Abstract
During the neonatal period, high protein breakdown rate is a metabolic process inherent to elevated rates of protein accretion in skeletal muscle. To determine the relationship between hindlimb net movements of essential and nonessential amino acids in the regulation of hindlimb protein breakdown during an overnight fasting-feeding cycle, we infused overnight-food-deprived 10- and 28-day-old piglets with [1-13C]phenylalanine and [ring-2H4]tyrosine over 7 h (during 3 h of fasting and then during 4 h of feeding). Extraction rates for aspartate and glutamate after an overnight fast were 15% and 51% in the 10-day-old compared with 6% and 25% in the 28-day-old (P < 0.05) piglets, suggesting an altered requirement for precursors of amino acids to shuttle nitrogen to the liver as early life progresses. This occurred simultaneously with marginal positive hindlimb net balance of essential amino acids after an overnight fast, with negative net release of many nonessential amino acids, such as alanine, asparagine, glutamine, glycine, and proline. This suggests that newborn muscle does not undergo significant protein mobilization after a short period of fasting in support of an elevated rate of protein accretion. Furthermore, tyrosine efflux from hindlimb breakdown between overnight fasting and feeding periods was not different in the 10-day-old piglets, for which tyrosine was limiting, but when tyrosine supply balanced requirements in the 28-day-old piglet, hindlimb efflux was increased (P = 0.01). The results of the present study indicate that proteolysis and net movements of amino acids are coordinated mechanisms that sustain the elevated rate of net protein accretion during overnight feeding-fasting cycles in the neonate.
Keywords: proteolysis, fasting-feeding cycles, tyrosine kinetics, hindlimb skeletal muscle, neonate
neonates have an inherent high capability to accrete protein to support neonatal growth and development. Their feeding pattern, i.e., frequent small meals, requires nutrient management that maximizes anabolism. Insulin is known to be a key regulator of neonatal postprandial metabolism, and the sensitivity of newborns to insulin enables rapid extraction of postprandial amino acids by the skeletal musculature and utilization of these amino acids for protein synthesis through activation of a translational apparatus initiating protein synthesis (11, 29, 30, 35). Individual components of the feeding process, such as amino acids, can also independently regulate muscle protein synthesis (12, 22), with the branched-chain amino acids, such as leucine, acting as nutrient signals to increase eIF4E availability for eIF4F complex assembly (15).
Apart from the regulation of protein synthesis by amino acids and insulin, proteolysis also plays an important role in the control of protein accretion. Whole body protein breakdown is downregulated with feeding in neonatal pigs (31) and in infants and rodents (17, 32). Neonatal regulation of whole body protein breakdown in full-term infants is considered to be primarily regulated through availability of amino acids, rather than insulin (23, 24). In contrast to whole body proteolysis, muscle protein breakdown is not reduced during feeding after an overnight fast in neonatal pigs but, rather, is increased (31). The possible physiological reasons for this muscle response are not fully understood but may include the need to maintain intracellular concentrations of free amino acids through alteration of protein breakdown to sustain feeding-induced elevation of protein synthesis (3, 4). Net uptake of amino acids may be coordinated with the rate of proteolysis to sustain the anabolic rate of the neonate during overnight fasting-feeding cycles. Adaptive uptake of amino acids or other compounds may occur to sustain nitrogen management through synthesis of nitrogen shuttles within the muscle and the whole body during the feeding-fasting cycle (2, 16, 20).
To define which of these possibilities are critical during neonatal development, direct in vivo assessment of proteolysis rates in neonatal hindlimb skeletal muscle in response to overnight food deprivation and feeding was undertaken. Chronically catheterized neonatal pigs were used in the present experiment for study of hindlimb net movements of amino acids and investigation of the association of these net movements of amino acids with the need to support the high rate of protein accretion in the neonate. These findings were then related to rates of proteolysis, assessed from tyrosine kinetics and net transfers across the hindlimb bed.
MATERIALS AND METHODS
Materials and methods are described elsewhere (31). Briefly, six 28-day-old piglets and five 10-day-old piglets were used in an incomplete block design to investigate hindlimb net movements of amino acids in the regulation of skeletal muscle metabolism after overnight food deprivation followed by a feeding period. The study was approved by the Animal Care and Use Committee of Baylor College of Medicine and was conducted in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals.
Experimental design.
Cross-bred sows (Yorkshire × Landrace × Hampshire × Duroc; Agriculture Headquarters, Texas Department of Criminal Justice, Huntsville, TX) were housed in lactation crates in individual environmentally controlled rooms. They were maintained on a commercial diet (Purina Laboratory Porcine Diet; 4–5 pounds/day) and had free access to water. Twelve newborn pigs (≥1.2 kg body wt) were selected and remained with their sow until 3 or 21 days of age. They were not given supplemental creep feed. At 3 or 21 days after birth, the animals were surgically prepared under sterile conditions after overnight food deprivation, with postsurgical care as previously described (31). A feeding catheter was placed in the duodenum, and a venous catheter for tracer infusion was inserted in a jugular vein. Catheters for measurement of hindlimb arteriovenous difference were placed in a carotid artery and the inferior vena cava. The catheter tip in the inferior vena cava was positioned caudal to the renal vein and superior to the common iliac vein (with the tip toward common iliac vein) (7, 19). Blood sampling of this venous site mainly represents muscle protein metabolism in the hindlimb but also includes metabolism in skin, bone marrow, and adipose tissue (19). A perivascular flow probe was placed around the caudal aorta (Transonic Systems, Ithaca, NY). Piglets did not receive food during the first 24 h after the surgery. To accelerate postsurgical recovery, the piglets were fed enterally an elemental diet (Table 1) through the duodenal catheter from 24 to 72 h after the surgery at a maximum fraction representing 10–15% of their nutritional requirements. Postsurgical feeding was managed using this approach to provide minimal nutrient amounts to the animal and allowed the natural recovery of hunger.
Table 1.
Composition of elemental nutrition solution
| Ingredient | Amount |
|---|---|
| Carbohydrate, g/l | 104 |
| Intralipid (20%), g/l | 21 |
| Amino acids, g/l | 55 |
| Alanine | 2.70 |
| Arginine | 2.34 |
| Aspartic acid | 4.15 |
| Glutamic acid | 5.19 |
| Glutamine | 4.15 |
| Cysteine HCl | 1.20 |
| Glycine | 2.03 |
| Histidine | 1.35 |
| Isoleucine | 3.01 |
| Leucine | 5.35 |
| Lysine | 5.05 |
| Methionine | 1.35 |
| Phenylalanine | 2.75 |
| Proline | 3.90 |
| Serine | 2.91 |
| Threonine | 3.26 |
| Tryptophan | 0.63 |
| Tyrosine | 0.63 |
| Valine | 3.28 |
| Electrolytes, mmol/l | |
| NaCl | 19.0 |
| NaOH | 30.0 |
| K acetate | 15.2 |
| KPO4 | 22.0 |
| MgSO4 | 3.2 |
| Ca gluconate | 5.0 |
Elemental diet formulated to meet or exceed requirements for neonatal pigs (∼215 kcal·kg−1·day−1, 13 g amino acid ·kg−1·day−1, 240 ml·kg−1·day−1 fluid intake) was started after a 3-h fasting period and maintained for the subsequent 4 h (feeding period). Dextrose was obtained from Sigma (St. Louis, MO), Intralipid (20% solution) from Baxter Healthcare (Deerfield, IL), and amino acids from Ajinomoto/Sigma.
At 72 h after surgery, a milk replacer (Litter Life, Merricks, Middleton, WI) was offered at ≥6.25% body weight to fulfill ≥100% of nutritional requirements for growth (21) from 6 to 9 days of age in young piglets and from 24 to 27 days of age in the older piglets. Piglets were maintained on the milk replacer until measurements were conducted at 10 (3.5 ± 0.4 kg body wt) or 28 (6.6 ± 0.9 kg body wt) days of age. Both groups were fasted overnight on days 9 and 27 to ensure that they would be in a fasting state at 10 and 28 days of age, respectively. (The arterial catheter of a 10-day-old piglet was not patent at the moment of the measurements; therefore, results for the 10-day-old piglets represent only five animals.)
Isotopes.
The tracers l-[1-13C]phenylalanine, l-[1-13C]tyrosine, NaH13CO3 [99 atom percent excess (APE); Cambridge Isotopes Laboratories], l-[ring-2H4]tyrosine (98 APE; Cambridge Isotopes Laboratories), and NaH14CO3 (Cambridge Isotope Laboratories) were dissolved in physiological saline.
Measurement day.
The present study reports amino acid net balances and tyrosine kinetics across the hindlimb bed; phenylalanine kinetics are published elsewhere (31). After an overnight food deprivation period, the onset of tracer infusions was preceded by 15 min of blood flow monitoring (Transonic Systems), as performed at 10 and 28 days of age for the young and older piglets, respectively. Three background blood samples were taken simultaneously from the artery and the vena cava for determination of natural abundance of metabolites. At time 0, body pools were primed with l-[1-13C]phenylalanine (22 μmol/kg), l-[1-13C]tyrosine (3.3 μmol/kg), l-[ring-2H4]tyrosine (6 μmol/kg), and NaH13CO3 (6 μmol/kg). Continuous infusions of l-[1-13C]phenylalanine (22 μmol·kg−1·h−1) and l-[ring-2H4]tyrosine (6 μmol·kg−1·h−1) were immediately initiated and maintained for 7 h. After an oral bolus (5 ml), an intraduodenal feeding of the elemental diet was initiated (10 ml·kg−1·h−1) at 3 h and continuously administered for the subsequent 4 h (6). This infusion rate was consistent with the recommended range of water-to-food intake ratios of 2.5:1–4.3:1 for pigs (25). The elemental diet (Table 1) described previously (6) was formulated to meet or exceed the nutritional requirements of neonatal pigs (energy = 215 kcal·kg−1·day−1, amino acids = 13 g·kg−1·day−1, fluid intake = 240 ml·kg−1·day−1). CO2 production was measured during the food deprivation and feeding periods using NaH14CO3, as described elsewhere (31). The amino acid composition of the elemental diet was consistent with the ideal ratios of amino acids to lysine for protein accretion in pigs (25). Breath and blood samples were taken every 30 min over the last 1.5 h of the food deprivation and feeding periods (4 blood samples per nutritional status). At 7 h, the infusions were stopped and the animals were killed by a lethal injection of pentobarbital sodium (50 mg/kg iv).
Plasma substrates and muscle amino acids in the free pool homogenate.
The packed cell volume of blood was determined by the microhematocrit method (28). The concentrations of individual amino acids from frozen plasma samples were measured by HPLC (Pico-Tag reverse-phase column, Waters, Milford, MA), as previously described (13). Frozen gastrocnemius muscle (50 mg) was homogenized in duplicates in 2 ml of ice-cold TCA [10% (wt/vol)], with the amount of TCA weighed. A weighed volume of 500 μl of homogenate was filtered through 10,000 cutoff filter at 15,000 rpm for 15 min; 50 μl of the filtrate were mixed with 50 μl of an internal standard of methionine sulfone on a weight basis and then processed according to the Pico-Tag method for HPLC analysis, as outlined previously (1).
Plasma tyrosine isotopic enrichments.
Plasma tyrosine was separated on a cation-exchange resin column (AGW50 resin, Bio-Rad) and converted to its n-propyl ester heptafluorobutyramide derivative according to the method of Reeds et al. (26). Because deuterated tyrosine derivatives are sensitive to heat, plasma tyrosine was derivatized at 30°C for 2 h. The recovery of the tracers was always verified by linear regression. GC-MS was carried out on a gas chromatograph (model 6890, Hewlett Packard) coupled with a quadrupole mass spectrometer mass selective detector (model 5973, Hewlett Packard) operating in the negative chemical ionization mode. Selective ion monitoring was carried out at mass-to-charge ratios of 595, 596, and 599 for tyrosine. Breath 13CO2 enrichment was determined on an isotope ratio mass spectrometer (ThermoQuest Finnigan Delta Plus XL, Thermo Finnigan MAT) monitoring ion masses 44, 45, and 46.
Calculations.
Plasma net uptakes of amino acids were calculated as the difference between plasma concentration in the carotid artery and plasma concentration in the vena cava (arteriovenous difference) multiplied by the respective blood flow corrected for hematocrit. Hindlimb fractional extraction was calculated using the arteriovenous difference divided by arterial concentration [(A − V)/A]. Hindlimb outflow of 3-methylhistidine was calculated by multiplying the vena cava concentration by the respective plasma flow and was further corrected for body weight (33).
Total whole body flux (ILR) of tyrosine was calculated from the steady-state dilution of l-[ring-2H4]tyrosine according to the tracer-dilution method, as described previously (7, 31). Hindlimb movements of [ring-2H4]tyrosine were calculated according to the tracer/mass balance technique (7)
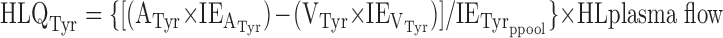 |
where HLQTyr is hindlimb tyrosine total flux, A is arterial, V is venous, IE is isotopic enrichment, and ppool is precursor pool (i.e., vena cava plasma).
In the absence of any further catabolism of tyrosine, HLQTyr represents hindlimb protein synthesis (HLPS). Then
 |
where hindlimb protein breakdown (HLPB) was derived from the hindlimb flux equation. No significant net release of 13CO2 from the hindquarters has been monitored previously in growing pigs when [1-13C]phenylalanine is infused (7). Furthermore, in the present study, no hydroxylation of phenylalanine to tyrosine occurred across the hindlimb bed of the piglets. The difference between the arterial and the venous ratios of [1-13C]tyrosine to [1-13C]phenylalanine isotopic enrichments was not different from zero in the food-deprived state according to a Student's t-test (0.0775 and 0.0722, respectively, SEM = 0.0155, P = 0.42), and the venous ratio was reduced compared with the arterial ratio in the fed state (0.2223 vs. 0.2042, SEM = 0.1525, P = 0.03). Phenylalanine was hydroxylated in the liver: after 4 h of enteral feeding, the average hepatic isotopic enrichment of free [1-13C]tyrosine in the hepatic homogenate was 3.67 mole percent excess (SEM 0.44), while the arterial [1-13C]tyrosine isotopic enrichment averaged 1.56 mole percent excess (SEM 0.22).
Statistical analyses.
The data were subjected to analysis of variance using the general linear model procedures of SAS (27). The statistical design was presented as a split-block design comparing two nonrandomized nutritional states with two age groups in an incomplete block design based on initial weight. This design allowed comparisons between nutritional state, age, and nutritional state-age interaction. The statistical model, including the overall mean (μ) and residual error (e) associated with ijkl observations, is described as follows
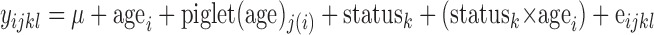 |
The error term for testing the age effect was derived from the variance within each age group. Type III sum of squares was interpreted. Values are least square means with pooled standard error of the mean. P ≤ 0.05 was considered statistically significant, and 0.05 < P ≤ 0.10 was considered a tendency. Regressions were performed using the general linear model procedures of SAS (27).
RESULTS
Previous studies demonstrated that neonatal pigs undergo a dramatic decline in the sensitivity of amino acid utilization for protein deposition to insulin (11, 35). Consistent with results from previous studies, the arterial concentration of most essential and nonessential amino acids, as well as the branched-chain amino acids and those used as nitrogen shuttles, increased with age (Table 2). As expected, feeding increased concentrations of arterial amino acids, but these represent a composite pool integrating absorption and the balance between protein synthesis and breakdown, with differential effects with feeding observed between the 10- and the 28-day-old piglets. The greater increase in total amino acid concentration with feeding in the 28-day-old group was due mainly to a greater increase in essential amino acids (76% more than in the 10-day-old piglets). Nonessential amino acids were also increased more with feeding in the 28-day-old piglets (254% of the increase in the 10-day-old piglets). Similarly, arterial branched-chain amino acids and amino acids shuttling nitrogen from the musculature to the liver were increased more with feeding in the 28- than in the 10-day-old piglets. The feeding-induced increase in branched-chain amino acids contributed to a similar proportion of the essential amino acids between the 10- and the 28-day-old piglets (+42% and +43%, respectively). The amino acids shuttling nitrogen also contributed similarly to nonessential amino acids in response to feeding (+52% and +41% for 10- and 28-day-old piglets, respectively). Alanine, glutamine, proline, and tyrosine were the nonessential amino acids most increased by feeding in the 28-day-old compraed with the 10-day-old piglets.
Table 2.
Arterial concentrations of individual and sums of amino acids in neonatal piglets
| 10 Days of Age (n = 5) |
28 Days of Age (n = 6) | SEM | P | |||||
|---|---|---|---|---|---|---|---|---|
| Food Deprived | Fed | Food Deprived | Fed | Age | Nutrition | Age × Nutrition | ||
| EAA | ||||||||
| Arginine | 130.6 | 203.0 | 130.7 | 259.7 | 15.6 | 0.17 | <0.001 | 0.09 |
| Histidine | 13.8 | 40.3 | 26.3 | 65.4 | 9.6 | 0.19 | 0.006 | 0.51 |
| Isoleucine | 99.9 | 218.2 | 114.4 | 321.1 | 20.5 | 0.005 | <0.001 | 0.05 |
| Leucine | 126.1 | 324.9 | 184.8 | 524.1 | 25.7 | <0.001 | <0.001 | 0.02 |
| Lysine | 146.2 | 365.6 | 143.7 | 505.7 | 24.1 | 0.05 | <0.001 | 0.01 |
| Phenylalanine | 103.0 | 196.5 | 156.3 | 396.4 | 16.7 | <0.001 | <0.001 | 0.001 |
| Methionine | 63.3 | 459.2 | 68.8 | 379.6 | 67.9 | 0.55 | <0.001 | 0.53 |
| Threonine | 306.4 | 232.9 | 295.4 | 491.9 | 59.5 | 0.16 | 0.30 | 0.04 |
| Valine | 259.8 | 483.9 | 277.9 | 692.1 | 26.8 | 0.002 | <0.001 | 0.005 |
| NEAA | ||||||||
| Alanine | 361.1 | 594.8 | 512.9 | 1,132.4 | 58.2 | 0.003 | <0.001 | 0.007 |
| Aspartic acid | 5.5 | 7.2 | 7.1 | 17.7 | 1.4 | 0.01 | 0.001 | 0.008 |
| Asparagine | 33.3 | 50.6 | 53.7 | 25.2 | 16.0 | 0.53 | 0.69 | 0.15 |
| Glycine | 1,226.6 | 1,038.4 | 848.9 | 875.0 | 70.6 | 0.005 | 0.26 | 0.15 |
| Glutamic acid | 106.8 | 103.8 | 143.8 | 173.5 | 11.4 | 0.006 | 0.25 | 0.17 |
| Glutamine | 382.6 | 517.6 | 443.3 | 702.3 | 30.6 | 0.09 | <0.001 | 0.06 |
| Proline | 176.1 | 416.1 | 237.4 | 699.3 | 34.1 | 0.002 | <0.001 | 0.008 |
| Serine | 156.2 | 227.4 | 131.0 | 272.3 | 13.5 | 0.39 | <0.001 | 0.02 |
| Tyrosine | 88.0 | 47.4 | 90.3 | 134.9 | 10.5 | <0.001 | 0.84 | 0.002 |
| Total EAA | 1,293 | 2,569 | 1,451 | 3,685 | 136 | <0.001 | <0.001 | 0.005 |
| Total NEAA | 2,529 | 2,986 | 2,468 | 4,021 | 159 | 0.03 | <0.001 | 0.006 |
| BCAA | 486 | 1,027 | 577 | 1,537 | 70 | <0.001 | <0.001 | 0.01 |
| N shuttles | 883 | 1,257 | 1,161 | 2,040 | 72 | 0.003 | <0.001 | 0.006 |
Values are expressed in μM. BCAA, branched-chain amino acids (Leu, Val, Ile); N shuttles, amino acids shuttling nitrogen in the body (Glu, Gln, Asp, Asn, Ala).
Hindlimb arterial extraction rates varied according to age and fed/fasted state (Table 3). The skeletal musculature of the young newborn extracted a greater fraction of arterial amino acid, particularly arginine, isoleucine, and leucine, to sustain hindlimb protein metabolism. In addition, large arterial fractional extractions of many nonessential amino acids, including aspartate, glutamate, serine, and tyrosine, were observed. Fractional extractions were 51% and 15% greater for glutamate and aspartate, respectively, during food deprivation in the 10-day-old piglets but declined to 25% and 6%, respectively, in the 28-day-old piglets. These fractional extractions occurred simultaneously with fractional release of alanine, asparagine, glycine, glutamine, and proline during food deprivation.
Table 3.
Hindlimb net extraction of arterial amino acid supplies in neonatal piglets
| 10 Days of Age (n = 5) |
28 Days of Age (n = 6) | SEM | P | |||||
|---|---|---|---|---|---|---|---|---|
| Food Deprived | Fed | Food Deprived | Fed | Age | Nutrition | Age × Nutrition | ||
| EAA | ||||||||
| Arginine | 6.4* | 15.5 | −5.4* | 6.9 | 2.5 | 0.01 | <0.001 | 0.49 |
| Histidine | −4.9† | 30.8 | −13.8† | 0.05 | 15.4 | 0.66 | 0.09 | 0.41 |
| Isoleucine | 2.7* | 13.1 | 1.4† | 9.0 | 1.1 | 0.02 | <0.001 | 0.18 |
| Leucine | 3.6* | 15.3 | 1.8† | 9.0 | 1.8 | 0.03 | <0.001 | 0.18 |
| Lysine | 1.4† | 10.6 | −0.6† | 5.6 | 1.4 | 0.07 | <0.001 | 0.24 |
| Phenylalanine | 0.2† | 8.1 | 1.9† | 5.0 | 4.4 | 0.13 | 0.14 | 0.46 |
| Methionine | −2.4† | 6.3 | 0.2† | 5.5 | 2.9 | 0.42 | 0.02 | 0.52 |
| Threonine | −0.10† | 6.9 | −1.1† | 3.9 | 2.2 | 0.16 | 0.01 | 0.59 |
| Valine | 2.1† | 10.1 | 2.7* | 7.9 | 1.4 | 0.60 | <0.001 | 0.27 |
| NEAA | ||||||||
| Alanine | −5.8* | 8.0 | −5.2* | 2.3 | 2.8 | 0.42 | 0.002 | 0.21 |
| Aspartic acid | 14.8* | 46.6 | 6.3† | 26.8 | 7.1 | 0.09 | 0.002 | 0.37 |
| Asparagine | −134.1* | −7.1 | −0.8† | 3.9 | 59.1 | 0.74 | 0.24 | 0.27 |
| Glycine | −2.2† | 3.8 | −0.6† | 1.9 | 1.7 | 0.75 | 0.02 | 0.24 |
| Glutamic acid | 51.0* | 51.6 | 24.7* | 25.8 | 3.4 | 0.01 | 0.73 | 0.97 |
| Glutamine | −5.6* | 3.2 | −4.1* | 0.88 | 2.3 | 0.58 | 0.01 | 0.37 |
| Proline | −3.5† | 8.2 | −0.3† | 5.2 | 2.1 | 0.43 | 0.003 | 0.12 |
| Serine | 8.3* | 16.0 | 3.9* | 8.3 | 1.7 | 0.007 | 0.004 | 0.33 |
| Tyrosine | 1.5† | 17.5 | −0.5† | 6.9 | 2.4 | 0.03 | 0.001 | 0.11 |
Values are expressed as percentages, calculated as follows: (arterial concentration − venous concentration)/arterial concentration.
Statistical significance (by Student's t-test) is as follows: *P < 0.05 difference vs. 0;
P > 0.05 difference vs. 0.
When normalized for body weight, age did not alter hindlimb net uptake of most amino acids (Table 4). Feeding increased net uptake for most amino acids, with the exception of phenylalanine and aspartate, and age did not further alter this response. The 10-day-old piglets remained in mild positive balance for most essential amino acids across the hindlimb, even during food deprivation. A similar observation was made when labeled phenylalanine was used to monitor mild net positive accretion rates during food deprivation across the piglet hindlimb bed (31). The reason for the positive balance of average essential amino acids during food deprivation may have been partly to provide nitrogen for mild net accretion plus endogenous synthesis of certain nonessential amino acids that exhibited negative net balance, such as alanine, asparagine, glycine, glutamine, and proline (2, 8, 9).
Table 4.
Hindlimb net fluxes of individual and different sums of amino acids in neonatal piglets
| 10 Days of Age (n = 5) |
28 Days of Age (n = 6) | SEM | P | |||||
|---|---|---|---|---|---|---|---|---|
| Food Deprived | Fed | Food Deprived | Fed | Age | Nutrition | Age × Nutrition | ||
| EAA | ||||||||
| Arginine | 12.9† | 57.1 | −22.7† | 64.2 | 14.1 | 0.43 | <0.001 | 0.10 |
| Histidine | 0.8† | 15.8 | −3.3† | 6.3 | 9.5 | 0.42 | 0.16 | 0.74 |
| Isoleucine | 4.8† | 61.2 | 3.3† | 99.7 | 14.3 | 0.19 | <0.001 | 0.13 |
| Leucine | 7.7† | 102.1 | 7.8† | 175.8 | 40.8 | 0.29 | 0.005 | 0.31 |
| Lysine | 3.4† | 74.2 | −3.7† | 97.7 | 15.0 | 0.66 | <0.001 | 0.26 |
| Phenylalanine | 2.9† | 29.7 | 4.4† | 63.9 | 7.0 | 0.06 | <0.01 | 0.02 |
| Methionine | −5.7† | 40.4 | 1.2† | 54.6 | 8.3 | 0.21 | <0.001 | 0.61 |
| Threonine | 2.2† | 41.3 | −2.5† | 77.9 | 24.5 | 0.51 | 0.02 | 0.34 |
| Valine | 9.8† | 100.5 | 18.6† | 191.1 | 25.3 | 0.13 | <0.001 | 0.09 |
| NEAA | ||||||||
| Alanine | −28.5† | 87.8 | −70.2* | 94.7 | 39.2 | 0.78 | 0.003 | 0.48 |
| Aspartic acid | 1.3† | 6.6 | 1.4† | 15.9 | 2.0 | 0.03 | <0.001 | 0.02 |
| Asparagine | −31.1† | 38.5 | −1.9† | 5.3 | 24.2 | 0.02 | 0.13 | 0.19 |
| Glycine | −44.4† | 67.7 | −12.0† | 58.7 | 38.4 | 0.82 | 0.02 | 0.53 |
| Glutamic acid | 92.2* | 112.3 | 98.2* | 146.0 | 16.4 | 0.51 | 0.04 | 0.34 |
| Glutamine | −34.3† | 16.5 | −44.9† | 31.5 | 31.4 | 0.75 | 0.04 | 0.63 |
| Proline | −10.6† | 70.7 | −22.5† | 136.8 | 26.7 | 0.05 | 0.001 | 0.14 |
| Serine | 22.8† | 74.4 | 13.6† | 84.4 | 13.9 | 0.91 | 0.0008 | 0.44 |
| Tyrosine | 2.4† | 16.4 | −2.28† | 33.7 | 6.6 | 0.25 | 0.002 | 0.08 |
| Total EAA | 39† | 532 | 5† | 872 | 121 | 0.25 | <0.001 | 0.10 |
| Total NEAA | −24† | 500 | −14† | 603 | 148 | 0.67 | 0.001 | 0.71 |
| BCAA | 22† | 264 | 30† | 467 | 76 | 0.18 | <0.001 | 0.16 |
| N shuttles | 6† | 271 | −17† | 289 | 76 | 0.89 | 0.002 | 0.75 |
Values are expressed in μmol·kg−1h−1. Statistical significance (by Student's t-test) is as follows:
P < 0.05 difference vs. 0;
P > 0.05 difference vs. 0.
The role of proteolysis in the regulation of muscle protein anabolism in the newborn was also investigated through hindlimb transfers of labeled tyrosine (Table 5) and phenylalanine (31). Feeding had a greater impact on hindlimb tyrosine irreversible loss rate in the 28- than in the 10-day-old piglets because of the greater musculature (25), as was previously highlighted with labeled phenylalanine (31). Although feeding increased hindlimb phenylalanine release from breakdown in both age groups (31), this was more extensive in the 28-day-old piglets for tyrosine. Net hindlimb outflow of 3-methylhistidine was not significantly affected by age and feeding state (Table 5). However, the absolute values of the net 3-methylhistidine outflows consistently decreased by 31% with age, along with the fractional protein turnover of these piglets (15.4 vs. 5.6%/day) (31).
Table 5.
Hindlimb and whole body tyrosine kinetics in neonatal piglets
| 10 Days of Age (n = 5) |
28 Days of Age (n = 6) | SEM | P | |||||
|---|---|---|---|---|---|---|---|---|
| Food Deprived | Fed | Food Deprived | Fed | Age | Nutrition | Age × Nutrition | ||
| Hindlimb | ||||||||
| Net flux, μmol·kg−1h·−1 | 2.41 | 16.36 | −2.29 | 33.73 | 6.57 | 0.002 | 0.08 | 0.23 |
| Tyrosine total flux, μmol·kg−1h·−1 | 16.93 | 31.98 | 31.46 | 71.44 | 9.93 | 0.01 | 0.17 | 0.03 |
| Protein breakdown, μmol·kg−1h·−1 | 14.51 | 15.62 | 33.75 | 37.71 | 9.25 | 0.75 | 0.86 | 0.01 |
| 3-Methylhistidine outflow, μmol·kg−1h·−1 | 9.5 | 11.4 | 8.4 | 6.0 | 2.4 | 0.40 | 0.92 | 0.34 |
| Whole body | ||||||||
| ILR, μmol·kg−1h·−1 | 135 | 133 | 109 | 140 | 2.4 | 0.0001 | <0.001 | 0.21 |
| IE, MPE | ||||||||
| Carotid artery | 4.37 | 5.56 | 5.46 | 4.22 | 0.09 | 0.14 | <0.001 | <0.001 |
| Vena cava | 4.00 | 3.89 | 4.80 | 3.91 | 0.17 | 0.008 | 0.03 | 0.17 |
ILR, irreversible loss rate; IE, isotopic enrichment; MPE, mole percent excess. With phenylalanine used as tracer, hindlimb protein breakdown was 19.9 and 26.5 μmol·kg−1·h−1 in fasted and fed states, respectively, in 10-day-old piglets and 29.4 and 37.1 μmol·kg−1·h−1 in fasted and fed states, respectively, in 28-day-old piglets (31).
In the fed state, the concentrations of many essential amino acids (e.g., isoleucine, phenylalanine, and valine) in muscle homogenates are greater in the 28- than in the 10-day old piglets (Fig. 1), as observed previously (1). Among nonessential amino acids, glycine and serine concentrations are increased in the more anabolic newborn muscle than in musculature of the older piglets, as monitored previously (1). The different changes in individual amino acid concentrations in muscle homogenates result in similar amounts of total amino acids. In this study, none of the sums investigated were significantly affected by age in the fed state. However, the ratio of essential to nonessential amino acids increased consistently with age (1) but did not reach significance in the present study.
Fig. 1.
Concentrations of free amino acids in gastrocnemius homogenates sampled at the end of a 4-h enteral feeding period in 10-day-old (n = 5) and 28-day-old (n = 6) piglets. A: individual essential amino acids. B: individual nonessential amino acids. C: total amino acids (Total), essential amino acids (EAA), nonessential amino acids (NEAA), branched-chain amino acids (BCAA), amino acids not oxidized within muscle (NoOx; i.e., Met, Phe, His), and amino acids shuttling nitrogen between muscle and liver (NShut; i.e., Ala, Glu, Gln, Asp). D: ratio of essential (E) to nonessential (NE) amino acids. Mean age effect: *P < 0.05; **P = 0.01; ***P = 0.0001; †P = 0.07. Values are least square means; error bars, SEM.
DISCUSSION
Perpetual remodeling of muscle proteins involves proteins that are degraded and resynthesized. As a consequence of this turnover, free amino acids are reincorporated into proteins, undergo catabolism, or flow out of the cells. The synchronicity of these processes is acutely regulated for different nutritional and physiological stages to maintain nitrogen homeostasis. Using stable isotopes, we previously showed that hindlimb muscle in the newborn piglet is resistant to a reduction in proteolysis during food ingestion and deprivation, as opposed to whole body protein breakdown, which was markedly decreased and increased, respectively (31). We hypothesized that the high protein turnover of the newborn musculature requires the maintenance of intracellular concentrations of free amino acids, potentially through protein breakdown adjustments, to sustain protein synthesis, as previously shown in skeletal muscle of exercising adults (4) and as suggested in infants, inasmuch as feeding amino acids during a short fasting period reduced whole body protein breakdown, whereas feeding glucose and lipid did not have the same effect (14, 23). The present study focuses on the net movements of amino acids and tyrosine metabolism across the hindlimb bed of newborn pigs to obtain more insights on this regulation.
In the present study, feeding increased arterial concentrations of most amino acids, but the increase in many amino acids was greater in the 28- than in the 10-day-old piglets. Because arterial concentrations mirror the balance between appearance in plasma and net retention, the age-related elevation of essential amino acids in older piglets probably relates to the reduced insulin-mediated utilization of amino acids, inasmuch as the enteral feeding rate was similar between age groups (11, 35). Moreover, the difference in the feeding-induced elevations of essential (+76%) and nonessential (+254%) amino acids in the 28-day-old piglets compared with the 10-day-old piglets has important implications. A lower skeletal muscle protein turnover in the older piglet associated with a reduced sensitivity to insulin elicited increased whole body transamination and endogenous synthesis of nonessential amino acids, probably as part of nitrogen homeostasis management. In support of this homeostasis, hindlimb extraction rates were elevated for glutamate and aspartate relative to other amino acids, particularly during food deprivation, but glutamate and aspartate represent only 16% and 10% of muscle protein, respectively, whereas the content of other amino acids in muscle protein varies from 3% to 9% (25). Management of nitrogen homeostasis helps conserve α-amino-nitrogen within the body (18) and includes extensive synthesis from gut metabolism (2), with endogenous synthesis for nitrogen shuttling between tissues to the liver in a nontoxic form (5). In this regard, hindlimb metabolism of the piglets requires a large amount of carbon-chain precursors for nitrogen management within cells as a result of transamination processes plus that required for net synthesis.
Feeding increases whole body protein synthesis and reduces proteolysis, while the hindlimb synthesis and breakdown are increased, but to different extents, dependent on age (31). From the latter response, muscle proteolysis was considered an essential mechanism in sustaining hindlimb protein synthesis through the maintenance of intracellular concentrations of free amino acids in the neonate. The direct measurement of hindlimb net movements of amino acids in the present study reveals certain aspects of this regulation. 1) Neonatal resistance to reduction of muscle proteolysis is shown after overnight food deprivation, during which mild net removal of most essential amino acids occurred, despite reduced arterial concentrations. Thus removal of essential amino acids during food deprivation occurred with the net hindlimb efflux of many nonessential amino acids, potentially in support of marginal net accretion in the skeletal muscle, while whole body protein was mobilized, and the splanchnic bed might be the main site at which this mobilization occurs (31). In addition to the requirement for net protein synthesis, hindlimb removal of essential amino acids probably involves the muscle transamination process, which produces mainly glutamine and alanine (2, 5, 34), which are then released from muscle during food deprivation. The transamination process, in muscle particularly, involves the branched-chain amino acids (2, 5, 34) and represents 50–56% of essential amino acids extracted across the hindlimb, although they represent only 38% of essential amino acids in porcine muscle proteins (25). 2) The requirement for nonessential amino acids, particularly glutamate, to sustain neonatal anabolism requires transminase reactions that utilize carbon-chain precursors (10, 20). Previous studies have highlighted a muscle adaptation for transport of inert nitrogen (10, 16, 34). Glutamate would be among the key substrates during fasting (20), and in the present study, glutamate was largely removed from arterial blood by the neonatal hindlimb bed during food deprivation.
In the present study, tyrosine kinetics across the hindlimb also provide evidence that proteolysis and movements of amino acids have a role in sustaining neonatal skeletal muscle anabolism during feeding. Hindlimb appearance of tyrosine from breakdown occurred to a lesser extent during feeding than during fasting in the 10-day-old piglets compared with the older piglets. In contrast, phenylalanine was sufficiently supplied in the 10-day-old piglets during the feeding period (31) and was largely released from hindlimb breakdown in the 10- and 28-day-old piglets. The enteral feeding provided tyrosine to a limited extent, due to low solubility, and the arterial concentration declined in the 10-day-old group upon feeding, suggesting a limitation in this amino acid, in contrast to other amino acids, for this age group. The differential response of proteolysis to feeding an unbalanced amount of tyrosine in the young neonates compared with the older piglets according to the tyrosine kinetics could be due to 1) lack of a large change in protein breakdown of hindlimb muscle or 2) lack of a large change in tyrosine outward transport from the cell while proteolysis is altered. Inasmuch as the rate of proteolysis was increased during feeding the elemental diet with a balanced amount of tracee, i.e., phenylalanine (31), assumption 1 is eliminated. Lack of a large change in tyrosine outward transport and efflux from the hindlimb bed appears to be a reliable occurrence. In support of this speculation, tyrosine in the cellular pool of muscle homogenate was not different between the 10- and the 28-day-old piglets during the enteral feeding, which appears to be consistent with a requirement for maintaining a cellular pool size to sustain a high rate of protein synthesis. This occurred while muscle phenylalanine concentration was largely increased in the 28-day-old piglets, and phenylalanine was largely provided by the elemental diet. A similar mechanism was previously observed (33): an increase in phenylalanine in muscle homogenate was accompanied by an augmentation of the outward transport after administration of glucose and amino acids to young and elderly humans. The present observations suggest a requirement for maintaining cellular concentrations of amino acids in skeletal muscle in the neonate to replenish the amino acid precursor pool and sustain a high rate of protein synthesis, which may occur in part through alteration of the transmembrane exchange constant. The hindlimb amino acid movements show that inflow and outflow are tightly controlled to sustain net synthesis during food-deprived and fed periods in the neonate but also suggest that cellular concentrations are maintained as part of a coordinated mechanism that sustains high protein turnover and accretion rate in the neonate.
Perspective
The net movements of amino acids and tyrosine kinetics across the hindlimb of the newborn pig support the hypothesis that skeletal muscle of the neonate is highly anabolic. Overall, muscle anabolism is sustained during fasting and feeding cycles by mild net uptakes of essential amino acids after an overnight food deprivation period, but this involves endogenous synthesis of nonessential amino acids released from the hindlimb bed to shuttle nitrogen to the liver. Net removal of glutamate potentially plays a key role in this nitrogen management. Using the tyrosine kinetic data, we have also provided evidence that the amino acids released from hindlimb protein breakdown are partly offset during an imbalance between exogenous supply and peripheral requirements. Coordination between alteration of hindlimb proteolysis rates and amino acid efflux from muscle appears to maintain cellular concentrations of free amino acids to sustain neonatal protein synthesis. The regulation of protein accretion in the neonate is a finely tuned process that involves tightly regulated synthesis and breakdown and cellular exchanges. In addition, the cellular management of nitrogen-like compounds is delicately regulated, and the hindlimb bed manages to selectively extract amino acids, thus facilitating protein accretion.
GRANTS
This work was supported by National Institutes of Health Grants R01-AR-44474 and T32 HD-074451 and the US Department of Agriculture/Agricultural Research Service under Cooperative Agreement 58-6250-6-001.
Acknowledgments
We thank W. Liu for technical assistance, J. Stubblefield for animal care, and L. Weiser for secretarial assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bergeron K, Julien P, Davis TA, Myre A, Thivierge MC. Long-chain n-3 fatty acids enhance neonatal insulin-regulated protein metabolism in piglets differentially altering muscle lipid composition. J Lipid Res 48: 2396–2410, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergman EN, Pell JM. Interorgan movement of amino acids. Proc Int Congr Nutr 1985, p. 370–373.
- 3.Biolo G, Williams BD, Fleming RYD, Wolfe RR. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes 48: 949–957, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Bolster DR, Pikosky MA, Gaine CG, Martin W, Wolfe RR, Tipton KD, Maclean D, Maresh CM, Rodriguez NR. Dietary protein intake impacts human skeletal muscle protein fractional synthetic rates after endurance exercise. Am J Physiol Endocrinol Metab 289: E678–E683, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Brosnan JT Interorgan amino acid transport and its regulation. J Nutr 133: 2068S–2072S, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Bush JA, Burrin DG, Suryawan A, O'Connor PMJ, Nguyen HV, Reeds PJ, Steele NC, Goudoever JBV, Davis TA. Somatotropin-induced amino acid conservation in pigs involves differential regulation of liver and gut urea cycle enzyme activity. J Nutr 132: 59–67, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Bush JA, Burrin DG, Suryawan A, O'Connor PMJ, Nguyen HV, Reeds PJ, Steele NC, Goudoever JBV, Davis TA. Somatotropin-induced protein anabolism in hindquarters and portal-drained viscera of growing pigs. Am J Physiol Endocrinol Metab 284: E302–E312, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Chang T, Goldberg A. The origin of alanine produced in skeletal muscle. J Biol Chem 253: 3677–3684, 1978. [PubMed] [Google Scholar]
- 9.Chang TW, Goldberg AL. The metabolic fates of amino-acids and the formation of glutamine in skeletal muscle. J Biol Chem 253: 3685–3695, 1978. [PubMed] [Google Scholar]
- 10.Davis EJ, Lee SHC. Amino acid metabolism by perfused rat hindquarter. Biochem J 229: 19–29, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis TA, Burrin DG, Fiorotto ML, Reeds PJ, Jahoor F. Roles of insulin and amino acids in the regulation of protein synthesis in the neonate. J Nutr 128: 347S–350S, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O'Connor PMJ. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab 282: E880–E890, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Enhanced response of muscle protein synthesis and plasma insulin to food intake in suckled rats. Am J Physiol Regul Integr Comp Physiol 265: R334–R340, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Denne SC, Karn CA, Wang J, Liechty EA. Effect of intravenous glucose and lipid on proteolysis and glucose production in normal newborns. Am J Physiol Endocrinol Metab 269: E361–E367, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am J Physiol Endocrinol Metab 290: E612–E621, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Fryburg DA, Barrett EJ, Louard RJ, Gelfand RA. Effect of starvation on human muscle protein metabolism and its response to insulin. Am J Physiol Endocrinol Metab 259: E477–E482, 1990. [DOI] [PubMed] [Google Scholar]
- 17.Goldspink DF, Kelly FJ. Protein turnover and growth in the whole body, liver and kidney of the rat from the foetus to senility. Biochem J 217: 507–516, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimble GF, Whitehead RG. Changes in the concentration of specific amino acids in the serum of experimentally malnourished pigs. Br J Nutr 24: 557–564, 1970. [DOI] [PubMed] [Google Scholar]
- 19.Hoskin SO, Savary-Auzeloux IC, Calder AG, Zuur G, Lobley GE. Effect of feed intake on amino acid transfers across the ovine hindquarters. Br J Nutr 89: 167–179, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Lee SHC, Davis EJ. Amino acid catabolism by perfused rat hindquarter. Biochem J 233: 621–630, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Research Council. Nutrient Requirements of Swine (version 10). Washington, DC: Natl Acad Sci, 1998.
- 22.O'Connor PMJ, Bush JA, Suryawan A, Nguyen HV, Davis TA. Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab 284: E110–E119, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Poindexter BB, Karn CA, Ahlrichs JA, Wang J, Leitch CA, Liechty EA, Denne SC. Amino acids suppress proteolysis independent of insulin throughout the neonatal period. Am J Physiol Endocrinol Metab 272: E592–E599, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Poindexter BB, Karn CA, Leitch CA, Liechty EA, Denne SC. Amino acids do not suppress proteolysis in premature neonates. Am J Physiol Endocrinol Metab 281: E472–E478, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Pond WG, Mersmann HJ. Biology of the Domestic Pig. Ithaca, NY: Cornell University Press, 2001.
- 26.Reeds PJ, Hachey DL, Patterson BW, Motil KJ, Kleige PD. VLDL apolipoprotein B-100, a potential indicator of the isotopic labeling of the hepatic synthetic precursor pool in humans: studies with multiple stable isotopically labeled amino acids. J Nutr 122: 457–466, 1992. [DOI] [PubMed] [Google Scholar]
- 27.SAS Institute. Statistical Analysis System. Cary, NC: SAS Institute, 2000.
- 28.Strumia MM, Sample AB, Hart ED. An improved micro hematocrit method. Am J Clin Pathol 24: 1016–1024, 1954. [DOI] [PubMed] [Google Scholar]
- 29.Suryawan A, Escobar J, Frank JW, Nguyen HV, Davis TA. Developmental regulation of the activation of signalling components leading to translation initiation in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab 291: E849–E859, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Suryawan A, Nguyen HV, Bush JA, Davis TA. Developmental changes in the feeding-induced activation of the insulin-signaling pathway in neonatal pigs. Am J Physiol Endocrinol Metab 281: E908–E915, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Thivierge MC, Bush JA, Suryawan A, Nguyen HV, Orellana RA, Burrin DG, Jahoor F, Davis TA. Whole body and hindlimb protein breakdown are differentially altered by feeding in neonatal piglets. J Nutr 135: 1430–1435, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Toledo-Eppinga LV, Kalhan SC, Kilik W, Jakobs C, Lafeber HN. Relative kinetics of phenylalanine and leucine in low birth weight infants during nutrient administration. Pediatr Res 40: 41–46, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab 85: 4481–4490, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolff JE, Bergman EN. Metabolism and interconversions of five plasma amino acids by tissues of the sheep. Am J Physiol 223: 447–454, 1972. [DOI] [PubMed] [Google Scholar]
- 35.Wray-Cahen D, Beckett PR, Nguyen HV, Davis TA. Insulin-stimulated amino acid utilization during glucose and amino acids clamps decreases with development. Am J Physiol Endocrinol Metab 273: E305–E314, 1997. [DOI] [PubMed] [Google Scholar]



