Abstract
Tendons are important for optimal muscle force transfer to bone and play a key role in functional ability. Changes in tendon properties with aging could contribute to declines in physical function commonly associated with aging. We investigated the in vivo mechanical properties of the patellar tendon in 37 men and women [11 young (27 ± 1 yr) and 26 old (65 ± 1 yr)] using ultrasonography and magnetic resonance imaging (MRI). Patella displacement relative to the tibia was monitored with ultrasonography during ramped isometric contractions of the knee extensors, and MRI was used to determine tendon cross-sectional area (CSA) and signal intensity. At peak force, patellar tendon deformation, stress, and strain were 13 (P = 0.05), 19, and 12% less in old compared with young (P < 0.05). Additionally, deformation, stiffness, stress, CSA, and length were 18, 35, 41, 28, and 11% greater (P < 0.05), respectively, in men compared with women. After normalization of mechanical properties to a common force, no age differences were apparent; however, stress and strain were 26 and 22% higher, respectively, in women compared with men (P < 0.05). CSA and signal intensity decreased 12 and 24%, respectively, with aging (P < 0.05) in the midregion of the tendon. These data suggest that differences in patellar tendon in vivo mechanical properties with aging are more related to force output rather than an age effect. In contrast, the decrease in signal intensity indirectly suggests that the internal milieu of the tendon is altered with aging; however, the physiological and functional consequence of this finding requires further study.
Keywords: ultrasonography, tendon injury, stress-strain relationship
in contrast to skeletal muscle, studies in humans evaluating the influence of aging on in vivo tendon properties have been relatively limited (27, 32, 40). This lack of data is surprising given that tendons, once thought to be largely inert structures, have been shown to have specific mechanical properties (14) that can directly influence skeletal muscle performance and functional ability (3, 6, 40), and thus, any changes in tendon with aging may contribute to age-related declines in physical function (17). Additionally, observations of Achilles tendon injury rates (19, 29, 38) suggest that the incidence of tendon injuries may be increased in elderly individuals. Therefore, defining the influence of aging on tendon is important and will advance our understanding of the role of tendon in aging-associated tendon injury and declining physical function (17).
Over the past several years, various groups (5, 11, 14, 30, 45) have pioneered the use of ultrasonography for the evaluation of tendinous tissues in vivo and have provided a means to noninvasively determine the elastic properties of tendon in humans. Using this methodology, maximal human gastrocnemius tendon (40) and vastus lateralis tendon-aponeurosis (27) stiffness have been shown to decrease with aging, suggesting that tendon may become more compliant, which could influence muscle performance (6). These studies, however, measure elongation at the level of the gastrocnemius or vastus lateralis muscle, and the total elongation measured is likely influenced by other muscles involved in plantarflexion or knee extension, respectively. Additionally, free tendon and aponeurosis have different properties (34); thus this approach is inherently problematic when assessing free tendon adaptations because it is unlikely a measure of free tendon alone.
In addition to ultrasound-based studies, several in vitro studies have reported reductions in tendon ultimate tensile strength (20, 57, 59) and changes in tendon ultrastructure (8, 39, 42, 48, 56, 61) with aging. Yet, to our knowledge, no data exist describing the influence of aging on the in vivo mechanical properties of the patellar tendon in humans, an important tendon for performance of normal locomotion and activities of daily living. Therefore, we chose to utilize ultrasonography and MRI to determine 1) if the in vivo mechanical properties of the patellar tendon are altered with aging, 2) if patellar tendon size is decreased with aging, and 3) if aging alters MRI-determined signal intensity. On the basis of previous findings in humans (27, 40), we hypothesized that patellar tendon stiffness, modulus, strain, and stress would be lower in older individuals. More recent evidence (41) also suggests that patellar tendon mechanical properties are sex specific; therefore, we recruited both men and women to participate in this investigation. Given the absence of data available in humans describing tendon adaptations to aging, the findings from this study will contribute significant new knowledge to the areas of aging and human tendon physiology and provide better guidance for patient care and injury prevention.
METHODS
Subjects.
Eleven young (6 men and 5 women) and 26 older (16 men and 10 women) individuals were recruited to participate in this investigation (Table 1). The Institutional Review Board of Ball State University and Ball Memorial Hospital approved this study, and informed consent was obtained from all subjects before participation. All subjects were sedentary to moderately active, nonsmokers, and apparently healthy as determined from a detailed medical history questionnaire and standard blood and urine chemistries. Additionally, all older individuals were given a medical exam and completed a resting and exercise electrocardiogram. Any individuals with known knee or tendon pathologies were excluded.
Table 1.
Subject characteristics
| Age, yr | Height, cm | Body Weight, kg | BMI, kg/m2 | 1RM, kg | 1RM/Body Weight | Quadriceps Muscle CSA, mm2 | |
|---|---|---|---|---|---|---|---|
| Young (n = 11) | 27±1 | 171±3 | 71.9±4.7 | 25±1 | 93±9* | 1.3±0.1* | 6,814±488* |
| Old (n = 26) | 65±1* | 171±2 | 84.0±2.5* | 29±1 | 68±5 | 0.8±0.1 | 5,684±460 |
| YM (n = 6) | 27±2 | 176±3 | 82.9±4.9 | 27±2 | 113±5 | 1.4±0.1 | 8,030±396 |
| OM (n = 16) | 65±1 | 179±1 | 89.4±2.6 | 28±1 | 85±3 | 1.0±0.1 | 6,713±223 |
| YW (n = 5) | 29±1 | 164±3 | 58.8±2.8 | 22±1† | 62±5 | 1.1±0.1 | 5,355±316 |
| OW (n = 10) | 66±2 | 160±4 | 75.2±3.4 | 30±1 | 40±2 | 0.5±0.1 | 4,038±156 |
Data are expressed as means ± SE; n = no of subjects. 1RM, one-repetition maximum; BMI, body mass index; CSA, cross-sectional area; YM, young men; OM, old men; YW, young women; OW, old women.
P < 0.05, Young vs. Old.
P < 0.05, YW different from OM and OW.
Muscle strength assessment: one-repetition maximum.
Quadriceps strength was evaluated by assessing each individual's bilateral one-repetition maximum (1RM) on a knee extension device (Cybex Eagle, Medway, MA). After 5 min of light cycling and two warm-up sets of five repetitions, several 1RM attempts were performed each separated by a 2-min rest period. The maximum weight lifted through a full range of motion was recorded as the subject's 1RM.
MRI: quadriceps femoris.
After lying supine for 1 h (2), axial images of the quadriceps femoris (55) were obtained [repetition time (TR)/echo time (TE) = 2,000/9; field of view (FOV) = 48 cm, 512 × 512 matrix; slice thickness = 8 mm; spacing = 0 mm] for the determination of muscle size. Muscle image files were transferred to a personal computer (iMac G5) and analyzed with NIH Image software (ImageJ, version 1.34) using manual planimetry, as done previously in our laboratory (54, 55). The same investigator performed all analyses.
MRI: patellar tendon.
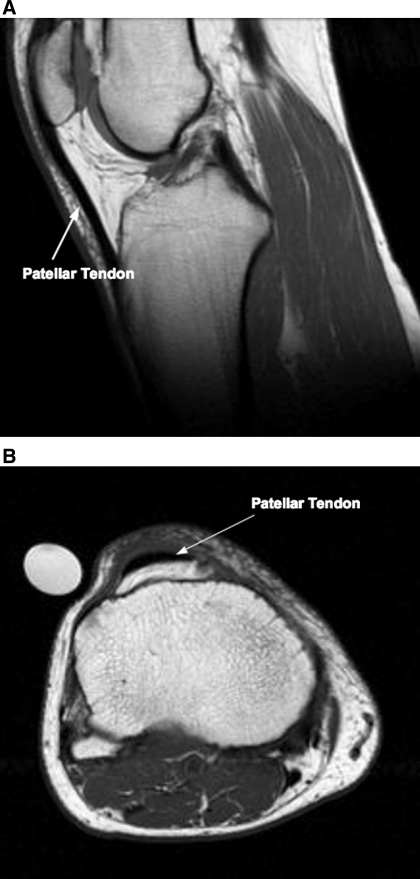
Immediately following the muscle scan, sagittal (Fig. 1A) and axial (Fig. 1B) images of the patellar tendon were obtained. While continuing to lie supine, the individual's right knee was placed in an extremity coil (GE 1.5 T, Quadrature Lower Extremity Coil 472GE-64, Invivo, Pewaukee, WI). Leg and joint positions were maintained by specific padding under the knee and resting the right foot in a nonmetallic support (55). A plastic tube containing 1.0% CuSO4 was placed in the FOV for normalization of tendon signal intensity. Sagittal images were obtained [TR/TE = 400/14, spin echo; echo train length (ETL) = 0; number of excitations (NEX) = 2; percent phase field of view (PPFOV) = 100; FOV = 16 × 16 cm, 256 × 256 matrix; slice thickness = 4 mm; spacing = 0 mm] beginning on the lateral most portion of the lateral condyle of the tibia, then moving medially (based on a coronal scout scan). Axial images of the patellar tendon were obtained (TR/TE = 550/12.4, fast spin echo train; ETL = 3; NEX = 3; PPFOV = 100; FOV = 16 × 16 cm, 256 × 256 matrix; slice thickness = 4 mm; spacing = 0 mm) beginning 8 mm (2 slices) proximal of the distal pole of the patella and proceeding distally (based on a sagittal scout scan). Tendon image files were then transferred to a personal computer (iMac G5) for analysis. For tendon length, sagittal images were reviewed with OsiriX (software version 2.7.5), and only slices with complete tendon from the distal pole of the patella to tibial insertion were chosen for analysis. ImageJ (version 1.34) was then used to determine tendon length via manual planimetry. The shortest measured distance from the most distal part of the patella to the first insertion point on the tibia along the posterior portion of the tendon was recorded as tendon length (22).
Fig. 1.
Representative sagittal [young man (23 yr old); A] and axial [older man (73 yr old); B] MRI images of the patellar tendon. The sagittal image is representative of a slice used to determined tendon length. The axial image is a representative slice from the distal portion of the patellar tendon.
Axial scans were also reviewed using OsiriX, and then ImageJ was used to manually circumscribe the patellar tendon. Specifically, tendon CSA and signal intensity [mean gray value (MGV)] were determined from an average of all slices beginning with the first slice not containing patella, then proceeding distally until just before insertion of the patellar tendon into the tibia (i.e., no indication of bone contacting the tendon). Tendon signal intensity was normalized to the 1% CuSO4 (tendon signal intensity/CuSO4 signal intensity). To determine region-specific CSA and signal intensity, the first slice not containing patella was considered the proximal tendon, and the slice just before insertion of the patellar tendon into the tibia was considered the distal tendon. The midregion was taken as the slice halfway between the proximal and distal slices. If a subject had an even number of slices, the values from the two slices nearest the midregion were averaged. An average of five traces per slice were used for the calculation of length, CSA, and signal intensity, and the same investigator completed all measurements. The coefficient of variation for trace-retrace was 1.1, 2.9, and 3.8% for tendon length, CSA, and signal intensity, respectively.
Tendon mechanical properties.
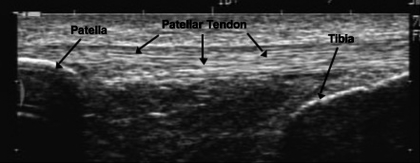
Patellar tendon mechanical properties were assessed as previously described (14, 22) with modifications. Specifically, subjects were firmly strapped into a rigid aluminum chair with the knee joint at 90° of flexion. A slightly padded cuff was attached to their lower leg ∼3 cm proximal to the medial malleolus. All participants completed three sessions, one familiarization and two testing. For each session, subjects performed four to seven “ramped” 10-s isometric quadriceps contractions to maximal effort with 90-s rest between contractions. The goal of each session was to obtain four attempts with linear increases in force; however, if during testing it was felt that there was an error during the contraction, e.g., reaching peak force too early, excessive subject movement, or issues with probe contact, an additional ramped contraction was performed after a 90-s rest period. During each contraction force was recorded (60-Hz sampling rate) via a strain-gauge load cell (Omegadyne, LC101-500) interfaced with a personal computer (Gateway E-4200). During ramped contractions an ultrasound probe (7.5 MHz, 70-mm B-mode linear array, Sonoline Sienna, Siemens, Erlangen, Germany) was mounted on the skin overlying the patellar tendon (Fig. 2) to monitor displacement of the patella and tibia during the ramped isometric effort (14). Video feed from the ultrasound unit was captured on a personal computer using frame grabber software (Matrox Inspector, Matrox Electronic Systems, Dorval, Quebec, Canada). To permit later synchronization of the ultrasound video and force output, a custom device was built that provided a visual signal on each ultrasound video allowing the user to select the specific frame of video at which force collection commenced.
Fig. 2.
Representative ultrasound image of the patellar tendon. Image taken before testing for tendon mechanical properties. Knee joint at 90°.
Displacement of the patella relative to the tibia was determined with custom-designed software (34), and deformation was defined as the change in distance between the tibia and patella, as previously described (14). Tendon force was calculated by dividing the total knee extension moment by the internal moment arm, which was estimated from femur length (14, 58). For each ramped contraction, deformation was determined three times and the highest observed deformation was used for analysis. After determining the deformation for each ramped contraction, the two contractions with the highest and lowest deformation were excluded. Force and deformation data from the remaining attempts were analyzed to a greatest common force and averaged, as previously outlined (14). Force-deformation curves were fitted with a second-order polynomial fit, and any curves with R2 < 0.97 were excluded from further analysis. Tendon stiffness and modulus were calculated from the final 10% of the force-deformation and stress-strain relationships, respectively, as previously described (14). Tendon stress was calculated by dividing tendon force by the average CSA of all slices along the length of the patellar tendon. Tendon strain was calculated as the change in tendon length relative to initial resting length.
The familiarization session was not used in the data analysis; thus the results of the second and third testing sessions were averaged. The same individual performed all mechanical analyses. The average coefficient of variation between the two included trials from each session (within day) was 4.4, 7.8, 7.8, 0.8, and 4.4% for tendon deformation, stiffness, modulus, stress, and strain, respectively. Average between-day coefficient of variation was 8.7, 7.0, 7.0, 0.6, and 8.7% for tendon deformation, stiffness, modulus, stress, and strain, respectively. The average values obtained between testing sessions 2 and 3 were not different (P > 0.05) for tendon deformation (2.6 ± 0.2 vs. 2.7 ± 0.2 mm), stiffness (2,383 ± 0.4 vs. 2,341 ± 0.4 N/mm), modulus (0.93 ± 0.33 vs. 0.91 ± 0.31 GPa), stress (23.5 ± 0.4 vs. 23.5 ± 0.4 MPa), and strain (5.5 ± 0.2 vs. 5.7 ± 0.2%). These values are similar to a previous evaluation of patellar tendon in vivo mechanical properties in young individuals (14).
Normalized force comparison.
For further comparison of mechanical properties, tendon deformation, stiffness, modulus, stress, and strain were determined for each individual at a common force. We initially chose to compare all groups at the lowest common mean force, i.e., the average tendon force of older women (1,650 N). However, given the large force differences between men and women, we also felt that comparing men and women separately was warranted. In this case, mechanical properties for young and old men were calculated at the mean tendon force of old men (3,014 N), and mechanical properties of young and old women were calculated at the mean force of old women (1,650 N).
Statistics.
A one-way ANOVA was used to compare tendon CSA and MGV at proximal, mid, and distal tendon. All other data were compared using two-way (age and sex) ANOVA. Tukey's honestly significant difference test was used to explore differences when a significant interaction was detected. Values were considered significant at an α-level of P < 0.05. All data were expressed as means ± SE.
RESULTS
No age and sex interactions were detected for any of the patellar tendon properties measured; therefore, data were collapsed and presented as young vs. old (age) and men vs. women (sex). Individual group data are also presented for general discussion.
MRI-determined tendon properties.
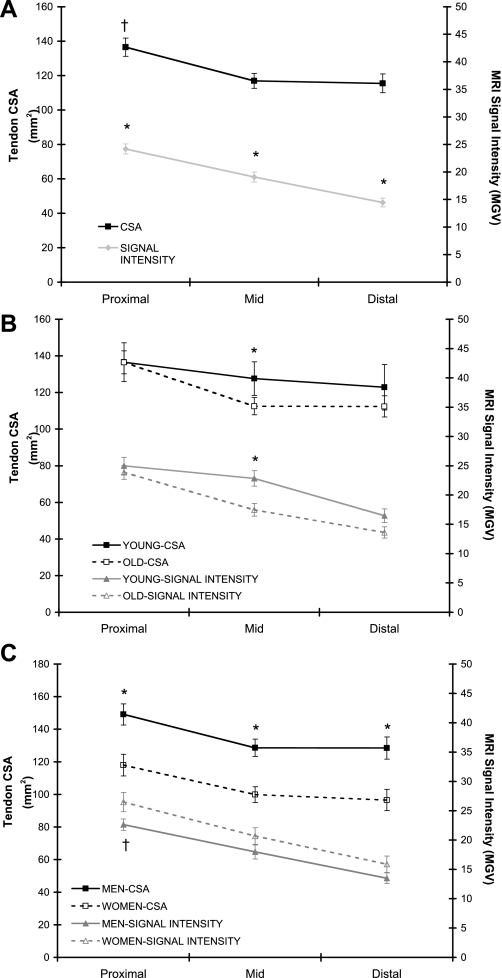
When all slices along the tendon were averaged, absolute patellar tendon CSA was not affected by aging (Table 2). In contrast, after normalization to body weight, tendon CSA was lower in the old individuals (P < 0.05, Table 2). Additionally, the CSA of the tendon midregion was 12% (P < 0.05) lower in older individuals (Fig. 3B). Averaged tendon CSA along the tendon was 28% greater (P < 0.05) in men compared with women (Table 2), and this difference was consistent along the tendon (Fig. 3C). However, this sex effect was eliminated after correction for body weight (Table 2). Tendon CSA was directly correlated (P < 0.05) to 1RM strength (P < 0.05, r = 0.68), quadriceps CSA (P < 0.05, r = 0.64), and body weight (P < 0.05, r = 0.41). Patellar tendon length was unaffected by aging but was 11% greater (P < 0.05) in men compared with women (Table 2).
Table 2.
MRI-determined patellar tendon properties
| Tendon CSA, mm2 | Tendon CSA/Body Weight, mm2/kg | Signal Intensity, MGV | Tendon Length, mm | |
|---|---|---|---|---|
| Young (n = 11) | 127.5±8.7 | 1.8±0.2* | 22.3±1.0* | 46.4±2.1 |
| Old (n = 26) | 117.5±4.7 | 1.4±0.2 | 18.3±0.9 | 45.8±1.2 |
| Men (n = 22) | 131.9±5.1† | 1.5±0.2 | 18.4±0.9 | 48.0±1.2† |
| Women (n = 15) | 103.1±4.6 | 1.5±0.2 | 21.1±1.2 | 43.1±1.5 |
| YM (n = 6) | 145.4±9.0 | 1.8±0.2 | 22.3±1.6 | 50.7±1.9 |
| OM (n = 16) | 126.8±5.8 | 1.4±0.2 | 17.0±0.9 | 47.0±1.5 |
| YW (n = 5) | 106.1±9.3 | 1.8±0.1 | 22.3±1.2 | 41.3±2.3 |
| OW (n = 10) | 101.6±5.4 | 1.4±0.1 | 20.5±1.8 | 44.0±2.0 |
Data are expressed as means ± SE; n = no. of subjects. MGV, mean gray value.
P < 0.05, Young vs. Old.
P < 0.05, men vs. women.
Fig. 3.
A: tendon MRI signal intensity [mean gray value (MGV)] and cross-sectional area (CSA) along the length of the patellar tendon: all subjects combined (n = 37). *P < 0.05, proximal > midregion (mid) > distal. †P < 0.05, proximal > mid and distal. B: tendon MRI signal intensity and CSA along the length of the patellar tendon: age comparison. Main effect for age: *P < 0.05, young > old. C: tendon MRI signal intensity and CSA along the length of the patellar tendon: sex comparison. Main effect for sex: *P < 0.05, men > women; †P = 0.054, women > men.
Independent of age or sex, tendon MRI signal intensity decreased from proximal to distal (P < 0.05; Fig. 3A). When all slices along the tendon were averaged, MRI signal intensity decreased 18% with aging (P < 0.05; Table 2). When compared in a region-specific manner, signal intensity decreased 5% (proximal), 24% (midregion), and 17% (distal) with aging, however, only the midregion was significant (P < 0.05; Fig. 3B). In general, signal intensity was not influenced by sex (Table 2), but there was a trend for signal intensity to be greater in women at the proximal tendon (P = 0.054, Fig. 3C).
Comparison at maximal tendon force.
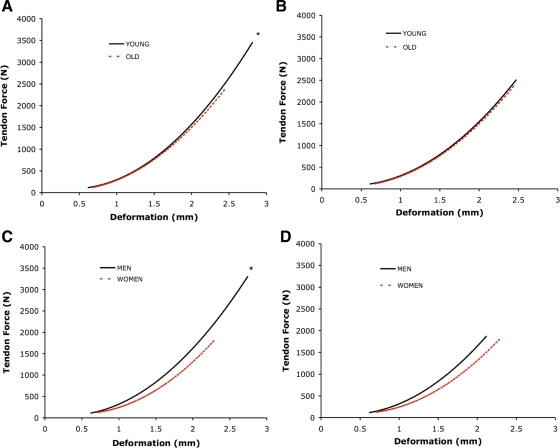
Young individuals were 37% stronger (P < 0.05, Table 1) than old subjects. At maximal levels of tendon force (Table 3), older individuals had 13% less patellar tendon deformation (P = 0.05; Fig. 4A) and lower peak stress (19%) and strain (12%, Fig. 5A) (P < 0.05) compared with young. Aging did not alter maximal tendon stiffness (Fig. 4A) or elastic modulus (Fig. 5A, Table 3) of the patellar tendon.
Table 3.
Tendon mechanical properties at peak force
| Deformation, mm | Stiffness, N/mm | Modulus, GPa | Stress, MPa | Strain, % | |
|---|---|---|---|---|---|
| Young (n = 11) | 2.8±0.2‡ | 2,552±218 | 0.9±0.1 | 26.6±2.4* | 6.0±0.2* |
| Old (n = 26) | 2.4±0.1 | 2,411±170 | 0.9±0.1 | 21.6±1.5 | 5.3±0.2 |
| Men (n = 22) | 2.7±0.1† | 2,744±178† | 1.0±0.1 | 26.2±1.7† | 5.6±0.2 |
| Women (n = 15) | 2.3±0.1 | 2,026±153 | 0.9±0.1 | 18.6±1.4 | 5.3±0.3 |
| YM (n = 6) | 3.1±0.2 | 2,924±332 | 1.0±0.1 | 30.3±3.6 | 6.0±0.2 |
| OM (n = 16) | 2.6±0.2 | 2,676±215 | 1.0±0.1 | 24.6±1.9 | 5.4±0.3 |
| YW (n = 5) | 2.4±0.2 | 2,106±64 | 0.8±0.1 | 22.1±1.7 | 6.0±0.4 |
| OW (n = 10) | 2.2±0.1 | 1,986±231 | 0.9±0.1 | 16.8±1.8 | 5.0±0.3 |
Data are expressed as means ± SE.
P < 0.05, Young vs. Old.
P < 0.05, men vs. women.
P = 0.05, Young vs. Old.
Fig. 4.
Mean tendon force (y-axes; in N) vs. deformation curves. Data fitted with 2nd-order polynomial regression. Slope of the line equals tendon stiffness (N/mm). A: young vs. old at maximal tendon force. *P < 0.05, force and deformation, young > old; for deformation P = 0.05, young > old. B: young vs. old at normalized tendon force. C: men vs. women at maximal tendon force. *P < 0.05, force, deformation, and stiffness, men > women. D: men vs. women at normalized tendon force.
Fig. 5.
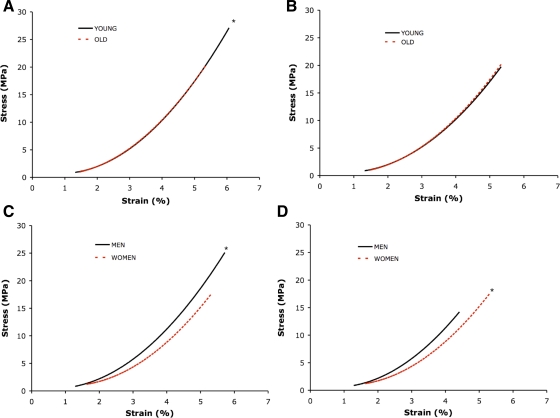
Stress (y-axes; in MPa) vs. strain curves. Data fitted with 2nd-order polynomial regression. Slope of the line equals tendon modulus (in GPa). A: young vs. old at maximal tendon force. *P < 0.05, stress and strain, young > old. B: young vs. old at normalized tendon force. C: men vs. women at maximal tendon force. *P < 0.05, stress, men > women. D: men vs. women at normalized tendon force. *P < 0.05, stress and strain, women > men.
Men (1RM = 93 kg) were 100% stronger (P < 0.05) than women (1RM = 46 kg). At maximal levels of tendon force, patellar tendon deformation (18%), stiffness (35%, Fig. 4C), and stress (41%, Fig. 5C) were greater in men compared with women (P < 0.05, Table 3). There was no difference between men and women in either maximal patellar tendon modulus or strain (Table 3, Fig. 5C).
Normalized force comparisons.
When calculated at relative levels of tendon force, no age differences in patellar tendon mechanical properties were apparent (Table 4; Figs. 4B and 5B). However, patellar tendon stress and strain were 26 and 22% higher (Table 4, Fig. 5D), respectively, in women compared with men regardless of age (P < 0.05).
Table 4.
Patellar tendon mechanical properties: force-normalized comparison
| Deformation, mm | Stiffness, N/mm | Modulus, GPa | Stress, MPa | Strain, % | % of Maximal Force | |
|---|---|---|---|---|---|---|
| All subjects at ∼1,650 N | ||||||
| Young (n = 11) | 2.0±0.1 | 1,818±83 | 0.7±0.1 | 13.6±1.0 | 4.5±0.3 | 54.8±5.5 |
| Old (n = 26) | 2.1±0.1 | 1,929±109 | 0.8±0.1 | 14.8±0.6 | 4.6±0.2 | 76.7±6.6 |
| Men (n = 22) | 2.0±0.1 | 1,922±97 | 0.7±0.1 | 13.1±0.5* | 4.2±0.2* | 52.8±3.0 |
| Women (n = 15) | 2.2±0.1 | 1,858±142 | 0.8±0.1 | 16.5±0.8 | 5.1±0.2 | 95.7±8.3 |
| Men at ∼3,014 N | ||||||
| YM (n = 6) | 2.6±0.2 | 2,472±203 | 0.9±0.1 | 21.3±1.2 | 5.2±0.3 | 73.7±6.6 |
| OM (n = 16) | 2.6±0.2 | 2,656±194 | 1.0±0.1 | 24.7±1.2 | 5.5±0.3 | 105.5±6.1 |
| Women at ∼1,650 N | ||||||
| YW (n = 5) | 2.1±0.1 | 1,772±63 | 0.7±0.1 | 16.0±1.5 | 5.2±0.4 | 73.2±1.8 |
| OW (n = 10) | 2.2±0.1 | 1,902±214 | 0.8±0.1 | 16.7±0.9 | 5.1±0.3 | 107.5±10.6 |
Data are expressed as means ± SE; n = no. of subjects.
P < 0.05, men vs. women.
DISCUSSION
Tendon properties and age.
To our knowledge, there are no data describing the influence of aging on in vivo patellar tendon properties in humans. Therefore, we evaluated the in vivo mechanical properties of the patellar tendon in young and old men and women. Consistent with mechanical data from other tendon structures (27, 32, 40), we demonstrate that tendon stress and strain at peak force output are reduced with aging. These differences, however, appear to be more related to force output rather than an age effect. Although tendon CSA and MRI signal intensity decreased with aging, aging does not appear to alter the slope of the force-deformation (stiffness) and stress-strain (modulus) relationships of the patellar tendon.
Our findings suggest that tendon elastic properties, at least of the patellar tendon, may not explain age-related declines in quadriceps muscle strength or functional performance (17). This lack of change is surprising given 1) the large decrease in muscle mass and strength associated with aging (also seen in this study); 2) the acute and chronic influence of skeletal muscle loading (24, 26–28, 37, 44, 46) and unloading (7, 23, 31, 43) on tendon in humans; and 3) several in vitro studies in humans (10, 48) and animals (18, 56), which have demonstrated changes in tendon cellular structure with aging. However, it is plausible that the reduced loading of the patellar tendon with age may result in structural alterations (e.g., changes in collagen cross-linking or content) within the tendon, influencing the integrity of the tendon, which were not detected with the ultrasound measures. Consistent with this argument, patellar tendon collagen content of animals has been shown to be altered with age (18, 56). Additionally, Johnson et al. (20) have reported that in vitro patellar tendon ultimate tensile strength is reduced with aging (young, 64.7 MPa; old, 53.6 MPa). These measurements, however, were taken at the failure point of the tendon and likely do not reflect the actual conditions (e.g., tensile forces) reached in older individuals during daily activities (21.6 ± 2.4 MPa in our older subjects at peak force). Although speculative, the lower force-generating capacity of sedentary older individuals may aid in minimizing the development of tendinopathies in these individuals by providing a buffer to excessive tendon strain. This concept, however, may only be applicable to the patellar tendon, as the incidence of Achilles tendon ruptures appears to be elevated in elderly individuals (19, 38).
Interestingly, although the average CSA along the tendon was not different with aging, we did observe regional tendon atrophy (Fig. 3B), the implications of which require further study. However, these data do suggest that stress may vary along the length of the tendon, potentially predisposing the midregion of the tendon to injury. Interestingly, after adjusting for body weight, average CSA along the tendon was lower in our older individuals. We can speculate that the greater body weight (Table 1) of the older individuals may act as a loading stimulus, minimizing any age-related decrease in absolute tendon CSA.
In addition to our measure of patellar tendon CSA, the MRI allows for the determination of signal intensity, which reflects the gray pixel density of a given tissue. Signal intensity has previously been shown to increase with tendinosis (50) and may indicate changes in extracellular matrix, tissue hydration, collagen fiber structure, or proteoglycan and glycosaminoglycan content (9, 12, 51, 62). Interestingly, we observed on 18% decline (P < 0.05) in MRI signal intensity of the patellar tendon (Table 2) with aging. This effect was also region specific (Fig. 3B). Several investigations (49, 52, 53) have shown tendon MRI signal to be altered with tendinosis or tissue injury and change with healing or chronic exercise (50), highlighting the potential physiological relevance of the signal intensity measurement. However, further biochemical studies of the tendon are needed to determine the physiological significance of the signal intensity measurement.
The region specificity of MRI signal intensity is consistent with previous studies of the patellar tendon, which have found regional difference in tendon CSA (22, 35, 60), mechanical properties (15), and collagen and proteoglycan mRNA expression (47). Interestingly, the decrease in signal intensity approximately mirrors the change in CSA along the tendon. Although speculative, the tendon may alter its properties at points along the tendon to compensate for lower CSA to prevent excessive tendon strain.
Tendon properties and sex.
Similar to previous studies (25, 41, 60), we found the in vivo properties of tendon to be different between men and women. Tendon stiffness and stress at maximal force were greater in men, which is consistent with previous reports (25, 41, 60). However, after normalization to a common force, the difference in stiffness was no longer observed. As with other studies of the patellar tendon, absolute tendon CSA (41, 60) and length (16) were greater in men compared with women. The sex difference in tendon CSA appears to be related to the greater body weight of men, as the sex difference in tendon CSA was eliminated after correction for body weight (Table 2). Therefore, the greater tendon CSA in men may be a body/muscle size effect rather than a true sex effect, which is supported by the correlations between body weight/muscle size and tendon CSA. The lack of difference in modulus (material stiffness) and MRI signal intensity would also suggest that the material properties of the tendon are similar between men and women. Therefore, sex differences in tendon mechanical properties appear to be more related to body size and muscle force output rather than an intrinsic difference in tendon. However, studies of isolated patellar tendon have shown that the modulus and ultimate stress of collagen fascicles are greater in men compared with fascicles from women (33). After normalization to a common force, patellar tendon stress and strain were greater in females compared with males, which may influence the risk of tendon injury. Based on our findings and those of others (33, 36, 60), further studies evaluating tendon properties in females are warranted.
Limitations.
Although our measurement of tendon mechanical properties provides a unique way to noninvasively study the human tendon in vivo, there are some limitations. The MRI and knee coil configuration utilized in this study do not allow for the knee joint and patellar tendon to be evaluated at 90° of flexion, i.e., the angle at which tendon mechanical testing is performed. Although this may prevent the determination of absolute mechanical parameters, all subjects are evaluated in the same manner; thus between-group comparisons should not be compromised. The inherent variability of the ultrasound technique may have prevented us from detecting more subtle differences in tendon mechanical properties, especially given our MRI findings. This combined with the smaller sample size in the young groups may have limited our ability to detect differences between groups.
Summary.
We demonstrate that although patellar tendon mechanical properties at maximal force are altered with aging, these differences are more related to force output rather than an age effect. Even with the large decrease in muscle mass and strength with aging, we demonstrate that the force-deformation (stiffness) and stress-strain (modulus) relationships are not altered, at least until the eighth decade of life, in healthy individuals. The lower stress and strain at maximal force in the old, due to a lower force output, suggests older individuals may have a lower risk of traumatic patellar tendon injury. However, one must consider the regional differences in tendon CSA and signal intensity, the latter of which may reflect structural alterations in the tendon, that were not detected by the ultrasound measures. Given the decrease in signal intensity with aging, we feel that additional studies are warranted to determine the physiological relevance of this measure.
In addition to our aging findings and in concurrence with previous studies (41, 60), in vivo patellar tendon mechanical properties are different in males compared with females. These differences appear to be largely related to anatomic difference (tendon size) in the patellar tendon between men and women and may have implications for the greater rate of tendon injury in physically active females (1, 4, 13, 21).
GRANTS
Funding for this study was provided by National Institute on Aging Grant R01-AG-020532 to T. A. Trappe and American Physiological Society Postdoctoral Initiative Fellowship to C. C. Carroll.
Acknowledgments
We thank the subjects for their participation; Beverly Slye, Sharon Meece, and Jamie Roberts of the Ball Memorial Hospital Radiology Department for expert assistance with the MRI procedures; the staff at Medical Consultants, Ball Memorial Hospital for screening of the older participants; and Jenny LeMoine for assistance with subject recruitment.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med 23: 694–701, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Berg HE, Tedner B, Tesch PA. Changes in lower limb muscle cross-sectional area and tissue fluid volume after transition from standing to supine. Acta Physiol Scand 148: 379–385, 1993. [DOI] [PubMed] [Google Scholar]
- 3.Biewener AA, Roberts TJ. Muscle and tendon contributions to force, work, and elastic energy savings: a comparative perspective. Exerc Sport Sci Rev 28: 99–107, 2000. [PubMed] [Google Scholar]
- 4.Bijur PE, Horodyski M, Egerton W, Kurzon M, Lifrak S, Friedman S. Comparison of injury during cadet basic training by gender. Arch Pediatr Adolesc Med 151: 456–461, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Bojsen-Moller J, Hansen P, Aagaard P, Kjaer M, Magnusson SP. Measuring mechanical properties of the vastus lateralis tendon-aponeurosis complex in vivo by ultrasound imaging. Scand J Med Sci Sports 13: 259–265, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Bojsen-Moller J, Magnusson SP, Rasmussen LR, Kjaer M, Aagaard P. Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structures. J Appl Physiol 99: 986–994, 2005. [DOI] [PubMed] [Google Scholar]
- 7.de Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV, Rennie MJ. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol 585: 241–251, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamant J, Keller A, Baer E, Litt M, Arridge RG. Collagen; ultrastructure and its relation to mechanical properties as a function of ageing. Proc R Soc Lond B Biol Sci 180: 293–315, 1972. [DOI] [PubMed] [Google Scholar]
- 9.Erickson SJ, Prost RW, Timins ME. The “magic angle” effect: background physics and clinical relevance. Radiology 188: 23–25, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Eriksen HA, Pajala A, Leppilahti J, Risteli J. Increased content of type III collagen at the rupture site of human Achilles tendon. J Orthop Res 20: 1352–1357, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Fukunaga T, Ito M, Ichinose Y, Kuno S, Kawakami Y, Fukashiro S. Tendinous movement of a human muscle during voluntary contractions determined by real-time ultrasonography. J Appl Physiol 81: 1430–1433, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Fullerton GD, Cameron IL, Ord VA. Frequency dependence of magnetic resonance spin-lattice relaxation of protons in biological materials. Radiology 151: 135–138, 1984. [DOI] [PubMed] [Google Scholar]
- 13.Geary KG, Irvine D, Croft AM. Does military service damage females? An analysis of medical discharge data in the British armed forces. Occup Med (Lond) 52: 85–90, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Hansen P, Bojsen-Moller J, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties of the human patellar tendon, in vivo. Clin Biomech (Bristol, Avon) 21: 54–58, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Haraldsson BT, Aagaard P, Krogsgaard M, Alkjaer T, Kjaer M, Magnusson SP. Region-specific mechanical properties of the human patella tendon. J Appl Physiol 98: 1006–1012, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Hashemi J, Chandrashekar N, Slauterbeck J. The mechanical properties of the human patellar tendon are correlated to its mass density and are independent of sex. Clin Biomech (Bristol, Avon) 20: 645–652, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol 103: 2068–2076, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Haut RC, Lancaster RL, DeCamp CE. Mechanical properties of the canine patellar tendon: some correlations with age and the content of collagen. J Biomech 25: 163–173, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Houshian S, Tscherning T, Riegels-Nielsen P. The epidemiology of Achilles tendon rupture in a Danish county. Injury 29: 651–654, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Johnson GA, Tramaglini DM, Levine RE, Ohno K, Choi NY, Woo SL. Tensile and viscoelastic properties of human patellar tendon. J Orthop Res 12: 796–803, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Jones BH, Bovee MW, Harris JM, Cowan DN. Intrinsic risk factors for exercise-related injuries among male and female army trainees. Am J Sports Med 21: 705–710, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Kongsgaard M, Reitelseder S, Pedersen TG, Holm L, Aagaard P, Kjaer M, Magnusson SP. Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol (Oxf) 191: 111–121, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Kubo K, Akima H, Ushiyama J, Tabata I, Fukuoka H, Kanehisa H, Fukunaga T. Effects of 20 days of bed rest on the viscoelastic properties of tendon structures in lower limb muscles. Br J Sports Med 38: 324–330, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubo K, Kanehisa H, Fukunaga T. Effects of resistance and stretching training programmes on the viscoelastic properties of human tendon structures in vivo. J Physiol 538: 219–226, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubo K, Kanehisa H, Fukunaga T. Gender differences in the viscoelastic properties of tendon structures. Eur J Appl Physiol 88: 520–526, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Kubo K, Kanehisa H, Fukunaga T. Influences of repetitive drop jump and isometric leg press exercises on tendon properties in knee extensors. J Strength Cond Res 19: 864–870, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Kubo K, Kanehisa H, Miyatani M, Tachi M, Fukunaga T. Effect of low-load resistance training on the tendon properties in middle-aged and elderly women. Acta Physiol Scand 178: 25–32, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Langberg H, Rosendal L, Kjaer M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol 534: 297–302, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maffulli N, Waterston SW, Squair J, Reaper J, Douglas AS. Changing incidence of Achilles tendon rupture in Scotland: a 15-year study. Clin J Sport Med 9: 157–160, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Maganaris CN Tensile properties of in vivo human tendinous tissue. J Biomech 35: 1019–1027, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Maganaris CN, Reeves ND, Rittweger J, Sargeant AJ, Jones DA, Gerrits K, De Haan A. Adaptive response of human tendon to paralysis. Muscle Nerve 33: 85–92, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Magnusson SP, Beyer N, Abrahamsen H, Aagaard P, Neergaard K, Kjaer M. Increased cross-sectional area and reduced tensile stress of the Achilles tendon in elderly compared with young women. J Gerontol A Biol Sci Med Sci 58: 123–127, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Magnusson SP, Hansen M, Langberg H, Miller B, Haraldsson B, Westh EK, Koskinen S, Aagaard P, Kjaer M. The adaptability of tendon to loading differs in men and women. Int J Exp Pathol 88: 237–240, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magnusson SP, Hansen P, Aagaard P, Brond J, Dyhre-Poulsen P, Bojsen-Moller J, Kjaer M. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol Scand 177: 185–195, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Magnusson SP, Kjaer M. Region-specific differences in Achilles tendon cross-sectional area in runners and non-runners. Eur J Appl Physiol 90: 549–553, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Miller BF, Hansen M, Olesen JL, Schwarz P, Babraj JA, Smith K, Rennie MJ, Kjaer M. Tendon collagen synthesis at rest and after exercise in women. J Appl Physiol 102: 541–546, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 567: 1021–1033, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moller A, Astron M, Westlin N. Increasing incidence of Achilles tendon rupture. Acta Orthop Scand 67: 479–481, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Moriguchi T, Fujimoto D. Age-related changes in the content of the collagen crosslink, pyridinoline. J Biochem 84: 933–935, 1978. [DOI] [PubMed] [Google Scholar]
- 40.Onambele GL, Narici MV, Maganaris CN. Calf muscle-tendon properties and postural balance in old age. J Appl Physiol 100: 2048–2056, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Onambele GN, Burgess K, Pearson SJ. Gender-specific in vivo measurement of the structural and mechanical properties of the human patellar tendon. J Orthop Res 25: 1635–1642, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Patterson-Kane JC, Firth EC, Goodship AE, Parry DA. Age-related differences in collagen crimp patterns in the superficial digital flexor tendon core region of untrained horses. Aust Vet J 75: 39–44, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Reeves ND, Maganaris CN, Ferretti G, Narici MV. Influence of 90-day simulated microgravity on human tendon mechanical properties and the effect of resistive countermeasures. J Appl Physiol 98: 2278–2286, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol 548: 971–981, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reeves ND, Narici MV, Maganaris CN. Myotendinous plasticity to ageing and resistance exercise. Exp Physiol 91: 483–498, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Reeves ND, Narici MV, Maganaris CN. Strength training alters the viscoelastic properties of tendons in elderly humans. Muscle Nerve 28: 74–81, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Robbins JR, Vogel KG. Regional expression of mRNA for proteoglycans and collagen in tendon. Eur J Cell Biol 64: 264–270, 1994. [PubMed] [Google Scholar]
- 48.Sargon MF, Ozlu K, Oken F. Age-related changes in human tendon calcaneus collagen fibrils. Saudi Med J 26: 425–428, 2005. [PubMed] [Google Scholar]
- 49.Schmid MR, Hodler J, Cathrein P, Duewell S, Jacob HA, Romero J. Is impingement the cause of jumper's knee? Dynamic and static magnetic resonance imaging of patellar tendinitis in an open-configuration system. Am J Sports Med 30: 388–395, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Shalabi A Magnetic resonance imaging in chronic Achilles tendinopathy. Acta Radiol 1–45, 2004. [DOI] [PubMed]
- 51.Shalabi A, Kristoffersen-Wiberg M, Papadogiannakis N, Aspelin P, Movin T. Dynamic contrast-enhanced MR imaging and histopathology in chronic Achilles tendinosis. A longitudinal MR study of 15 patients. Acta Radiol 43: 198–206, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Shalabi A, Kristoffersen-Wilberg M, Svensson L, Aspelin P, Movin T. Eccentric training of the gastrocnemius-soleus complex in chronic Achilles tendinopathy results in decreased tendon volume and intratendinous signal as evaluated by MRI. Am J Sports Med 32: 1286–1296, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Shalabi A, Svensson L, Kristoffersen-Wiberg M, Aspelin P, Movin T. Tendon injury and repair after core biopsies in chronic Achilles tendinosis evaluated by serial magnetic resonance imaging. Br J Sports Med 38: 606–612, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trappe TA, Burd NA, Louis ES, Lee GA, Trappe SW. Influence of concurrent exercise or nutrition countermeasures on thigh and calf muscle size and function during 60 days of bed rest in women. Acta Physiol (Oxf) 191: 147–159, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Trappe TA, Lindquist DM, Carrithers JA. Muscle-specific atrophy of the quadriceps femoris with aging. J Appl Physiol 90: 2070–2074, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Vailas AC, Pedrini VA, Pedrini-Mille A, Holloszy JO. Patellar tendon matrix changes associated with aging and voluntary exercise. J Appl Physiol 58: 1572–1576, 1985. [DOI] [PubMed] [Google Scholar]
- 57.Vilarta R, Vidal Bde C. Anisotropic and biomechanical properties of tendons modified by exercise and denervation: aggregation and macromolecular order in collagen bundles. Matrix 9: 55–61, 1989. [DOI] [PubMed] [Google Scholar]
- 58.Visser JJ, Hoogkamer JE, Bobbert MF, Huijing PA. Length and moment arm of human leg muscles as a function of knee and hip-joint angles. Eur J Appl Physiol Occup Physiol 61: 453–460, 1990. [DOI] [PubMed] [Google Scholar]
- 59.Vogel HG Species differences of elastic and collagenous tissue—influence of maturation and age. Mech Ageing Dev 57: 15–24, 1991. [DOI] [PubMed] [Google Scholar]
- 60.Westh E, Kongsgaard M, Bojsen-Moller J, Aagaard P, Hansen M, Kjaer M, Magnusson SP. Effect of habitual exercise on the structural and mechanical properties of human tendon, in vivo, in men and women. Scand J Med Sci Sports 18: 23–30, 2008. [DOI] [PubMed] [Google Scholar]
- 61.Wilmink J, Wilson AM, Goodship AE. Functional significance of the morphology and micromechanics of collagen fibres in relation to partial rupture of the superficial digital flexor tendon in racehorses. Res Vet Sci 53: 354–359, 1992. [DOI] [PubMed] [Google Scholar]
- 62.Yoon JH, Halper J. Tendon proteoglycans: biochemistry and function. J Musculoskelet Neuronal Interact 5: 22–34, 2005. [PubMed] [Google Scholar]