Abstract
HAMP domains, found in many bacterial signal transduction proteins, generally transmit an intramolecular signal between an extracellular sensory domain and an intracellular signaling domain. Studies of HAMP domains in proteins where both the input and output signals occur intracellularly are limited to those of the Aer energy taxis receptor of Escherichia coli, which has both a HAMP domain and a sensory PAS domain. Campylobacter jejuni has an energy taxis system consisting of the domains of Aer divided between two proteins, CetA (HAMP domain containing) and CetB (PAS domain containing). In this study, we found that the CetA HAMP domain differs significantly from that of Aer in the predicted secondary structure. Using similarity searches, we identified 55 pairs of HAMP/PAS proteins encoded by adjacent genes in a diverse group of microorganisms. We propose that these HAMP/PAS pairs form a new family of bipartite energy taxis receptors. Within these proteins, we identified nine residues in the HAMP domain and proximal signaling domain that are highly conserved, at least three of which are required for CetA function. Additionally, we demonstrated that CetA contributes to the invasion of human epithelial cells by C. jejuni, while CetB does not. This finding supports the hypothesis that members of HAMP/PAS pairs possess the capacity to act independently of each other in cellular traits other than energy taxis.
HAMP domains (named for their presence in histidine kinases, adenylyl cyclases, methyl-accepting chemotaxis proteins, and phosphatases) (6) represent a common element in numerous bacterial signal transduction proteins. More than 11,700 known or predicted proteins containing HAMP domains are identified in the SMART database (39). The vast majority of these proteins are eubacterial, but HAMP domains have also been identified in archaea and lower eukaryotic organisms. HAMP domains are thought to play a role in intramolecular communication between the input and output domains of a single protein (4-6). HAMP domains have been studied predominantly in transmembrane receptors that translate a signal originating extracellularly to an intracellular signal transduction domain.
Our understanding of how HAMP domains function has been hampered by considerable sequence divergence among these domains and a paucity of structural data. Sequence analysis and mutagenesis studies have indicated that HAMP domains consist of two amphipathic helices (AS-1 and AS-2), which are joined by a flexible loop region to form a coiled-coil (5, 14, 47). Recently, the structure of the HAMP domain from the Archaeoglobus fulgidus protein Af1503 was solved (31). Af1503 is atypical of HAMP domain-containing proteins in that it lacks an output signal transduction domain (31). This structure consists of two amphipathic helices that come together in a parallel coiled-coil. These helices form a four-helix bundle in a HAMP domain dimer. This four-helix bundle adopts an unusual knobs-to-knobs conformation. These findings gave rise to a model where a shift in two transmembrane helices is translated into a gear-like 26° rotation of the helices relative to one another within the HAMP dimer four-helix bundle (31).
HAMP domains are often found in transmembrane receptors with extracellular input and intracellular output domains, although this is not true of all HAMP domains. A search of the SMART database reveals more than 400 known or predicted proteins with a HAMP domain that lack a predicted transmembrane domain. Even accounting for the fact that some of these proteins may actually have transmembrane domains that the SMART tool missed, there are clearly a number of proteins in nature that have HAMP domains and lack transmembrane domains. The mechanism by which HAMP domains might function in such proteins has not been extensively probed. Studies of a HAMP domain-containing protein in which the input and output signals both occur in the cytoplasm are limited to Escherichia coli Aer. Aer, the major energy taxis receptor of E. coli (10, 45), possesses four major domains: (i) a PAS domain (named after the three proteins [Per, ARNT, and Sim] where it was first identified) (51) that binds flavin adenine dinucleotide (FAD), the redox state of which is thought to reflect the redox state of an element(s) of the electron transport system, (ii) two transmembrane domains separated by a short periplasmically accessible region, (iii) a HAMP domain, and (iv) a conserved signaling domain present in all methyl-accepting chemotaxis proteins (MCPs) (1, 50). The PAS domain of Aer has been predicted to interact directly with the HAMP domain to transmit an energy taxis signal parallel to, rather than across, the inner membrane (50).
An energy taxis system consisting of a variation on the domain arrangement of Aer was previously identified in Campylobacter jejuni (27). C. jejuni, a microaerophilic, gram-negative bacterium commonly found in the gastrointestinal tracts of chickens and other livestock, is one of the most common causes of food-borne gastroenteritis in the United States. The flagellar motility of this bacterium has proven essential for both its commensal and its pathogenic lifestyle (25, 57). An energy taxis system of C. jejuni was identified in a screen of a transposon library for mutants defective in flagellar motility (27). This system consists of two proteins, CetA and CetB (formerly known as Cj1190c and Cj1189c, respectively), which together contain all of the domains of the single protein Aer. CetA, a predicted membrane-bound protein, possesses a predicted HAMP domain and the signaling domain. CetB, a predicted cytoplasmic protein, possesses a predicted PAS domain (27).
CetA and CetB are proposed to interact with one another directly to transduce an energy taxis signal via a mechanism similar to that of the single protein Aer (27). Since the HAMP domain of Aer is proposed to interact directly with the PAS domain, we hypothesize that the HAMP domain of CetA may mediate an interaction between CetA and CetB. Separation of these domains into distinct proteins may enable CetA and/or CetB to interact with other proteins and participate independently in alternate signaling pathways (27).
In this study, we determined that the HAMP domain of CetA differs from that of Aer in predicted secondary structure. Based on similarity with the CetA HAMP domain, we identified other members of a new family of putative bipartite energy taxis transducers. We found that the CetA homologs in this family possess highly conserved HAMP domain residues, at least three of which are required for wild-type function of CetA in energy taxis. Finally, we determined that the ΔcetA mutant, but not the ΔcetB mutant, has a defect in invasion of human epithelial cells, supporting the hypothesis that CetA and/or CetB may function independently of one another in cellular processes other than energy taxis.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
All bacterial strains and plasmids used in this study are listed in Table 1. DRH212, a spontaneous streptomycin-resistant mutant of the clinical isolate C. jejuni 81-176 (27), was the background strain for all mutants studied and is referred to as the wild type. C. jejuni was routinely grown on Mueller-Hinton (MH) agar with 10 μg/ml trimethoprim under microaerobic conditions (85% N2, 10% CO2, 5% O2) in a tri-gas incubator. For C. jejuni, the following antibiotic concentrations were used: 10 μg/ml trimethoprim, 30 μg/ml cefoperazone, 50 μg/ml kanamycin, 20 μg/ml chloramphenicol, and 0.1 or 2 mg/ml streptomycin. E. coli was grown in Luria-Bertani (LB) agar or broth. For E. coli, the following antibiotic concentration was used: 50 μg/ml kanamycin or 100 μg/ml ampicillin.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Bacteria | ||
| E. coli | ||
| JM101 | F′ traD36 proA+B+ lacIq Δ(lacZ)M15/Δ(lac-proAB) glnV thi | New England Biolabs |
| DH5α/pRK212.1 | Contains conjugative plasmid for transfer of plasmid into C. jejuni | 20 |
| C. jejuni | ||
| DRH212 | 81-176 Smr; spontaneous mutant | 27 |
| DRH304 | cetB::cat-rpsL; intermediate strain for deletion mutagenesis | 27 |
| DRH307 | ΔcetB | 27 |
| DRH321 | ΔrpoN | 27 |
| DRH333 | ΔcetA | 27 |
| KYCj172 | ΔcetAB | This study |
| Plasmids | ||
| pUC19 | Ampr | New England Biolabs |
| pRY108 | Kmr; E. coli/C. jejuni shuttle vector | 56 |
| pKTY60 | pUC19 with a 3.5-kb fragment containing cj1191c, cetA (cj1190c), and cetB (cj1189c) regions cloned into the KpnI site | This study |
| pKTY62 | pKTY60 with a deletion from the first codon of cetA to the last codon of cetB | This study |
| pKTY152 | pKTY60 with the D94A mutation in the cetA coding sequence | This study |
| pKTY153 | pKTY60 with the E102A mutation in the cetA coding sequence | This study |
| pKTY154 | pKTY60 with the E97A mutation in the cetA coding sequence | This study |
| pKTY155 | pKTY60 with the K118A mutation in the cetA coding sequence | This study |
| pKTY156 | pKTY60 with the R101A mutation in the cetA coding sequence | This study |
| pKTY157 | pKTY60 with the R117A mutation in the cetA coding sequence | This study |
| pKTY158 | pKTY60 with the R71A mutation in the cetA coding sequence | This study |
| pKTY159 | pKTY60 with the Y116A mutation in the cetA coding sequence | This study |
| pKTY160 | pKTY60 with the Y99A mutation in the cetA coding sequence | This study |
| pKTY360 | pRY108 with a 2.4-kb fragment containing the cetA and cetB coding sequences cloned into the XmnI site | This study |
| pKTY361 | pKTY360 with the R71A mutation in the cetA coding sequence | This study |
| pKTY362 | pKTY360 with the D94A mutation in the cetA coding sequence | This study |
| pKTY363 | pKTY360 with the E97A mutation in the cetA coding sequence | This study |
| pKTY364 | pKTY360 with the Y99A mutation in the cetA coding sequence | This study |
| pKTY365 | pKTY360 with the R101A mutation in the cetA coding sequence | This study |
| pKTY366 | pKTY360 with the E102A mutation in the cetA coding sequence | This study |
| pKTY367 | pKTY360 with the Y116A mutation in the cetA coding sequence | This study |
| pKTY368 | pKTY360 with the R117A mutation in the cetA coding sequence | This study |
| pKTY369 | pKTY360 with the K118A mutation in the cetA coding sequence | This study |
Bioinformatic analysis.
The HAMP domain in CetA was identified by PSI-BLAST (2). Secondary-structure predictions were obtained using the PSA server (http://bmerc-www.bu.edu/psa/request.htm). Proteins that contained a HAMP domain and proximal signaling domain similar to those of CetA (residues 44 to 139) were identified by a BLAST search of the nonredundant protein database. Those proteins identified in this BLAST search that also possessed a neighboring open reading frame (ORF) containing a PAS domain were further analyzed for the presence of other functional domains using the SMART tool (39). These proteins were also assessed for the presence of transmembrane domains using the DAS (dense alignment surface) method (17). Proteins predicted to contain one long transmembrane helix with a strong dip in hydrophobicity in the middle of this region, corresponding to helical hairpin residues (42, 43), were designated as possessing two transmembrane domains on the basis of topology analysis of CetA (19). The G+C percentage of each HAMP-containing bipartite family member gene was plotted against the G+C percentage of the genome in which it was found. Using Excel, a linear regression trend line and associated R2 value were calculated. HAMP domains and adjacent regions from homologous proteins were aligned using CLUSTALX (52) with default parameters. Conserved residues were identified using Consensus script (available at www.bork.embl-heidelberg.de/Alignment/consensus.html).
Modeling of CetA structure.
The structure of the HAMP domain from Archaeoglobus fulgidus (Protein Data Bank ID, 2ASW) was used as the foundation for modeling the structure of CetA. To model the HAMP domain of CetA, the amino acid sequence of 2ASW was virtually mutated into that of CetA by using the graphics program O (33), and the single amino acid insertion was fit using the program's lego-loop option. The resulting CetA model was then placed in a box of waters containing a minimum of two shells of water, minimized, and put through simulated annealing using torsion angle dynamics in the Crystallography & NMR system (12).
Construction of the ΔcetAB deletion mutant.
The ΔcetAB deletion mutant was constructed essentially as described by Hendrixson et al. (27). The cetA and cetB coding sequences with 1,036 bp upstream and 595 bp downstream were amplified by PCR with primers designed with KpnI sites at their 5′ ends for cloning into pUC19. The resulting plasmid was pKTY60. A deletion from the first codon of cetA to the last codon of cetB was created via Pfu mutagenesis (55). The resulting plasmid, pKTY62, was electroporated into DRH304, which harbors the cat-rpsL cassette in the cetB coding sequence. Transformants were selected on 2 mg/ml streptomycin and screened for sensitivity on 20 μg/ml chloramphenicol. The deletion was confirmed by PCR analysis and chromosomal sequencing.
Construction of a plasmid to complement the ΔcetAB mutant.
pKTY60 was digested with ApaLI and BsrBI. The resulting fragment, containing the cetA and cetB coding sequences along with 299 bases upstream and 202 bases downstream, was blunted by T4 DNA polymerase. This fragment was then cloned into the XmnI site in the E. coli/C. jejuni shuttle vector pRY108 (56).
Site-directed mutagenesis.
Point mutations in the cetA coding sequence leading to alanine substitutions (R71A, D94A, E97A, Y99A, R101A, E102A, Y116A, R117A, and K118A) were made in pKTY60 by using Pfu mutagenesis (55). The DNA sequences of the resulting plasmids were determined in order to confirm the presence of the point mutations and ensure the absence of additional mutations. These plasmids were then digested with ApaLI and BsrBI, and the resulting fragments were cloned into the XmnI site of pRY108 as described above. The orientation of the insertions into pRY108 was checked by multiple restriction digests to confirm that the resulting plasmids, pKTY361 to pKTY369, were identical to pKTY360 except for the indicated point mutations.
Conjugation of plasmids into C. jejuni.
Plasmids were conjugated into C. jejuni as described by Guerry et al. (26). Briefly, C. jejuni was grown on MH agar with 10 μg/ml trimethoprim for 16 to 20 h and resuspended in MH broth to an optical density at 600 nm (OD600) of 1.0. Overnight cultures of the E. coli donor strain [DH5α(pRK212.1), containing the plasmid to be conjugated into C. jejuni] were diluted into fresh LB broth and grown to an OD600 of 0.5. Five hundred microliters of the donor culture was centrifuged, and the pellet was first washed twice with MH broth and then resuspended in 1 ml of the C. jejuni recipient culture. This mixture was spotted onto MH agar with no antibiotics. After 5 h at 37°C under microaerophilic conditions, the bacteria were resuspended and spread onto MH agar containing 10 μg/ml trimethoprim, 30 μg/ml cefoperazone, 2 mg/ml streptomycin, and 50 μg/ml kanamycin. PCR was used to verify the transfer of the plasmid to the recipient C. jejuni strain.
Motility assays.
C. jejuni was first grown on MH agar containing 10 μg/ml trimethoprim and 50 μg/ml kanamycin for 16 to 20 h and then resuspended in MH broth to an OD600 of 0.4. A 0.4-μl aliquot of each strain was injected into MH motility medium containing 0.4% agar. Plates were incubated for 28 h under microaerophilic conditions. The diameter of the outermost motility ring was measured with calipers. The average and standard deviation of six replicates were calculated, and the assay was repeated three times.
Tissue culture.
The human epithelial cell line INT 407 was used in invasion experiments. INT 407 cells were cultured in Dulbecco's modified Eagle medium (DMEM) plus 10% fetal bovine serum supplemented with GIBCO MEM nonessential amino acids and 2 mM glutamine (referred to below as DMEM) in a 37°C, 5% CO2 incubator. When cells were cultured in the absence of C. jejuni, the DMEM was supplemented with 10 U/ml penicillin and 10 μg/ml streptomycin.
Invasion assays.
For invasion assays, INT 407 cells were seeded at approximately 105/well in each well of a 24-well plate and were incubated in the absence of antibiotics for an additional 12 to 18 h. For the inoculum, C. jejuni strains were grown on MH agar for 16 to 20 h and resuspended in DMEM. The INT 407 cells were rinsed twice with phosphate-buffered saline (PBS) and inoculated with C. jejuni at a multiplicity of infection of ∼200. The 24-well plates were centrifuged at 150 × g for 5 min and then incubated in a 37°C, 5% CO2 incubator. To determine the number of total cell-associated bacteria, the cells were incubated for 2 h, rinsed twice with PBS, and lysed in PBS plus 0.1% Triton X-100, and serial dilutions were plated onto MH agar to obtain CFU. To determine the number of intracellular bacteria, the cells were incubated for 2 h, rinsed twice with PBS, and incubated for an additional 2.5 h in DMEM plus 100 μg/ml gentamicin. The cells were then rinsed twice with PBS and lysed in PBS plus 0.1% Triton X-100, and serial dilutions were plated onto MH agar to obtain CFU. For invasion time course experiments, the cells were infected with C. jejuni as described above. At 0.5 h, 1 h, 1.5 h, 2 h, or 4 h postinfection, the numbers of total cell-associated and intracellular bacteria were determined as described above. The percentage of total cell-associated bacteria that were intracellular was calculated. The average and standard deviation of three replicates were obtained, and each invasion assay and time course assay was repeated a minimum of three times.
RESULTS
CetA and Aer differ in their predicted HAMP domain secondary structures.
Using PSI-BLAST, the CetA HAMP domain was identified as residues 47 to 101. This region was not identified as a HAMP domain by SMART analysis. This is not surprising, because there is a high degree of divergence among various HAMP domains. Current HAMP domain models miss more than 30% of HAMP homologs (B. P. Rekapalli and I. B. Zhulin, unpublished data).
The predicted structures of the HAMP domains of Aer and CetA differ substantially from one another (40). While the canonical HAMP domain consists of two amphipathic helices (AS-1 and AS-2), Aer possesses one amphipathic helix (AS-1) and one hydrophobic helix (AS-2) (Fig. 1A) (40). In contrast to the unusual secondary structure of Aer, CetA is predicted to have the more common HAMP domain structure of two amphipathic helices (Fig. 1B).
FIG. 1.
Secondary-structure analysis of the Aer and CetA HAMP domains (PSA server). The probability of each structural possibility (listed on the left) is indicated for each residue by using contour line increments of 0.1. Regions with many contour lines have a high probability of the indicated structure at that residue. Shown are Aer (A) and CetA (B) HAMP domain contour plots of secondary-structure probabilities at the indicated residues.
CetA and CetB found a new family of proposed bipartite energy taxis receptors.
Aer and CetA/CetB contain the same domains and are hypothesized to transduce energy taxis signals via similar mechanisms (27). However, the differences in HAMP domain primary and secondary structures between Aer and CetA led us to examine the CetA HAMP domain in more detail. Using the HAMP domain and proximal signaling domain (residues 44 to 139) of CetA in a BLASTP search of the nonredundant database, we identified 63 proteins with domain arrangements similar to that of CetA. Fifty-five of these proteins were encoded by genes with a neighboring ORF encoding a PAS domain and lacking other functional domains, a pattern similar to that of cetA and cetB, which are adjacent in the C. jejuni genome. We refer to these pairs of proteins encoded by adjacent genes as HAMP/PAS pairs (Table 2).
TABLE 2.
Members of the bipartite family of energy taxis transducers identified by similarity to the CetA HAMP and proximal signaling domains
| Species and strain | Bacterial phylum or class | Accession no. of gene encoding the HAMP domain | E valuea | Class of HAMP-containing bipartite energy taxis receptorb | Accession no. of neighboring ORFc |
|---|---|---|---|---|---|
| Azoarcus sp. strain BH72 | Betaproteobacteria | YP_934977 | 9.00E-04 | I | YP_934978 |
| Bradyrhizobium japonicum USDA 110 | Alphaproteobacteria | NP_769616 | 2.00E-06 | Id | NP_769615 |
| Caminibacter mediatlanticus TB-2 | Epsilonproteobacteria | ZP_01871323 | 0.001 | I | ZP_01871324 |
| ZP_01870851 | 0.002 | I | ZP_01870852 | ||
| Campylobacter coli RM2228 | Epsilonproteobacteria | ZP_00367208 | 8.00E-51 | Id | ZP_00367209, ZP_00367207 |
| Campylobacter concisus 13826 | Epsilonproteobacteria | YP_001466825 | 2.00E-06 | III | YP_001466824 |
| Campylobacter curvus 525.92 | Epsilonproteobacteria | YP_001408340e | 0.001 | III | YP_001408339 |
| Campylobacter jejuni | Epsilonproteobacteria | ||||
| 11168 | NP_282337 (cetA) | 7.00E-51 | Id | NP_282336 (cetB), NP_282338 | |
| 81-176 | YP_001000865 | 7.00E-51 | Id | YP_001000864, YP_001000866 | |
| HB93-13 | ZP_01070888 | 7.00E-51 | I | ZP_01070815, ZP_01071201 | |
| CG8486 | ZP_01809879 | 7.00E-51 | Id | ZP_01809880, ZP_01809878 | |
| 260.94 | ZP_01069112 | 7.00E-51 | Id | ZP_01069301, ZP_01069270 | |
| CF93-6 | ZP_01068608 | 7.00E-51 | Id | ZP_01068595, ZP_01068567 | |
| 81116 | YP_001482710 | 7.00E-51 | Id | YP_001482711, YP_001482709 | |
| RM1221 | YP_179311 | 7.00E-51 | Id | YP_179312, YP_179310 | |
| 84-25 | ZP_01099629 | 8.00E-51 | Id | ZP_01099329, ZP_01099967 | |
| Campylobacter jejuni subsp. doylei 269.97 | Epsilonproteobacteria | YP_001397715 | 2.00E-49 | Id | YP_001397716 |
| Campylobacter lari RM2100 | Epsilonproteobacteria | ZP_00368322 | 7.00E-34 | I | ZP_00368323, ZP_00368324 |
| Campylobacter upsaliensis RM3195 | Epsilonproteobacteria | ZP_00371342 | 1.00E-38 | I | ZP_00371343, ZP_00371341 |
| Chromobacterium violaceum ATCC 12472 | Betaproteobacteria | NP_900065 | 3.00E-04 | I | NP_900066 |
| Dechloromonas aromatica RCB | Betaproteobacteria | YP_287205 | 7 | I | YP_287206 |
| Kineococcus radiotolerans SRS30216 | Actinobacteria | YP_001363132 | 0.009 | III | YP_001363131 |
| Magnetospirillum gryphiswaldense MSR-1 | Alphaproteobacteria | CAM75236 | 0.003 | Id | CAM75237 |
| CAM75032 | 0.005 | V | CAM75033 | ||
| CAM78133 | 0.065 | VI | CAM78134, CAM78135f | ||
| Magnetospirillum magneticum AMB-1 | Alphaproteobacteria | YP_419949g | 3.00E-04 | I | YP_419948 |
| YP_423371 | 0.002 | I | YP_423372 | ||
| YP_423064 | 0.06 | I | YP_423063 | ||
| YP_420357 | 0.07 | I | YP_420358 | ||
| YP_421559 | 0.13 | I | YP_421560 | ||
| YP_419547 | 1.9 | I | YP_419548 | ||
| Magnetospirillum magnetotacticum MS-1 | Alphaproteobacteria | ZP_00055894 | 4.00E-04 | IV | ZP_00055893 |
| ZP_00207863 | 9.00E-04 | I | ZP_00207862 | ||
| ZP_00208993 | 0.003 | VIII | ZP_00208994, ZP_00051415f | ||
| ZP_00054945h | 0.43 | VII | ZP_00054944 | ||
| Oceanobacter sp. strain RED65 | Gammaproteobacteria | ZP_01306652 | 0.32 | I | ZP_01306653 |
| Oceanospirillum sp. strain MED92 | Gammaproteobacteria | ZP_01166142 | 0.051 | I | ZP_01166143 |
| Reinekea sp. strain MED297 | Gammaproteobacteria | ZP_01113626 | 0.88 | I | ZP_01113627 |
| Rhodopseudomonas palustris | Alphaproteobacteria | ||||
| BisA53 | YP_783562 | 1.00E-04 | I | YP_783563 | |
| BisB5 | YP_568532 | 4.00E-04 | I | YP_568531 | |
| YP_571029 | 0.26 | I | YP_571030 | ||
| BisB18 | YP_534502 | 2.00E-06 | I | YP_534503 | |
| CGA009 | NP_949817i | 8.00E-05 | I | NP_949818i | |
| NP_949819i | 1.00E-04 | II | NP_949820i | ||
| NP_949538 | 1.00E-04 | Id | NP_949539 | ||
| NP_949647 | 0.45 | I | NP_949648 | ||
| HaA2 | YP_484693 | 3.00E-07 | II | YP_484692 | |
| YP_485035 | 8.00E-04 | I | YP_485034 | ||
| YP_484934 | 0.16 | I | YP_484933 | ||
| Rhodospirillum rubrum ATCC 11170 | Alphaproteobacteria | YP_428546j | 0.011 | I | YP_428545 |
| Stappia aggregata IAM 12614 | Alphaproteobacteria | ZP_01546232 | 2.00E-04 | Id | ZP_01546231 |
| Sulfurimonas denitrificans DSM 1251 | Epsilonproteobacteria | YP_392559 | 1.00E-05 | III | YP_392560 |
| Wolinella succinogenes DSM 1740 | Epsilonproteobacteria | NP_907800 | 1.00E-11 | I | NP_907801 |
| NP_906923 | 6.00E-09 | Id | NP_906922 | ||
| NP_907510 | 3.00E-05 | Id | NP_907511 |
Obtained from a BLAST search with the CetA HAMP and proximal signaling domains (amino acids 44 to 139).
See Fig. 2.
The neighboring ORF encodes a PAS domain, but no other domains unless so indicated. Where two accession numbers are given, the gene encoding the HAMP-containing protein is flanked by two genes encoding PAS domain proteins.
Transmembrane helix prediction programs predict one transmembrane helix, but we propose that there are two transmembrane helices present in a helical hairpin (see Materials and Methods).
Misannotated as a DNA binding response regulator.
The neighboring ORF contains additional predicted functional domains.
Misannotated as a sensory rhodopsin II transducer.
Misannotated as an MCP but does not contain the highly conserved domain.
These HAMP/PAS pairs are adjacent to one another.
Misannotated as a response regulator.
The CetA-like (HAMP-containing) members of this family can be divided into eight classes on the basis of predicted topology and functional domains (Fig. 2). Most CetA homologs fall into classes I to III, which contain a HAMP domain, a signaling domain, and two, one, or no transmembrane domains, respectively. Only two proteins contain a single predicted transmembrane domain. Single pass transmembrane proteins are unusual in bacteria but have been identified (16, 41). Further examination is necessary, however, to ascertain whether this topology prediction holds true or whether, perhaps, the predicted start site of translation is incorrect. Variations on the domain arrangement of CetA, with proteins containing additional domains or lacking the signaling and/or transmembrane domain, were also identified (classes IV to VIII). In all, we identified 55 HAMP/PAS pairs, 40 of which are in genera other than Campylobacter (Table 2). The majority of organisms containing HAMP/PAS pairs belong to the epsilon class of Proteobacteria, but HAMP/PAS pairs are also found in the alpha, beta, and gamma classes of Proteobacteria as well as in one gram-positive organism, the actinobacterium Kineococcus radiotolerans (Table 2). The GC content of the HAMP genes correspond well to those of the genomes in which they are found (Fig. 3). Several strains contain more than one HAMP/PAS pair. In those strains that contain multiple HAMP/PAS pairs, the HAMP members of different HAMP/PAS pairs may also fall into more than one of the classes defined in Fig. 2. We propose that many of these HAMP/PAS pairs identified by similarity to the CetA HAMP domain comprise a new family of bipartite energy taxis receptors.
FIG. 2.
Classes of HAMP-containing bipartite family members. HAMP-containing proteins from Table 2 were analyzed by the SMART and DAS tools and separated into different classes on the basis of predicted topology and functional domains. SD indicates the signaling domain conserved in MCPs. PilZ domains are cyclic di-GMP effector domains.
FIG. 3.
The G+C percentage of each gene encoding a HAMP protein in the bipartite family was plotted against the G+C percentage of the genome in which it resides. A linear regression trend line and R2 value are shown. The data point for the Kineococcus radiotolerans HAMP protein is circled. An arrow indicates the data point for the Magnetospirillum magneticum AMB-1 HAMP protein found in the magnetosome island (see Discussion).
Bipartite family members contain conserved HAMP and proximal domain residues.
The HAMP domain and proximal signaling domain of representative CetA-like bipartite family members were aligned using CLUSTALX (Fig. 4A). Eleven residues are highly conserved within this subfamily but not within the HAMP consensus sequence. HAMP domains, in general, share very low levels of sequence conservation, so the presence of such highly conserved residues is notable. Most of these highly conserved residues are either charged or aromatic, consistent with the possibility that they could play roles in protein-protein interactions.
FIG. 4.
Conserved residues within the HAMP domain and a proximal connector to the signaling domain of the HAMP-containing bipartite family members. This multiple alignment of representative CetA homologs includes species from different divisions of Proteobacteria and the actinobacterium. Both representative orthologs and paralogs are shown. The boundaries of the predicted HAMP domain and the N-terminal start of the signaling domain are shown above the alignment. We refer to the region between the HAMP and signaling domains as a connector. Each sequence is identified by its NCBI locus tag (e.g., Cj1190c is the locus tag for the CetA protein). Initial characters in locus tags identify species and/or strain names: Cj, Campylobacter jejuni NCTC 11168; CMTB2, Caminibacter mediatlanticus TB-2; WS, Wolinella succinogenes DSM 1740; azo, Azoarcus sp. strain BH72; MED92, Oceanospirillum sp. strain MED92; MED297, Reinekea sp. strain MED297; CV, Chromobacterium violaceum ATCC 12472; Daro, Dechloromonas aromatica RCB; RPB, Rhodopseudomonas palustris HaA2; blr, Bradyrhizobium japonicum USDA 110; amb, Magnetospirillum magneticum AMB-1; Rru, Rhodospirillum rubrum ATCC 11170; SIAM614, Stappia aggregata IAM 12614; Krad, Kineococcus radiotolerans SRS30216. Strongly conserved positions likely to contribute to protein-protein interactions are highlighted in blue (negative charge), red (positive charge), or green (aromatic). Strongly conserved positions likely to contribute primarily to structure and mildly conserved positions are highlighted in gray. The conservation consensus (cons.) at 100% and 80% (calculated using the Consensus script, available at http://coot.embl.de/Alignment//consensus.html) is shown below the alignment. The types of residues are abbreviated as follows: h, hydrophobic; t, turn-like; p, polar; s, small; l, aliphatic; c, charged; a, aromatic. Residues that are conserved at 100% make up the signature of the entire bipartite family (conserved at 90% within the entire family).
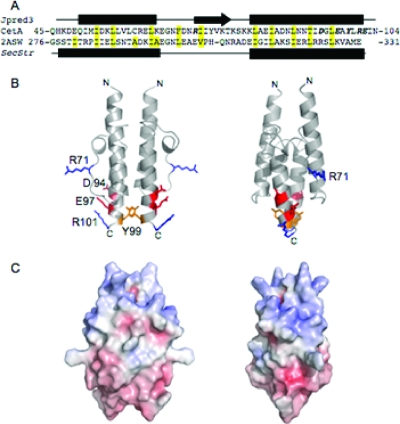
To understand the placement of these conserved residues in the protein, a model of the CetA HAMP domain was created. The amino acid sequence of the CetA HAMP domain was threaded onto the averaged nuclear magnetic resonance structure of Archaeoglobus fulgidus (2ASW), the only HAMP domain present in the Protein Data Bank, using the graphics program O (33) and the alignment shown in Fig. 5A. Four of the five conserved residues that fall within the HAMP domain (R71, D94, E97, and R101) appear to cluster near one another on the surface of the HAMP four-helix bundle (Fig. 5B). The fifth conserved residue in the HAMP domain (Y99), however, is located between the two monomers and is predicted to be involved in tyrosine stacking interactions between HAMP monomers at the base of the four-helix bundle (Fig. 5B). The six remaining conserved residues (E102, F115, Y116, R117, K118, and F128) fall outside of the Af1503 structure used to model the CetA HAMP domain, and therefore, their location within the protein cannot be predicted. This model of the CetA HAMP domain structure shows an overall dipole moment, as the N-terminal half of the structure has a net positive charge, while the C-terminal half of the structure has a net negative charge (Fig. 5C).
FIG. 5.
Model of CetA. (A) The sequence alignment of the CetA HAMP domain and Af1503 from Clustal W2 (15) (http://www.ebi.ac.uk/Tools/clustalw2/index.html) is shown with the hydrophobic residues of the heptad repeat and linker region boxed in yellow. The secondary-structure prediction for CetA (18) (http://www.compbio.dundee.ac.uk/∼www-jpred/) and the published secondary structure of Af1503 are shown above and below their amino acid sequences, respectively. Helices and β-strands are represented as solid rectangles and arrows, respectively. Residues mutated in CetA are italicized. This alignment was used in the creation of the structural model of the CetA HAMP domain shown in panels B and C. (B) The model of CetA is depicted as a ribbon diagram, with the mutated residues shown as sticks. The views for individual molecules are separated by a 90° rotation about the y axis. (C) Electrostatic surface potentials for the modeled structure of CetA were calculated using APBS (7) and mapped onto their respective solvent-accessible surfaces by using the PyMOL molecular graphics system (http://www.pymol.org/). Negative potentials (−10 kT/e) are shown in red, positive potentials (10 kT/e) in blue. The views are the same as in panel B. The protein structures are shown at the same magnification for each view.
Conserved HAMP domain residues are important for CetA function.
Attempts to complement a ΔcetA mutant with a plasmid expressing cetA from a constitutive promoter were unsuccessful (data not shown), likely because the relative levels of CetA and CetB, as well as the levels of these proteins relative to other MCPs, are important for proper energy taxis signal transduction. A double-deletion strain was constructed with the deletion extending from the first codon of cetA to the last codon of cetB. We also constructed a plasmid, pKTY360, consisting of the cetA and cetB genes, as well as 299 bases upstream and 202 bases downstream, cloned into the pRY108 E. coli/C. jejuni shuttle vector (56). cetAB expression in pKTY360 presumably originates from the native promoter, since there is no promoter to drive the expression of cloned DNA in pRY108. pKTY360 complements the motility defect of the ΔcetAB double mutant in MH motility agar but has no effect on the motility defect of the ΔrpoN mutant, which is nonmotile and lacks flagella (Fig. 6).
FIG. 6.
Influence of point mutations on the function of CetA in motility. Motility assays were performed on wild-type (wt), ΔcetAB, and ΔrpoN strains containing an empty vector, pRY108, or pRY108::cetAB (with or without the indicated HAMP and proximal signaling domain point mutations).
Alanine substitutions were made for nine of the conserved residues in CetA in the context of pKTY360. Most of these substitutions led to a growth defect (D94A, E97A, R101A, E102A, and R117A) or loss of CetA stability (Y116A) (data not shown). Three alanine substitution mutants (R71A, Y99A, and K118A [pKTY361, pKTY364, and pKTY369, respectively]) retained growth kinetics and CetA expression levels at or near those for the ΔcetAB mutant complemented with pKTY360 (data not shown). Mutant proteins with these substitutions exhibited an abrogated or reduced ability to rescue the motility defect of the ΔcetAB double-deletion strain (Fig. 6). The motility pattern of the HAMP mutants on MH agar may also be altered in shape or ring structure, possibly reflecting CetA/CetB sensing. However, unlike the media used for E. coli motility plates, the MH motility medium used for C. jejuni is too opaque for such distinctions to be observed. Still, these results indicate that at least some of the conserved residues within the bipartite family HAMP and proximal signaling domains are required for wild-type function.
The ΔcetA mutant has an epithelial cell invasion defect, but the ΔcetB mutant does not.
CetA and CetB are each required for energy taxis in C. jejuni, suggesting a functional interaction whose mechanism may be similar to that of Aer (27). However, the separation of Aer domains into two proteins in CetA and CetB, as well as in other members of this family, raises the possibility that each member of a HAMP/PAS pair may contribute independently to different traits. Because C. jejuni is a common commensal of chickens, we previously tested ΔcetA and ΔcetB mutants in a chick colonization model, and both mutants colonized to wild-type levels (28).
C. jejuni actively invades human epithelial cells, a trait associated with its pathogenicity (37). To determine whether or not CetA or CetB contributes to this phenotype, we tested mutants in a tissue culture model of invasion (29, 44). INT 407 cells were infected with the wild type (DRH212) or with a ΔcetA, ΔcetB, or ΔcetAB mutant at a multiplicity of infection of ∼200. Immediately upon infection, bacteria were centrifuged onto the INT 407 cells in order to rule out any effects of motility on the invasion assay. After 2 h, the number of total cell-associated bacteria was determined in half of the wells (see Materials and Methods), and gentamicin was added to the remaining wells to kill extracellular bacteria. After a further 2.5 h, the number of intracellular bacteria was determined as described in Materials and Methods, and the percentage of total cell-associated bacteria that were intracellular (i.e., that had invaded) was calculated. Strains lacking cetA alone, or lacking both cetA and cetB, invaded INT 407 cells approximately 5 times less efficiently than the wild type, whereas a ΔcetB mutant invaded at wild-type levels (Fig. 7A). C. jejuni does not grow significantly in DMEM or intracellularly over the time period of these invasion assays, and there were no differences in survival between strains during the course of these experiments (K. T. Elliott and V. J. DiRita, unpublished data).
FIG. 7.
Effects of cetA and cetB mutations on epithelial cell invasion. (A) INT 407 cells were infected with the wild type (wt) or the ΔcetB, ΔcetA, or ΔcetAB mutant. The percentages of total cell-associated bacteria that were intracellular following a 2-h infection were calculated. (B) INT 407 cells were infected with the wt or the ΔcetA mutant. The percentages of total cell-associated bacteria that were intracellular following a 30-min, 1-h, 1.5-h, 2-h, or 4-h infection are shown.
The invasion defect exhibited by the ΔcetA mutant was investigated further by analyzing the kinetics of invasion. The percentage of total cell-associated bacteria that had invaded the INT 407 cells was determined at various times between 30 min and 4 h postinfection (Fig. 7B). The level of invasion by the ΔcetA mutant remained lower than that of the wild type at all times. The rate of invasion by the ΔcetA mutant was initially much lower than that by the wild type. By 2 to 4 h postinfection, however, the rate of invasion by the ΔcetA mutant reached near-wild-type levels. These results indicate that the ΔcetA mutant lags behind the wild type in the initiation of invasion.
DISCUSSION
We identified a new family of apparent bipartite energy taxis receptors that contain HAMP and proximal signaling domains homologous to CetA. These domains have several highly conserved residues, at least three of which are required for CetA function in C. jejuni motility. We propose that these conserved residues, several of which cluster on the exterior of the base of the HAMP domain four-helix bundle, make up the PAS interaction surface.
In further studies of the CetA/CetB HAMP/PAS pair, we demonstrated that CetA is required for the invasion of human epithelial cells by C. jejuni while CetB is dispensable. These findings support the hypothesis that CetA (and perhaps other HAMP-containing members of the bipartite family) can act independently of its PAS partner to regulate traits other than energy taxis. CetA could control invasion either alone or through interactions with as yet unknown proteins (Fig. 8). Similarly, CetB could perhaps regulate traits independently of CetA, although we have yet to identify a CetB-dependent phenotype other than energy taxis. Thus, this work identified a new family of apparent bipartite energy taxis receptors and provided evidence of a functional consequence of having the domains of Aer separated into distinct proteins within this family.
FIG. 8.
Proposed model for CetA/CetB function compared to Aer function. CetA and CetB are proposed to transduce an energy taxis signal via a mechanism similar to that of Aer. However, CetA is proposed to interact with another, unidentified protein to promote invasion, while CetB does not. See Discussion for details.
Unique features of the CetA HAMP domain suggest that it functions differently from that of Aer.
The HAMP domains of Aer and CetA differ substantially from one another. Aer has a hydrophobic helix (AS-2) where most HAMP domains have a second amphipathic helix (Fig. 1A) (40). The hydrophobic nature of the Aer AS-2 is proposed to reflect the fact that this helix is involved both in HAMP-HAMP interactions (between Aer monomers in a dimer), and in HAMP-PAS interactions (40). Since Aer and CetA/CetB are proposed to transduce an energy taxis signal via similar mechanisms, we predict that the HAMP domain of CetA would also be involved in both HAMP-HAMP and HAMP-PAS interactions. If so, then the fact that the CetA HAMP domain, in contrast to that of Aer, contains two amphipathic helices (Fig. 1B) is unexpected. Assuming that the CetA AS-2 interacts directly with CetB, the molecular nature of this interaction must differ substantially from that of the interaction between the HAMP and PAS domains of Aer.
A newly identified family of HAMP/PAS protein pairs.
Based on similarity with the CetA HAMP and proximal signaling domains and genome context, numerous HAMP/PAS pairs homologous to CetA and CetB were identified. We hypothesize that most of the members of this protein family function as bipartite energy taxis receptors. Based on their bipartite nature and on the alternative domain architectures present within this family (Fig. 2), some pairs may have other functions in addition to, or instead of, energy taxis (see below). The species containing HAMP/PAS pairs represent a broad range of bacteria—alphaproteobacteria, betaproteobacteria, epsilonproteobacteria, gammaproteobacteria, and one gram-positive actinobacterium (Table 2)—including human pathogens, animal commensals, plant symbionts, and species found in marine, aquatic, and soil environments. Together, these observations suggest that these HAMP/PAS pairs have been conserved under a diverse set of selection pressures. Our analysis of the GC contents of the HAMP-containing bipartite family genes suggests that they were not spread recently by horizontal gene transfer. There are a few possible exceptions to this, with slightly lower GC contents in the HAMP gene than in the overall genome. One of these (Fig. 3) occurs in a known genomic island associated with magnetoaerotaxis (22). GC content comparisons, however, represent only one criterion for gene acquisition by horizontal transfer. Further bioinformatic evaluation of the bipartite family genes and the genomes in which they reside is necessary in order to more definitively ascertain whether these genes have been propagated by such a mechanism.
Other than CetA and CetB, none of the HAMP/PAS pairs has been studied beyond sequence analysis. However, the various classes of CetA-like HAMP-containing proteins present some novel functional possibilities (Fig. 2). Class IV has a domain that has been implicated in osmotic stress response, resistance to reactive oxygen species, and redox regulation (3, 32, 46). Class V proteins in this family have a PilZ domain, thought to be a cyclic diguanylate effector domain (8). Class VI has the same domains as CetA, but with a larger periplasmic loop, perhaps enabling it to sense an extracellular signal in addition to the signal that originates in the PAS domain. We predict that each of these classes possesses increased functional flexibility, with the additional domains noted above providing alternative means of input to or output from the HAMP domain.
In several Campylobacter species, there are two PAS neighbors encoded by genes flanking the gene encoding the HAMP protein. These are likely homologous to cetB and cj1191c, which flank cetA in the C. jejuni 11168 genome (27). The role of cj1191c remains unclear. Multiple attempts at constructing an in-frame deletion in this ORF have been unsuccessful (Elliott and DiRita, unpublished data). Whether or not the PAS domains of each of these proteins interact with their HAMP neighbors is unknown.
Conserved HAMP domain residues are required for CetA function.
Several highly conserved residues that are not conserved in a canonical HAMP domain are found in the HAMP and proximal signaling domains of the bipartite family members (Fig. 4A). These are in the connector between AS-1 and AS-2, in AS-2, and in the proximal signaling region. Alanine substitutions at nine of these positions in CetA were investigated, and only three of the point mutations (R71A, Y99A, and K118A) enabled growth kinetics and CetA expression levels comparable to those of wild-type cells. These three mutant proteins were unable to restore motility when expressed in the ΔcetAB mutant (Fig. 6), prompting our conclusion that these residues are required for wild-type function of CetA in motility. Complementation tests with these mutant plasmids for the invasion phenotype of the ΔcetA mutant have been difficult to interpret, perhaps due to expression of the complementing alleles on a multiple-copy replicon (Elliott and DiRita, data not shown). Testing of these residues for their role in C. jejuni invasion may require that we place the mutant alleles on the chromosome, a process that works with varying efficiency in C. jejuni. We cannot draw any conclusions about the role of the other six conserved residues in CetA function. Other substitutions may be required in order to probe the role of these residues.
Structural implications for signaling transduction through CetA and CetB.
The current model of signal transduction through Aer includes a direct interaction between the PAS and HAMP domains, allowing the FAD redox signal to be transmitted parallel to the inner membrane (50). Current evidence for such an interaction is thus far indirect. Deletion of, or mutations in, the Aer HAMP domain disrupt Aer maturation and FAD binding (9, 13, 40). A point mutation in the HAMP domain that abrogated aerotaxis by Aer could be specifically suppressed by a second-site mutation in the PAS domain (54). We propose that the CetA HAMP domain and CetB interact, similarly to the proposed PAS-HAMP interaction in Aer, and studies to investigate this proposed interaction are currently in progress. Further, we predict that the other HAMP/PAS pairs in the bipartite family identified in this study also interact with one another.
Modeling the structure of the Aer HAMP domain onto that previously determined for Af1503 led to the observation that point mutations resulting in a constant “signal-on” state of Aer cluster together at the base of the four-helix bundle formed by the HAMP dimer (54). These point mutations may strengthen the proposed PAS-HAMP interaction and define the PAS-HAMP interaction surface (54). Most of the conserved residues within the HAMP domains of the bipartite family members are predicted to be located at the base of the HAMP dimer four-helix bundle, near one another on the surface of this dimer (Fig. 5B). We hypothesize that this region of the HAMP domain dimer plays a role in HAMP-PAS interactions between members of the HAMP/PAS pairs in this family of proteins.
The one residue in the CetA HAMP domain model that does not fall on the same surface as the others is Y99. While the role of this tyrosine in HAMP domain function remains unclear, the location of these tyrosines in our model lends some support to the model of HAMP domain signaling proposed by Hulko et al. (31). Specifically, if the helices of the HAMP domain four-helix bundle do rotate relative to one another, they must do so in the direction proposed by Hulko et al. (31); rotation in the opposite direction is prevented by steric hindrance due to the location of Y99.
Our model suggests that the HAMP domain of CetA is a polar structure, with a positively charged N terminus and a negatively charged C terminus (Fig. 5C). This dipole moment is apparent, but less pronounced, in the Af1503 structure (data not shown) and may be a previously unrecognized feature of HAMP domains. Since the N termini of HAMP domains are generally proximal to the inner membrane, it may be that the net positive charge in this region acts as an attractive force, further tethering the HAMP domain to the membrane. The role of the net negative charge of the base of the HAMP domain is more speculative. Since this region has been implicated in the PAS-HAMP interaction in Aer, we propose that there may be a cognate positive surface on the CetB PAS domain facilitating interaction between CetA and CetB. Since the AS-2 helix of Aer is hydrophobic, we would predict that the equivalent surface of the Aer PAS domain would comprise a hydrophobic patch.
CetA, but not CetB, contributes to cell invasion.
C. jejuni is one of the most prevalent causes of bacterial gastroenteritis in the United States (21). While not considered a highly invasive organism compared to bacteria such as Salmonella and Shigella spp., C. jejuni actively invades nonphagocytic human epithelial cells in tissue culture models (34, 44). Our studies indicate that the ΔcetA mutant and the ΔcetAB mutant have an approximately fivefold defect in invasion compared to the wild type (Fig. 7A). Compared to those of some other known C. jejuni invasion mutants, the magnitude of the invasion defect of the ΔcetA mutant is relatively small (24, 36, 49). What is striking about our observations regarding invasion is not the magnitude of the ΔcetA effect but the fact that a cetB mutation shows no defect (Fig. 7A). If CetA and CetB function solely as partners to transduce an energy taxis signal, we would expect the ΔcetA and ΔcetB mutants to have similar phenotypes. It should be noted that CetB levels are quite low in the ΔcetA mutant (19). However, the lack of an invasion defect in the ΔcetB mutant directly rules out a role for CetB in invasion. We conclude that CetA and CetB function independently of one another to regulate invasion. A previous study found that a transposon insertion in cetB resulted in increased adherence to INT 407 cells and decreased invasion of INT 407 cells (23). These results clearly differ from our findings that the ΔcetB mutant is not affected in adherence (Elliott and DiRita, data not shown) or invasion. The source of the discrepancy between those findings and ours is not clear, although that study used a transposon insertion mutant, as opposed to the in-frame deletion used in our study.
One explanation for the invasion defect of the ΔcetA mutant is that it results from the motility defect of this mutant. To reduce the contribution of motility to invasion in these experiments, the bacteria were brought into contact with the INT 407 cells by low-speed centrifugation at the beginning of the assay. Further, we eliminated this potential contribution of motility to our results by determining the percentage of total cell-associated bacteria that were intracellular. By this method of calculation, the ΔcetA and ΔcetAB mutants still have an approximately fivefold defect in invasion (Fig. 7A). Given these considerations, we conclude that the invasion defect of the ΔcetA mutant cannot be attributed solely to the motility defect.
The invasion defect of the ΔcetA mutant results from an initial lag in the rate of invasion. By 2 to 4 h postinfection, the ΔcetA mutant invades at rates near or at that of the wild type (Fig. 7B). The mechanism of C. jejuni invasion is still being dissected but appears to be mainly microtubule dependent (37), a feature that differentiates the C. jejuni invasion process from those of many invasive pathogens that use host cell actin for internalization. Proteins secreted from the flagellum are also required for C. jejuni invasion (35, 36, 48). Metabolic labeling experiments showed no significant changes in the ability of the ΔcetA mutant to secrete proteins associated with invasion (S. A. Pacheco, M. E. Konkel, K. T. Elliott, and V. J. DiRita, unpublished data). Additionally, C. jejuni invasion involves host cell protein kinase activity as well as a subset of small Rho GTPases (11, 30, 38), and we speculate that the ΔcetA mutant has a defect in the ability to initiate these signaling events. A recent study observed that C. jejuni can migrate beneath (“subvade”) epithelial cells in tissue culture prior to invasion (53). However, it was observed that increased subvasion efficiency correlated with decreased CheW expression and decreased motility in soft agar (53). Therefore, if CetA contributes to subvasion, it would appear that it must do so independently of CetB and the chemotactic machinery. Until more mechanistic details about both subvasion and the initiation of epithelial cell signaling events are known, the molecular mechanisms behind the invasion defect of the ΔcetA mutants will remain to be elucidated.
In summary, we identified a new family of proposed bipartite energy taxis receptors, similar to the CetA/CetB system in C. jejuni. Although we suggest that the HAMP/PAS pairs in this family transduce an energy taxis signal via a mechanism similar to that of Aer, there are clear departures from the Aer model. Differences between CetA and Aer in their predicted HAMP domains, as well as the presence of highly conserved residues within the HAMP and proximal domains of the CetA family members, suggest that the nature of the PAS-HAMP interaction within this family is mechanistically different from that in Aer. Finally, the involvement of CetA, but not CetB, in epithelial cell invasion supports our hypothesis that the members of HAMP/PAS pairs may act independently of each other to control phenotypes other than energy taxis.
Acknowledgments
We thank Jian-guo Zhu for help in the construction of the HAMP domain point mutations. We also thank David Friedman, Eric Krukonis, and Phil Hanna for thoughtful suggestions on this work.
This work was supported by grants from the USDA Food Safety Program (to V.J.D.) and by National Institutes of Health grant GM72285 (to I.B.Z.). K.T.E. was supported by a Howard Hughes Medical Institute Predoctoral Fellowship and a Willison Predoctoral Fellowship.
Footnotes
Published ahead of print on 24 October 2008.
REFERENCES
- 1.Alexander, R. P., and I. B. Zhulin. 2007. Evolutionary genomics reveals conserved structural determinants of signaling and adaptation in microbial chemoreceptors. Proc. Natl. Acad. Sci. USA 1042885-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ansell, R., K. Granath, S. Hohmann, J. M. Thevelein, and L. Adler. 1997. The two isoenzymes for yeast NAD+-dependent glycerol 3-phosphate dehydrogenase encoded by GPD1 and GPD2 have distinct roles in osmoadaptation and redox regulation. EMBO J. 162179-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appleman, J. A., L. L. Chen, and V. Stewart. 2003. Probing conservation of HAMP linker structure and signal transduction mechanism through analysis of hybrid sensor kinases. J. Bacteriol. 1854872-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appleman, J. A., and V. Stewart. 2003. Mutational analysis of a conserved signal-transducing element: the HAMP linker of the Escherichia coli nitrate sensor NarX. J. Bacteriol. 18589-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aravind, L., and C. P. Ponting. 1999. The cytoplasmic helical linker domain of receptor histidine kinase and methyl-accepting proteins is common to many prokaryotic signalling proteins. FEMS Microbiol. Lett. 176111-116. [DOI] [PubMed] [Google Scholar]
- 7.Baker, N. A., D. Sept, S. Joseph, M. J. Holst, and J. A. McCammon. 2001. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 9810037-10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benach, J., S. S. Swaminathan, R. Tamayo, S. K. Handelman, E. Folta-Stogniew, J. E. Ramos, F. Forouhar, H. Neely, J. Seetharaman, A. Camilli, and J. F. Hunt. 2007. The structural basis of cyclic diguanylate signal transduction by PilZ domains. EMBO J. 265153-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bibikov, S. I., L. A. Barnes, Y. Gitin, and J. S. Parkinson. 2000. Domain organization and flavin adenine dinucleotide-binding determinants in the aerotaxis signal transducer Aer of Escherichia coli. Proc. Natl. Acad. Sci. USA 975830-5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bibikov, S. I., R. Biran, K. E. Rudd, and J. S. Parkinson. 1997. A signal transducer for aerotaxis in Escherichia coli. J. Bacteriol. 1794075-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biswas, D., H. Niwa, and K. Itoh. 2004. Infection with Campylobacter jejuni induces tyrosine-phosphorylated proteins into INT-407 cells. Microbiol. Immunol. 48221-228. [DOI] [PubMed] [Google Scholar]
- 12.Brunger, A. T., P. D. Adams, G. M. Clore, W. L. DeLano, P. Gros, R. W. Grosse-Kunstleve, J. S. Jiang, J. Kuszewski, M. Nilges, N. S. Pannu, R. J. Read, L. M. Rice, T. Simonson, and G. L. Warren. 1998. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54905-921. [DOI] [PubMed] [Google Scholar]
- 13.Buron-Barral, M. C., K. K. Gosink, and J. S. Parkinson. 2006. Loss- and gain-of-function mutations in the F1-HAMP region of the Escherichia coli aerotaxis transducer Aer. J. Bacteriol. 1883477-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler, S. L., and J. J. Falke. 1998. Cysteine and disulfide scanning reveals two amphiphilic helices in the linker region of the aspartate chemoreceptor. Biochemistry 3710746-10756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chenna, R., H. Sugawara, T. Koike, R. Lopez, T. J. Gibson, D. G. Higgins, and J. D. Thompson. 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 313497-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crawford, J. A., E. S. Krukonis, and V. J. DiRita. 2003. Membrane localization of the ToxR winged-helix domain is required for TcpP-mediated virulence gene activation in Vibrio cholerae. Mol. Microbiol. 471459-1473. [DOI] [PubMed] [Google Scholar]
- 17.Cserzo, M., E. Wallin, I. Simon, G. von Heijne, and A. Elofsson. 1997. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 10673-676. [DOI] [PubMed] [Google Scholar]
- 18.Cuff, J. A., and G. J. Barton. 2000. Application of multiple sequence alignment profiles to improve protein secondary structure prediction. Proteins 40502-511. [DOI] [PubMed] [Google Scholar]
- 19.Elliott, K. T., and V. J. DiRita. 2008. Characterization of CetA and CetB, a bipartite energy taxis system in Campylobacter jejuni. Mol. Microbiol. 691091-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 761648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foodnet. 2007. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 states, 2006. MMWR Morb. Mortal. Wkly. Rep. 56336-339. [PubMed] [Google Scholar]
- 22.Fukuda, Y., Y. Okamura, H. Takeyama, and T. Matsunaga. 2006. Dynamic analysis of a genomic island in Magnetospirillum sp. strain AMB-1 reveals how magnetosome synthesis developed. FEBS Lett. 580801-812. [DOI] [PubMed] [Google Scholar]
- 23.Golden, N. J., and D. W. Acheson. 2002. Identification of motility and autoagglutination Campylobacter jejuni mutants by random transposon mutagenesis. Infect. Immun. 701761-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goon, S., C. P. Ewing, M. Lorenzo, D. Pattarini, G. Majam, and P. Guerry. 2006. A σ28-regulated nonflagella gene contributes to virulence of Campylobacter jejuni 81-176. Infect. Immun. 74769-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerry, P. 2007. Campylobacter flagella: not just for motility. Trends Microbiol. 15456-461. [DOI] [PubMed] [Google Scholar]
- 26.Guerry, P., R. Yao, R. A. Alm, D. H. Burr, and T. J. Trust. 1994. Systems of experimental genetics for Campylobacter species. Methods Enzymol. 235474-481. [DOI] [PubMed] [Google Scholar]
- 27.Hendrixson, D. R., B. J. Akerley, and V. J. DiRita. 2001. Transposon mutagenesis of Campylobacter jejuni identifies a bipartite energy taxis system required for motility. Mol. Microbiol. 40214-224. [DOI] [PubMed] [Google Scholar]
- 28.Hendrixson, D. R., and V. J. DiRita. 2004. Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol. Microbiol. 52471-484. [DOI] [PubMed] [Google Scholar]
- 29.Hu, L., and D. J. Kopecko. 1999. Campylobacter jejuni 81-176 associates with microtubules and dynein during invasion of human intestinal cells. Infect. Immun. 674171-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu, L., J. P. McDaniel, and D. J. Kopecko. 2006. Signal transduction events involved in human epithelial cell invasion by Campylobacter jejuni 81-176. Microb. Pathog. 4091-100. [DOI] [PubMed] [Google Scholar]
- 31.Hulko, M., F. Berndt, M. Gruber, J. U. Linder, V. Truffault, A. Schultz, J. Martin, J. E. Schultz, A. N. Lupas, and M. Coles. 2006. The HAMP domain structure implies helix rotation in transmembrane signaling. Cell 126929-940. [DOI] [PubMed] [Google Scholar]
- 32.Jeong, D. W., T. S. Kim, I. T. Cho, and I. Y. Kim. 2004. Modification of glycolysis affects cell sensitivity to apoptosis induced by oxidative stress and mediated by mitochondria. Biochem. Biophys. Res. Commun. 313984-991. [DOI] [PubMed] [Google Scholar]
- 33.Jones, T. A., J. Y. Zou, S. W. Cowan, and M. Kjeldgaard. 1991. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47110-119. [DOI] [PubMed] [Google Scholar]
- 34.Konkel, M. E., and L. A. Joens. 1989. Adhesion to and invasion of HEp-2 cells by Campylobacter spp. Infect. Immun. 572984-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konkel, M. E., B. J. Kim, V. Rivera-Amill, and S. G. Garvis. 1999. Bacterial secreted proteins are required for the internalization of Campylobacter jejuni into cultured mammalian cells. Mol. Microbiol. 32691-701. [DOI] [PubMed] [Google Scholar]
- 36.Konkel, M. E., J. D. Klena, V. Rivera-Amill, M. R. Monteville, D. Biswas, B. Raphael, and J. Mickelson. 2004. Secretion of virulence proteins from Campylobacter jejuni is dependent on a functional flagellar export apparatus. J. Bacteriol. 1863296-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kopecko, D. J., L. Hu, and K. J. Zaal. 2001. Campylobacter jejuni—microtubule-dependent invasion. Trends Microbiol. 9389-396. [DOI] [PubMed] [Google Scholar]
- 38.Krause-Gruszczynska, M., M. Rohde, R. Hartig, H. Genth, G. Schmidt, T. Keo, W. Konig, W. G. Miller, M. E. Konkel, and S. Backert. 2007. Role of the small Rho GTPases Rac1 and Cdc42 in host cell invasion of Campylobacter jejuni. Cell. Microbiol. 92431-2444. [DOI] [PubMed] [Google Scholar]
- 39.Letunic, I., R. R. Copley, S. Schmidt, F. D. Ciccarelli, T. Doerks, J. Schultz, C. P. Ponting, and P. Bork. 2004. SMART 4.0: towards genomic data integration. Nucleic Acids Res. 32D142-D144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma, Q., M. S. Johnson, and B. L. Taylor. 2005. Genetic analysis of the HAMP domain of the Aer aerotaxis sensor localizes flavin adenine dinucleotide-binding determinants to the AS-2 helix. J. Bacteriol. 187193-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Missiakas, D., M. P. Mayer, M. Lemaire, C. Georgopoulos, and S. Raina. 1997. Modulation of the Escherichia coli σE (RpoE) heat-shock transcription-factor activity by the RseA, RseB and RseC proteins. Mol. Microbiol. 24355-371. [DOI] [PubMed] [Google Scholar]
- 42.Monne, M., M. Hermansson, and G. von Heijne. 1999. A turn propensity scale for transmembrane helices. J. Mol. Biol. 288141-145. [DOI] [PubMed] [Google Scholar]
- 43.Monne, M., I. Nilsson, A. Elofsson, and G. von Heijne. 1999. Turns in transmembrane helices: determination of the minimal length of a “helical hairpin” and derivation of a fine-grained turn propensity scale. J. Mol. Biol. 293807-814. [DOI] [PubMed] [Google Scholar]
- 44.Oelschlaeger, T. A., P. Guerry, and D. J. Kopecko. 1993. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc. Natl. Acad. Sci. USA 906884-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rebbapragada, A., M. S. Johnson, G. P. Harding, A. J. Zuccarelli, H. M. Fletcher, I. B. Zhulin, and B. L. Taylor. 1997. The Aer protein and the serine chemoreceptor Tsr independently sense intracellular energy levels and transduce oxygen, redox, and energy signals for Escherichia coli behavior. Proc. Natl. Acad. Sci. USA 9410541-10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen, W., Y. Wei, M. Dauk, Y. Tan, D. C. Taylor, G. Selvaraj, and J. Zou. 2006. Involvement of a glycerol-3-phosphate dehydrogenase in modulating the NADH/NAD+ ratio provides evidence of a mitochondrial glycerol-3-phosphate shuttle in Arabidopsis. Plant Cell 18422-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh, M., B. Berger, P. S. Kim, J. M. Berger, and A. G. Cochran. 1998. Computational learning reveals coiled coil-like motifs in histidine kinase linker domains. Proc. Natl. Acad. Sci. USA 952738-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song, Y. C., S. Jin, H. Louie, D. Ng, R. Lau, Y. Zhang, R. Weerasekera, S. Al Rashid, L. A. Ward, S. D. Der, and V. L. Chan. 2004. FlaC, a protein of Campylobacter jejuni TGH9011 (ATCC 43431) secreted through the flagellar apparatus, binds epithelial cells and influences cell invasion. Mol. Microbiol. 53541-553. [DOI] [PubMed] [Google Scholar]
- 49.Szymanski, C. M., D. H. Burr, and P. Guerry. 2002. Campylobacter protein glycosylation affects host cell interactions. Infect. Immun. 702242-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor, B. L. 2007. Aer on the inside looking out: paradigm for a PAS-HAMP role in sensing oxygen, redox and energy. Mol. Microbiol. 651415-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor, B. L., and I. B. Zhulin. 1999. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 63479-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 254876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Alphen, L. B., N. M. Bleumink-Pluym, K. D. Rochat, B. W. van Balkom, M. M. Wosten, and J. P. van Putten. 2008. Active migration into the subcellular space precedes Campylobacter jejuni invasion of epithelial cells. Cell. Microbiol. 1053-66. [DOI] [PubMed] [Google Scholar]
- 54.Watts, K. J., M. S. Johnson, and B. L. Taylor. 2008. Structure-function relationships in the HAMP and proximal signaling domains of the aerotaxis receptor Aer. J. Bacteriol. 1902118-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weiner, M. P., G. L. Costa, W. Schoettlin, J. Cline, E. Mathur, and J. C. Bauer. 1994. Site-directed mutagenesis of double-stranded DNA by the polymerase chain reaction. Gene 151119-123. [DOI] [PubMed] [Google Scholar]
- 56.Yao, R., R. A. Alm, T. J. Trust, and P. Guerry. 1993. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene 130127-130. [DOI] [PubMed] [Google Scholar]
- 57.Young, K. T., L. M. Davis, and V. J. DiRita. 2007. Campylobacter jejuni: molecular biology and pathogenesis. Nat. Rev. Microbiol. 5665-679. [DOI] [PubMed] [Google Scholar]