Abstract
Akt substrate of 160 kDa (AS160/TBC1D4) is associated with insulin and contraction-mediated glucose uptake. Human skeletal muscle AS160 phosphorylation is increased during aerobic exercise but not immediately following resistance exercise. It is not known whether AS160 phosphorylation is altered during recovery from resistance exercise. Therefore, we hypothesized that muscle AS160/TBC1D4 phosphorylation and glucose uptake across the leg would be increased during recovery following resistance exercise. We studied 9 male subjects before, during, and for 2 h of postexercise recovery. We utilized femoral catheterizations and muscle biopsies in combination with indirect calorimetry and immunoblotting to determine whole body glucose and fat oxidation, leg glucose uptake, muscle AMPKα2 activity, and the phosphorylation of muscle Akt and AS160/TBC1D4. Glucose oxidation was reduced while fat oxidation increased (∼35%) during postexercise recovery (P ≤ 0.05). Glucose uptake increased during exercise and postexercise recovery (P ≤ 0.05). Akt phosphorylation was increased at 1 h and AMPKα2 activity increased at 2 h postexercise (P ≤ 0.05). Phospho(Ser/Thr)-Akt substrate (PAS) phosphorylation (often used as a marker for AS160) was unchanged immediately postexercise and increased at 1 h (P ≤ 0.05) and 2 h postexercise (P = 0.07). The PAS antibody is not always specific for AS160/TBC1D4 and can detect proteins at a similar molecular weight. Therefore, we immunoprecipitated AS160/TBC1D4 and then blotted with the PAS antibody, which confirmed that PAS phosphorylation is occurring on AS160/TBC1D4. There was also a positive correlation between PAS phosphorylation and leg glucose uptake during recovery (P < 0.05). We conclude that resistance exercise increases AS160/TBC1D4 phosphorylation in association with an increase in leg glucose uptake during postexercise recovery.
Keywords: AMP-activated protein kinase, fat oxidation, GLUT4, Akt
akt substrate of 160 kDa (AS160/TBC1D4) is associated with the regulation of glucose uptake in both adipocytes and skeletal muscle (4, 16, 18, 27). It has been suggested that AS160 may serve as a converging point for both insulin-dependent and insulin-independent (muscle contraction) signaling pathways on GLUT4 translocation and subsequent glucose uptake into skeletal muscle (5). For example, insulin signaling has been shown to lead to increased AS160 phosphorylation via the phosphoinositide 3-kinase (PI3K)-Akt pathway in both rat (2, 4) and human skeletal muscle (6, 19). The phosphorylation of AS160 by insulin appears to be mediated by Akt since incubation of muscle with wortmannin, use of Akt2 null mice, or suppression of IRS-1 or Akt2 prevents insulin's ability to phosphorylate AS160 (3, 4, 20). On the other hand, muscle contraction-induced phosphorylation of AS160 in isolated rat epitrochlearis muscle is inhibited by wortmannin (4), whereas wortmannin only partially inhibits the contraction-induced increase in AS160 phosphorylation in isolated mouse extensor digitorum longus muscle (20). AMP-activated protein kinase (AMPK), an important regulator of contraction-mediated GLUT4 translocation (10, 33), was first suggested as the kinase responsible for phosphorylating AS160 in skeletal muscle when it was shown that both muscle contraction and AICAR (an AMPK activator) incubation in isolated rat skeletal muscle increased AS160 phosphorylation (4). Subsequent studies inhibited AMPK (via AMPKα2 knockout or α2-AMPK kinase dead mice) and found that contraction-induced AS160 phosphorylation is significantly reduced; however, elimination of AMPK activity does not completely abolish all AS160 phosphorylation following contraction (20). Interestingly, concurrent phosphorylation of Akt and AMPK activation induced an additive increase in AS160 phosphorylation (31). Taken together the data suggest that Akt and AMPK participation in AS160 phosphorylation may be dependent on the type of exercise or muscle contraction stimulus and also that results may be species specific.
Exercise in human subjects also increases muscle AS160 phosphorylation immediately following 60 or 90 min of moderate-intensity cycling exercise (30) but not after 1, 10, or 30 min of moderate-intensity cycling exercise (30). Only a few studies have looked at the effects of resistance exercise in humans, and those studies found that AS160 phosphorylation did not increase when measured immediately following exercise (6, 14). Currently, it is not known whether AS160 phosphorylation is altered during recovery from resistance exercise in human skeletal muscle. The previous resistance exercise studies used the phospho(Ser/Thr)-Akt substrate (PAS) antibody as a marker of AS160/TBC1D4 phosphorylation. However, recently it has been shown that the PAS antibody also interacts with a novel protein referred to as TBC1D1 (29). Therefore, it is also important to determine whether changes detected using the PAS antibody reflect changes in AS160/TBC1D4 phosphorylation in human skeletal muscle.
We have previously shown that AMPK activity is elevated during postexercise recovery in rats concurrently with an increase in fat oxidation (24). In addition to stimulating fat oxidation, AMPK activation during postexercise recovery may also play a role in restoring muscle glycogen levels by promoting GLUT4 translocation (34). For example, glucose transport and glycogen repletion in skeletal muscle are increased during the recovery period following aerobic exercise in both rodents and humans (1, 15, 21–23). Resistance exercise also decreases glycogen stores in human muscle, which are gradually replenished during recovery (15, 21–23). AMPK may facilitate muscle glycogen repletion by phosphorylating AS160 during recovery (increased glucose transport into muscle cells) and by stimulating fat oxidation to provide the necessary energy to sustain the ATP-consuming process of glycogen synthesis. Therefore, we hypothesized that muscle AS160/TBC1D4 phosphorylation, glucose uptake across the leg, and fat oxidation would be elevated during recovery following a bout of resistance exercise in young men.
MATERIALS AND METHODS
Subjects.
We studied nine young men. All subjects were healthy and physically active but were not currently engaged in an exercise training program. All subjects gave informed written consent before participating in the study, which was approved by the Institutional Review Board of the University of Texas Medical Branch (which is in compliance with the Declaration of Helsinki). Screening of subjects was performed with clinical history, physical exam, and laboratory tests, including complete blood count with differential, liver and kidney function tests, coagulation profile, fasting blood glucose and oral glucose tolerance test (OGTT), hepatitis B and C screening, HIV test, thyroid-stimulating hormone (TSH), lipid profile, urinalysis, drug screening, and ECG. Subject characteristics are summarized in Table 1.
Table 1.
Physical characteristics of the subjects
| Value | |
|---|---|
| Age, yr | 27±2 |
| Height, cm | 176±3 |
| Weight, kg | 78±5 |
| Body mass index, kg/m2 | 25.2±1.4 |
| Lean body mass, kg | 61±3 |
| Body fat, % | 19±2 |
| Leg lean mass, kg | 10.4±0.7 |
Values are means ± SE; n = 9.
Study design.
Details of the study design have previously been published (8). Seven of the nine subjects were included in a previous publication that presented AMPK, Akt, and glucose uptake data (8). On two separate occasions (>5 days apart) and more than 5 days before conducting the study, each subject was tested for muscle strength by measuring their 1 repetition maximum (1RM) on a leg extension machine (Cybex-VR2, Medway, MA) located within the General Clinical Research Center (GCRC) Exercise Laboratory. The higher of the two 1RM values obtained was used to determine the starting weight (70% of 1RM) for the resistance exercise portion of our study. On the second visit a dual-energy X-ray absorptiometry (DEXA) scan (Hologic QDR 4500W, Bedford, MA) was performed to measure body composition and lean mass.
Each subject was admitted to the GCRC of the University of Texas Medical Branch the day before the exercise study. The subjects were then fed a standard dinner, and a snack was given at 2200. The subjects were studied following an overnight fast under basal conditions and refrained from exercise for 24 h before study participation. The subjects were all fed a standardized meal (12 kcal/kg body wt; 60% carbohydrate, 20% fat, and 20% protein) prepared by the Bionutrition Division of the GCRC. The morning of the study, polyethylene catheters were inserted into a forearm vein, in the contralateral hand vein, which was heated for arterialized blood sampling, and in the femoral artery and vein (retrograde placement) of the leg for blood sampling. The femoral lines were placed in the same leg from which muscle biopsies were obtained. The arterial catheter was also used for the infusion of indocyanine green (ICG, Akorn, Buffalo Grove, IL) to determine blood flow.
Subjects were studied during four time periods: first period (basal), second period (exercise), third period (the first hour postexercise; 1 h Post), and fourth period (the second hour postexercise; 2 h Post). The second period was performed in the exercise lab within the GCRC, and the first, third, and fourth periods were all conducted in the special procedures room, also within the GCRC.
Marking the beginning of the basal period, and 2 h following study initiation, the first muscle biopsy was obtained from the lateral portion of the vastus lateralis of the leg with the biopsy site between 15 and 25 cm from the midpatella. The biopsy was performed using a 5-mm Bergström biopsy needle, under sterile procedure and local anesthesia (1% lidocaine). The muscle sample was immediately blotted and frozen in liquid nitrogen (within seconds) and stored at −80°C until analysis. Immediately after the first biopsy, continuous breath analysis (indirect calorimetry) was begun to measure inspired O2 and expired CO2 for O2 uptake (V̇o2) and CO2 production (V̇co2) determination. At the same time a continuous infusion of indocyanine green (ICG) was started in the femoral artery (0.5 mg/min) and maintained for 50 min for the purpose of measuring leg blood flow. Ten minutes after ICG infusion was started, blood samples were drawn 4 times, at 10-min intervals, from the femoral vein and the arterialized hand vein to measure ICG concentration (Fig. 1). In addition to the blood obtained for ICG measurement, blood samples were also taken from the femoral artery and vein and from the arterialized hand vein to measure insulin and glucose concentrations. At the end of the basal period, a second muscle biopsy was obtained; however, the biopsy needle was inclined at a different angle so that the second biopsy was taken ∼5 cm apart from the first. This second biopsy was used to measure the basal rate of muscle protein synthesis from our previous publication (8). In the present study, all baseline data reported in the results section were obtained from the first basal muscle biopsy.
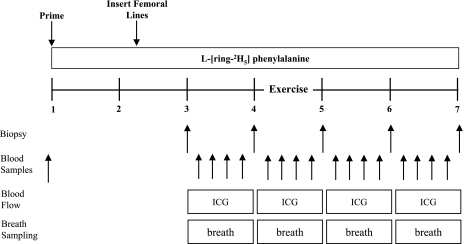
Fig. 1.
Study design and timeline in hours. Schematic displaying the study design used to measure the effect of resistance exercise on the regulation of whole body fat and glucose oxidation, glucose uptake, and cell signaling in male subjects. The study design consisted of a baseline, exercise, 1-h postexercise, and 2-h postexercise period. Continuous breath sampling was obtained with a Sensor Medics Vmax series metabolic cart. Indocyanine green (ICG) was infused to measure blood flow during each period. Blood samples were collected to measure plasma glucose and insulin and blood flow and to calculate glucose uptake. Muscle biopsies were used to measure muscle cell signaling, specifically components upstream and partially responsible for the activation of Akt substrate of 160 kDa (AS160).
Following the second biopsy, the subject was transported to the exercise lab within the GCRC for the entire second period. The subjects performed 10 sets of 10 repetitions of leg extension exercises on a Cybex leg extension machine (Cybex International, Medway, MA) set to 70% of their 1RM. All subjects started out at 70% of 1RM; however, for a few of the subjects the weight was slightly reduced (60–65% of 1RM) to achieve 10 repetitions per set. The rest period between sets was 3 min, except during blood collection, which required a few more minutes. As during the basal period, indirect calorimetry was performed and ICG was infused. Blood samples were collected immediately after the third, sixth, eighth, and tenth sets. Following the last blood collection, subjects performed one final set of 10 repetitions and a third muscle biopsy was obtained through the same incision within seconds of completing the last muscle contraction. The subjects were then transported back to the special procedures room of the GCRC for the duration of the study.
During the third period (the first hour postexercise), ICG was again infused continuously (as during the first and second periods) to measure leg blood flow, and blood was drawn for the measurement of insulin and glucose concentrations. Samples were obtained every 10 min (as during the first and second periods). At the end of the first hour postexercise, a fourth muscle biopsy was obtained through a new incision site ∼5 cm from the first incision.
During the fourth period (second hour postexercise), blood samples were collected in the same manner as during the previous periods. At the end of the second hour postexercise, a final muscle biopsy was collected as described above from the second incision; however, the biopsy needle was inclined at a different angle again so that the biopsy was taken ∼5 cm apart from the prior biopsy.
Indirect calorimetry.
During each period of the study, V̇o2, V̇co2, and the respiratory quotient (RQ) were obtained by continuous breath sample analysis with a metabolic cart (Sensor Medics, Yorba Linda, CA). Whole body fat and carbohydrate oxidation were calculated from these data using established equations (35).
Blood flow, serum lactate, glucose, glucose uptake across the leg, and insulin.
Serum ICG concentration for the determination of leg blood flow was measured spectrophotometrically (Beckman Coulter, Fullerton, CA) at λ = 805 nm (17). Plasma lactate and glucose concentration were measured using an automated glucose analyzer (YSI, Yellow Springs, OH). Leg glucose utilization was calculated as net glucose uptake across the leg:
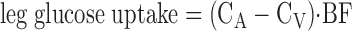 |
where BF is blood flow and CA and CV are the blood glucose concentrations in the femoral artery and vein, respectively, and glucose utilization is expressed as micromoles of glucose utilized per minute per kilogram of fat free mass (FFM) of the leg (μmol·min−1·kg leg FFM−1). Plasma insulin concentrations were determined by ELISA (Linco Research, St. Charles, MO).
AMPK activity assay.
Specific details of the AMPKα2 activity assay have been previously described (8). Activity is expressed in picomoles of phosphate incorporated per milligram of muscle protein subjected to immunoprecipitation per minute (pmol·mg−1·min−1).
SDS PAGE and immunoblotting.
Specific details of the immunoblotting procedures have been previously described (7) with slight modifications for the present paper. Aliquots from homogenates were loaded (equal amount of protein per lane) in duplicate and separated by SDS-PAGE. All proteins were run on 7.5% gels (Bio-Rad, Hercules, CA) for 60 min at 150 V. Following SDS-PAGE, proteins were transferred to polyvinylidene diflouride membranes (PVDF) (Hybond-P, Amersham Biosciences, Piscataway, NJ) at 50 V for 1 h. We confirmed equal loading on each gel and that an equivalent amount of protein was transferred to the membrane by Coomassie and/or Ponceau S staining. Once transferred, PVDF membranes were placed in blocking buffer [5% nonfat dry milk (NFDM) in Tris-buffered saline-0.1% Tween-20 (TBST)] for 1 h. Following serial washes the membranes were incubated with primary antibody in 5% NFDM in TBST overnight at 4°C with constant agitation. The next morning, the blots were washed in TBST twice and incubated with secondary antibody for 1 h in 5% NFDM in TBST at room temperature with constant agitation. After serial washes the blots were then incubated for 5 min with enhanced chemiluminescence reagent (ECL plus Western Blotting Detection System, Amersham Biosciences, Piscataway, NJ) to detect horseradish peroxidase activity. Images were obtained with a ChemiDoc XRS imaging system (Bio-Rad). Once the appropriate image was captured, densitometric analysis was performed using Quantity One 1-D analysis Software (version 4.5.2) (Bio-Rad). All data for each band minus a representative background sample from the membrane were normalized to an internal loading control.
AS160 /TBC1D4 immunoprecipitation.
To verify the specificity of the PAS antibody, we performed the following immunoprecipitation assay. Frozen muscle was homogenized in CHAPS buffer [40 mM HEPES (pH 7.5), 120 mM β-glycerolphosphate, 40 mM NaF, 1.5 mM sodium vanadate, 0.3% CHAPS, 0.1 mM PMSF, 1 mM benzamadine, and 1 mM DTT], and the homogenate was rocked for 20 min at 4°C and then centrifuged at 1,000 g for 3 min at 4°C. To an aliquot of supernatant containing 300 μg of protein, we added 1 μl of AS160 (Rab GAP) antibody [gene symbol TBC1D4 (no. 07–741, Upstate Cell Signaling Solutions, Charlottesville, VA)], and this protein-antibody complex was mixed by rocking overnight at 4°C. The protein-antibody complex was isolated by adding BioMag goat anti-rabbit IgG (Qiagen) bead slurry and rocked for 1 h at 4°C. Just before using the BioMag beads, they were washed twice with CHAPS buffer without protease inhibitors, and one-half of the volume was resuspended in CHAPS buffer with 0.1% NFDM and rocked for 1 h at 4°C. Following incubation the bead-antibody-protein complex was isolated and collected by utilizing a magnetic stand (Qiagen) washed twice with CHAPS buffer with protease inhibitors and once in CHAPS buffer containing 150 mM of NaCl and 50 mM of HEPES. The bead-antibody-protein complex was eluted with 2× sample buffer, boiled for 5 min at 100°C, and separated with SDS PAGE using a 7.5% gel. Blots were probed using the PAS antibody to determine whether the increase in PAS phosphorylation was occurring on AS160/TBC1D4.
Antibodies.
The following primary antibodies used were purchased from Cell Signaling (Beverly, MA): phospho-Akt (Ser473; 1:500); phospho-ACC (Ser79; 1:500; this antibody detects both the ACC1 and ACC2 isoforms; however, ACC2 is primarily expressed in skeletal muscle and the phospho-site in human muscle is Ser221, as shown in Fig. 4); total Akt (1:1,000); total ACC (1:1,000); phospho-AMPKα (Thr172; 1:1,000); and PAS antibody (1:1,000). Total AS160/TBC1D4 (1:1,000) antibody was purchased from Upstate Cell Signaling Solutions (Lake Placid, NY). Anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody was purchased from Amersham Bioscience (1:2,000).
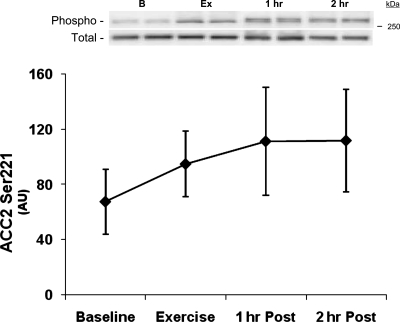
Fig. 4.
ACC phosphorylation. Data are from each biopsy taken at baseline, immediately following resistance exercise (Exercise), 1 h following exercise, and 2 h following resistance exercise. Data are expressed as means ± SE. *P ≤ 0.05 vs. baseline (n = 7). Insets: representative blots for each time point: baseline (B), exercise (Ex), 1 h postexercise (1 h), and 2 h postexercise (2 h).
Statistical analysis.
All values are expressed as means ± SE. Comparisons were performed using ANOVA with repeated measures, the effects being subject and time (basal, exercise, 1 h postexercise, and 2 h postexercise). The exercise data from indirect calorimetry were not used because the RQ was consistently over 1, which invalidates the substrate oxidation calculations during this time period. Variables tended to have SDs that were proportional to the means (which causes equality tests to fail), so we ran statistical tests on the natural log of each outcome variable, which resulted in equality tests passing for all outcome variables. Post hoc testing was performed using Bonferroni's test for multiple comparisons. Power calculations were performed for variables with significance ≤0.20 and >0.05. Pearson product-moment correlations were run on variables of interest (glucose uptake and PAS phosphorylation). Significance was set at P ≤ 0.05.
RESULTS
Whole body RQ, glucose, and fat oxidation.
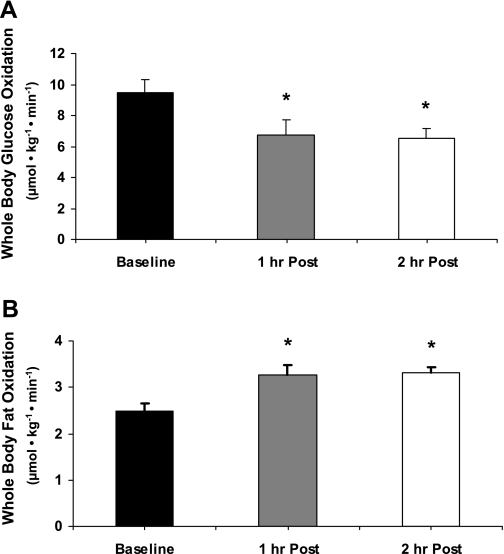
Whole body basal RQ was 0.84 ± 0.01 and decreased during the 1 and 2 h postexercise. Whole body glucose oxidation rates decreased during the 1- to 2-h postexercise recovery period (P ≤ 0.05 vs. baseline; Fig. 2A). Fat oxidation increased during the 1–2 h of postexercise recovery (P ≤ 0.05 vs. baseline; Fig. 2B).
Fig. 2.
Whole body glucose oxidation and postexercise fat oxidation. Breath samples were continuously obtained with the Vmax series metabolic cart during each period of the study. Whole body glucose and fat oxidation during exercise are not reported due to a respiratory quotient (RQ) being greater than 1. A: whole body glucose oxidation. B: whole body fat oxidation. Data are expressed as means ± SE. 1 h Post, 1 h postexercise; 2 h Post, 2 h postexercise. *P ≤ 0.05 vs. baseline.
Blood flow, glucose uptake across the leg, glucose, and insulin.
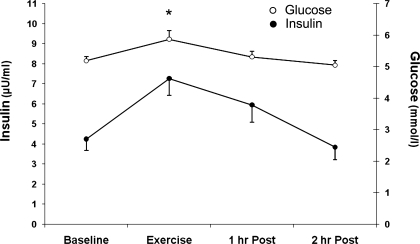
Blood flow was significantly increased during exercise (baseline, 3.81 ± 0.41; exercise, 14.65 ± 1.42 ml·min−1·100 ml leg volume−1; P ≤ 0.05 vs. baseline). Blood flow values during the 1–2 h of postexercise recovery were not different from basal values (1 h Post, 5.43 ± 0.96; 2 h Post, 4.48 ± 0.69 ml·min−1·100 ml leg volume−1; P > 0.05 vs. baseline). Glucose uptake increased during exercise and remained elevated relative to baseline (baseline, 3.50 ± 0.67; exercise, 23.75 ± 4.08; 1 h Post, 9.93 ± 2.38; 2 h Post, 9.46 ± 2.42 μmol·min−1·FFM−1; P ≤ 0.05 vs. baseline;). Glucose uptake remained elevated during the first hour (P ≤ 0.05 vs. baseline) and second hour (P = 0.057 vs. baseline) (power = 0.47) of postexercise recovery. Serum glucose concentration increased during exercise (P ≤ 0.05 vs. baseline) and returned to baseline values during postexercise recovery (P > 0.05 vs. baseline; Fig. 3). Serum insulin concentration were not different during exercise or the 1–2 h of postexercise recovery (P > 0.05 vs. baseline; Fig. 3).
Fig. 3.
Blood insulin and glucose concentrations. Blood samples were obtained 4 times at regular intervals (approximately every 10 min.) during each period of the study: baseline, exercise, 1 h postexercise, and 2 h postexercise. Venous insulin and glucose concentrations. Data are expressed as means ± SE. *P ≤ 0.05 vs. baseline.
AMPKα2 activity and ACC phosphorylation.
AMPKα2 activity tended to increase immediately and at 1 h postexercise (P = 0.16) (power = 0.26) but did not reach significance (baseline, 1.70 ± 0.40; exercise, 2.54 ± 0.57; 1 h Post, 2.77 ± 0.058 pmol·min−1·mg protein−1; P > 0.05 vs. baseline). However, AMPKα2 activity was significantly elevated at 2 h postexercise (2 h Post, 2.60 ± 0.43 pmol·min−1·mg protein−1; P ≤ 0.05 vs. baseline). AMPKα phosphorylation (n = 6) was not significantly elevated at any time point (baseline, 0.35 ± 0.10; exercise, 0.29 ± 0.06; 1 h Post, 0.46 ± 0.11; 2 h Post, 0.37 ± 0.10 AU; P > 0.05 vs. baseline) (power = 0.18). ACC2 Ser221 phosphorylation also tended to increase at 1–2 h postexercise (P = 0.19 vs. baseline; Fig. 4) (power = 0.23).
Akt, PAS, and AS160/TBC1D4 phosphorylation.
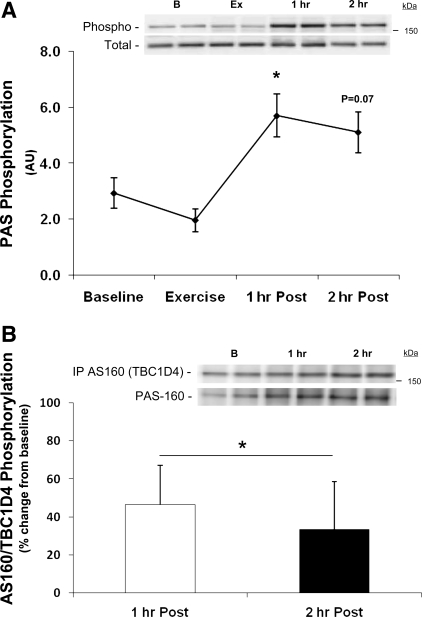
The phosphorylation of Akt at Ser473 was increased only at 1 h postexercise (baseline, 30 ± 8; 1 h Post, 68 ± 19 AU; P ≤ 0.05). PAS phosphorylation was unchanged during exercise (P > 0.05 vs. baseline; Fig. 5A). However, PAS phosphorylation was increased at 1 and 2 h of postexercise recovery (P ≤ 0.05 vs. baseline at 1 h Post and P = 0.07 vs. baseline at 2 h Post; Fig. 5A) (power for 2 h Post = 0.43). The specificity of the PAS antibody was verified by immunoprecipitating samples (n = 6) with anti-AS160/TBC1D4 using methods adopted from Ref. 32. Following immunoprecipitation, membranes were blotted with the PAS antibody to determine AS160/TBC1D4 phosphorylation. AS160/TBC1D4 phosphorylation increased during postexercise recovery (time effect P ≤ 0.05; Fig. 5B).
Fig. 5.
Phospho(Ser/Thr)-Akt substrate (PAS) phosphorylation (A) and immunoprecipitation of AS160/TBC1D4 (B). A: data are from each biopsy taken at baseline, immediately following resistance exercise, 1 h following exercise, and 2 h following resistance exercise for PAS160 phosphorylation. *P ≤ 0.05 vs. baseline; n = 7. B: AS160/TBC1D4 was first immunoprecipitated and then blotted with the PAS antibody. Data are expressed as means ± SE. *P ≤ 0.05 time effect; n = 6. Insets: representative blots for each time point [baseline (B), exercise (Ex), 1 h postexercise (1 h), and 2 h postexercise (2 h)].
Correlation between glucose uptake across the leg and PAS phosphorylation.
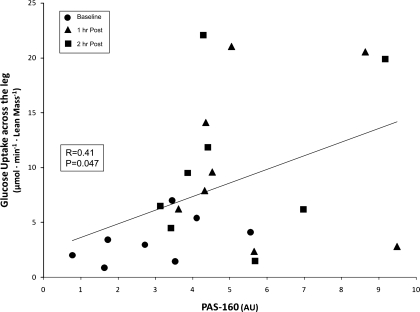
PAS phosphorylation was positively correlated with glucose uptake across the leg (R = 0.41; P ≤ 0.05; Fig. 6) during the 2 h of postexercise recovery. Data from baseline and 1 and 2 h postexercise were included in the analysis.
Fig. 6.
Correlation of PAS phosphorylation and glucose uptake across the leg. Data points from baseline and 1 h and 2 h post-resistance exercise are included in the Pearson product-moment correlation (24 data points). PAS phosphorylation was positively correlated with glucose uptake across the leg (R = 0.41; P ≤ 0.05).
DISCUSSION
The primary and novel finding from this study is that a bout of resistance exercise increased human skeletal muscle AS160/TBC1D4 phosphorylation in association with an elevated glucose uptake across the leg during postexercise recovery. In addition, muscle AMPKα2 activity and whole body fat oxidation also increased during postexercise recovery. We propose that the increase in glucose uptake across the leg during postexercise recovery may be due to AMPKα2 phosphorylation of AS160. Further, the stimulation of fat oxidation following resistance exercise may also be due to AMPK activation. However, Akt phosphorylation was also increased at 1 h postexercise, and thus we cannot exclude a role for Akt in enhancing AS160/TBC1D4 phosphorylation during postexercise recovery.
Several studies in human subjects (6, 13, 28) have demonstrated that muscle AS160 phosphorylation is significantly elevated immediately following a single 60-min bout of aerobic exercise, which may remain elevated for up to 15 h following the last bout of endurance training exercise (9). However, only two studies (6, 14) have measured muscle AS160 phosphorylation following a bout of resistance exercise. Of those studies, Deshmukh et al. (6) found no change in AS160 phosphorylation immediately following a bout of resistance exercise while Howlett et al. (14) showed a significant decline. In the present study, we found that muscle PAS phosphorylation tended to decrease immediately following exercise and then increased during the first couple of hours of recovery. A key difference between these two previous human studies and the present study is that we examined AS160/TBC1D4 phosphorylation during postexercise recovery. As such, our results are unique in that they clearly show that PAS (specific for AS160/TBC1D4) phosphorylation is elevated during postexercise recovery.
In the present study we put forward the notion that glucose uptake in the first few hours following a bout of resistance is associated with an increase in AS160/TBC1D4 phosphorylation. However, our findings may also be relevant for enhanced insulin-stimulated glucose transport that is commonly seen following acute exercise bouts (2). Future work is needed to determine whether enhanced insulin-stimulated glucose uptake following resistance exercise is also associated with changes in AS160/TBC1D4 phosphorylation.
Leg glucose uptake was significantly elevated during the resistance exercise bout while PAS phosphorylation was unaltered at the end of exercise. This may appear to contradict our conclusion suggesting that signaling via AS160/TBC1D4 is responsible, in part, for the elevated glucose uptake during recovery. However, the increase in glucose uptake during exercise seems to be dependent on the large increase in blood flow and muscle perfusion. We suspect that the large increase in glucose delivery to the muscle cell likely led to an increase in glucose uptake via a mass-action type effect; however, an increase in GLUT4 translocation may also be playing a role “during” resistance exercise. During the postexercise recovery period, however, blood flow was not different from baseline values, and glucose uptake was increased in association with increased PAS phosphorylation (see Fig. 6).
A portion of the data included in this manuscript has been previously published (8), and findings from that study showed that glucose uptake was not significantly elevated during post-resistance exercise recovery. However, that study included four female subjects who may have introduced bias with regard to glucose uptake across the leg as recent findings suggest that there may be a sex-based difference in glucose kinetics during post-resistance exercise recovery (Dreyer and Rasmussen, unpublished observations). Furthermore, the post-resistance exercise recovery glucose uptake values from that study (8), which included females, were elevated above baseline to similar levels but did not reach statistical significance due to greater variability.
AMPK activation is one potential mechanism responsible for the increase in muscle AS160/TBC1D4 phosphorylation during postexercise recovery. For example, it has previously been shown that AS160/TBC1D4 phosphorylation is associated with AMPK activation (4, 20, 30) and translocation of GLUT4 (26, 27). An increase in skeletal muscle AMPK activity is associated with an increase in GLUT4 translocation to the sarcolemma and the subsequent uptake of glucose by muscle (11, 12). Currently, it is not known how AMPK is activated during postexercise recovery. It is unlikely that significant changes occurred in AMP and ATP concentrations; however, one possible explanation may be that glycogen depletion during exercise may account for the modest increase in AMPK activity (34). It should be noted that some of the AMPK activity data used for comparisons in this study was included in a previous publication (8). That study (8), however, included 11 subjects (7 male and 4 female), while this study utilized data generated from males only, which may account for the slight differences in the AMPK activation time course postexercise. Thus the present study may have been underpowered to detect significant changes in some variables of interest (e.g., AMPK activity and ACC2). It is likely that future work with a larger sample size (based on our power values, which are shown in results) will provide additional support of our original hypothesis.
As previously mentioned, insulin signaling has been shown to lead to increased PAS phosphorylation in both rat (2, 4) and human skeletal muscle (6, 19). In the present study, plasma insulin concentration tended to increase during exercise and then returned to baseline during the first hour of postexercise recovery. The changes in circulating insulin levels measured during exercise were most likely a response to the small increase in blood glucose concentrations that were a consequence of enhanced liver glycogenolysis during exercise. Therefore, we cannot exclude the possibility that small changes in circulating insulin levels during and immediately following exercise may have played a role in activating Akt during the first hour of postexercise recovery. However, the changes in circulating insulin and glucose concentrations were short lived and rapidly returned to basal values during the first hour of recovery. Additionally, exercise may have increased the sensitivity of muscle cells to insulin, which may also potentially explain the phosphorylation of Akt at 1 h. On the other hand, Akt phosphorylation was unaltered immediately following exercise, which may indicate that the small changes in plasma insulin were insufficient to alter Akt phosphorylation since it has previously been shown that Akt can be rapidly phosphorylated by insulin (i.e., within minutes) (4). Alternatively, changes in intracellular Ca2+ may also have phosphorylated Akt at Ser473, but why this occurred at 1 h postexercise as opposed to immediately following exercise is unclear as work in rat muscle has demonstrated that Akt at Ser473 can be rapidly phosphorylated (25).
Another interesting finding from this study was the change in whole body fuel selection during postexercise recovery. Whole body glucose oxidation was significantly depressed during the 1 and 2 h of postexercise recovery (−29 and −31%, respectively). The change in glucose oxidation from baseline was not a result of decreased availability of substrate as serum glucose concentration following exercise was not different from baseline. Moreover, glucose uptake across the leg (likely indicating uptake into skeletal muscle during recovery) remained elevated 177% (1 h postexercise) and 164% (2 h postexercise) above basal values. AMPK is not only involved in the regulation of glucose uptake but also in regulating fuel selection in skeletal muscle (10, 33). Specifically, we have previously shown that the exercise-induced activation of AMPK is associated with the stimulation of fat oxidation during postexercise recovery in rodents (24). These data suggest that AMPK stimulation of fat oxidation may be playing a role in partitioning glucose toward storage during recovery to replenish diminished glycogen levels. For example, several previous studies have shown that resistance exercise also decreases glycogen stores in human muscle, which are gradually replenished during recovery (15, 21–23). We did not measure muscle glycogen concentrations in the present study due to tissue limitations; however, our data are consistent with this hypothesis since we demonstrate that the increase in glucose uptake across the leg during recovery is associated with an ∼30% increase in fat oxidation during postexercise recovery.
Recently, it was reported that the PAS antibody may bind to a newly discovered protein, TBC1D1, which is an AS160/TBC1D4 paralog (29). The discovery of this protein, which is potentially the major phospho-Akt-substrate detectable at a molecular mass of ∼160 kDa in skeletal muscle, suggests that our results must include the possibility that our PAS antibody may detect AS160/TBC1D4 and other Akt substrates including TBC1D1. Thus, any conclusions based on data using this antibody must acknowledge this potential caveat. However, when we first immunoprecipitated AS160/TBC1D4 and then followed by blotting with the PAS antibody we show that PAS phosphorylation during postexercise is occurring on AS160/TBC1D4 (Fig. 5B). This does not exclude the possibility that our PAS phosphorylation data in Fig. 5A included both phosphorylation of TBC1D1 and TBC1D4. Further research is necessary to delineate the effects of resistance exercise on potential changes in AS160/TBC1D4 and TBC1D1 phosphorylation.
In summary, our data show that skeletal muscle AS160/TBC1D4 phosphorylation and glucose uptake across the leg increased during the postexercise recovery period following heavy resistance exercise. These changes were concurrent with an increase in muscle Akt phosphorylation (1 h postexercise), AMPKα2 activation (2 h postexercise), and enhanced whole body fat oxidation (1–2 h postexercise). We conclude that an increase in skeletal muscle AMPKα2 activity, Akt phosphorylation, and AS160/TBC1D4 phosphorylation following a bout of resistance exercise are associated with an increase in leg glucose uptake. AMPK activation during postexercise recovery may promote skeletal muscle glycogen repletion by increasing substrate (glucose uptake) and energy (ATP via fat oxidation) availability.
GRANTS
This study was supported by Grant R01-AR-049877 from the National Institute for Arthritis and Musculoskeletal and Skin Diseases, Grant S10-RR-16650 from the Shared Instrumentation Grant Program, and Grant M01-RR-00073 from the General Clinical Research Branch, National Center for Research Resources, National Institutes of Health, and by National Institute on Aging Grant P30-AG-024832. H. C. Dreyer was supported by Grant H133-P040003 from the National Institute on Disability and Rehabilitation Research, Department of Education.
Acknowledgments
We thank the nurses and personnel of the General Clinical Research Center of the University of Texas Medical Branch for help with the conduct of the clinical portion of this study.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahlborg G, Felig P. Lactate and glucose exchange across the forearm, legs, and splanchnic bed during and after prolonged leg exercise. J Clin Invest 69: 45–54, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab 292: E1191–E1200, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bouzakri K, Karlsson HK, Vestergaard H, Madsbad S, Christiansen E, Zierath JR. IRS-1 serine phosphorylation and insulin resistance in skeletal muscle from pancreas transplant recipients. Diabetes 55: 785–791, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes 54: 41–50, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Cartee GD, Wojtaszewski JF. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab 32: 557–566, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Deshmukh A, Coffey VG, Zhong Z, Chibalin AV, Hawley JA, Zierath JR. Exercise-induced phosphorylation of the novel Akt substrates AS160 and filamin A in human skeletal muscle. Diabetes 55: 1776–1782, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab 294: E392–E400, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 576: 613–624, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frosig C, Rose AJ, Treebak JT, Kiens B, Richter EA, Wojtaszewski JF. Effects of endurance exercise training on insulin signaling in human skeletal muscle: interactions at the level of phosphatidylinositol 3-kinase, Akt, and AS160. Diabetes 56: 2093–2102, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Hardie DG, Carling D. The AMP-activated protein kinase—fuel gauge of the mammalian cell? Eur J Biochem 246: 259–273, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology 21: 48–60, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes 49: 527–531, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Howlett KF, Mathews A, Garnham A, Sakamoto K. The effect of exercise and insulin on AS160 phosphorylation and 14-3-3 binding capacity in human skeletal muscle. Am J Physiol Endocrinol Metab 294: E401–E407, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Howlett KF, Sakamoto K, Garnham A, Cameron-Smith D, Hargreaves M. Resistance exercise and insulin regulate AS160 and interaction with 14-3-3 in human skeletal muscle. Diabetes 56: 1608–1614, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Ivy JL, Holloszy JO. Persistent increase in glucose uptake by rat skeletal muscle following exercise. Am J Physiol Cell Physiol 241: C200–C203, 1981. [DOI] [PubMed] [Google Scholar]
- 16.Jessen N, Goodyear LJ. Contraction signaling to glucose transport in skeletal muscle. J Appl Physiol 99: 330–337, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci 41: 459–473, 1971. [DOI] [PubMed] [Google Scholar]
- 18.Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, Lienhard GE. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem 277: 22115–22118, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson HK, Ahlsen M, Zierath JR, Wallberg-Henriksson H, Koistinen HA. Insulin signaling and glucose transport in skeletal muscle from first-degree relatives of type 2 diabetic patients. Diabetes 55: 1283–1288, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes 55: 2067–2076, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Pascoe DD, Costill DL, Fink WJ, Robergs RA, Zachwieja JJ. Glycogen resynthesis in skeletal muscle following resistive exercise. Med Sci Sports Exerc 25: 349–354, 1993. [PubMed] [Google Scholar]
- 22.Pascoe DD, Gladden LB. Muscle glycogen resynthesis after short term, high intensity exercise and resistance exercise. Sports Med 21: 98–118, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Peters Futre EM, Noakes TD, Raine RI, Terblanche SE. Muscle glycogen repletion during active postexercise recovery. Am J Physiol Endocrinol Metab 253: E305–E311, 1987. [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen BB, Hancock CR, Winder WW. Postexercise recovery of skeletal muscle malonyl-CoA, acetyl-CoA carboxylase, and AMP-activated protein kinase. J Appl Physiol 85: 1629–34, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Sakamoto K, Hirshman MF, Aschenbach WG, Goodyear LJ. Contraction regulation of Akt in rat skeletal muscle. J Biol Chem 277: 11910–11917, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Sano H, Eguez L, Teruel MN, Fukuda M, Chuang TD, Chavez JA, Lienhard GE, McGraw TE. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab 5: 293–303, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278: 14599–14602, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, Eagan PA, Jenkinson CP, Cersosimo E, DeFronzo RA, Sakamoto K, Musi N. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes 56: 836–848, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, Bowles N, Hirshman MF, Xie J, Feener EP, Goodyear LJ. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem 283: 9787, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Treebak JT, Birk JB, Rose AJ, Kiens B, Richter EA, Wojtaszewski JF. AS160 phosphorylation is associated with activation of α2β2γ1 but not α2β2γ3 AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab 292: E715–E722, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jorgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes 55: 2051–2058, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Williamson DL, Kubica N, Kimball SR, Jefferson LS. Exercise-induced alterations in extracellular signal-regulated kinase 1/2 and mammalian target of rapamycin (mTOR) signalling to regulatory mechanisms of mRNA translation in mouse muscle. J Physiol 573: 497–510, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol Endocrinol Metab 277: E1–E10, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Wojtaszewski JF, MacDonald C, Nielsen JN, Hellsten Y, Hardie DG, Kemp BE, Kiens B, Richter EA. Regulation of 5′-AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am J Physiol Endocrinol Metab 284: E813–E822, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. Hoboken, NJ: Wiley, 2005.