Abstract
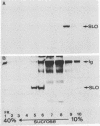
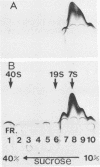
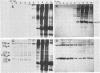
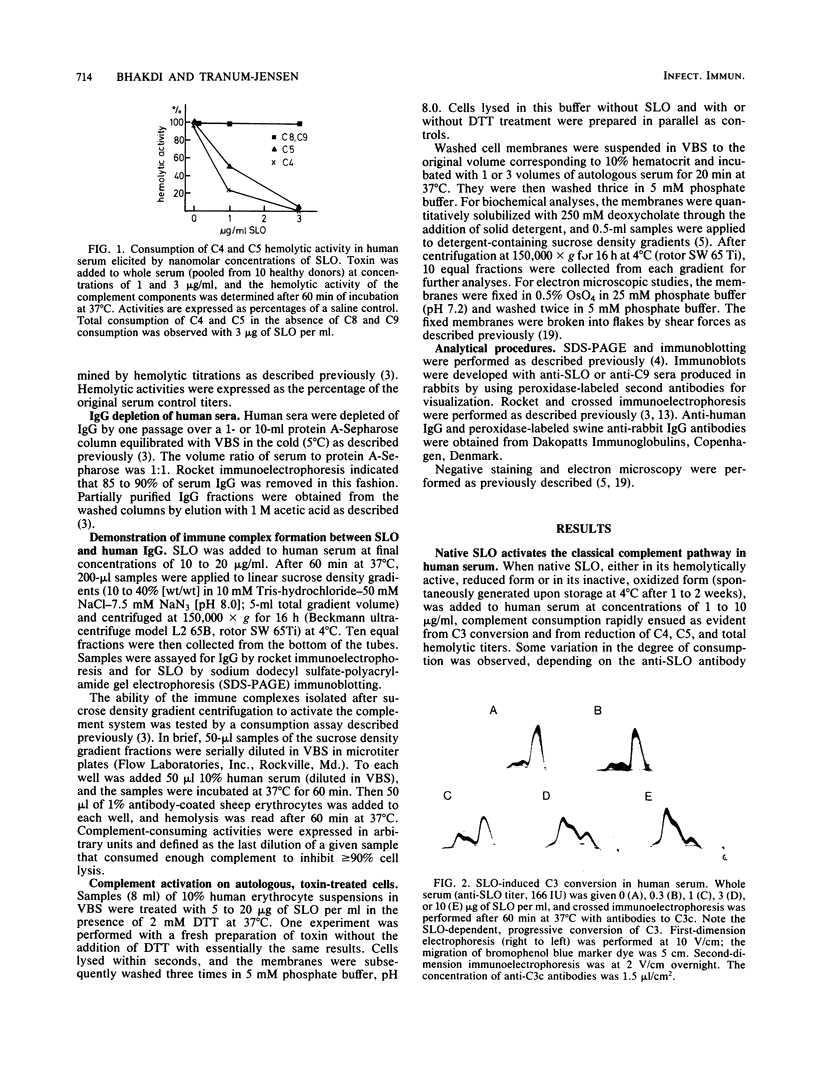
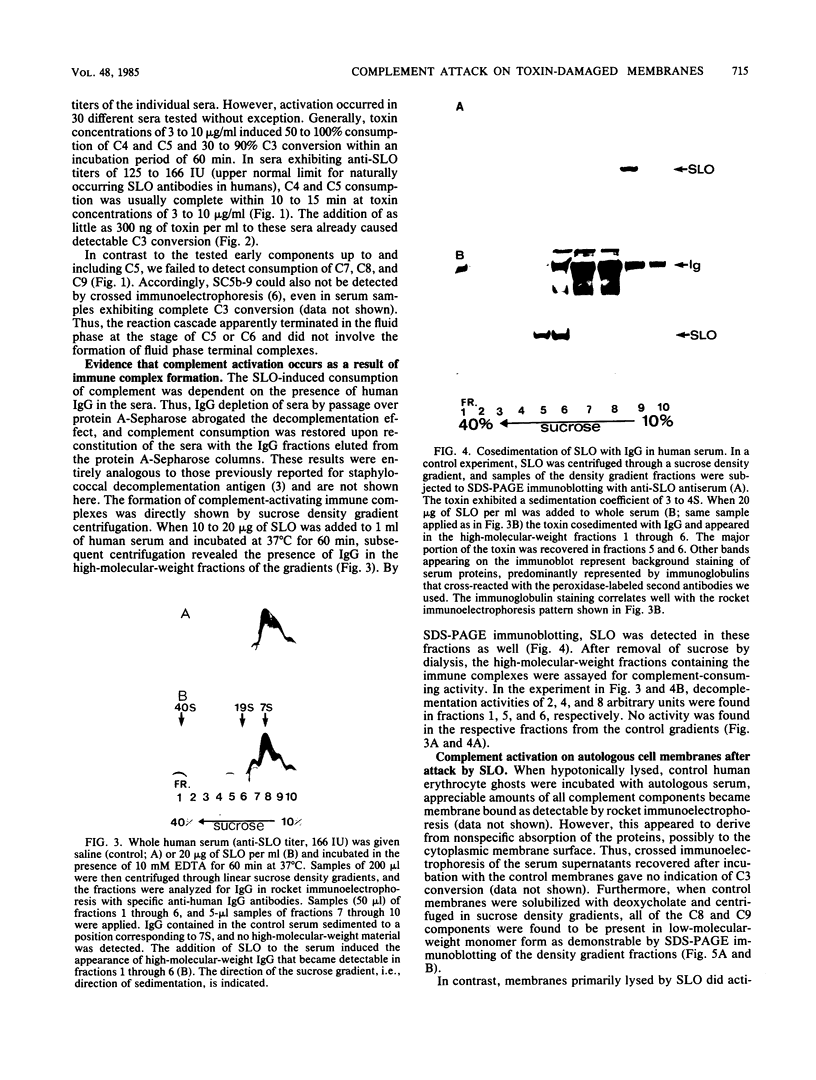
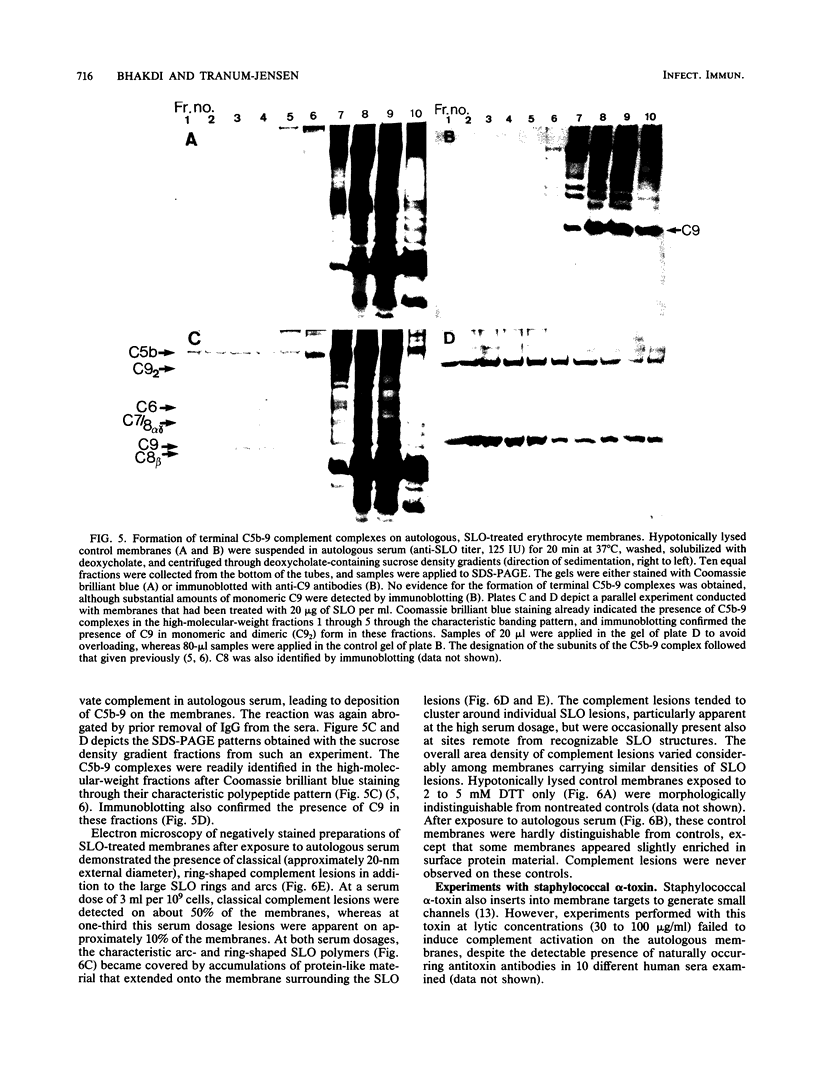
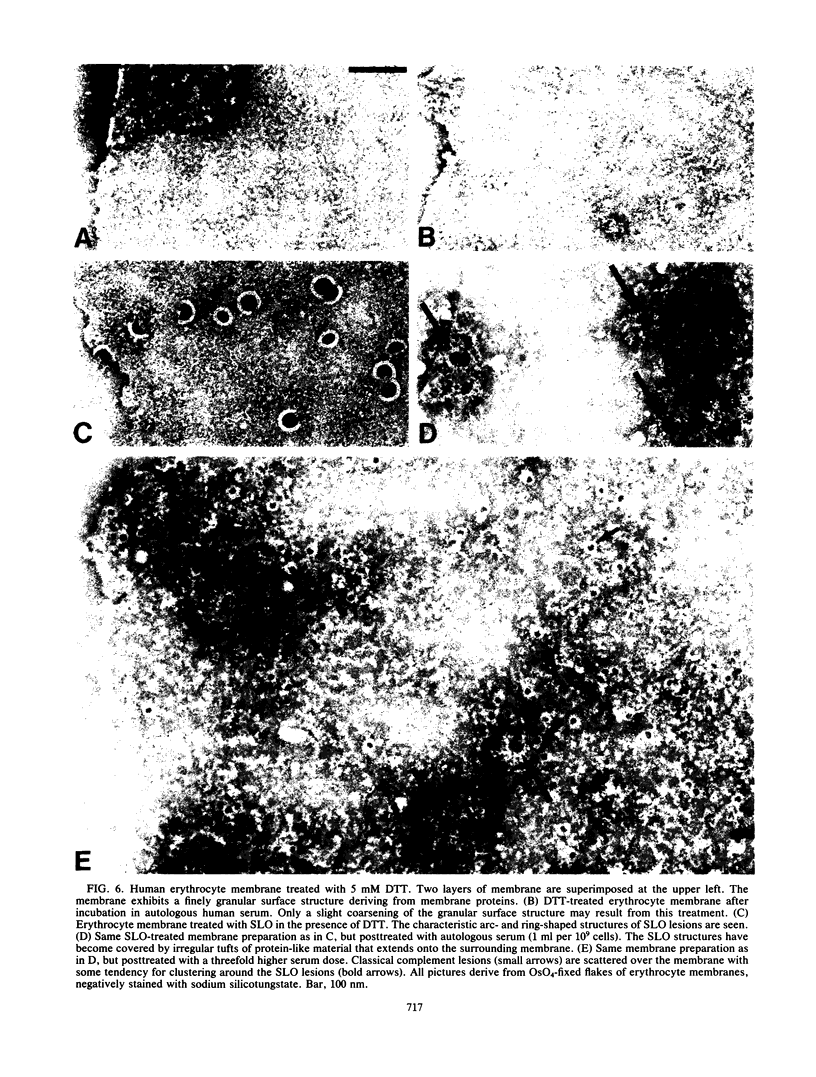
Streptolysin-O damages mammalian membranes through generation of large transmembrane channels formed by membrane-inserted polymers of the toxin (S. Bhakdi et al., Infect. Immun. 47:52-60, 1985). We here report that the native toxin binds naturally occurring human serum immunoglobulin G antibodies to form immune complexes with potent complement-activating capacity. Nanomolar concentrations of toxin added to antibody-containing serum cause rapid consumption of C4 and C5 hemolytic activity and 30 to 90% C3 conversion within 10 to 60 min at 37 degrees C. After binding to target membranes, streptolysin-O polymers serve as foci for antibody-dependent complement activation, which proceeds to completion with the formation of terminal C5b-9 complexes on the autologous cells. The binding and insertion of a primarily water-soluble bacterial product into a host cell membrane has thus been shown to generate a stable and hyperactive focus for activation of and self-attack by the complement system. We suggest that this process perpetuates local tissue damage, deviates host complement action away from the invading bacteria, and may possibly play a role in the pathogenesis of poststreptococcal disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alouf J. E. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxin). Pharmacol Ther. 1980;11(3):661–717. doi: 10.1016/0163-7258(80)90045-5. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M. Decomplementation antigen, a possible determinant of staphylococcal pathogenicity. Infect Immun. 1985 Jan;47(1):41–46. doi: 10.1128/iai.47.1.41-46.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Roth M., Sziegoleit A., Tranum-Jensen J. Isolation and identification of two hemolytic forms of streptolysin-O. Infect Immun. 1984 Nov;46(2):394–400. doi: 10.1128/iai.46.2.394-400.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Mechanism of complement cytolysis and the concept of channel-forming proteins. Philos Trans R Soc Lond B Biol Sci. 1984 Sep 6;306(1129):311–324. doi: 10.1098/rstb.1984.0092. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Membrane damage by complement. Biochim Biophys Acta. 1983 Aug 11;737(3-4):343–372. doi: 10.1016/0304-4157(83)90006-0. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J., Sziegoleit A. Mechanism of membrane damage by streptolysin-O. Infect Immun. 1985 Jan;47(1):52–60. doi: 10.1128/iai.47.1.52-60.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Terminal membrane C5b-9 complex of human complement: transition from an amphiphilic to a hydrophilic state through binding of the S protein from serum. J Cell Biol. 1982 Sep;94(3):755–759. doi: 10.1083/jcb.94.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. L. Characteristics of streptolysin O hemolysis: kinetics of hemoglobin and 86rubidium release. Infect Immun. 1974 Jun;9(6):1022–1027. doi: 10.1128/iai.9.6.1022-1027.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. L., Schlegel R. Effect of streptolysin O on erythrocyte membranes, liposomes, and lipid dispersions. A protein-cholesterol interaction. J Cell Biol. 1975 Oct;67(1):160–174. doi: 10.1083/jcb.67.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5867–5871. doi: 10.1073/pnas.76.11.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J., van de Rijn I., Ohkuni H., Fischetti V. A., Zabriskie J. B. Immunological studies of post-streptococcal sequelae. Evidence for presence of streptococcal antigens in circulating immune complexes. J Clin Invest. 1984 Sep;74(3):1027–1034. doi: 10.1172/JCI111470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugli T. E., Müller-Eberhard H. J. Anaphylatoxins: C3a and C5a. Adv Immunol. 1978;26:1–53. doi: 10.1016/s0065-2776(08)60228-x. [DOI] [PubMed] [Google Scholar]
- Kazatchkine M. D., Nydegger U. E. The human alternative complement pathway: biology and immunopathology of activation and regulation. Prog Allergy. 1982;30:193–234. [PubMed] [Google Scholar]
- Lange K., Seligson G., Cronin W. Evidence for the in situ origin of poststreptococcal glomerulonephritis: glomerular localization of endostreptosin and the clinical significance of the subsequent antibody response. Clin Nephrol. 1983 Jan;19(1):3–10. [PubMed] [Google Scholar]
- Tranum-Jensen J., Bhakdi S. Freeze-fracture analysis of the membrane lesion of human complement. J Cell Biol. 1983 Sep;97(3):618–626. doi: 10.1083/jcb.97.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal H., Jr, Fischetti V. A., van de Rijn I., Zabriskie J. B. The occurrence of a protein in the extracellular products of streptococci isolated from patients with acute glomerulonephritis. J Exp Med. 1979 Feb 1;149(2):459–472. doi: 10.1084/jem.149.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B. The role of streptococci in human glomerulonephritis. J Exp Med. 1971 Sep 1;134(3 Pt 2):180s–192s. [PubMed] [Google Scholar]