Abstract
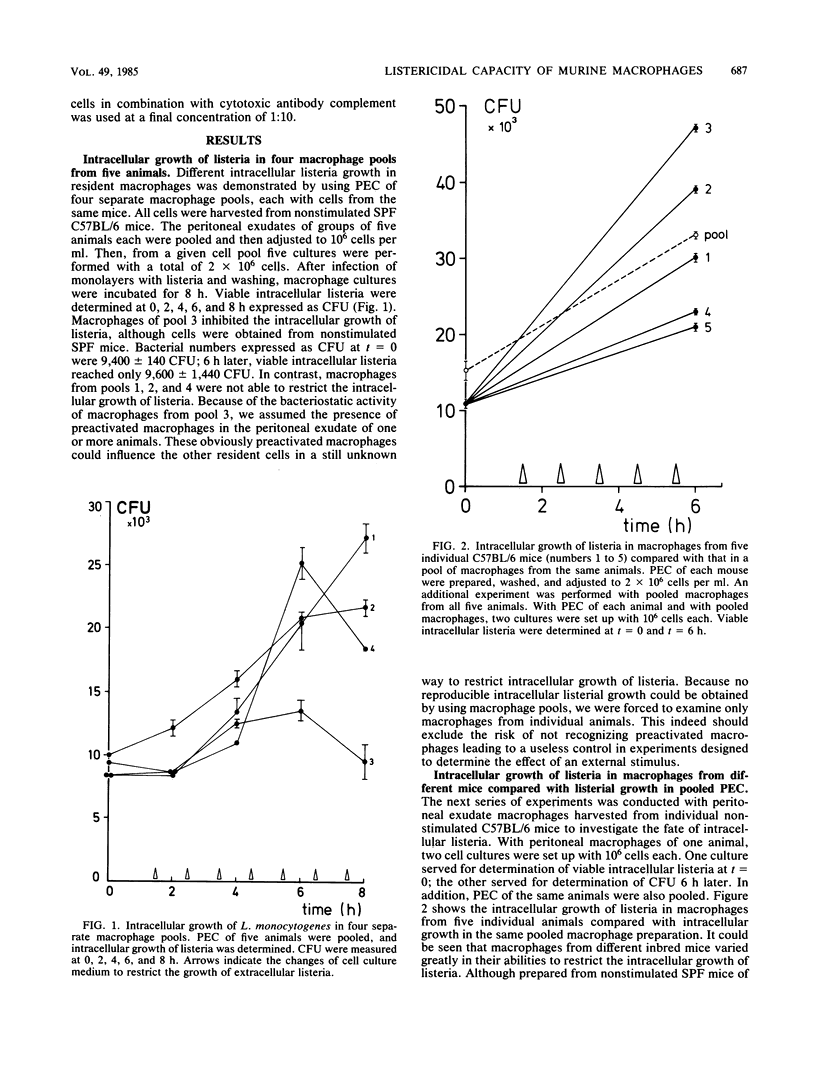
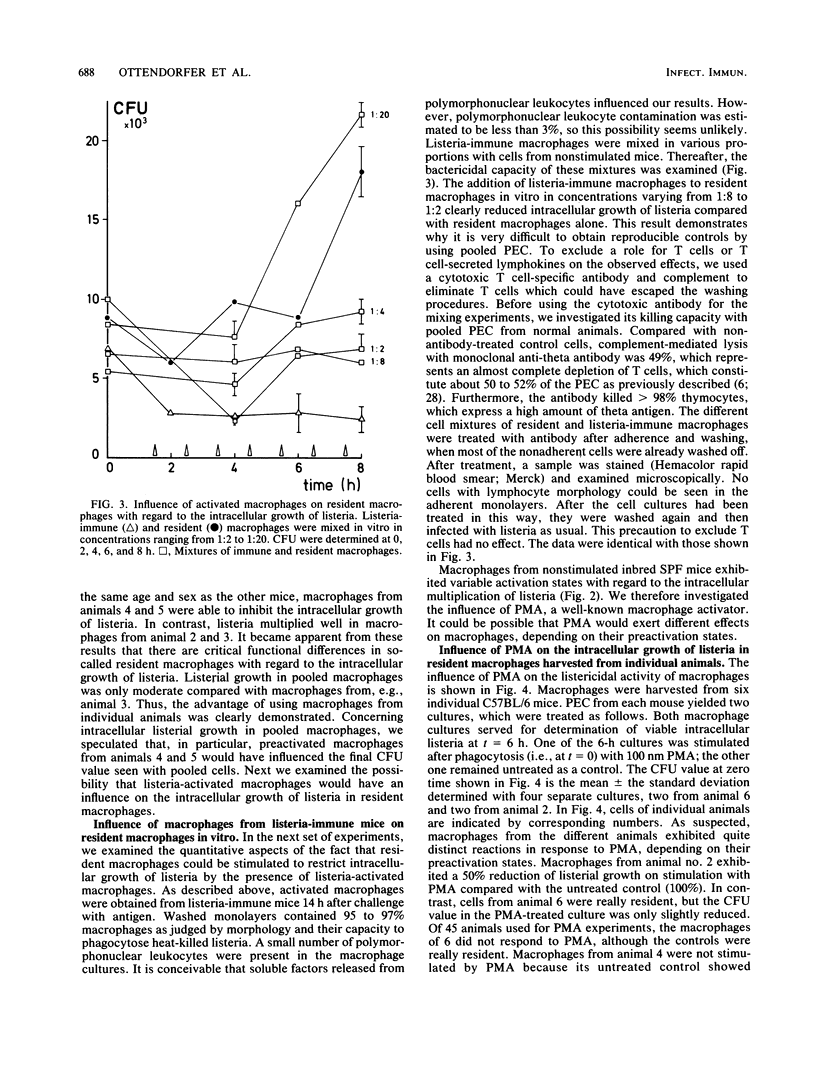
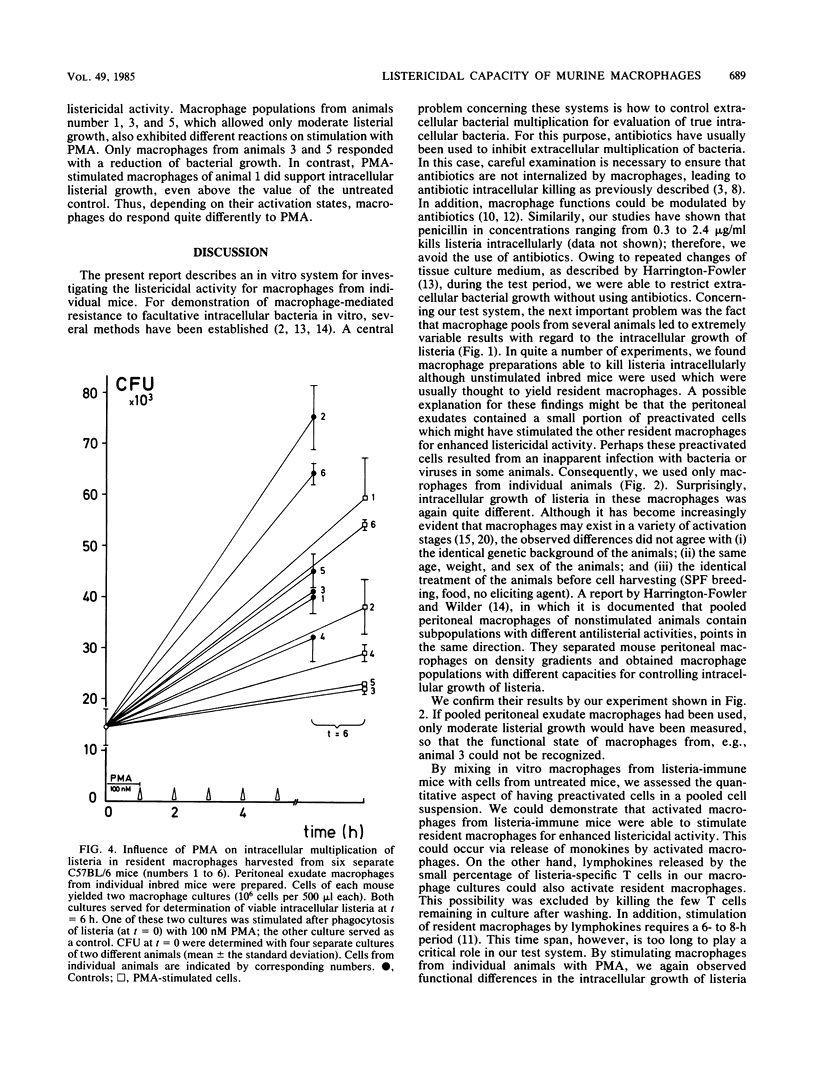
An in vitro system with macrophages from individual mice was established to study their listericidal capacity. Because no antibiotics were used, bacterial killing was really due to macrophages in short-term culture. To restrict the extracellular growth of bacteria, cell culture medium was changed at 1-h intervals. We demonstrated that intracellular growth of listeria in macrophage pools from untreated animals varies considerably. Obviously, preactivated macrophages are constantly present, so that the common procedure of using macrophage pools from several animals is no longer acceptable. In addition, we demonstrated that in vitro mixtures of listeria-immune macrophages of one animal with cells from untreated animals at different ratios exhibit enhanced bacterial killing above a mere additive effect. Consequently, by using macrophages from individual untreated mice, we found that cells of different animals exhibited various activation stages, although unstimulated, inbred specific-pathogen-free mice of the same age, weight, and sex were used. When equal numbers of macrophages from untreated separate animals were mixed in vitro, intracellular growth of listeria was only moderate; that is, the number of preactivated macrophages of the individual animals determined listerial growth in the pooled preparation. Furthermore, we showed that identical doses of phorbol myristate acetate exerted different effects on the listericidal activities of macrophages as a function of their preactivation states. These experiments clearly demonstrate the advantage of using macrophages from individual mice for in vitro studies of macrophage activation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beller D. I., Kiely J. M., Unanue E. R. Regulation of macrophage populations. I. Preferential induction of Ia-rich peritoneal exudates by immunologic stimuli. J Immunol. 1980 Mar;124(3):1426–1432. [PubMed] [Google Scholar]
- Bonventre P. F., Imhoff J. G. Uptake of h-dihydrostreptomycin by macrophages in culture. Infect Immun. 1970 Jul;2(1):89–95. doi: 10.1128/iai.2.1.89-95.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. J. Bactericidal Activity of Human Macrophages: Analysis of Factors Influencing the Killing of Listeria monocytogenes. Infect Immun. 1970 Aug;2(2):156–161. doi: 10.1128/iai.2.2.156-161.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole P. Activation of mouse peritoneal cells to kill Listeria monocytogenes by T-lymphocyte products. Infect Immun. 1975 Jul;12(1):36–41. doi: 10.1128/iai.12.1.36-41.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole P., Brostoff J. Intracellular killing of Listeria monocytogenes by activated macrophages (Mackaness system) is due to antibiotic. Nature. 1975 Aug 7;256(5517):515–517. doi: 10.1038/256515a0. [DOI] [PubMed] [Google Scholar]
- Fowles R. E., Fajardo I. M., Leibowitch J. L., David J. R. The enhancement of macrophage bacteriostasis by products of activated lymphocytes. J Exp Med. 1973 Oct 1;138(4):952–964. doi: 10.1084/jem.138.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmell C. G., Peterson P. K., Schmeling D., Kim Y., Mathews J., Wannamaker L., Quie P. G. Potentiation of opsonization and phagocytosis of Streptococcus pyogenes following growth in the presence of clindamycin. J Clin Invest. 1981 May;67(5):1249–1256. doi: 10.1172/JCI110152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey R. W., Horton P. G., Wilder M. S. Time course of antilisterial activity by immunologically activated murine peritoneal macrophages. Infect Immun. 1983 Feb;39(2):532–539. doi: 10.1128/iai.39.2.532-539.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L., Johnson J. D. Influence of bacterial-antibiotic interactions on subsequent antimicrobial activity of alveolar macrophages. J Infect Dis. 1984 Feb;149(2):271–276. doi: 10.1093/infdis/149.2.271. [DOI] [PubMed] [Google Scholar]
- Harrington-Fowler L., Henson P. M., Wilder M. S. Fate of Listeria monocytogenes in resident and activated macrophages. Infect Immun. 1981 Jul;33(1):11–16. doi: 10.1128/iai.33.1.11-16.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington-Fowler L., Wilder M. S. Fate of Listeria monocytogenes in murine peritoneal macrophage subpopulations. Infect Immun. 1982 Jan;35(1):124–132. doi: 10.1128/iai.35.1.124-132.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Chapman H. A., Jr, Weinberg J. B. Macrophage tumor killing: influence of the local environment. Science. 1977 Jul 15;197(4300):279–282. doi: 10.1126/science.327547. [DOI] [PubMed] [Google Scholar]
- Hoover D. L., Nacy C. A. Macrophage activation to kill Leishmania tropica: defective intracellular killing of amastigotes by macrophages elicited with sterile inflammatory agents. J Immunol. 1984 Mar;132(3):1487–1493. [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H., Simon M. M. T-cell subsets induced in Listeria monocytogenes-immune mice. Ly phenotypes of T cells interacting with macrophages in vitro. Scand J Immunol. 1982 Dec;16(6):539–542. doi: 10.1111/j.1365-3083.1982.tb00756.x. [DOI] [PubMed] [Google Scholar]
- Lee K. C., Wong M., McIntyre D. Characterization of macrophage subpopulations responsive to activation by endotoxin and lymphokines. J Immunol. 1981 Jun;126(6):2474–2479. [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- Masuda A., Bennett M. Concanavalin A-induced resistance to Listeria monocytogenes and activation of macrophages: defect in mice treated with 89Sr. Eur J Immunol. 1981 Jul;11(7):556–561. doi: 10.1002/eji.1830110707. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S., Occhionero M., Ruco L. P. Macrophage activation for tumor cytotoxicity: regulatory mechanisms for induction and control of cytotoxic activity. Fed Proc. 1982 Apr;41(6):2198–2205. [PubMed] [Google Scholar]
- Newell S. L., Atkinson J. P. Biosynthesis of C4 by mouse peritoneal macrophages. II. Comparison of C4 synthesis by resident and elicited cell populations. J Immunol. 1983 Feb;130(2):834–838. [PubMed] [Google Scholar]
- Pedersen J. K., Bennedsen J., Rhodes J. M., Larsen S. O. In vitro effect of mitogen-activated speen cells and their culture supernatants on mouse peritoneal macrophages as assayed by intracellular killing of Listeria monocytogenes and content of beta-galactosidase. J Reticuloendothel Soc. 1979 May;25(5):469–477. [PubMed] [Google Scholar]
- Serio C., Gandour D. M., Walker W. S. Macrophage functional heterogeneity: evidence for different antibody-dependent effector cell activities and expression of Fc-receptors among macrophage subpopulations. J Reticuloendothel Soc. 1979 Feb;25(2):197–206. [PubMed] [Google Scholar]
- Sethi K. K., Teschner M., Brandis H. In vitro antilisterial activity of soluble product(s) released from Listeria-immune murine peritoneal macrophages. Infect Immun. 1974 Oct;10(4):960–962. doi: 10.1128/iai.10.4.960-962.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Enhancement of macrophage bactericidal capacity by antigenically stimulated immune lymphocytes. Cell Immunol. 1972 Jun;4(2):163–174. doi: 10.1016/0008-8749(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Tsunawaki S., Nathan C. F. Enzymatic basis of macrophage activation. Kinetic analysis of superoxide production in lysates of resident and activated mouse peritoneal macrophages and granulocytes. J Biol Chem. 1984 Apr 10;259(7):4305–4312. [PubMed] [Google Scholar]
- Tucker S. B., Pierre R. V., Jordon R. E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J Immunol Methods. 1977;14(3-4):267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]
- Walker L., Lowrie D. B. Killing of Mycobacterium microti by immunologically activated macrophages. Nature. 1981 Sep 3;293(5827):69–71. doi: 10.1038/293069a0. [DOI] [PubMed] [Google Scholar]
- Yen S. E., Walker W. S. Selective induction of enhanced complement receptor 3 activity on murine macrophages. J Cell Physiol. 1983 Apr;115(1):61–66. doi: 10.1002/jcp.1041150110. [DOI] [PubMed] [Google Scholar]
- Zimmer B., Hartung H. P., Scharfenberger G., Bitter-Suermann D., Hadding U. Quantitative studies of the secretion of complement component C3 by resident, elicited and activated macrophages. Comparison with C2, C4 and lysosomal enzyme release. Eur J Immunol. 1982 May;12(5):426–430. doi: 10.1002/eji.1830120513. [DOI] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]


