Abstract
Herpes simplex virus 2 (HSV-2) and, to a lesser extent, HSV-1 cause the majority of sexually transmitted genital ulcerative disease. No effective prophylactic vaccine is currently available. Replication-defective HSV stimulates immune responses in animals but produces no progeny virus, making it potentially useful as a safe form of live vaccine against HSV. Because it does not replicate and spread in the host, however, replication-defective virus may have relatively limited capacity to solicit professional antigen presentation. We previously demonstrated that in mice devoid of B7-1 and B7-2 costimulation molecules, replication-defective HSV-2 encoding B7-1 or B7-2 induces stronger immune responses and protection against HSV-2 challenge than immunization with replication-defective virus alone. Here, we vaccinated wild-type mice fully competent to express endogenous B7 costimulation molecules with replication-defective HSV-2 or replication-defective virus encoding B7-2 and compared their capacities to protect against vaginal HSV-2 infection and disease. Replication-defective virus encoding B7-2 induced more IFN-γ-producing CD4 T cells than did replication-defective virus alone. Immunization with B7-2-expressing virus decreased challenge virus replication in the vaginal mucosa, genital and neurological disease, and mortality more effectively than did immunization with the parental replication-defective virus. Prior immunization with B7-expressing, replication-defective virus also effectively suppressed infection of the nervous system compared to immunization with the parental virus. Thus, B7 costimulation molecules expressed at the site of HSV infection can enhance vaccine efficacy even in a fully immunocompetent host.
Herpes simplex virus (HSV) types 1 and 2 perpetrate most genital ulcerative disease. Approximately 17% of individuals in the United States (39) and up to 75% worldwide (13, 25) are infected with HSV-2. HSV-2 infects primarily the genital epithelium, where foci of replication cause vesicles to form and ulcerate. The virus also rapidly ascends sensory nerve fibers terminating in the mucosa and enters a latent state in the sensory nerve ganglia, from which it periodically reactivates and travels within axons back to the mucosal epithelium to cause asymptomatic shedding or recurrent disease (15). HSV-2 infections typically are sexually transmitted, but babies may also become infected when born to women who experience peripartum primary or recurrent infection. In newborns, HSV can widely disseminate, causing sometimes fatal disease and leaving survivors with long-term sequelae. Vaccines to prevent or treat HSV-2 infections and alleviate disease burden have been sought for decades. One adjuvanted preparation of gD2 glycoprotein has shown some promise, but its efficacy is limited to HSV-seronegative women (31). Methods to improve current vaccines under development or new approaches that combine safety with superior efficacy are needed.
Induction of immune responses to virus or antiviral vaccine normally requires a signal delivered to T cells by costimulation molecules whose expression is limited to so-called professional antigen-presenting cells (APC). Costimulation molecules B7-1 and B7-2 (encoded by CD80 and CD86, respectively) activate T cells by ligation of CD28, adding a second signal to the essential first signal provided by T-cell receptor binding to a specific antigen-MHC complex (20). APCs constitutively express low levels of B7-2, and B7-2 is upregulated upon host contact with a pathogen (1, 5, 12). Pathogen exposure also induces expression of B7-1 (1, 12), but with slower kinetics (6, 11, 16). B7-1- and B7-2-mediated costimulation significantly enhances cytokine production, proliferation, cytotoxicity, and antibody production (10, 28, 29, 30). While induction of T-cell responses to virus infections does not absolutely depend on B7 costimulation, CD28-B7 interactions markedly augment T-cell activation (19, 29, 32, 34, 38), with significant consequences for the host. For example, HSV-2 causes more severe genital and neurologic disease and higher mortality in mice lacking both B7-1 and B7-2 costimulation molecules (B7KO) than in wild-type mice (33), indicating that B7-1 and B7-2 costimulation molecules enhance the generation of anti-HSV immune responses which resolve the infection.
If B7 costimulation plays a pivotal role in expanding nascent antiviral immune responses, then development of optimal immunity following vaccination also likely requires ample B7-mediated costimulation. Live attenuated vaccines may effectively stimulate expression of costimulation molecules and vigorous immunity because they closely mimic natural infection, but their safety in the case of HSV is suspect. Replication-defective viruses may satisfy the need for a safe and effective alternative to live, attenuated virus vaccines (4). Immunization of mice with a replication-defective (ICP8−) HSV-2 reduces primary replication of challenge virus and development of genital disease (22). However, relatively few APC are likely to contact and become activated by nonreplicating virus as a consequence of immunization (41), which may limit costimulation and, hence, the induction of immune responses to the vaccine.
Expression of B7-1 and/or B7-2 by transfection or infection with B7-encoding recombinant virus can remodel infected cells into cells with full antigen-presenting capacity (8, 17, 26, 37). We recently constructed replication-defective (ICP8−) HSV-2 strains which encode murine B7-1 or B7-2 to determine whether expression of B7 costimulation molecules could augment vaccine-induced immune responses to HSV-2 itself (35). In B7KO mice, immunization with B7-1- or B7-2-expressing virus slightly increased the numbers of IFN-γ-secreting cells in draining lymph nodes and markedly elevated HSV-specific antibody titers compared with mice immunized with the parental replication-defective virus. Replication of HSV-2 in the vaginal epithelium, genital inflammation, weight loss, neurologic disease, and mortality were all reduced in mice immunized with replication-defective virus encoding B7-1 or B7-2 compared to those immunized with the replication-defective vaccine prototype. These findings in mice lacking endogenous B7 costimulation molecules unequivocally indicated that the enhanced immunogenicity and protection from disease resulted from expression of B7 costimulation molecules encoded by the replication-defective virus. For purposes of an antiviral vaccine, however, the benefits of expressing exogenous B7 costimulation to reduce genital and neurological HSV-2 infection and pathology must be demonstrated in a normal, immunocompetent host. Therefore, we determined whether B7-2-expressing, replication-defective virus would provide greater protective efficacy against HSV-2 genital infection than would the replication-defective parental strain in wild-type (BALB/c) mice.
MATERIALS AND METHODS
Cells and viruses.
The replication-defective mutant of HSV-2 strain 186, 5BlacZ, does not produce the essential viral gene product ICP8 due to insertion of the lacZ gene into the UL29 open reading frame (3). 5BlacZ was further mutated to contain the murine B7-2 (CD86) open reading frame driven by the human cytomegalovirus immediate-early enhancer/promoter, which was inserted into a KpnI-KpnI deletion in the thymidine kinase gene, creating 5B86 (35). 5BlacZ and 5B86 were propagated in S2 cells, a Vero cell line stably expressing ICP8 upon infection (7). HSV-2 strain G-6 (24), a plaque-purified derivative of strain G, was propagated in Vero cells. Virus used for immunizations was produced free of cell debris by isolation from the supernatant of infected cell monolayers, using high-speed centrifugation as previously described (23). Virus titers were determined on S2 or Vero cells by standard plaque assay (14).
Immunization of mice.
Female BALB/c mice were purchased from the National Cancer Institute. Mice were housed at Saint Louis University under specific-pathogen-free conditions in accordance with institutional and federal guidelines. All mice were used at 6 weeks of age. For immunization, hind flanks of the mice were injected subcutaneously (s.c.) with 1 × 106 PFU (high), 2 × 105 PFU (medium), or 4 × 104 PFU (low) doses of virus suspended in a 40-μl total volume of normal saline. Some mice received an equivalent amount of supernatant concentrated from uninfected cell cultures as a negative control for immunization.
T-cell assays.
For assessment of T-cell responses to immunization, inguinal and paraaortic lymph nodes were harvested from mice 5 days after immunization with 4 × 104 PFU 5BlacZ, 5B86, or control supernatant. Single-cell suspensions were prepared and stimulated for 4 h in the presence of phorbol myristate acetate (PMA; 50 ng/ml), calcium ionophore A23187 (1 μg/ml), and GolgiStop (0.67 μl/ml; Pharmingen). Cell surface staining was performed using anti-CD3-PerCP Cy5.5 (1:160), anti-CD4-Pacific Blue (1:300), and anti-CD8-Alexa Fluor 700 (1:80) (all from Pharmingen). A cytostain kit (Pharmingen) was used according to the manufacturer's instructions to perform intracellular staining. Anti-IFN-γ-PE (Pharmingen) was used at a 1:80 dilution, and staining was visualized by flow cytofluorometric analysis on a FACSCalibur cytometer. Fifty thousand to 100,000 cells in the lymphocyte gate were analyzed for each sample by using CellQuest software (Becton Dickinson). The percentage of CD4+IFN-γ+ or CD8+IFN-γ+ cells from unstimulated samples was subtracted from the percentage in PMA-stimulated samples, and the difference was multiplied by the total number of lymph node cells recovered to yield the number of CD4+IFN-γ+ or CD8+IFN-γ+ cells. To assess memory CD4 T-cell responses, functional HSV-specific T cells were enumerated by IFN-γ enzyme-linked immunospot (ELISPOT) assay. The immunodominant HSV-2 peptide gD245-259 (2) was used as a CD4 T-cell stimulus. Mononuclear cells were isolated from the spleen 1 month after s.c. immunization. Splenocytes (1 × 106 or 3 × 105 cells per well) were cultured in the presence of 2.5 μM gD peptide for 20 h on multiscreen-HA plates (Millipore) coated with anti-IFN-γ capture antibody. UV-inactivated HSV-2 was added to some wells at a multiplicity of infection of 0.3 (prior to inactivation). Spots were visualized with anti-IFN-γ detection antibody, followed by streptavidin-alkaline phosphatase and BCIP (5-bromo-4-chloro-3-indolylphosphate)-nitroblue tetrazolium substrate and quantitated using an Immunospot plate reader (version 5.0; Cellular Technology, Ltd.).
Quantitation of serum antibodies.
To determine the titer of HSV-specific serum antibodies induced by vaccination, mice were unimmunized or immunized with 5BlacZ, 5B86, or control supernatant. Blood samples were collected from the tail vein of mice 22 days after immunization. Serum was prepared by clot retraction and analyzed by enzyme-linked immunosorbent assay (ELISA) as previously described (22). Anti-mouse immunoglobulin G (IgG) biotin (R & D Systems, Minneapolis, MN) was used as secondary antibody and detected using streptavidin-horseradish peroxide followed by O-phenylenediamine dihydrochloride substrate (Sigma, St. Louis, MO). Plates were read at 490 nm on a Bio-Rad 680 plate reader. Antibody titers were determined by comparison to standard curves generated with serum containing known concentrations of IgG captured on plates coated with goat-anti-kappa light-chain antibody (Caltag).
In vivo challenge.
Mice were challenged with virulent HSV-2 4 weeks after immunization. At 7 days and 1 day prior to challenge, mice were injected s.c. in the neck ruff with 3 mg Depo-Provera (Pharmacia & Upjohn) suspended in a 100-μl volume of normal saline. For challenge, mice were anesthetized by intraperitoneal injection of ketamine/xylazine and infected by intravaginal (i.vag.) inoculation of 5 × 105 PFU G-6 in a 5-μl volume. To measure virus replication in the genital mucosa, vaginal vaults were swabbed twice with calcium alginate swabs at 9 h and at 1 to 5 days postinfection. Duplicate swabs for each time point were placed together in 1 ml phosphate-buffered saline and stored frozen until use. Virus was quantified on Vero cell monolayers by standard plaque assay. After challenge, body weight, signs of disease, and survival were monitored on a daily basis. Mice were weighed individually, and the mean change from initial body weight was calculated daily for each group. Disease scores were assigned in a blinded fashion based on the following scale: 0, no apparent signs of disease; 1, slight erythema and edema of the external genitals; 2, prominent erythema and edema of the genitals; and 3, severe erythema and edema with lesions on the genitals. The mean daily disease score was calculated for each group. Hind limb paralysis was also assessed. Virus replication in neural tissues was analyzed by dissection of brains, brainstems, and spinal cords from a cohort of mice 5 days after challenge. Tissues were stored frozen until use. For virus titer determination, the tissues were thawed and disrupted using a mini-bead beater (BioSpec, Inc.) and then diluted for standard plaque assay.
Statistics.
Significance of difference in virus or antibody titers on individual days was determined by Student's t test, as was the difference in number of IFN-γ-producing T cells. Proportions of mice with hind-limb paralysis or surviving infection were compared using the Fisher exact method. The Kruskal-Wallis nonparametric test was used to assess the significance of difference in disease scores on individual days postchallenge.
RESULTS
The potential of B7-2-expressing, replication-defective HSV-2 as an HSV vaccine must be tested in a normal environment containing endogenous B7 costimulation molecules. We therefore immunized immunocompetent BALB/c mice with the B7-2-expressing virus 5B86 or the parental replication-defective virus 5BlacZ and assessed immune responses elicited by immunization and the efficacy of these responses in protecting against HSV-2 challenge.
Virus-encoded expression of B7-2 moderately enhances immune responses to HSV-2.
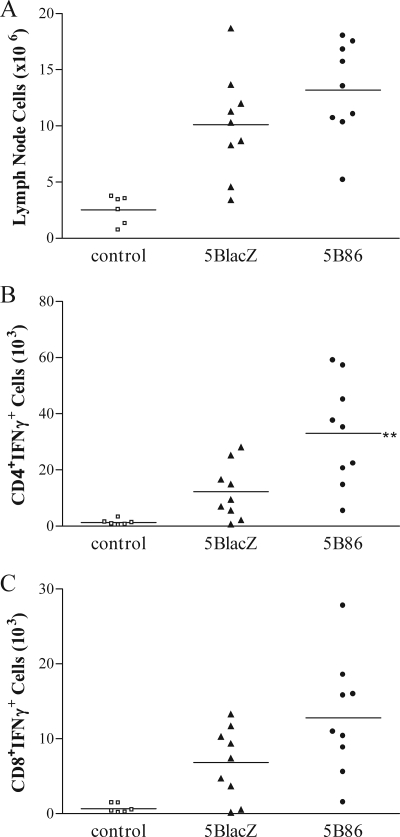
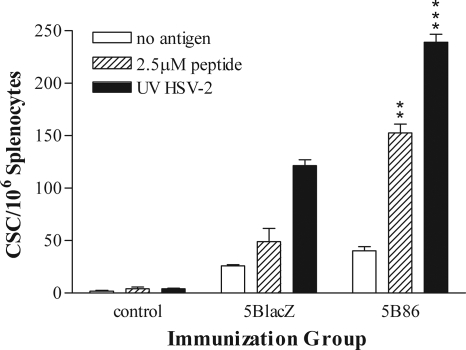
T cells activated in response to immunization were enumerated among mononuclear cells isolated from the draining lymph nodes 5 days after s.c. immunization in the hind flank. Cells stimulated with PMA and calcium ionophore were stained for intracellular IFN-γ. 5B86 caused fivefold more cells to accumulate in the draining lymph nodes than did control immunizations with uninfected cell supernatant, whereas 5BlacZ induced a fourfold expansion (Fig. 1A). Immunization with 5B86 stimulated significantly more activated, IFN-γ-producing CD4 T cells than did 5BlacZ (P = 0.0096) (Fig. 1B). More activated, IFN-γ-producing CD8 T cells were also found after immunization with 5B86 than with 5BlacZ, though the difference was not statistically significant (P = 0.0668) (Fig. 1C). To determine whether a more robust HSV-specific T-cell response persists to the time of challenge in mice immunized with 5B86 than in those immunized with 5BlacZ, splenocytes were isolated 1 month after immunization and assessed by an IFN-γ ELISPOT assay. Splenocytes from mice immunized with 5B86 yielded many more spot-forming cells when stimulated with the immunodominant gD245-259 peptide (2) than did cells from mice immunized with 5BlacZ (Fig. 2). More memory CD4 T cells from 5B86-immunized mice also produced IFN-γ in response to UV-inactivated HSV-2, indicating that the results using an immunodominant peptide were representative of the response to whole virus. Thus, a stronger memory CD4 T-cell response was maintained after immunization with B7-2-expressing virus than with replication-defective virus alone.
FIG. 1.
IFN-γ-producing T cells induced by immunization. Groups of BALB/c mice were immunized with 4 × 104 PFU of 5BlacZ, 5B86, or an equivalent amount of control supernatant. After 5 days, mononuclear cells isolated from the draining lymph nodes of individual mice were stimulated for 4 h with PMA and calcium ionophore in the presence of GolgiStop. Cells were simultaneously stained for expression of surface markers CD3, CD4, and CD8, then fixed and permeabilized, stained for intracellular IFN-γ, and analyzed by flow cytometry. Gates were set on activated CD3-expressing cells, and the numbers of CD4+IFN-γ+ (B) and CD8+IFN-γ+ (C) cells were determined by multiplying the percentage of positive cells by the total number of lymphocytes recovered (A). Data represent the number of T cells from six to nine individual mice compiled from three independent experiments, with the mean for each group shown as a horizontal bar. **, P value of 0.0096 for 5B86 compared with 5BlacZ.
FIG. 2.
Maintenance of HSV-specific, memory CD4 T cells. Groups of BALB/c mice were immunized with 2 × 105 PFU of 5BlacZ, 5B86, or an equivalent amount of control supernatant. After 1 month, splenocytes were cultured with or without HSV-2 gD245-259 peptide or UV-inactivated HSV-2 (UV HSV-2) for 20 h on plates coated with anti-IFN-γ antibody. Cytokine-secreting cells (CSC) were then enumerated by an ELISPOT assay. Data represent the mean ± the standard error of the mean (SEM) of triplicate wells. The experiment was repeated once. **, P value of 0.0024; ***, P value of 0.0002 for 5B86 compared with 5BlacZ.
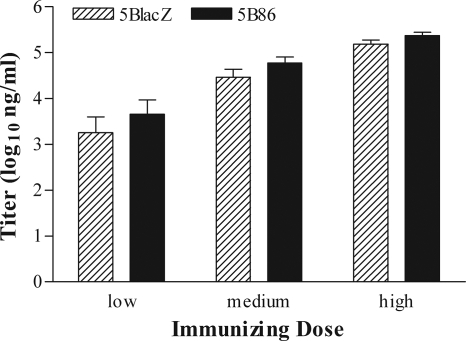
The capacity of 5B86 to augment HSV-specific antibody responses was assessed by ELISA using serum samples collected from immunized mice 22 days after immunization. Mice immunized with 5BlacZ produced HSV-specific IgG in a dose-dependent fashion (Fig. 3). Immunization with 5B86 induced slightly more antibody production at each dose level, although the differences with 5BlacZ did not achieve statistical significance. Thus, replication-defective HSV-2 encoding B7-2 causes the expansion of greater numbers of IFN-γ-producing T cells in an immunocompetent host than does replication-defective virus alone, but yields similar peak titers of HSV-specific serum IgG.
FIG. 3.
HSV-specific antibody in the sera of immunized mice. Blood samples were collected 22 days after immunization of mice receiving low, medium, or high doses of 5BlacZ or 5B86. Titer of HSV-specific IgG was determined by ELISA. Data represent the geometric mean ± SEM of 15 samples compiled from three independent experiments.
B7-expressing virus improves protection from challenge.
For vaccine efficacy studies, groups of mice were immunized with incremental doses (1 × 106 PFU [high], 2 × 105 PFU [medium], or 4 × 104 PFU [low]) of 5BlacZ or 5B86. An additional group received control supernatant in an amount equivalent to the high dose of virus as a negative control for protection. All mice were challenged 1 month later with a virulent heterologous strain of HSV-2, and the protective capacity of 5B86 vaccination compared with that of 5BlacZ vaccination was assessed using several parameters.
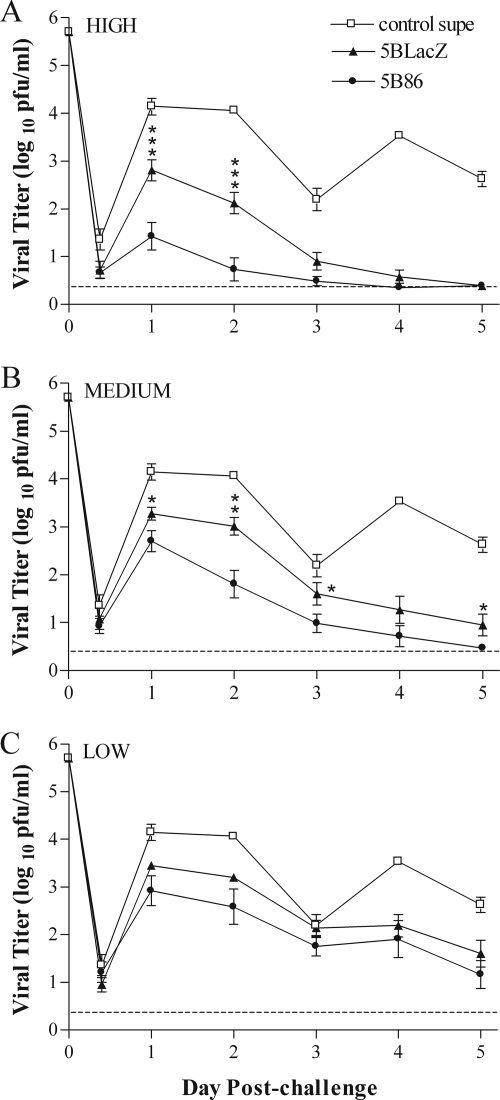
Replication of challenge virus in the vaginal mucosa was determined over the first 5 days postinfection by an assay of material collected on mucosal swabs (Fig. 4). Mice immunized with control supernatant showed high levels of replication during the first 2 days postchallenge. The titer of virus shed from the mucosa of these mice declined on day 3 postinfection, as is typically observed (21, 35), but then rebounded to high levels 4 and 5 days postchallenge (Fig. 4). As expected, mice immunized with either replication-defective virus shed significantly less challenge virus at all time points than did control-immunized mice, and this reduction was dose dependent. In mice immunized with the high dose of 5BlacZ, virus shedding was eliminated by day 5 postchallenge (Fig. 4A). However, immunization of mice with 5B86 dramatically reduced replication of challenge virus in the mucosa over the first 2 days postchallenge. Titers were more than 1,000-fold lower than those in mice immunized with control supernatant, and elimination of detectable virus occurred by day 3 postchallenge (Fig. 4A). Although immunization with the medium or low dose of 5BlacZ reduced challenge virus replication in the mucosa compared to immunization with control supernatant (Fig. 4B), immunization with B7-2-expressing virus resulted in still-lower titers than those in 5BlacZ-immunized mice. The low immunization dose of 5BlacZ yielded consistent reductions of 5- to 22-fold in challenge virus titers compared to immunization with control supernatant, except at day 3 postchallenge (Fig. 4C), and titers in mice immunized with low doses of 5B86 were two- to fourfold lower still. Importantly, 5B86 immunization inhibited challenge virus replication more effectively than 5BlacZ at every dose, virtually eliminating replication of HSV-2 in mice receiving the highest immunization dose (Fig. 4A). Thus, provision of B7-2 by the vaccine virus in an immunocompetent host positively impacts protection from initial replication of HSV-2 in the genital mucosa.
FIG. 4.
Titers of challenge virus shed from the genital mucosa. Groups of BALB/c mice were immunized with high (A), medium (B), or low (C) doses of supernatant-derived 5BlacZ or 5B86. Data for a group of mice immunized with control supernatant (control supe) in an amount equivalent to the high dose of virus are shown on all three graphs for comparison. All mice were challenged 1 month after immunization by i.vag. infection with HSV-2 strain G-6. Titers of virus collected by vaginal swab at the indicated times postinfection were determined by standard plaque assay. Data represent the geometric mean ± SEM for 10 samples compiled from two independent experiments. *, P value between 0.0495 and 0.0381; **, P value of 0.0022; ***, P value between 0.0013 and 0.0005 for 5B86 compared with 5BlacZ.
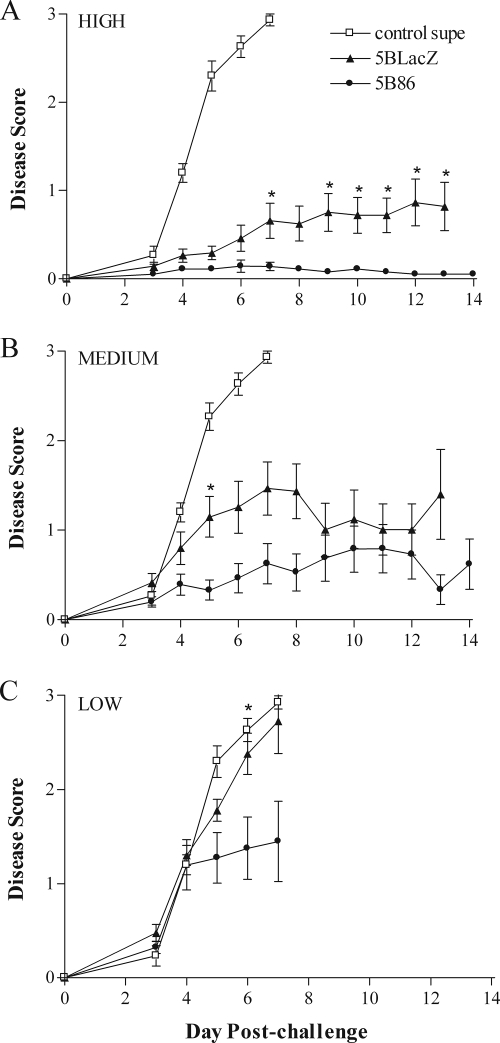
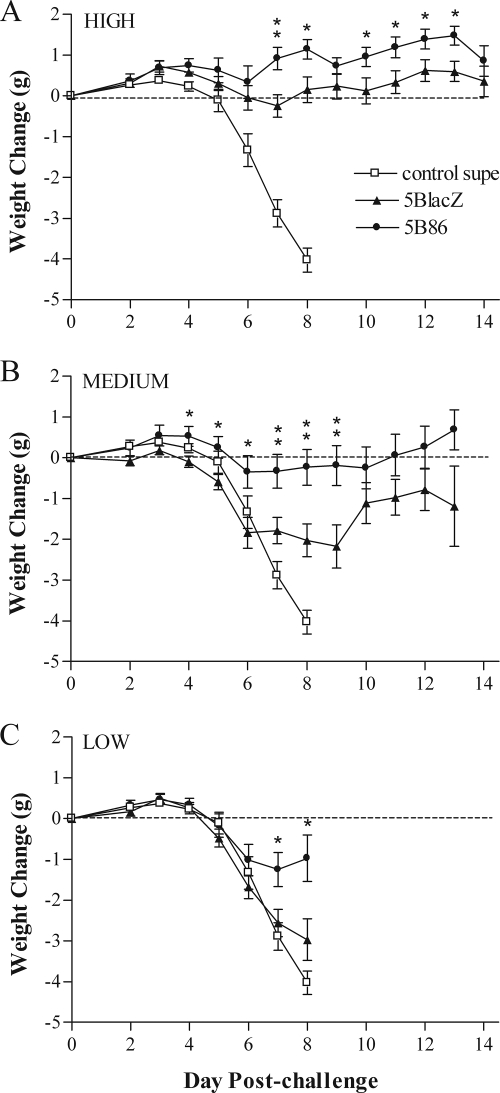
The protective effect of immunization on disease induced by challenge infection with HSV-2 was assessed in three ways. First, inflammation of the external genitalia was monitored daily postchallenge (Fig. 5). Mice immunized with control supernatant rapidly showed signs of inflammation that worsened to lesion development by days 5 to 6 postchallenge. In stark contrast, mice immunized with the high dose of 5BlacZ showed only mild inflammation of the external genitalia, and mice immunized with 5B86 showed virtually no signs of inflammation (Fig. 5A). The protective effect of vaccination waned with decreasing doses of immunizing virus. The medium immunizing dose of either 5BlacZ or 5B86 still significantly ameliorated inflammatory signs compared with those in control-immunized mice (Fig. 5B), but protection against genital inflammation and lesion formation was lost with low-dose immunization with 5BlacZ (Fig. 5C). Significantly, prior immunization with any dose of 5B86 left mice much better protected from inflammation of the external genitalia than did 5BlacZ. In addition, even the low dose of 5B86 afforded substantial protection, and very few mice immunized with low-dose 5B86 developed external genital lesions (Fig. 5C) (5/20 for 5B86 versus 13/20 for 5BlacZ; P = 0.0248). As a second measure of protection from disease, body weight was monitored daily postchallenge (Fig. 6). Whereas mice immunized with control supernatant rapidly lost weight beginning on day 5 postchallenge, mice immunized with the high dose of 5BlacZ maintained their body weight, and 5B86-immunized mice gained weight over the same period postchallenge (Fig. 6A). Maintenance of body weight depended on the immunization dose. Mice immunized with medium or low doses of 5BlacZ lost weight at a rate closely resembling that of control-immunized mice (Fig. 6B and C). In contrast, mice immunized with the medium dose of 5B86 maintained their weight postchallenge (Fig. 6B), and those immunized with the low dose lost an average of only 1 g over days 5 to 8 postchallenge (Fig. 6C). As a third method of disease assessment, mice were observed for signs of hind-limb paralysis. The incidence of paralysis among mice receiving the low dose of 5BlacZ was nearly as great as the 80% of control mice that became paralyzed (Table 1). In contrast, only 25% of mice immunized with the low dose of 5B86 developed paralysis (P = 0.0248, compared with 5BlacZ). Thus, by three measures of local and systemic health, 5B86 provided significantly better protection from challenge than did 5BlacZ.
FIG. 5.
Genital inflammation and disease after i.vag. challenge of immunized mice. Mice immunized and challenged as described in the legend to Fig. 4 were observed daily for signs of inflammation and lesions on their external genitalia. Mice were immunized with high (A), medium (B), or low (C) doses of 5BlacZ or 5B86 or an amount of control supernatant corresponding to the high dose of virus. Data represent the arithmetic mean ± SEM for all samples compiled from four independent experiments (n = 15 for control; n = 20 for 5BlacZ and 5B86). *, P value of ≤0.027 for 5B86 compared with 5BlacZ.
FIG. 6.
Body weights of immunized mice after i.vag. challenge with HSV-2. Mice immunized and challenged as described in the legend to Fig. 4 were monitored daily for change in weight. Mice were immunized with high (A), medium (B), or low (C) doses of 5BlacZ or 5B86 or an amount of control supernatant (control supe) corresponding to the high dose of virus. Data represent the arithmetic mean ± SEM for all samples compiled from four independent experiments (n = 15 for control; n = 20 for 5BlacZ and 5B86). *, P value between 0.045 and 0.0107; **, P value between 0.0093 and 0.0047 for 5B86 compared with 5BlacZ.
TABLE 1.
Incidence of hind-limb paralysisa
| Immunization type | Proportion paralyzed (%) | Mean no. of days to onset ± SEM |
|---|---|---|
| Control supernatant | 12/15 (80) | 7.17 ± 0.94 |
| 5BlacZ | 13/20 (65) | 6.85 ± 0.69 |
| 5B86 | 5/20 (25)b | 6.80 ± 0.84 |
Data from mice immunized with the low dose and challenged 1 month later.
P = 0.0248, compared with immunization with 5BlacZ.
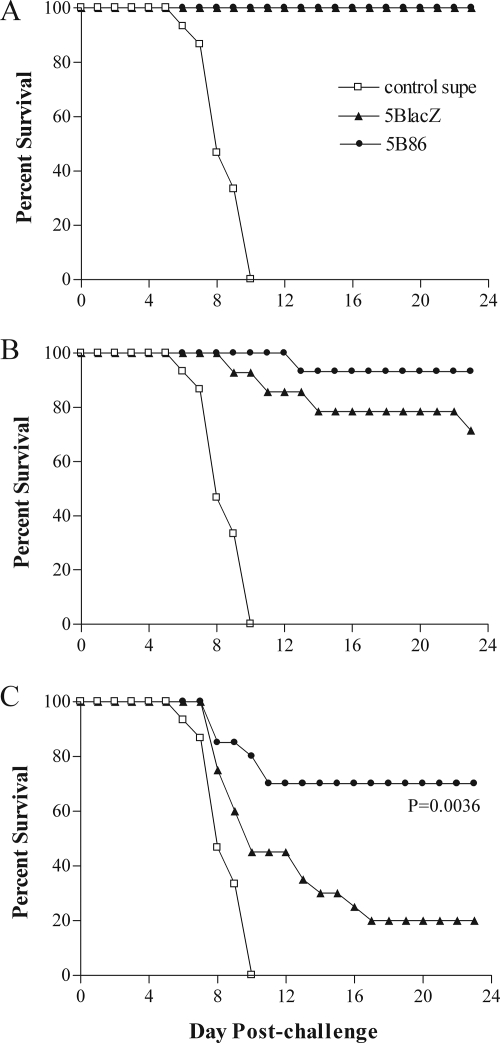
Immunization of mice with 5BlacZ or 5B86 also had an impact on survival rate after challenge with virulent HSV-2 (Fig. 7). Mice receiving the control immunization began to die as early as 6 days postchallenge, and all had succumbed to infection by 10 days postchallenge. In sharp contrast, all mice immunized with the high dose of 5BlacZ or 5B86 survived (Fig. 7A). The medium immunizing dose of either virus was not universally effective in preventing lethal infection (Fig. 7B) but was still markedly more protective than control immunization. Among mice receiving the low immunization dose, only one-third of those immunized with 5BlacZ survived challenge virus infection, whereas almost 80% of 5B86-immunized mice survived (Fig. 7C).
FIG. 7.
Survival of immunized mice after i.vag. challenge with HSV-2. Mice immunized and challenged as described in the legend to Fig. 3 were monitored daily for survival. Mice were immunized with high (A), medium (B), or low (C) doses of 5BlacZ or 5B86 or an amount of control supernatant (control supe) corresponding to the high dose of virus. Data represent the percentage of surviving mice out of 15 to 20 mice from four independent experiments. *, P value of 0.0036 for 5B86 compared with 5BlacZ.
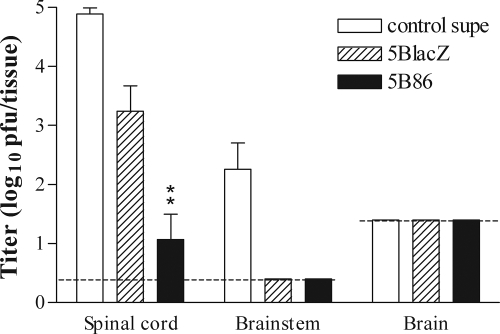
An important aspect of HSV pathogenesis is the establishment of infection in the nervous system. An efficacious vaccine must therefore be capable of restricting HSV-2 access to sensory neurons. To determine whether B7-1 or B7-2 expression by the vaccine virus enhances protection of the nervous system compared with that of the replication-defective parental virus, we examined acute and latent infection of the nervous system. We attempted to quantify latent viral genomes in the dorsal root ganglia 2 months after challenge of mice previously immunized with the medium dose of 5BlacZ or 5B86. However, we were unable to reproducibly detect quantifiable levels of viral DNA by using a real-time PCR assay (J. E. Schrimpf and L. A. Morrison, unpublished data). To determine whether increased survival in mice immunized with 5B86 could be related to better vaccine-mediated protection of the nervous system, we immunized groups of mice with the low dose of 5BlacZ, 5B86, or control supernatant and determined virus titers in regions of the central nervous system (CNS) 5 days postchallenge, a time when CNS infection in control animals is typically robust. Spinal cords and brainstems of mice immunized with control supernatant contained large quantities of challenge virus (Fig. 8). Spinal cords of mice immunized with 5BlacZ yielded significantly smaller amounts challenge virus than did those of control mice (P = 0.0038). However, challenge virus could barely be detected in the spinal cords of mice previously immunized with 5B86 (P value of <0.0001 for 5B86-immunized mice versus control mice; P value of 0.0049 for 5B86-immunized mice versus 5BlacZ-immunized mice), and virus was not found in the brainstem (Fig. 8) (P value of 0.002 for 5B86-immunized mice versus control mice). These results indicate that prior immunization with 5B86 significantly improved protection of the nervous system from acute infection by HSV-2. Thus, we conclude that immunization of mice with 5B86 significantly reduced infection of the nervous system after challenge compared with that with 5BlacZ, at least as determined by measures of acute infection, namely the incidence of hind-limb paralysis and the level of replicating virus in neural tissue.
FIG. 8.
Levels of challenge virus replication in the nervous system. Mice were immunized with the low dose of 5BlacZ or 5B86 or with control supernatant and challenged i.vag. with HSV-2 1 month later. After 5 days, mice were sacrificed, the indicated regions of the CNS were dissected and homogenized, and the virus titer in them was determined by standard plaque assay. Data represent the geometric mean ± SEM for six samples per group. **, P value of 0.0049 for 5B86 compared with 5BlacZ.
DISCUSSION
We have demonstrated that replication-defective HSV-2 encoding within its genome murine B7-2 costimulation molecules can enhance the immunogenicity and protective capacity of a replication-defective virus vaccine, even in a B7-sufficient host environment. Specifically, immunization with B7-2-expressing virus 5B86 stimulated more IFN-γ-producing T cells than did immunization with 5BlacZ. The enhanced cellular immune response correlated with lower rates of HSV-2 shedding from the vaginal mucosa and reduced genital inflammation. The capacity to protect the nervous system is most noteworthy as a desirable characteristic of vaccine-mediated protection against sexually transmitted herpesviral disease, and we observed nearly complete protection of the CNS from acute infection after genital challenge of immunized mice. We were unable to accurately assess latent genome loads in dorsal root ganglia, but genome loads of challenge virus in immunized immunodeficient mice are very low (24) and may be even lower in the fully immunocompetent mice used in this study.
We had previously demonstrated that 5B86 and a B7-1-expressing replication-defective virus, 5B80, could partially restore the ability to develop immune responses and to fend off HSV-2 challenge in mice lacking both B7-1 and B7-2. We have now extended these findings to the fully immunocompetent host. It was not certain whether mice with a normal ability to upregulate endogenous costimulation molecules in response to replication-defective virus would benefit from the additional exogenous expression of B7-2. Graded doses of vaccine demonstrated that vaccine virus-encoded B7 molecules could indeed enhance protection against the homologous pathogen over what was achieved with replication-defective vaccine alone, particularly at low immunizing doses where the total amount of viral antigen and/or vaccine virus-infected cells may otherwise be limiting. The mechanism underlying enhanced protective immune responses may involve B7-2 expression on nonprofessional APCs or increased B7-2 expression on infected professional APCs. Further research will be required to resolve this question.
We do not yet know the role of individual immune effectors stimulated by B7-expressing vaccine in providing the protection from challenge that we observed. Interestingly, virus-encoded B7-2 expression in wild-type mice significantly improved CD4 T-cell but not antibody responses to HSV. The greatest impact on the replication of HSV-2 in the vaginal mucosa, particularly over the first 2 days postchallenge, was observed in mice receiving the high immunizing dose of 5B86. Previous work with replication-defective HSV-2 indicated that immune antibody and CD4 T cells work synergistically to protect the genital tract from HSV-2 replication during this period but are not independently effective (21, 24). Thus, improved CD4 T-cell responses in 5B86-immunized mice may assist antibody to better control early replication of challenge virus in the vaginal mucosa. CD4 T cells and antibody stimulated by replication-defective virus also work cooperatively to ameliorate genital and neurological disease (24). The lowest immunizing dose of 5B86 stimulated an immune response that did not reduce challenge virus replication in the mucosa significantly more than did that of 5BlacZ but, nonetheless, greatly limited genital and neurological disease compared with 5BlacZ. Once again, synergy between immune antibody and enhanced CD4 T-cell responses in 5B86-immunized mice may explain the increased protection we observed. Studies utilizing serum transfer and T-cell depletion will be needed to yield a more precise understanding of the underlying immune basis for protection afforded by vaccination with virus expressing B7-2.
Most viruses engineered to express B7 molecules have been used to enhance responses to coexpressed heterologous antigens such as tumor-specific epitopes (18, 27, 36, 40). We have notably extended this work by demonstrating that virus-encoded B7 costimulation molecules enhance protective immune responses to the homologous viruses. Although we had previously constructed both B7-1- and B7-2-expressing, replication-defective viruses, we chose the virus expressing B7-2 for further study because our previous work had revealed its slightly greater capacity to curtail challenge virus replication in the genital mucosa and protect against genital inflammation in B7KO mice (35). Thus, it appeared most likely to possess the qualities desirable in an effective vaccine against HSV-2. It is possible, however, that a B7-1-expressing virus may prove more efficacious in protecting against HSV infections at another site, for example the eye. The B7 family of costimulation molecules has expanded in recent years with the discovery of additional members (9). Some of these other costimulation molecules, encoded within virus genomes, may modulate additional aspects of the nascent or memory antiviral response, permitting a vaccine to be tailored to the unique requirements for protection against individual viral pathogens.
Acknowledgments
This work was supported by award VRD-15/181/414 from the World Health Organization and by institutional funds.
We thank Hong Wang for expert technical assistance. Helpful discussions with Keril Blight, Mike Diamond, Robyn Klein, David Leib, Paul Olivo, Andy Pekosz, Pat Stuart, David Wang, Dong Yu, and members of their laboratories are greatly appreciated.
Footnotes
Published ahead of print on 5 November 2008.
REFERENCES
- 1.Bertram, E. M., W. Dawicki, and T. H. Watts. 2004. Role of T cell costimulation in anti-viral immunity. Semin. Immunol. 16185-196. [DOI] [PubMed] [Google Scholar]
- 2.Cooper, D., J. C. Mester, M. Guo, F. Nasar, V. Souza, S. Dispoto, M. Sidhu, M. Hagen, J. H. Eldridge, R. J. Natuk, and M. W. Pride. 2006. Epitope mapping of full-length glycoprotein D from HSV-2 reveals a novel CD4+ CTL epitope located at the transmembrane-cytoplasmic junction. Cell Immunol. 239113-120. [DOI] [PubMed] [Google Scholar]
- 3.Da Costa, X. J., N. Bourne, L. R. Stanberry, and D. M. Knipe. 1997. Construction and characterization of a replication-defective herpes simplex virus 2 ICP8 mutant strain and its use in immunization studies in a guinea pig model of genital disease. Virology 2321-12. [DOI] [PubMed] [Google Scholar]
- 4.Dudek, T., and D. M. Knipe. 2006. Replication-defective viruses as vaccines and vaccine vectors. Virology 344230-239. [DOI] [PubMed] [Google Scholar]
- 5.Elloso, M. M., and P. Scott. 1999. Expression and contribution of B7-1 (CD80) and B7-2 (CD86) in the early immune response to Leishmania major infection. J. Immunol. 1626708-6715. [PubMed] [Google Scholar]
- 6.Freeman, G. J., G. S. Gray, C. D. Gimmi, D. B. Lombard, L. J. Zhou, M. White, J. D. Fingeroth, J. G. Gribben, and L. M. Nadler. 1991. Structure, expression, and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7. J. Exp. Med. 174625-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao, M., and D. M. Knipe. 1989. Genetic evidence for multiple nuclear functions of the herpes simplex virus ICP8 DNA-binding protein. J. Virol. 635258-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaspari, A. A., B. Ferbel, Z. Chen, F. Razvi, and R. Polakowska. 1993. Accessory and alloantigen-presenting cell functions of A431 keratinocytes that stably express the B7 antigen. Cell Immunol. 149291-302. [DOI] [PubMed] [Google Scholar]
- 9.Greenwald, R. J., G. J. Freeman, and A. H. Sharpe. 2005. The B7 family revisited. Annu. Rev. Immunol. 23515-548. [DOI] [PubMed] [Google Scholar]
- 10.Harding, F. A., and J. P. Allison. 1993. CD28-B7 interactions allow the induction of CD8+ cytotoxic T lymphocytes in the absence of exogenous help. J. Exp. Med. 1771791-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hathcock, K. S., G. Laszlo, C. Pucillo, P. Linsley, and R. J. Hodes. 1994. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J. Exp. Med. 180631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janeway, C. A., Jr., and K. Bottomly. 1994. Signals and signs for lymphocyte responses. Cell 76275-285. [DOI] [PubMed] [Google Scholar]
- 13.Kamali, A., A. J. Nunn, D. W. Mulder, E. Van Dyck, J. G. Dobbins, and J. A. Whitworth. 1999. Seroprevalence and incidence of genital ulcer infections in a rural Ugandan population. Sex. Transm. Infect. 7598-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knipe, D. M., and A. E. Spang. 1982. Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. J. Virol. 43314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koelle, D. M., and A. Wald. 2000. Herpes simplex virus: the importance of asymptomatic shedding. J. Antimicrob. Chemother. 45(Suppl. T3)1-8. [DOI] [PubMed] [Google Scholar]
- 16.Lenschow, D. J., A. I. Sperling, M. P. Cooke, G. Freeman, L. Rhee, D. C. Decker, G. Gray, L. M. Nadler, C. C. Goodnow, and J. A. Bluestone. 1994. Differential up-regulation of the B7-1 and B7-2 costimulatory molecules after Ig receptor engagement by antigen. J. Immunol. 1531990-1997. [PubMed] [Google Scholar]
- 17.Marti, W. R., D. Oertli, J. B. Meko, J. A. Norton, and K. Tsung. 1997. Induction of antigen-presenting capacity in tumor cells upon infection with nonreplicating recombinant vaccinia virus encoding murine MHC class II and costimulatory molecules. J. Immunol. Methods 200191-198. [DOI] [PubMed] [Google Scholar]
- 18.Marti, W. R., P. Zajac, G. Spagnoli, M. Heberer, and D. Oertli. 1997. Nonreplicating recombinant vaccinia virus encoding human B-7 molecules elicits effective costimulation of naive and memory CD4+ T lymphocytes in vitro. Cell. Immunol. 179146-152. [DOI] [PubMed] [Google Scholar]
- 19.McAdam, A. J., E. A. Farkash, B. E. Gewurz, and A. H. Sharpe. 2000. B7 costimulation is critical for antibody class switching and CD8+ cytotoxic T-lymphocyte generation in the host response to vesicular stomatitis virus. J. Virol. 74203-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAdam, A. J., A. N. Schweitzer, and A. H. Sharpe. 1998. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol. Rev. 165231-247. [DOI] [PubMed] [Google Scholar]
- 21.Morrison, L. A. 2008. Replication-defective virus vaccine-induced protection of mice from genital herpes simplex virus 2 requires CD4 T cells. Virology 376205-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison, L. A., X. J. Da Costa, and D. M. Knipe. 1998. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology 243178-187. [DOI] [PubMed] [Google Scholar]
- 23.Morrison, L. A., and D. M. Knipe. 1996. Mechanisms of immunization with a replication-defective mutant of herpes simplex virus 1. Virology 220402-413. [DOI] [PubMed] [Google Scholar]
- 24.Morrison, L. A., L. Zhu, and L. G. Thebeau. 2001. Vaccine-induced serum immunoglobin contributes to protection from herpes simplex virus type 2 genital infection in the presence of immune T cells. J. Virol. 751195-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obasi, A., F. Mosha, M. Quigley, Z. Sekirassa, T. Gibbs, K. Munguti, J. Todd, H. Grosskurth, P. Mayaud, J. Changalucha, D. Brown, D. Mabey, and R. Hayes. 1999. Antibody to herpes simplex virus type 2 as a marker of sexual risk behavior in rural Tanzania. J. Infect. Dis. 17916-24. [DOI] [PubMed] [Google Scholar]
- 26.Oertli, D., W. R. Marti, J. A. Norton, and K. Tsung. 1996. Non-replicating recombinant vaccinia virus encoding murine B-7 molecules elicits effective costimulation of naive CD4+ splenocytes in vitro. J. Gen. Virol. 773121-3125. [DOI] [PubMed] [Google Scholar]
- 27.Petrulio, C. A., and H. L. Kaufman. 2006. Development of the PANVAC-VF vaccine for pancreatic cancer. Expert Rev. Vaccines 59-19. [DOI] [PubMed] [Google Scholar]
- 28.Schweitzer, A. N., and A. H. Sharpe. 1998. Studies using antigen-presenting cells lacking expression of both B7-1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J. Immunol. 1612762-2771. [PubMed] [Google Scholar]
- 29.Shahinian, A., K. Pfeffer, K. P. Lee, T. M. Kundig, K. Kishihara, A. Wakeham, K. Kawai, P. S. Ohashi, C. B. Thompson, and T. W. Mak. 1993. Differential T cell costimulatory requirements in CD28-deficient mice. Science 261609-612. [DOI] [PubMed] [Google Scholar]
- 30.Sigal, L. J., H. Reiser, and K. L. Rock. 1998. The role of B7-1 and B7-2 costimulation for the generation of CTL responses in vivo. J. Immunol. 1612740-2745. [PubMed] [Google Scholar]
- 31.Stanberry, L. R., S. L. Spruance, A. L. Cunningham, D. I. Bernstein, A. Mindel, S. Sacks, S. Tyring, F. Y. Aoki, M. Slaoui, M. Denis, P. Vandepapeliere, and G. Dubin. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 3471652-1661. [DOI] [PubMed] [Google Scholar]
- 32.Suresh, M., J. K. Whitmire, L. E. Harrington, C. P. Larsen, T. C. Pearson, J. D. Altman, and R. Ahmed. 2001. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J. Immunol. 1675565-5573. [DOI] [PubMed] [Google Scholar]
- 33.Thebeau, L. G., and L. A. Morrison. 2002. B7 costimulation plays an important role in protection from herpes simplex virus type 2-mediated pathology. J. Virol. 762563-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thebeau, L. G., and L. A. Morrison. 2003. Mechanism of reduced T-cell effector functions and class-switched antibody responses to herpes simplex virus type 2 in the absence of B7 costimulation. J. Virol. 772426-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thebeau, L. G., S. P. Vagvala, Y. M. Wong, and L. A. Morrison. 2007. B7 costimulation molecules expressed from the herpes simplex virus 2 genome rescue immune induction in B7-deficient mice. J. Virol. 8112200-12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Todo, T., R. L. Martuza, M. J. Dallman, and S. D. Rabkin. 2001. In situ expression of soluble B7-1 in the context of oncolytic herpes simplex virus induces potent antitumor immunity. Cancer Res. 61153-161. [PubMed] [Google Scholar]
- 37.Williams, I. R., R. J. Ort, and T. S. Kupper. 1994. Keratinocyte expression of B7-1 in transgenic mice amplifies the primary immune response to cutaneous antigens. Proc. Natl. Acad. Sci. USA 9112780-12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu, Y., and Y. Liu. 1994. Viral induction of co-stimulatory activity on antigen-presenting cells bypasses the need for CD4+ T-cell help in CD8+ T-cell responses. Curr. Biol. 4499-505. [DOI] [PubMed] [Google Scholar]
- 39.Xu, F., M. R. Sternberg, B. J. Kottiri, G. M. McQuillan, F. K. Lee, A. J. Nahmias, S. M. Berman, and L. E. Markowitz. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296964-973. [DOI] [PubMed] [Google Scholar]
- 40.Zajac, P., A. Schutz, D. Oertli, C. Noppen, C. Schaefer, M. Heberer, G. C. Spagnoli, and W. R. Marti. 1998. Enhanced generation of cytotoxic T lymphocytes using recombinant vaccinia virus expressing human tumor-associated antigens and B7 costimulatory molecules. Cancer Res. 584567-4571. [PubMed] [Google Scholar]
- 41.Zimmermann, C., P. Seiler, P. Lane, and R. M. Zinkernagel. 1997. Antiviral immune responses in CTLA4 transgenic mice. J. Virol. 711802-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]