Abstract
Dengue virus (DENV) is an ∼10.7-kb positive-sense RNA virus that circularizes via RNA-RNA interactions between sequences in the 5′ and 3′ terminal regions. Complementarity between the cyclization sequence (CS) and the upstream AUG region (UAR) has been shown to be necessary for viral replication. Here, we present the solution structure of the 5′ end of DENV type 2 in the presence and absence of the 3′ end. We demonstrate that hybridization between the 5′ and 3′ CSs is independent of the UAR while the 5′ UAR-3′ UAR hybridization is dependent upon the 5′ CS-3′ CS interaction.
Dengue virus (DENV) is a mosquito-borne, positive-stranded RNA virus within the Flavivirus genus. The four serotypes (DENV1 to DENV4) cause major global public health problems, with tens of millions of dengue fever cases and hundreds of thousands of cases of life-threatening dengue hemorrhagic fever/dengue shock syndrome annually. The 10.7-kb viral RNA is translated into a single polyprotein, which is subsequently cleaved into three structural and seven nonstructural proteins by viral and cellular proteases. The open reading frame is flanked by an ∼100-nucleotide (nt) 5′ untranslated region (UTR) which contains a type I cap, and an ∼450-nt 3′ UTR lacking a poly(A) tail. The 5′ and 3′ ends interact via RNA-RNA hybridization of two regions, the cyclization sequence (CS) (6) and the upstream AUG region (UAR) (1), resulting in circularization of the viral genome. The CS has previously been shown to be crucial for DENV replication and RNA synthesis (18, 19), and the UAR is proposed to play a similar role (1-3). RNA folding algorithms predict conserved secondary structures in the Flavivirus 5′ UTR, and the CS is a highly conserved sequence among flaviviruses, suggesting an important function for these elements (4, 16). Both the CS and the UAR have recently been shown to play a role in 5′-to-3′ end-to-end interaction and replication of the related West Nile virus (8, 20, 21).
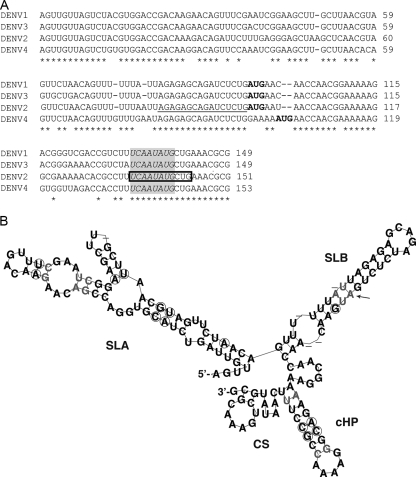
To define the solution structure of the 5′ end and its interaction with the 3′ UTR, we chemically and enzymatically probed the DENV2 5′ end alone or hybridized to the 3′ UTR. We resolved the structure of the 96-nt 5′ UTR, as well as the 5′ UTR including 55 nt of the capsid gene (5′UTR+55). The additional 55 nt in the coding region contain the CS region and the capsid hairpin (cHP), previously shown to be involved in viral translation start site selection (7) and RNA synthesis (6). Sequences were derived from the infectious clone of the prototype DENV2 strain 16681 (pD2/IC-30P-A; GenBank accession no. U87411) (11) and cloned into Litmus 28 (New England BioLabs, Beverly, MA) to generate the 5′ UTR (104 nt, including AUG and an AflIII overhang) or 5′UTR+55 (151 nt, including an MluI overhang) downstream of the T7 RNA polymerase promoter. A predicted consensus structure for the 5′ end of DENV1 to DENV4 (DENV1, GenBank accession no. U88536; DENV2, accession no. U87411; DENV3, accession no. M93130; DENV4, accession no. AF326825), corresponding to 5′UTR+55, was obtained by multiple alignment using CLUSTAL W v.1.83 (Fig. 1A) (15), followed by consensus structure prediction using Alifold (Fig. 1B) (12). Additional consensus structure prediction with MAFFT v.5.8 alignment (10) rendered an equivalent structure (data not shown).
FIG. 1.
Predicted and solution structures of the 5′ end of DENV2. (A) CLUSTAL W (v.1.83) alignment of the first ∼150 nt of DENV1 to DENV4. The CS region is boxed, and conserved nucleotides among the flaviviruses (dengue, yellow fever, Murray Valley encephalitis, West Nile, and St. Louis encephalitis viruses) (6) are in italics in the shaded area. The start codon is indicated in boldface, and the UAR is underlined. Asterisks indicate conserved nucleotides among all DENV serotypes. (B) Consensus structure prediction of DENV serotypes from panel A, generated by Alifold. Circles indicate sequence variation supporting the consensus structure. Pairs with noncompatible sequence variation are shown in gray, and dashes represent variable positions. The arrow indicates the start codon.
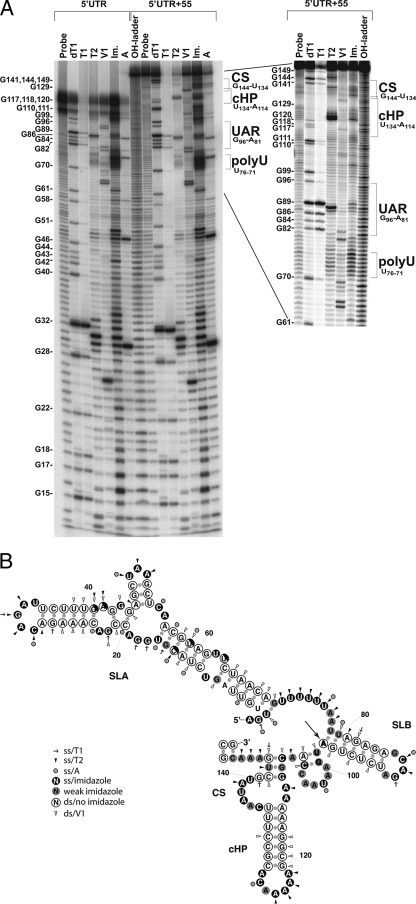
To determine the solution structure, 100 pmol of the 5′ UTR or 5′UTR+55 in vitro-transcribed RNA was dephosphorylated with alkaline phosphatase and subsequently labeled with [γ-32P]ATP (Amersham Biosciences, Piscataway, NJ) by using polynucleotide kinase. The probe was separated on a 6% denaturing polyacrylamide gel; the correct product was excised and eluted in 0.3 M sodium acetate with 0.1% sodium dodecyl sulfate, phenol extracted, and precipitated with isopropanol. The purified probe was heated to 95°C and then cooled to 75°C (5°C decrease/min), followed by slower cooling to 25°C (1°C decrease/min) in a thermocycler to allow for the refolding of secondary structures. Labeled RNAs were digested with RNase T1 (Ambion, Austin, TX) and RNase T2 and RNase A (Sigma-Aldrich, St. Louis, MO) for 15 min at room temperature (RT) in buffer A (10 mM Tris, pH 7.0, 20 mM MgCl2); digestion with RNase V1 (Ambion) was performed for 10 min at RT in buffer A with 200 mM NaCl. For sequence orientation purposes, heat-denatured RNA was OH hydrolyzed (10 min at 95°C in hydrolysis buffer [Ambion]) or digested with RNase T1 for 10 min in sequence buffer (V1 RNase kit; Ambion). All RNase digestions were performed in the presence of 1 μg yeast RNA and with nonlimiting MgCl2 concentrations (3) and were subsequently phenol-chloroform extracted and ethanol precipitated in the presence of glycogen-tRNA. As strong secondary structures can be resistant to enzymatic hydrolysis (13), we also performed chemical cleavage with 0.4 M imidazole overnight at RT in 40 mM NaCl, 1 mM EDTA, and 10 mM MgCl2, followed by precipitation with lithium perchlorate-sodium acetate and acetone (17). Imidazole cleaves only single-stranded RNA, while double-stranded RNA remains protected. The samples were analyzed by denaturing 10% or 12% polyacrylamide gel electrophoresis and imaged using a Typhoon phosphorimager (GE Healthcare, Piscataway, NJ). The structure of the 5′ UTR was deduced from at least 10 independent experiments (representative gel shown in Fig. 2A) and was found to adopt the same conformation in the presence of an additional 55 nt of the capsid gene (Fig. 2A and B).
FIG. 2.
Solution structure of the 5′ end of DENV2. (A) Phosphorimage of the DENV2 5′ UTR and 5′UTR+55 digested with RNase T1 (7 U), RNase T2 (0.1 U), RNase V1 (0.7 mU), RNase A (0.1 ng), or imidazole (Im.) (0.4 M) separated on a 10% native polyacrylamide gel. Probe, untreated probe; OH-ladder, hydrolyzed probe; dT1, RNase T1 digestion of denatured probe; T1, RNase T1; T2, RNase T2; V1, RNase V1; A, RNase A. Structural elements are indicated on the right, and a G-sequence ladder (based on T1 digestion) is shown on the left. The right panel shows a 10% native polyacrylamide gel with greater resolution of the 3′ end of 5′UTR+55. (B) Secondary structure of the DENV2 5′ end based on chemical and enzymatic probing. SLA, SLB, the cHP, and the CS are indicated. The large arrow indicates the start codon. Single-stranded (ss) and double-stranded digests are indicated corresponding to the activity observed at each site. Arrow, RNase T1 digestion (ss); arrowhead, RNase T2 digestion (ss); gray dot, RNase A digestion (ss). Open circles indicate no imidazole activity (ds), black circles indicate strong imidazole cleavage (ss), and gray circles indicate imidazole cleavage above background but not as strong as that indicated by black circles. Half-white and half-black circles indicate different cleavage patterns in different gels (of a total of ∼10 examined), suggesting that these positions may be more dynamic than others.
The experimentally determined solution structure of stem-loop A (SLA) supports the predicted structure, with a few exceptions (Fig. 1B and 2B). In our analysis, imidazole digestion invariably shows G43 as single stranded, giving rise to a conformationally stable central, bulged G in a GGG triplet. This structure shortens the stem of the computationally predicted side hairpin consisting of nt 45 to 53 in SLA by 1 bp (Fig. 2B). Furthermore, in the experimentally determined structure, the poly(U) tract (spanning nt 71 to 76) is consistently single stranded, shortening the stem of the predicted UAR-harboring hairpin (stem-loop B [SLB]). Also, DENV2 forms a larger cHP loop than those predicted for DENV1, DENV3, and DENV4 (7), thus forming a slightly shorter stem (7 bp) than the 8-bp stem of the consensus prediction. Both the predicted and experimentally determined structures reveal a single-stranded CS region, although the experimental model displays a longer bulge than the prediction.
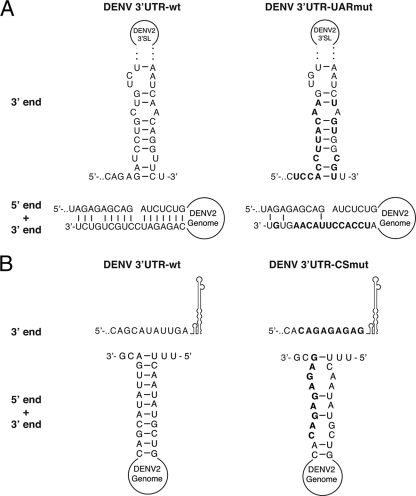
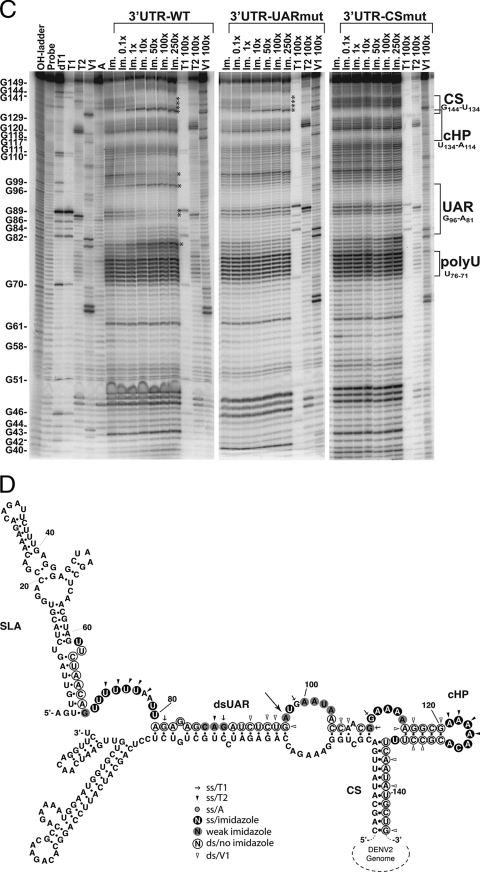
To characterize the RNA-RNA interaction between the DENV2 5′ end and the 3′ UTR, labeled 5′UTR+55 was hybridized with (i) wild-type 3′ UTR (3′UTR-wt), (ii) 3′ UTR with a mutated UAR that interferes with the 5′ UAR-3′ UAR interaction (3′UTR-UARmut) (Fig. 3A), or (iii) 3′ UTR with a mutated CS (3′UTR-CSmut) (Fig. 3B) as described previously (17), disrupting the 5′ CS-3′ CS interaction (Fig. 3A and B). Increasing amounts of each 3′ UTR variant were annealed to labeled 5′UTR+55 and digested with imidazole and RNases as described above (Fig. 3C). Upon binding of 3′UTR-wt, a clear shift in 5′UTR+55 from single-stranded to double-stranded RNA (dsRNA) can be noted in the 5′ CS region (Fig. 3C). This shift is apparent even at equimolar ratios of the 5′ UTR and 3′ UTR (Fig. 3C, lane “Im. 1x”), indicating a strong interaction. Structural changes were also observed in the UAR, converting from a hairpin to a 5′-to-3′ dsRNA form (Fig. 3C and D). Specifically, the changes observed upon binding of 3′UTR-wt include the following: (i) for the CS (spanning nt 134 to 144), nt 135 to 140 become double stranded upon hybridization with the 3′ CS, and nt 133, outside the 3′ CS, becomes highly susceptible to specific single-stranded digestion; (ii) for the UAR (spanning nt 81 to 96), nt 88 and 89, at the top of SLB, become more protected against imidazole cleavage, which is the sole change in the cleavage pattern of the UAR as this region changes from a hairpin to an end-to-end double-stranded region; (iii) for nt 98 to 102, downstream of the UAR, nt U98 is part of the first AUG codon and is no longer protected from imidazole cleavage upon 3′ UTR binding, while nt 101 and 102 become more protected; and (iv) nt 80, upstream of the UAR, shows increased imidazole susceptibility in the presence of the 3′ UTR (Fig. 3C). The changes observed in the 5′ end structure outside the UAR-CS interaction points are consistent with structural rearrangements that could occur upon end-to-end interaction and thus are further indications of 5′-to-3′ hybridization.
FIG. 3.
Experimentally determined solution structure of the DENV2 5′ end in the presence of the 3′ UTR. (A) Predicted 3′ UAR structure of 3′UTR-wt (left) and 3′UTR-UARmut (right), showing the 3′ end alone (top) or 5′-to-3′ end-to-end interaction (bottom). (B) Predicted 3′ CS structure of 3′UTR-wt (left) and 3′UTR-CSmut (right), showing the 3′ UTR alone (top) or 5′-to-3′ end-to-end interaction (bottom). Mutated nucleotides are marked in boldface letters in panels A and B. (C) Phosphorimages of chemical and enzymatic probing of the DENV2 5′ end (151 nt) in the presence of increasing amounts of the DENV 3′ UTR (3′UTR-wt) or 3′ UTR with mutations in the UAR (3′UTR-UARmut) or the CS region (3′UTR-CSmut) separated on a 10% polyacrylamide gel with 8 M urea. Numbers correspond to molar excesses of the 3′ UTR over 5′UTR+55 in lanes treated with 0.4 M imidazole or T1 (7 U), T2 (0.1 U), or V1 (0.2 mU) RNase. OH-ladder, hydrolyzed probe; Probe, untreated probe; dT1, RNase T1 (7 U) digestion of denatured probe; T1, RNase T1 digestion; T2, RNase T2 digestion; V1, RNase V1 digestion; A, RNase A digest (0.1 ng); Im., imidazole treatment (0.4 M). Structural elements are indicated on the right, and a G-sequence ladder (based on RNase T1 digestion) is shown on the left. Asterisks indicate major 5′ end structural changes upon binding of the 3′ UTR constructs. (D) Structure of the DENV2 5′ end hybridized with 3′UTR-wt, as determined for panel B. Single-stranded (ss) and double-stranded digests are indicated corresponding to the activity observed at each site (see key and Fig. 2 legend). Structural elements are indicated. The large arrow indicates the start codon.
As expected, the addition of 3′UTR-UARmut prevented the formation of the 5′ UAR-3′ UAR interaction (nt 80, 88 to 89, 98, and 101 and 102) while allowing the 5′ CS-3′ CS interaction to form readily (nt 133 and 135 to 144), starting at a 10-fold molar excess of 3′UTR-UARmut (Fig. 3C). Similarly, the presence of 3′UTR-CSmut abrogated the formation of the double-stranded CS. However, 3′UTR-CSmut severely compromised the formation of the 5′ UAR-3′ UAR interaction, even in the presence of a large molar excess of 3′UTR-CSmut (Fig. 3C). Overall, this experimentally determined structure of the 5′ end hybridized to the 3′ UTR in trans confirms previously predicted structures for genome cyclization of DENV (3, 9) and indicates that the 5′ CS-3′ CS interaction is necessary for the formation of 5′-to-3′ end-to-end hybridization. Interestingly, the SLB hairpin formed at the 5′ UAR bears a GNRA-like motif (GNRXA; nt 84 to 88, GCAGA) which may potentially stabilize the hairpin and be a candidate site for a possible protein interaction, as has been demonstrated for bacteriophage λ and P22 RNAs (5, 14). Furthermore, such a structurally stabilizing motif is consistent with the apparent reduced ability of the 5′ UAR in SLB to hybridize to the 3′ UAR in the absence of the 5′ CS-3′ CS interaction.
In summary, our experimentally determined model of the DENV 5′ end is similar to the predicted Alifold consensus and Mfold structures. By adding the 3′ UTR in trans, we demonstrate a strong hybridization between the 5′ and 3′ CSs and a relatively weaker interaction between the 5′ and 3′ UARs. Since these experiments were performed with genome fragments, naturally there may be additional factors affecting these interactions in the context of the entire genome in vivo. However, we show that 5′-to-3′ RNA-RNA interactions alone are sufficient to induce structural rearrangement of the 5′ UTR and mediate stable interactions known to be essential to the viral life cycle. Under the conditions tested, the 5′-to-3′ dsRNA CS interaction is necessary for the 5′-to-3′ UAR interaction. Therefore, we hypothesize that the CS is the primary site of cyclization, which then stabilizes the hybridization of the UARs, possibly in association with other structures, such as the cHP.
Acknowledgments
We thank W. Allen Miller and Ian G. Goodfellow for technical advice, Karen Clyde and Magnus Strandh for bioinformatic assistance and editorial comments, and Richard Kinney for the generous gift of pD2/IC.
Footnotes
Published ahead of print on 12 November 2008.
REFERENCES
- 1.Alvarez, D. E., A. L. De Lella Ezcurra, S. Fucito, and A. V. Gamarnik. 2005. Role of RNA structures present at the 3′UTR of dengue virus on translation, RNA synthesis, and viral replication. Virology 339200-212. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez, D. E., C. V. Filomatori, and A. V. Gamarnik. 2008. Functional analysis of dengue virus cyclization sequences located at the 5′ and 3′UTRs. Virology 375223-235. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez, D. E., M. F. Lodeiro, S. J. Luduena, L. I. Pietrasanta, and A. V. Gamarnik. 2005. Long-range RNA-RNA interactions circularize the dengue virus genome. J. Virol. 796631-6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinton, M. A., and J. H. Dispoto. 1988. Sequence and secondary structure analysis of the 5′-terminal region of flavivirus genome RNA. Virology 162290-299. [DOI] [PubMed] [Google Scholar]
- 5.Cai, Z., A. Gorin, R. Frederick, X. Ye, W. Hu, A. Majumdar, A. Kettani, and D. J. Patel. 1998. Solution structure of P22 transcriptional antitermination N peptide-boxB RNA complex. Nat. Struct. Biol. 5203-212. [DOI] [PubMed] [Google Scholar]
- 6.Clyde, K., J. Barrera, and E. Harris. 2008. The capsid-coding region hairpin element (cHP) is a critical determinant of dengue virus and West Nile virus RNA synthesis. Virology 379314-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clyde, K., and E. Harris. 2006. RNA secondary structure in the coding region of dengue virus type 2 directs translation start codon selection and is required for viral replication. J. Virol. 802170-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong, H., B. Zhang, and P. Y. Shi. 2008. Terminal structures of West Nile virus genomic RNA and their interactions with viral NS5 protein. Virology 381123-135. [DOI] [PubMed] [Google Scholar]
- 9.Hahn, C. S., Y. S. Hahn, C. M. Rice, E. Lee, L. Dalgarno, E. G. Strauss, and J. H. Strauss. 1987. Conserved elements in the 3′ untranslated region of flavivirus RNAs and potential cyclization sequences. J. Mol. Biol. 19833-41. [DOI] [PubMed] [Google Scholar]
- 10.Katoh, K., K. Kuma, H. Toh, and T. Miyata. 2005. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 33511-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinney, R. M., S. Butrapet, G. J. Chang, K. R. Tsuchiya, J. T. Roehrig, N. Bhamarapravati, and D. J. Gubler. 1997. Construction of infectious cDNA clones for dengue 2 virus: strain 16681 and its attenuated vaccine derivative, strain PDK-53. Virology 230300-308. [DOI] [PubMed] [Google Scholar]
- 12.Mathews, D. H., J. Sabina, M. Zuker, and D. H. Turner. 1999. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol. 288911-940. [DOI] [PubMed] [Google Scholar]
- 13.Stanley, J., and S. Vassilenko. 1978. A different approach to RNA sequencing. Nature 27487-89. [DOI] [PubMed] [Google Scholar]
- 14.Su, L., J. T. Radek, L. A. Labeots, K. Hallenga, P. Hermanto, H. Chen, S. Nakagawa, M. Zhao, S. Kates, and M. A. Weiss. 1997. An RNA enhancer in a phage transcriptional antitermination complex functions as a structural switch. Genes Dev. 112214-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 224673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thurner, C., C. Witwer, I. L. Hofacker, and P. F. Stadler. 2004. Conserved RNA secondary structures in Flaviviridae genomes. J. Gen. Virol. 851113-1124. [DOI] [PubMed] [Google Scholar]
- 17.Vlassov, V. V., G. Zuber, B. Felden, J. P. Behr, and R. Giege. 1995. Cleavage of tRNA with imidazole and spermine imidazole constructs: a new approach for probing RNA structure. Nucleic Acids Res. 233161-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.You, S., B. Falgout, L. Markoff, and R. Padmanabhan. 2001. In vitro RNA synthesis from exogenous dengue viral RNA templates requires long range interactions between 5′- and 3′-terminal regions that influence RNA structure. J. Biol. Chem. 27615581-15591. [DOI] [PubMed] [Google Scholar]
- 19.You, S., and R. Padmanabhan. 1999. A novel in vitro replication system for dengue virus: initiation of RNA synthesis at the 3′-end of exogenous viral RNA templates requires 5′- and 3′-terminal complementary sequence motifs of the viral RNA. J. Biol. Chem. 27433714-33722. [DOI] [PubMed] [Google Scholar]
- 20.Zhang, B., H. Dong, D. A. Stein, P. L. Iversen, and P. Y. Shi. 2008. West Nile virus genome cyclization and RNA replication require two pairs of long-distance RNA interactions. Virology 3731-13. [DOI] [PubMed] [Google Scholar]
- 21.Zhang, B., H. Dong, D. A. Stein, and P. Y. Shi. 2008. Co-selection of West Nile virus nucleotides that confer resistance to an antisense oligomer while maintaining long-distance RNA/RNA base pairings. Virology 38298-106. [DOI] [PMC free article] [PubMed] [Google Scholar]