Abstract
Adenoviruses (Ads) are icosahedral, nonenveloped viruses with a double-stranded DNA genome. The 51 known Ad serotypes exhibit profound variations in cell tropism and disease types. The number of observed Ad infections is steadily increasing, sometimes leading to fatal outcomes even in healthy individuals. Species B Ads can cause kidney infections, hemorrhagic cystitis, and severe respiratory infections, and most of them use the membrane cofactor protein CD46 as a cellular receptor. The crystal structure of the human Ad type 11 (Ad11) knob complexed with CD46 is known; however, the determinants of CD46 binding in related species B Ads remain unclear. We report here a structural and functional analysis of the Ad11 knob, as well as the Ad7 and Ad14 knobs, which are closely related in sequence to the Ad11 knob but have altered CD46-binding properties. The comparison of the structures of the three knobs, which we determined at very high resolution, provides a platform for understanding these differences and allows us to propose a mechanism for productive high-affinity engagement of CD46. At the center of this mechanism is an Ad knob arginine that needs to switch its orientation in order to engage CD46 with high affinity. Quantum chemical calculations showed that the CD46-binding affinity of Ad11 is significantly higher than that of Ad7. Thus, while Ad7 and Ad14 also bind CD46, the affinity and kinetics of these interactions suggest that these Ads are unlikely to use CD46 productively. The proposed mechanism is likely to determine the receptor usage of all CD46-binding Ads.
The nonenveloped adenoviruses (Ads) typically cause respiratory infections, with symptoms ranging from the common cold to pneumonia, but they can also infect the eye, urinary tract, and intestine. Individuals with compromised immune systems are especially prone to severe and life-threatening infections caused by Ads (24). The virion measures about 800 Å in diameter and carries a double-stranded DNA genome. Its icosahedral capsid is primarily formed by 240 copies of the hexon and 12 copies of the penton proteins (3). A trimeric, elongated protein called “fiber” protrudes from each of the 12 vertices of the capsid and mediates the initial attachment to target cells by interacting with cell surface receptors. The fiber consists of a globular head, a fibrous shaft, and a tail. The knob mediates interactions with receptors, whereas the tail anchors the fiber to the penton base. The engagement of receptors is followed by viral internalization via clathrin-coated endocytosis (54).
A group of highly pathogenic species B Ads use the membrane cofactor protein CD46 as their cellular receptor (18, 20, 25, 44, 50). The heavily glycosylated extracellular portion of CD46 is composed of four short consensus repeat (SCR) modules and a 25-amino-acid sequence rich in serine, threonine, and proline residues (the STP region). CD46 inhibits complement activation by binding separately to C3b or C4b and stabilizes them for proteolytic cleavage by factor I, thus preventing continued complement activation. In addition to Ads, measles virus, human herpesvirus 6, group A Streptococcus pyogenes, Neisseria gonorrhoeae, and Neisseria meningitidis all use CD46 as a specific cellular receptor (10).
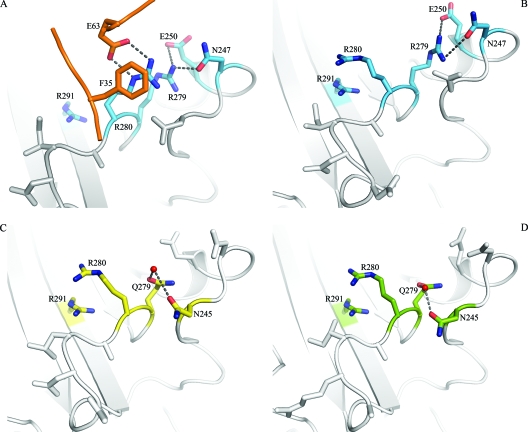
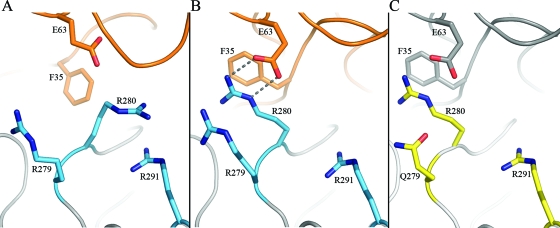
Crystallization of the Ad type 11 (Ad11) knob in complex with the N-terminal two SCRs of CD46 (SCR1 and SCR2) established the structural basis for the interaction of species B Ads with CD46 (37). Each knob binds three copies of CD46 with high affinity via large, contiguous surfaces that span almost the entire length of each SCR1-SCR2 fragment. Surprisingly, binding to the Ad11 knob alters the conformation of CD46 by realigning the previously V-shaped two repeats into a linear, rod-like structure. Central to this rearrangement is the Arg280 side chain of Ad11, which is wedged into the SCR1-SCR2 interface of CD46, forming a cation-π orbital contact with CD46 Phe35, as well as a salt bridge with CD46 Glu63. These interactions alter the conformation of Phe35 and Glu63 at the SCR1-SCR2 interface, making the realignment of the two CD46 repeats possible. Remarkably, the CD46 binding Ad11 Arg280 lies parallel to another arginine, Arg279, with the two guanidinium groups stacked against each other (Fig. 1A).
FIG. 1.
Structures of Ad11, Ad7, and Ad14 knobs and comparison with the liganded Ad11 knob. (A) Close-up view of the binding interface between the Ad11 knob (blue) and CD46 (orange), centered at the HI and DG loops of the knob. (B to D) Close-up views of the same regions in the unliganded Ad11 (blue) (B), Ad7 (yellow) (C), and Ad14 (green) (D) knobs. Oxygens and nitrogens are red and blue, respectively. Water molecules are represented by red spheres.
There is some controversy about which species B Ads can engage CD46 productively and establish an infection. It has been clearly established that Ad11, as well as Ad35, causes infections by engaging CD46 with high affinity (36, 37, 51). Moreover, transfection with human CD46 cDNA rendered Chinese hamster ovary cells more permissive to infection by all species B Ads, with the exception of Ad3 and Ad7 (25, 50), indicating that most species B Ads use CD46 as a receptor. However, Ad3 and Ad7 were also reported to bind and infect CD46-expressing cells in two recent studies (17, 18). Finally, a second receptor (receptor X; species B Ad receptor [sBAR]) is thought to interact with some species B Ads (43, 50).
In order to define the mechanism of CD46 binding by Ads, we performed a structural and functional analysis of three highly similar Ads (25): (i) Ad11, which is known to use CD46 as a high-affinity receptor and for which a crystal structure of the knob in complex with CD46 has been determined; (ii) Ad14, which can bind and infect CD46-expressing cells (25); and (iii) Ad7, which has been reported to bind and infect CD46-expressing cells in one study (17). However, others showed that Ad14 (50) and Ad7 (25, 50) do not use CD46 for attachment but rather use the unknown receptor X (50), which shows characteristics similar to those of the species B Ad receptor (43). The knob proteins of these three Ads are exceptionally similar in sequence and therefore ideally suited for a comparison of structural and functional features. The crystal structures of all three knobs were determined at high resolution. In addition, the interaction with CD46 was in each case characterized with steady-state and solution affinity measurements using surface plasmon resonance (SPR). In the case of Ad11, kinetic measurements were also carried out. The affinity data were then complemented with cell binding assays using whole virions. We also performed quantum chemical calculations in order to explore energetically favorable contacts between Ad knobs and CD46 and analyzed two Ad knob mutants with altered binding affinities. Our results have relevance for the design of Ad knobs with altered binding specificities, as well as for an improved understanding of the parameters that guide the formation of protein-protein contact surfaces.
MATERIALS AND METHODS
Expression and purification of Ad knobs.
Amino acids 118 to 325 of the fiber of the human Ad11 strain Slobitsky were cloned into the pET15b vector (Novagen) for expression of the knob domain. The expression construct contained an N-terminal thrombin-cleavable hexahistidine tag. Expression in Escherichia coli Rosetta2 DE3 was induced by adding 0.1 mM isopropyl-β-d-thiogalactopyranoside at an optical density at 600 nm of 0.6, followed by growth at 20°C for 16 h. Cells were harvested using 50 mM NaH2PO4 (pH 8.0), 300 mM NaCl, 20 mM imidazole and were lysed using a homogenizer (with 1 mM protease inhibitor cocktail). The clarified supernatant was applied to a His-Trap HP column (GE Healthcare) for affinity purification, and the Ad11 knob was eluted using a gradient from 50 to 500 mM imidazole. Thrombin (Sigma) cleavage was performed with 1 U thrombin/mg of recombinant protein at 20°C for 16 h. After concentration, the cleaved protein was further purified by gel filtration using a Superdex 200 (GE Healthcare) and buffer containing 20 mM HEPES at pH 7.4 and 150 mM NaCl. Small amounts of uncleaved protein were removed with a second His-Trap column. The same protocol was used to overexpress knobs from Ad7 (strain Gomen; amino acids 118 to 325) and Ad14 (strain De Wit; amino acids 123 to 325), as well as two mutants. The mutations were introduced using overlap extension PCR with a second set of primers containing a mismatch, introducing the mutation in two steps.
Expression and purification of CD46 SCR1-SCR2 and SCR1-SCR4.
The expression and purification of CD46 SCR1-SCR2 (referred to here as D2) was performed as described previously (9). Production of CD46 SCR1-SCR4 (referred to as D4) followed a similar strategy. cDNA encoding amino acids 35 to 288 of human CD46 was cloned into the pBJ5-GS expression vector, which contains glutamine synthetase for selection. The vector was transfected into CHO-Lec 3.2.8.1 cells (48). Stable transfectants were selected by increasing amounts of methionine sulfoxime. The cells were grown in glutamine synthetase-supplemented Ex-Cell 302 CHO serum-free medium (SAFC Biosciences), 5% fetal calf serum (Gibco), and penicillin/streptavidin. D4 was purified by first applying 1 liter cell culture supernatant to concanavalin A-Sepharose (GE Healthcare). Beads were washed with 20 mM HEPES at pH 7.4, 500 mM NaCl, 1 mM CaCl2, 1 mM MnCl2, 1 mM MgCl2, and bound protein was eluted with 12% (wt/vol) methyl α-d-mannopyranoside (Sigma) in wash buffer. After the volume of the eluate was reduced to 2 ml using a Centricon Plus-70 device (Millipore), the protein was further purified using gel filtration on a Superdex 200 column (GE Healthcare), followed by ion-exchange chromatography on a MonoQ 5/50 column (GE Healthcare).
35S labeling of virions and binding studies.
Human respiratory epithelial A549 cells were grown in Dulbecco's modified Eagle medium (Sigma Chemical Co., St. Louis, MO) containing 10% fetal calf serum, HEPES, and penicillin/streptomycin (all from Sigma). 35S-labeled Ad11 (strain Slobitski) virions were propagated in A549 cells and purified as described elsewhere (22). In order to quantify binding, 2 × 105 A549 cells were preincubated with increasing amounts of soluble recombinant knobs on ice in binding buffer (Dulbecco's modified Eagle medium, 2% bovine serum albumin, 0.02% sodium azide). After 1 hour, 2 × 109 35S-labeled virions (104 virions/cell) were added to a final volume of 100 μl, and the mixtures were incubated on ice for another hour. Nonbound virions were removed by washing, and the cell-associated radioactivity was measured using a Wallac 1406 liquid scintillation counter (Perkin-Elmer).
Crystallization and structure determination.
Crystallization of the Ad7, Ad11, and Ad14 knobs was performed at 20°C using the hanging-drop method and concentrations of about 7 to 10 mg/ml protein. In all cases, well-diffracting crystals were obtained after 4 to 15 days. The crystallization solutions were as follows: for Ad11 knobs, 18% (wt/vol) polyethylene glycol (PEG) 6000, 0.1 M HEPES, pH 7.0; for Ad7 knobs, 23% (wt/vol) PEG 3350, 0.1 M HEPES, pH 7.6; for Ad14 knobs, 20% (wt/vol) PEG 8000, 0.1 M cyclohexyl-2-aminoethanesulfonic acid (CHES), pH 9.0, 200 mM NaCl. In all cases, crystals were grown by mixing 1 μl crystallization solution and 1 μl protein and letting the mixture equilibrate against a larger reservoir of crystallization solution in a sealed compartment. All crystals were flash frozen in liquid nitrogen, and X-ray data were collected at the Swiss Light Source (Villigen Switzerland) beamline X06SA using either a MarCCD (Ad7 and Ad11 knobs) or a Pilatus 6 M detector (Ad14 knob). Data for the Ad11 and Ad7 knobs were integrated and scaled using HKL/DENZO (35), whereas the Ad14 knob data were processed with XDS (23). Initial phases for Ad11 were obtained by molecular replacement using AMoRe (29) and the trimeric Ad3 knob structure (Protein Data Bank [PDB] code 1H7Z) as a search model. The additional knob structures were then solved with PHASER (38) using the Ad11 knob structure as a search model. Initial rigid-body refinement of the solutions was performed with CNS (6, 7), and subsequent refinement was carried out in REFMAC5 (28). Data statistics are given in Table 1. Structural figures were prepared with PyMOL (14).
TABLE 1.
Data collection and refinement statistics
| Statistic | Value
|
||
|---|---|---|---|
| Ad11 knob | Ad7 knob | Ad14 knob | |
| Data collection | |||
| Space group | I23 | P21212 | P3212 |
| No. of monomers in asymmetric unit | 1 | 3 | 9 |
| Unit cell dimensions | |||
| a, b, c (Å) | 100.6, 100.6, 100.6 | 83.31, 88.05, 79.17 | 106.47, 106.47, 311.71 |
| α, β, γ (°) | 90.00, 90.00, 90.00 | 90.00, 90.00, 90.00 | 90.00, 90.00, 120.00 |
| Resolution (Å) | 71.1-1.45 (1.45-1.50)a | 79-1.75 (1.75-1.81)a | 47.4-1.8 (1.80-1.90)a |
| Rmergeb | 4.5 (38.8) | 5.0 (36.4) | 7.8(27.0) |
| I/σI | 13.2 (1.56) | 10.17 (1.34) | 11.91 (4.83) |
| Completeness (%) | 99.9 (100.0) | 99.1 (95.1) | 99.1 (94.4) |
| Redundancy | 6.0 (5.4) | 5.1 (4.6) | |
| Refinement | |||
| Resolution (Å) | 71.1-1.45 | 79.06-1.75 | 47.4-1.8 |
| No. of reflections | 30,079 | 59,433 | 185,428 |
| Rwork/Rfreec | 19.2/22.4 | 19.1/22.9 | 18.8/21.5 |
| No. of atoms | 1,764 | 5,128 | 15,866 |
| Protein | 1,579 | 4,636 | 14,044 |
| Water | 185 | 492 | 1,803 |
| B factors (Å2) | |||
| Protein | 13.66 | 21.79 | 18.74 |
| Waters | 24.91 | 36.57 | 24.4 |
| R.m.s. deviations | |||
| Bond lengths (Å) | 0.005 | 0.008 | 0.004 |
| Bond angles (°) | 1.15 | 0.945 | 0.926 |
The highest-resolution shell is shown in parentheses.
Rmerge = ΣhklI − <I>/ΣhklI , where I is the intensity of a reflection hkl, and <I> is the average over symmetry-related observations of hkl.
Rwork = Rfree = ΣhklFobs - Fcalc/ΣhklFobs, where Fobs and Fcalc are observed and calculated structure factors, respectively. The free set contains 5 or 10% of reflections.
Affinity measurements using SPR.
All kinetic, steady-state, and solution competition interaction experiments between different Ad knobs and their monomeric binding partner CD46 (either D2 or D4) were performed at 25°C using a Biacore 2000 instrument (Biacore, Uppsala, Sweden). The data collection rate was 1 Hz if not otherwise stated. The Ad knob can bind up to three copies of CD46. To avoid influencing the binding parameters through avidity effects, all Ad knobs were immobilized to the biosensor surface. CM5 and nitrilotriacetic acid (NTA) sensor chips, an amine-coupling kit, and surfactant P20 were purchased from GE Healthcare. HBS-EP (10 mM HEPES, 150 mM NaCl, 3 mM EDTA, 0.005% [vol/vol] surfactant P20, pH 7.4, at 25°C) was used as a running buffer for the CM5 chips, while HBS-P (10 mM HEPES, 150 mM NaCl, 0.005% [vol/vol] surfactant P20, pH 7.4, at 25°C) containing 50 μΜ EDTA was used for the NTA chips. Protein concentrations were calculated by using absorbance values at 280 nm and theoretical extinction coefficients.
Kinetic experiments.
Kinetic experiments were performed with an Ni-NTA sensor chip. The surfaces of two consecutive flow cells were coated with nickel, followed by immobilization of Ad knobs (Ad11-His6) to the surface of the downstream experimental flow cell via their hexahistidine tags. The Ni-coated surface of the upstream flow cell was used as a reference. The analytes (D2 and D4) were serially diluted in running buffer and injected in series over the reference and experimental biosensor surfaces at a flow rate of 50 μl/min. A sample containing pure running buffer was injected as well under the same conditions. Sensorgrams of each sample were double referenced by subtraction of sensorgrams obtained from the reference surface of each cycle to remove bulk effects, followed by further subtraction of the referenced sensorgram obtained from the running buffer sample injection to remove drift and system noise. The association (on-rate) and dissociation (off-rate) constants (ka and kd) were determined simultaneously by globally fitting double-referenced sensorgrams of the entire titration series to a “1:1 binding with mass transfer” model with BIAevaluation software 4.1 (Biacore). The dissociation constant, KD, was calculated by the following relation: KD = kd/ka. If applicable, kinetic sensorgram series were also analyzed by performing a steady-state analysis to cross-validate the obtained KD values calculated from rate constants. In all kinetic and steady-state experiments, surface regeneration was not necessary, since all interactions showed either very rapid or moderate dissociation rates. However, the NTA chip was stripped of nickel-ligand complex after each interaction cycle using HBS-P containing 350 mM EDTA, followed by nickel coating and ligand immobilization at the beginning of a new cycle.
In cases where micromolar concentrations of CD46 had to be used, NTA chips were replaced by CM5 chips, since a strong nonspecific binding of both D2 and D4 to the Ni-coated biosensor surface in the absence of knobs was observed. The knobs were covalently immobilized to the CM5 biosensor surface of the downstream flow cell by amine-coupling chemistry following the manufacturer's instructions, while the upstream surface was subjected to the coupling reaction in the absence of knobs. The on- and off-rate constants were determined as described for Ni-NTA chips.
To determine the rate constants of interactions that showed high on and off rates, the flow rate and data collection rate were altered. The analyte was serially diluted in running buffer and injected in series over the control and experimental flow cells at a flow rate of 75 μl/min and a data collection rate of 5 Hz. The sensorgrams were double referenced, the rate constants were determined, and the KD was calculated as described above, and the sensorgram series were also analyzed by performing a steady-state analysis to cross-validate the obtained KD values calculated from the rate constants. Note that for all kinetic experiments, low surface immobilization levels were used that, combined with high flow rates and the model used, were supposed to minimize mass transfer effects.
Equilibrium analysis experiments (steady-state experiments).
The experimental and reference surfaces were prepared as described above for CM5 sensor chips. For interactions that exhibit extremely high on and off rates, clearly visible as “square-wave” profiles of individual sensorgrams, both the on- and off-rate constants can be outside the measurable range for reliable quantitative evaluation (Biacore 2000 system specifications). If an interaction was not amenable to kinetic experiments, a steady-state experiment approach was used instead for the KD value determination. Analytes were serially diluted in running buffer and injected in series over the experimental and reference surfaces at a flow rate of 5 μl/min. Sensorgrams were double-referenced as described above. The equilibrium binding levels were determined by averaging the response at equilibrium and plotted against the injected CD46 concentration. The KD values were determined by nonlinear curve fitting of a “1:1 Langmuir isotherm” model with BIAevaluation software 4.1 (Biacore).
Solution competition SPR studies (1, 2, 30).
All competition assays to determine the affinity in solution were performed with a CM5 sensor chip and a flow rate of 5 μl/min. Experimental and reference surfaces were prepared as described above for CM5 sensor chips. A high-density Ad35 trimer biosensor surface (2,000 resonance units [RU]) was prepared to measure the concentration of unliganded CD46 in solution. The biosensor surface was calibrated by determination of the response level 30 s after the end of injection (“Response30”) with a series of known CD46 concentrations. A plot of “Response30” against the concentration of CD46 was fitted using a four-parameter logistic function provided by BIAevaluation 4.1 (Biacore). The biosensor surface was regenerated after each cycle with 1.5 M MgCl2 (Sigma) for 1 minute. Soluble Ad knobs were serially diluted in HBS-EP buffer and mixed with a constant concentration of CD46 (13.6 nM). The mixture was allowed to reach chemical equilibrium at 25°C, and the equilibrated samples were then injected over the biosensor surface and analyzed individually for the remaining unliganded CD46 concentration by determination of the “Response30” values and use of the standard curve. The biosensor surface was regenerated after each cycle with 1.5 M MgCl2 (Sigma) for 1 minute. Injection of equilibrated samples containing micromolar concentrations of fiber knobs exhibited negative response values during association, which were caused by a refractive index artifact that is related to different immobilization levels of the reference and experimental flow cells. This artifact, however, does not affect the dissociation phase and “Response30” values, respectively. A plot of the measured unliganded CD46 concentrations versus the total concentration of added soluble Ad knob protomers (equal to the Ad knob binding site concentration) was fitted to the “solution affinity” model provided by BIAevaluation software 4.1 (Biacore) to obtain the KD of the interaction between one CD46 and one soluble Ad knob protomer in solution.
Computational details.
To generate protein structures suitable for the calculations, hydrogen atoms were added to the coordinates of the Ad11 knob-D2 complex (37) using the REFMAC5 tool (28) of the CCP4 package (11) and optimized with a static variant of the Merck molecular force field (MMFF) while all non-hydrogen atom coordinates were taken from the X-ray data without further computational optimization. One monomeric Ad11-CD46 complex was chosen for all calculations. In order to generate a putative Ad7 knob-D2 complex, residues of Ad11 that were not conserved in the Ad7 knob were replaced with the respective Ad7 counterparts.
Since the reliable description of solvent effects is still a major challenge in quantum chemistry, all calculations were performed in vacuo. Although the missing solvent will certainly affect the total binding energies, we expect that relative energies and, in particular, those following the interchange of various amino acids are much less influenced (due to a systematic cancellation of influences).
While recently developed linear-scaling quantum chemical methods (reference 32 and references therein) allow the calculation of systems with 1,000 and more atoms, the calculation of about 5,000 atoms for the total Ad11-CD46 system is extremely demanding. For this reason, we studied smaller subsystems of the two complexes with CD46. When choosing subsystems of the structure, the N termini were saturated as formamide (—NH—CHO), while the C termini were saturated as amide (—CO—NH2). The saturation of the subsystems was carried out using the program package Maestro 7.5 (41). Whenever possible, the positions of the atoms of saturating groups were taken from the neighboring units within the respective superstructure so that only the positions of newly attached hydrogen atoms had to be reoptimized.
Quantum chemical calculations were performed at the Hartree-Fock level (49) and improved by second-order many-body perturbation theory (MP2) (49) using the resolution of identity (RI) approximation (15, 16). The RI-MP2 method allows accounting for the often highly important dispersion-type effects, which are neglected in the simpler Hartree-Fock or common density-functional-theory approaches. The RI-MP2 results are the most accurate in the present study, while the force field data deviate up to 55 kJ/mol from these results for the present systems. The basis set SVP (40) was used in the RI-MP2 and Hartree-Fock calculations, and only pure spherical harmonic Gaussians were employed. Interaction energies were corrected for basis set superposition errors using the counterpoise scheme (4). All ab initio calculations were performed using a developmental version of the program package Q-Chem (Q-Chem, Inc., Pittsburgh, PA) employing the CFMM (46, 52) and LinK (33, 34) methods. For MMFF force field calculations, the Macromodel program (27) was used.
Protein structure accession numbers.
The structures have been deposited with the Protein Data Bank (http://www.rcsb.org) with accession numbers 3EXW (Ad7 knob), 3EXV (Ad11 knob), and 3F0Y (Ad14 knob).
RESULTS
Structure of unliganded Ad11 knob and comparison with the complex.
The structural analysis of the Ad11 knob-D2 complex revealed that CD46 undergoes a profound conformational change upon engaging the Ad11 knob. In order to determine whether structural changes also occur in the Ad11 knob as it binds CD46, we first determined the structure of the unliganded Ad11 knob at 1.45-Å resolution (Table 1). The overall structures of the unliganded and liganded Ad11 knobs are very similar, and the trimers can be superimposed with a root mean square (r.m.s.) difference of 0.420 Å (all atoms), including the CD46-binding loops. However, rearrangements of side chains occur in the region that contacts CD46. The most profound rearrangement is seen for the Arg280 side chain in the HI loop. In the complex, Arg280 mediates key contacts with CD46 and lies parallel to Arg279, with the two guanidinium groups stacked against each other (Fig. 1A). This arrangement is stabilized by two interactions involving Arg280: a hydrophobic contact with CD46 Phe35 and a salt bridge with CD46 Glu63. No water molecules participate in these interactions. In the unliganded Ad11 knob, the Arg280 side chain has flipped 170°, pointing away from Arg279. Its guanidinium group is now stacked against that of the Arg291 side chain (Fig. 1B). Both the Arg280 and Arg291 side chains are solvent exposed and surrounded by water molecules. Thus, the Arg280 guanidinium group is stacked against an arginine side chain, albeit a different one, in both structures. The distances between the central carbon atoms of the guanidinium groups are similar, with 4.1 Å for the Arg279-Arg280 pair and 3.9 Å for the Arg280-Arg291 pair. These distances suggest that π-π contacts between the guanidinium groups occur in both pairs.
In addition to Arg280, residue Asp284 in the Ad11 HI loop also undergoes a rotation as it engages His43 in CD46. The remaining CD46-contacting residues of the Ad11 knob occupy essentially the same conformations before and after binding.
Structures of the Ad7 and Ad14 fiber knobs.
Ad11 uses CD46 as a high-affinity receptor (36, 37, 51). It is unclear whether the closely related Ad7 and Ad14 also do so (17, 18, 20, 21, 25, 44, 50). The knobs of all three Ads are highly similar in sequence, indicating that the differences in binding are due to specific amino acid exchanges. In order to provide a structural basis for the observed differences in CD46 binding, we determined the high-resolution crystal structures of the Ad7 and Ad14 knobs with excellent geometry (Table 1). The two structures can be superimposed onto that of the Ad11 knob with an r.m.s. deviation of about 0.3 to 0.4 Å using all Cα atoms (Ad7 protomer, 0.218 Å, and trimer, 0.296 Å; Ad14 protomer, 0.445 Å, and trimer, 0.387 Å), indicating a high degree of structural similarity. The HI, DG, and IJ loops, which engage CD46 in the Ad11-CD46 complex, have very similar conformations in all three unliganded knobs. This is somewhat surprising, as the shape complementarity between the Ad11 and CD46 surfaces appeared to be a hallmark of the complex (37). The mutation of Arg279 in the Ad11 HI loop to Gln was shown earlier to abolish binding to CD46-expressing cells (21). Both Ad7 and Ad14 carry a glutamine at position 279. Surprisingly, this substitution does not change the CD46-binding surface and does not alter the conformations of the HI and DG loops. In Ad11, Arg279 forms direct hydrogen bonds to Asn247 and Glu250 in the DG loop (Fig. 1B). In Ad7 and Ad14, the Gln279 side chain also contacts the DG loop, but in this case Asn245, albeit via a water molecule (Fig. 1C and D). Thus, in all three cases, polar interactions between the DG and HI loop exist, holding them in essentially the same conformation. Most importantly, the Arg280 side chain would also be able to rotate next to Gln279 and engage CD46 in Ad7 or Ad14.
The differences in binding to CD46 must therefore be due to altered contacts between CD46 and Ad knob side chains and not to larger structural differences. As was seen in the unliganded Ad11 knob (Fig. 1B), the Arg280 side chain stacks against Arg291 in both Ad7 and Ad14 (Fig. 1C and D). This unusual interaction places two positive charges next to each other and must be energetically favorable for three reasons: (i) it is seen in all three unliganded knobs, which were crystallized under different conditions; (ii) it is seen despite the fact that Arg280 could adopt several other rotamer conformations; and (iii) it is not caused by crystal contacts, as there are none in the vicinity of Arg280. However, both arginine side chains are fully solvent exposed in all three cases. We therefore think it likely that solvent molecules and ions present in the buffer solutions contribute to the stability of the interaction by absorbing some of the charges and that the two guanidinium groups form stabilizing π-π interactions.
The Ad7, Ad11, and Ad14 knobs all bind to CD46, but with differences in complex stability.
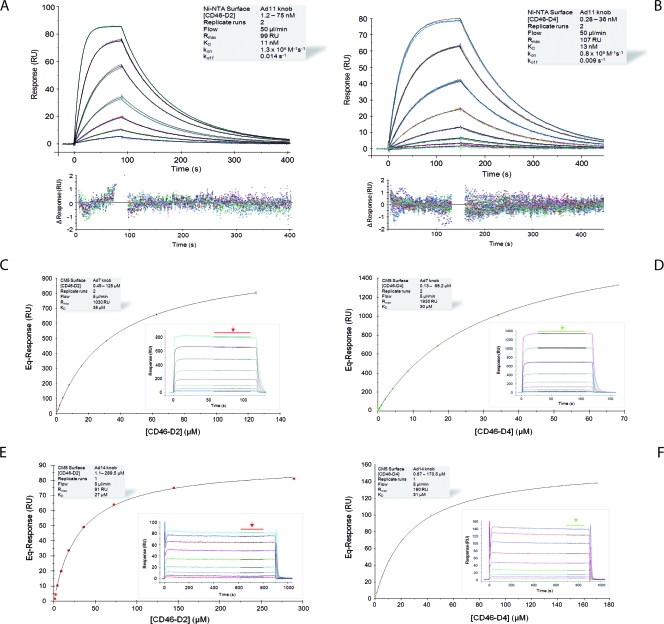
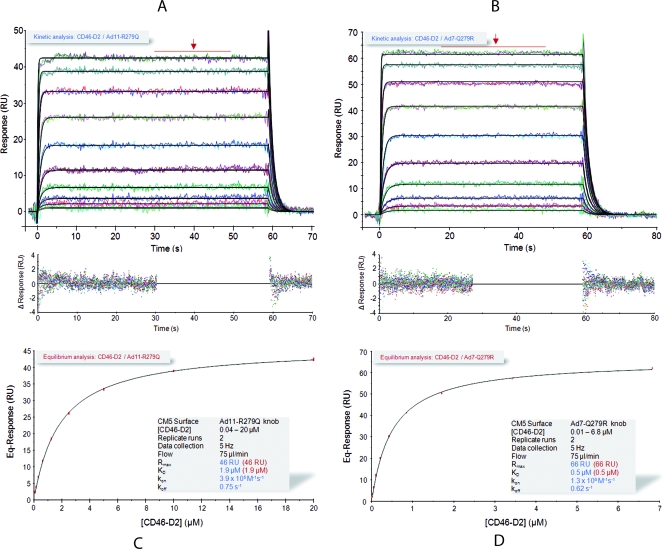
We next used steady-state and kinetic SPR analysis to determine the binding characteristics of all three knobs to CD46. Both the D2 and D4 versions of CD46 were used. Kinetic analysis of the Ad11-D2 and Ad11-D4 (Fig. 2A and B) interactions revealed KD values of 11 and 13 nM, respectively. These values are similar to the published KD value of 15.5 nM for the Ad35-D2 interaction (51), indicating that Ad11 and Ad35 bind CD46 with similarly high affinities. In addition, the highly similar on-rate (ka values of 1.3 × 106 M−1 s−1 and 0.8 × 106 M−1 s−1, respectively) and off-rate (kd values of 0.014 s−1 and 0.009 s−1, respectively) (Table 2 and Fig. 2A and B) constants of the Ad11-D2 and Ad11-D4 complexes, which match the binding mechanism, provide clear evidence that the third and fourth repeats of CD46 do not contribute to Ad11 binding (Fig. 2A and B). This result is in good agreement with the crystal structure of the Ad11-D2 complex (37).
FIG. 2.
Kinetic and equilibrium SPR analyses of CD46-knob interactions. (A and B) Kinetic analysis of CD46-D2 (A) and -D4 (B) binding to immobilized Ad11 knobs. Double-referenced sensorgrams (shown in color) are overlaid with fits of a “1:1 binding with mass transfer” model (black lines) on top over the corresponding residual values showing the kinetic-fit range and absolute deviation (Δ) of data points from curve fit values. (C to F) Equilibrium analysis of CD46-D2 (C and E) or -D4 (D and F) binding to immobilized Ad7 (C and D) and Ad14 (E and F) knobs. Equilibrium (Eq) response values (colored squares) are plotted against CD46 concentrations and fitted to a “1:1 Langmuir isotherm” model (black line). The insets show overlay plots of double-referenced sensorgrams (shown in color) with overlaid average fits (black lines; indicated by arrows) of equilibrium response values. The shaded boxes contain additional information about setup details and measured parameters.
TABLE 2.
Affinity data determined by SPR
| Protein interaction ligand/analyte | Equilibrium analysis (KD [M]) | Kinetic analysis
|
Solution competition analysis (KD [M]) | ||
|---|---|---|---|---|---|
| KD (M) | ka (M−1 s−1) | kd (s−1) | |||
| Ad35/CD46-D2 | 17 × 10−9 | 17 × 10−9 | 1.5 × 106 | 0.025 | 21 × 10−9 |
| Ad35/CD46-D4 | 16 × 10−9 | 16 × 10−9 | 1.1 × 106 | 0.017 | 27 × 10−9 |
| Ad11/CD46-D2 | ND | 11 × 10−9 | 1.3 × 106 | 0.014 | 23 × 10−9 |
| Ad11/CD46-D4 | ND | 13 × 10−9 | 0.8 × 106 | 0.009 | 19 × 10−9 |
| Ad7/CD46-D2 | 35 × 10−6 | −a | − | − | 58 × 10−6 |
| Ad7/CD46-D4 | 30 × 10−6 | − | − | − | 65 × 10−6 |
| Ad14/CD46-D2 | 27 × 10−6 | − | − | − | 73 × 10−6 |
| Ad14/CD46-D4 | 31 × 10−6 | − | − | − | 63 × 10−6 |
| Ad7-279R/CD46-D2 | 0.5 × 10−6 | 0.5 × 10−6 | 1.3 × 106 | 0.62 | 0.1 × 10−6 |
| Ad11-R279Q/CD46-D2 | 1.9 × 10−6 | 1.9 × 10−6 | 3.9 × 105 | 0.75 | 0.8 × 10−6 |
−, on and off rates were outside the measurable range for reliable quantitative evaluation of rate constants.
We then performed a similar kinetic analysis for the Ad7 and Ad14 knobs (Table 2 and Fig. 2C to F). The sensorgrams obtained exhibited “square-wave-like” shapes (31) that were typical of transient interactions, i.e., interactions with high dissociation rate constants. Because of this, the durations of both the association and dissociation phases were extremely short, which did not produce enough curvature in the sensorgram to allow a reliable kinetic fitting of the data in order to obtain a unique set of rate constants (39). Therefore, we performed a steady-state analysis that allowed the calculation of KD values based on equilibrium response levels instead of on kinetic rate constants obtained from fitting the association and dissociation phases (31, 42). The KD values for the Ad7 knob and Ad14 knob interactions with D2 were 35 μM and 27 μM, respectively (Fig. 2C to F and Table 2). Thus, both Ad7 and Ad14 do bind D2, albeit with a significantly lower affinity than the Ad11 knobs (Fig. 2A and B and Table 2). As was the case for the Ad11 knobs, the KD values for the interaction of the Ad7 and Ad14 knobs with D4 are similar to those for D2 (Fig. 2C to F and Table 2), indicating that only the first two repeats of CD46 mediate binding.
Although no absolute values for rate constants can be obtained in steady-state analysis, dissociation of the different knobs from CD46 can at least be qualitatively ranked in terms of their kd values. An increase in kd values generally reflects a loss of energetically favorable short-range interactions at the binding interface of the two interacting molecules, which makes the kd value a direct measure of the stability/lifetime of the complex (12, 31, 45, 55). Compared to the Ad11 knob, the Ad7 and Ad14 knobs dissociate significantly faster and must therefore have considerably higher kd values (Fig. 2). Thus, the Ad7 and Ad14 interactions with CD46 are probably based on fewer stabilizing interactions than those seen in the Ad11 complex.
Ad11, Ad14, and Ad7 knobs bind the same epitope on CD46.
The weaker affinity and faster dissociation of the Ad7-CD46 and Ad14-CD46 complexes compared to Ad11 could result from (i) different binding areas on the knobs for CD46, (ii) conformational changes of loops that were shown to be important for CD46 binding in the Ad11-CD46 complex, and (iii) different binding areas on D2 for the individual knobs. To determine whether the three knobs bind the same epitope of D2, we performed SPR solution competition analysis (Table 2 and Fig. 3). The crystal structure of the Ad11 knob in complex with D2 showed that each trimeric knob binds three D2 molecules and that there are no contacts between the D2 subunits. Accordingly, all models used for fitting the SPR data are based on the assumption of a simple 1:1 interaction between knob protomers and CD46.
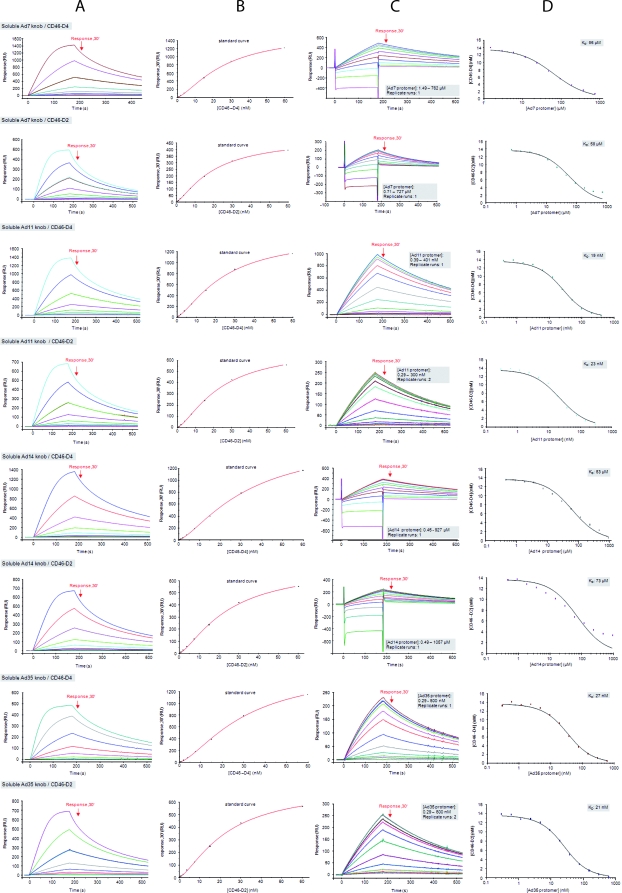
FIG. 3.
Solution competition SPR analysis with soluble Ad11, Ad7, and Ad14 knobs. Shown are solution competition analyses of four soluble Ad knobs competing with surface-immobilized Ad35 knob for binding to CD46. Starting on the left, the columns (A to D) show the four successive steps of a competition experiment. (A) Double-referenced sensorgrams (shown in color) generated with 10 different concentrations of CD46 (0.12 to 60 nM) binding to an Ad35 knob biosensor surface with “Response30” values indicated (red arrows). (B) “Response30” values (black squares) plotted against the CD46 concentration and fitted using a four-parameter logistic function (red line) to create a standard curve. (C) Double-referenced sensorgrams generated from equilibrium mixtures of 10 different concentrations of soluble Ad knob with 13.6 nM CD46. “Response30” values are indicated (red arrows). The gray shaded boxes show setup details. (D) Plots of unbound CD46 against the total concentration of soluble-knob protomers (colored squares) fitted to a “solution affinity” model. The gray shaded boxes show the determined solution KD values of the CD46-soluble-knob interaction.
In addition to Ad11, Ad7, and Ad14, we also included Ad35 knobs in this analysis as a positive control. The solution affinities of the Ad11 and Ad35 knobs for CD46 are comparable (Table 2) to those of our own kinetic SPR analysis, which also revealed that Ad35 (Table 2) behaves mechanistically similarly to Ad11 knobs, judged by the nearly identical rate constants for either D2 or D4 (Table 2). The experimental setup was based on immobilized Ad35 knobs and soluble Ad11, Ad14, Ad7, or Ad35 (positive control) knobs, all of which can compete with immobilized Ad35 knobs for D2 or D4 binding. The concentrations of D2 or D4 were kept constant, whereas the total concentration of soluble knob was increased stepwise. This leads to lower concentrations of unbound D2 or D4 when binding has reached equilibrium. The concentration of unbound D2 or D4 at equilibrium is then calculated by using a standard curve obtained from sensorgram series of different concentrations of D2 or D4 binding to the Ad35 biosensor surface. Plotting the unbound D2 or D4 concentration at chemical equilibrium against the total concentration of soluble-knob binding sites results in a sigmoid-shaped curve if both immobilized and soluble knobs recognize overlapping epitopes on D2 or D4. As shown in Fig. 3, the analysis produces a sigmoidal curve for soluble Ad7, Ad14, Ad35, and Ad11 knobs. By fitting these curves to a “solution affinity” model, solution KD values of D2 or D4 binding to knobs in solution could be obtained. These values were in all cases very similar to the KD values obtained with surface-based kinetic or steady-state SPR studies (Table 2). Thus, the competition analysis showed that all three soluble knobs (and Ad35) compete with immobilized Ad35 for binding to CD46 and must therefore bind the same epitope on D2 or D4 (Fig. 4). Furthermore, given their high degree of structural conservation, it is highly unlikely that the three knobs have different binding sites for CD46 or that binding involves the rearrangement of surface loops. The significantly lower affinity of the Ad7 and Ad14 knobs for D2 or D4 must therefore be due to differences in sequence in the D2-binding surface of the knob.
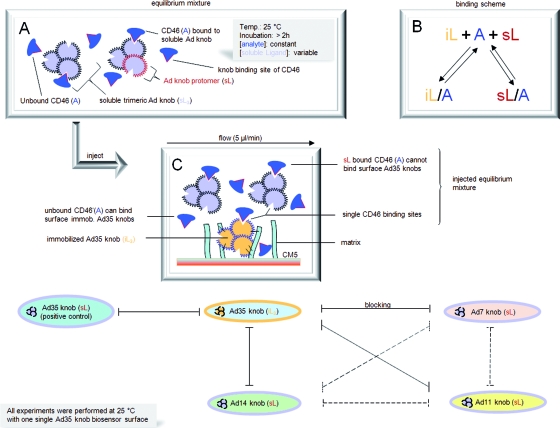
FIG. 4.
SPR epitope mapping of CD46. Shown is an overview of solution competition SPR experiments. Soluble Ad7, Ad14, and Ad11 knobs were each tested for the ability to block binding of CD46 to immobilized Ad35 knobs. Blocking is shown as black lines, while the dashed lines indicate blocking interactions that were not experimentally determined but are a logical consequence of the experimentally determined blocking results. Soluble Ad35 knob was used as a positive control. (A) Schematic snapshot showing an equilibrium mixture of CD46 and soluble Ad knob. The gray shaded box shows setup details. A, analytic; L, ligand. (B) Scheme of binding possibilities of CD46 if both soluble (s) and immobilized (i) knobs recognize the same binding epitope of CD46. (C) Schematic snapshot of the equilibrium mixture when injected over the Ad35 biosensor surface. Unbound CD46 binds immobilized Ad35 and generates the response signal used for analysis.
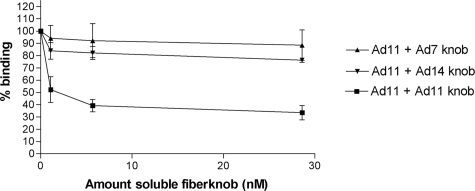
Ad11, but not Ad7 and Ad14, block binding of 35S-labeled Ad11 particles.
We next asked whether each of the three knobs, which are highly similar in sequence, can compete for Ad11 binding to human respiratory cells. A previously established binding assay was used for these studies (22). As expected, binding of 35S-labeled Ad11 was substantially reduced upon preincubation with Ad11 knobs (Fig. 5). Interestingly, neither Ad7 nor Ad14 knobs were able to efficiently compete with the binding of 35S-labeled Ad11 virions. This is most likely due to the significantly lower affinity of the Ad7 and Ad14 knobs for CD46, which would require micromolar concentrations of fiber knobs to achieve a measurable blockage of Ad11 binding. Total blockage of Ad11 binding was never observed, probably due to nonspecific binding or binding to secondary or additional receptors (50, 53). Ad-blocking experiments usually do not result in complete blocking, as was also observed by others (43, 44).
FIG. 5.
Binding of 35S-labeled Ad11 virions to human respiratory epithelial (A549) cells. Soluble Ad11, but not Ad7 or Ad14, knobs block binding of 35S-labeled Ad11 virions to A549 cells in a dose-dependent manner. The error bars indicate standard deviations.
An arginine at position 279 is required for high-affinity binding of CD46.
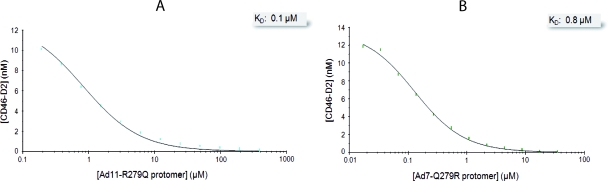
A recent study showed that mutation of the Ad11 knob residue Arg279 to glutamine essentially abolishes Ad11 binding to CD46-expressing cells (21). Although this residue does not contact CD46 directly, it is located directly next to the CD46-contacting Arg280 side chain. We therefore produced an Arg279Gln mutant of Ad11 (Ad11-R279Q) to mimic the situation in the Ad7 and Ad14 knobs, which both contain glutamines at position 279. In addition, we also produced the reverse mutation in the Ad7 knob (Ad7- Q279R) in order to test whether high-affinity binding to CD46 can be restored by introducing an arginine at position 279. Both mutants were subjected to kinetic and steady-state SPR analyses. The KD values for the Ad11-R279Q and Ad7-Q279R mutants were 1.9 μM and 0.5 μM, respectively (Fig. 6 and Table 2). These affinity values were also confirmed by solution affinity measurement (Fig. 7 and Table 2). We also note that the Ad11-R279Q mutant shows a considerably higher kd value (0.75 s−1) than the Ad11 wild type (wt) (0.014 s−1), which clearly indicates that Arg279 is a key residue for stabilizing the complex with CD46.
FIG. 6.
Kinetic and equilibrium SPR analyses of CD46 binding to Ad11-R279Q and Ad7-Q279R mutant knobs. (A and B) Kinetic analysis of CD46 binding to immobilized Ad11- R279Q (A) and Ad7-Q279R (B) mutant knobs. Double-referenced sensorgrams (shown in color) are overlaid with fits of a “1:1 binding with mass transfer” model (black lines) on top over the corresponding residual values showing the kinetic-fit range and absolute deviation (Δ) of data points from curve fit values. The arrows indicate data used for equilibrium analysis. (C and D) Equilibrium analysis of CD46 binding to Ad11-R279Q (C) and Ad7-Q279R (D) mutant knobs. Equilibrium (Eq) response values (colored squares) obtained from data in panels A and B were plotted against the CD46 concentration and fitted to a “1:1 Langmuir isotherm” model (black line). The gray shaded boxes show setup details (black font) and measured parameters of the kinetic (blue font) and equilibrium (red font) analyses.
FIG. 7.
Solution competition SPR analysis with soluble Ad11-R279Q and Ad7-Q279R knobs. Shown are solution competition analyses of Ad11-R279Q (A) and Ad7-Q279R (B) knobs competing with surface-immobilized Ad35 knob for binding to CD46. Unbound CD46-D2 was plotted against the total concentration of soluble-knob protomers (colored squares) and fitted to a “solution affinity” model. The gray shaded boxes show the determined solution KD values of the CD46-D2-soluble-knob interaction. The plots were obtained as described in the legend to Fig. 3.
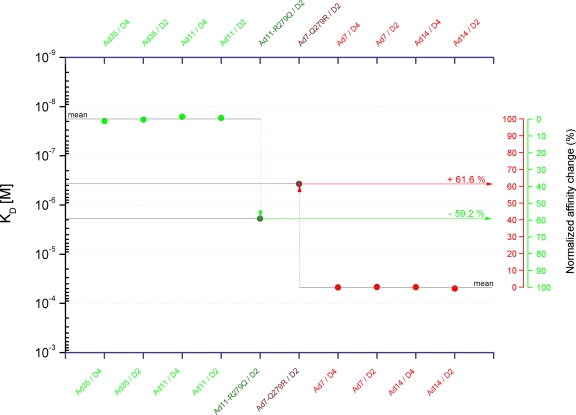
Figure 8 provides a schematic summary of the collected affinity data plotted as KD values on a log10 scale. The KD values represent averaged values from kinetic, steady-state, and solution competition SPR experiments performed with both CD46-D2 and -D4 species for individual knobs. The logarithmic presentation was chosen (i) to display values that range over several orders of magnitude; (ii) to directly visualize the significance of an affinity change in proportion to the affinity value itself, rather than a change in the absolute quantity of an affinity value; and (iii) to define the y distance between the high-affinity ligands (Ad11 and Ad35 knobs) and the low-affinity ligands (Ad7 and Ad14 knobs) for CD46 as a 100% change in affinity where an equal amount of space within this range corresponds to an equal percent change in affinity. Analysis of our data showed that the affinity of Ad11-R279Q for CD46 is reduced by about 60% compared to the Ad11 wt. Conversely, the Ad7-Q279R mutation restores about 60% of the affinity for CD46 compared to the Ad7 wt. We conclude that the presence of an arginine side chain at position 279 accounts for most of the affinity for CD46. However, this alone is not sufficient to mediate high-affinity binding.
FIG. 8.
Overview of knob-CD46 interactions. Shown is an overview of KD values of knob-CD46 interactions evaluated in this study. The KD values are plotted on a log10 scale and represent averaged values from kinetic, steady-state, and solution competition SPR experiments. Percent changes of affinity are indicated.
The CD46-contacting surface in Ad11 involves a number of side chains other than the Arg279-Arg280 pair (37). In addition to R279Q, the Ad7 knob carries two other amino acid substitutions in the vicinity of the CD46-binding surface. (i) Ad11 residue Asp284, which forms a salt bridge with the CD46 residue His43 in the complex, is replaced with an asparagine in Ad7 and an alanine in Ad14. Neither Ad7 nor Ad14 can form a similar salt bridge with CD46. (ii) Ile282 forms contacts with CD46 main-chain atoms. It is replaced with a leucine in Ad7, which could result in less favorable contacts with CD46. However, since Ad14, like Ad11, carries an isoleucine at this position, we speculate that the residue does not contribute substantially to binding.
Computational studies.
To gain further insight into the factors governing the different affinities of Ad11 and Ad7 knobs for CD46, quantum chemical calculations were performed. As the experimental X-ray structures for the Ad11 and Ad7 knobs suggest a strong similarity, it is useful to compare the stability of the Ad11-CD46 complex to that of a hypothetical Ad7-CD46 complex (see “Computational details” above).
The first evidence supporting the preferred binding of Ad11 is provided at the simple force field level, indicating that Ad11 binds CD46 more strongly by 348 kJ/mol than Ad7 (Table 3). In order to gain more reliable information, quantum chemical calculations were performed to explore which parts of the Ad11 and Ad7 knobs control the interaction energies. For this purpose, we studied smaller subsystems of the two complexes with CD46. To study the strong dependence of binding effects on Arg279, we chose a subsystem containing the stacked Arg279 and Arg280 residues of Ad11, as well as the Glu63 and Phe35 residues of CD46. Since charge delocalization is expected to be important, parts of the protein environment were taken into consideration by inclusion of five additional amino acids (Asn245, Asp246, Asn247, and Glu250 of Ad11 and Thr64 of CD46). This subsystem containing overall 9 amino acids was denoted S9-Ad11.
TABLE 3.
Interaction energies (ΔE) of CD46 with Ad11 and Ad7
| System | RI/MP2/SVP ΔE (kJ/mol)
|
MMFF force field ΔE (kJ/mol)
|
||
|---|---|---|---|---|
| Ad11-CD46 | Ad7-CD46 | Ad11-CD46 | Ad7-CD46 | |
| Total | −1,329.4 | −981.3 | ||
| S9a | −354.4 | −275.6 | −410.4 | −299.3 |
| S7b | −263.3 | −267.5 | −274.3 | −283.7 |
Residues 35, 63, 64, 245, 246, 247, 250, 279, 280.
Residues 35, 63, 64, 245, −, 247, 250, −, 280.
Likewise, the subsystem S9-Ad7 from the Ad7-CD46 complex consists of the same residues as S9-Ad11, with two exceptions: Gln279 replaces Arg279, and Val246 replaces Asp246. As expected, the interaction energies for the subsystems S9-Ad11 and S9-Ad7 differ strongly from those of the corresponding total systems (see the force field results in Table 3), but similarly to the total systems, the interaction energy within S9-Ad11 is stronger by 79 kJ/mol than that for S9-Ad7. Most importantly, starting from the S9 subsystems it is possible to estimate the influence of the Arg279 group using quantum chemical methods: If the Arg279 and Asp246 groups are removed (the latter to avoid influences by the overall charge of the Ad11 fragment), leading to subsystem S7-Ad11, the binding energy to the same CD46 part decreases by 91 kJ/mol (RI-MP2). Analogous calculations performed for the substructures of the hypothetical Ad7/CD46 complex, S9-Ad11 and S7-Ad11, indicate a much smaller change in binding energy of 8 kJ/mol (RI-MP2) upon removing the residues Gln279 and Val246 in Ad7 (S7-Ad7). This shows that the Arg279 and Asp246 groups in Ad11 have a much larger influence on binding than the corresponding mutated groups in Ad7, Gln279 and Val246.
Overall, the quantum chemical calculations are in line with the experimental observations, indicating that there is a strongly preferred binding of CD46 to Ad11 compared with Ad7, and a significant part of the interaction process can be attributed to the presence of the Arg279 group.
DISCUSSION
It is currently not clear which species B Ads can use CD46 as a receptor, as somewhat contradictory reports exist (17, 20, 25, 44, 50). While there is universal agreement that some Ads do use CD46 (e.g., Ad11 and Ad35), whether others (e.g., Ad7 and Ad14) also do so remains unresolved. A different receptor (receptor X) appears to be used by a number of Ads, including Ad14 and Ad7, although there is evidence that Ad11 is able to use both CD46 and receptor X (50). Here, we define the structural, energetic, and functional parameters for the interactions of CD46 with the three highly similar knobs of Ad11, Ad7, and Ad14.
We first determined the structures of all three knobs to very high resolution. The three structures superimpose very well, including the regions that serve as the binding site for CD46 in Ad11 (37). This provides evidence that differences in binding are not due to differences in the arrangement of surface loops. The structure of the complex between CD46 and the Ad11 knob showed that Arg280 mediates central contacts with CD46. This residue is conserved in all three knobs, and it also has the same conformation, with its guanidinium group stacked against that of the Arg291 side chain in unliganded knobs. Comparison with the structure of the Ad11 knob-CD46 complex showed that the Arg280 side chain must flip 170° in order to engage CD46. Intriguingly, Arg280 stacks against another arginine, Arg279, once the complex has formed. Thus, Arg280 is in an “off position” in all three unliganded knobs and switches to an “on position” in Ad11 upon engagement of CD46 (Fig. 9A and B).
FIG. 9.
Proposed mechanism for binding to CD46. Shown is a view into the central Ad11-CD46 contact area around Arg280. CD46 and the Ad11 knob are shown in orange and blue, respectively. As CD46 approaches the Ad11 knob, Arg280 flips over from its “off position” near Arg291 (A) and swings next to CD46 Phe35 and Ad11 Arg279 into the “on position” (B). A theoretical model for an Ad7 knob-CD46 complex is shown in panel C, with the Ad7 knob in yellow and CD46 in grey. A Gln at position 279 would not interfere sterically with a rotating Arg280.
The solution competition SPR studies clearly demonstrated that all three knobs (but also Ad35) bind to the same epitope on the N-terminal two repeats of CD46. However, the affinities are drastically different (Fig. 2 and Table 2). We found that Ad11 binds CD46 with an affinity in the range of 10 to 15 nM and exhibits similar rate constants comparable to what was found for the Ad35 knob-CD46 interaction (Table 2) (51). In contrast, both the Ad7 and Ad14 knobs bind CD46 with significantly lower affinity and also exhibit very high off-rate constants. Although a micromolar affinity could still lead to a productive interaction, the kinetic off-rate constants suggest that complexes form only transiently and fall apart quickly. Thus, structural features that transform initial contacts into a stable complex are absent in Ad7 and Ad14. The most obvious candidate for such a feature is Arg279, which was shown earlier by mutational studies to play a key role in CD46 binding (21). However, Arg279 does not contact CD46 directly but rather serves as a platform for the CD46-engaging residue Arg280.
It seems plausible to suggest that the replacement of Arg279 with Gln, as seen in Ad7 and Ad14, does not favor the flip of the Arg280 side chain into the “on position.” Comparison of the liganded Ad11 knob and the unliganded Ad7 knob (Fig. 9B and C) structures clearly demonstrates that there are no clashes with Gln279 that would prevent a similar flip in Ad7. In fact, there is ample space to accommodate the Arg280 side chain next to Gln279 in the Ad7 knob, as well as in the Ad14 knob.
The crystal structures of all three unliganded knobs uniformly show that the Arg280 side chain stacks against Arg291, indicating a preferred arrangement of these two arginine residues. Energetically favorable π-π interactions between the guanidinium groups likely stabilize this arrangement. Only the Ad11 knob offers a second arginine residue at position 279, with which a similar π-π interaction can be formed. Quantum chemical calculations show a much larger binding energy between CD46 and Ad knobs if an arginine at position 279 is present. The Ad7 and Ad14 knobs would be able to make some initial contacts with CD46, but lacking a correctly positioned Arg280 to engage the CD46 interface, they would not be able to elicit the conformational change in CD46 that straightens the receptor. As this conformational change enables additional stabilizing interactions between the knob and CD46, a high-affinity interaction with low off-rate constants, as seen with Ad11, becomes impossible. This interpretation is in excellent agreement with the SPR data, which show a dramatic increase in the KD (in line with an increase in the kd) when Arg279 is mutated to a glutamine in Ad11. However, the SPR data also clearly demonstrate that a single substitution at position 279 is not sufficient to transform the CD46-binding properties of Ad11 into those of Ad7, or vice versa, showing that other amino acid side chains of the Ad11 knob interface with CD46 contribute to binding.
Taken together our data indicate that all three knobs interact with CD46 but probably use different binding mechanisms. However, whether this translates into productive infection of cells is questionable, as it appears unlikely that Ad7 and Ad14 would use CD46 as their sole receptor due to the significantly weaker complex stability (12) than with the Ad11 and Ad35 knobs. It is certainly possible that CD46, in addition to Ad11, also functions as a cellular receptor for Ad7 and Ad14 on certain cell types that carry especially high densities of CD46, which would increase the avidity of the virus for the cell, perhaps triggering entry. In addition, CD46 could function as an adhesion-strengthening receptor that is engaged only once another receptor, such as receptor X (50), is engaged or that facilitates the engagement of receptor X.
Arginine residues are often found at protein interfaces, and a comparative analysis of protein complexes has shown that, among all amino acids, arginines are the most abundant residues in such contacts, accounting on average for about 10% of the total contact interface area (5, 13). Furthermore, many contacts involving arginine residues are interactions that in more than 50% of the observed cases feature a coplanar arrangement of the positively charged guanidinium group of the arginine and the π-electron system of aromatic ring systems in amino acids (8, 13). The energy gain of such interactions can even exceed that of a salt bridge (19). By comparison, the stacking of two similarly charged arginine residues, as observed here, is rare. A search of the PDB revealed only a small number of such contacts. For example, an arginine stacking is present at the interface of the glutathione-S-transferase homodimer (PDB code 1GUH) (47), and it contributes to the recognition of a substrate-based inhibitor in the human immunodeficiency virus type 1 protease (PDB code 4HVP) (26). Our analysis points to a key role of such an arginine stacking in the high-affinity recognition of at least one viral receptor. As structural information about virus-receptor complexes is still very limited, we think it possible, and perhaps quite likely, that arginine pairs will emerge as receptor-binding determinants for more viruses and also for other pathogens. Taken together, our data contribute to a better understanding of the Ad life cycle, provide a target for the development of novel antiviral drugs, and form the basis for more efficient design of Ad-based vectors with altered receptor-binding properties for gene and cancer therapy approaches.
Acknowledgments
We thank the Swiss Light Source (Villigen, Switzerland) for beam time and the staff at beamline X06SA for support.
Financial support for this study was provided by Deutsche Forschungsgemeinschaft (DFG) grants STE-1463 and SFB-685 (T.S.) and Swedish Research Council grants 2003-6008, 2004-6174, and 2007-3402 (N.A.). B.B.T.S. is supported by a graduate fellowship from the Cusanuswerk-Bischöfliche Studienförderung. C.V.S. thanks the Fonds der Chemischen Industrie (FCI) for a graduate fellowship. S.S. is grateful to the Studienstiftung des deutschen Volkes for a graduate fellowship. C.O. acknowledges financial support by the DFG.
Footnotes
Published ahead of print on 5 November 2008.
REFERENCES
- 1.Adamczyk, M., J. C. Gebler, A. H. Gunasekera, P. G. Mattingly, and Y. Pan. 1997. Immunoassay reagents for thyroid testing. 2. Binding properties and energetic parameters of a T4 monoclonal antibody and its Fab fragment with a library of thyroxine analog biosensors using surface plasmon resonance. Bioconjug. Chem. 8133-145. [DOI] [PubMed] [Google Scholar]
- 2.Adamczyk, M., J. A. Moore, and Z. Yu. 2000. Application of surface plasmon resonance toward studies of low-molecular-weight antigen-antibody binding interactions. Methods 20319-328. [DOI] [PubMed] [Google Scholar]
- 3.Berk, A. J. 2007. Adenoviridae: the viruses and their replication, p. 2301-2326. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 4.Boys, S., and F. Bernardi. 1970. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 19440-467. [Google Scholar]
- 5.Brinda, K. V., and S. Vishveshwara. 2005. Oligomeric protein structure networks: insights into protein-protein interactions. BMC Bioinform. 6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunger, A. T. 2007. Version 1.2 of the Crystallography and NMR System. Nat. Protoc. 22728-2733. [DOI] [PubMed] [Google Scholar]
- 7.Brunger, A. T., P. D. Adams, G. M. Clore, W. L. DeLano, P. Gros, R. W. Grosse-Kunstleve, J. S. Jiang, J. Kuszewski, M. Nilges, N. S. Pannu, R. J. Read, L. M. Rice, T. Simonson, and G. L. Warren. 1998. Crystallography and NMR System: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54905-921. [DOI] [PubMed] [Google Scholar]
- 8.Burley, S. K., and G. A. Petsko. 1986. Amino-aromatic interactions in proteins. FEBS Lett. 203139-143. [DOI] [PubMed] [Google Scholar]
- 9.Casasnovas, J. M., M. Larvie, and T. Stehle. 1999. Crystal structure of two CD46 domains reveals an extended measles virus-binding surface. EMBO J. 182911-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cattaneo, R. 2004. Four viruses, two bacteria, and one receptor: membrane cofactor protein (CD46) as pathogens' magnet. J. Virol. 784385-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collaborative Computing Project No. 4. 1994. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50760-763. [DOI] [PubMed] [Google Scholar]
- 12.Copeland, R. A., D. L. Pompliano, and T. D. Meek. 2006. Drug-target residence time and its implications for lead optimization. Nat. Rev. Drug Discov. 5730-739. [DOI] [PubMed] [Google Scholar]
- 13.Crowley, P. B., and A. Golovin. 2005. Cation-pi interactions in protein-protein interfaces. Proteins 59231-239. [DOI] [PubMed] [Google Scholar]
- 14.DeLano, W. L. 2002. The PyMOL Molecular Graphics System. DeLano Scientific, San Carlos, CA.
- 15.Eichkorn, K., F. Weigend, O. Treutler, and R. Ahlrichs. 1997. Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theor. Chem. Acc. 97119-124. [Google Scholar]
- 16.Feyereisen, M., G. Fitzgerald, and A. Komornicki. 1993. Use of approximate integrals in ab initio theory. An application in MP2 energy calculation. Chem. Phys. Lett. 208359-363. [Google Scholar]
- 17.Fleischli, C., D. Sirena, G. Lesage, M. J. Havenga, R. Cattaneo, U. F. Greber, and S. Hemmi. 2007. Species B adenovirus serotypes 3, 7, 11 and 35 share similar binding sites on the membrane cofactor protein CD46 receptor. J. Gen. Virol. 882925-2934. [DOI] [PubMed] [Google Scholar]
- 18.Fleischli, C., S. Verhaagh, M. Havenga, D. Sirena, W. Schaffner, R. Cattaneo, U. F. Greber, and S. Hemmi. 2005. The distal short consensus repeats 1 and 2 of the membrane cofactor protein CD46 and their distance from the cell membrane determine productive entry of species B adenovirus serotype 35. J. Virol. 7910013-10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flocco, M. M., and S. L. Mowbray. 1994. Planar stacking interactions of arginine and aromatic side-chains in proteins. J. Mol. Biol. 235709-717. [DOI] [PubMed] [Google Scholar]
- 20.Gaggar, A., D. M. Shayakhmetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 91408-1412. [DOI] [PubMed] [Google Scholar]
- 21.Gustafsson, D. J., A. Segerman, K. Lindman, Y. F. Mei, and G. Wadell. 2006. The Arg279Gln substitution in the adenovirus type 11p (Ad11p) fiber knob abolishes EDTA-resistant binding to A549 and CHO-CD46 cells, converting the phenotype to that of Ad7p. J. Virol. 801897-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson, S. M., E. C. Nilsson, M. Elofsson, N. Ahlskog, J. Kihlberg, and N. Arnberg. 2007. Multivalent sialic acid conjugates inhibit adenovirus type 37 from binding to and infecting human corneal epithelial cells. Antivir. Res. 7392-100. [DOI] [PubMed] [Google Scholar]
- 23.Kabsch, W. 1993. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr. 26795-800. [Google Scholar]
- 24.Kojaoghlanian, T., P. Flomenberg, and M. S. Horwitz. 2003. The impact of adenovirus infection on the immunocompromised host. Rev. Med. Virol. 13155-171. [DOI] [PubMed] [Google Scholar]
- 25.Marttila, M., D. Persson, D. Gustafsson, M. K. Liszewski, J. P. Atkinson, G. Wadell, and N. Arnberg. 2005. CD46 is a cellular receptor for all species B adenoviruses except types 3 and 7. J. Virol. 7914429-14436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, M., J. Schneider, B. K. Sathyanarayana, M. V. Toth, G. R. Marshall, L. Clawson, L. Selk, S. B. Kent, and A. Wlodawer. 1989. Structure of complex of synthetic HIV-1 protease with a substrate-based inhibitor at 2.3 Å resolution. Science 2461149-1152. [DOI] [PubMed] [Google Scholar]
- 27.Mohamadi, F. R., M. G. J. Richards, W. C. Guida, R. Liskamp, M. Lipton, C. Caufield, G. Chang, T. Hendrickson, and W. C. Still. 1990. Macromodel—an integrated software system for modeling organic and bioorganic molecules using molecular mechanics. J. Comp. Chem. 11440-467. [Google Scholar]
- 28.Murshudov, G. N., A. A. Vagin, and E. J. Dodson. 1997. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D. 53240-255. [DOI] [PubMed] [Google Scholar]
- 29.Navaza, J. 1994. AMoRe: an automated package for molecular replacement. Acta Crystallogr. A 50157-163. [Google Scholar]
- 30.Nieba, L., A. Krebber, and A. Pluckthun. 1996. Competition BIAcore for measuring true affinities: large differences from values determined from binding kinetics. Anal. Biochem. 234155-165. [DOI] [PubMed] [Google Scholar]
- 31.Nordin, H., M. Jungnelius, R. Karlsson, and O. P. Karlsson. 2005. Kinetic studies of small molecule interactions with protein kinases using biosensor technology. Anal. Biochem. 340359-368. [DOI] [PubMed] [Google Scholar]
- 32.Ochsenfeld, C., J. Kussmann, and D. S. Lambrecht. 2007. Linear-scaling methods in quantum chemistry. Rev. Comp. Chem. 231-82. [Google Scholar]
- 33.Ochsenfeld, C. 2000. Linear scaling exchange gradients for Hartree-Fock and hybrid density funtional theory. Chem. Phys. Lett. 327216-223. [Google Scholar]
- 34.Ochsenfeld, C., C. A. White, and M. J. Head-Gordon. 1998. Linear and sub-linear scaling formation of Hartree-Fock-type exchange matrices. J. Chem. Phys. 1091663-1669. [Google Scholar]
- 35.Otwinowski, Z., and W. Minor. 1997. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276307-326. [DOI] [PubMed] [Google Scholar]
- 36.Pache, L., S. Venkataraman, V. S. Reddy, and G. R. Nemerow. 2008. Structural variations in species B adenovirus fibers impact CD46 association. J. Virol. 827923-7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Persson, B. D., D. M. Reiter, M. Marttila, Y. F. Mei, J. M. Casasnovas, N. Arnberg, and T. Stehle. 2007. Adenovirus type 11 binding alters the conformation of its receptor CD46. Nat. Struct. Mol. Biol. 14164-166. [DOI] [PubMed] [Google Scholar]
- 38.Read, R. 2001. Pushing the boundaries of molecular replacement with maximum likelihood. Acta Crystallogr. D 571373-1382. [DOI] [PubMed] [Google Scholar]
- 39.Rich, R. L., and D. G. Myszka. 2006. Survey of the year 2005 commercial optical biosensor literature. J. Mol. Recognit. 19478-534. [DOI] [PubMed] [Google Scholar]
- 40.Schäfer, A., H. Horn, and R. Ahlrichs. 1992. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 972571-2577. [Google Scholar]
- 41.Schrödinger, Inc. 2005. Maestro 7.5. Schrödinger Inc., Portland, OR.
- 42.Schuck, P. 1997. Reliable determination of binding affinity and kinetics using surface plasmon resonance biosensors. Curr. Opin. Biotechnol. 8498-502. [DOI] [PubMed] [Google Scholar]
- 43.Segerman, A., N. Arnberg, A. Erikson, K. Lindman, and G. Wadell. 2003. There are two different species B adenovirus receptors: sBAR, common to species B1 and B2 adenoviruses, and sB2AR, exclusively used by species B2 adenoviruses. J. Virol. 771157-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segerman, A., J. P. Atkinson, M. Marttila, V. Dennerquist, G. Wadell, and N. Arnberg. 2003. Adenovirus type 11 uses CD46 as a cellular receptor. J. Virol. 779183-9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Selzer, T., S. Albeck, and G. Schreiber. 2000. Rational design of faster associating and tighter binding protein complexes. Nat. Struct. Biol. 7537-541. [DOI] [PubMed] [Google Scholar]
- 46.Shao, Y., C. A. White, and M. Head-Gordon. 2001. Efficient evaluation of the Coulomb force in density-functional theory calculations. J. Chem. Phys. 1146572-6577. [Google Scholar]
- 47.Sinning, I., G. J. Kleywegt, S. W. Cowan, P. Reinemer, H. W. Dirr, R. Huber, G. L. Gilliland, R. N. Armstrong, X. Ji, P. G. Board, et al. 1993. Structure determination and refinement of human alpha class glutathione transferase A1-1, and a comparison with the Mu and Pi class enzymes. J. Mol. Biol. 232192-212. [DOI] [PubMed] [Google Scholar]
- 48.Stanley, P. 1989. Chinese hamster ovary cell mutants with multiple glycosylation defects for production of glycoproteins with minimal carbohydrate heterogeneity. Mol. Cell. Biol. 9377-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szabo, A., and N. S. Ostlund. 1989. Modern quantum chemistry. Dover Publications, Mineola, NY.
- 50.Tuve, S., H. Wang, C. Ware, Y. Liu, A. Gaggar, K. Bernt, D. Shayakhmetov, Z. Li, R. Strauss, D. Stone, and A. Lieber. 2006. A new group B adenovirus receptor is expressed at high levels on human stem and tumor cells. J. Virol. 8012109-12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, H., Y. C. Liaw, D. Stone, O. Kalyuzhniy, I. Amiraslanov, S. Tuve, C. L. Verlinde, D. Shayakhmetov, T. Stehle, S. Roffler, and A. Lieber. 2007. Identification of CD46 binding sites within the adenovirus serotype 35 fiber knob. J. Virol. 8112785-12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White, C. A., B. G. Johnson, P. M. W. Gill, and M. Head-Gordon. 1994. The continuous fast multipole method. Chem. Phys. Lett. 2308-16. [Google Scholar]
- 53.Wickham, T. J., R. R. Granados, H. A. Wood, D. A. Hammer, and M. L. Shuler. 1990. General analysis of receptor-mediated viral attachment to cell surfaces. Biophys. J. 581501-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73309-319. [DOI] [PubMed] [Google Scholar]
- 55.Wild, M. K., M. C. Huang, U. Schulze-Horsel, P. A. van der Merwe, and D. Vestweber. 2001. Affinity, kinetics, and thermodynamics of E-selectin binding to E-selectin ligand-1. J. Biol. Chem. 27631602-31612. [DOI] [PubMed] [Google Scholar]