Abstract
Cutaneous human betapapillomaviruses (beta-HPVs) are widespread in the general population and have been associated with skin cancer. To evaluate the impact of continuous person-to-person contact within families on an individual's beta-HPV type spectrum, we collected serial skin swab samples from parents and children from 10 families. All participants were found to be beta-HPV DNA positive, with 1 to 13 types at study entry (median, 4.0 types). Initial and cumulative (2 to 16 types) HPV type multiplicities varied widely between different families but only a little between family members. The high intrafamilial correlation of HPV multiplicity is already obvious for babies aged 10 days to 10 months. Family members typically displayed similar spectra of HPV types. More than 75% of the HPV types in babies were also detected in their parents. This indicates that HPV transmission mainly results from close contact between family members. Type-specific persistence for at least 9 months was more prevalent in parents (92%) than in children (66%). Of the types detected throughout the study, 24% turned out to persist in the parents and only 11% in the children. Interestingly, about one-half of the HPV types found to persist in one of the parents occurred less frequently or even only sporadically in the spouse. Similarly, only one-third of the persisting parental types also persisted in their children. This indicates that even regular exposure to cutaneous HPV does not necessarily lead to the establishment of a persistent infection, which may point to type-specific susceptibilities of different individuals.
Cutaneous human papillomaviruses (HPV) cluster in the genera Betapapillomavirus (beta-HPV), Gammapapillomavirus, Mupapillomavirus, and Nupapillomavirus of the family Papillomaviridae, and several types are classified in genus Alphapapillomavirus together with mostly mucosal types (7). Common, plantar, and flat warts are induced by representatives of genera Alphapapillomavirus, Gammapapillomavirus, Mupapillomavirus, and Nupapillomavirus most frequently in children and adolescents 12 to 16 years of age (13). Beta-HPV causes flat warts and red-brown plaque-like and pityriasis versicolor-like lesions in patients with epidermodysplasia verruciformis but no characteristic pathology in the general population (14). Clinically inapparent infections by members of genera beta-HPV and Gammapapillomavirus have been found to be extremely widespread in the general population when testing skin swabs or plucked eyebrow hairs for HPV DNA (1, 3, 4). These HPV types seem to be acquired already in the first days of life (2), but everybody will be exposed throughout life to multiple beta-HPV and gamma-HPV types in view of their high prevalence.
Follow-up studies showed that a part of the transmitted HPV is able to establish persistent infections in the new host. Type-specific persistence was observed over 5 to 7 years in forehead skin and for up to 24 months in eyebrow hairs (6, 10).
Beta-HPVs deserve special interest because of compelling evidence for the carcinogenicity of beta-HPV types 5 and 8 in the skin of patients with epidermodysplasia verruciformis and because of growing evidence for the carcinogenicity of beta-HPV in the skin in general (12). Beta-HPV DNA was detected in up to 85% of precancerous actinic keratoses (15), in 30% to 50% of cutaneous squamous cell carcinomas of immunocompetent patients, and in up to 90% of cutaneous squamous cell carcinomas of immunosuppressed patients (reviewed in reference 14).
We became interested in the impact of continuous person-to-person contact within a family on an individual's beta-HPV type spectrum. To address this question, we collected serial samples from the foreheads, the backs of the hands, and the buttocks of parents and children. Since young children were involved, easy and painless skin swabs were used as study material instead of plucked hairs. To ensure a comprehensive and sensitive analysis, we chose a recent HPV detection and typing method which allows the parallel identification of the 25 established betapapillomavirus (beta-PV) genotypes (5).
MATERIALS AND METHODS
Study population.
Ten families with young children voluntarily applied to participate in this study after local announcement. The study population comprised 40 individuals: 14 children (median age at study entry, 9.6 months; range, 10 days to 8.6 years), 10 mothers (median age, 31.5 years; range, 29.1 to 44.5 years), 10 fathers (median age, 33.2 years; range, 25.9 to 44.5 years), and 6 grandparents (median age, 65.8 years; range, 57.3 to 77.8 years). The sampling period spanned a mean of 13.7 months (range, 0 to 28.5 months), with a median of 4.4 sampling episodes (range, 1 to 9 episodes). Altogether 484 swab samples from the forehead, back of the right hand, and right side of the buttock were collected. The study protocol was approved by the ethics review board of the University of Cologne, and the study was conducted according to the Declaration of Helsinki principles. The parents gave written informed consent to participate in the study and answered a questionnaire about the presence/absence of psoriasis or neurodermatitis or other acute or chronic diseases. No diseases were present in any of the participants, and none of them suffered from clinically apparent warts. Samples were assigned pseudonyms, and all analyses were performed blinded to any information about the samples.
Test procedures.
Tubes including wood sticks with cotton (PS swab tube; Greiner Bio-One, Solingen, Germany) and filled with 2 ml phosphate-buffered saline (PBS) were used for swab sampling. Samples within one family were taken by one or both of the parents. Participants were advised to take samples in the evening. Swabs were soaked in saline solution (PBS) and first used to moisten a skin area of 4 by 5 cm. After 1 minute, swabs were firmly drawn back and forth 15 times over the moistened area and then suspended in the remaining PBS. The samples were stored at 4°C and processed within 72 h. Suspensions were centrifuged at 14,000 × g for 5 min, and DNA extraction of pellets (QIAamp DNA minikit; Qiagen, Hilden, Germany) was carried out according to the manufacturer's protocol. Beta-HPV detection and genotyping were carried out by a broad-spectrum PCR with subsequent analysis of amplimers with a reverse hybridization assay that permitted specific detection and identification of the 25 established beta-PV genotypes as described previously (5).
Statistical analysis.
All analyses (Pearson test, paired t test) were performed two-sided using SPSS 15.0 (SPSS Inc., Chicago, IL).
RESULTS
HPV DNA prevalence and multiplicity in cutaneous swabs.
All 40 participants of this study were found to be beta-HPV DNA positive, although to highly variable degrees. The positivity rates of the samples from different individuals ranged from 7 to 97%. Considering site-specific prevalence per individual, HPV positivity was on average higher on samples from the forehead (79%) and the back of the hand (81%) than on those from the buttock (64%). One to 13 HPV types were detected per individual in the three swabs taken at study entry (median, 4.0 types), and the cumulative HPV type multiplicity over all samples from a participant ranged from 1 to 16 types (Fig. 1).
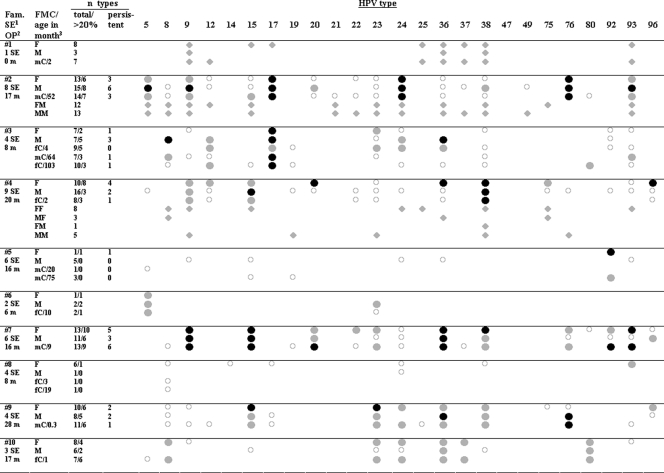
FIG. 1.
Persistent and nonpersistent HPV infections and family-specific type spectra. Black circles, persistently found types (for definition of persistence see text); gray circles, types detected in >20% of the samples but not persistent according to the definition; open circles, types detected in <20% of the samples; gray diamonds, types in participants tested only once. F, father; M, mother; fC, female child; mC, male child; MM, mother of the mother; FM, father of the mother; MF, mother of the father; FF father of the father. Footnotes: 1, number of sampling episodes (SE); 2, observation period (OP) in months; 3, age in months of children at study entry.
The HPV type multiplicity varied widely between different families (2 to 18 types), but only a little between family members (Fig. 1). For example, high HPV type multiplicities could be observed for all members of families 2 and 7, with 12 to 15 and 11 to 13 HPV types, respectively (Fig. 1). In contrast, all members of family 5 had low type multiplicities (one to five types). There was some concern that differences in multiplicity between families were related to systematic differences in sampling. Despite the specified protocol, the intensity of swabbing and the size of the swabbed area may have varied between the different families, and this may have resulted in differences in the number of squames and cells per swab. We therefore tested whether type multiplicity correlated with the amount of cellular DNA but observed no such correlation (R = −0.059; paired t test) in 28 randomly chosen samples. When the samples were divided into three equally large groups according to cellular DNA input, the positivity rates of the 18 specific types present in these swabs did not appear to differ between these groups. Even HPV types with low prevalence could be detected in the group with the smallest amount of cellular DNA. Furthermore, no impact of the individual sampling procedure on HPV multiplicity was observed after two individuals from families with low type multiplicities were swabbed by an individual who obtained a high type multiplicity by self-swabbing (data not shown). This experiment suggests additionally that the sampling technique is not prone to contamination by the person taking the swab. Altogether, these observations indicate that the characteristic HPV multiplicities observed for different families are not biased by self-sampling.
To compare the prevalences of specific HPV types in the study population, we considered only the samples from parents and their youngest children taken at study entry to avoid any bias due to different numbers of family members and samples per participant. The most prevalent HPV types were 93, 23, 17, 76, 9, 24, 38, and 36 in ascending sequence (15.6% to 34.4%).
It was most interesting to note that the high intrafamilial correlation of HPV type multiplicity is already obvious for babies aged 10 days to 10 months (babies versus mothers: R = 0.915, P = 0.001; babies versus fathers: R = 0.662, P = 0.07; Pearson test). All the babies showed at least 1 beta-HPV type and up to 10 different beta-HPV types in their first swabs (Table 1). More than 75% of the HPV types detected in babies were also detected in at least one of their parents. Specifically, six of eight HPV types detected in the 10-day-old baby were found in its mother or father.
TABLE 1.
HPV DNA in skin swabs from babies and parents collected at study entry
| Childa/age in mo | Family | HPV type(s)b in:
|
||
|---|---|---|---|---|
| Child | Mother | Father | ||
| mC/0.3 | 9 | 9, 12, 17, 23, 24, 36, 38, 76 | 17, 23, 36, 38, 76, 96 | 15, 23, 24, 36, 38, 96 |
| fC/1 | 10 | 8, 23, 36, 37, 80 | 23, 24, 36, 38 | 36 |
| mC/2 | 1 | 9, 12, 25, 36, 37, 38, 93 | 9, 36, 38 | 9, 15, 17, 25, 36, 37, 38, 93 |
| fC/2 | 4 | 22, 38, 96 | 15, 19, 38 | 12, 15, 20, 36, 38, 93, 96 |
| fC/3 | 8 | Xc | 24 | 8, 14, 24, 93 |
| fC/4 | 3 | 17, 23, 24, 36 | 8, 17, 24, 36 | 17, 92, 93 |
| mC/9 | 7 | 9, 15, 19, 23, 24, 36, 38, 76, 92, 93 | 9, 15, 23, 36, 38, 76, 92, 96 | 9, 15, 20, 24, 36, 38, 76, 93, 96 |
| fC/10 | 6 | 5 | 5 | 5 |
fC, female child; mC, male child.
HPV types in children that were also detected in at least one of the parents at study entry are in boldface.
X, indeterminate HPV type detected with a universal probe for beta-HPV types (5).
HPV persistence and family-specific beta-HPV spectra.
HPV persistence could be analyzed in parents and all children of six families (families 2, 3, 4, 5, 7, and 9) for whom samples from at least four points in time and a follow-up period of at least nine months were available (mean follow-up time: 17.7 months; range, 9 to 28.5 months; mean number of time points, 6.0; range, 4 to 9 time points). Type-specific persistence was defined as HPV DNA being site specifically detectable in more than two-thirds of the samples or over 9 months with at most one negative sample in between. HPV types found in less than 20% of samples were defined as sporadic. Of the infections classified as sporadic, 38% occurred on the back of the hand, 36% on the forehead, and 26% on the buttocks. Persistent HPV types could be observed in 92% of 12 adults and 66% of 9 children, with a maximum of 6 types and a median of 2.5 HPV types in parents and 1.0 HPV type in children (Fig. 1). This corresponds on average to 30% of the detectable HPV types in parents and to 12% in children.
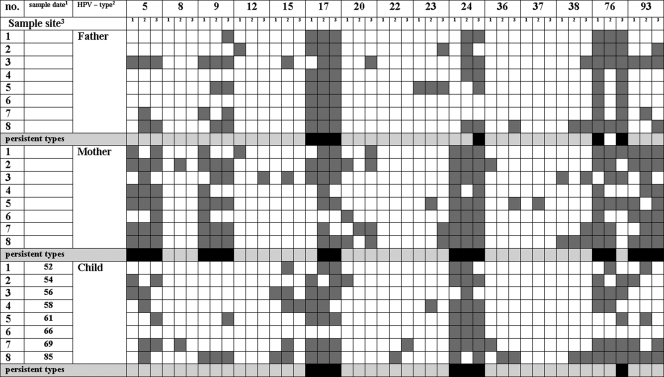
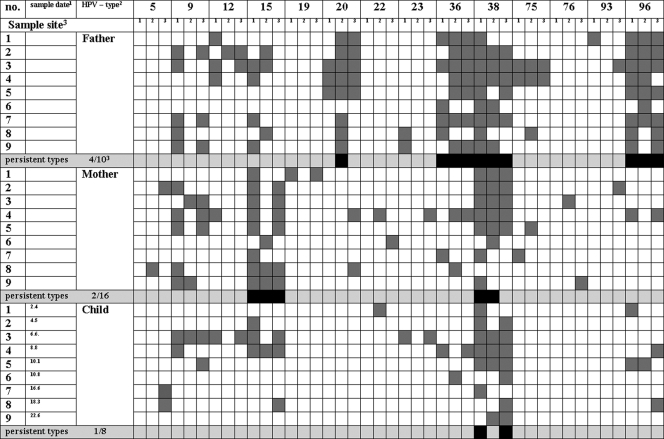
Overall, one-half of the persisting HPV types differed between the parents of a family. In family 2 (Fig. 2), HPV types 17, 24, and 76 fulfilled the definition of persistence, both in the father and mother, whereas HPV types 5, 9, and 93 appear to persist only in the mother. The persistent HPV types 5, 9, 24, and 93 found on the mother were furthermore detected on both parents of the mother. For the father of family 4 (Fig. 3), HPV types 20, 36, 38, and 96 could be identified as persistent. The mother persistently harbored HPV types 15 and 38. Thus, except for type 38, different HPV types persisted in the parents. HPV types 20, 36, and 96, persistently detected on the father, were only sporadically detected in swabs from the mother. HPV type 15, which persists on the mother, was also detected in 7 of 27 swabs from the father. Five of eight HPV types frequently or persistently detected in the father or mother were also detected in the respective grandparents (Fig. 1).
FIG. 2.
Persistent HPV types and types detected at different sampling dates in parents and the child of family 2. Footnotes: 1, age of child in months; 2, HPV type 49 was detected once in the mother, and HPV types 21 and 80 were detected in the child and are not included in the figure; 3, sample sites were forehead (1), buttock (2), and back of the hand (3).
FIG. 3.
Persistent HPV types and types detected at different sampling dates in parents and the child of family 4. Footnotes: 1, age of child in months; 2, HPV types 24 and 92 and an unspecified HPV type were detected once in the mother and are not included in the figure; 3, sample sites were forehead (1), buttock (2), and back of the hand (3).
Eleven out of 13 HPV types that were found to persist in children of all age groups were also persistently detected in at least one of the parents. No child had a persistent type that was only sporadically present or not present in the parents. However, not all persistent HPV types on the parents were persistent on children, too. HPV types 17, 24, and 76, which persist on both parents of family 2, were also persistently found in the child. In contrast, types 5, 9, and 93 were not persistently detected on the child even though they dominated in one of the parents. For the child of family 4, the only persisting HPV type was 38, which is the only common persistent type found on the parents.
Several HPV types appeared to be clearly overrepresented in the samples from families 2, 3, 4, 7, and 9 (Fig. 1) compared to the samples collected at study entry from parents and the youngest children of the nine other families. For instance, in family 2, the prevalence ratios of HPV types 5, 17, 24, 76, and 93 were 4.5 (49% versus 11%), 4.4 (71% versus 16%), 3.9 (78% versus 20%), 3.9 (67% versus 17%), and 3.1 (50% versus 16%), respectively. The impression of family-specific spectra is further supported by the fact that HPV types which are prevalent in general may be underrepresented in specific families, e.g., type 36 in family 2 (13% versus 34%) and types 17, 24, 76, and 93 in family 4 (0% versus 16%, 2% versus 20%, 4% versus 17%, and 4% versus 16%, respectively).
DISCUSSION
This study describes the first comprehensive analysis of beta-HPV spectra in healthy families. We observed a high HPV DNA prevalence and often a high type multiplicity on the skin, which increased with the number of samples analyzed. The type multiplicities found here, with up to 13 different types at a single point in time, exceed the numbers observed in earlier studies, which differed in methods and material. Antonsson et al. (1) also analyzed multiple skin swabs of both healthy individuals and dialysis patients but used a different primer set for PCR and typed by sequencing 4 to 10 clones of PCR products of each sample. They found up to seven different HPV types or type candidates per individual. It can be assumed that more HPV types would have been found if additional clones had been analyzed. de Koning et al. (6) used the detection and typing method applied in this study but analyzed eight samples of 8 to 10 plucked eyebrow hairs collected over 2 years from 23 adults. They observed a maximum number of six to nine types at different time points and median HPV type multiplicities ranging from 0.5 to 2. When comparing these data with a maximum of 13 different types and a median of 4.0 types in the current study, it must be realized that detection of HPV DNA in plucked hairs and skin swabs reflects to different degrees either established infections, transient infections, or just surface contamination. Hair follicles have been proposed as a possible reservoir of beta-HPV (reviewed in reference 14), and thus HPV detection in follicles more likely reflects persistent infections. In contrast, in the interfollicular epidermis one may expect a higher proportion of transient infections. Furthermore, rather large skin areas are sampled by swabbing, which makes this material more sensitive to surface contamination than plucked hairs. By the analysis of swabs, it is not possible to distinguish between an established infection and surface contamination in individual cases.
An established infection appears to be more likely if a type is repeatedly detected in consecutive swabs. We therefore used a very stringent definition for HPV DNA persistence: the DNA must be site specifically detectable in more than two-thirds of the samples or over 9 months with at most one negative sample in between. According to this definition, two-thirds of the children and more than 90% of the adults had at least one persistent HPV type. de Koning et al. (6) showed type-specific persistence of beta-HPV in consecutively plucked eyebrow hairs for at least one-half of a year in 74% of adults.
Different families showed characteristic HPV multiplicities, both when sporadically detected HPV types are included and when they are excluded. Furthermore, similar type spectra among family members allowed the identification of family-specific HPV types. These findings most likely reflect continuous HPV transmission by close direct contact between family members and via virus-contaminated surfaces in the common household. High multiplicities in specific families may result from a high multiplicity in at least one of the parents.
As noted before (2), beta-HPV was already detectable on the skin of children in the first months of life. In babies less than 1 month old, detection of beta-HPV DNA in skin swabs primarily reflects exposure and not-yet-established infections (2). In the current study, all children who were less than 1 year old already showed a type multiplicity strongly correlated with that of their parents. Finding the vast majority of HPV types from the children also in one or both parents strongly argues for a predominantly intrafamilial transmission. A few HPV types that were present on infants and not on parents may have been transmitted from other contact persons such as nurses or grandparents or may just have escaped detection in parents at study entry.
HPV types detected sporadically in any family member mostly occurred only sporadically or not at all in other members of that family. In about 30% of the cases sporadic HPV types in one family member were found more frequently or even persistently in at least one other family member. Sporadically detected HPV types may therefore result from transmission within the family but may also be from outside.
It was interesting that about one-half of the HPV types found to persist in one of the parents occurred less frequently or even only sporadically in the spouse. This indicates that even continuous transmission of cutaneous HPV over years through close physical contact does not necessarily result in a persistent or at least frequently detected infection in spouses. Similarly, only a fraction of HPV types found persistently in one of the parents established a persistent infection in the children. On the other hand, HPV types 20 and 92 were persistently detected in the child of family 7 and only less frequently in the parents. This is consistent with finding unique beta-PV profiles in plucked-hair samples from individuals sharing a student household for 6 to 12 months (6). Although the contact with non-family members is in general certainly less intense than that within families, one may nevertheless expect a regular exposure to cutaneous HPV. In certain families of this study we could even confirm exposure to several prevalent HPV types by sporadic detection without finding evidence for persistent infections.
There are numerous examples of HPV types that are abundant in one individual of a common household but only infrequently detected on other family members. This may point to type-specific beta-HPV susceptibility of different individuals. This concept would be in line with finding individual-specific HPV types in different immunosuppressed renal transplant recipients, which prevail in multiple benign and malignant lesions located on various anatomical sites and partially removed up to 7 years apart (8, 9, 11).
There are no data so far on a molecular basis of type-specific susceptibility. Intracellular or intercellular control mechanisms for the replication and transcription of the viral genome and the activity of viral proteins, as well as immune reactions against HPV-harboring cells, may be responsible. Individual immunologic determinants such as HLA alleles may be of major importance for the clearance of inapparent or overt HPV infections. It would be interesting to analyze possible correlations between host genetics and individual spectra of persisting beta-HPV types.
Acknowledgments
We thank Monika Junk for excellent technical assistance and W. Lehmacher, H. Christ, and Z. Tolman for helpful discussions. Furthermore, we are very much obliged to the families who participated in this study.
Footnotes
Published ahead of print on 5 November 2008.
REFERENCES
- 1.Antonsson, A., O. Forslund, H. Ekberg, G. Sterner, and B. G. Hansson. 2000. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. J. Virol. 7411636-11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonsson, A., S. Karanfilovska, P. G. Lindqvist, and B. G. Hansson. 2003. General acquisition of human papillomavirus infections of skin occurs in early infancy. J. Clin. Microbiol. 412509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astori, G., D. Lavergne, C. Benton, B. Hockmayr, K. Egawa, C. Garbe, and E. M. de Villiers. 1998. Human papillomaviruses are commonly found in normal skin of immunocompetent hosts. J. Investig. Dermatol. 110752-755. [DOI] [PubMed] [Google Scholar]
- 4.Boxman, I. L., R. J. Berkhout, L. H. Mulder, M. C. Wolkers, J. N. Bouwes Bavinck, B. J. Vermeer, and J. ter Schegget. 1997. Detection of human papillomavirus DNA in plucked hairs from renal transplant recipients and healthy volunteers. J. Investig. Dermatol. 108712-715. [DOI] [PubMed] [Google Scholar]
- 5.de Koning, M., W. Quint, L. Struijk, B. Kleter, P. Wanningen, L. J. van Doorn, S. J. Weissenborn, M. Feltkamp, and J. ter Schegget. 2006. Evaluation of a novel highly sensitive, broad-spectrum PCR-reverse hybridization assay for detection and identification of beta-papillomavirus DNA. J. Clin. Microbiol. 441792-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Koning, M. N. C., L. Struijk, J. N. Bouwes Bavinck, B. Kleter, J. ter Schegget, W. G. V. Quint, and M. C. W. Feltkamp. 2007. Betapapillomaviruses frequently persist in the skin of healthy individuals. J. Gen. Virol. 881489-1495. [DOI] [PubMed] [Google Scholar]
- 7.de Villiers, E. M., C. Fauquet, T. R. Broker, H. U. Bernard, and H. zur Hausen. 2004. Classification of papillomaviruses. Virology 32417-27. [DOI] [PubMed] [Google Scholar]
- 8.de Villiers, E. M., D. Lavergne, K. McLaren, and E. C. Benton. 1997. Prevailing papillomavirus types in non-melanoma carcinomas of the skin in renal allograft recipients. Int. J. Cancer 73356-361. [DOI] [PubMed] [Google Scholar]
- 9.Harwood, C. A., T. Surentheran, J. M. McGregor, P. J. Spink, I. M. Leigh, J. Breuer, and C. M. Proby. 2000. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J. Med. Virol. 61289-297. [DOI] [PubMed] [Google Scholar]
- 10.Hazard, K., A. Karlsson, K. Andersson, H. Ekberg, J. Dillner, and O. Forslund. 2007. Cutaneous human papillomaviruses persist on healthy skin. J. Investig. Dermatol. 127116-119. [DOI] [PubMed] [Google Scholar]
- 11.Hopfl, R., G. Bens, U. Wieland, A. Petter, B. Zelger, P. Fritsch, and H. Pfister. 1997. Human papillomavirus DNA in non-melanoma skin cancers of a renal transplant recipient: detection of a new sequence related to epidermodysplasia verruciformis associated types. J. Investig. Dermatol. 10853-56. [DOI] [PubMed] [Google Scholar]
- 12.IARC. 2007. Human papillomaviruses. IARC Monogr. Eval. Carcinog. Risks Hum. 90476-477. [PMC free article] [PubMed] [Google Scholar]
- 13.Jablonska, S., S. Majewski, S. Obalek, and G. Orth. 1997. Cutaneous warts. Clin. Dermatol. 15309-319. [DOI] [PubMed] [Google Scholar]
- 14.Pfister, H. 2003. Human papillomavirus and skin cancer. J. Natl. Cancer Inst. Monogr. 200352-56. [DOI] [PubMed] [Google Scholar]
- 15.Pfister, H., P. G. Fuchs, S. Majewski, S. Jablonska, I. Pniewska, and M. Malejczyk. 2003. High prevalence of epidermodysplasia verruciformis-associated human papillomavirus DNA in actinic keratoses of the immunocompetent population. Arch. Dermatol. Res. 295273-279. [DOI] [PubMed] [Google Scholar]