Abstract
In this study, we report the DNA sequence and biological analysis of a mycobacterial mercury resistance operon encoding a novel Hg2+ transporter. MerH was found to transport mercuric ions in Escherichia coli via a pair of essential cysteine residues but only when coexpressed with the mercuric reductase.
Narrow-spectrum mercury resistance (i.e., resistance to mercury salts) requires transport of mercuric ions across the membrane by specific transporters, typically MerT and MerP (10), cytoplasmic reduction to elemental mercury Hg(0) by mercuric reductase (MR) and passive diffusion of the volatile metal out of the cell (2). In broad-spectrum mercury resistance, the organomercurial lyase MerB catalyzes the breakdown of carbon-mercury bonds and, with the transport and reductase proteins, confers resistance to a wide range of organomercurials (12). Both mechanisms are subject to tight regulation by a mercury-responsive transcriptional regulator, MerR (5).
Although mercury resistance has been extensively studied and characterized in different bacterial genera, there are few reports of mycobacterial mercury resistance genes, and no mer operons have been described in mycobacteria. Most studies consist of the isolation and identification of mercury-resistant mycobacterial strains in which the mercuric reductase gene has been detected by PCR and MR activity has been shown by volatilization of radioactive mercury (11, 21). Some strains were also positive for amplification of the merB gene. Mercury resistance was also demonstrated in mycobacteria expressing mer genes from Tn501, and mycobacterial expression vectors have been developed with mercury resistance as the only selectable marker (3).
Mycobacterium marinum, which causes a tuberculosis-like disease in fish and skin infections in humans, is closely related to Mycobacterium tuberculosis and is a good model for mycobacterial infection studies (6). The genome of a clinical isolate of M. marinum (strain ATCC BAA-535) (14) carrying a plasmid encoding potential mercury resistance genes was recently published (22). Here, we report the DNA sequence and analysis of the first Mycobacterium mercury resistance operon. We characterized a previously unknown membrane protein (MerH) from M. marinum, which we found transports mercuric ions across the inner membrane of Escherichia coli via a pair of cysteine residues located in the first transmembrane (TM) region but only when coexpressed with MR. In Mycobacterium smegmatis, MerH and MR confer resistance to mercuric chloride, which may be a useful, naturally occurring, nonantibiotic selection marker in mycobacteria.
A 3.6-kb mer operon confers mercury resistance to M. marinum strain ATCC BAA-535.
M. marinum strain ATCC BAA-535 (GenBank accession number NC_010612) contains a 23-kb plasmid (pMM23; GenBank accession number NC_010604) which harbors a 3.6-kb mer operon (Fig. 1) (22) containing five genes likely to be involved in mercury resistance (Table 1). The operon is predicted to contain genes encoding functions associated with regulation, transport, and reduction of mercuric ions and organomercurial breakdown, but the gene arrangement is unusual, with two of the genes being divergently transcribed from the remaining three (Fig. 1). These mer genes are surrounded by three other genes that may not participate directly in mercury resistance—a predicted glutathione reductase and two recombinases that may promote mobility of the genetic element.
FIG. 1.
M. marinum mer operon and surrounding genes. Gray-filled arrows represent surrounding genes that are unlikely to participate directly in Hg resistance.
TABLE 1.
Annotation of mercury resistance genes located on pMM23 from M. marinuma
| ORF no. | Gene name | BLAST resultb | Reference/observation |
|---|---|---|---|
| pMM23.08 | Belongs to the superfamily of serine DNA recombinases; 99% identical to DNA resolvase of Mycobacterium abscessus | May be involved in mobility of the genetic element | |
| pMM23.09 | Belongs to the family of FAD-dependent pyridine nucleotide-disulphide oxidoreductases; 43% identical to glutathione reductase of Gramella forsetii | Unlikely to be involved in mercury resistance | |
| pMM23.10 | merB | 64% identical to the organomercurial lyase from Streptomyces lividans | 18 |
| pMM23.11 | merH | No statistically significant match | Hypothetical membrane protein |
| pMM23.12 | merA | 67% identical to the mercuric ion reductase from Streptomyces sp. strain CHR28 | 15 |
| pMM23.13 | merR | 62% identical to the MerR protein from Streptomyces lividans pJOE796 | 18 |
| pMM23.14 | merT | 54% identical to the MerT protein encoded by the mer operon of Streptomyces lividans | 18 |
| pMM23.15 | Belongs to the superfamily of serine DNA recombinases; 99% identical to DNA invertase/resolvase of M. abscessus | May be involved in mobility of the genetic element |
The genes were compared against the non-redundant databases using FASTA and BLAST, and protein motifs were identified using Pfam, Prosite TMHMM and SignalP.
FAD, flavin adeninie dinucleotide.
M. marinum strain ATCC BAA-535 was found to be three to four times more resistant to mercuric chloride than was M. smegmatis mc2155 in two different growth media (see Table 5), and similar levels of mercury resistance were observed for M. marinum and E. coli expressing mer genes from Tn501 on LB agar, which suggests that mercury resistance genes located on the pMM23 plasmid from M. marinum are functional.
TABLE 5.
Comparison of mercury resistance levels of E. coli (expressing mer genes from Tn501), M. marinum, and M. smegmatis mc2155 (parental strain or strain expressing mer genes from the M. marinum mer operon)
| Strain | HgCl2 MIC (μM)a in:
|
||
|---|---|---|---|
| L agar containing 25 μg/ml kanamycin | L agar | 7H11 agar | |
| E. coli | 15 ± 4 | ||
| E. coli (Tn501) | 118 ± 8 | ||
| M. marinum | 110 ± 8 | 177 ± 8 | |
| M. smegmatis mc2155 | 30 ± 4 | 59 ± 4 | |
| M. smegmatis mc2155 (pVV16) | 4 ± 2 | 59 ± 8 | |
| M. smegmatis mc2155 (pVV16-merA) | 15 ± 4 | 103 ± 8 | |
| M. smegmatis mc2155 (pVV16-merAH) | 37 ± 4 | 110 ± 8 | |
Values are mean MIC ± the standard deviation.
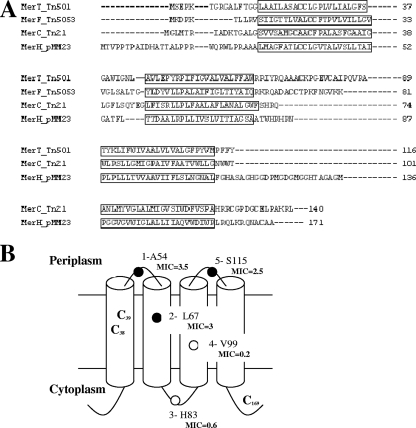
One gene from the mer operon in pMM23.11 has no significant similarity to any protein in the nonredundant database but was predicted to be a membrane protein with four putative transmembrane helices. We hypothesized that this gene, designated merH, was involved in mercuric ion transport (Fig. 2B).
FIG. 2.
Sequence and topology of MerH. (A) Predicted protein sequence of MerH aligned with MerT, MerC, and MerF based on TM domain locations. Note that MerH has no significant similarity to the others at the primary sequence level. TM regions are shown in boxes and conserved cysteine residues in boldface type. (B) Topology of MerH determined by fusion to β-lactamase. Filled and open circles show fusions where the β-lactamase was found active and inactive, respectively. Carbenicillin MICs of strains expressing MerH-β-lactamase hybrid proteins are indicted in boldface type in mg ml−1.
Characterization of the merH gene product in E. coli.
When expressed in E. coli in place of MerT within a minimal mercury resistance operon, MerH could be recovered in the membrane protein fraction, indicating that it is inserted in the membrane (data not shown). Five fragments of merH were PCR amplified using primers listed in Table 2 and cloned using NcoI/BstBII in pYZ-TBL (4), making β-lactamase fusions at five different locations in MerH. (Fig. 2B). β-Lactamase activity of the resulting hybrid proteins was measured in E. coli C43 cells (cultured, washed, and diluted in 0.85% saline solution before plating onto L agar containing increasing concentrations of carbenicillin). β-Lactamase confers high-level resistance to ampicillin or carbenicillin only when located in the periplasmic compartment (9). As indicated by the carbenicillin MICs in Fig. 2B, β-lactamase protein fusions at residues alanine 54, leucine 67, and serine 115 of MerH resulted in a carbenicillin-resistant phenotype for E. coli cells. This suggests that loops between TM regions 1 to 2 and 3 to 4 of MerH are located in the periplasm. In contrast, fusions at residues histidine 83 and valine 99 led to inactive β-lactamase and a carbenicillin-sensitive phenotype in E. coli. These data are compatible with the SOSUI model for MerH topology, which predicts both N-terminal and C-terminal regions to be located in the cytoplasm.
TABLE 2.
List of primers used in this study
| Primer name | Primer sequence (5′-3′)a | Purpose |
|---|---|---|
| MerH_F | 5′ CCA TTC GAAAGG AGA ACC CTG ATG 3′ | merH amplification |
| MerH_R | 5′ ACT AGA GAT CTG ATA TCA AGC GGC GC 3′ | |
| MerH_C38S_F | 5′ GCC ACT TTG AGC TGC CTC GGT GTG 3′ | merH mutagenesis |
| MerH_C38S_R | 5′ CAC ACC GAG GCA GCT CAA AGT GGC 3′ | |
| MerH_C39S_F | 5′ GCC ACT TTG TGC AGC CTC GGT GTG 3′ | |
| MerH_C39S_R | 5′ CAC ACC GAG GCT GCA CAA AGT GGC 3′ | |
| MerH_C3839S_F | 5′ GCC ACT TTG AGC AGC CTC GGT GTG 3′ | |
| MerH_C3839S_R | 5′ CAC ACC GAG GCT GCT CAA AGT GGC 3′ | |
| MerH_C169S_R | 5′ ACT AGA GAT CTG ATA TCA AGC GGC GCT 3′ | |
| MerH_NcoI_F | 5′ AAG GAG AAC CCC CAT GGC TGT G 3′ | MerH topology |
| HBL1 | 5′ AGT CAG GAG GTT ACC GCC GAT GG 3′ | |
| HBL2 | 5′ GAT CAC CAG GTT ACC GAT CAG CAG C 3′ | |
| HBL3 | 5′ GGG TTG CGG TGA CCA TGC CAG 3′ | |
| HBL4 | 5′ GGA GAA GAG GTT ACC CAC CGC GG 3′ | |
| HBL5 | 5′ GGC GCT GGG GTG ACC GAA CAG 3′ | |
| MerA_NdeI_F | 5′ GAG ACG CCA TAT GGG CTA TGA CTT GGC 3′ | Cloning in pVV16 |
| MerA_HindIII_R | 5′ GGT GGG AAG CTTTCA GCT GGC GCA G 3′ | |
| MerH_NdeI_F | 5′ GAG AAC CCA TAT GAC TGT GCC CCC CAC 3′ | |
| MerH_HindIII_R | 5′ AGG GAT AAG CTTTCA AGC GGC GCA GG 3′ |
Boldface type indicates particular sequence features (start codons, stop codons, and ribosome-binding sites). Italicized residues represent introduced restriction sites. Underlined sequences show differences from the original sequence for point mutations.
MerH was expressed in E. coli by replacing merT with merH within a cloned minimal Tn501 mer operon containing the merR, merT, merP, and merA genes in plasmid pBRmerT (Table 3). When merT was replaced by merH in plasmid pBRmerH, the mercuric chloride MIC for E. coli cells decreased from 111 to 81 μM but remained much higher than that for control cells expressing no mercuric ion transporters (MIC of 15 μM). This result suggests that MerH acts as a mercuric ion transporter in E. coli.
TABLE 3.
List of plasmids and strains used in this study
| Strain or plasmid | Use | Genotype or description | Reference |
|---|---|---|---|
| Strains | |||
| E. coli TG2 | supE hsdΔ5 thi Δ(lac-proAB)F′ [traD36 proAB+lacIqlacZΔM15] Δ(srl-recA)306::Tn10 Tetr | 16 | |
| E. coli C43 | Derived from BL21(DE3), tolerant for lethal protein expression | 1 | |
| M. smegmatis MC2155 | Mutant of M. smegmatis mc26 with increased efficiency for electroporation of plasmid DNA; Ept− Kans | 19 | |
| M. marinum ATCC BAA-535 | Hgr; human patient isolate; genome sequence available at http://www.sanger.ac.uk/Projects/M_marinum/ | 14 | |
| Plasmids | |||
| pBRmer BS2 | Mercury resistance and transport studies in E. coli | Apr; Hgr; the EcoRI-NruI fragment of pUB3466, containing Tn501, cloned into pBR322 (ΔEcoRI-NruI) | 24 |
| pBRmerT | Apr; NotI-NruI deletion in pBRmer BS2 to remove merD, merE, and urf2 from the end of Tn501 | This work | |
| pBRmerH | merH from M. marinum cloned in pBRmerT by using BstBI-BglII ligation | This work | |
| pBRmerΔT | Apr; NotI-NruI deletion in pBRmerΔT BS2 to remove merD, merE and urf2 from the end of Tn501 | This work | |
| pBRmerC | Apr; NotI-NruI deletion in pBRmerC BS2 to remove merD, merE, and urf2 from the end of Tn501 | This work | |
| pBRmerF | Apr; NotI-NruI deletion in pBRmerF BS2 to remove merD, merE, and urf2 from the end of Tn501 | This work | |
| pBRmerH CxxS | Apr; cysteine (Cxx changed to serine) mutants of merH cloned into pBRmerT by using BstBI-BglII ligation | This work | |
| pHBL 1 to 5 | MerH topology study | merH amplified with MerH_Nco I_F and HBL1 to -5 and cloned into the pYZ-TBL vector by using NcoI/BstEII restriction sites; this changed the second amino acid of MerH from T to A. | This work |
| 8d06 | Mercury resistance in mycobacteria | Cmr; pBACe3.6 derivative with fragment of pMM23 plasmid DNA containing the putative mer operon | 7 |
| pVV16 | Kanr; HygBr; expression vector used for constitutive protein expression from a PHsp60 promoter | 23 | |
| pVV16-MerA | merA gene from 8d06 cloned with NdeI/HindIII in pVV16 to allow expression of MerA from PHsp60 | This work | |
| pVV16-MerH | merH gene from 8d06 cloned with NdeI/HindIII in pVV16 to allow expression of MerA from PHsp60 | This work | |
| pVV16-MerAH | merH and merA gene from 8d06 cloned with NdeI/HindIII in pVV16 to allow expression of MerA from PHsp60 | This work |
In vivo 203Hg volatilization assays were performed as described elsewhere (17) in E. coli TG2 strains expressing either MerT, MerC, MerF, or MerH as the sole mercuric ion transporter within a minimal Tn501 mer operon (Fig. 3A). Mercury volatilization observed in the absence of a mercuric ion transporter was probably the result of cell permeabilization and was subtracted from all samples. MerT conferred the highest rate of mercury volatilization in E. coli, while MerH conferred intermediate rates, between the volatilization rates achieved with and without MerT, that were equivalent to the mercury volatilization rates achieved with MerF and MerC, mercuric ion transport proteins from Tn5053 and Tn21, respectively (8, 24). These data show that MerH is able to import mercuric ions into the E. coli cytoplasm for reduction by MR.
FIG. 3.
Functional characterization of MerH. Mercury resistance in E. coli was determined by volatilization of 203Hg (A and B) and by HgCl2 hypersensitivity tests (C). MerX represents the transporter expressed in a minimal mer operon (merRXPA) in E. coli TG2 cells. Wild-type proteins are shown in black (A) and cysteine MerH mutants in white (B). Background volatilization in the absence of mer transporters has been substracted.
The following five combinations of cysteine-to-serine mutations were created in MerH using a two-step PCR method: C38S, C39S, C169S, C38S/C39S, and C38S/C39S/C169S (20) (Table 2). These were confirmed by sequencing both DNA strands. Mutants were cloned in place of merT in the pBRmerT plasmid and expressed from the PmerT promoter in E. coli TG2 upon induction with 0.4 μM HgCl2. Expression and membrane localization of the cysteine mutants were confirmed by 35S labeling of plasmid-encoded proteins (data not shown).
The effects of the cysteine mutations on mercuric ion resistance and transport were tested by HgCl2 MICs (Table 4) and 203Hg2+ volatilization assays (Fig. 3B) using E. coli TG2 cells expressing the different MerH cysteine mutants. The cysteine residues in MerH did not play an equal role in mercury resistance. Cysteine 169 did not participate in mercuric ion transport, since its mutation to serine did not decrease mercury resistance or mercuric ion transport activity compared to wild-type MerH. Mutation of cysteine 38 decreased mercury resistance by approximately 25%, whereas mutation of cysteine 39 completely abolished resistance. Both were predicted to be in the first TM region of MerH. Cells expressing the MerH double (C38S/C39S) or triple (C38S/C39S/C169S) mutants were as sensitive to mercuric chloride as were cells expressing no mercuric ion transport proteins (Table 4).
TABLE 4.
Effects of cysteine-to-serine mutations in MerH upon mercury resistance conferred on E. coli TG2 expressing a minimal mer operon (merRHPA)
| MerH protein or mutant or plasmida | HgCl2 MIC (μM)b |
|---|---|
| Wild type | 81 ± 4 |
| C38S mutant | 71 ± 4 |
| C39S mutant | 22 ± 4 |
| C38S/C39S mutant | 22 ± 4 |
| C169S mutant | 81 ± 4 |
| C38S/C39S/C169S mutant | 22 ± 4 |
| — | 18 ± 4 |
| Empty vector pBR322 | 18 ± 4 |
—, no transporter expressed.
Values are the mean MIC ± the standard deviation.
Similarly, mutation of cysteine 38 significantly decreased Hg volatilization, and mutation of cysteine 39 completely abolished volatilization (Fig. 3B). As expected, the double and triple cysteine mutants of MerH were not able to transport mercuric ions.
MerH cannot transport Hg2+ across the cytoplasmic membrane of E. coli without MR.
Cells expressing mercuric ion transporters in the absence of MR were expected to show a hypersensitive phenotype to mercuric chloride, since they specifically import mercuric ions (21). Plasmids expressing different mercuric ion transporters (MerT, MerC, MerF, and MerH) without expression of MR were constructed and transformed into E. coli TG2 cells (Table 3) to test the HgCl2 sensitivity of resulting strains (Fig. 3C). Expression of MerT or MerC in the absence of MR resulted in an HgCl2-hypersensitive phenotype in TG2. Their sensitivity to mercuric chloride increased by approximately fourfold compared to that of cells expressing no mercury resistance proteins or no transporters. In the case of MerF, a 1.5-fold increase in HgCl2 sensitivity was observed. However, for MerH, this hypersensitive phenotype was not observed. Cells expressing MerH unexpectedly showed a slight increase in mercury resistance (1.2-fold increase) compared to cells expressing no mer genes. These data suggest that MerH transports mercuric ions across the inner membrane of E. coli cells only when MR is present in the cytoplasm.
Apart from the predicted length and number of TM regions, the major difference between MerH and other mercuric ion transporters is that MerH lacks a second pair of cysteine residues predicted to be located in the cytoplasm (Fig. 2A). In MerT, MerC, and MerF, this pair of cysteine residues has been shown to be involved in mercury transport, since their mutation resulted in a reduced rate of mercury volatilization (13, 24). These data suggest that MerH is not able to import mercuric ions to the E. coli cytoplasm because it lacks the cysteine pair in this compartment. In order to achieve the transport of Hg2+ into the cytoplasm, MerH may require MR cysteine residues to act as acceptors for mercuric ions, or MR may be required to cause a productive conformational change in MerH. It is also possible that these cytoplasmic cysteines are required for Hg2+ transport in E. coli and not in the natural host, M. marinum.
merA and merH from M. marinum confer mercury resistance on M. smegmatis.
The merH and merA genes were separately and jointly cloned in pVV16 (an E. coli-Mycobacterium shuttle vector) and constitutively expressed in M. smegmatis mc2155 (Table 3). Transformants were selected on 25 μg ml−1 kanamycin and 50 μg ml−1 hygromycin. After 4 days of incubation at 37°C, single colonies of mc2155 expressing different mer genes were observed under the microscope. Expression of merA slightly affected the mc2155 colony morphology but did not affect the growth rate. Expression of merA with merH resulted in a significant change in the morphology of the colonies (which were much smoother) but did not affect the growth rate. However, expression of merH from the constitutive hsp60 promoter of pVV16 was toxic to the cells. Ring-shaped colonies appeared on L agar plates, and cells could not be grown in L broth. The effects of merA and merAH expression upon mercury resistance in M. smegmatis were determined by MIC assays. Levels of mercury resistance of mc2155 strains expressing merA or merA and merH from the hsp60 promoter in the pVV16 vector were determined on L agar with antibiotic selection or 7H11 agar without antibiotic selection pressure after 3 to 4 days of incubation at 37°C. (Table 5). Expression of merA in M. smegmatis resulted in a fourfold increase in mercury resistance, while coexpression of merA and merH in resulted in a 10-fold increase. These data show that the merA gene product is sufficient to confer some mercury resistance on M. smegmatis but that expression of the putative Hg2+ transport gene (merH) is required for maximal mercury resistance. On 7H11 agar plates, M. smegmatis also showed maximal mercury resistance upon coexpression of merA and merH but with much greater MICs, suggesting that the kanamycin selection of pVV16 strongly decreased mercury resistance. However, expression of merAH in mc2155 conferred sufficient mercury resistance (>30 μM) to allow selection of recombinant M. smegmatis cells on HgCl2, indicating that these two genes can be used as a non-antibiotic-selectable marker in mycobacteria.
Acknowledgments
We are grateful to Delon Barfuss of Georgia State University, who provided us with 1 mCi of 203HgCl2, and to Jon Hobman for discussion and advice.
This work was supported by a studentship awarded to M.S. from the Darwin Trust of Edinburgh, United Kingdom. G.S.B. acknowledges support from James Bardrick in the form of a Personal Chair, from the Lister Institute as a former Jenner Research Fellow, from the Medical Research Council (United Kingdom), and from the Wellcome Trust.
Footnotes
Published ahead of print on 17 October 2008.
REFERENCES
- 1.Arechaga, I., B. Miroux, S. Karrasch, R. Huijbregts, B. de Kruijff, M. J. Runswick, and J. E. Walker. 2000. Characterisation of new intracellular membranes in Escherichia coli accompanying large scale overproduction of the b subunit of F(1)F(o) ATP synthase. FEBS Lett. 482215-219. [DOI] [PubMed] [Google Scholar]
- 2.Barkay, T., S. M. Miller, and A. O. Summers. 2003. Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiol. Rev. 27355-384. [DOI] [PubMed] [Google Scholar]
- 3.Baulard, A., V. Escuyer, N. Haddad, L. Kremer, C. Locht, and P. Berche. 1995. Mercury resistance as a selective marker for recombinant mycobacteria. Microbiology 141(Pt. 4)1045-1050. [DOI] [PubMed] [Google Scholar]
- 4.Broome-Smith, J. K., and B. G. Spratt. 1986. A vector for the construction of translational fusions to TEM beta-lactamase and the analysis of protein export signals and membrane protein topology. Gene 49341-349. [DOI] [PubMed] [Google Scholar]
- 5.Brown, N. L., J. V. Stoyanov, S. P. Kidd, and J. L. Hobman. 2003. The MerR family of transcriptional regulators. FEMS Microbiol. Rev. 27145-163. [DOI] [PubMed] [Google Scholar]
- 6.Decostere, A., K. Hermans, and F. Haesebrouck. 2004. Piscine mycobacteriosis: a literature review covering the agent and the disease it causes in fish and humans. Vet. Microbiol. 99159-166. [DOI] [PubMed] [Google Scholar]
- 7.Frengen, E., D. Weichenhan, B. Zhao, K. Osoegawa, M. van Geel, and P. J. de Jong. 1999. A modular, positive selection bacterial artificial chromosome vector with multiple cloning sites. Genomics 58250-253. [DOI] [PubMed] [Google Scholar]
- 8.Hamlett, N. V., E. C. Landale, B. H. Davis, and A. O. Summers. 1992. Roles of the Tn21 merT, merP, and merC gene products in mercury resistance and mercury binding. J. Bacteriol. 1746377-6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herzberg, O., and J. Moult. 1987. Bacterial resistance to beta-lactam antibiotics: crystal structure of beta-lactamase from Staphylococcus aureus PC1 at 2.5 A resolution. Science 236694-701. [DOI] [PubMed] [Google Scholar]
- 10.Lund, P. A., and N. L. Brown. 1987. Role of the merT and merP gene products of transposon Tn501 in the induction and expression of resistance to mercuric ions. Gene 52207-214. [DOI] [PubMed] [Google Scholar]
- 11.Meissner, P. S., and J. O. Falkinham III. 1984. Plasmid-encoded mercuric reductase in Mycobacterium scrofulaceum. J. Bacteriol. 157669-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller, S. M. 1999. Bacterial detoxification of Hg(II) and organomercurials. Essays Biochem. 3417-30. [DOI] [PubMed] [Google Scholar]
- 13.Morby, A. P., J. L. Hobman, and N. L. Brown. 1995. The role of cysteine residues in the transport of mercuric ions by the Tn501 MerT and MerP mercury-resistance proteins. Mol. Microbiol. 1725-35. [DOI] [PubMed] [Google Scholar]
- 14.Ramakrishnan, L., and S. Falkow. 1994. Mycobacterium marinum persists in cultured mammalian cells in a temperature-restricted fashion. Infect. Immun. 623222-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravel, J., J. DiRuggiero, F. T. Robb, and R. T. Hill. 2000. Cloning and sequence analysis of the mercury resistance operon of Streptomyces sp. strain CHR28 reveals a novel putative second regulatory gene. J. Bacteriol. 1822345-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 17.Schue, M., K. J. Glendinning, J. L. Hobman, and N. L. Brown. 2008. Evidence for direct interactions between the mercuric ion transporter (MerT) and mercuric reductase (MerA) from the Tn501 mer operon. Biometals 21107-116. [DOI] [PubMed] [Google Scholar]
- 18.Sedlmeier, R., and J. Altenbuchner. 1992. Cloning and DNA sequence analysis of the mercury resistance genes of Streptomyces lividans. Mol. Gen. Genet. 23676-85. [DOI] [PubMed] [Google Scholar]
- 19.Snapper, S. B., R. E. Melton, S. Mustafa, T. Kieser, and W. R. Jacobs, Jr. 1990. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 41911-1919. [DOI] [PubMed] [Google Scholar]
- 20.Stanssens, P., C. Opsomer, Y. M. McKeown, W. Kramer, M. Zabeau, and H. G. Fritz. 1989. Efficient oligonucleotide-directed construction of mutations in expression vectors by the gapped duplex DNA method using alternating selectable markers. Nucleic Acids Res. 174441-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steingrube, V. A., R. J. Wallace, Jr., L. C. Steele, and Y. J. Pang. 1991. Mercuric reductase activity and evidence of broad-spectrum mercury resistance among clinical isolates of rapidly growing mycobacteria. Antimicrob. Agents Chemother. 35819-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stinear, T. P., T. Seemann, P. F. Harrison, G. A. Jenkin, J. K. Davies, P. D. Johnson, Z. Abdellah, C. Arrowsmith, T. Chillingworth, C. Churcher, K. Clarke, A. Cronin, P. Davis, I. Goodhead, N. Holroyd, K. Jagels, A. Lord, S. Moule, K. Mungall, H. Norbertczak, M. A. Quail, E. Rabbinowitsch, D. Walker, B. White, S. Whitehead, P. L. Small, R. Brosch, L. Ramakrishnan, M. A. Fischbach, J. Parkhill, and S. T. Cole. 2008. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res. 18729-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stover, C. K., V. F. de la Cruz, T. R. Fuerst, J. E. Burlein, L. A. Benson, L. T. Bennett, G. P. Bansal, J. F. Young, M. H. Lee, G. F. Hatfull, et al. 1991. New use of BCG for recombinant vaccines. Nature 351456-460. [DOI] [PubMed] [Google Scholar]
- 24.Wilson, J. R., C. Leang, A. P. Morby, J. L. Hobman, and N. L. Brown. 2000. MerF is a mercury transport protein: different structure but a common mechanism for mercuric ion transporters? FEBS Lett. 47278-82. [DOI] [PubMed] [Google Scholar]