Abstract
Akt substrate of 160 kDa (AS160), the most distal insulin signaling protein known to be important for insulin-stimulated glucose transport, becomes phosphorylated with skeletal muscle contraction. Akt, AMP-activated protein kinase (AMPK), and Ca2+/calmodulin-dependent kinase II (CaMKII) have been implicated in regulating AS160 and/or glucose transport. Our primary aim was to assess time courses for contraction's effects on glucose transport and phosphorylation of Akt, AMPK, CaMKII, and AS160. Isolated rat epitrochlearis muscles were studied without or with contraction (5, 10, 20, 40, 60 min). Phospho-Akt substrate (PAS) antibody was used to measure AS160 PAS phosphorylation by quantifying the ∼160-kDa band on PAS immunoblots (PAS-160); a separate band at 150 kDa (PAS-150) that responded similarly to contraction was also identified. Using specific antibodies for AS160 or TBC1D1 on immunoblots, the molecular mass of PAS-160 was found to correspond with that of AS160 and not TBC1D1, whereas PAS-150 corresponded with TBC1D1 and not AS160. Furthermore, supernatant of sample immunodepleted with anti-AS160 had greatly reduced PAS-160, whereas supernatant of sample immunodepleted with anti-TBC1D1 had greatly reduced PAS-150, providing further evidence that PAS-160 and PAS-150 correspond with PAS-AS160 and PAS-TBC1D1, respectively. Contraction induced transient increases in PAS-160, PAS-150, phospho-glycogen synthase kinase 3 (an Akt substrate) and phospho-CaMKII; glucose transport and phospho-AMPK increases were maintained for 60 min of contraction. These data suggest the following: 1) PAS-160 (AS160) and PAS-150 (TBC1D1) respond to contraction transiently, despite sustained stimulation; 2) continual AMPK activation was insufficient for sustained increase in PAS-160 or PAS-150; and 3) sustained elevation of PAS-160 or PAS-150 was unnecessary to maintain contraction-stimulated glucose transport for up to 60 min.
Keywords: exercise, adenosine 5′-monophosphate-activated protein kinase, Ca2+/calmodulin-dependent kinase II, Akt
insulin or contractile activity each result in a rapid increase in glucose transport by isolated rat skeletal muscle that can be sustained for at least 60 min with continuous stimulation (11, 20). Although each stimulus induces the redistribution of GLUT4 glucose transporters from the cell's interior to its surface, multiple lines of evidence indicate that they trigger translocation by distinct mechanisms (5, 8). For example, combining maximally effective insulin and contractile activity results in an essentially additive increase in glucose transport (7, 19), and concentrations of the phosphatidylinositol 3-kinase inhibitor wortmannin that completely inhibit insulin-stimulated glucose transport do not alter contraction-stimulated glucose transport (16, 36).
Sano et al. (26) demonstrated that Akt substrate of 160-kDa (AS160) phosphorylation is a key step linking the insulin signaling pathway with GLUT4 translocation in 3T3L1 adipocytes. Subsequently, Bruss et al. (3), using isolated rat skeletal muscle, found rapid (half-time of ∼2.5 min) and sustained increases in the phosphorylation of Akt (pAkt) and AS160 [measured using the phospho-Akt substrate (PAS) antibody, half-time ∼7 min], consistent with the time course for insulin's effect on glucose transport in isolated skeletal muscle (11). Experiments using wortmannin (3), an Akt inhibitor (10), Akt knockdown by short hairpin RNA (10), or Akt2 null mice (24) provide substantial evidence for Akt being the major insulin-stimulated kinase that phosphorylates AS160.
Insulin and contractile activity appear to regulate glucose transport by distinct mechanisms, but, because muscle contraction can activate Akt (25), it was not completely unexpected that contraction by isolated muscle in the absence of insulin also increased AS160 PAS phosphorylation (3). Incubation of isolated skeletal muscle with 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) resulted in increased PAS-AS160 (3), suggesting that AMP-activated protein kinase (AMPK) might also be capable of phosphorylating AS160, which was confirmed by Treebak et al. (32), who reported that incubation of recombinant AMPK with AS160 caused an increase in PAS-AS160.
Activation of Akt is not essential for contraction-stimulated glucose transport (24, 36), whereas AMPK and Ca2+/calmodulin-dependent kinase II (CaMKII) have each been implicated to be involved in contraction-stimulated glucose transport (34). Previous research has demonstrated transient, contraction-mediated activation of Akt (17, 25) and CaMKII (22, 23), but sustained AMPK activation during contraction (28, 30). However, because the time courses for activation of these kinases were determined in separate studies using different contraction protocols, it would be valuable to assess the activation of each kinase, together with PAS-AS160, in the same muscles stimulated by the same contraction protocol. Accordingly, the primary aim of this study was to evaluate the time courses for contraction-stimulated effects on phosphorylation of Akt, AMPK, CaMKII, and AS160 in isolated rat skeletal muscle. To better understand contraction-induced modulation of the three contraction-stimulated kinases, we also evaluated the phosphorylation of glycogen synthase kinase 3 (GSK3), acetyl-CoA carboxylase (ACC), and serum response factor (SRF), substrates for Akt, AMPK, and CaMKII, respectively.
Our laboratory previously found that wortmannin could completely eliminate the contraction-induced increase in PAS-AS160 of isolated rat epitrochlearis muscles, suggesting that Akt may be the dominant kinase for increasing PAS-AS160 under these conditions (3). Therefore, we hypothesized that contractile activity would result in a transient increase in pAkt and PAS-AS160. We further hypothesized that the same contraction protocol would transiently activate CaMKII, but induce a sustained activation of AMPK and glucose transport.
METHODS
Materials.
Reagents and apparatus for SDS-PAGE and immunoblotting, including Precision Plus Protein Dual Color Standards (no. 161-0734), were from Bio-Rad (Hercules, CA). Bicinchoninic acid protein assay reagent (no. 23227) and T-PER tissue protein extraction reagent (no. 78510) were from Pierce Biotechnology (Rockford, IL). Anti-phospho-Thr308 Akt (pThrAkt, no. 9275), anti-phospho-Ser473 Akt (pSerAkt, no. 9271), anti-phospho-Ser21/9 GSK3-α/β (pGSK3, no 9331), anti-phospho-Thr172 AMPK (pAMPK, no. 2531), anti-phospho-Ser79 ACC (pACC, no. 3661), anti-phospho-Thr286 CaMKII (pCaMKII, no. 3361), anti-phospho-Ser103 SRF (pSRF, no. 4261), anti-phospho-(Ser/Thr) Akt substrate (PAS, no. 9611), and goat anti-rabbit IgG horseradish peroxidase (HRP) conjugate (no. 7074) were purchased from Cell Signaling Technology (Danvers, MA). PAS recognizes pAkt motif peptide sequences (RXRXXpT/S). TBC1D1 polyclonal antibody was provided by Dr. Jianxin Xie (Cell Signaling Technology). AS160 antibody (no. 07-741) was purchased from Upstate USA (Charlottesville, VA). Preclearing Matrix F (no. 45057) and ExactaCruz F-HRP (no. 45043) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). SuperSignal (West Dura Extended Duration Substrate; Pierce, no. 34075) was used to visualize immunoblots. 3-O-methyl-[3H]glucose ([3H]3-MG) was from Sigma-Aldrich (St. Louis, MO), and [14C]mannitol was from Perkin Elmer (Waltham, MA). Other reagents were from Sigma-Aldrich and Fisher Scientific (Pittsburgh, PA).
Animal treatment.
Procedures for animal care were approved by the University of Michigan Committee on Use and Care of Animals. Male Wistar rats (∼150–200 g; Harlan, Indianapolis, IN) were provided with rodent chow (Lab Diet; PMI Nutritional International, Brentwood, MO), and water ad libitum until 1700 the night before experiment and did not have access to food thereafter. On the next day, between 1000 and 1300, rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (∼60 mg/kg wt). While rats were under deep anesthesia, both epitrochlearis muscles were rapidly dissected out.
Muscle treatment.
Epitrochlearis muscles were incubated in glass vials containing Krebs-Henseleit buffer (KHB) + 0.1% bovine serum albumin (BSA) + 8 mM glucose (solution 1) and were shaken for 30 min in a water bath at 35°C with continuous gassing (95% O2-5% CO2). Muscles were then mounted in a water-jacketed glass vessel that was warmed using a temperature-controlled bath (35°C). The distal end of the muscle was attached to a glass rod, and the proximal end was attached to a force transducer (Radnoti, Litchfield, CT), as previously described (9). The mounted muscles were incubated in KHB + BSA + glucose with continuous gassing (95% O2-5% CO2) and were stimulated to contract (Grass S48 Stimulator; Grass Instruments, Quincy, MA) for 5, 10, 20, 40, or 60 min (2-ms twitch, 120 twitches/min) or rested (0, 5, or 60 min). Subsequently, some muscles were rapidly blotted, trimmed of connective tissue, and freeze-clamped with aluminum tongs cooled to the temperature of liquid N2 and then stored at −80°C until homogenization and analysis. Other muscles were transferred to vials containing KHB + 2 mM pyruvate + 36 mM mannitol (solution 2) at 30°C for 10 min before being used for determination of glucose transport rate.
Measurement of glucose transport.
After the 10-min incubation in solution 2, muscles were transferred to flasks containing KHB, 0.1% BSA with 8 mM 3-MG (including [3H]3-MG 0.25 mCi/mmol), and 2 mM mannitol (including [14C]mannitol 0.1 mCi/mmol). After incubation with 3-MG for 10 min, the muscles were rapidly blotted on filter paper dampened with incubation media, trimmed, freeze-clamped, and stored at −80°C until processed as described below.
Homogenization.
Frozen muscles used for glucose transport and immunoblotting (PAS, pSerAkt, pThrAkt, pGSK3, pAMPK, pACC, pCaMKII, and pSRF) were homogenized in 1 ml ice-cold homogenization buffer (20 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1% IGEPAL, 2 mM Na3VO4, 2 mM EDTA, 2 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM phenylmethanesulphonyl fluoride, and 1 μg/ml leupeptin) using glass-on-glass tubes (Kontes, Vineland, NJ). Homogenates were subsequently rotated at 4°C for 1 h before being centrifuged (12,000 g for 10 min at 4°C). Aliquots of the supernatant from muscles used for the 3-MG analysis were pipetted into vials for scintillation counting, and 3-MG accumulation was determined as previously described (4). A portion of the supernatant was used to determine protein concentration by the bicinchoninic acid assay (27), and the remainder was stored at −80°C until it was further analyzed.
Immunoprecipitation.
Frozen muscles to be immunoprecipitated with anti-AS160 or anti-TBC1D1 were homogenized in T-PER supplemented homogenization buffer (2 mM Na3VO4, 2 mM EDTA, 2 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM phenylmethanesulphonyl fluoride, and 1 μg/ml leupeptin in T-PER). Homogenized muscle (300–500 μg protein) was precleared in preclearing matrix F for 30 min, and the resulting supernatant was immunoprecipitated with 1.5–2 μg of anti-AS160 or anti-TBC1D1 at 4°C using ExactaCruz F-HRP. After gentle rotation overnight, the immunoprecipitation mix was centrifuged at 4,000 g, and the immunodepleted supernatant was aspirated and subsequently used for immunoblotting. After washing (four times with 500-μl phosphate-buffered saline), the protein bound to the beads was eluted with 2 × SDS loading buffer and boiled before loading on a polyacrylamide gel.
Immunoblotting.
Immunoprecipitates, immunodepleted supernatants, or homogenized muscle lysates in the SDS loading buffer were separated and electrophoretically transferred to nitrocellulose. Samples were then rinsed with Tris-buffered saline plus Tween (TBST) (0.14 mol/l NaCl, 0.02 mol/l Tris base, pH 7.6, and 0.1% Tween), blocked with 5% nonfat dry milk in TBST for 1 h at room temperature, washed 3 × 5 min at room temperature, and treated with the relevant primary antibody (1:1,000 in TBST + 5% BSA) overnight at 4°C. Blots were then washed 3 × 5 min with TBST, incubated with the secondary antibody, goat anti-rabbit IgG horseradish peroxidase conjugate (1:20,000 in TBST + 5% milk), for 1 h at room temperature, washed again 3 × 5 min with TBST, and developed with SuperSignal reagent. Protein bands were quantitated by densitometry (Alpha Innotech, San Leandro, CA). The mean values for resting samples on each blot were normalized to equal 1.0, and then all samples on the blot were expressed relative to the normalized resting value.
Statistical analysis.
Statistical analyses were done using Sigma Stat version 2.0 (San Rafael, CA). Data are expressed as means ± SE. P ≤ 0.05 was considered statistically significant. One-way ANOVA was used to determine significant differences with contraction, and the source of significant variance was detected using the Dunnett post hoc test (vs. resting control). When data failed the Levene median test for equal variance, the data were transformed (base 10 logarithm) before ANOVA was performed. A Pearson product-moment correlation was used to assess the relationship between variables. For correlations determined between two signaling proteins, each correlated pair of signaling measurements was from the same muscle. Correlations determined with tension measurements also used signaling or glucose transport values from the same muscle. For correlations determined between glucose transport and a signaling value, the data were from the contralateral muscles from the same rat.
RESULTS
Rested muscles.
There were no significant effects of incubation time (0, 5, or 60 min) for protein phosphorylation or glucose transport in the rested muscles. Therefore, values for resting muscles were pooled for statistical analyses and are represented as 0-min time point in the figures (see Figs. 2, 4, and 5).
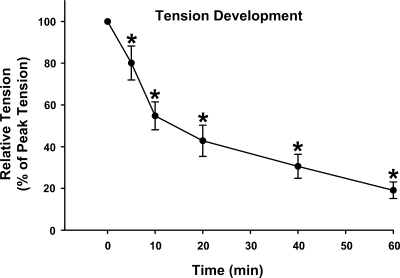
Tension development.
Peak tension was 117.2 ± 8.7 g/g wt muscle. There was a progressive decline in tension development reaching ∼50% of the peak value at ∼15 min and 19.1 ± 4.0% of peak tension at 60 min of contraction (Fig. 1). The peak tension (represented at 0 min) was significantly greater than tension development at 5, 10, 20, 40, and 60 min (P < 0.05).
Fig. 1.
Time course for tension development in isolated rat epitrochlearis muscles that were contracted for 60 min. Values are means ± SE; n = 15 per group. Post hoc analysis: *P < 0.05 vs. peak tension (at 0 min).
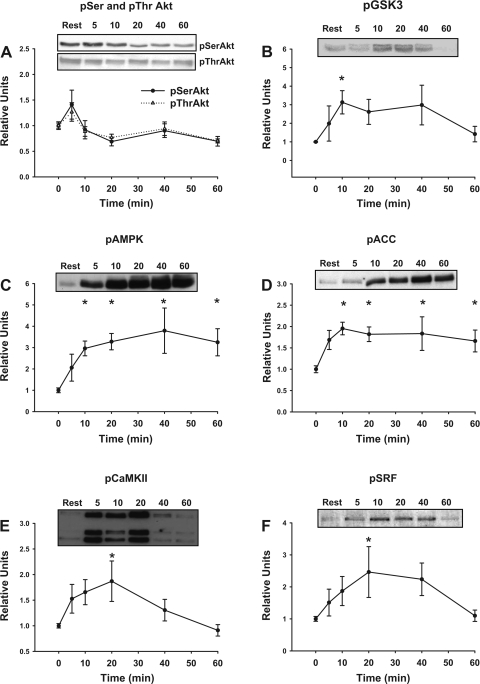
Contraction-activated kinases and phosphorylated substrates.
There was a transient trend for level of pSerAkt and pThrAkt to increase above resting at 5 min of contraction (Fig. 2A). The level of pGSK3, an Akt substrate, was significantly increased above resting at 10 min (half-time of ∼5 min) and returned to baseline by 60 min (Fig. 2B). There was a significant contraction-induced increase in both pAMPK (10, 20, 40, and 60 min, half time of ∼7.5 min, Fig. 2C) and its substrate pACC (10, 20, 40, and 60 min, half time of ∼4 min, Fig. 2D). Contraction caused a transient increase in pCaMKII (20 min, half-time of ∼4 min, Fig. 2E) and its substrate pSRF (20 min, half-time of ∼7 min, Fig. 2F), and each returned to baseline by 60 min.
Fig. 2.
Time course for contraction-stimulated phosphorylation (p) of AktSer473 and AktThr308 (A), glycogen synthase kinase 3 (GSK3)Ser21/9 (B), AMP-activated protein kinase (AMPK)Thr172 (C), acetyl-CoA carboxylase (ACC)Ser79 (D), Ca2+/calmodulin-dependent kinase II (CaMKII)Thr286 (E), and serum response factor (SRF)Ser103 (F) in isolated rat epitrochlearis muscles. The values at 0 min are from rested muscles. Representative blots are shown. Values are means ± SE; n = 7–12 per group. Post hoc analysis: *P < 0.05 vs. basal.
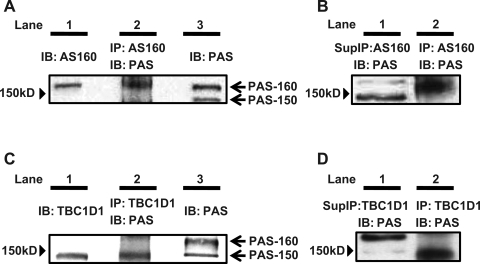
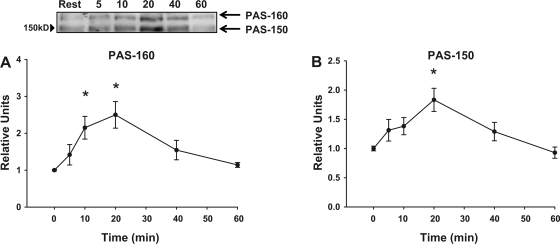
PAS-160 and PAS-150.
When lysates prepared from rat epitrochlearis muscles were immunoblotted using anti-AS160, the AS160 band migrated above the 150-kDa molecular mass marker (Fig. 3A, lane 1). In samples that were immunoprecipitated using anti-AS160 before immunoblotting with anti-PAS, the contraction-responsive PAS band was also visualized above the 150-kDa molecular mass marker (PAS-AS160, Fig. 3A, lane 2). For samples that were immunoblotted using anti-PAS without prior immunoprecipitation, a contraction-responsive PAS band was visible at a site corresponding to the location of the AS160 (Fig. 3A, lane 1) and PAS-AS160 (Fig. 3A, lane 2) bands, and it was designated as PAS-160 (Fig. 3A, lane 3). There was strong PAS immunoreactivity above the 150-kDa marker in the anti-AS160 immunoprecipitate of contraction-stimulated muscle (PAS-AS160; Fig. 3B, lane 2), but the PAS-160 signal was barely detectable in the adjacent lane, which had been loaded with supernatant of the anti-AS160 immunodepleted sample (Fig. 3B, lane 1). These results suggest that PAS-AS160 accounts for the PAS-160 band of samples that had not been immunoprecipitated. PAS-160 was significantly greater than the resting value at 10 min of contraction (half time of ∼7 min), reached its peak value at 20 min, and returned to baseline by 60 min of contraction (Fig. 4A).
Fig. 3.
Phospho-Akt substrate (PAS)-160 and PAS-150. Lysates from isolated rat epitrochlearis muscle that were stimulated to contract for 20 min were subjected to immunoblotting (IB) [with or without prior immunoprecipitation (IP)]. The samples in each panel (A, B, C, or D) were run on the same gel and transferred to the same blot, and then the blots were cut into strips that were separately incubated in the different primary antibodies as indicated. A: the band in the anti-Akt substrate of 160 kDa (AS160) IB migrated above 150 kDa (lane 1). The band for the sample undergoing anti-AS160 IP before anti-PAS IB was also visualized above 150 kDa (PAS-AS160, lane 2). The anti-PAS IB of muscle lysates without prior IP had a PAS band visible at a site corresponding to the location of the AS160 (lane 1) and PAS-AS160 (lane 2) bands and was designated as PAS-160 (lane 3). B: AS160-immunodepleted supernatant had a greatly reduced PAS-160 signal (lane 1), indicating that PAS-AS160 (lane 2) accounts for the PAS-160 band of samples that had not been IP. C: on the anti-PAS IB, we also observed a PAS band at ∼150 kDa (A, lane 3 and C, lane 3, designated PAS-150). Anti-TBC1D1 IB (lane 1) and anti-TBC1D1 IP before anti-PAS IB (PAS-TBC1D1, lane 2) each had a band at ∼150 kDa, corresponding to the location of PAS-150. D: TBC-1D1-immunodepleted supernatant had a greatly reduced PAS-150 signal (lane 1), indicating that PAS-TBC1D1 (lane 2) accounts for the PAS-150 band of samples that had not been IP.
Fig. 4.
Time course for contraction-stimulated PAS-160 (A) and PAS-150 (B) in isolated rat epitrochlearis muscles. The values at 0 min are from rested muscles. Representative blots are shown. Values are means ± SE; n = 7–16 per group. Post hoc analysis: *P < 0.05 vs. basal.
On the same immunoblots, we also observed a contraction-responsive PAS band at ∼150 kDa (Fig. 3A, lane 3, and Fig. 3C lane 3, designated PAS-150). Recently, a paralog protein of AS160 called TBC1D1 has been identified as a novel substrate of Akt that may also be involved in insulin-stimulated GLUT-4 translocation in adipocytes (21). TBC1D1 has been reported to have a slightly lower apparent molecular mass compared with AS160, and, therefore, it seemed possible that PAS-150 was TBC1D1 (29). Supporting this idea, when lysates were immunoblotted using anti-TBC1D1, the TBC1D1 band migrated at ∼150 kDa (Fig. 3C, lane 1). In samples that were immunoprecipitated using anti-TBC1D1 before immunoblotting with anti-PAS, the contraction-responsive PAS band corresponded to PAS-150 (PAS-TBC1D1, Fig. 3C, lane 2), providing supporting evidence that PAS-150 may include PAS-TBC1D1. Furthermore, the supernatant of anti-TBC1D1 immunoprecipitated sample had only a barely detectable PAS-150 signal (Fig. 3D, lane 1) compared with the strong PAS signal in the anti-TBC1D1 immunoprecipitate (PAS-TBC1D1; Fig. 3D, lane 2). These findings provide additional evidence that PAS-TBC1D1 accounts for the PAS-150 band of samples that had not been immunoprecipitated. PAS-150 peaked at 20 min of contraction (half time of ∼10 min), at which time it was significantly greater than resting values. PAS-150 returned to baseline at 60 min (Fig. 4B).
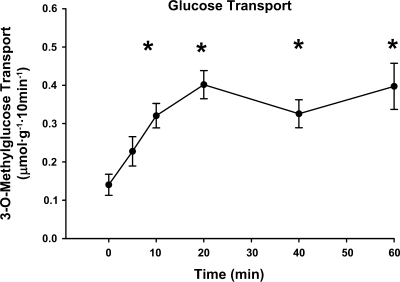
Glucose transport.
Contraction resulted in a rapid (half-time of ∼8 min) and significant increase in glucose transport (10, 20, 40, and 60 min). The peak value occurred at 20 min and plateaued thereafter (Fig. 5).
Fig. 5.
Time course for contraction-stimulated glucose transport in isolated rat epitrochlearis muscles. The values at 0 min are from rested muscles. The rate of glucose transport was measured using 3H-labeled 3-O-methylglucose (3-MG). Values are means ± SE; n = 12–18 per group. Post hoc analysis: *P < 0.05 vs. basal.
Correlations.
Pearson correlation analyses revealed (Table 1) that PAS-160 was significantly (P < 0.01) correlated only with pGSK3 (R = 0.629), pCaMKII (R = 0.724), and PAS-150 (R = 0.776). 3-MG transport was significantly (P < 0.01) correlated only with pAMPK (R = 0.350). Tension was not significantly correlated with 3-MG transport or any of the signaling proteins studied.
Table 1.
R values for Pearson product-moment correlation analyses
| pGSK3 | pAMPK | pCaMKII | PAS-160 | PAS-150 | |
|---|---|---|---|---|---|
| PAS-160 | 0.629* | −0.053 | 0.724* | 0.776* | |
| PAS-150 | 0.660* | 0.044 | 0.666* | 0.776* | |
| Glucose transport | −0.033 | 0.350* | 0.257 | 0.118 | 0.078 |
The R values for Pearson product-moment correlations are indicated in the matrix. Each point on correlations between two signaling proteins is from the same muscle. Each point used for the correlation between a signaling protein and glucose transport is from paired muscles. n = 41–51. pGSK3, phosphorylation of glycogen synthase kinase 3; pAMPK, phosphorylation of AMP-activated protein kinase; pCaMKII, phosphorylation of Ca2+/calmodulin-dependent kinase II; PAS, phospho-Akt substrate.
P < 0.01.
DISCUSSION
The primary aim of this study was to assess the time courses for contractile activity on tension development, phosphorylation of three contraction-stimulated kinases (Akt, AMPK, CaMKII), PAS-160, and glucose transport. In an earlier study, our laboratory found that a brief and discontinuous tetanic stimulation protocol (10-s tetanus duration, 2 tetani/min for 5 min), which induced a large activation of Akt, can also increase AS160 phosphorylation (3). For the present study, we instead used a twitch contraction protocol (2-ms twitch, 120 twitch/min) because 1) discontinuous stimulation with 20-s recovery periods between tetani would complicate the interpretation of a time course analysis; 2) the time course for glucose transport by rat epitrochlearis had previously been characterized using a similar twitch contraction protocol (20); and 3) the twitch vs. tetanic protocol resulted in a slower fatigue rate. This approach revealed some useful new insights regarding contraction effects on skeletal muscle glucose transport, including the following: 1) identification of a contraction-responsive phosphorylated protein band (PAS-150) that appears to correspond with PAS-TBC1D1 and that migrated at a slightly lower apparent molecular mass than AS160; 2) demonstration that a sustained increase in neither PAS-150 nor PAS-160 (which appears to correspond with PAS-AS160) was essential for maintenance of elevated glucose transport with 40 or 60 min of stimulation; and 3) recognition that the values for PAS-150 and PAS-160 from muscles after contraction correlated with each other and with pGSK3 (an Akt substrate) or pCaMKII, but not with pAMPK or glucose transport.
The commercially available PAS antibody was designed to identify unknown Akt substrates by reacting with proteins that are phosphorylated on Akt consensus motifs (RXRXXpS/T). PAS immunoblots prepared using homogenates of rat epitrochlearis muscles had multiple PAS-reactive bands at various molecular masses, including two that were consistently increased for contraction vs. resting samples at an apparent molecular mass of ∼150 kDa and >150 kDa. The band visible above the 150-kDa marker presumably includes AS160, because immunoreactivity against the AS160 antibody corresponds to the same location on immunoblots. Immunoprecipitation using anti-AS160 followed by immunoblotting with PAS also identified an AS160-associated PAS band at the same location (Fig. 3A). Furthermore, the supernatant of sample immunodepleted with the AS160 antibody had a greatly reduced PAS-160 signal (Fig. 3B). The PAS-150 band responded similarly to PAS-160 in response to the contraction protocol, peaking at 20 min and reversing to resting values by 60 min of contraction. During the preparation of this paper, Chavez et al. (6) and Taylor et al. (29) reported that TBC1D1 protein is much more abundant in skeletal muscle than in adipose tissue and that TBC1D1 becomes phosphorylated in response to AICAR (an AMPK activator) or contractile activity. The location on immunoblots for reactivity against the TBC1D1 antibody corresponded to the location of PAS-150 band, suggesting that it includes TBC1D1 (Fig. 3C). Supporting this idea, the supernatant of sample immunodepleted using TBC1D1 antibody had a greatly reduced PAS-150 signal (Fig. 3D). AS160 was apparently not part of the PAS-150 band based on the slower migration of the band visualized using the AS160 antibody and the apparent lack of reduced PAS-150 signal in the AS160-immunodepleted supernatant. Furthermore, the lack of immunoreactivity against the TBC1D1 antibody on immunoblots at the location of PAS-160 and the apparent lack of reduced PAS-160 signal in the TBC1D1-immunodepleted supernatant suggest that TBC1D1 was not part of that protein band.
The reversal of the contraction-stimulated increase in PAS-160 at 40 and 60 min, despite continued electrical stimulation, is in contrast to the sustained increase in PAS-160 that was previously found with 60 min of in vitro insulin stimulation of rat epitrochlearis muscle (3). It is conceivable that the reversal of the increase in PAS-160 was related, at least in part, to muscle fatigue, although the time courses were different for the decrements in contraction-stimulation of PAS-160 and tension development. These results with in vitro contraction were also in contrast to the published results for in vivo exercise, which have indicated that 1) in muscle biopsies taken during in vivo exercise by humans, PAS-160 is increased at 60 and 90 min, but not at 1, 10, or 30 min (31); and 2) after in vivo exercise, increase in PAS-160 is maintained for at least 2.5–4 h after cessation of exercise in rats (1) or humans (13, 28). Even if reversal of PAS-160 is not typical during in vivo exercise, in vitro contraction data may reveal useful clues regarding the regulatory processes that modulate phosphorylation of AS160 and/or TBC1D1.
To begin evaluating the mechanisms that regulate AS160 phosphorylation during contraction, we compared the time courses for contraction effects on several key kinases concomitant with PAS-160 in the same muscles. As hypothesized, contraction resulted in transient enhancement of pGSK3 and pCaMKII, together with a sustained increase in pAMPK. The patterns of contraction effects on pGSK3, pCaMKII, and pAMPK were consistent with previous reports (17, 22, 23, 25, 28, 30). Although it is possible that pAMPK was involved in the initial increase in PAS-160, the reversal of the increase in PAS-160 at 40 and 60 min of stimulation indicates that increased pAMPK was not sufficient for a sustained increase in PAS-160. The significant correlation between pGSK3 (an Akt substrate) and PAS-160 is consistent with the idea that Akt is the primary in vitro contraction-stimulated AS160 kinase in rat epitrochlearis, at least as recognized with the PAS antibody. This result is also in agreement with our demonstration that the phosphatidylinositol 3-phosphate inhibitor wortmannin causes full inhibition of contraction-stimulation of pAkt, PAS-AS160 (3), and PAS-160 (E. B. Arias and G. D. Cartee, unpublished observations) in rat epitrochlearis. Wortmannin does not inhibit the activity of purified AMPK (2) or AMPK phosphorylation in contraction-stimulated cardiomyocytes (35). Nonetheless, kinases other than Akt may be relevant, and phosphorylation may also be occurring on sites not recognized by the PAS antibody. Furthermore, protein phosphorylation reflects the action of both kinases and phosphatases, and currently little is known about the role of phosphatases in the effects of contraction on AS160 phosphorylation. A novel result was the significant correlation between pCaMKII and PAS-160, as well as PAS-150. Both rat AS160 and rat TBC1D1 proteins contain several motifs that are potential sites of CaMKII phosphorylation (scansite.mit.edu). Of these, AS160 Ser597 and TBC1D1 Thr590 are Akt phospho-motifs (RXRXXpS/T), and the remaining CaMKII phospho-sites have arginine at the −3 position (XXRXXpS/T). Therefore, it is conceivable that CaMKII phosphorylates AS160 and TBC1D1 on PAS sites. It is also possible that CaMKII phosphorylates AS160 and/or TBC1D1 on non-PAS sites and thereby affects contraction-stimulated increases in PAS-AS160, PAS-TBC1D1, and/or glucose transport. The relatively high correlation between PAS-160 and PAS-150 suggests that these proteins may share mechanisms that regulate the phosphorylation on PAS sites (kinases and/or phosphatases).
The only statistically significant correlation that was found between a signaling protein and glucose transport was the modest relationship between pAMPK and glucose transport. This association supports a great deal of previous evidence that AMPK is involved in contraction-stimulated glucose transport (5, 12, 18). In contrast, a persistent increase in pGSK3 or pCaMKII was not required for a sustained increase in glucose transport. The pGSK3 data are not surprising, because many studies have indicated that Akt is not essential for contraction-stimulated glucose transport. However, some studies using inhibitors have suggested that CaMKII may play a role in a portion of contraction-mediated glucose transport (33, 34). These earlier studies used relatively brief periods of contraction, and their findings are consistent with the current data with regard to an activation of pCaMKII during the initial minutes of contraction. Different processes may be required for initiation compared with the maintenance of elevated glucose transport.
Compelling evidence indicates that a contraction-induced increase in PAS-160 is not essential for increased muscle glucose transport, including 1) the complete inhibition of contraction-stimulated PAS-AS160 in isolated rat epitrochlearis muscle (3) by concentrations of wortmannin, which have no effect on contraction-stimulated glucose transport in isolated muscle (16, 36); and 2) the failure for in vitro contraction to elevate PAS-160 in muscles from α2-AMPK null mice (32), even though contraction-stimulated glucose uptake is not attenuated in the null compared with wild-type control mice (14). Furthermore, PAS-AS160 can remain elevated above basal levels for several hours after the cessation of in vivo exercise by rats (1), despite reversal of exercise-stimulated increase in insulin-independent glucose transport, indicating that elevated PAS-AS160 alone is not sufficient to increase glucose transport. A new finding that is consistent with these earlier studies was that sustained contraction resulted in temporal uncoupling of PAS-160 from contraction-stimulated glucose transport. Importantly, the current data demonstrate that sustained increase in PAS-150 (apparently TBC1D1) was also not required for maintained increase in contraction-stimulated glucose transport at 40 or 60 min.
The PAS antibody appears to have differential immunoreactivity with AS160 phosphorylated on some Akt phospho-motifs relative to others (26), and PAS immunoreactivity would presumably not be a sensitive indicator of increased phosphorylation of AS160 or TBC1D1 on phospho-motifs for other kinases. Furthermore, there is evidence that AS160 may modulate contraction-stimulated glucose transport by a mechanism related to its calcium/calmodulin binding domain (15), so the current findings do not preclude roles for AS160 in regulating contraction-stimulated glucose transport. It remains to be determined if TBC1D1 plays a role in contraction-stimulated glucose transport.
In conclusion, the present data using an in vitro twitch contraction protocol with rat epitrochlearis muscle demonstrate that 1) PAS-160 (apparently AS160) and PAS-150 (apparently TBC1D1) both transiently respond to a sustained stimulation protocol; 2) continual activation of AMPK was not sufficient for sustained increase in PAS-160 or PAS-150; 3) temporal relationships suggest that Akt and possibly CaMKII may be involved in the contraction-stimulated increase in PAS-160 and/or PAS-150; and 4) sustained elevation of PAS-160 or PAS-150 is not necessary for contraction-stimulated glucose transport. Other approaches will be essential to clarify the mechanisms whereby contraction regulates AS160 and/or TBC1D1 function and to reveal the specific roles of these Rab GAP proteins in the initiation, maintenance, and reversal of contraction-stimulated glucose transport.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-071771 (to G. D. Cartee).
Acknowledgments
We thank David R. Blair for assistance in tension measurements.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab 292: E1191–E1200, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J 408: 297–315, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes 54: 41–50, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Cartee GD, Bohn EE. Growth hormone reduces glucose transport but not GLUT-1 or GLUT-4 in adult and old rats. Am J Physiol Endocrinol Metab 268: E902–E909, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Cartee GD, Wojtaszewski JF. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab 32: 557–566, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Chavez JA, Roach WG, Keller SR, Lane WS, Lienhard GE. Inhibition of GLUT4 translocation by Tbc1d1, a Rab GTPase-activating protein abundant in skeletal muscle, is partially relieved by AMP-activated protein kinase activation. J Biol Chem 283: 9187–9195, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Constable SH, Favier RJ, Cartee GD, Young DA, Holloszy JO. Muscle glucose transport: interactions of in vitro contractions, insulin, and exercise. J Appl Physiol 64: 2329–2332, 1988. [DOI] [PubMed] [Google Scholar]
- 8.Douen AG, Ramlal T, Rastogi S, Bilan PJ, Cartee GD, Vranic M, Holloszy JO, Klip A. Exercise induces recruitment of the “insulin-responsive glucose transporter”. Evidence for distinct intracellular insulin- and exercise-recruitable transporter pools in skeletal muscle. J Biol Chem 265: 13427–13430, 1990. [PubMed] [Google Scholar]
- 9.Dumke CL, Kim J, Arias EB, Cartee GD. Role of kallikrein-kininogen system in insulin-stimulated glucose transport after muscle contractions. J Appl Physiol 92: 657–664, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez E, McGraw TE. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol Biol Cell 17: 4484–4493, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimditch GK, Barnard RJ, Kaplan SA, Sternlicht E. Insulin binding and glucose transport in rat skeletal muscle sarcolemmal vesicles. Am J Physiol Endocrinol Metab 249: E398–E408, 1985. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 47: 1369–1373, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Howlett KF, Mathews A, Garnham A, Sakamoto K. The effect of exercise and insulin on AS160 phosphorylation and 14-3-3 binding capacity in human skeletal muscle. Am J Physiol Endocrinol Metab 294: E401–E407, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside but not contraction-induced glucose uptake in skeletal muscle. J Biol Chem 279: 1070–1079, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Kramer HF, Taylor EB, Witczak CA, Fujii N, Hirshman MF, Goodyear LJ. Calmodulin-binding domain of AS160 regulates contraction- but not insulin-stimulated glucose uptake in skeletal muscle. Diabetes 56: 2854–2862, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Lee AD, Hansen PA, Holloszy JO. Wortmannin inhibits insulin-stimulated but not contraction-stimulated glucose transport activity in skeletal muscle. FEBS Lett 361: 51–54, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Markuns JF, Wojtaszewski JF, Goodyear LJ. Insulin and exercise decrease glycogen synthase kinase-3 activity by different mechanisms in rat skeletal muscle. J Biol Chem 274: 24896–24900, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Mu J, Brozinick JT Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7: 1085–1094, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Nesher R, Karl IE, Kipnis DM. Dissociation of effects of insulin and contraction on glucose transport in rat epitrochlearis muscle. Am J Physiol Cell Physiol 249: C226–C232, 1985. [DOI] [PubMed] [Google Scholar]
- 20.Nesher R, Karl IE, Kipnis DM. Epitrochlearis muscle. II. Metabolic effects of contraction and catecholamines. Am J Physiol Endocrinol Metab 239: E461–E467, 1980. [DOI] [PubMed] [Google Scholar]
- 21.Roach WG, Chavez JA, Miinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J 403: 353–358, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose AJ, Alsted TJ, Kobbero JB, Richter EA. Regulation and function of Ca2+-calmodulin-dependent protein kinase II of fast-twitch rat skeletal muscle. J Physiol 580: 993–1005, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose AJ, Kiens B, Richter EA. Ca2+-calmodulin-dependent protein kinase expression and signaling in skeletal muscle during exercise. J Physiol 574: 889–903, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakamoto K, Arnolds DE, Fujii N, Kramer HF, Hirshman MF, Goodyear LJ. Role of Akt2 in contraction-stimulated cell signaling and glucose uptake in skeletal muscle. Am J Physiol Endocrinol Metab 291: E1031–E1037, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Sakamoto K, Hirshman MF, Aschenbach WG, Goodyear LJ. Contraction regulation of Akt in rat skeletal muscle. J Biol Chem 277: 11910–11917, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278: 14599–14602, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–85, 1985. [DOI] [PubMed] [Google Scholar]
- 28.Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, Eagan PA, Jenkinson CP, Cersosimo E, DeFronzo RA, Sakamoto K, Musi N. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes 56: 836–848, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, Bowles N, Hirshman MF, Xie J, Feener EP, Goodyear LJ. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem 283: 9787–9796, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toyoda T, Tanaka S, Ebihara K, Masuzaki H, Hosoda K, Sato K, Fushiki T, Nakao K, Hayashi T. Low-intensity contraction activates the alpha1-isoform of 5′-AMP-activated protein kinase in rat skeletal muscle. Am J Physiol Endocrinol Metab 290: E583–E590, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Treebak JT, Birk JB, Rose AJ, Kiens B, Richter EA, Wojtaszewski JF. AS160 phosphorylation is associated with activation of α2β2γ1- but not α2β2γ 3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab 292: E715–E722, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jorgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes 55: 2051–2058, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Wright DC, Geiger PC, Holloszy JO, Han DH. Contraction- and hypoxia-stimulated glucose transport is mediated by a Ca2+-dependent mechanism in slow-twitch rat soleus muscle. Am J Physiol Endocrinol Metab 288: E1062–E1066, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Wright DC, Hucker KA, Holloszy JO, Han DH. Ca(2+) and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes 53: 330–335, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Holman GD. Insulin and contraction stimulate exocytosis, but increased AMP-activated protein kinase activity resulting from oxidative metabolism stress slows endocytosis of GLUT4 in cardiomyocytes. J Biol Chem 280: 4070–4078, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Yeh JI, Gulve EA, Rameh L, Birnbaum MJ. The effects of wortmannin on rat skeletal muscle. Dissociation of signaling pathways for insulin- and contraction-activated hexose transport. J Biol Chem 270: 2107–2111, 1995. [DOI] [PubMed] [Google Scholar]