS containing 0.1% bovine serum albumin, 0.01% thimerosal, and 750 Kullikrein inhibitor units of aprotinin as previously described (16). Samples were stored at −70°C until analyzed.
Analysis of binding antibodies.
Serum antibody to SIV gp120 was evaluated by direct enzyme-linked immunosorbent assay (ELISA) as previously described (6). Antibody titer was defined as the reciprocal of the serum dilution at which the optical density of the test serum was two times greater than that of a naive control macaque serum diluted 1:50.
For determination of binding antibody in mucosal secretions, serially diluted samples (100 μl of each dilution) were applied to a 96-well plate, which was previously coated with 300 ng of native SIVgp120/well and blocked with skim milk. After 2 h of incubation at 37°C, the plate was washed with PBS-Tween, reacted with peroxidase-conjugated anti-monkey immunoglobulin G (IgG) antibody (Fc) or peroxidase-conjugated anti-monkey IgA antibody (Fc), and incubated for another hour. After washing, TMB (3,3′,5,5′-tetramethylbenzidine) peroxidase substrate solution was added to each well, followed by incubation at room temperature until the color developed. The reaction was stopped by adding 1 M H3PO4, and the plate was read at 450 nm with a reference at 650 nm within 30 min.
Total IgG and IgA of BAL, nasal, and rectal samples were assayed by using a sandwich ELISA. The method is similar to the direct ELISA described above, except that serially diluted mucosal samples were applied to an anti-monkey IgG or IgA antibody-coated plate. Sets of monkey IgG or IgA standards were included in each plate, respectively. Total IgG and IgA levels were determined based on standard curves of known antibody concentrations measured in the same plate.
The specific activity of antibody responses was calculated by dividing the antibody titer by the amount of total IgG or IgA in each sample. The results are reported as the fold increase in specific activity compared to the specific activity of antibody in the preimmunization sample.
Neutralizing antibody assay.
Neutralizing antibodies against rhesus PBMC-grown primary SIVmac251 were assessed by using the TZM-bl assay as described elsewhere (28) both at Duke University and in house. Neutralizing antibodies against SIVmac251 grown on H9 cells were assayed using the M7-Luc assay as previously described (37). Neutralizing titers are expressed as the reciprocal of the serum dilution at which relative luminescence units were reduced 50% compared to results for virus control wells (no test sample).
ADCC.
The rapid fluorometric ADCC (RF-ADCC) assay was performed as previously described (15). Briefly, CEM-NKr cells (AIDS Research and Reference Reagent Program, National Institutes of Allergy and Infectious Diseases) coated with native SIV gp120 were used as targets. The target cells were dually labeled with the membrane dye, PKH-26 (Sigma-Aldrich), and CFSE (Molecular Probes), a vital dye that is rapidly lost when cell membranes are damaged. Labeled targets were resuspended in RPMI 1640 medium containing 10% fetal calf serum (R-10) and allowed to react with heat-inactivated (56°C, 30 min) serially diluted plasma in a 96-well microtiter plate for 30 min at room temperature. Human PBMC used as effector cells were added at a 50:1 effector/target ratio. The reaction mixture was incubated for 4 h at 37°C in 5% CO2, after which the cells were fixed with 3.7% paraformaldehyde for flow cytometry. Controls included unstained and single-stained target cells. Nongated events (n = 50,000) in duplicate wells were acquired within 18 h by using a FACSCalibur instrument (BD Biosciences). Acquisition was done by using CellQuest software, and data analysis was performed with WinMDI 2.0. The percent ADCC cell killing is reported as the percentage of membrane-labeled target cells having lost the viability dye, i.e., the percentage of CFSE− cells within the PKH-26high gate. ADCC titers are defined as the reciprocal dilution at which the percent ADCC killing was greater than the mean percent killing of the negative control plus three standard deviations.
ADCVI.
The ADCVI assay was based on methods previously described (8, 10). Rhesus PBMC target cells were stimulated with phytohemagglutinin (2 μg/ml) and recombinant interleukin-2 (0.5 ng/ml) for 72 h, washed, and infected with primary SIVmac251 (50% tissue culture infective dose of 200). After adsorption for 1 h, rhesus PBMC were washed and incubated in 5% CO2 at 37°C for 48 h in medium. Infected target cells (5 × 104 per well) were plated in 96-well round-bottom microtiter plates, and 1:100 dilutions of test serum were added to target cells, along with rhesus PBMC effector cells at an effector/target ratio of 10:1. Serum in the absence of effector cells was also tested. Target cells without serum and effector cells were used as reference control. After 7 days incubation at 37°C in 5% CO2, supernatant fluid was collected and assayed for p27 by antigen-capture ELISA (ABL, Inc., Kensington, MD). Virus inhibition due to ADCVI was calculated as the percentage of the decrease in p27 concentration between conditions without and with effector cells, compared to the p27 concentration in the absence of both serum and effector cells by using the following formula:
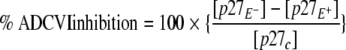 |
where [p27c] is the p27 concentration of control, and [p27E+] and [p27E−] are the p27 concentrations in the presence or absence of effector cells, respectively.
Inhibition of transcytosis.
SIV transcytosis across epithelial cells was performed as previously described (5) with some modifications. Briefly, the intestinal epithelial HT-29 cell line was grown as a tight, polarized monolayer on 24-Transwell polycarbonate permeable membranes (6.5-mm diameter, 0.4-μm pore size; Costar, Corning, Inc., Corning, NY) for 7 to 10 days in RPMI 1640 containing 10% fetal calf serum. The tightness of the monolayer was monitored by transepithelial resistance (>250 Ω/cm2) with a Millicell ohmmeter (Millipore Corp., Billerica, MA) and confirmed by a [14C]inulin leakage test. The epithelial monolayer divided the well into two separate chambers: the apical (luminal) surface and the basolateral (serosal) surface. Primary SIVmac251-infected rhesus PBMC (106 cells) that had been washed three times were added to the apical chamber with or without sera or rectal secretions (1:10 dilution). Transcytosis was assessed after 4 h by measuring p27 antigen in the basal chamber by antigen-capture ELISA (ABL, Inc., Kensington, MD). Inhibition of SIV transcytosis was expressed as the percentage of p27 antigen recovered in the basal chamber in the presence of serum/rectal sample, compared to the amount of p27 antigen recovered in the absence of serum per rectal sample.
Statistical analysis.
Analyses of specific humoral immune responses used the exact Kruskal-Wallis test for testing the null hypothesis that the three groups have the same mean, the exact Wilcoxon rank sum test for tests of two groups, and the Wilcoxon signed-rank test for changes from one time to a later time. Antibody-viral load correlations were obtained by using Spearman rank correlation coefficients and the Jonckheere-Terpstra test for trends between specific time points. Analysis of transcytosis inhibition between immunization and control groups at several time points used repeated measures analysis of variance.
RESULTS
Systemic humoral immune responses.
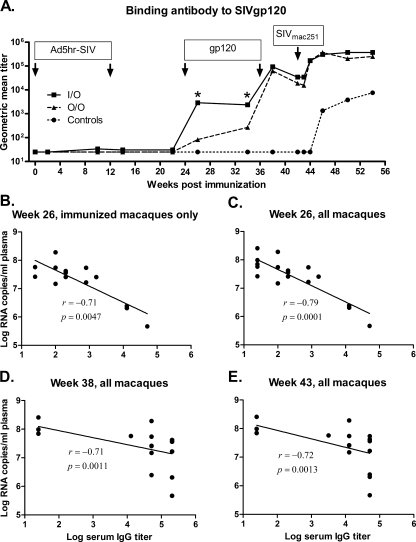
As previously reported (60) and summarized in Fig. 1B, both the O/O and the I/O immunization regimens elicited significant protection following SIVmac251 challenge compared to the controls. Levels of viremia reduction were similar in both immunization groups during the chronic phase of infection. Both groups also exhibited significantly reduced acute viremia compared to controls, however, the I/O group displayed lower acute viremia at 2 weeks postchallenge compared to the O/O group (P = 0.026). In general, strong anamnestic cellular responses were not observed at the 2 week time point but only later postchallenge. Further, SIV-specific central and effector memory CD8+ and CD4+ T-cell responses in blood and BAL were similar between the two immunization groups 2 weeks postchallenge (60). Since the cellular immune responses did not correlate with the viral load outcomes during the acute phase of infection, we evaluated humoral immunity elicited by the vaccines to investigate a possible association of systemic and/or mucosal antibody responses with the better control of acute viremia in the I/O group. Ad5hr-SIV recombinant priming is intended primarily to elicit cellular immune responses. In line with this, strong antibody responses were not detected until 2 weeks after the first SIV gp120 protein boost (week 26) but subsequently were maintained at least 10 weeks postboost to week 34. All animals in the I/O group exhibited high SIVgp120-specific IgG binding titers at weeks 26 and 34, significantly different (P = 0.0017 and P = 0.047, respectively) from those of the O/O group (Fig. 2A). The second SIV gp120 boost significantly increased the binding titers in both the O/O and I/O groups (P = 0.016 for each) to comparable levels. Prior to challenge, the binding titers of both groups slightly decreased, but a rapid anamnestic response appeared in most animals in both immunization groups within 2 weeks postchallenge. Subsequently, responses peaked at 4 weeks after challenge, exhibiting a significant increase for each change compared to prechallenge values (P = 0.016), and remained high for at least 8 weeks thereafter (Fig. 2A). The unimmunized control animals only began exhibiting gp120-specific responses at week 4 postchallenge.
FIG. 2.
Serum IgG anti-SIVmac251 titers. (A) Geometric mean titers for the two immunization groups and controls over the duration of the study. Arrows indicate Ad5hr-SIV recombinant immunizations, protein boosts, and time of challenge. *, I/O titers are significantly different compared to the O/O group at weeks 26 and 34 (P values of 0.0017 and 0.047, respectively). (B to E) The binding antibody titer of individual animals at the indicated time points is plotted against week 2 viremia. The straight line in each graph is the result of linear regression analysis of the data set, and the correlation coefficients (r) and P values are from Spearman rank analysis.
Taking all 18 immunized and control macaques together, the binding antibody titers at weeks 26 and 38 prechallenge and week 43 (1 week postchallenge) were significantly correlated with reduced viremia at week 2 postchallenge (Spearman rank correlation coefficients of −0.79, −0.71, and −0.72; P values of 0.0001, 0.0011, and 0.0013, respectively; Fig. 2C to E). A more rigorous analysis of just the 14 immunized macaques showed the correlation of week 26 binding antibody titer with reduced week 2 viremia remained significant (correlation coefficient of −0.71, P = 0.0047; Fig. 2B).
Neutralizing antibody.
In line with the high serum binding titers achieved, both the O/O and the I/O groups developed antibodies able to neutralize T-cell-line-adapted SIVmac251, with titers ranging from 420 to 18,756 (geometric mean titer of 2,064) for the O/O group and from 476 to 23,722 (geometric mean titer of 5,361) for the I/O group, at the time of challenge. No significant difference between groups was obtained. Furthermore, neutralization against primary SIVmac251 was not achieved at a starting serum dilution of 1:80 (data not shown). At serum dilution of 1:15, in-house assays against primary SIVmac251 also were negative for neutralization. At weeks 38, 42 (time of challenge), and 44, mean percent neutralization values for the O/O group were 29, 29, and 35%, respectively, and for the I/O group were 18, 28, and 41%, respectively.
ADCC.
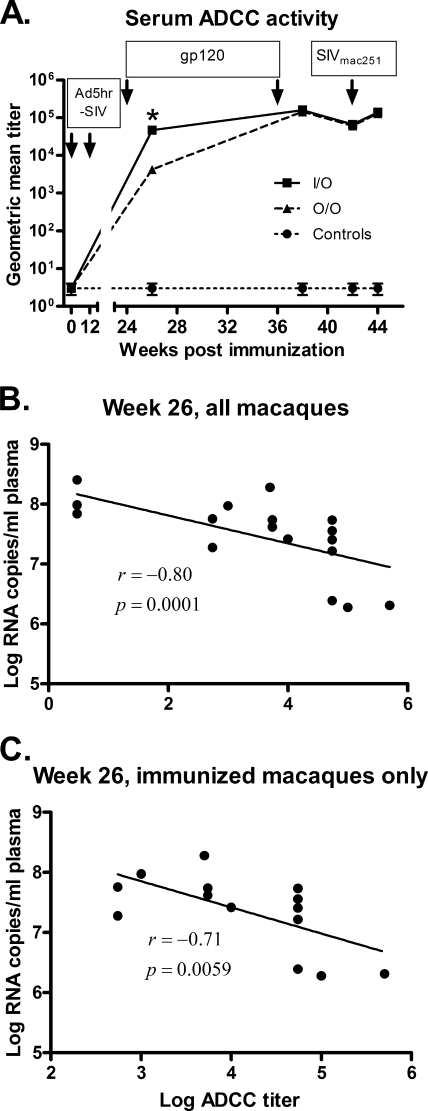
Since significant neutralizing antibody activity against primary SIVmac251 was not observed, we explored other functional activities of SIV-specific antibodies in serum samples. Initially, we investigated ADCC activity specific for the SIV envelope using native SIV gp120-coated CEM-NKr cells as targets. Consistent with the higher binding titers in the I/O group at week 26, we also observed higher ADCC titers in these samples compared to those of the O/O group (P = 0.016) (Fig. 3A). ADCC titers were boosted significantly in both immunization groups together after the second gp120 immunization (P = 0.0009), peaking at week 38. There were no significant differences in ADCC titers between immunization groups upon completion of the vaccination regimens or after challenge.
FIG. 3.
ADCC activity induced by the vaccine regimens. (A) Sera collected at the indicated time points were tested for ADCC activity to SIVmac251 gp120-coated target cells. The geometric mean ADCC titers of each group are shown. Arrows indicate Ad5hr-SIV recombinant immunizations, protein boosts, and time of challenge. *, The geometric mean ADCC titer of the I/O group is significantly higher than that of the O/O group (P = 0.016). The straight lines in graphs B and C result from linear regression analysis of week 26 ADCC titers versus peak acute viremia of all macaques (B) and immunized macaques only (C). The correlation coefficients (r) and P values are from Spearman rank analysis.
Similar to the binding antibody titers, the week 26 ADCC titers were significantly correlated with reduced peak viremia, both when all 18 macaques were included and when only the 14 immunized macaques were analyzed (correlation coefficients of −0.80 and −0.71; P values of 0.0001 and 0.0059, respectively; Fig. 3B and C).
ADCVI.
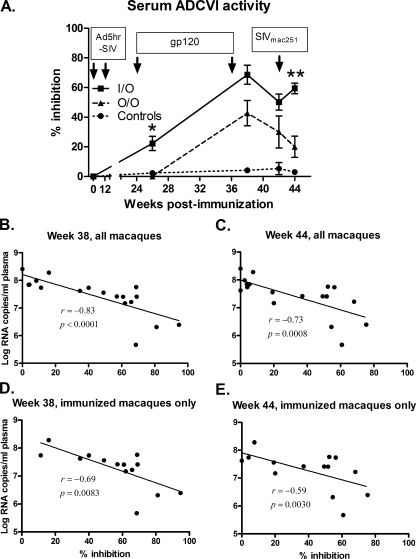
Since the SIV-envelope-specific ADCC results did not provide a basis for the significant difference between the I/O and O/O virus loads during the acute infection phase, we broadened our investigations to measure the ability of vaccine-elicited, nonneutralizing antibodies to inhibit SIVmac251 replication in the presence of effector cells in the ADCVI assay. As with ADCC activity, ADCVI activity in the I/O group was first detectable at week 26 after the initial protein boost and was significantly elevated compared to the O/O group (P = 0.0006; Fig. 4A). After the second protein boost, the ADCVI activities in both groups greatly increased, leading to a significant difference between the vaccinated groups and controls at week 38, the time of challenge (week 42), and 2 weeks postchallenge (P values of 0.0007, 0.017, and 0.0082, respectively). The mean percent ADCVI observed in the I/O group was consistently higher over time than in the O/O group. In addition to the week 26 time point, a significant difference between the two immunization groups was observed at week 44 (2 weeks postchallenge) when an anamnestic response in the I/O group led to a greater percent inhibition than that of the O/O group (P = 0.0023; Fig. 4A).
FIG. 4.
Vaccine-induced ADCVI activity. (A) Sera collected at the indicated time points were measured for ADCVI activity. Arrows indicate Ad5hr-SIV recombinant immunizations, protein boosts, and time of challenge. Error bars indicate the standard error of the mean. Inhibition by the I/O group was significantly greater than that by the O/O group at week 26 (*, P = 0.0006) and at week 44 (**, P = 0.0023). (B to E) The straight line in each graph is the result of linear regression analysis of serum ADCVI activity at the indicated time point versus week 2 viremia. The correlation coefficients (r) and P values are from Spearman rank analysis.
The ADCVI percent inhibition values of all 18 macaques at weeks 38 and 44 were significantly correlated with decreased week 2 viremia (correlation coefficients of −0.83 and −0.73; P values of <0.0001 and 0.0008, respectively; Fig. 4B and C). At the time of challenge (week 42) the ADCVI activity decreased slightly in both immunization groups; however, all 18 macaques retained a significant correlation with reduced week 2 viral load (correlation coefficient = −0.59, P = 0.011). When only the 14 immunized macaques were analyzed at these three time points, correlations with weeks 38 and 44 (2 weeks postchallenge) remained significant (coefficients of −0.69 and −0.59; P values of 0.0083 and 0.030, respectively), suggesting a role of this nonneutralizing antibody response in acute protection (Fig. 4D and E).
Mucosal antibody responses.
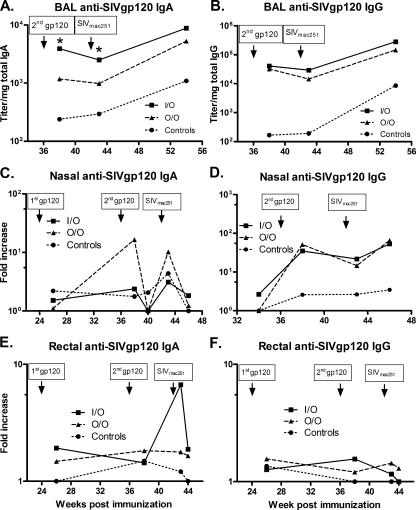
An efficacious vaccine against HIV/SIV is expected to require both mucosal and systemic immunity. Since administration of the Ad5hr recombinants either through intranasal or oral routes should enhance mucosal immunity, we next investigated whether SIVmac251 specific antibodies were elicited at mucosal sites. The lung is not a typical mucosal site but is an effector site similar to the upper gastrointestinal tract. Therefore, we initially measured specific antibody responses in BAL samples. Due to possible variation arising from sample dilution during collections, all titers were standardized to the total amount of IgA or IgG present in the same samples. BAL samples were not collected prior to immunization, so calculation of the fold increase relative to the zero time baseline was not possible. The SIV gp120-specific IgA antibody titers of the three groups at week 38 postimmunization were significantly different (P = 0.0007), with the I/O group titers higher than those of the O/O and control groups combined (P = 0.0013, corrected for multiple tests) and also significantly higher than the O/O group (P = 0.0023) (Fig. 5A). The difference between groups remained significant at 1 week after challenge (week 43, P = 0.040). Anamnestic responses in the immunized macaques were not seen between weeks 38 and 43. Nevertheless, specific binding activities in all macaques increased due to SIV infection. By week 54, 12 weeks postchallenge, the titer of the control group remained significantly lower than that of the vaccinated groups combined (P = 0.0039).
FIG. 5.
SIVmac251 gp120 specific antibodies in mucosal samples. Mucosal samples collected at the indicated time points were assayed for SIVmac251 gp120 specific IgA (A, C, and E) or IgG (B, D, and F). Titers of each sample were normalized to the total IgA or IgG of the corresponding samples. Values for nasal and rectal secretions were further compared to values prior to immunization and reported as fold increase. The data are reported as geometric means. *, In panel A, the BAL anti-SIV gp120 IgA titer of the I/O group is significantly higher than that of the O/O group at weeks 38 (P = 0.0023) and 43 (P = 0.040).
Higher IgG specific activity was also observed in BAL samples of immunized animals compared to the control group (P < 0.008) at weeks 38, 43, and 54, but the titers of O/O and I/O groups were not significantly different (Fig. 5B). As with the IgA specific activities, IgG specific activities increased after SIV infection between weeks 43 and 54 (P < 0.0001 for all macaques; P = 0.0001 for the immunized macaques only).
Specific mucosal antibodies were also investigated at sites where the Ad5hr recombinant vaccines (nasal and gut/rectal mucosa) and challenge virus (rectal mucosa) were administered. Values were computed as fold increases in antibody specific activity compared to the initial level present in the same animals before vaccination. Values of twofold or more are considered positive. The O/O group showed high levels of SIV gp120-specific nasal IgA at week 38 but, considering the overall pattern of responses, these values may represent a random fluctuation (Fig. 5C). However, taking all of the vaccinated animals together, there was a boost in anti-gp120 IgA levels from week 26 to week 38 (P = 0.0009). Similarly, there was also a significant increase in antibody level from week 40 to week 43 in the immunized animals (P = 0.0005), indicating anamnestic responses after challenge, although no differences between the I/O and O/O groups were observed.
With regard to SIV gp120-specific IgG in nasal samples, both vaccinated groups exhibited higher IgG levels than the control animals (P < 0.035) at weeks 38, 43, and 46. Overall, antibody levels in both O/O and I/O groups were comparable (Fig. 5D).
Prior to challenge, rectal samples exhibited low SIV-gp120-specific IgA or IgG antibody, and no statistically significant differences were seen between groups. However, the level of IgA antibody increased in vaccinated macaques compared to controls from week 38 to week 43 due to the I/O group (P = 0.049; Fig. 5E). A similar anamnestic response was not observed for rectal IgG (Fig. 5F). In fact, rectal anti-gp120 IgG values rarely rose above the positive threshold of a twofold increase.
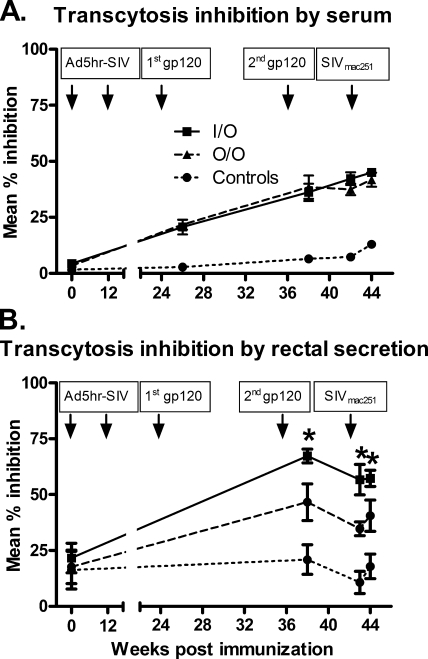
Transcytosis inhibition.
In a further test of whether functional antibody activity could have influenced the acute-phase challenge outcome, inhibition of transcytosis of SIV across a tight epithelial cell barrier was assessed. Sera were initially evaluated to establish the assay system. As shown in Fig. 6A, sera from macaques in both the O/O and the I/O immunization regimens were able to modestly inhibit SIV transcytosis and at levels greater than those of control macaque sera. Notably, however, when rectal secretions were evaluated, greater transcytosis inhibition was consistently seen over weeks 38, 43, and 44 in samples from macaques immunized by the I/O route compared to the O/O route (P = 0.0042; Fig. 6B). Transcytosis inhibition by the I/O group was also consistently higher than the controls (P < 0.0001), as was that by the O/O group compared to the controls (P = 0.0029).
FIG. 6.
Transcytosis inhibition. Serum (A) and rectal (B) samples collected at the indicated time points were measured for inhibition of SIV transcytosis across a tight epithelial cell barrier. Arrows indicate Ad5hr-SIV recombinant immunizations, protein boosts, and time of challenge. Error bars indicate the standard error of the mean. *, Rectal secretions of the I/O group exhibited greater transcytosis inhibition over weeks 38, 43, and 44 compared to the O/O group (P = 0.0042).
DISCUSSION
Humoral immunity is important in preventing the establishment of early viral infection. Neutralizing antibodies provide sterilizing immunity against intravenous and mucosal HIV challenge as shown by passive-transfer studies (3, 34, 35). These antibodies can prevent viral interaction with receptor or interfere with the fusion process (23). In many viral infections, such as influenza, measles, varicella, Epstein-Barr virus, and herpes simplex virus, humoral immunity is also able to mediate clearance of intracellular virus from infected cells (27) and block virus production or release (7, 12, 48, 49, 51). After HIV entry across mucosal barriers and the establishment of initial foci of infection, a window of time exists before widespread viral dissemination to underlying lymph nodes and eventual systemic infection (19, 36). During this period of several hours (39) to several days, nonneutralizing antibodies with other functional activities might participate in control of HIV infection. Neutralizing antibodies may also mediate additional functions associated with FcR binding, as shown for the broadly neutralizing monoclonal antibody, b12 (20), and therefore also limit viral spread from infectious foci.
As previously reported (60) and summarized above, comparison of O/O and I/O Ad5hr-SIV recombinant priming/envelope boosting regimens revealed a statistically significant difference in acute viremia between groups with the I/O regimen, resulting in lower viral burdens. Previous studies have reported that vaccine regimens lacking envelope immunogens have elicited CTL responses able to blunt acute viremia after challenge with either SHIV89.6P or pathogenic SIVmac239 (53, 55) in the absence of anti-envelope antibodies. Here, cellular immunity induced by the I/O and O/O regimens undoubtedly contributed to acute-phase protection of these immunized macaques. However, since the cellular immune responses postchallenge did not correlate with the greater reduction in acute viremia in the I/O compared to the O/O group (60), other factors likely contributed to the outcome. Here we provide evidence that in the absence of detectable neutralizing antibodies against primary SIVmac251, vaccine-induced nonneutralizing, functional antibody activities may have contributed to this improved outcome.
The I/O regimen was better than the O/O regimen in priming systemic humoral responses as seen by the early appearance and significantly higher titers of anti-envelope serum antibody 2 weeks (week 26) after the first SIV gp120 immunization. Unexpectedly, the binding titers at week 26 but not later time points were significantly correlated with reduced viremia at 2 weeks postchallenge (Fig. 2B). It will be of interest in future studies to explore whether antibody characteristics resulting from earlier antibody elicitation, such as induction of memory T cells, antibody maturation, or alteration of antibody quality, will correlate with reduced viremia.
In the presence of effector cells, virus-specific antibodies can eliminate virus-infected cells through the ADCC mechanism. A better clinical outcome has been associated with ADCC activity in HIV-infected individuals (1, 4, 11). Moreover, both serum antibodies and purified IgG from immunized rhesus macaques have mediated ADCC activity significantly correlated with reduction in acute viremia following SIVmac251 challenge (17). In the present study both vaccination groups developed SIV envelope-specific antibodies that mediated ADCC activity. The I/O group again showed a significantly elevated response compared to the O/O group after the first protein boost. Similarly to the anti-envelope binding titers, ADCC titers at week 26 were also significantly correlated with reduced peak viremia during the acute phase. The high ADCC activity in all immunized animals at the time of challenge and 2 weeks postchallenge might have contributed to their reduced peak viremia compared to controls; however, the similarity of responses in both immunization groups fail to explain the significantly lower viremia demonstrated by the I/O group at week 2 postchallenge compared to the O/O group.
Use of SIV-infected target cells in the ADCC assay might have allowed recognition of additional SIV epitopes by the vaccine-elicited antibodies, including those on envelope trimers, Gag, and Nef. We chose to examine this broader spectrum of reactivities by investigating ADCVI. By forming a bridge between infected target cells and FcR-bearing effector cells, specific antibody can block further transmission of virus particles from infected to uninfected cells (8, 10) by direct cell lysis (ADCC), secretion of soluble antiviral factors, or FcγR-mediated phagocytosis of immune complexes (10, 22). High ADCVI activity in early HIV infection has been correlated with lower viral load (10). Further, passive transfer of vaccine-elicited anti-SIV antibodies with strong ADCVI activity to newborn rhesus macaques has prevented infection by oral SIVmac251 challenge (9, 54). Here, both vaccination regimens induced potent ADCVI activity against primary SIVmac251 replication, although the I/O group consistently displayed higher activity. Significantly higher ADCVI in the I/O group was exhibited at weeks 38, 42, and 44. The significant correlation of ADCVI activity in the immunized macaques at weeks 38 and 44 with reduced peak viremia clearly suggests that this systemic antibody response might have played a role in protection against the mucosal challenge.
The immunization regimens also elicited mucosal antibody responses. Both the O/O and the I/O groups developed strong SIV Env-specific mucosal IgA and IgG in BAL fluids. The I/O group exhibited higher IgA titers at week 38 and soon after challenge in comparison to both the O/O and the control groups. The better priming of IgA responses in BAL by the I/O regimen is not surprising, since the first immunization was to the upper respiratory tract. However, whether BAL antibody plays a direct role in controlling early HIV infection is unknown.
Both immunization regimens induced variable antibody responses at nasal and rectal sites, but overall, none were significantly correlated with reduced acute viremia. However, the rectal anti-envelope IgA response in the I/O-immunized macaques showed a strong anamnestic response 1 week postchallenge, suggestive of a role for mucosal antibody in viremia control. Therefore, we examined rectal antibodies for their ability to inhibit transcytosis across an epithelial monolayer. Notably, rectal secretions of the I/O group of macaques exhibited greater transcytosis inhibition compared to the O/O group, reflecting the consistent pattern of better antibody responses in this group of immunized macaques. Although a statistically significant correlation with the reduced acute-phase plasma viremia of the I/O group was not observed, it will be important to explore tissue viral loads in the future to determine whether this parameter plays a role in mucosal protection by retarding viral dissemination from the initial infected foci.
While the I/O regimen was superior in eliciting systemic and mucosal antibodies, we cannot conclude that this was due to the immunization route. The Ad5hr vector used in our studies contains a single mutation in the DNA-binding protein which allows replication in monkey cells (24, 25). Nevertheless, replication of Ad5hr recombinants in rhesus macaques does not appear to be as robust as that of wild-type Ad5 in humans (6, 16, 60). Further, replication of Ad5hr recombinants following administration to the upper respiratory tract in macaques is more prolonged compared to replication following oral immunization (16, 42). Comparative immunogenicity studies of replication-competent Ad vectors in humans will be needed to accurately evaluate the two routes. In this regard, we are advancing a replication-competent Ad4-based HIV vaccine candidate to phase I clinical trial. Oral Ad4 wild-type vaccine was used for over 25 years in the military and exhibited strong protective efficacy against Ad4-induced acute respiratory disease (13). Furthermore, seropositivity to Ad4 in the population is low (30), so prior immunity is not expected to be a problem.
The data presented here strengthen the potential of replicating Ad recombinants as HIV vaccines. Mucosal delivery of the Ad5hr-SIV recombinants induced both SIV-specific cellular immunity (60) and robust systemic and mucosal antibodies. Although neither immunization regimen elicited neutralizing antibodies, most likely due to the suboptimal envelope immunogen, nonneutralizing antibodies with several functional activities were induced. The significant correlation of systemic ADCVI with reduced acute viremia suggests a mechanism that explains the better control of peak viremia in the I/O group. The vaccine-induced mucosal antibody in rectal secretions that mediated greater transcytosis inhibition in the I/O compared to the O/O group is of particular interest. Further investigation of this response, together with quantification of mucosal tissue viral levels, will help elucidate its role in mucosal vaccine protection.
Acknowledgments
We are very grateful to Marisa St. Claire, Steve Harbaugh, and Jeff Harbaugh (Bioqual, Inc., Gaithersburg, MD) for excellent care of the rhesus macaques and expert execution of all animal technical procedures. We thank Nancy Miller (DAIDS, NIAID, NIH) and Ronald C. Desrosiers, New England National Primate Research Center, Boston, MA, for the titrated SIVmac251 challenge stock. We also thank Ruth H. Florese for assistance with the RF-ADCC assay. The CEM-NKr cells were obtained from Peter Cresswell through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
This study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
Published ahead of print on 29 October 2008.
REFERENCES
- 1.Ahmad, A., and J. Menezes. 1996. Antibody-dependent cellular cytotoxicity in HIV infections. FASEB J. 10258-266. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad, R., S. T. Sindhu, E. Toma, R. Morisset, J. Vincelette, J. Menezes, and A. Ahmad. 2001. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J. Clin. Immunol. 21227-233. [DOI] [PubMed] [Google Scholar]
- 3.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nature medicine 6200-206. [DOI] [PubMed] [Google Scholar]
- 4.Banks, N. D., N. Kinsey, J. Clements, and J. E. Hildreth. 2002. Sustained antibody-dependent cell-mediated cytotoxicity (ADCC) in SIV-infected macaques correlates with delayed progression to AIDS. AIDS Res. Hum. Retrovir. 181197-1205. [DOI] [PubMed] [Google Scholar]
- 5.Bomsel, M. 1997. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat. Med. 342-47. [DOI] [PubMed] [Google Scholar]
- 6.Buge, S. L., E. Richardson, S. Alipanah, P. Markham, S. Cheng, N. Kalyan, C. J. Miller, M. Lubeck, S. Udem, J. Eldridge, and M. Robert-Guroff. 1997. An adenovirus-simian immunodeficiency virus env vaccine elicits humoral, cellular, and mucosal immune responses in rhesus macaques and decreases viral burden following vaginal challenge. J. Virol. 718531-8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowdle, W. R., J. C. Downie, and W. G. Laver. 1974. Inhibition of virus release by antibodies to surface antigens of influenza viruses. J. Virol. 13269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forthal, D. N., and G. Landucci. 1998. In vitro reduction of virus infectivity by antibody-dependent cell-mediated immunity. J. Immunol. Methods 220129-138. [DOI] [PubMed] [Google Scholar]
- 9.Forthal, D. N., G. Landucci, K. S. Cole, M. Marthas, J. C. Becerra, and K. Van Rompay. 2006. Rhesus macaque polyclonal and monoclonal antibodies inhibit simian immunodeficiency virus in the presence of human or autologous rhesus effector cells. J. Virol. 809217-9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forthal, D. N., G. Landucci, and E. S. Daar. 2001. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J. Virol. 756953-6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forthal, D. N., G. Landucci, and B. Keenan. 2001. Relationship between antibody-dependent cellular cytotoxicity, plasma HIV type 1 RNA, and CD4+ lymphocyte count. AIDS Res. Hum. Retrovir. 17553-561. [DOI] [PubMed] [Google Scholar]
- 12.Fujinami, R. S., and M. B. Oldstone. 1979. Antiviral antibody reacting on the plasma membrane alters measles virus expression inside the cell. Nature 279529-530. [DOI] [PubMed] [Google Scholar]
- 13.Gaydos, C. A., and J. C. Gaydos. 1995. Adenovirus vaccines in the U.S. military. Mil. Med. 160300-304. [PubMed] [Google Scholar]
- 14.Girard, M. P., S. K. Osmanov, and M. P. Kieny. 2006. A review of vaccine research and development: the human immunodeficiency virus (HIV). Vaccine 244062-4081. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Román, V. R., R. H. Florese, L. J. Patterson, B. Peng, D. Venzon, K. Aldrich, and M. Robert-Guroff. 2006. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J. Immunol. Methods 30853-67. [DOI] [PubMed] [Google Scholar]
- 16.Gómez-Román, V. R., G. J. Grimes, G. K. Potti, B. Peng, T. Demberg, L. Gravlin, J. Treece, R. Pal, E. M. Lee, W. G. Alvord, P. D. Markham, and M. Robert-Guroff. 2006. Oral delivery of replication-competent adenovirus vectors is well tolerated by SIV- and SHIV-infected rhesus macaques. Vaccine 245064-5072. [DOI] [PubMed] [Google Scholar]
- 17.Gómez-Román, V. R., L. J. Patterson, D. Venzon, D. Liewehr, K. Aldrich, R. Florese, and M. Robert-Guroff. 2005. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J. Immunol. 1742185-2189. [DOI] [PubMed] [Google Scholar]
- 18.Gómez-Román, V. R., and M. Robert-Guroff. 2003. Adenoviruses as vectors for HIV vaccines. AIDS Rev. 5178-185. [PubMed] [Google Scholar]
- 19.Haase, A. T. 2005. Perils at mucosal front lines for HIV and SIV and their hosts. Nat. Rev. Immunol. 5783-792. [DOI] [PubMed] [Google Scholar]
- 20.Hessell, A. J., L. Hangartner, M. Hunter, C. E. Havenith, F. J. Beurskens, J. M. Bakker, C. M. Lanigan, G. Landucci, D. N. Forthal, P. W. Parren, P. A. Marx, and D. R. Burton. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449101-104. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann-Lehmann, R., J. Vlasak, A. L. Williams, A. L. Chenine, H. M. McClure, D. C. Anderson, S. O'Neil, and R. M. Ruprecht. 2003. Live attenuated, nef-deleted SIV is pathogenic in most adult macaques after prolonged observation. AIDS 17157-166. [DOI] [PubMed] [Google Scholar]
- 22.Holl, V., M. Peressin, T. Decoville, S. Schmidt, S. Zolla-Pazner, A. M. Aubertin, and C. Moog. 2006. Nonneutralizing antibodies are able to inhibit human immunodeficiency virus type 1 replication in macrophages and immature dendritic cells. J. Virol. 806177-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klasse, P. J., and Q. J. Sattentau. 2002. Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J. Gen. Virol. 832091-2108. [DOI] [PubMed] [Google Scholar]
- 24.Klessig, D. F., and T. Grodzicker. 1979. Mutations that allow human Ad2 and Ad5 to express late genes in monkey cells map in the viral gene encoding the 72K DNA binding protein. Cell 17957-966. [DOI] [PubMed] [Google Scholar]
- 25.Klessig, D. F., and M. P. Quinlan. 1982. Genetic evidence for separate functional domains on the human adenovirus specified, 72 kd, DNA binding protein. J. Mol. Appl. Genet. 1263-272. [PubMed] [Google Scholar]
- 26.Letvin, N. L. 2006. Progress and obstacles in the development of an AIDS vaccine. Nat. Rev. Immunol. 6930-939. [DOI] [PubMed] [Google Scholar]
- 27.Levine, B., J. M. Hardwick, B. D. Trapp, T. O. Crawford, R. C. Bollinger, and D. E. Griffin. 1991. Antibody-mediated clearance of alphavirus infection from neurons. Science 254856-860. [DOI] [PubMed] [Google Scholar]
- 28.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 7910108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lubeck, M. D., R. Natuk, M. Myagkikh, N. Kalyan, K. Aldrich, F. Sinangil, S. Alipanah, S. C. Murthy, P. K. Chanda, S. M. Nigida, Jr., P. D. Markham, S. Zolla-Pazner, K. Steimer, M. Wade, M. S. Reitz, Jr., L. O. Arthur, S. Mizutani, A. Davis, P. P. Hung, R. C. Gallo, J. Eichberg, and M. Robert-Guroff. 1997. Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat. Med. 3651-658. [DOI] [PubMed] [Google Scholar]
- 30.Ludwig, S. L., J. F. Brundage, P. W. Kelley, R. Nang, C. Towle, D. P. Schnurr, L. Crawford-Miksza, and J. C. Gaydos. 1998. Prevalence of antibodies to adenovirus serotypes 4 and 7 among unimmunized US Army trainees: results of a retrospective nationwide seroprevalence survey. J. Infect. Dis. 1781776-1778. [DOI] [PubMed] [Google Scholar]
- 31.Malkevitch, N., L. J. Patterson, K. Aldrich, E. Richardson, W. G. Alvord, and M. Robert-Guroff. 2003. A replication competent adenovirus 5 host range mutant-simian immunodeficiency virus (SIV) recombinant priming/subunit protein boosting vaccine regimen induces broad, persistent SIV-specific cellular immunity to dominant and subdominant epitopes in Mamu-A*01 rhesus macaques. J. Immunol. 1704281-4289. [DOI] [PubMed] [Google Scholar]
- 32.Malkevitch, N. V., L. J. Patterson, M. K. Aldrich, Y. Wu, D. Venzon, R. H. Florese, V. S. Kalyanaraman, R. Pal, E. M. Lee, J. Zhao, A. Cristillo, and M. Robert-Guroff. 2006. Durable protection of rhesus macaques immunized with a replicating adenovirus-SIV multigene prime/protein boost vaccine regimen against a second SIVmac251 rectal challenge: role of SIV-specific CD8+ T-cell responses. Virology 35383-98. [DOI] [PubMed] [Google Scholar]
- 33.Malkevitch, N. V., and M. Robert-Guroff. 2004. A call for replicating vector prime-protein boost strategies in HIV vaccine design. Expert Rev. Vaccines 3S105-S117. [DOI] [PubMed] [Google Scholar]
- 34.Mascola, J. J. R., G. G. Stiegler, T. T. C. VanCott, H. H. Katinger, C. C. B. Carpenter, C. C. E. Hanson, H. H. Beary, D. D. Hayes, S. S. S. Frankel, D. D. L. Birx, and M. M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6207-210. [DOI] [PubMed] [Google Scholar]
- 35.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 734009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, C. J., Q. Li, K. Abel, E. Y. Kim, Z. M. Ma, S. Wietgrefe, L. La Franco-Scheuch, L. Compton, L. Duan, M. D. Shore, M. Zupancic, M. Busch, J. Carlis, S. Wolinsky, and A. T. Haase. 2005. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J. Virol. 799217-9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montefiori, D. C. 2005. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr. Protoc. Immunol. Chapter 12:Unit 12.11. [DOI] [PubMed]
- 38.Mori, K., Y. Yasutomi, S. Ohgimoto, T. Nakasone, S. Takamura, T. Shioda, and Y. Nagai. 2001. Quintuple deglycosylation mutant of simian immunodeficiency virus SIVmac239 in rhesus macaques: robust primary replication, tightly contained chronic infection, and elicitation of potent immunity against the parental wild-type strain. J. Virol. 754023-4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishimura, Y., T. Igarashi, N. L. Haigwood, R. Sadjadpour, O. K. Donau, C. Buckler, R. J. Plishka, A. Buckler-White, and M. A. Martin. 2003. Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: implications for HIV-1 vaccine development. Proc. Natl. Acad. Sci. USA 10015131-15136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parren, P. W., J. P. Moore, D. R. Burton, and Q. J. Sattentau. 1999. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS 13(Suppl. A)S137-S162. [PubMed] [Google Scholar]
- 41.Patterson, L. J., J. Beal, T. Demberg, R. H. Florese, N. Malkevich, D. Venzon, K. Aldrich, E. Richardson, V. S. Kalyanaraman, I. Kalisz, E. M. Lee, D. C. Montefiori, F. A. Robey, and M. Robert-Guroff. 2008. Replicating adenovirus HIV/SIV recombinant priming alone or in combination with a gp140 protein boost results in significant control of viremia following a SHIV(89.6P) challenge in Mamu-A01-negative rhesus macaques. Virology 374322-337. [DOI] [PubMed] [Google Scholar]
- 42.Patterson, L. J., N. Malkevitch, J. Pinczewski, D. Venzon, Y. Lou, B. Peng, C. Munch, M. Leonard, E. Richardson, K. Aldrich, V. S. Kalyanaraman, G. N. Pavlakis, and M. Robert-Guroff. 2003. Potent, persistent induction and modulation of cellular immune responses in rhesus macaques primed with Ad5hr-simian immunodeficiency virus (SIV) env/rev, gag, and/or nef vaccines and boosted with SIV gp120. J. Virol. 778607-8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patterson, L. J., N. Malkevitch, D. Venzon, J. Pinczewski, V. R. Gomez-Roman, L. Wang, V. S. Kalyanaraman, P. D. Markham, F. A. Robey, and M. Robert-Guroff. 2004. Protection against mucosal simian immunodeficiency virus SIVmac251 challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. J. Virol. 782212-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patterson, L. J., and M. Robert-Guroff. 2008. Replicating adenovirus vector prime/protein boost strategies for HIV vaccine development. Expert Opin. Biol. Ther. 81347-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng, B., L. R. Wang, V. R. Gómez-Román, A. Davis-Warren, D. C. Montefiori, V. S. Kalyanaraman, D. Venzon, J. Zhao, E. Kan, T. J. Rowell, K. K. Murthy, I. Srivastava, S. W. Barnett, and M. Robert-Guroff. 2005. Replicating rather than nonreplicating adenovirus-human immunodeficiency virus recombinant vaccines are better at eliciting potent cellular immunity and priming high-titer antibodies. J. Virol. 7910200-10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reynolds, M. R., A. M. Weiler, K. L. Weisgrau, S. M. Piaskowski, J. R. Furlott, J. T. Weinfurter, M. Kaizu, T. Soma, E. J. Leon, C. Macnair, D. P. Leaman, M. B. Zwick, E. Gostick, S. K. Musani, D. A. Price, T. C. Friedrich, E. G. Rakasz, N. A. Wilson, A. B. McDermott, R. Boyle, D. B. Allison, D. R. Burton, W. C. Koff, and D. I. Watkins. 2008. Macaques vaccinated with live-attenuated SIV control replication of heterologous virus. J. Exp. Med. doi: 10.1084/jem.20081524. [DOI] [PMC free article] [PubMed]
- 47.Robert-Guroff, M., H. Kaur, L. J. Patterson, M. Leno, A. J. Conley, P. M. McKenna, P. D. Markham, E. Richardson, K. Aldrich, K. Arora, L. Murty, L. Carter, S. Zolla-Pazner, and F. Sinangil. 1998. Vaccine protection against a heterologous, non-syncytium-inducing, primary human immunodeficiency virus. J. Virol. 7210275-10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez, J. E., T. Moninger, and C. Grose. 1993. Entry and egress of varicella virus blocked by same anti-gH monoclonal antibody. Virology 196840-844. [DOI] [PubMed] [Google Scholar]
- 49.Sairenji, T., P. S. Reisert, R. C. Spiro, T. Connolly, and R. E. Humphreys. 1985. Inhibition of Epstein-Barr virus (EBV) release from the P3HR-1 Burkitt's lymphoma cell line by a monoclonal antibody against a 200,000 dalton EBV membrane antigen. J. Exp. Med. 1611097-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwartlander, B., J. Stover, N. Walker, L. Bollinger, J. P. Gutierrez, W. McGreevey, M. Opuni, S. Forsythe, L. Kumaranayake, C. Watts, and S. Bertozzi. 2001. AIDS: resource needs for HIV/AIDS. Science 2922434-2436. [DOI] [PubMed] [Google Scholar]
- 51.Shariff, D. M., J. Hallworth, M. Desperbasques, A. Buchan, and G. R. Skinner. 1988. Immune inhibition of virus release from herpes simplex virus-infected cells by human sera. Intervirology 29125-132. [DOI] [PubMed] [Google Scholar]
- 52.Shattock, R. J., and J. P. Moore. 2003. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 125-34. [DOI] [PubMed] [Google Scholar]
- 53.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415331-335. [DOI] [PubMed] [Google Scholar]
- 54.Van Rompay, K. K., C. J. Berardi, S. Dillard-Telm, R. P. Tarara, D. R. Canfield, C. R. Valverde, D. C. Montefiori, K. S. Cole, R. C. Montelaro, C. J. Miller, and M. L. Marthas. 1998. Passive immunization of newborn rhesus macaques prevents oral simian immunodeficiency virus infection. J. Infect. Dis. 1771247-1259. [DOI] [PubMed] [Google Scholar]
- 55.Vogel, T. U., M. R. Reynolds, D. H. Fuller, K. Vielhuber, T. Shipley, J. T. Fuller, K. J. Kunstman, G. Sutter, M. L. Marthas, V. Erfle, S. M. Wolinsky, C. Wang, D. B. Allison, E. W. Rud, N. Wilson, D. Montefiori, J. D. Altman, and D. I. Watkins. 2003. Multispecific vaccine-induced mucosal cytotoxic T lymphocytes reduce acute-phase viral replication but fail in long-term control of simian immunodeficiency virus SIVmac239. J. Virol. 7713348-13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wyand, M. S., K. H. Manson, M. Garcia-Moll, D. Montefiori, and R. C. Desrosiers. 1996. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J. Virol. 703724-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 2801884-1888. [DOI] [PubMed] [Google Scholar]
- 58.Zhao, J., Y. Lou, J. Pinczewski, N. Malkevitch, K. Aldrich, V. S. Kalyanaraman, D. Venzon, B. Peng, L. J. Patterson, Y. Edghill-Smith, R. Woodward, G. N. Pavlakis, and M. Robert-Guroff. 2003. Boosting of SIV-specific immune responses in rhesus macaques by repeated administration of Ad5hr-SIVenv/rev and Ad5hr-SIVgag recombinants. Vaccine 214022-4035. [DOI] [PubMed] [Google Scholar]
- 59.Zhao, J., J. Pinczewski, V. R. Gomez-Roman, D. Venzon, V. S. Kalyanaraman, P. D. Markham, K. Aldrich, M. Moake, D. C. Montefiori, Y. Lou, G. N. Pavlakis, and M. Robert-Guroff. 2003. Improved protection of rhesus macaques against intrarectal simian immunodeficiency virus SIV(mac251) challenge by a replication-competent Ad5hr-SIVenv/rev and Ad5hr-SIVgag recombinant priming/gp120 boosting regimen. J. Virol. 778354-8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou, Q., R. Hidajat, B. Peng, D. Venzon, M. K. Aldrich, E. Richardson, E. M. Lee, V. S. Kalyanaraman, G. Grimes, V. R. Gomez-Roman, L. E. Summers, N. Malkevich, and M. Robert-Guroff. 2007. Comparative evaluation of oral and intranasal priming with replication-competent adenovirus 5 host range mutant (Ad5hr)-simian immunodeficiency virus (SIV) recombinant vaccines on immunogenicity and protective efficacy against SIV(mac251). Vaccine 258021-8035. [DOI] [PubMed] [Google Scholar]