Abstract
While the smallpox vaccine, Dryvax or Dryvax-derived ACAM2000, holds potential for public immunization against the spread of smallpox by bioterror, there is serious concern about Dryvax-mediated side effects. Here, we report that a single-dose vaccination regimen comprised of Dryvax and an antiviral agent, cidofovir, could reduce vaccinia viral loads after vaccination and significantly control Dryvax vaccination side effects. However, coadministration of cidofovir and Dryvax also reduced vaccine-elicited immune responses of antibody and T effector cells despite the fact that the reduced priming could be boosted as a recall response after monkeypox virus challenge. Evaluations of four different aspects of vaccine efficacy showed that coadministration of cidofovir and Dryvax compromised the Dryvax-induced immunity against monkeypox, although the covaccinated monkeys exhibited measurable protection against monkeypox compared to that of naïve controls. Thus, the single-dose coadministration of cidofovir and Dryvax effectively controlled vaccination side effects but significantly compromised vaccine-elicited immune responses and vaccine-induced immunity to monkeypox.
The development of safe and effective vaccines to defend against the spread of smallpox by bioterror remains one of the most important biodefense countermeasures (6, 9, 14, 15, 19, 21, 23, 36). Dryvax or Dryvax-derived ACAM2000, the vaccine from vaccinia virus formulations that is associated with the global eradication of smallpox, may hold potential for public immunization against the spread of smallpox through bioterror (9, 11, 24, 25), but there is concern about Dryvax vaccination-induced side effects. A severe skin rash at the Dryvax vaccination site occurs quite often; the painful skin lesions inevitably resolve with visible scars. Even touching the skin rash or vaccination site can result in the spread of the vaccinia virus to persons in contact with it (contact transmission). Some Dryvax-vaccinated persons can even develop serious side effects, such as lymphadenopathy, vaccinia dissemination, eye infection, postvaccinial encephalitis, permanent disability, life-threatening illness, or death (19, 20, 34, 35). Furthermore, recent data from clinical monitoring suggest that vaccination with replicating vaccinia virus can induce adverse cardiovascular events (30, 33). Due to its complications, Dryvax is contraindicated for the vaccination of immune-compromised persons and for use in many other clinical settings (2, 3, 10, 27). It is therefore important to develop a useful vaccination regimen that can reduce the side effects of Dryvax but maintain the vaccine efficacy.
Cidofovir is a potent antiviral drug that is currently being investigated for treating deadly smallpox (variola) and monkeypox, although it is licensed for human immunodeficiency virus-associated cytomegalovirus retinitis (1, 5, 26, 31). Given the possibility that cidofovir or other antiviral drugs can limit initial active vaccinia virus replication, cidofovir and Dryvax (cidofovir+Dryvax) coadministration may reduce Dryvax-mediated vaccination complications. However, it is important to determine whether cidofovir+Dryvax coadministration, while potentially reducing Dryvax-mediated vaccination toxicity, can preserve a certain degree of the Dryvax-elicited immune responses and Dryvax-induced immunity against smallpox. These important scientific and clinical questions regarding cidofovir+Dryvax coadministration should be readily addressed by using a nonhuman primate model in which Dryvax-elicited immunity against monkeypox could be evaluated. Monkeypox may be the best substitute for smallpox, as monkeypox virus (Orthopoxvirus) shares some biologic features with smallpox virus, and monkeypox infection is clinically similar to smallpox in humans (9, 16, 32). We therefore employed a cynomolgus monkey model to examine whether cidofovir+Dryvax coadministration can reduce Dryvax vaccination side effects and preserve some levels of Dryvax-elicited immune responses and Dryvax-induced immunity against monkeypox.
MATERIALS AND METHODS
Monkeys and immunization.
Eighteen female cynomolgus monkeys (Macaca fascicularis, approximately 3 to 6 years old, with body weights of 2.0 to 4.0 kg) were provided by a National Institute of Allergy and Infectious Diseases-sponsored facility colony and divided by randomization into three groups of equal sizes (group 1, mock; group 2, Dryvax alone; group 3, cidofovir+Dryvax). Experiments were done according to American Association for the Accreditation of Laboratory Animal Care standards and U.S. NIH Animal Care and Use Committee guidelines. Animal and biosafety protocols for animal use and experiments were approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee and Institutional Biosafety Committee.
For vaccinations, monkey groups 2 and 3 were vaccinated with approximately 2 × 105 PFU/animal of Dryvax vaccine (lot no. 4020075; L. Wyatt) using 15 jabs of a bifurcated needle by the standard dermal scarification technique on the shaved back between the shoulder blades on day 0. Group 3 animals were intravenously injected with 20 mg of cidofovir/kg of body weight (lot no. 692343; Gilead Sciences), and as controls, monkeys in groups 1 and 2 were treated similarly with an equal volume of saline at the time of vaccination. After vaccination, all monkeys were assessed daily for skin rashes, rectal temperature, and body weight and were given routine physicals. The skin rashes on the vaccination sites were measured and documented with digital photographs at least three times per week until the scabs fell off. Blood samples were collected from individual animals at day 2 before vaccination, day 0, and days 7, 14, 28, 35, and 55 after vaccination. Plasma and peripheral blood mononuclear cells (PBMC) were isolated from whole blood by Ficoll-Paque density gradient centrifugation as previously described (4, 28) and then used for measuring vaccine-elicited antibody and T-cell responses, respectively.
Monkeypox virus challenge of monkeys.
Monkeypox virus strain Zaire 79 (catalog no. NR-2324, lot no. 4729797) was obtained from the Biodefense and Emerging Infections Research Resources Repository (BEI Resources) and stocked at −80°C. Vero (ATCC CRL-1587) and LLC-MK2 (ViroMed Laboratories) cells were maintained in Eagle minimal essential medium (MEM) containing 5% heat-inactivated fetal bovine serum (FBS), 10 mM HEPES, and 2 mM glutamine in a humidified air-5% CO2 atmosphere at 37°C and used to determine monkeypox virus titration by a standard plaque assay. On day 55 after immunization, each monkey was intravenously infected with 5.0 × 107 PFU of monkeypox virus at a Biologic Resources Laboratory Annex biosafety level 3 monkey facility. The infected monkeys were assessed daily, with documentation of the development of skin lesions on the different body parts and monitoring of other clinical signs of monkeypox. Every 2 to 3 days after the monkeypox virus challenge, blood samples were collected to isolate plasma and PBMC. Monkeys that developed fatal monkeypox syndromes leading to moribundity were sacrificed for a complete necropsy. At necropsy, different organs and tissues were thoroughly examined for gross pathological changes, and organs/tissues were collected for virus isolation and histopathological analysis. On day 28 after challenge, all surviving monkeys were sacrificed for necropsy of gross and histological pathology as well as tissue collection to isolate viruses as described below.
Measurement of vaccine-elicited antibodies.
Anti-B5R and anti-L1R antibodies were measured by enzyme-linked immunosorbent assay to investigate Dryvax vaccine-elicited antibody responses against poxvirus extracellular enveloped virion (B5R) and intracellular mature virion (L1R), respectively. Recombinant vaccinia virus B5R protein (catalog no. NR-546) and L1R protein (catalog no. NR-2625) were obtained from BEI Resources and used to coat 96-well plates (Costar 9018; Corning) at a concentration of 10 ng/ml in coating buffer (100 μl per well) at 4°C overnight. After the plates were washed three times with washing buffer (KPL) and then blocked for 1 to 2 h, 50 μl of serial two- or fourfold dilutions (from 1:4 to 1:16,400) prepared from the heat-treated plasma was added in triplicate wells and incubated for 1 to 2 h. The washed plates were incubated for 1 h at 37°C with peroxidase-labeled goat anti-monkey immunoglobulin G (KPL) diluted 1:3,000 in blocking buffer, and the colorimetric reaction was developed by using 100 μl of 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonate peroxidase substrate (ABTS; KPL) per well for 10 min at room temperature. After the reaction was stopped with 100 μl of stopping buffer, the optical density at 405 nm (OD405) was detected by using a Multiskan Ascent reader (Thermo). Plasma samples from eight healthy, uninfected monkeys were used in the control experiments to calculate the mean OD405 (0.11), standard deviation (SD; 0.03), and interassay (9%) and intraassay (14%) coefficients of variation. The mean OD405 plus 3.0 SD was employed to determine end point dilution titers of antibodies.
Measurement of monkeypox virus-neutralizing antibody.
A standard plaque reduction neutralization assay was performed to measure neutralizing antibody levels. Briefly, 1,000 PFU/ml of monkeypox virus working solution was prepared from the virus stock by serial 10-fold dilutions in MEM on an ice bath. The plasma samples were inactivated complements at 56°C for 30 min and then diluted serially as described above. In the plaque reduction neutralization assay, 100 μl MEM containing 100 PFU of monkeypox virus was mixed with an equal volume of the plasma dilutions and incubated at 37°C for 1 h. Each dilution of the virus-plasma mixture was added to three petri dishes containing 2 × 105 Vero cell monolayers and then incubated at 37°C for 1 h in a 5% CO2 atmosphere. The virus plasma supernatant was removed from the petri dishes and overlaid with MEM medium containing 2.5% heat-inactivated FBS and 1.2% carboxymethylcellulose (Sigma) and incubated for 4 to 6 days. The overlaid layer was removed, the dishes were stained with 0.5% crystal violet and washed thoroughly, and then the PFU in each dish were counted. The mean numbers of PFU from the triplicate dishes of each dilution were calculated, and end point titers of neutralizing antibody were determined as the highest dilutions of monkey plasma which reduced the virus plaque formation by 50% (ND50).
ELISPOT detection of protective antigen H3L-specific IFN-γ-producing cells.
PBMC isolated from the blood were used to measure cellular immune responses before and after vaccination or virus challenge as we previously described (17). To detect the viral H3L peptide-specific gamma interferon-positive (IFN-γ+) cells in the PBMC, an enzyme-linked immunospot assay (ELISPOT) plate (Millipore) was coated with 10 μg/ml of purified mouse anti-human IFN-γ antibody (B27; Pharmingen) at 100 μl per well and incubated at 4°C overnight. The plate was washed three times with 0.25% Tween 20-phosphate-buffered saline (PBS) and blocked with 5% FBS-PBS at 37°C for 2 h. After the plate was seeded with 2 × 105/well of PBMC, 10 μg/ml of a pool of 15-mer peptides overlapping by 12-mer, spanning the entire H3L protein (synthesized by GenScript), was added to stimulate the cells, and cells were incubated at 37°C in 5% CO2 for 18 h. PBMC incubations with 10 μg/ml of phytohemagglutinin (Sigma) and 10% FBS-RPMI 1640 were designated the positive and negative controls, respectively. The plate was washed nine times with Tween 20-PBS, and 200 μl of double-distilled H2O was added for cell lysis. Fifty microliters (2 μg/ml) of biotinylated rabbit polyclonal anti-human IFN-γ antibody (BioSource) was distributed into each well for a 2-h incubation. After the plate was washed thoroughly with Coulter wash buffer, 100 μl/well of streptavidin (Southern Biotechnology) was added, and the plates were incubated for 2 h. The wells were washed five times with Coulter wash buffer and once with PBS, and 100 μl/well of 1-Step NBT/BCIP (Pierce) was added to develop visible spots at room temperature for 10 min. The spots on the dried plates were counted by using an automated ELISPOT reader system (CTL Analyzers) with ImmunoSpot software. The mean number of spots from triplicate wells was adjusted to 1 × 106 PBMC, and the ELISPOT data were expressed as the mean ± SD. The H3L peptide-specific IFN-γ responses were calculated by subtracting the number of spots formed in negative control medium wells from the number of spots formed in response to the H3L peptide pool used in the stimulation.
ICS for measuring H3L-specific IFN-γ+ CD4 and CD8 T effector cells.
PBMC (1 × 106) were distributed to measure the viral H3L peptide-specific IFN-γ+ CD4 and CD8 T effector cells using intracellular cytokine staining (ICS), as we previously described (17). Anti-CD28 (clone CD28.2, 0.1 μg; BD Pharmingen) and anti-CD49d (clone 9F10, 0.1 μg; BD Pharmingen) were added to the PBMC suspension, which was then incubated with 10 μg/ml of overlapping H3L peptides at 37°C in 5%CO2 for 1 h. PBMC stimulated with 10% FBS-RPMI 1640 only and those with phorbol myristate acetate (200 ng/ml)/ionomycin (1 μg/ml) served as negative and positive controls, respectively. The cells were incubated for 5 h with 1 μl of GolgiPlug (BFA) at 37°C with shaking, washed once with 3 ml of 2% FBS-PBS, and stained with fluorescein isothiocyanate-conjugated CD3 (clone L200; BD Pharmingen), CD4 PB (clone OKT4; eBiosource), and CD8 PE-Cy5 (clone PRAT8; BD Pharmingen). After being washed twice with 3 ml of 2% FBS-PBS, 200 μl of Cytofix/Cytoperm solution was added and the mixture was stored at 4°C in the dark for 45 min. The cells were stained with 1 μl of IFN-γ phycoerythrin (clone 4S,B3; BD Pharmingen), incubated at room temperature in the dark for 45 min, washed twice with Perm/Wash buffer, and fixed with 250 μl of 2% formalin, and H3L-specific IFN-γ+ CD4 and CD8 T effector cells were measured by flow cytometry.
Real-time quantitative PCR to detect monkeypox virus gene transcripts.
Copies of the vaccinia or monkeypox virus A33R gene in PMBC were measured by real-time quantitative PCR at days 4, 7, 14, and 28 postvaccination and at days 4, 7, 14, 21, or 28 or at the time of early death after virus challenge. RNA from 1 × 106 PBMC was extracted using the TRIzol-based isolation method, and cDNA was synthesized using a cDNA synthesis kit (Clontech). A specific pair of primers, 5-TGTTAAATACTTGTCTGGAG-3′ and 5′-AGATCATTAATTGTTACCTT-3′, were used to amplify the viral A33R gene combined with the synthesized probe, 5′-(6-carboxyfluorescein)TCATGATGATTTGGTTGTAT-3′. The real-time PCR was performed using a PE Applied Biosystems 7700 single-reporter sequence detection system, and all amplifications were carried out with a MicroAmp optical 96-well reaction plate with an optical membrane cover (PE Applied Biosystems), as previously described (17, 29). To minimize variation, RNA extraction, cDNA synthesis, and real-time quantitative PCR were performed using cell pellets collected and stored longitudinally at different time points from each monkey and run together in a plate for detection of copies of the A33R gene. All PCR data were analyzed using GeneAmp 7700 SDS software.
Determination of monkeypox virus titration in the tissues.
At necropsy, fresh lungs, lymph nodes, and lesion-containing skin tissues were collected from each monkey to determine monkeypox virus titrations using a plaque-forming assay with Vero cells. The tissues stored at −80°C were thawed rapidly and homogenized 10 s for a total of 2 min on ice and then spun in a microcentrifuge at 10,000 × g for 5 s to pellet cell debris. The supernatants were collected and serially diluted from 10−1 to 10−7 with serum-free MEM. A 0.1-ml sample of the dilution was mixed with 900 ml of MEM, added to the six-well plates in triplicate containing Vero cell monolayers, cultured at 37°C for 5 days, and stained for plaques with 0.5% crystal violet. The PFU in each dilution were counted, and the monkeypox virus titration was expressed as PFU per gram of tissue (PFU/g).
Gross and histological pathology evaluation.
At necropsy, each monkey was thoroughly evaluated in detail by a senior pathologist for gross pathology of organs and tissues. To quantitate the pathological changes, organs or tissues were carefully removed, measured, weighed, and imaged with a fluorescence ruler using a digital camera. Grayish-white monkeypox lesions and other macroscopic changes were counted, and their numbers and sizes were documented. Multiple tissue sections collected from up to three different locations of each organ were prepared through routine procedures. Routine microscopic analyses of tissue sections of organs were also carried out by the senior pathologist.
Statistical analysis.
Mean geometric end-point titers (GMT) were employed to express antibody responses at different time points after vaccination or virus challenge in each of the three groups. Analysis of variance was used as previously described (28) to statistically analyze the data for differences among the three groups; a P value of <0.05 was the criterion for statistical significance.
RESULTS
Cidofovir+Dryvax coadministration controlled Dryvax-mediated skin lesions and reduced vaccinia (Dryvax) viral loads in PBMC after vaccination.
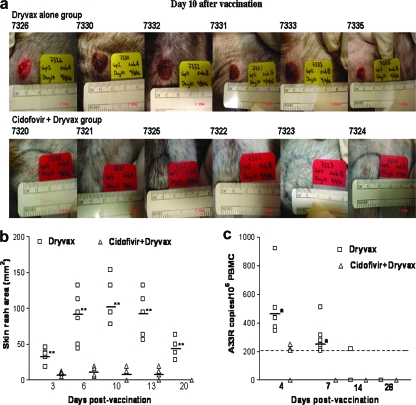
To examine whether cidofovir treatment could reduce Dryvax vaccination side effects, three groups of monkeys (six in each group) were vaccinated with saline, Dryvax alone, and cidofovir+Dryvax, respectively. All the animals vaccinated with Dryvax alone developed skin rashes at the vaccination site. The skin rashes occurred as red bumps on day 3 postvaccination and then progressed to swollen and large pus-filled blisters. The skin blisters reached maximal sizes, with a mean of about 120 mm2, on day 10 after vaccination (Fig. 1a and b). The pustular skin rashes persisted for 18 days prior to scabbing and then resolved with visible scars on the skin. In contrast, the monkeys vaccinated simultaneously with cidofovir+Dryvax developed no or very small skin rashes after the coadministration. The mean size of skin rashes as measured at any time point was <20 mm2, with a P value of <0.01 at each of the time points compared to those seen for the monkeys vaccinated with Dryvax alone (Fig. 1b). In addition, minor skin rashes seen in the group vaccinated with cidofovir+Dryvax were less indurated and healed more rapidly (mean, 11.8 days) than those seen for the Dryvax-alone group (mean, 19.2 days; P < 0.01). These data demonstrated that cidofovir+Dryvax coadministration could significantly reduce the size and severity of Dryvax-induced skin rashes.
FIG. 1.
Cidofovir+Dryvax vaccination regimen significantly controlled Dryvax-induced skin lesions and reduced vaccinia (Dryvax) viral loads in PBMC. (a) Representative photos show the skin rashes formed at the vaccination sites of individual monkeys in the Dryvax-alone and cidofovir+Dryvax groups on day 10 after vaccination. All animals vaccinated with Dryvax alone developed large and severe skin blisters, whereas the monkeys vaccinated with the cidofovir+Dryvax regimen developed no or very small skin rashes. (b) Comparison of sizes of skin rashes at the vaccination sites between Dryvax alone and cidofovir+Dryvax groups at different time points postvaccination. The horizontal bars indicate mean skin rash areas (mm2) for the groups; the cidofovir+Dryvax group had a much smaller skin rash than that of the Dryvax-alone group on days 3, 6, 10, 13, and 20 postvaccination (**, P < 0.01). (c) Comparison of numbers of A33R copies in the PMBC between the Dryvax alone and cidofovir+Dryvax groups using real-time quantitative PCR. Viral A33R mRNA was detected at days 4 and 7 in PBMC from all monkeys vaccinated with Dryvax alone but was low or undetectable in the monkeys covaccinated with cidofovir+Dryvax. The mean number of copies of the A33R gene in PBMC of the Dryvax-alone group (horizontal bars) is much higher than that of the cidofovir+Dryvax group at days 4 and 7 postvaccination (*, P < 0.05). The dashed line indicates the detection limit for the real-time quantitative PCR.
It was likely that cidofovir in the cidofovir+Dryvax regimen reduced productive vaccinia virus infection after vaccination and therefore controlled the vaccination side effects. We presumed that productive vaccinia (Dryvax) infection could transiently be detected in the blood of Dryvax-vaccinated monkeys after the vaccination but would be reduced in animals covaccinated with cidofovir and Dryvax. To test this possibility, the expression levels of viral A33R mRNA in the PMBC were measured using real-time quantitative PCR, and those from the Dryvax-alone and cidofovir+Dryvax groups were compared. Interestingly, viral A33R mRNA was detected at days 4 and 7 in PBMC from all monkeys vaccinated with Dryvax alone (Fig. 1c). In contrast, three of six monkeys covaccinated with cidofovir+Dryvax had very low levels of viral mRNA in PBMC; the other three covaccinated monkeys exhibited undetectable viral mRNA (Fig. 1c, P < 0.05). These results suggested that the cidofovir-mediated reduction in vaccinia viral loads might contribute to the control of Dryvax-mediated vaccination side effects.
Cidofovir+Dryvax coadministration resulted in a significant decrease in Dryvax-elicited antibody and T-cell immune responses despite the fact that the reduced priming could be boosted as a recall response after monkeypox challenge.
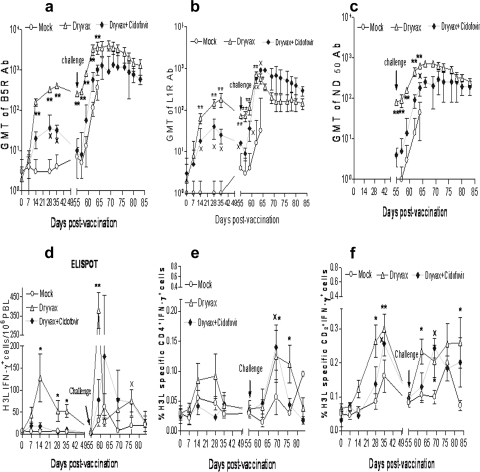
We then sought to determine the extent to which the cidofovir+Dryvax coadministration, while reducing Dryvax vaccination side effects, could affect Dryvax-elicited antibody and T-cell immune responses. Dryvax-elicited immune responses of anti-B5R (a neutralizing antibody-reactive protein in the extracellular enveloped virion) and anti-L1R (a protein in the intracellular mature virion) antibodies were measured by enzyme-linked immunosorbent assays, and GMT of the three groups were compared. cidofovir+Dryvax coadministration clearly resulted in the reduction of Dryvax-elicited antibody responses compared to immunization with Dryvax alone (Fig. 2a to c). Although five of six monkeys in the cidofovir+Dryvax group developed B5R- and L1R-specific antibody responses 1 or 2 weeks after the vaccination, the mean titers of the vaccine-elicited antibodies were about one log lower than those of the Dryvax-alone group over time after the vaccination (Fig. 2a to c). Monkey 7322 did not develop any detectable vaccine-elicited immune responses, a finding consistent with nondetectable vaccinia mRNA in PBMC (Fig. 1c). All the monkeys except animal 7322 in the cidofovir+Dryvax group were able to develop appreciable recall immune responses of anti-B5R, anti-L1R, and neutralizing antibodies after the monkeypox virus challenge. Within 7 days after the challenge, titers of anti-B5R, anti-L1R, and neutralizing antibodies rapidly increased to levels close to those seen for the Dryvax-alone group (Fig. 2a to c). The mock control group exhibited a primary immune response of anti-L1R and neutralizing antibodies following virus challenge despite a rapid increase in anti-B5R antibody titers (Fig. 2a to c). Importantly, our data showed a potential correlation between vaccine viral mRNA and vaccine-elicited neutralizing antibody levels after vaccination (before monkeypox challenge) for individual monkeys in the cidofovir+Dryvax or Dryvax-alone group (Table 1).
FIG. 2.
Cidofovir+Dryvax coadministration resulted in marked decreases in Dryvax-elicited antibody and T-cell immune responses despite the fact that the reduced priming could be boosted as a recall response after monkeypox challenge. (a to c) Mean GMT levels of anti-B5R, anti-L1R antibodies (Ab), and neutralizing antibodies (GMT ND50), respectively, before and after vaccination as well as after monkeypox virus challenge, as indicated. The virus-specific antibody titers in the cidofovir+Dryvax group were lower than those in the Dryvax-group but higher than those in the mock control group (×, P < 0.05 for days 28, 35, and 55 when results for the cidofovir+Dryvax group are compared to those for the mock control group). Note that up to 2 log increases in mean GMT within 7 days after monkeypox virus challenge were seen for the cidofovir+Dryvax group, with a P value of <0.05 for anti-L1R and ND50 antibodies on day 7 after challenge compared to the mock control group. (d to f) Mean absolute numbers of viral H3L-specific IFN-γ+ cells detected by ELISPOT (d) and mean percentages of H3L-specific IFN-γ+ CD4+ (e) and CD8+ T cells (f) by ICS for each group before and after vaccination as well as after monkeypox virus challenge. The numbers of H3L-specific IFN-γ+ CD8+ T cells in the cidofovir+Dryvax group were greater than those in the mock control group after vaccination (×, P < 0.05). At day 35, two naïve monkeys showed a high background of IFN-γ+ CD8+ T cells. H3L-specific IFN-γ+ lymphocytes and CD4+ and CD8+ T effector cells in the cidofovir+Dryvax group demonstrated some extent of recall expansion after monkeypox virus challenge. * and ** denote that P values for mean GMT levels of anti-B5R, anti-L1R antibodies, and neutralizing antibodies or mean percentage numbers of H3L-specific IFN-γ+ CD4+ and CD8+ T effector cells in the Dryvax-alone group were <0.05 and <0.01, respectively, compared with the mock control group.
TABLE 1.
Outcomes for individual monkeys in the postvaccination phase
| Group and animal no. | Skin rash area (mm2) on day:
|
A33R copies/106 PBMC on day:
|
ND50 (day 55) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 6 | 10 | 13 | 20 | 4 | 7 | 14 | 20 | ||
| Dryvax | ||||||||||
| 7332 | 45.8 | 132.7 | 132.7 | 132.7 | 50.2 | 920 | 590 | 238 | <200 | 128 |
| 7326 | 19.2 | 44.2 | 78.5 | 63.6 | 28.3 | 440 | 280 | <200 | <200 | 64 |
| 7330 | 39.3 | 113.0 | 132.7 | 113.0 | 63.6 | 370 | 230 | <200 | <200 | 64 |
| 7331 | 32.1 | 95.0 | 95.0 | 95.0 | 38.5 | 423 | 245 | <200 | <200 | 64 |
| 7333 | 37.4 | 86.5 | 153.9 | 113.0 | 63.6 | 505 | 314 | <200 | <200 | 128 |
| 7335 | 18.7 | 50.2 | 78.5 | 56.7 | 28.3 | 345 | 205 | <200 | <200 | 64 |
| Mean ± SD | 32 ± 11.1 | 86.9 ± 34.7 | 112 ± 32.1 | 95.7 ± 30.1 | 45.4 ± 16.3 | 499 ± 86 | 297 ± 45 | <200 | <200 | 81 ± 13 |
| Cidofovir+Dryvax | ||||||||||
| 7321 | 11.3 | 19.6 | 12.6 | 12.6 | 0.0 | 220 | <200 | <200 | <200 | 8 |
| 7325 | 12.2 | 19.6 | 19.6 | 12.6 | 0.0 | 250 | <200 | <200 | <200 | 8 |
| 7320 | 11.4 | 19.6 | 19.6 | 12.6 | 0.0 | 205 | <200 | <200 | <200 | 4 |
| 7322 | 3.1 | 5.3 | 0 | 0 | 0.0 | <200 | <200 | <200 | <200 | 0 |
| 7324 | 4.8 | 7.1 | 12.6 | 12.6 | 0.0 | <200 | <200 | <200 | <200 | 4 |
| 7323 | 9.3 | 15.9 | 12.6 | 7.1 | 0.0 | <200 | <200 | <200 | <200 | 2 |
| Mean ± SD | 8.7 ± 3.8 | 14.5 ± 6.0 | 12.8 ± 7.1 | 9.6 ± 5.2 | 0 | <200 | <200 | <200 | <200 | 4 ± 2 |
| P | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.05 | <0.05 | <0.01 | ||
Similarly, cidofovir+Dryvax coadministration resulted in decreased levels of Dryvax-elicited H3L-specific IFN-γ+ cellular responses. ELISPOT data showed that the cidofovir+Dryvax group exhibited significantly lower numbers of H3L-specific IFN-γ+ cells than the Dryvax-alone group after the vaccination (Fig. 2d). Consistently, ICS data showed decreased numbers of H3L-specific IFN-γ+ CD4+ and CD8+ T cells in the cidofovir+Dryvax group compared to those in the Dryvax-alone group (Fig. 2e and f). Although all the monkeys except animal 7322 in the cidofovir+Dryvax group developed rapid recall immune responses of H3L-specific IFN-γ+ T cells after the monkeypox virus challenge compared to the slow responses for the mock control, the magnitude of their recall responses was lower than that seen for the Dryvax-alone group (Fig. 2e and f). These results therefore demonstrated that cidofovir+Dryvax coadministration significantly decreased Dryvax vaccine-elicited antibody and T-cell immune responses despite the fact that the reduced priming could be boosted as recall responses after monkeypox challenge.
Cidofovir+Dryvax coadministration significantly compromised Dryvax-induced anti-monkeypox immunity, although the cidofovir+Dryvax group exhibited measurable protection against monkeypox compared to the naïve control.
Finally, we sought to determine whether cidofovir+Dryvax coadministration impaired Dryvax-induced immunity against monkeypox. The following four aspects of vaccine-induced immunity against monkeypox were evaluated.
(i) Monkeypox skin lesion counts.
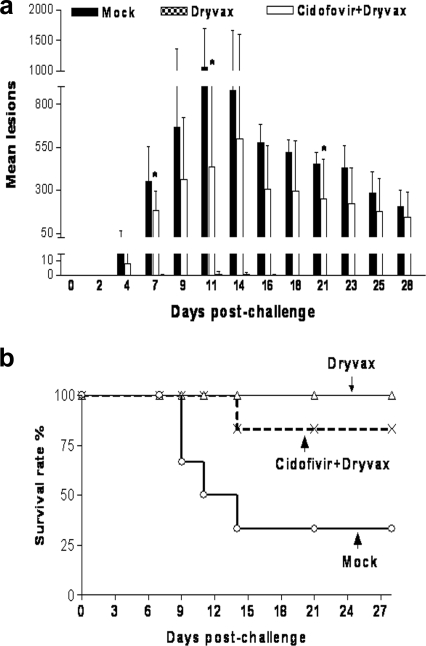
While the mock control group developed typical smallpox-like skin rashes on day 4 (day 59 after Dryvax vaccination), rapidly spreading up to a mean of 1,000 lesions per animal on days 9 and 12 after the monkeypox virus challenge, the Dryvax-alone group showed no or only one or two small skin lesions after virus challenge (Fig. 3a; Table 2). Significant differences in skin lesion numbers between the cidofovir+Dryvax group (all six animals included) and the mock control group were found only on days 7 and 11 after the monkeypox virus challenge (Fig. 3a; Table 2). Of note, the cidofovir+Dryvax group (all six monkeys) exhibited a 1- or 2-day delay in the occurrence of skin lesions compared to the mock control. However, the cidofovir+Dryvax group exhibited significantly more skin lesions than the Dryvax group (Fig. 3a; Table 2).
FIG. 3.
The cidofovir+Dryvax vaccination regimen impaired Dryvax-induced immunity to monkeypox and conferred poor, although measurable, protection against monkeypox. (a) All the monkeys vaccinated with Dryvax alone showed no or a few small skin lesions after virus challenge. It seemed that the cidofovir+Dryvax-vaccinated group developed fewer skin lesions than did mock control animals on days 7, 11, and 21 after monkeypox virus challenge (*, P < 0.05), but this group did not have significantly fewer lesions than the mock control group at other time points. (b) All the monkeys vaccinated with Dryvax alone survived the lethal monkeypox virus challenge. There was no significant difference in survival rate between the cidofovir+Dryvax group and the mock control group (P > 0.05), although the cidofovir+Dryvax-vaccinated group had a longer mean survival time than the mock control group during the 28-day follow-up after the monkeypox virus challenge (P < 0.032).
TABLE 2.
Clinical outcomes for individual monkeys in each group after monkeypox virus challengea
| Group and animal no. | No. of skin lesion on indicated day postchallenge
|
Lesions fused | Temp change | % Weight loss (day postchallenge) | ND50 on day 9 | Other CS | Day of death | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 7 | 9 | 11 | 14 | 21 | 28 | |||||||
| Mock | |||||||||||||
| 7337b | 2 | 65 | 19 | + | 9.1 (9) | 4 | Yes | 9b | |||||
| 7329 | 97 | 473 | 778 | 1,124 | 1,283 | ++++ | Decreased | 6.3 (14) | 32 | Yes | 14 | ||
| 7327 | 2 | 520 | 1,195 | ++++ | No | 11.2 (9) | 64 | Yes | 9 | ||||
| 7328 | 21 | 587 | 1,138 | 1,936 | ++++ | No | 8.2 (11) | 64 | Yes | 11 | |||
| 7334 | 32 | 202 | 462 | 603 | 679 | 500 | 272 | +++ | No | 7.1 (7) | 64 | Yes | Survived |
| 7336 | 4 | 256 | 406 | 559 | 671 | 410 | 140 | +++ | No | 7.1 (9) | 64 | Yes | Survived |
| Mean ± SD | 26 ± 27 | 351 ± 206 | 666 ± 4,566 | 1,056 ± 641 | 878 ± 351 | 455 ± 64 | 206 ± 93 | 7.0 (9) | |||||
| Dryvax | |||||||||||||
| 7332 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | No | No | <2 | 1,024 | No | Survived |
| 7326 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | No | No | <2 | 512 | No | Survived |
| 7330 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | No | No | <2 | 512 | No | Survived |
| 7331 | 0 | 2 | 0 | 5 | 2 | 0 | 0 | No | No | <2 | 512 | No | Survived |
| 7333 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | No | No | <2 | 1,024 | No | Survived |
| 7335 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | No | No | <2 | 512 | No | Survived |
| Mean ± SD | 0 | 0 ± 1 | 0 | 1 ± 1 | 1 ± 1 | 0 | 0 | No | |||||
| Pc | 0.103 | 0.006 | 0.028 | 0.037 | 0.031 | 0.035 | 0.048 | ||||||
| Cidofovir+Dryvax | |||||||||||||
| 7321 | 0 | 113 | 145 | 121 | 124 | 21 | 3 | + | No | 4.3 (4) | 256 | Yes | Survived |
| 7325 | 0 | 78 | 95 | 69 | 97 | 23 | 3 | + | No | 5.2 (4) | 512 | No | Survived |
| 7320 | 0 | 144 | 283 | 424 | 607 | 548 | 342 | +++ | No | 8.3 (9) | 256 | No | Survived |
| 7322 | 4 | 403 | 1062 | 1367 | 1758 | ++++ | Decreased | 11 (11) | 32 | Yes | 14 | ||
| 7324 | 33 | 137 | 203 | 223 | 395 | 307 | 126 | ++ | No | 5.5 (11) | 256 | No | Survived |
| 7323 | 10 | 215 | 383 | 422 | 606 | 361 | 240 | +++ | No | 11 (28) | 128 | No | Survived |
| Mean ± SD | 8 ± 13 | 182 ± 117 | 362 ± 356 | 438 ± 478 | 598 ± 610 | 252 ± 228 | 143 ± 149 | 6.4 (14) | |||||
| Pd | 0.167 | 0.031 | 0.055 | 0.031 | 0.135 | 0.047 | 0.119 | ||||||
CS, clinical signs, including depression, weakness, cough, unresponsiveness, dyspnea, dehydration, and anorexia. +, mild; ++, moderate; +++, marked; ++++, severe.
This monkey developed disseminated intravascular coagulation, which might lead to the absence of full-blown skin lesions after challenge.
P values for mean lesion counts from the Dryvax-alone group were compared with those from the cidofovir+Dryvax group.
P values for mean lesion counts from the cidofovir+Dryvax group were compared with those from the mock control group.
(ii) Survival.
Four monkeys in the mock control group developed severe clinical syndromes comprised of anorexia (>3 days), extremely low temperature (<94°F), shock, disseminated intravascular coagulation, or respiratory distress (an acute respiratory distress syndrome-like state) that led to moribundity on days 9, 11, or 14 (Table 2; Fig. 3b). In contrast, all the animals in the Dryvax-alone group survived the monkeypox virus infection without any notable clinical signs during the 28-day follow-up (Fig. 4b). Five of six cidofovir+Dryvax-vaccinated monkeys survived up to the lethal monkeypox virus challenge; the single animal (7322) which did not have any detectable Dryvax-elicited immune responses after cidofovir+Dryvax coadministration developed the severe syndrome and became moribund (Table 2). The cidofovir+Dryvax group (all six monkeys) showed significantly longer survival times (Fig. 3b, P < 0.03), but not a significantly higher survival rate, than the mock control group during the 28-day follow-up.
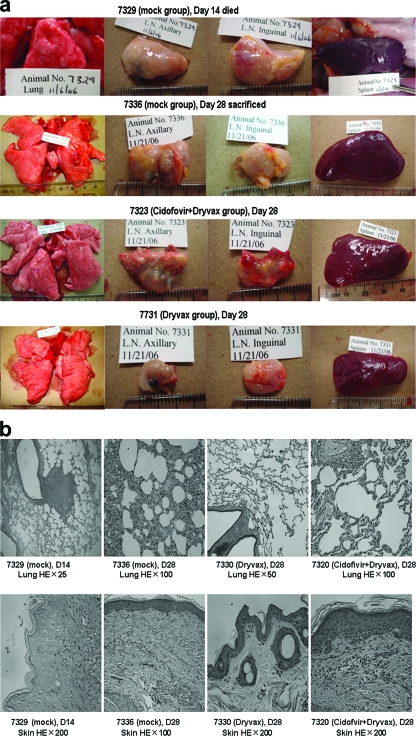
FIG. 4.
The Dryvax-alone group did not have any notable pathological findings, and the cidofovir+Dryvax group appeared to exhibit less-severe pathology than the mock control group (Table 3). (a) Representative gross pathology photographs demonstrating that the Dryvax-alone group was “lesion-free,” whereas the cidofovir+Dryvax group had noticeable monkeypox lesions that were less severe than those seen with the mock group. An early necropsied monkey, 7329, and a survivor, 7336, in the mock control group exhibited apparent lung congestion and edema with many large grayish-white lesions on the surface. No apparent lung lesions were seen for monkey 7331 and others vaccinated with Dryvax alone. Monkey 7325 and other survivors in the cidofovir+Dryvax group showed no lung congestion/edema and had fewer and smaller grayish-white lesions in the lungs than the two surviving monkeys in the mock group. The monkeys, including 7322, in the cidofovir+Dryvax group exhibited less-inflamed and less-congested spleens (splenomegaly) and other organs than those of animals in the mock group (P < 0.05, see Table 3 for organ weights). (b) Representative histological photographs demonstrating that the cidofovir+Dryvax group had less-severe lung and skin monkeypox lesions than the mock control. An early necropsied monkey, 7329, developed acutely exudative inflammation with central necrosis and destruction of the involved bronchiolar wall; monkey 7336 (mock group) surviving the 28-day follow-up still showed evident histopathologic changes in which some alveoli were filled with edema fluid, macrophages, degenerative neutrophils, and necrotic cellular debris, whereas hyperplastic fibroblasts and fibrous proliferation were seen in thickened alveolar walls. The monkey in the Dryvax-alone group exhibited “normal” histology in the lungs and skin. Monkey 7320 in the cidofovir+Dryvax group had less severe histopathology, as mild fibrous proliferation and thickening were seen only in a few alveoli in the lungs, and less-severe residual lesions in the skin were seen on day 28 after challenge. The representative data shown from gross pathology and histology seem to suggest that the pathology in animals from the cidofovir+Dryvax group looks similar to that found in survivors of the mock group, while net differences in pathology are visible in comparison to the Dryvax-only animals. HE, hematoxylin and eosin.
(iii) Gross pathology and histology.
The thorough and systemic examination/dissection of viscera and carcasses at necropsy showed that four monkeys from the mock control group that underwent early necropsies (early necropsied monkeys) exhibited full-blown severe pathology characterized by widespread monkeypox lesions on the skin and oral mucosa, remarkable lymphadenopathy and splenomegaly, and lung congestion/edema with grayish-white macroscopic lesions (Fig. 4a; Table 3). Even the two monkeys in the mock group that survived the monkeypox virus challenge during a 28-day follow-up still had evident lymphadenopathy/splenomegaly and lung congestion/edema at necropsies done on day 28 after the monkeypox virus challenge (Fig. 4a). In contrast, all monkeys in the Dryvax-alone group showed “normal” tissues/organs. Of note, five animals in the cidofovir+Dryvax group showed no or a few small grayish-white lesions in the lungs with mild or moderate lymphadenopathy and splenomegaly (Fig. 4a); the other monkey, 7322, which did not develop Dryvax-elicited immune responses, displayed apparent monkeypox pathology. Statistical analyses of both the numbers of grayish-white lung lesions and the weights of livers, kidneys and spleens indicated that the cidofovir+Dryvax group (all six monkeys) had less severe monkeypox pathology than the mock control group (Table 3, P < 0.05, respectively). However, the cidofovir+Dryvax group exhibited significantly worse pathology than the Dryvax group (Table 3). Overall, histology results were consistent with the gross pathology seen at necropsy (Fig. 4b).
TABLE 3.
Results of quantitative pathology and virus loads after monkeypox virus challenge
| Group and animal no. | Weight of organ (g)
|
No. of lesions in lungs | Virus titer (PFU/g)
|
A33R copies/106 PBMC on day:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lungs | Liver | Kidneys | Spleen | Lungs | Skin | Lymph nodes | 4 | 7 | 11 | 16 | 21 | 28 | ||
| Mock | ||||||||||||||
| 7337 | 9.8 | 79.9 | 16.6 | 10.5 | 20 | 2.3 × 109 | 2.0 × 108 | 1.7 × 106 | 1.3 × 104 | 1.3 × 104 | 2.9 × 106a | |||
| 7329 | 15.7 | 80.3 | 15.7 | 5.2 | 30 | 3.3 × 106 | 3.7 × 106 | 6.7 × 104 | 3.6 × 104 | 1.9 × 105 | 1.0 × 106 | 5.6 × 106a | ||
| 7327 | 31.5 | 128.6 | 18.6 | 9.3 | 18 | 2.7 × 108 | 4.3 × 107 | 5.0 × 105 | 7.4 × 104 | 3.9 × 105 | 3.0 × 106a | |||
| 7328 | 16.8 | 106.4 | 19.0 | 7.1 | 24 | 2.3 × 107 | 4.0 × 107 | 1.3 × 105 | 2.6 × 104 | 2.6 × 104 | 1.7 × 106 | |||
| 7334 | 17.6 | 62.9 | 11.6 | 5.7 | 8 | Negd | Neg | Neg | 5.0 × 103 | 1.3 × 104 | 3.5 × 104 | 2.0 × 103 | <2 × 102 | <2 × 102 |
| 7336 | 17.9 | 73.6 | 13.8 | 7.8 | 6 | Neg | Neg | Neg | 1.5 × 104 | 2.9 × 104 | 2.5 × 104 | 2.0 × 103 | 2.8 × 102 | <2 × 102 |
| Mean ± SD | 19.9 ± 5.9 | 88.6 ± 24.3 | 15.9 ± 2.8 | 7.6 ± 2.1 | 18 ± 9 | 4.1 × 104 | 4.6 × 104 | 4.8 × 105 | ||||||
| Dryvax | ||||||||||||||
| 7332 | 13.9 | 57.0 | 8.1 | 3.9 | 0 | Neg | Neg | Neg | <2 × 102 | <2 × 102 | <2 × 102 | <2 × 102 | <2 × 102 | <2 × 102 |
| 7326 | 15.0 | 87.1 | 12.6 | 4.1 | 0 | Neg | Neg | Neg | <2 × 102 | <2 × 102 | <2 × 102 | <2 × 102 | <2 × 102 | <2 × 102 |
| 7330 | 13.8 | 55.1 | 9.7 | 4.9 | 0 | Neg | Neg | Neg | <2 × 102 | <2 × 102 | <2 × 102 | <2 × 102 | <2 × 102 | <2 × 102 |
| 7331 | 17.4 | 74.1 | 10.8 | 3.1 | 0 | Neg | Neg | Neg | <2 × 102 | <2 × 102 | <2 × 102 | <2 × 102 | <2 × 102 | <2 × 102 |
| 7333 | 19.3 | 59.9 | 11.1 | 5.1 | 0 | Neg | Neg | Neg | <2 × 102 | <2 × 102 | <2 × 102 | <2 × 102 | <2 × 102 | <2 × 102 |
| 7335 | 17.5 | 62.1 | 10.1 | 4.3 | 0 | Neg | Neg | Neg | <2 × 102 | <2 × 102 | <2 × 102 | 3.8 × 102 | <2 × 102 | <2 × 102 |
| Mean ± SD | 16.1 ± 2.2 | 65.9 ± 12.3 | 10.4 ± 1.5 | 4.3 ± 0.7 | 0 | <2 × 102 | <2 × 102 | <2 × 102 | 2 × 102 | <2 × 102 | <2 × 102 | |||
| Pb | 0.39 | 0.44 | 0.12 | 0.004 | 0.014 | |||||||||
| Cidofovir+Dryvax | ||||||||||||||
| 7321 | 17.1 | 78.4 | 11.9 | 5.3 | 3 | Neg | Neg | Neg | 1.7 × 103 | 2.9 × 103 | <2 × 102 | <2 × 102 | <2 × 102 | <2 × 102 |
| 7325 | 14.2 | 43.8 | 9.4 | 5.4 | 4 | Neg | Neg | Neg | 8.1 × 102 | <2 × 102 | <2 × 102 | <2 × 102 | <2 × 102 | <2 × 102 |
| 7320 | 18.0 | 68.2 | 13.8 | 5.3 | 2 | Neg | Neg | Neg | 4.2 × 103 | 6.0 × 102 | 4.5 × 103 | <2 × 102 | <2 × 102 | <2 × 102 |
| 7322 | 17.1 | 84.5 | 15.4 | 5.2 | 12 | 1.7 × 108 | 7.3 × 108 | 2.3 × 106 | 3.6 × 104 | 3.5 × 104 | 1.2 × 105 | 1.0 × 105a | ||
| 7323 | 13.2 | 66.9 | 10.1 | 5.6 | 10 | 1.3 × 104 | 2.7 × 104 | Neg | 3.2 × 103 | 1.3 × 105 | 1.9 × 104 | <2 × 102 | <2 × 102 | <2 × 102 |
| 7324 | 14.4 | 62.7 | 11.9 | 5.4 | 2 | Neg | Neg | Neg | 2.2 × 103 | 9.8 × 103 | 8.1 × 103 | <2 × 102 | <2 × 102 | <2 × 102 |
| Mean ± SD | 15.7 ± 2.0 | 67.4 ± 14.1 | 12.1 ± 2.2 | 5.4 ± 0.1 | 6 ± 5 | 3.4 × 103 | 2.1 × 103 | 9.7 × 102 | ||||||
| Pc | 0.044 | 0.042 | 0.002 | 0.023 | 0.015 | 0.16 | 0.163 | 0.027 | ||||||
Day 9 data are shown for monkeys 7337 and 7327. Day 14 data are shown for monkeys 7329 and 7322.
P values for mean weights of organs, numbers of lung lesions, copies of the A33R gene, or live MPV titers in tissues of the Dryvax-alone group were compared with those of the cidofovir+Dryvax group.
P values for mean weights of organs, numbers of lung lesions, copies of the A33R gene, or live MPV titers in tissues of the cidofovir+Dryvax group were compared with those of the mock control group.
Neg, negative.
(iv) Viral loads (infection levels).
High expression levels of viral A33R genes in PBMC were seen for all the monkeys in the mock control group (Table 3). Low or moderate levels of A33R expression were seen for all the monkeys in the cidofovir+Dryvax-vaccinated group except for the high viral load of animal 7322, which did not develop a Dryvax-elicited immune response after vaccination (Table 3). The cidofovir+Dryvax group (all six monkeys) had significantly lower mean viral copies of A33R than the mock control group only at day 11 (P < 0.05, Table 3). Consistently, quantitation of live monkeypox virus isolated from fresh tissues at the time monkeys became moribund or on day 28 showed that the cidofovir+Dryvax group had lower geometrical mean levels (2 to 3 log) of live monkeypox virus in fresh lung, skin, and lymph node tissues than the mock control group but higher levels than the Dryvax-alone group (Table 3), as no virus was isolated from samples from the Dryvax group.
Thus, Dryvax alone conferred full protection against the lethal monkeypox virus challenge, whereas cidofovir+Dryvax coadministration significantly compromised the Dryvax-induced immunity, although the cidofovir+Dryvax group exhibited measurable protection against monkeypox compared to that of the naïve control.
DISCUSSION
Our data clearly indicate that the single-dose vaccination regimen comprised of cidofovir+Dryvax is very effective for reducing or eliminating Dryvax-mediated skin lesions at the vaccination site. However, our studies also indicate that cidofovir+Dryvax coadministration significantly decreases Dryvax-elicited antibody and T-cell responses and impairs Dryvax-induced immunity against monkeypox. The potential nephrotoxicity of cidofovir (18, 22) is another concern with the cidofovir+Dryvax vaccination approach. Thus, the single-dose cidofovir+Dryvax coadministration does not appear to be a useful vaccination strategy against smallpox. This seems to be in contrast to what has been described for the vaccination approach using the highly attenuated modified vaccinia virus Ankara, which has been reported as safe and effective against monkeypox in nonhuman primates (8, 32) and may potentially serve as a smallpox vaccine (7, 37).
The reduction of Dryvax-mediated skin lesions by cidofovir+Dryvax coadministration corresponds with impairment of Dryvax-elicited antibody and T-cell immune responses. The monkeys vaccinated with cidofovir+Dryvax developed weak Dryvax-elicited antibody and T-cell responses compared to those vaccinated with Dryvax alone. The attenuating effects on both skin lesions and Dryvax-elicited immune responses in the cidofovir+Dryvax coadministration setting are likely attributed to the cidofovir-mediated anti-vaccinia virus activities. As a nucleotide analog, cidofovir can inhibit vaccinia virus replication and spread after Dryvax vaccination and therefore reduce or contain virus-associated inflammation at the vaccination site. On the other hand, cidofovir-mediated antiviral effects can reduce viral antigen exposure to the immune system for immune priming after Dryvax vaccination. In fact, our studies of vaccinia virus mRNA expression in PBMC after vaccination suggest that cidofovir in the cidofovir+Dryvax regimen appears to reduce the productive infection of vaccinia virus after vaccination and then contribute to control Dryvax vaccination side effects. The findings also suggested that transiently productive vaccinia virus infection after Dryvax vaccination appears to contribute to its sterilizing anti-monkeypox immunity.
Cidofovir+Dryvax coadministration does not appear to affect the ability of Dryvax-elicited B (antibody) and T effector cells to mount rapid recall responses after monkeypox virus challenge. Despite cidofovir's effect on priming, Dryvax-elicited B and T effector cells in the monkeys vaccinated with cidofovir+Dryvax can mount faster and greater recall responses after monkeypox virus challenge than from the initial Dryvax priming. These recall immune responses are similar in pattern to the typical memory responses seen for the monkeys vaccinated with Dryvax alone. It is likely that cidofovir+Dryvax coadministration is still able to prime enough numbers of B and T cells that these effector cells can expand rapidly in response to monkeypox virus infection. The rapid recall immune responses of the decreased Dryvax-elicited immune cells appear to be the immune elements conferring some extent of protection against the fatal monkeypox syndrome.
The single-dose cidofovir+Dryvax vaccination regimen leads to a significant reduction in protection against monkeypox compared to the full immunity induced by vaccination with Dryvax alone. Difference in anti-monkeypox immunity between these two groups may be explained by the lower prechallenge levels of Dryvax-elicited antibodies and T effector cells in the whole cidofovir+Dryvax group. It is worth mentioning that the cidofovir+Dryvax group was evaluated for vaccine efficacy using the data from the whole group of six monkeys, in which one animal (7322) actually did not develop any detectable Dryvax-elicited immune responses after vaccination. Neither vaccinia virus mRNA in the PMBC nor a notable skin rash was detected after the cidofovir+Dryvax vaccination for monkey 7322, and we speculate that a vaccine failure (a nontake) occurred in this monkey, likely caused by cidofovir's “overkill” of vaccinia virus infection after Dryvax vaccination. This also implies a potential drawback for cidofovir+Dryvax coadministration, because one could speculate further that cases of vaccine failure (nontakes) like that with monkey 7322 may be proportionally increased when more outbred monkeys or humans are recruited for evaluation of the cidofovir+Dryvax vaccination approach. It is worthy of mention that with or without this single monkey included in the evaluation, similar conclusions are reached between the cidofovir+Dryvax and Dryvax-alone groups or between the cidofovir+Dryvax and unvaccinated groups. On the other hand, it is also worth noting that our proof-of-concept studies of vaccine efficacy were undertaken by intravenous challenge of vaccinated monkeys with extremely large doses of poxviruses (5 × 107 PFU). Given the possibility that natural smallpox or monkeypox infection is generally introduced by aerosol with a limited amount of virus, vaccine-elicited antibodies and T effector cells after cidofovir+Dryvax coadministration may confer substantial protection against the respiratory invasion by the monkeypox virus.
Since the newly FDA-licensed smallpox vaccine, ACAM2000 (12), is derived from Dryvax, this clonal vaccinia virus grown in cell culture may retain some of the Dryvax-mediated vaccine side effects. Because the vaccinia-derived vaccine can confer immunity, efforts are continuously being made to reduce vaccination toxicity but maintain vaccine efficacy (38). In fact, topical administration of povidone iodine in the Dryvax vaccination site can effectively block virus shedding after traditional smallpox vaccination and reduce the risks of autoinoculation or contact spread, although its ability to decrease Dryvax-mediated skin lesions remains to be characterized (13). Better approaches in combining a novel antiviral agent and Dryvax or Dryvax-derived ACAM2000 may reduce vaccinia virus-induced side effects after vaccination but still confer nearly full protection against monkeypox/smallpox.
Acknowledgments
We thank the Toxicology Research Laboratory and Biological Resource Laboratory of the University of Illinois at Chicago for supporting this work and Karen Hagen, Jewell Graves, and Brenda Paige for technical advice on flow cytometry analysis.
This work was supported by NIH award N01 AI50016 (to Z.W.C.).
All authors listed in the manuscript declare no conflicts of interest or financial interests.
The following authors made specific contributions to the manuscript. Huiyong Wei performed research and wrote the paper, Dan Huang performed research, Jeff Fortman contributed vaccination and monkeypox virus challenges, Richard Wang performed research, Linyun Shao performed research, and Zheng W. Chen designed research and wrote the paper.
Footnotes
Published ahead of print on 12 November 2008.
REFERENCES
- 1.Baker, R. O., M. Bray, and J. W. Huggins. 2003. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antivir. Res. 5713-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casey, C. G., J. K. Iskander, M. H. Roper, E. E. Mast, X. J. Wen, T. J. Torok, L. E. Chapman, D. L. Swerdlow, J. Morgan, J. D. Heffelfinger, C. Vitek, S. E. Reef, L. M. Hasbrouck, I. Damon, L. Neff, C. Vellozzi, M. McCauley, R. A. Strikas, and G. Mootrey. 2005. Adverse events associated with smallpox vaccination in the United States, January-October 2003. JAMA 2942734-2743. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2004. Update: adverse events following civilian smallpox vaccination—United States, 2003. MMWR Morb. Mortal. Wkly. Rep. 53106-107. [PubMed] [Google Scholar]
- 4.Chen, Z. W., Y. Shen, D. Zhou, M. Simon, Z. Kou, D. Lee-Parritz, L. Shen, P. Sehgal, and N. L. Letvin. 2001. In vivo T-lymphocyte activation and transient reduction of viral replication in macaques infected with simian immunodeficiency virus. J. Virol. 754713-4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Clercq, E. 2002. Cidofovir in the treatment of poxvirus infections. Antivir. Res. 551-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Giulio, D. B., and P. B. Eckburg. 2004. Human monkeypox: an emerging zoonosis. Lancet Infect. Dis. 415-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drexler, I., C. Staib, W. Kastenmuller, S. Stevanovic, B. Schmidt, F. A. Lemonnier, H. G. Rammensee, D. H. Busch, H. Bernhard, V. Erfle, and G. Sutter. 2003. Identification of vaccinia virus epitope-specific HLA-A*0201-restricted T cells and comparative analysis of smallpox vaccines. Proc. Natl. Acad. Sci. USA 100217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Earl, P. L., J. L. Americo, L. S. Wyatt, L. A. Eller, J. C. Whitbeck, G. H. Cohen, R. J. Eisenberg, C. J. Hartmann, D. L. Jackson, D. A. Kulesh, M. J. Martinez, D. M. Miller, E. M. Mucker, J. D. Shamblin, S. H. Zwiers, J. W. Huggins, P. B. Jahrling, and B. Moss. 2004. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 428182-185. [DOI] [PubMed] [Google Scholar]
- 9.Edghill-Smith, Y., H. Golding, J. Manischewitz, L. R. King, D. Scott, M. Bray, A. Nalca, J. W. Hooper, C. A. Whitehouse, J. E. Schmitz, K. A. Reimann, and G. Franchini. 2005. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 11740-747. [DOI] [PubMed] [Google Scholar]
- 10.Edghill-Smith, Y., D. Venzon, T. Karpova, J. McNally, J. Nacsa, W. P. Tsai, E. Tryniszewska, M. Moniuszko, J. Manischewitz, L. R. King, S. J. Snodgrass, J. Parrish, P. Markham, M. Sowers, D. Martin, M. G. Lewis, J. A. Berzofsky, I. M. Belyakov, B. Moss, J. Tartaglia, M. Bray, V. Hirsch, H. Golding, and G. Franchini. 2003. Modeling a safer smallpox vaccination regimen, for human immunodeficiency virus type 1-infected patients, in immunocompromised macaques. J. Infect. Dis. 1881181-1191. [DOI] [PubMed] [Google Scholar]
- 11.Enserink, M. 2004. Smallpox vaccines: looking beyond the next generation. Science 304809. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg, R. N., and J. S. Kennedy. 2008. ACAM2000: a newly licensed cell culture-based live vaccinia smallpox vaccine. Expert Opin. Investig. Drugs 17555-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammarlund, E., M. W. Lewis, J. M. Hanifin, E. L. Simpson, N. E. Carlson, and M. K. Slifka. 2008. Traditional smallpox vaccination with reduced risk of inadvertent contact spread by administration of povidone iodine ointment. Vaccine 26430-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson, D. A. 1998. Bioterrorism as a public health threat. Emerg. Infect. Dis. 4488-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson, D. A., T. V. Inglesby, J. G. Bartlett, M. S. Ascher, E. Eitzen, P. B. Jahrling, J. Hauer, M. Layton, J. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. Perl, P. K. Russell, K. Tonat, et al. 1999. Smallpox as a biological weapon: medical and public health management. JAMA 2812127-2137. [DOI] [PubMed] [Google Scholar]
- 16.Hooper, J. W., E. Thompson, C. Wilhelmsen, M. Zimmerman, M. A. Ichou, S. E. Steffen, C. S. Schmaljohn, A. L. Schmaljohn, and P. B. Jahrling. 2004. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 784433-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, D., L. Qiu, R. Wang, X. Lai, G. Du, P. Seghal, Y. Shen, L. Shao, L. Halliday, J. Fortman, L. Shen, N. L. Letvin, and Z. W. Chen. 2007. Immune gene networks of mycobacterial vaccine-elicited cellular responses and immunity. J. Infect. Dis. 19555-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izzedine, H., V. Launay-Vacher, and G. Deray. 2005. Antiviral drug-induced nephrotoxicity. Am. J. Kidney Dis. 45804-817. [DOI] [PubMed] [Google Scholar]
- 19.Lane, J. M., and V. A. Fulginiti. 2003. Transmission of vaccinia virus and rationale for measures for prevention. Clin. Infect. Dis. 37281-284. [DOI] [PubMed] [Google Scholar]
- 20.Lorich, M. F., S. B. Smith, G. T. Bessinger, and J. W. Olivere. 2004. Conjugal transfer vaccinia. J. Am. Acad. Dermatol. 51460-462. [DOI] [PubMed] [Google Scholar]
- 21.Meslin, F. X., K. Stohr, and D. Heymann. 2000. Public health implications of emerging zoonoses. Rev. Sci. Tech. 19310-317. [DOI] [PubMed] [Google Scholar]
- 22.Ortiz, A., P. Justo, A. Sanz, R. Melero, C. Caramelo, M. F. Guerrero, F. Strutz, G. Muller, A. Barat, and J. Egido. 2005. Tubular cell apoptosis and cidofovir-induced acute renal failure. Antivir. Ther. 10185-190. [PubMed] [Google Scholar]
- 23.Perkins, S. 2003. Monkeypox in the United States. Contemp. Top. Lab. Anim. Sci. 4270-72. [PubMed] [Google Scholar]
- 24.Poland, G. A., J. D. Grabenstein, and J. M. Neff. 2005. The US smallpox vaccination program: a review of a large modern era smallpox vaccination implementation program. Vaccine 232078-2081. [DOI] [PubMed] [Google Scholar]
- 25.Poland, G. A., and J. M. Neff. 2003. Smallpox vaccine: problems and prospects. Immunol. Allergy Clin. N. Am. 23731-743. [DOI] [PubMed] [Google Scholar]
- 26.Quenelle, D. C., D. J. Collins, and E. R. Kern. 2003. Efficacy of multiple- or single-dose cidofovir against vaccinia and cowpox virus infections in mice. Antimicrob. Agents Chemother. 473275-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sejvar, J. J., R. J. Labutta, L. E. Chapman, J. D. Grabenstein, J. Iskander, and J. M. Lane. 2005. Neurologic adverse events associated with smallpox vaccination in the United States, 2002-2004. JAMA 2942744-2750. [DOI] [PubMed] [Google Scholar]
- 28.Shen, Y., L. Shen, P. Sehgal, D. Huang, L. Qiu, G. Du, N. L. Letvin, and Z. W. Chen. 2004. Clinical latency and reactivation of AIDS-related mycobacterial infections. J. Virol. 7814023-14032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen, Y., D. Zhou, L. Qiu, X. Lai, M. Simon, L. Shen, Z. Kou, Q. Wang, L. Jiang, J. Estep, R. Hunt, M. Clagett, P. K. Sehgal, Y. Li, X. Zeng, C. T. Morita, M. B. Brenner, N. L. Letvin, and Z. W. Chen. 2002. Adaptive immune response of Vγ2Vδ2+ T cells during mycobacterial infections. Science 2952255-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sniadack, M. M., L. J. Neff, D. L. Swerdlow, R. A. Schieber, M. M. McCauley, and G. T. Mootrey. 2008. Follow-up of cardiovascular adverse events after smallpox vaccination among civilians in the United States, 2003. Clin. Infect. Dis. 46(Suppl. 3)S251-S257. [DOI] [PubMed] [Google Scholar]
- 31.Stittelaar, K. J., J. Neyts, L. Naesens, G. van Amerongen, R. F. van Lavieren, A. Holy, E. De Clercq, H. G. Niesters, E. Fries, C. Maas, P. G. Mulder, B. A. van der Zeijst, and A. D. Osterhaus. 2006. Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection. Nature 439745-748. [DOI] [PubMed] [Google Scholar]
- 32.Stittelaar, K. J., G. van Amerongen, I. Kondova, T. Kuiken, R. F. van Lavieren, F. H. Pistoor, H. G. Niesters, G. van Doornum, B. A. van der Zeijst, L. Mateo, P. J. Chaplin, and A. D. Osterhaus. 2005. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J. Virol. 797845-7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swerdlow, D. L., M. H. Roper, J. Morgan, R. A. Schieber, L. S. Sperling, M. M. Sniadack, L. Neff, J. W. Miller, C. R. Curtis, M. E. Marin, J. Iskander, P. Moro, P. Hightower, N. H. Levine, M. McCauley, J. Heffelfinger, I. Damon, T. J. Torok, M. Wharton, E. E. Mast, and G. T. Mootrey. 2008. Ischemic cardiac events during the Department of Health and Human Services Smallpox Vaccination Program, 2003. Clin. Infect. Dis. 46(Suppl. 3)S234-S241. [DOI] [PubMed] [Google Scholar]
- 34.Talbot, T. R., J. Peters, L. Yan, P. F. Wright, and K. M. Edwards. 2006. Optimal bandaging of smallpox vaccination sites to decrease the potential for secondary vaccinia transmission without impairing lesion healing. Infect. Control Hosp. Epidemiol. 271184-1192. [DOI] [PubMed] [Google Scholar]
- 35.Talbot, T. R., E. Ziel, J. K. Doersam, B. LaFleur, S. Tollefson, and K. M. Edwards. 2004. Risk of vaccinia transfer to the hands of vaccinated persons after smallpox immunization. Clin. Infect. Dis. 38536-541. [DOI] [PubMed] [Google Scholar]
- 36.Tegnell, A., B. Wahren, and F. Elgh. 2002. Smallpox—eradicated, but a growing terror threat. Clin. Microbiol. Infect. 8504-509. [DOI] [PubMed] [Google Scholar]
- 37.Wyatt, L. S., P. L. Earl, L. A. Eller, and B. Moss. 2004. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc. Natl. Acad. Sci. USA 1014590-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang, G., D. C. Pevear, M. H. Davies, M. S. Collett, T. Bailey, S. Rippen, L. Barone, C. Burns, G. Rhodes, S. Tohan, J. W. Huggins, R. O. Baker, R. L. Buller, E. Touchette, K. Waller, J. Schriewer, J. Neyts, E. DeClercq, K. Jones, D. Hruby, and R. Jordan. 2005. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J. Virol. 7913139-13149. [DOI] [PMC free article] [PubMed] [Google Scholar]