Abstract
ISG15 functions as a critical antiviral molecule against influenza virus, with infection inducing both the conjugation of ISG15 to target proteins and production of free ISG15. Here, we report that mice lacking the ISG15 E1 enzyme UbE1L fail to form ISG15 conjugates. Both UbE1L−/− and ISG15−/− mice display increased susceptibility to influenza B virus infection, including non-mouse-adapted strains. Finally, we demonstrate that ISG15 controls influenza B virus infection through its action within radioresistant stromal cells and not bone marrow-derived cells. Thus, the conjugation of ISG15 to target proteins within stromal cells is critical to its activity against influenza virus.
One of the earliest host responses to viral infection is the production of type I interferons (IFN-α and -β) and the subsequent upregulation of IFN-stimulated genes (ISGs). These ISGs generate an antiviral state in nearby cells and also play an important role in shaping the host innate and adaptive immune response (26, 28). We and others have recently identified ISG15 as a critical IFN-induced antiviral molecule. Overexpression of ISG15 by a recombinant Sindbis virus protected IFN-αβ receptor-deficient mice from lethality (13). In a cell culture system, the overexpression of ISG15 also inhibited the release of human immunodeficiency virus virions (20) and decreased alphavirus replication (32). Finally, mice lacking ISG15 are susceptible to several human pathogens, including influenza A and B viruses, herpesviruses, and Sindbis viruses (14). Though it is clear that ISG15 functions as an antiviral molecule, its mechanism and site of action remain poorly understood.
ISG15, a 17-kDa ubiquitin-like molecule, contains two ubiquitin domains, including a carboxy-terminal LRLRGG motif, through which it forms conjugates with target proteins (15, 19). ISG15 conjugation of target proteins (ISGylation) utilizes an IFN-induced conjugation cascade which includes an E1 (UbE1L/Uba7), an E2 (UbcH8), several E3 ligases (EFP, HHARI, and Herc5), and a deconjugating protease (UBP43/USP18) (1, 11, 17, 22, 31, 33, 35). Activation of this pathway results in the conjugation of ISG15 to over 100 known target proteins that encompass multiple biological pathways (8, 16, 30, 34). In addition to forming conjugates, free ISG15 also accumulates within cells and is released into the sera of patients following stimulation with IFN (5). Recombinant ISG15 has been reported to function as a cytokine, stimulating IFN-γ production, NK cell proliferation, neutrophil chemotaxis, and dendritic cell maturation (4, 23-25). During viral infection in mice, ISG15 exists in three forms: (i) unconjugated within cells, (ii) conjugated to target proteins, and (iii) released into the serum (14). Studies with Sindbis virus suggest that the conjugated form of ISG15 mediates its antiviral activity. The increased lethality seen in ISG15−/− mice can be rescued by a recombinant virus expressing wild-type ISG15 but not mutant ISG15, which cannot form conjugates in vitro (14). In contrast, two recent reports showed that free ISG15, in the absence of its conjugation cascade, inhibited the release of Ebola virus-like particles by interfering with the activity of Nedd4 (18, 21). Thus, evidence supports the antiviral activity of both conjugated and unconjugated ISG15. While mice lacking ISG15 are susceptible to influenza B virus infection, the form of ISG15 mediating this antiviral activity has yet to be determined.
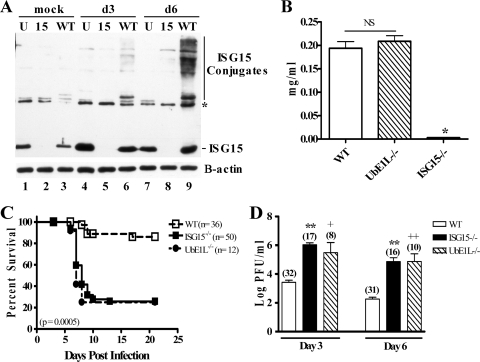
We took advantage of mice lacking the ISG15 E1 enzyme UbE1L (12) to genetically evaluate the requirement of ISG15 conjugation for its antiviral activity in vivo. First, we evaluated the requirement for UbE1L in the generation of ISG15 conjugates and in its release into the serum during viral infection. Six- to nine-week-old mice were mock treated or infected intranasally (i.n.) with influenza B/Lee/40 virus (B/Lee) at a dose of 1 × 106 PFU. At days 3 and 6 postinfection, their lung were removed and homogenates were analyzed by Western blotting for the expression of ISG15 and ISG15 conjugates, and their serum was analyzed for released ISG15 by enzyme-linked immunosorbent assay, as previously described (13, 14). Within 3 days postinfection, free ISG15 was upregulated in both wild-type (WT) and UbE1L−/− (U) mice (Fig. 1A, lanes 4 and 6) but not in ISG15−/− (15) mice (Fig. 1A, lane 5). While WT mice displayed low levels of ISG15 conjugates on day 3 (Fig. 1A, lane 6), and increasing levels by 6 days postinfection (Fig. 1A, lane 9), UbE1L−/− mice displayed no detectable conjugates, even at 6 days postinfection (Fig. 1A, lane 7). Therefore, even during viral infection, we were unable to detect ISG15 conjugates in mice lacking UbE1L. In contrast, both UbE1L−/− and WT mice released equivalent amounts of ISG15 into their serum (Fig. 1B). Our results indicate that UbE1L functions as the dominant E1 for ISGylation within the lung during influenza virus infection but is not required for the release of ISG15 into the serum.
FIG. 1.
UbE1L−/− mice fail to produce ISG15 conjugates and are more susceptible to influenza B virus infection than WT mice. Mice were infected i.n. with B/Lee at 1 × 106 PFU and analyzed for ISG15 conjugation, lethality, and viral load. (A) Lung lysates from either mock-treated or B/Lee-infected UbE1L−/− (U), ISG15−/− (15), or WT mice were analyzed for ISG15 expression by Western blotting on the indicated days postinfection. Parallel blots were probed with anti-β-actin as a loading control. The asterisk denotes a background band. The blot is representative of three independent experiments. (B) Sera from B/Lee-infected WT (n = 17), UbE1L−/− (n = 10), and ISG15−/− (n = 5) mice were analyzed by enzyme-linked immunosorbent assay as previously described (14) to detect released ISG15. The difference between WT and UbE1L−/− mice was not statistically significant. *, P = 0.0129 (versus WT) and P = 0.0070 (versus UbE1L−/−). (C) UbE1L−/−, ISG15−/−, and WT mice were infected as described above and monitored for lethality. The P value refers to the comparison between WT and UbE1L−/− mice. P was <0.0001 for the comparison of WT and ISG15−/− mice. There was no statistically significant difference between ISG15−/− and UbE1L−/− mice. (D) Lungs were harvested at 3 and 6 days postinfection from WT, ISG15−/−, and UbE1L−/− mice and analyzed for viral titer by standard plaque assay. **, P < 0.0001 for WT versus ISG15−/− mice; +, P = 0.0149, and ++, P = 0.0011 for WT versus UbE1L−/− mice. There was no statistical difference between ISG15−/− and UbE1L−/− mice. WT and ISG15−/− survival rates (C) and titers (D) include historical data from reference 14.
We previously demonstrated that ISG15−/− mice displayed increased susceptibility to influenza virus infection. To determine if ISGylation of target proteins mediates ISG15's antiviral activity against influenza B virus, we evaluated the response of UbE1L−/− mice to infection. WT, ISG15−/−, and UbE1L−/− mice were infected with 1 × 106 PFU of B/Lee i.n. as described previously (14). Mice were either monitored for lethality or evaluated for viral titers within their lungs by standard plaque assay. UbE1L−/− mice displayed markedly increased susceptibility to infection, with only 25% of UbE1L−/− mice surviving after 21 days (Fig. 1C, P = 0.0005), compared to 86% survival in WT mice. Both the kinetics of lethality and the overall survival in UbE1L−/− mice were identical to those observed in the ISG15−/− mice. In addition, UbE1L−/− mice, similar to ISG15−/− mice, displayed viral titers in their lungs that were 2 to 3 logs higher than those in WT mice at both 3 and 6 days postinfection (Fig. 1D). There was no significant difference between viral titers in ISG15−/− and UbE1L−/− mice. This is the first phenotype described for the UbE1L−/− mouse, which was previously found to have no defects in handling vesicular stomatitis virus or lymphocytic choriomenigitis virus infection (12). Importantly, unlike the data seen with Ebola virus-like particles, where conjugation does not seem to be required for the antiviral action of ISG15 (18, 21), nearly all of the antiviral activity of ISG15 against influenza B virus is mediated through its conjugation to target proteins.
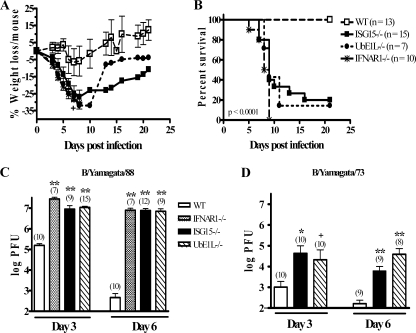
Non-mouse-adapted influenza B viruses cause a subclinical infection in WT mice, with rapid clearance of the virus from the respiratory tract. Since the mouse-adapted B/Lee strain caused significant lethality in ISG15−/− and UbE1L−/− mice, we next evaluated their response to non-mouse-adapted strains of influenza B virus. WT mice, ISG15−/− mice, UbE1L−/− mice, and mice lacking the type I IFN receptor (IFNAR1−/−) were infected i.n. with 3 × 105 PFU of the non-mouse-adapted strain influenza B/Yamagata/88 virus and monitored for morbidity and mortality. As expected, this nonadapted influenza B virus did not cause significant weight loss (Fig. 2A) or any lethality (Fig. 2B) in WT mice. In contrast, mice lacking either ISG15 or UbE1L were profoundly susceptible to B/Yamagata/88 virus infection, losing up to 30% of their body weight (Fig. 2A) and displaying significant lethality, with 80% of the ISG15−/− and 85.7% of the UbE1L−/− mice dying from the infection (Fig. 2B). The weight loss and lethality were similar to those observed in IFNAR1−/− mice, in which 100% of the mice succumbed to infection with B/Yamagata/88 virus (Fig. 2A and B), with similar kinetics to that observed for both the ISG15−/− and UbE1L−/− mice. We also evaluated the viral titers in the lungs of infected mice. At both 3 and 6 days postinfection, we found increased viral titers in the mice lacking either ISG15 or UbE1L. These titers were equivalent to those observed in IFNAR1−/− mice (Fig. 2C). By 6 days postinfection, WT mice were clearing the infection, while animals lacking ISG15, UbE1L, or IFNAR1 continued to have viral titers that were 4 logs greater than those seen in WT mice. A similar increase in viral titers was observed in ISG15−/− and UbE1L−/− mice following infection with influenza B/Yamagata/73 virus, another non-mouse-adapted strain (Fig. 2D). These results show that the type I IFN response contributes significantly to limiting the host specificity of influenza B virus infection, with ISG15 and its conjugation to target proteins accounting for a large portion of this effect.
FIG. 2.
ISG15−/− and UbE1L−/− mice are susceptible to non-mouse-adapted influenza B virus infection. (A and B) WT, ISG15−/−, UbE1L−/−, and IFNAR1−/− mice were infected i.n. with 3 × 105 PFU influenza B/Yamagata/88 virus and monitored for weight loss (A) and lethality (B). (A) +, less than 30% of mice remained. (B) Differences between WT mice and ISG15−/−, UbE1L−/−, and IFNAR1−/− mice were statistically significant (P < 0.0001). The difference between ISG15−/− and IFNAR1−/− mice was significant (P = 0.0219). Differences between UbE1L−/− and IFNAR1−/− mice and between UbE1L−/− and ISG15−/− mice were not statistically significant. (C and D) WT, ISG15−/−, UbE1L−/−, and IFNAR1−/− mice were infected with influenza B/Yamagata/88 virus (C) or B/Yamagata/73 virus (D) as described above, and their lungs were removed at 3 or 6 days postinfection and assayed for viral titer by standard plaque assay. The numbers of mice per group are in parentheses. +, P < 0.05; *, P < 0.005; **, P ≤ 0.0002 (WT mice versus the other groups).
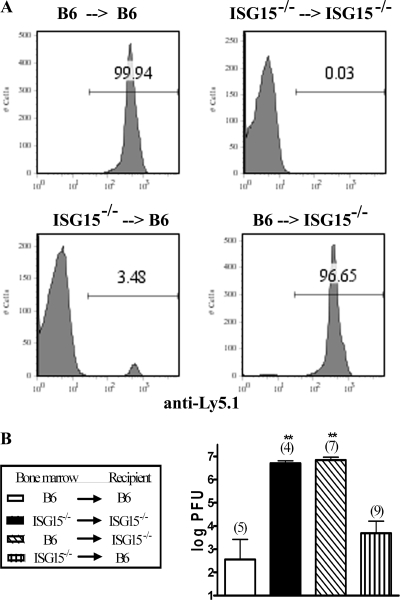
Many IFN-stimulated genes function as antiviral molecules by directly inhibiting viral replication (26). Yet previous studies revealed no significant alterations in the growth of influenza virus or additional viruses (herpes simplex virus 1, Sindbis virus, γHV68) in murine embryonic fibroblasts lacking ISG15 (14), despite ISG15−/− mice displaying increased susceptibility to these viruses in vivo (14). Since IFNs, in addition to their direct antiviral activity, can also modulate the function of immune cells involved in both the innate and adaptive response (26, 28), we wanted to determine if the antiviral action of ISG15 was mediated by its activity in stromal or immune cells. Toward this end, we generated bone marrow-chimeric mice by irradiating either C57BL/6 (B6) mice (Ly5.1+) or ISG15−/− mice (Ly5.2+) with 9.5 Gy. Irradiated B6 or ISG15−/− mice were injected via tail vein with 8.0 × 106 bone marrow cells isolated from either B6 (Ly5.1+) or ISG15−/− (Ly5.2+) mice. Six weeks after bone marrow transfer, the mice were assessed for reconstitution by fluorescence-activated cell sorting analysis of peripheral blood lymphocytes. The chimeric mice (ISG15−/− → B6 and B6 → ISG15−/−) displayed between 95 and 98% reconstitution, as determined by staining of peripheral blood lymphocytes with anti-Ly5.1 antibodies (Fig. 3A). Mice were then infected with influenza virus B/Lee at 1 × 106 PFU i.n., and 6 days postinfection, their lungs were harvested to determine viral loads by plaque assay. As expected, B6 → B6 mice displayed minimal signs of disease and were able to control viral replication within their lungs (Fig. 3B). In contrast, ISG15−/− → ISG15−/− mice displayed clinical signs of disease, with all of the mice showing ruffling of the fur and one animal dying by day 6 postinfection (data not shown). They also had viral titers that were nearly 4 logs greater than those observed in the B6 → B6 mice (Fig. 3B). Analysis of the chimeric mice revealed that, similar to the ISG15−/− mice, ISG15−/− mice reconstituted with bone marrow cells expressing ISG15 (B6 → ISG15−/−) appeared sick, with ruffling of the fur, and one death occurred by 6 days postinfection (data not shown). These B6 → ISG15−/− chimeric mice had viral loads that were equal to those of the ISG15−/− mice, with titers that were 4 logs greater than those observed in B6 mice (Fig. 3B). In contrast, B6 mice reconstituted with ISG15-deficient bone marrow (ISG15−/− → B6) showed minimal signs of disease and had greatly reduced viral loads, similar to those observed in the B6 mice (Fig. 3B). These results provide evidence that the expression of ISG15 in nonhematopoietic cells is critical for controlling acute influenza B virus replication.
FIG. 3.
The antiviral activity of ISG15 against influenza B virus is mediated by radioresistant stromal cells. Chimeric mice were generated by injecting B6 (Ly5.1) or ISG15−/− (Ly5.2) bone marrow into lethally irradiated B6 (Ly5.1) or ISG15−/− (Ly5.2) recipients. (A) Peripheral blood lymphocytes were obtained 6 weeks after bone marrow transfer and stained for expression of Ly5.1 to assess reconstitution. Representative histograms gating on the lymphocyte population are shown for each group. ISG15−/− → B6 and B6 → ISG15−/− mice displayed reconstitution rates between 95 and 98%. (B) Chimeric mice were infected i.n. with influenza virus B/Lee at 1 × 106 PFU and monitored for signs of disease and lethality. On day 6 postinfection, the mice were sacrificed, and their lungs were assayed by plaque assay for viral load. The numbers in parentheses are numbers of mice per group. **, P < 0.05 compared to B6 → B6 mice or P < 0.005 compared to ISG15−/− → B6 mice. The difference between B6 → B6 and ISG15−/− → B6 mice was not statistically significant.
ISG15 has been reported to have both conjugation-dependent and non-conjugation-dependent antiviral functions. In this study, we demonstrate that the conjugation of ISG15 to target proteins is responsible for its antiviral activity during influenza B virus infection. As has been seen in other viral systems (29), we also found that the type I IFN response played a significant role in regulating the host specificity of influenza B virus. Surprisingly, a large part of this response appears to be mediated by the conjugation of ISG15 to target proteins, providing further support for the importance of this innate antiviral immune response in influenza B virus pathogenesis. Additional support for this hypothesis comes from the targeting of this pathway by several viral immune evasion molecules. Both nairoviruses and arteriviruses encode ovarian tumor domain-containing viral proteases that deconjugate ISGylated proteins and abrogate the antiviral activity of ISG15 (7). The vaccinia virus E3 protein has recently been found to also bind to ISG15 and disrupt its antiviral activity (9). Finally, the NS1 protein of influenza B virus (B/NS1) binds to human ISG15 and inhibits its interaction with UbE1L, preventing the formation of ISG15 conjugates but not the upregulation of free ISG15 (31). Despite these inhibitory effects, influenza B virus still remains susceptible to the ISG15 system (14; also this study), indicating that the inhibition of ISG15 by influenza B virus is not complete, at least in mice. The B/NS1 protein employs multiple strategies to evade the host IFN response (2, 6). Whether the actions of B/NS1 as an immune evasion molecule differ between mouse and human will be of interest in future studies.
We also found that the predominant site of action of ISG15 during influenza virus infection resides within a radioresistant stromal cell population. A likely candidate is the respiratory epithelium, since it is the site of influenza virus replication. Previous work with Sendai virus found that the function of Stat-1 in the respiratory epithelium, as opposed to the immune cell compartment, was critical in the antiviral response (27). Whether ISG15 plays a significant antiviral role against additional respiratory pathogens and which proteins are targeted for ISGylation in the respiratory epithelium remain to be determined.
Recent studies have identified over 100 cellular target proteins that can be modified by ISG15, yet the fate of these ISGylated proteins is largely unknown, and the role that many of these targets play during viral infection has yet to be explored (8, 10, 16, 30, 34). These targets span multiple cellular pathways, including carbohydrate metabolism, cell cycle regulation, protein translation, signal transduction, and DNA damage. Also included among these target proteins are known antiviral molecules (RIG-I, MDA-5, Mx1, and PKR) and signaling molecules critical to the innate immune response (STAT-1 and JAK-1). Modification of the functional activity or localization of these host proteins by ISG15 conjugation may alter the antiviral response mediated by the host. Alternatively, ISG15 conjugation to viral proteins might impair viral replication in vivo. Determining which host and viral proteins are modified and critical to the host antiviral response may allow the development of targeted therapeutics against many important human pathogens.
Acknowledgments
This work was partly supported by NIAID grants K08 AI059390 and R21 AI077008 to D.J.L., grant Wo554/3-2 of Deutsche Forschungsgemeinschaft to T.W., NIAID grants R01 AI46954 and UC19 AI62623 (CIVIA, Center for Investigating Viral Immunity and Antagonism) to A.G.-S., and NIH grant GM 66955 to D.-E.Z.
We thank Darren Kreamalmayer for his outstanding expertise in animal care and Richard Cadagan for excellent technical assistance.
Footnotes
Published ahead of print on 12 November 2008.
REFERENCES
- 1.Dastur, A., S. Beaudenon, M. Kelley, R. M. Krug, and J. M. Huibregtse. 2006. Herc5, an interferon-induced HECT E3 enzyme, is required for conjugation of ISG15 in human cells. J. Biol. Chem. 2814334-4338. [DOI] [PubMed] [Google Scholar]
- 2.Dauber, B., G. Heins, and T. Wolff. 2004. The influenza B virus nonstructural NS1 protein is essential for efficient viral growth and antagonizes beta interferon induction. J. Virol. 781865-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dauber, B., J. Schneider, and T. Wolff. 2006. Double-stranded RNA binding of influenza B virus nonstructural NS1 protein inhibits protein kinase R but is not essential to antagonize production of alpha/beta interferon. J. Virol. 8011667-11677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Cunha, J., E. Knight, Jr., A. L. Haas, R. L. Truitt, and E. C. Borden. 1996. Immunoregulatory properties of ISG15, an interferon-induced cytokine. Proc. Natl. Acad. Sci. USA 93211-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Cunha, J., S. Ramanujam, R. J. Wagner, P. L. Witt, E. Knight, Jr., and E. C. Borden. 1996. In vitro and in vivo secretion of human ISG15, an IFN-induced immunomodulatory cytokine. J. Immunol. 1574100-4108. [PubMed] [Google Scholar]
- 6.Donelan, N. R., B. Dauber, X. Wang, C. F. Basler, T. Wolff, and A. Garcia-Sastre. 2004. The N- and C-terminal domains of the NS1 protein of influenza B virus can independently inhibit IRF-3 and beta interferon promoter activation. J. Virol. 7811574-11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frias-Staheli, N., N. V. Giannakopoulos, M. Kikkert, S. L. Taylor, A. Bridgen, J. J. Paragas, J. A. Richt, R. R. Rowland, C. S. Schmaljohn, D. J. Lenschow, E. J. Snijder, A. Garcia-Sastre, and H. W. Virgin. 2007. Ovarian tumor (OTU)-domain containing viral proteases evade ubiquitin- and ISG15-dependent innate immune responses. Cell Host Microbe 2404-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giannakopoulos, N. V., J. K. Luo, V. Papov, W. G. Zou, D. J. Lenschow, B. S. Jacobs, E. C. Borden, J. Li, H. W. Virgin, and D. E. Zhang. 2005. Proteomic identification of proteins conjugated to ISG15 in mouse and human cells. Biochem. Biophys. Res. Commun. 336496-506. [DOI] [PubMed] [Google Scholar]
- 9.Guerra, S., A. Caceres, K. P. Knobeloch, I. Horak, and M. Esteban. 2008. Vaccinia virus E3 protein prevents the antiviral action of ISG15. PLoS Pathogen 4e100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamerman, J. A., F. Hayashi, L. A. Schroeder, S. P. Gygi, A. L. Haas, L. Hampson, P. Coughlin, R. Aebersold, and A. Aderem. 2002. Serpin 2a is induced in activated macrophages and conjugates to a ubiquitin homolog. J. Immunol. 1682415-2423. [DOI] [PubMed] [Google Scholar]
- 11.Kim, K. I., N. V. Giannakopoulos, H. W. Virgin, and D. E. Zhang. 2004. Interferon-inducible ubiquitin E2, Ubc8, is a conjugating enzyme for protein ISGylation. Mol. Cell. Biol. 249592-9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, K. I., M. Yan, O. Malakhova, J. K. Luo, M. F. Shen, W. Zou, J. C. de la Torre, and D. E. Zhang. 2006. Ube1L and protein ISGylation are not essential for alpha/beta interferon signaling. Mol. Cell. Biol. 26472-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenschow, D. J., N. V. Giannakopoulos, L. J. Gunn, C. Johnston, A. K. O'Guin, R. E. Schmidt, B. Levine, and H. W. Virgin. 2005. Identification of interferon-stimulated gene 15 as an antiviral molecule during Sindbis virus infection in vivo. J. Virol. 7913974-13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenschow, D. J., C. Lai, N. Frias-Staheli, N. V. Giannakopoulos, A. Lutz, T. Wolff, A. Osiak, B. Levine, R. E. Schmidt, A. Garcia-Sastre, D. A. Leib, A. Pekosz, K. P. Knobeloch, I. Horak, and H. W. Virgin. 2007. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc. Natl. Acad. Sci. USA 1041371-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loeb, K. R., and A. L. Haas. 1992. The interferon-inducible 15-kDa ubiquitin homolog conjugates to intracellular proteins. J. Biol. Chem. 2677806-7813. [PubMed] [Google Scholar]
- 16.Malakhov, M. P., K. I. Kim, O. A. Malakhova, B. S. Jacobs, E. C. Borden, and D. E. Zhang. 2003. High-throughput Immunoblotting. Ubiquitin-like protein ISG15 modifies key regulators of signal transduction. J. Biol. Chem. 27816608-16613. [DOI] [PubMed] [Google Scholar]
- 17.Malakhov, M. P., O. A. Malakhova, K. I. Kim, K. J. Ritchie, and D. E. Zhang. 2002. UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J. Biol. Chem. 2779976-9981. [DOI] [PubMed] [Google Scholar]
- 18.Malakhova, O. A., and D. E. Zhang. 2008. ISG15 inhibits Nedd4 ubiquitin E3 activity and enhances the innate antiviral response. J. Biol. Chem. 2838783-8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narasimhan, J., M. Wang, Z. Fu, J. M. Klein, A. L. Haas, and J. J. Kim. 2005. Crystal structure of the interferon-induced ubiquitin-like protein ISG15. J. Biol. Chem. 28027356-27365. [DOI] [PubMed] [Google Scholar]
- 20.Okumura, A., G. Lu, I. Pitha-Rowe, and P. M. Pitha. 2006. Innate antiviral response targets HIV-1 release by the induction of ubiquitin-like protein ISG15. Proc. Natl. Acad. Sci. USA 1031440-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okumura, A., P. M. Pitha, and R. N. Harty. 2008. ISG15 inhibits Ebola VP40 VLP budding in an L-domain-dependent manner by blocking Nedd4 ligase activity. Proc. Natl. Acad. Sci. USA 1053974-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okumura, F., W. Zou, and D. E. Zhang. 2007. ISG15 modification of the eIF4E cognate 4EHP enhances cap structure-binding activity of 4EHP. Genes Dev. 21255-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owhashi, M., Y. Taoka, K. Ishii, S. Nakazawa, H. Uemura, and H. Hambara. 2003. Identification of a ubiquitin family protein as a novel neutrophil chemotactic factor. Biochem. Biophys. Res. Commun. 309533-539. [DOI] [PubMed] [Google Scholar]
- 24.Padovan, E., L. Terracciano, U. Certa, B. Jacobs, A. Reschner, M. Bolli, G. C. Spagnoli, E. C. Borden, and M. Heberer. 2002. Interferon stimulated gene 15 constitutively produced by melanoma cells induces e-cadherin expression on human dendritic cells. Cancer Res. 623453-3458. [PubMed] [Google Scholar]
- 25.Recht, M., E. C. Borden, and E. Knight, Jr. 1991. A human 15-kDa IFN-induced protein induces the secretion of IFN-gamma. J. Immunol. 1472617-2623. [PubMed] [Google Scholar]
- 26.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shornick, L. P., A. G. Wells, Y. Zhang, A. C. Patel, G. Huang, K. Takami, M. Sosa, N. A. Shukla, E. Agapov, and M. J. Holtzman. 2008. Airway epithelial versus immune cell Stat 1 function for innate defense against respiratory viral infection. J. Immunol. 1803319-3328. [DOI] [PubMed] [Google Scholar]
- 28.Stetson, D. B., and R. Medzhitov. 2006. Type I interferons in host defense. Immunity 25373-381. [DOI] [PubMed] [Google Scholar]
- 29.Wang, F., Y. Ma, J. W. Barrett, X. Gao, J. Loh, E. Barton, H. W. Virgin, and G. McFadden. 2004. Disruption of Erk-dependent type I interferon induction breaks the myxoma virus species barrier. Nat. Immunol. 51266-1274. [DOI] [PubMed] [Google Scholar]
- 30.Wong, J. J., Y. F. Pung, N. S. Sze, and K. C. Chin. 2006. HERC5 is an IFN-induced HECT-type E3 protein ligase that mediates type I IFN-induced ISGylation of protein targets. Proc. Natl. Acad. Sci. USA 10310735-10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan, W., and R. M. Krug. 2001. Influenza B virus NS1 protein inhibits conjugation of the interferon (IFN)-induced ubiquitin-like ISG15 protein. EMBO J. 20362-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang, Y., C. W. Burke, K. D. Ryman, and W. B. Klimstra. 2007. Identification and characterization of interferon-induced proteins that inhibit alphavirus replication. J. Virol. 8111246-11255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao, C., S. L. Beaudenon, M. L. Kelley, M. B. Waddell, W. Yuan, B. A. Schulman, J. M. Huibregtse, and R. M. Krug. 2004. The UbcH8 ubiquitin E2 enzyme is also the E2 enzyme for ISG15, an IFN-alpha/beta-induced ubiquitin-like protein. Proc. Natl. Acad. Sci. USA 1017578-7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao, C., C. Denison, J. M. Huibregtse, S. Gygi, and R. M. Krug. 2005. Human ISG15 conjugation targets both IFN-induced and constitutively expressed proteins functioning in diverse cellular pathways. Proc. Natl. Acad. Sci. USA 10210200-10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou, W., and D. E. Zhang. 2006. The interferon-inducible ubiquitin-protein isopeptide ligase (E3) EFP also functions as an ISG15 E3 ligase. J. Biol. Chem. 2813989-3994. [DOI] [PubMed] [Google Scholar]