Abstract
Lipoteichoic acid (LTA) is one of two anionic polymers on the surface of the gram-positive bacterium Staphylococcus aureus. LTA is critical for the bacterium-host cell interaction and has recently been shown to be required for cell growth and division. To determine additional biological roles of LTA, we found it necessary to identify permissive conditions for the growth of an LTA-deficient mutant. We found that an LTA-deficient S. aureus ΔltaS mutant could grow at 30°C but not at 37°C. Even at the permissive temperature, ΔltaS mutant cells had aberrant cell division and separation, decreased autolysis, and reduced levels of peptidoglycan hydrolases. Upshift of ΔltaS mutant cells to a nonpermissive temperature caused an inability to exclude Sytox green dye. A high-osmolarity growth medium remarkably rescued the colony-forming ability of the ΔltaS mutant at 37°C, indicating that LTA synthesis is required for growth under low-osmolarity conditions. In addition, the ΔltaS mutation was found to be synthetically lethal with the ΔtagO mutation, which disrupts the synthesis of the other anionic polymer, wall teichoic acid (WTA), at 30°C, suggesting that LTA and WTA compensate for one another in an essential function.
Staphylococcus aureus is a major pathogenic agent of opportunistic infections. Indeed, S. aureus can cause endocarditis, pneumonia, and septic shock, particularly in immunocompromised patients. The appearance and spread of multiple-drug-resistant strains of S. aureus make these infections difficult to treat (18). Thus, understanding the physiology of staphylococcal cell growth and pathogenesis is important for knowing how to treat infectious diseases and develop novel antibiotics.
On the surfaces of gram-positive S. aureus cells are two negatively charged polymers: lipoteichoic acid (LTA) and wall teichoic acid (WTA). Together with the peptidoglycan, they form a polyanionic network or matrix. While not all gram-positive bacteria have conventional LTA and WTA, those without these polymers generally have functionally similar anionic polymers, suggesting that cell wall anionic polymers are important for the growth of these organisms (36). WTA in S. aureus consists of repeating units of 1,5-d-ribitol phosphate and is attached via a linkage unit to the C-6 position of MurNAc residues of the peptidoglycan. The repeating units of d-ribitol phosphate are joined via phosphodiester linkages to form linear chains and are substituted with GlcNAc residues and d-alanyl esters (2, 4). WTA is critical for cell surface hydrophobicity, maintenance of cell shape, and adhesion to host cells (36, 51). WTA is also thought to be dispensable for bacterial cell growth, however, because a WTA-depleted ΔtagO mutant is viable (50).
Staphylococcal LTA consists of about 25 repeating units of poly(1-3)-glycerolphosphate linked to a membrane anchor, a diglycosyldiacylglycerol (12). Additionally, about 60 to 70% of the glycerol residues are d-alanylated. LTA is a major macroamphiphile of gram-positive bacteria that constitutes 1/10 to 1/5 of the outer leaflet of the cell membrane. Physicochemical studies have shown that LTA increases the phase transition temperature of the phospholipid membrane by stabilizing the membrane (16). In addition, LTA has been proposed to have roles in the localization and activity of peptidoglycan hydrolases on the cell surface (53). Moreover, LTA functions in bacterium-host cell interaction, with roles in directing cell adhesion, phagocytosis, and the induction of inflammatory cytokines (14, 34, 51).
Biochemical analyses have revealed that the polyglycerol phosphate is synthesized by a membrane protein that uses a membrane phospholipid, phosphatidylglycerol (PG), as a substrate (5, 28, 39). Recently, the ltaS (lipoteichoic acid synthase) gene was identified as a gene encoding a polyglycerolphosphate synthase of LTA in S. aureus (15). When LtaS expression was limited in S. aureus cells at 37°C, the amount of LTA in the cells decreased, cell growth was arrested, and abnormalities in cell division and separation appeared (15). Nonetheless, the exact physiological roles of LTA in bacterial cell growth and host cell interaction remain unclear.
We have previously isolated mutant temperature-sensitive S. aureus strains to identify essential genes in S. aureus (22, 31). Here we describe how the ltaS gene was identified via a different experimental approach; we obtained three mutant strains with a temperature sensitivity that could be complemented by the hypothetical gene SA0674 (ltaS). We show that the ltaS gene encodes a polyglycerolphosphate synthase of LTA that uses PG as a substrate and that the ΔltaS mutation results in a complete depletion of LTA from the cells. Notably, we identified permissive growth conditions for ΔltaS mutant cells: the ltaS gene is dispensable for cell growth at 30°C, though cells still exhibit irregular cell division and separation. At 37°C or higher, ltaS is essential for colony formation, viability, and resistance to low-osmolarity conditions. In addition, we found that synthetic lethality results from the combination of the ΔltaS mutation with the ΔtagO mutation, which eliminates WTA, suggesting that LTA and WTA have complementing essential functions.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
S. aureus and Escherichia coli strains were grown in Luria-Bertani (LB) medium (1% tryptone, 0.5% yeast extract, and 1% NaCl) containing, where appropriate, 12.5 μg/ml chloramphenicol (Cm), 20 μg/ml phleomycin (Phleo), 100 μg/ml ampicillin (Amp), 50 μg/ml kanamycin (Km), or 50 μg/ml thymine at 30°C or 37°C. The bacterial strains and plasmids used in this study are listed in Table 1. S. aureus temperature-sensitive mutant cells were derived from RN4220 (37). The plasmid pKE515-derived library and the E. coli-S. aureus shuttle vector (30) were as described previously (21, 22, 31). Genetic engineering of S. aureus cells (including electroporation, plasmid extraction, insertional deletion of genes, and phage transduction) was conducted as described previously (23, 24, 33, 35). To prepare a phage lysate from the ΔtagO mutant cells, a complementing strain was used, since tagO is required for phage absorption (50). To insert the Km resistance gene near the ltaS gene, we amplified the SA0667 and SA0668 gene region (5 kb away from ltaS) by PCR using primers ACTTCTTTGAAATGTTTTTT and TTATCAATACCAACAACTTG, and the resultant fragment was inserted at the SmaI site of pSF151. The resultant plasmid was introduced into temperature-sensitive mutant cells harboring point mutations in ltaS, and Km-resistant transformants, generated by site homologous recombination, were selected. The chromosomal ltaS gene was replaced with a Phleo resistance gene as described previously (9) but with some modifications. First, a DNA fragment of approximately 1.5 kbp from upstream or downstream of the ltaS gene was amplified by PCR using primers SA0674-P1 (TTCGAAATATGGTCCGGATA) and SA0674-P2 (TATTGGATCCGGAAATTATTTGCATCGGACTC) or SA0674-P3 (GTTCAGCAATCGGCATAGTCATCAAAGCCTTATCAA) and SA0674-P4 (GCTTTTGCTCTCCACCAGAT), respectively. Second, the Phleo resistance gene was amplified using primers Phleo-P1 (GGATCCAATAGACCAGTTGCA) and Phleo-P2 (CGATTGCTGAACAGATTAATAA), containing sequences complementary to SA0674-P2 and SA0674-P3, respectively, and to the pUC19-upp-phleo template sequence. Next, the three amplified fragments were connected by joining PCR (9), and the resultant fragment was inserted at the SmaI site of pSF151, creating pSFltaS. The pSFltaS plasmid was introduced into RN4220 cells harboring pM102-ltaS, and Km-resistant transformants, generated by first-site homologous recombination, were selected. From these cells, Km-sensitive strains with both Phleo resistance and Cm resistance, which were formed by a second-site homologous recombination event, were selected and named M0674/pM102-ltaS. The pM102 vector (M. Matsuo et al., unpublished data) is a shuttle vector that can be propagated in E. coli (Cmr) or S. aureus (Cmr), facilitated by replicons of E. coli pUC19 and S. aureus pN315, respectively. The pM102-ltaS plasmid is a pM102 derivative that harbors the ltaS gene. Deletion of the chromosomal ltaS gene in M0674/pM102-ltaS was confirmed by PCR using primers SA0674-P1 and SA0674-P4 (data not shown). M0674/pM102-ltaS was transformed with pM101 (Matsuo et al., unpublished), which is incompatible with pM102, since they possess the same replicon, but has a different marker from pM102 (namely, Km resistance). A Km-resistant transformant, M0674/pM101, was obtained at 30°C. The loss of pM102-ltaS in M0674/pM101 was confirmed by Cm sensitivity (data not shown). In M0107, the spa gene, which encodes protein A, has been disrupted in RN4220 cells by replacement with a Phleo resistance gene by double-crossover recombination as described above using primers spa-P1 (GGGTCTAGAAAAAAGTCAAGCCTGAAGTCG), spa-P2 (TATTGGATCCAAAGTGGGGCTTTGAATGTG), spa-P3 (CCCGGGTACCTGCAGCGTTATTAGCTGGAC), spa-P4 (GGGGAATTCTAATTGGTGCAACTGGGACA), and Phleo-P1 and Phleo-P3 (GGTACCCGGGCGATTGCTGAA). The pNDX1 vector is a derivative of the E. coli-S. aureus shuttle vector pND50 (54); it has a xyl-tet promoter-operator fusion and the tetR gene from pWH353 (13). pNDXltaS-His is a derivative of pNDX1 that contains a C-terminally His-tagged LtaS open reading frame (ORF) under the control of the xyl-tet promoter-operator. pSltaS was a derivative of pKE515 obtained from a pKE515-based genomic DNA library. pSF151 is a so-called suicide vector for S. aureus (23, 47).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| JM109 | recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 Δ(lac-proAB)/F′[traD36 proAB+lacIqlacZ ΔM15] | TakaraBio |
| JE5513Tc | Hfr man1 pps lpp2 thyA uvrC279::Tn10 | 3 |
| YA5513Tc | JE5513Tc pgsA3 | 3 |
| S. aureus | ||
| RN4220 | Restriction minus wild type | 37 |
| TS2519 | RN4220 ltaS2519 | This work |
| TS2947 | RN4220 ltaS2947 | This work |
| TS8643 | RN4220 ltaS8643 | This work |
| TS2519kan | TS2519 Ω(SA0667-SA0668::pSF151kan) | This work |
| TS2947kan | TS2947 Ω(SA0667-SA0668::pSF151kan) | This work |
| TS8643kan | TS8643 Ω(SA0667-SA0668::pSF151kan) | This work |
| M0674/pM101 | RN4200 ΔltaS::phleo/pM101 | This work |
| M0674/pM101-ltaS | RN4200 ΔltaS::phleo/pM101-ltaS | This work |
| M0674/pM102-ltaS | RN4200 ΔltaS::phleo/pM102-ltaS | This work |
| M0107 | RN4220 Δspa::phleo | This work |
| M0702 | RN4220 ΔtagO::pT0702 | 24 |
| M0875 | RN4220 ΔypfP::pT0875 | 24 |
| YO1001/pM101 | M0674 ΔypfP::pT0875/pM101 | This work |
| YO1001/pM102-ltaS | M0674 ΔypfP::pT0875/pM102-ltaS | This work |
| YO1003/pM102-ltaS | M0674 ΔtagO::pT0702/pM102-ltaS | This work |
| Plasmids | ||
| pND50 | S. aureus-E. coli shuttle vector; Cmr | 54 |
| pKE515 | S. aureus-E. coli shuttle vector, Cmr Ampr | 30 |
| pSltaS | pKE515 carrying the ltaS gene | This work |
| pSF151 | E. coli pUC plasmid; Kmr | 47 |
| pSF-ltaS | pSF151 carrying ΔltaS::phleo | This work |
| pM101 | S. aureus-E. coli shuttle vector; Kmr | Matsuo et al., unpublished |
| pM102 | S. aureus-E. coli shuttle vector; Cmr | Matsuo et al., unpublished |
| pM101-ltaS | pM101 carrying the ltaS gene | This work |
| pM102-ltaS | pM102 carrying the ltaS gene | This work |
| pHW353 | S. aureus-E. coli shuttle vector carrying TetR and xyl-tet | 13 |
| pNDX1 | pND50-based S. aureus-E. coli shuttle vector carrying TetR and xyl/tet from pWH353 | This work |
| pNDXltaSHis | pNDX1 carrying His-tagged LtaS ORF | This work |
| pBAD24 | Inducible expression vector in E. coli | 17 |
| pBADltaSHis | pBAD24 carrying His-tagged LtaS ORF | This work |
Localization analysis of LtaS.
To examine the localization of the LtaS protein via subcellular fractionation, the protein A-deficient M0107 (Δspa::phleo) strain harboring pNDX1 or pNDXltaS-His was first grown at 37°C to an optical density at 600 nm (OD600) of 0.5. Strain M0107 was used to diminish any unwanted signal from the interaction of protein A with immunoglobulin G during Western blotting. Next, up to 0.5 μg/ml anhydrotetracycline (ATc) (Sigma-Aldrich) was added, and the cells were further incubated for 2 h. To prepare the culture medium protein, cells were centrifuged; the supernatant fractions were collected, brought to a final concentration of 10% trichloroacetic acid, and incubated overnight at 4°C. Precipitates were collected by centrifugation at 10,000 × g for 10 min, washed twice with ice-cold ethanol, dried, and dissolved in 8 M urea and 2 M thiourea as previously described (55). To prepare membrane proteins, precipitated cells were washed, resuspended in 1 ml of phosphate-buffered saline (PBS), and treated with 200 μg/ml lysostaphin (Wako) at 37°C for 30 min. The resulting cell lysate was sonicated and then centrifuged at 100,000 × g for 30 min at 4°C. The pellet was resuspended in PBS by sonication. The protein concentration was determined by the Lowry method using bovine serum albumin as a standard (32). Membrane (30 μg) and culture medium (3 μg) proteins were analyzed by immunoblotting with a peroxidase-conjugated anti-His monoclonal antibody (Nacalai Tesque).
Analysis of LTA using 32Pi.
LTA was extracted from S. aureus cells with phenol and was purified using Octyl-Sepharose as described previously (11). In brief, S. aureus cells were grown at 30°C overnight in LB medium containing up to 10 μCi/ml 32Pi (GE Healthcare). 32Pi-labeled cells were suspended in TSS buffer (50 mM Tris-HCl [pH 7.9], 150 mM NaCl, 20% sucrose) and lysed with 200 μg/ml lysostaphin at 37°C for 1 h. Spheroplasts were collected by centrifugation and resuspended in PBS. The LTA fraction was extracted with an equal volume of 80% phenol at 70°C for 1 h; the extract was treated with chloroform; and finally the extract was dried under a vacuum. The LTA fraction was absorbed to 250 μl of Octyl-Sepharose Fast Flow slurry, washed twice with 500 μl of 15% 1-propanol, and eluted with 50% 1-propanol. 32P-labeled LTA was dried and dissolved in MilliQ water. 32P-labeled LTA was separated by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (15% SDS-PAGE). The gels were then dried, and the radioactive bands were detected using BAS1800 (Fuji Film).
Quantification of LTA by ELISA.
An enzyme-linked immunosorbent assay (ELISA) of LTA was performed as described previously (48). In brief, S. aureus cells (1 ml of culture) were first collected and resuspended in 1 ml of PBS. For E. coli harboring pBAD24 (17) or pBADltaS-His, cells were grown at 37°C to an OD600 of 0.5; then l-(+)-arabinose (Sigma-Aldrich) was added to a final concentration of 1%, and the cells were further incubated for 2 h, collected, and resuspended in 1 ml of PBS. Cells were lysed with 200 μg/ml lysostaphin for S. aureus or 200 μg/ml lysozyme for E. coli. LTA was extracted from the resultant cell lysate with phenol at 70°C for 1 h. The extract was then treated with chloroform, dried under a vacuum, and dissolved in 1 ml of water. Samples were diluted with PBS, and 100 μl of sample was incubated on an Immulon 1B ELISA plate (Dynatech) at 4°C overnight. After three washes with 200 μl of PBST (PBS containing 0.05% Tween 20), the plate was treated with 200 μl of 3% bovine serum albumin in PBST at room temperature for 1 h. After five washes with PBST, a 200-fold-diluted anti-S. aureus LTA monoclonal antibody (clone 55; Hycult Biotech) was added and incubated for 2 h at room temperature. The plate was washed three times and then incubated with alkaline phosphatase-conjugated anti-mouse immunoglobulin G (Promega) at room temperature for 2 h. Then the plates were washed three times with PBST and incubated with 1 mg/ml p-nitrophenylphosphate (Sigma) in buffer AP (50 mM Tris-HCl [pH 9.0] and 1 mM MgCl2). The OD405 was measured using a microplate reader. LTA from S. aureus (Sigma-Aldrich) was used as a standard.
Preparation of WTA.
WTA was prepared as described previously (41). In brief, 200 ml of cells grown in LB medium was harvested by centrifugation and washed with 0.9% NaCl. Cells were then resuspended in water, boiled for 30 min, washed with water, and washed twice with acetone. The cells were resuspended in PBS and disrupted using a French press (35,000 lb/in2; Aminco). The resultant crude cell wall fraction was collected by centrifugation and treated with 1 μg/ml DNase I and 10 μg/ml RNase for 18 h, followed by treatment with 200 μg/ml trypsin for 18 h. The cell wall fraction was collected by centrifugation and washed twice with 0.9% NaCl. WTA was solubilized from the insoluble cell wall fraction by treatment with 5% trichloroacetic acid for 18 h at room temperature, precipitated with 3 volumes of acetone, and dissolved in water. WTA was separated on an 18% polyacrylamide gel and visualized using 0.5% alcian blue 8GX (Sigma-Aldrich) in 5% acetic acid.
Determining the rate of turnover of PG.
S. aureus cells in liquid culture were diluted in 10 ml of LB medium containing 12.5 μg/ml Cm and were grown at 30°C to an OD600 of 0.1. Then the culture was brought to 30 μCi/ml 32Pi (GE Healthcare) and incubated at 30°C for 1 h. Cells were collected by centrifugation, washed twice with 1 ml of LB medium, released, and further incubated at 30°C in fresh LB medium. Aliquots (1 ml) of the culture were sampled periodically. Total lipids were extracted by the Bligh-Dyer method and spotted onto a silica gel 60 thin-layer chromatography plate (Merck). The plate was developed using chloroform-methanol-acetic acid (65:25:10) and dried, and the radioactivity of 32P-labeled PG was quantified using BAS1500 (Fuji Film) (38).
Autolysis assay.
The autolysis assay was performed as described previously (40). In brief, cells growing exponentially in 5 ml of LB medium were collected by centrifugation, washed, and suspended in 5 ml of buffer containing 50 mM Tris-HCl (pH 7.4) and 0.05% Triton X-100. The resultant cell suspension was incubated at 30°C, and OD600 values were monitored.
Zymographic analyses.
Bacteriolytic activities were measured, using polyacrylamide gels containing heat-killed Micrococcus luteus cells, by a slight modification of a previously described method (45). The stacking gel contained 3% acrylamide, 0.08% bisacrylamide, 0.125 M Tris-HCl (pH 6.8), 0.2% SDS, 0.25% N,N,N′,N′-tetramethylethyenediamine, and 0.05% ammonium persulfate. The separating gel contained 7.5% acrylamide, 0.135% bisacrylamide, 0.125 M Tris-HCl (pH 8.8), 0.2% SDS, 0.1% N,N,N′,N′-tetramethylethyenediamine, 0.1% ammonium persulfate, and 1.0 mg (wet weight)/ml heat-killed M. luteus cells. The sample loading buffer contained 25 mM Tris-HCl (pH 6.8), 2% SDS, 5% glycerol, and 0.04% bromophenol blue. Cell surface fractions (4% SDS extract) corresponding to 2.5 ml of culture at an OD600 of 1 were used. Electrophoresis was performed at a constant voltage. After electrophoresis, gels were washed in 400 ml of water for 45 min and then incubated in 0.2 M phosphate buffer (pH 7.0) for 1 to 4 h. Visualization of the zone cleared by cell lysis was enhanced by staining with 0.5% methylene blue, and gels were imaged using a GT-8700 scanner (Epson).
Microscopic analysis.
For Sytox Green staining, cells growing exponentially in LB medium at 30°C were shifted to 37°C or 43°C. Aliquots of the cells were sampled and stained with 5 μM Sytox green nucleic acid stain (Molecular Probes) in LB medium. Cells were spread onto glass slides coated with aminopropylsilane (Matsunami) and were observed with a DM4000B fluorescent microscope (Leica) using a 100× objective. Images were taken with a charge-coupled device camera (Leica) and processed by Adobe Photoshop Elements. At least 200 cells were counted. Electron microscopic analysis was performed as described previously (46). In brief, cells growing exponentially in 200 ml of LB medium at 30°C were sampled directly or shifted to 43°C for 3 h. Harvested cells were washed twice and doubly fixed with 2.5% glutaraldehyde and 1% OsO4. The samples were then dehydrated using an ethanol series and were embedded in Spurr's Epon. Ultrathin sections were cut with an ultramicrotome and examined using a JEOL JEM-2000 EXII electron microscope at 80 or 100 kV.
RESULTS
Identification of the ltaS gene.
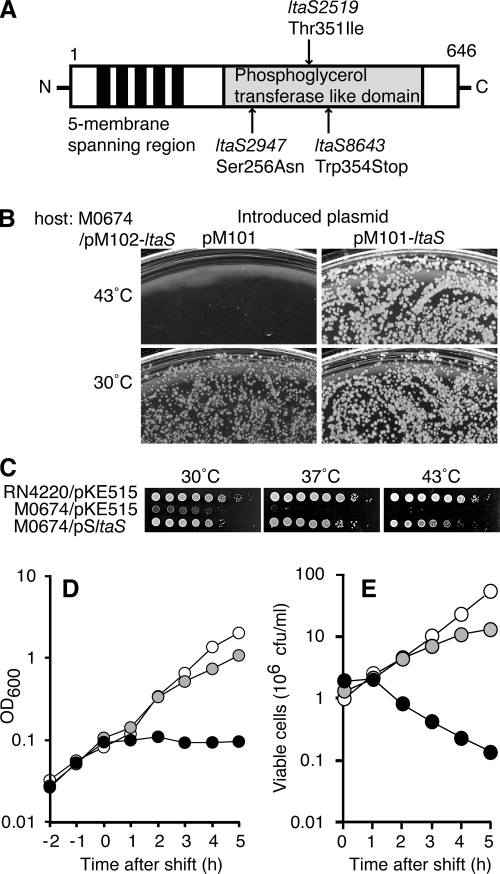
To identify genes essential for S. aureus cell growth, we collected about 750 temperature-sensitive mutants that grow at 30°C but not at 43°C. Genes that complement temperature-sensitive cell growth in these mutant strains were identified by introducing a pKE515-based plasmid library containing chromosomal DNA fragments derived from S. aureus strain RN4220. We found three temperature-sensitive mutant strains that could be complemented by pSltaS, a plasmid harboring the hypothetical gene SA0674 (ltaS), but not by the empty vector, pKE515 (Table 2). (The gene identification number was assigned in S. aureus strain N315 [29].) The pSltaS genomic insert did not contain any genes other than ltaS; this gene appears to be encoded in a monocistron. We next compared the nucleotide sequences of the chromosomal ltaS gene and its flanking regions in the mutant strains. We found that each strain had a different single transition or transversion mutation in the LtaS coding region that resulted in an amino acid substitution. Three distinct lesions were identified: G768A (Ser256Asn; strain TS2947), C1055T (Thr351Ile; strain TS2519), and G1062T (Trp354opal; strain TS8643) (Fig. 1A). We named these mutations ltaS2947, ltaS2519, and ltaS8643, respectively.
TABLE 2.
Complementation of temperature-sensitive mutant strainsa
| Strain | No. of transformants (103) with the following plasmid at 43°C/30°C:
|
|
|---|---|---|
| pSltaS | pKE515 | |
| TS2519 | 2.1/1.3 | 0.0/0.8 |
| TS2947 | 1.0/2.0 | 0.0/2.6 |
| TS8643 | 5.0/4.4 | 0.0/1.6 |
Competent cells of each temperature-sensitive mutant strain of S. aureus (50 μl) were electroporated with 250 ng of pSltaS or the empty vector pKE515, followed by incubation at 43°C or 30°C for 24 h on LB agar plates containing 12.5 μg/ml Cm. The number of transformants was counted. Data are representative of more than three independent experiments.
FIG. 1.
Isolation of mutations in S. aureus ltaS. (A) Primary structure of LtaS protein. Filled bars and shaded box indicate membrane-spanning regions and the phosphoglycerol transferase-like domain, respectively. Each mutation site identified in temperature-sensitive ltaS mutant strains is indicated by an arrow. (B) Colony formation of ΔltaS mutant cells. M0674 (ΔltaS::phleo) harboring pM102-ltaS (Cmr) was transformed with an incompatible plasmid, pM101 (Kmr) or pM101-ltaS (Kmr). Cells were spread onto LB agar plates containing 50 μg/ml Km and 20 μg/ml Phleo and were incubated at 30°C and 43°C for 24 h. (C) Temperature-sensitive cell growth of ΔltaS mutant cells. RN4220/pKE515, M0674/pM101/pKE515, or M0674/pM101/pSltaS cells at 30°C in LB medium were serially diluted 10-fold, and each 2-μl aliquot was spotted onto LB agar plates. Plates were incubated at 30°C, 37°C, or 43°C for 48 h. (D and E) Changes in turbidity and viability of ΔltaS mutant cells (M0674/pM101) after a temperature shift. Cells were diluted and incubated at 30°C in 5 ml of LB medium. At the exponential-growth phase, the temperature either was shifted to 43°C (filled symbols) or 37°C (shaded symbols) or was maintained at 30°C (open symbols). Turbidity was measured (D), and viable cell numbers were counted (E). Data are representative of at least three independent experiments.
To determine if these mutations were really responsible for the temperature-sensitive growth of these mutant strains, we performed phage transduction experiments using phage 80alpha. We inserted a selection marker (a kanamycin resistance gene) into a noncoding region between the SA0667 and SA0668 genes, a region that is proximal to ltaS in each of the mutant strains (TS2519, TS2947, and TS8643). For TS2519, 27 of 136 kanamycin-resistant transductants showed temperature-sensitive cell growth, whereas the remaining 109 transductants remained temperature resistant (Table 3). A similar link between kanamycin resistance and temperature sensitivity was observed in TS2947 and TS8643 (Table 3). As with the parental mutant strains, pSltaS was able to complement the temperature sensitivity of the mutant transductants (data not shown). These results strongly suggest that these ltaS point mutations are responsible for the temperature-sensitive phenotype.
TABLE 3.
Results of phage transductiona
| Donor | No. of transductants with:
|
Linkage (%) between Kmr and TS phenotype | |
|---|---|---|---|
| Kmr | TS phenotype | ||
| TS2519kan | 136 | 27 | 20 |
| TS2947kan | 104 | 10 | 10 |
| TS8643kan | 174 | 29 | 16 |
In each temperature-sensitive mutant strain identified in this study, a Km resistance gene was inserted near the ltaS gene locus, and the resultant mutant was used as a donor for transduction analysis using phage 80alpha. The recipient was M0107 in all transductions. The Km-resistant (Kmr) transductants were selected at 30°C and tested for temperature-sensitive cell growth (TS phenotype) at 43°C. Data are representative of two independent experiments.
The ltaS gene encodes a 68-kDa protein with an N-terminal five membrane-spanning region and a C-terminal phosphoglycerol transferase-like (or anion binding, sulfatase, alkaline phosphatase-like) domain (Fig. 1A). Notably, each of the three mutations is located within the phosphoglycerol transferase-like domain. Sequence alignment of LtaS orthologues in related species, such as Listeria monocytogenes, Streptococcus pyogenes, and Bacillus subtilis (Table 4), revealed that Ser256 has been well conserved in S. aureus LtaS and that Thr352 is Thr or Ser in the other organisms. The conservation of these amino acids is consistent with their importance for LtaS protein function.
TABLE 4.
Conservation of the ltaS gene among bacteria
| Phylum and species | LTAa | Presence or absence of ltaSb |
|---|---|---|
| Firmicutes | ||
| Staphylococcus aureus | GroP | + |
| Bacillus subtilis | GroP | +c |
| Listeria monocytogenes | GroP | + |
| Lactobacillus casei | GroP | + |
| Streptococcus pyogenes | GroP | + |
| Streptococcus agalactiae | GroP | + |
| Streptococcus pneumoniae | RibP + tetrasaccharide | − |
| Mycoplasma genitalium | − | − |
| Proteobacteria (Escherichia coli) | − | − |
| Actinobacteria (Mycobacterium tuberculosis) | − | − |
GroP, glycerolphosphate polymer; RibP + tetrasaccharide, polymer of ribitol phosphate and a tetrasaccharide unit; −, no LTA.
+, presence; −, absence.
Four ltaS orthologues are found in Bacillus subtilis.
Because ltaS8643 is a nonsense mutation, it seems plausible to imagine that ltaS is dispensable for cell growth at 30°C. To test this hypothesis, the chromosomal ltaS gene was replaced with a Phleo resistance gene in a strain harboring pM102-ltaS, a complementing plasmid. Plasmid pM102 is a vector containing a replicon of the S. aureus pN315 plasmid and a Cm resistance gene (Matsuo et al., unpublished). This strain, M0674/pM102-ltaS, was transformed with pM101, a Km-resistant plasmid containing the same pN315 replicon region as pM102, so that pM101 is incompatible with pM102-ltaS. Km-resistant transformants (M0674/pM101) were obtained at 30°C but not at 43°C (Fig. 1B). The loss of the preexisting pM102-ltaS in M0674/pM101 was confirmed. Colonies obtained at 30°C grew more slowly than those of M0674/pM101-ltaS. Furthermore, the colony-forming ability of the M0674/pM101 strain dramatically decreased (to less than 10−5) on agar plates when cells were cultured at 37°C or 43°C but was recovered by the introduction of pSltaS (Fig. 1C). Upon a temperature upshift of M0674/pM101 cells from 30°C in liquid medium, a turbidity increase was detected at 37°C, but this increase was smaller than that observed at 30°C. When the culture was shifted to 43°C, there was no further increase in turbidity (Fig. 1D). The number of viable cells tapered off at 37°C and began to decrease at 43°C (Fig. 1E). Taken together, these results indicate that the ltaS gene is dispensable for S. aureus cell growth at 30°C but essential at temperatures above 37°C.
Loss of LTA synthesis in the ΔltaS mutant.
We next sought to reveal the biochemical function of the LtaS protein. We identified four ltaS orthologues (yflE, yfnI, yqgS, and yvgJ) in Bacillus subtilis. The C-terminal regions of two of the four proteins, the yflE and yfnI gene products, have been reported to be extracellular (1, 19). Consistent with this result, the C-terminal region of the S. aureus LtaS protein was identified among extracellular proteins in a proteomics-based analysis of S. aureus (55). Thus, we postulated that the LtaS C-terminal region containing the phosphoglycerol transferase-like domain was located outside the cell membrane. Additionally, it seemed likely that the region is cleaved and released to the culture medium.
To test this possibility, we expressed a version of LtaS with a His tag on the C terminus under the control of the ATc-inducible promoter in a protein A-deficient S. aureus strain (M0107). Immunoblotting revealed that a 68-kDa protein could be detected in the membrane fraction in a manner dependent on the presence of ATc (see Fig. S1A in the supplemental material). This size is consistent with the predicted molecular mass of a full-length LtaS-His, and this result supports the idea that LtaS is a membrane protein. As expected, a C-terminal 48-kDa protein fragment containing the phosphoglycerol transferase-like domain was detected in an extracellular protein fraction (see Fig. S1A in the supplemental material). The ratio of the amount of full-length protein present in the membrane fraction to the amount of the C-terminal fragment in the extracellular protein fraction was about 6:1 in exponentially growing cells. These results suggest that the C-terminal region, containing the phosphoglycerol transferase-like domain, is oriented toward the extracellular space on the cell membrane and that this region is cleaved and released into the culture medium (see Fig. S1B in the supplemental material).
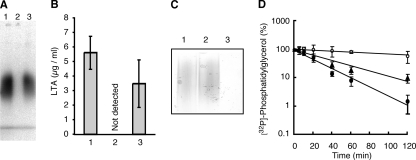
Since the phosphoglycerol transferase-like domain of LtaS appears to be oriented to the extracellular space, we next asked if the ΔltaS mutant strain was depleted of any cell surface constituents, including LTA. The mutant and parent cells were metabolically labeled with 32Pi, and the 32P-labeled LTA was extracted by phenol and purified by Octyl-Sepharose column chromatography as described previously (11). In the ΔltaS mutant (M0674/pM101/pKE515), no 32P-labeled LTA was detected (Fig. 2A, lane 2), and its loss was complemented by the introduction of plasmid pSltaS into the mutant (Fig. 2A, lane 3). The loss of LTA in the ΔltaS mutant was also confirmed by an ELISA (Fig. 2B), in which an anti-LTA monoclonal antibody directed against polyglycerolphosphate was used. In contrast, the amount of WTA in the ΔltaS mutant strain (M0674/pM101) was similar to that in the parental strain (Fig. 2C). These results indicate that ltaS is required for the biosynthesis of the polyglycerolphosphate chain present in LTA. The extracellular localization of the phosphoglycerol transferase-like domain of LtaS is consistent with the idea that polyglycerolphosphate of LTA is synthesized on the outer leaflet of the cell membrane in gram-positive bacteria (36).
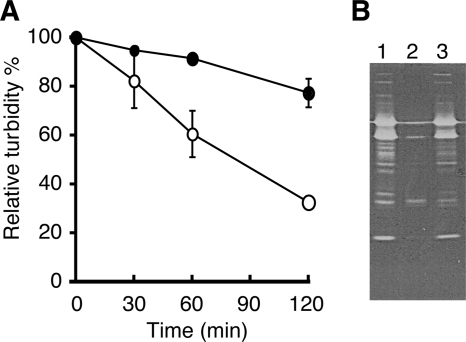
FIG. 2.
Essential nature of the ltaS gene for LTA biosynthesis. (A) Loss of LTA in ΔltaS mutant cells. Cells were metabolically labeled with 32Pi, and 32P-labeled LTA was extracted, separated by 15% SDS-PAGE, and detected by autoradiography. Lanes: 1, RN4220/pKE515; 2, M0674 (ΔltaS)/pM101/pKE515; 3, M0674/pM101/pSltaS. (B) Quantification of LTA by ELISA. Levels of LTA in S. aureus cell culture were quantified by ELISA using an anti-polyglycerolphosphate antibody. Means from three independent experiments are presented along with standard deviations. Bars: 1, RN4220/pKE515; 2, M0674/pM101/pKE515; 3, M0674/pM101/pSltaS. (C) The ltaS gene is dispensable for WTA biosynthesis. WTA was extracted from stationary-phase S. aureus cells, separated by PAGE (18%), and visualized by 0.5% alcian blue staining. Lanes: 1, RN4220; 2, M0674/pM101; 3, M0702 (ΔtagO). (D) Decreased turnover rate of membrane PG in an S. aureus ΔltaS mutant. Cells were metabolically pulse-labeled with 32Pi for 1 h and incubated further in fresh medium for the indicated periods. Total lipids were extracted and separated by silica gel thin-layer chromatography (chloroform-methanol-acetic acid, 65:25:10). 32P-labeled PG was quantified by autoradiography. Filled circle, RN4220/pKE515; open circle, M0674/pM101/pKE515; filled triangle, M0674/pM101/pSltaS. Data are means and standard deviations from three independent experiments.
Previous biochemical studies have demonstrated that membrane PG is a substrate for the biosynthesis of the polyglycerolphosphate present in LTA. We tested this idea by using the ΔltaS mutant strain. By a pulse-chase experiment using 32Pi (Fig. 2D), the half-life (t1/2) of PG in the parental strain (RN4220/pKE515) was found to be 19 min, consistent with previously reported data (28). In contrast, in the ΔltaS mutant (M0674/pM101/pKE515), the t1/2 for PG was more than 120 min. The introduction of pSltaS into the ΔltaS mutant strain decreased the t1/2 to 32 min. These results are consistent with the previous biochemical observations and suggest that PG is really a substrate for polyglycerolphosphate biosynthesis of LTA. Additionally, our data clearly reveal that a large proportion of the PG in S. aureus cells is used for the biosynthesis of LTA.
Induction of LTA synthesis in E. coli cells via ectopic expression of LtaS.
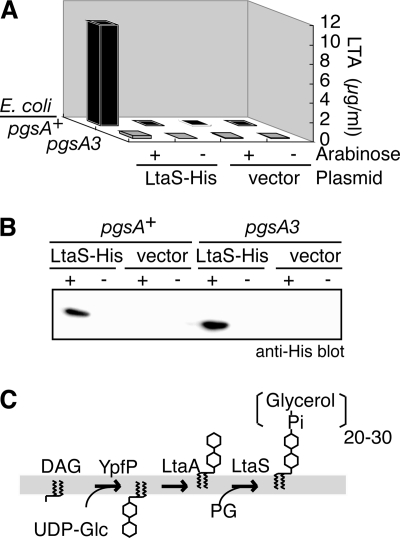
Next, we tested if the ectopic expression of LtaS in E. coli cells could induce the biosynthesis of polyglycerolphosphate. Since E. coli has the substrate PG but not LTA and the S. aureus ΔypfP mutant strain lacks the glycolipid anchor of LTA and instead is able to synthesize polyglycerolphosphate on diacylglycerol (10, 27), we postulated that the expression of LtaS in E. coli might induce the synthesis of polyglycerolphosphate. To test this idea, an E. coli pgsA3 mutant strain, which has severe defects in PG biosynthesis, and its isogenic parent strain (3) were transformed with pBADltaS-His harboring the LtaS ORF with a C-terminal His tag under the control of the arabinose promoter. The induction of LtaS in the parent E. coli strain by the addition of l-arabinose caused synthesis of polyglycerolphosphate (Fig. 3A), as measured by an ELISA for LTA as described for Fig. 2B. In contrast, much lower levels of LTA synthesis were induced in the pgsA3 mutant (Fig. 3A). Arabinose-dependent expression of LtaS in both strains was confirmed by immunoblotting for the His tag (Fig. 3B). These results strongly suggest that ltaS encodes a polyglycerolphosphate synthase that uses PG as a substrate. The role of the LtaS protein in the LTA biosynthetic pathway is diagramed in Fig. 3C.
FIG. 3.
PG-dependent induction of LTA synthesis in E. coli cells by ectopic expression of S. aureus LtaS. (A) pgsA-dependent LTA synthesis in E. coli. Exponentially growing E. coli cells were brought to a final concentration of 1% l-arabinose and were further incubated for 3 h. LTA levels in a phenol extract of the E. coli cells were quantified by ELISA as described for Fig. 2B. The results shown are representative of at least three independent experiments. Strains used were JE5513Tc (pgsA+) and YA5513Tc (pgsA3), which is deficient in PG biosynthesis, each harboring pBADltaS-His (LtaS-His) or pBAD24 (vector). (B) Expression of LtaS-His in E. coli cells. Whole-cell extracts of E. coli cells were prepared as described for panel A, and LtaS-His protein was detected by immunoblotting with an anti-His antibody. (C) Proposed LTA biosynthetic pathway. YpfP catalyzes the transfer of two glucose molecules to diacylglycerol (DAG) from UDP-glucose (UDP-Glc), and the resultant diglycosyldiacylglycerol is transported to the outer leaflet of the membrane by the putative membrane permease, LtaA. Glycerol phosphate residues are transferred by LtaS using PG as a donor.
The ltaS gene has been conserved among low-GC-content gram-positive bacteria, which have glycerol phosphate type LTA, including staphylococci, bacilli, listeriae, lactobacilli, enterococci, and streptococcal groups A and B (Table 4; also data not shown). Streptococcus pneumoniae, in which LTA has a repeating unit of ribitol phosphate and tetrasaccharide instead of polyglycerolphosphate (8), does not have an identifiable ltaS orthologue (Table 4). Thus, the conservation of the gene is consistent with the idea that LtaS catalyzes polyglycerolphosphate synthesis of LTA.
Aberrant cell division and separation in ΔltaS mutant cells.
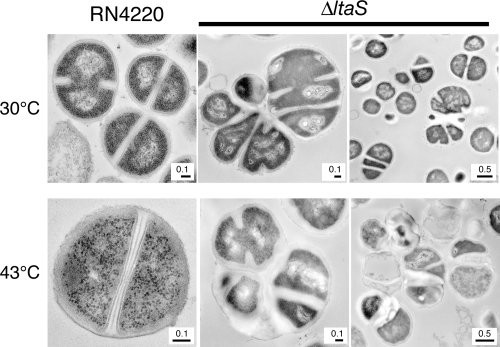
To further characterize the physiological roles of the ltaS gene, analyses of the mutant cells were performed by electron microscopy. When grown at 30°C, ΔltaS mutant cells irregularly positioned the division septa; they had parallel but not perpendicular division septa (Fig. 4). In addition, daughter cells often remained connected to each other. Furthermore, cells were enlarged, and their cell walls were thicker than those of wild-type cells (Fig. 4). A similar phenotype was observed for ΔltaS mutant cells cultured at 43°C for 4 h (Fig. 4). Fluorescent light microscopy of 4′,6-diamidino-2-phenylindole (DAPI)-stained temperature-sensitive ltaS point mutant cells revealed that cells were enlarged and harbored multiple nucleoids at their restrictive temperature (data not shown). The introduction of pSltaS into these strains suppressed these phenotypes (data not shown). These results clearly indicate that LTA synthesis by LtaS is required for complete cell division and separation and for proper cell wall metabolism.
FIG. 4.
Aberrant cell division and separation in ΔltaS mutant cells. Exponentially growing cells at 30°C (top) or cells after a shift to 43°C for 3 h (bottom) were fixed and observed by transmission electron microscopy. (Left) RN4220; (center and right) M0674/pM101 (ΔltaS).
Decreased autolysis in ΔltaS mutant cells.
The thickened cell wall and irregular cell separation in ΔltaS mutant cells resemble phenotypes that have been observed for Δatl and Δsle1 peptidoglycan hydrolase mutants (25, 46). Thus, we supposed that a ΔltaS mutant might express lower peptidoglycan hydrolase activity on its cell surface. To test this hypothesis, we performed a Triton X-100-mediated autolysis assay. Cells growing exponentially at 30°C were harvested and suspended in a buffer containing 0.05% Triton X-100; then changes in turbidity due to autolysis were monitored. There was an observable decrease in turbidity in ΔltaS mutant cells; this decrease was smaller than that for the parent strain (Fig. 5A). In addition, a zymography assay, in which peptidoglycan hydrolase activity is monitored by in-gel digestion of heat-killed Micrococcus luteus cells, revealed that the levels of peptidoglycan hydrolases in the cell wall fraction were lower in ΔltaS mutant cells than in parent cells (Fig. 5B). The introduction of pSltaS into the ΔltaS mutant rescued the peptidoglycan hydrolase activity (Fig. 5B). Thus, LTA is also critical for maintaining normal levels of peptidoglycan hydrolase activity on the bacterial cell surface.
FIG. 5.
Decrease in peptidoglycan hydrolase activity in ΔltaS mutant cells. (A) Monitoring of autolysis of ΔltaS mutant cells. Exponentially growing cells at 30°C were harvested, suspended in 50 mM Tris-HCl (pH 7.4) buffer containing 0.05% Triton X-100, and incubated at 30°C. The OD600 was determined at the indicated times. Open circles, RN4220; filled circles, M0674/pM101 (ΔltaS). Data are means and standard deviations for at least three independent experiments. (B) Zymographic analysis of peptidoglycan hydrolases. A cell wall fraction was prepared and separated by SDS-PAGE (10%) with a gel containing 1 mg/ml heat-killed M. luteus cells. The gel was incubated in 0.2 M phosphate buffer, and visualization of the cleared zone was enhanced by staining with 0.5% methylene blue. Lanes: 1, RN4220/pKE515; 2, M0674/pM101/pKE515; 3, M0674/pM101/pSltaS.
Inability to exclude a dye from ΔltaS mutant cells at high temperatures.
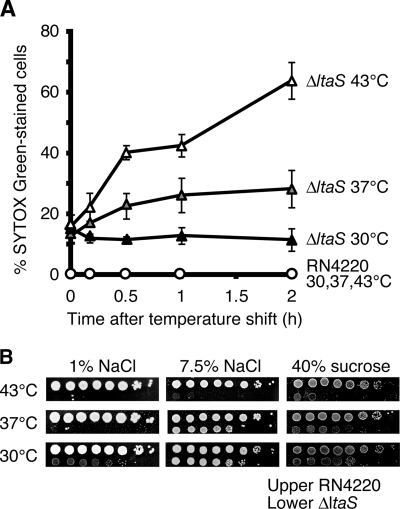
The aberrant cell division and separation and the decrease in peptidoglycan hydrolase activity in ΔltaS mutant cells were observed under permissive conditions (i.e., at 30°C). Because ΔltaS mutant cells stop the increase in turbidity after a temperature upshift to 43°C, we supposed that another defect in cellular function, in addition to the phenotypes described above, contributes to the loss of colony formation under nonpermissive conditions (i.e., at 37°C or 43°C). Based on previous reports that LTA occupies 1/10 to 1/5 of the available lipid molecules in the outer leaflet of the cell membrane and that LTA increases the phase transition temperature of the cell membrane, leading to stabilization of the membrane (16), we speculated that loss of the stabilizing effect of LTA on the cell membrane at high temperatures would damage the cell membrane severely enough to account for the decrease in cell viability. To test for membrane damage in ΔltaS mutant cells, we used the fluorescent dye Sytox green, which is excluded from intact cell membranes but can permeate membrane-damaged cells and render the cells fluorescent by binding to nucleic acids inside the cell (42). As shown in Fig. 6A, parent cells did not fluoresce at either temperature; about 10% of the ΔltaS mutant cells fluoresced at 30°C, and this percentage of Sytox green-positive cells increased after a temperature upshift (Fig. 6A). To lessen the membrane stress, we added 7.5% NaCl or 40% sucrose to the nutrient agar plates and found that either treatment remarkably suppressed the growth defect of ΔltaS mutant cells at 37°C (Fig. 6B). One possible interpretation of these results is that the growth defect of the ΔltaS mutant at high temperatures is caused, at least in part, by loss of membrane integrity due to the loss of LTA.
FIG. 6.
Inability to exclude Sytox green dye at high temperatures in ΔltaS mutant cells. (A) Exponentially growing RN4220 and M0674/pM101 (ΔltaS) cells at 30°C were shifted to the indicated temperatures and sampled at the indicated times. Cells were then stained with 5 μM Sytox green, and the percentage of Sytox green-stained cells was determined under a microscope. At least 200 cells were counted. Data are means and standard deviation from three independent experiments. (B) Effect of high osmolarity on colony formation by ΔltaS mutant cells. Fully grown RN4220 and M0674/pM101 cells at 30°C in LB medium were serially 10-fold diluted, and each 2-μl aliquot was spotted onto LB agar (1% NaCl), LB agar with 7.5% NaCl, or LB agar with 40% sucrose. Plates were incubated at 30°C, 37°C, or 43°C for 48 h.
Accumulation of LTA intermediates cannot explain the growth defect of ΔltaS mutant cells.
It has been proposed that the accumulation of intermediates in WTA synthesis leads to impaired cell growth in B. subtilis and S. aureus (6, 7). This idea is based on the fact that the tagO gene, which catalyzes the first reaction of WTA biosynthesis, is dispensable for cell growth, whereas the tagBDF genes, which act in subsequent steps of WTA biosynthesis, are indispensable. We thought that it would be interesting to know if a similar situation exists for LTA synthesis—that is, if the accumulation of LTA intermediates (specifically glycolipids) in ΔltaS mutant cells may cause the growth defect at high temperatures. To test this possibility, we generated an ltaS ypfP double mutant; the ypfP gene is responsible for the synthesis of the glycolipid anchor of LTA and is dispensable for cell growth (27). Specifically, we deleted the chromosomal ypfP gene in the M0674/pM102-ltaS strain via phage transduction, which created the YO1001/pM102-ltaS strain; then we replaced pM102-ltaS with pM101 to remove ltaS function. The resultant ΔltaS ΔypfP double-mutant strain YO1001/pM101 formed colonies at 30°C but not at 43°C, similar to what was seen for the ΔltaS single-mutant strain M0674/pM101 (data not shown). This result demonstrates that the ΔypfP mutation cannot suppress the growth defect of ΔltaS mutant cells at high temperatures, thus arguing against the rationale that the temperature sensitivity of ΔltaS is related to the accumulation of glycolipid intermediates of LTA biosynthesis.
Loss of both LTA and WTA leads to synthetic lethality in S. aureus.
Wall anionic polymers of bacteria have been thought to be important for growth (36). To examine the requirement of WTA for the growth of LTA-depleted mutant cells, we next tested the effect of removing WTA from the S. aureus ΔltaS mutant. To perform this experiment, a ΔtagO::erm construct was transduced into M0674/pM102-ltaS by using phage 80alpha. The resultant YO1003/pM102-ltaS strain was transformed with pM101 to remove ltaS function. The ΔtagO ΔltaS double mutant, in which pM102-ltaS was successfully replaced by pM101, could not be obtained under any condition tested, including growth at 30°C on LB agar alone and on LB agar with 40% sucrose (Table 5). This result suggests that the simultaneous depletion of LTA and WTA leads to synthetic lethality at 30°C, thus providing evidence that S. aureus requires the presence of at least one of these two anionic polymers for growth at 30°C.
TABLE 5.
Deficiency of both LTA and WTA leads to synthetic lethality
| Temp (°C) | No. of transformantsa on the following medium with the indicated plasmid:
|
|||
|---|---|---|---|---|
| LB
|
LB + sucrose
|
|||
| pM101 | pM101-ltaS | pM101 | pM101-ltaS | |
| 30 | 20b | 1,581 | 0 | 868 |
| 37 | 4b | 1,670 | 0 | 929 |
| 43 | 0 | 1,250 | 0 | 920 |
YO1003 (ΔltaS ΔtagO) harboring pM102-ltaS was transformed with pM101 or pM101-ltaS and selected with Km on LB agar plates with or without 40% sucrose for 48 h. Data are representative of at least three independent experiments.
Tiny colonies appeared but failed to regrow to detectable levels under the same agar plate conditions after being picked from the original agar plates.
DISCUSSION
The polyglycerolphosphate chain of LTA was recently shown to be synthesized by LtaS. Because of the limited study of LTA-depleted cells, however, the biological roles of LTA in cell growth and the pathogenic-bacterium-host cell interaction have not been determined clearly. In this study, we show that a ΔltaS mutation in S. aureus resulted in the depletion of LTA and a dramatic decrease in the turnover of membrane PG. We also show that ectopic expression of the S. aureus LtaS protein in E. coli induced polyglycerolphosphate synthesis in a manner dependent on pgsA, which is required for PG production. Taken together, these data strongly suggest that ltaS encodes the polyglycerolphosphate synthase of LTA and uses PG as a substrate. The ΔltaS mutant cells were viable at 30°C but not at 37°C or 43°C; thus, LTA synthesis is required for S. aureus cell growth at temperatures of 37°C and higher.
When ΔltaS mutant cells were shifted from 30°C to 43°C, turbidity did not continue to increase as it would for normally growing cells, and the number of viable cells decreased. It should be noted, however, that cell lysis caused by a general attenuation of peptidoglycan biosynthesis or its degradation does not necessarily account for the decrease in cell viability, because ΔltaS mutant cells at 43°C maintained a constant turbidity and a lower autolysis activity. Moreover, levels of peptidoglycan hydrolase activity on the cell surface were also decreased. Another finding of note is that ΔltaS mutant cells became permeable to Sytox green fluorescent dye after the temperature upshift. Furthermore, we observed that conditions of high osmolarity remarkably suppressed the cell growth defects of ΔltaS mutant cells at 37°C. Therefore, LTA is required for the resistance of low osmolarity. We also observed this phenomenon at 30°C; indeed, we found that the growth of ΔltaS mutant cells could be largely attenuated by decreasing the concentration of NaCl in the LB medium from 1% to 0.5% (data not shown).
One explanation both for the inability to exclude the dye at high temperatures and for the high-osmolarity requirement of the ΔltaS mutant is that a protein(s) involved directly or indirectly in systems for excluding the dye requires LTA or high osmolarity for its structure or function. Another explanation is that LTA is required for membrane integrity. Because LTA is one of the constituents of membrane lipids and has been reported to stabilize cell membranes by increasing the phase transition temperature (16), this cell membrane-stabilizing effect of LTA might function in maintaining the membrane integrity, and thus the cell viability, of S. aureus at higher temperatures. Alternatively, LTA in cooperation with peptidoglycan and WTA might be needed to compose a cell wall structure adequate to resist intracellular osmotic pressure across the cell membrane. The high-osmolarity requirement of the ΔltaS mutant seems consistent with this notion.
An idea for the role of LTA in resisting low osmolarity is that LTA is important for osmotic regulation in S. aureus and other LTA-containing gram-positive bacteria, a role similar to that of membrane-derived oligosaccharides (MDOs) in E. coli and related gram-negative bacteria. E. coli MDOs are located in the periplasmic space and are a family of soluble glucans of 5 to 12 glucose molecules that are substituted with phosphoglycerol and phosphoethanolamine groups (43). It is thought that MDOs release fixed anions into the periplasmic space and that negatively charged MDOs and their counter-ions are the principal source of the osmotic pressure in the space of cells (26). LTA contains anionic polymers of phosphoglycerol and thus releases fixed anions on the outside of the cell membrane. LTA and its counter-ions could provide local osmotic pressure on the outside of the cell membrane and could thus reduce osmotic stress; this idea could explain the high-osmolarity requirement of the ΔltaS mutant. The expected roles of LTA described above might function in combination. Further studies to uncover the roles of LTA in resisting low osmolarity are needed.
LTA and WTA are negatively charged polymers and are thought to have similar functions on the cell surface. We have shown that deletion of both ltaS and tagO leads to synthetic lethality at 30°C. Thus, WTA appears to compensate for a loss of LTA, and vice versa. This genetic evidence demonstrates the essential nature of wall anionic polymers for the growth of S. aureus.
While LTA-depleted S. aureus mutant cells were temperature sensitive, no increase in the LTA content was observed in normal cells after a temperature upshift (data not shown). Recently, it was reported that LTA was reduced to 1/10 of its normal levels in ΔypfP mutant cells, which nevertheless grew normally (10). Thus, the amount of LTA required for normal cell growth is much smaller than its typical content in wild-type cells; this finding could help explain why the LTA content did not increase at higher temperatures.
The ΔltaS mutation resulted in aberrant cell division and separation, lower levels of peptidoglycan hydrolase activity on the cell surface than in parent cells, and decreased autolysis. Interestingly, the irregularity in cell separation that we observed in ΔltaS cells is similar to that observed for the Δatl peptidoglycan hydrolase autolysin mutant and the Δsle1 peptidoglycan amidase mutant (25, 46). The S. aureus Atl protein is known to localize to the cell division plane and the predicted cell division site; the binding of Atl to LTA is predicted to be involved in septal localization (53). Thus, it seems reasonable that LTA might be involved in cell separation by maintaining the amount and localization of peptidoglycan hydrolases on the cell surface. Moreover, we speculate that LtaS may localize to the cell division plane, where it would act to synthesize nascent LTA chains at the septum. Support for this idea comes from a previous report that YpfP, which is required for glycolipid anchor synthesis, is localized to the cell division plane (49).
Our measurement of the t1/2 of PG revealed that LTA synthesis has a dramatic effect on membrane phospholipid metabolism, suggesting that about half the PG molecules on the cell membrane are used for LTA synthesis in one doubling time. Concomitant with LTA synthesis, a large quantity of diacylglycerol is produced on the outer leaflet of the cell membrane. These molecules must be translocated to the cytoplasmic side of the cell membrane so that they can be used for phosphatidic acid production. This process requires the translocation of a large number of molecules. Thus, phospholipid metabolism might have a role in the maintenance of cell physiology and cell cycle events, since the activity of the replication initiator protein DnaA is sensitive to acidic phospholipids (20, 44, 52). In the future, it will be interesting to learn more about the role of LTA synthesis in cell division and separation.
Because the catalytic domain of LtaS is oriented toward the extracellular space, inhibitors of LtaS would not need to permeate the bacterial cell membrane; thus, soluble compounds, which have favorable pharmacological properties, could be considered in the design of therapeutics. In addition, since S. aureus is a normal constituent of the resident flora in animals, the fact that ΔltaS mutant cells cannot grow at the physiological temperature of 37°C is interesting. Moreover, the LtaS protein has been conserved among those gram-positive pathogenic bacteria that have glycerol phosphate-type LTA. These factors make LtaS an attractive target for the development of antimicrobial agents.
We have uncovered permissive conditions for ΔltaS mutant cells. The identification of the temperature-sensitive nature of ΔltaS mutant cells is important, because it enables us to culture LTA-depleted mutant cells and thus makes it possible to analyze the physiological roles of LTA in bacterium-host cell interactions.
Supplementary Material
Acknowledgments
We thank T. Santa and R. Taguchi (University of Tokyo) for their kind help with HPLC and MS analyses of lipids, respectively. We also thank W. Hillen, S. Yasuda, K. Hiramatsu, and J. J. Ferretti for kindly providing pWH353, pBAD24, pN315, and pSF151, respectively.
This work was supported in part by the Japan Science and Technology Agency (2005) and by the Industrial Technology Research Grant Program of the New Energy and Industrial Technology Development Organization of Japan (2004).
Footnotes
Published ahead of print on 24 October 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Antelmann, H., H. Tjalsma, B. Voigt, S. Ohlmeier, S. Bron, J. M. van Dijl, and M. Hecker. 2001. A proteomic view on genome-based signal peptide predictions. Genome Res. 111484-1502. [DOI] [PubMed] [Google Scholar]
- 2.Araki, Y., and E. Ito. 1989. Linkage units in cell walls of gram-positive bacteria. Crit. Rev. Microbiol. 17121-135. [DOI] [PubMed] [Google Scholar]
- 3.Asai, Y., Y. Katayose, C. Hikita, A. Ohta, and I. Shibuya. 1989. Suppression of the lethal effect of acidic-phospholipid deficiency by defective formation of the major outer membrane lipoprotein in Escherichia coli. J. Bacteriol. 1716867-6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baddiley, J. 1972. Teichoic acids in cell walls and membranes of bacteria. Essays Biochem. 835-77. [PubMed] [Google Scholar]
- 5.Chiu, T. H., B. Arnold, S. R. Kim, and L. L. Yeh. 1985. Phosphatidyl glycerolphosphate serves as glycerolphosphate donor in polymer synthesis. Biochem. Biophys. Res. Commun. 128906-912. [DOI] [PubMed] [Google Scholar]
- 6.D'Elia, M. A., K. E. Millar, T. J. Beveridge, and E. D. Brown. 2006. Wall teichoic acid polymers are dispensable for cell viability in Bacillus subtilis. J. Bacteriol. 1888313-8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Elia, M. A., M. P. Pereira, Y. S. Chung, W. Zhao, A. Chau, T. J. Kenney, M. C. Sulavik, T. A. Black, and E. D. Brown. 2006. Lesions in teichoic acid biosynthesis in Staphylococcus aureus lead to a lethal gain of function in the otherwise dispensable pathway. J. Bacteriol. 1884183-4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draing, C., M. Pfitzenmaier, S. Zummo, G. Mancuso, A. Geyer, T. Hartung, and S. von Aulock. 2006. Comparison of lipoteichoic acid from different serotypes of Streptococcus pneumoniae. J. Biol. Chem. 28133849-33859. [DOI] [PubMed] [Google Scholar]
- 9.Fabret, C., S. D. Ehrlich, and P. Noirot. 2002. A new mutation delivery system for genome-scale approaches in Bacillus subtilis. Mol. Microbiol. 4625-36. [DOI] [PubMed] [Google Scholar]
- 10.Fedtke, I., D. Mader, T. Kohler, H. Moll, G. Nicholson, R. Biswas, K. Henseler, F. Gotz, U. Zahringer, and A. Peschel. 2007. A Staphylococcus aureus ypfP mutant with strongly reduced lipoteichoic acid (LTA) content: LTA governs bacterial surface properties and autolysin activity. Mol. Microbiol. 651078-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer, W., H. U. Koch, and R. Haas. 1983. Improved preparation of lipoteichoic acids. Eur. J. Biochem. 133523-530. [DOI] [PubMed] [Google Scholar]
- 12.Fournier, B., and D. J. Philpott. 2005. Recognition of Staphylococcus aureus by the innate immune system. Clin. Microbiol. Rev. 18521-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geissendorfer, M., and W. Hillen. 1990. Regulated expression of heterologous genes in Bacillus subtilis using the Tn10 encoded tet regulatory elements. Appl. Microbiol. Biotechnol. 33657-663. [DOI] [PubMed] [Google Scholar]
- 14.Ginsburg, I. 2002. Role of lipoteichoic acid in infection and inflammation. Lancet Infect. Dis. 2171-179. [DOI] [PubMed] [Google Scholar]
- 15.Grundling, A., and O. Schneewind. 2007. Synthesis of glycerol phosphate lipoteichoic acid in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 1048478-8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutberlet, T., J. Frank, H. Bradaczek, and W. Fischer. 1997. Effect of lipoteichoic acid on thermotropic membrane properties. J. Bacteriol. 1792879-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose pBAD promoter. J. Bacteriol. 1774121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiramatsu, K., L. Cui, M. Kuroda, and T. Ito. 2001. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 9486-493. [DOI] [PubMed] [Google Scholar]
- 19.Hirose, I., K. Sano, I. Shioda, M. Kumano, K. Nakamura, and K. Yamane. 2000. Proteome analysis of Bacillus subtilis extracellular proteins: a two-dimensional protein electrophoretic study. Microbiology 14665-75. [DOI] [PubMed] [Google Scholar]
- 20.Ichihashi, N., K. Kurokawa, M. Matsuo, C. Kaito, and K. Sekimizu. 2003. Inhibitory effects of basic or neutral phospholipid on acidic phospholipid-mediated dissociation of adenine nucleotide bound to DnaA protein, the initiator of chromosomal DNA replication. J. Biol. Chem. 27828778-28786. [DOI] [PubMed] [Google Scholar]
- 21.Inoue, R., C. Kaito, M. Tanabe, K. Kamura, N. Akimitsu, and K. Sekimizu. 2001. Genetic identification of two distinct DNA polymerases, DnaE and PolC, that are essential for chromosomal DNA replication in Staphylococcus aureus. Mol. Genet. Genomics 266564-571. [DOI] [PubMed] [Google Scholar]
- 22.Ishibashi, M., K. Kurokawa, S. Nishida, K. Ueno, M. Matsuo, and K. Sekimizu. 2007. Isolation of temperature-sensitive mutations in murC of Staphylococcus aureus. FEMS Microbiol. Lett. 274204-209. [DOI] [PubMed] [Google Scholar]
- 23.Kaito, C., K. Kurokawa, Y. Matsumoto, Y. Terao, S. Kawabata, S. Hamada, and K. Sekimizu. 2005. Silkworm pathogenic bacteria infection model for identification of novel virulence genes. Mol. Microbiol. 56934-944. [DOI] [PubMed] [Google Scholar]
- 24.Kaito, C., and K. Sekimizu. 2007. Colony spreading in Staphylococcus aureus. J. Bacteriol. 1892553-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kajimura, J., T. Fujiwara, S. Yamada, Y. Suzawa, T. Nishida, Y. Oyamada, I. Hayashi, J. Yamagishi, H. Komatsuzawa, and M. Sugai. 2005. Identification and molecular characterization of an N-acetylmuramyl-l-alanine amidase Sle1 involved in cell separation of Staphylococcus aureus. Mol. Microbiol. 581087-1101. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy, E. P. 1982. Osmotic regulation and the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. Proc. Natl. Acad. Sci. USA 791092-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiriukhin, M. Y., D. V. Debabov, D. L. Shinabarger, and F. C. Neuhaus. 2001. Biosynthesis of the glycolipid anchor in lipoteichoic acid of Staphylococcus aureus RN4220: role of YpfP, the diglucosyldiacylglycerol synthase. J. Bacteriol. 1833506-3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koch, H. U., R. Haas, and W. Fischer. 1984. The role of lipoteichoic acid biosynthesis in membrane lipid metabolism of growing Staphylococcus aureus. Eur. J. Biochem. 138357-363. [DOI] [PubMed] [Google Scholar]
- 29.Kuroda, M., T. Ohta, I. Uchiyama, T. Baba, H. Yuzawa, I. Kobayashi, L. Cui, A. Oguchi, K. Aoki, Y. Nagai, J. Lian, T. Ito, M. Kanamori, H. Matsumaru, A. Maruyama, H. Murakami, A. Hosoyama, Y. Mizutani-Ui, N. K. Takahashi, T. Sawano, R. Inoue, C. Kaito, K. Sekimizu, H. Hirakawa, S. Kuhara, S. Goto, J. Yabuzaki, M. Kanehisa, A. Yamashita, K. Oshima, K. Furuya, C. Yoshino, T. Shiba, M. Hattori, N. Ogasawara, H. Hayashi, and K. Hiramatsu. 2001. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 3571225-1240. [DOI] [PubMed] [Google Scholar]
- 30.Li, Y., K. Kurokawa, M. Matsuo, N. Fukuhara, K. Murakami, and K. Sekimizu. 2004. Identification of temperature-sensitive dnaD mutants of Staphylococcus aureus that are defective in chromosomal DNA replication. Mol. Genet. Genomics 271447-457. [DOI] [PubMed] [Google Scholar]
- 31.Li, Y., K. Kurokawa, L. Reutimann, H. Mizumura, M. Matsuo, and K. Sekimizu. 2007. dnaB and dnaI temperature-sensitive mutants of Staphylococcus aureus: evidence for involvement of DnaB and DnaI in synchrony regulation of chromosome replication. Microbiology 1533370-3379. [DOI] [PubMed] [Google Scholar]
- 32.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193265-275. [PubMed] [Google Scholar]
- 33.Matsuo, M., K. Kurokawa, S. Nishida, Y. Li, H. Takimura, C. Kaito, N. Fukuhara, H. Maki, K. Miura, K. Murakami, and K. Sekimizu. 2003. Isolation and mutation site determination of the temperature-sensitive murB mutants of Staphylococcus aureus. FEMS Microbiol. Lett. 222107-113. [DOI] [PubMed] [Google Scholar]
- 34.Morath, S., A. Stadelmaier, A. Geyer, R. R. Schmidt, and T. Hartung. 2002. Synthetic lipoteichoic acid from Staphylococcus aureus is a potent stimulus of cytokine release. J. Exp. Med. 1951635-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murai, N., K. Kurokawa, N. Ichihashi, M. Matsuo, and K. Sekimizu. 2006. Isolation of a temperature-sensitive dnaA mutant of Staphylococcus aureus. FEMS Microbiol. Lett. 25419-26. [DOI] [PubMed] [Google Scholar]
- 36.Neuhaus, F. C., and J. Baddiley. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67686-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novick, R. P., H. F. Ross, S. J. Projan, J. Kornblum, B. Kreiswirth, and S. Moghazeh. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 123967-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oku, Y., K. Kurokawa, N. Ichihashi, and K. Sekimizu. 2004. Characterization of the Staphylococcus aureus mprF gene, involved in lysinylation of phosphatidylglycerol. Microbiology 15045-51. [DOI] [PubMed] [Google Scholar]
- 39.Op den Camp, H. J., A. Oosterhof, and J. H. Veerkamp. 1985. Phosphatidylglycerol as biosynthetic precursor for the poly(glycerol phosphate) backbone of bifidobacterial lipoteichoic acid. Biochem. J. 228683-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peschel, A., C. Vuong, M. Otto, and F. Gotz. 2000. The d-alanine residues of Staphylococcus aureus teichoic acids alter the susceptibility to vancomycin and the activity of autolytic enzymes. Antimicrob. Agents Chemother. 442845-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenthal, R. S., and R. Dziarski. 1994. Isolation of peptidoglycan and soluble peptidoglycan fragments. Methods Enzymol. 235253-285. [DOI] [PubMed] [Google Scholar]
- 42.Roth, B. L., M. Poot, S. T. Yue, and P. J. Millard. 1997. Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl. Environ. Microbiol. 632421-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulman, H., and E. P. Kennedy. 1979. Localization of membrane-derived oligosaccharides in the outer envelope of Escherichia coli and their occurrence in other gram-negative bacteria. J. Bacteriol. 137686-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sekimizu, K., and A. Kornberg. 1988. Cardiolipin activation of DnaA protein, the initiation protein of replication in Escherichia coli. J. Biol. Chem. 2637131-7135. [PubMed] [Google Scholar]
- 45.Sugai, M., T. Akiyama, H. Komatsuzawa, Y. Miyake, and H. Suginaka. 1990. Characterization of sodium dodecyl sulfate-stable Staphylococcus aureus bacteriolytic enzymes by polyacrylamide gel electrophoresis. J. Bacteriol. 1726494-6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi, J., H. Komatsuzawa, S. Yamada, T. Nishida, H. Labischinski, T. Fujiwara, M. Ohara, J. Yamagishi, and M. Sugai. 2002. Molecular characterization of an atl null mutant of Staphylococcus aureus. Microbiol. Immunol. 46601-612. [DOI] [PubMed] [Google Scholar]
- 47.Tao, L., D. J. LeBlanc, and J. J. Ferretti. 1992. Novel streptococcal-integration shuttle vectors for gene cloning and inactivation. Gene 120105-110. [DOI] [PubMed] [Google Scholar]
- 48.van Langevelde, P., E. Ravensbergen, P. Grashoff, H. Beekhuizen, P. H. Groeneveld, and J. T. van Dissel. 1999. Antibiotic-induced cell wall fragments of Staphylococcus aureus increase endothelial chemokine secretion and adhesiveness for granulocytes. Antimicrob. Agents Chemother. 432984-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weart, R. B., A. H. Lee, A. C. Chien, D. P. Haeusser, N. S. Hill, and P. A. Levin. 2007. A metabolic sensor governing cell size in bacteria. Cell 130335-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weidenmaier, C., J. F. Kokai-Kun, S. A. Kristian, T. Chanturiya, H. Kalbacher, M. Gross, G. Nicholson, B. Neumeister, J. J. Mond, and A. Peschel. 2004. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 10243-245. [DOI] [PubMed] [Google Scholar]
- 51.Weidenmaier, C., and A. Peschel. 2008. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat. Rev. Microbiol. 6276-287. [DOI] [PubMed] [Google Scholar]
- 52.Xia, W., and W. Dowhan. 1995. In vivo evidence for the involvement of anionic phospholipids in initiation of DNA replication in Escherichia coli. Proc. Natl. Acad. Sci. USA 92783-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamada, S., M. Sugai, H. Komatsuzawa, S. Nakashima, T. Oshida, A. Matsumoto, and H. Suginaka. 1996. An autolysin ring associated with cell separation of Staphylococcus aureus. J. Bacteriol. 1781565-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamagishi, J., T. Kojima, Y. Oyamada, K. Fujimoto, H. Hattori, S. Nakamura, and M. Inoue. 1996. Alterations in the DNA topoisomerase IV grlA gene responsible for quinolone resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 401157-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ziebandt, A. K., H. Weber, J. Rudolph, R. Schmid, D. Hoper, S. Engelmann, and M. Hecker. 2001. Extracellular proteins of Staphylococcus aureus and the role of SarA and sigma B. Proteomics 1480-493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.