Abstract
End-stage renal disease (ESRD) is characterized by resting sympathetic overactivity. Baseline muscle sympathetic nerve activity (MSNA), which is governed by baroreflexes and chemoreflexes, is elevated in ESRD. Whether resting skin sympathetic nerve activity (SSNA), which is independent from baroreflex and chemoreflex control, is also elevated has never been reported in renal failure. The purpose of this study was to determine whether sympathetic overactivity of ESRD is generalized to include the skin distribution. We measured sympathetic nerve activity to both muscle and skin using microneurography in eight ESRD patients and eight controls. MSNA was significantly (P = 0.025) greater in ESRD (37.3 ± 3.6 bursts/min) when compared with controls (23.1 ± 4.4 bursts/min). However, SSNA was not elevated in ESRD (ESRD vs. controls, 17.6 ± 2.2 vs. 16.1 ± 1.7 bustst/min, P = 0.61). Similar results were obtained when MSNA was quantified as bursts per 100 heartbeats. We report the novel finding that although sympathetic activity directed to muscle is significantly elevated, activity directed to skin is not elevated in ESRD. The differential distribution of sympathetic outflow to the muscle vs. skin in ESRD is similar to the pattern seen in other disease states characterized by sympathetic overactivity such as heart failure and obesity.
Keywords: dialysis
sympathetic nervous system (SNS) activity is chronically elevated in patients with end-stage renal disease (ESRD) and contributes to hypertension and cardiovascular risk in these patients. The mechanisms underlying sympathetic overactivity in renal failure are largely unknown. Direct microneurographic measurements of sympathetic nerve activity in ESRD patients have revealed marked increases in sympathetic traffic directed to skeletal muscle (3); however, it is unknown whether skin sympathetic nerve activity is also elevated. Sympathetic output to the muscle and skin is regulated by different mechanisms; whereas muscle sympathetic nerve activity (MSNA) is tightly regulated by baroreflexes and chemoreflexes, skin sympathetic nerve activity (SSNA) is free from baroreflex and chemoreflex control, and it is instead regulated by sensory stimuli and thermoregulatory input (4–6, 12, 22). Examining potential differences in the distribution of sympathetic activity to muscle and skin may provide insights into whether sympathetic overactivation is widespread in ESRD, or reflects independent regulatory patterns, because the regulation of sympathetic nerve traffic to these body distributions are different.
A dissociation between MSNA and SSNA has been found in other disease states characterized by chronic sympathetic overactivity, including heart failure, essential hypertension, and obesity (8, 23). In these conditions, heightened MSNA was found, without concomitant elevations in SSNA. The differential distribution of MSNA and SSNA in heart failure may be explained by diminished baroreceptor sensitivity, because it is known that baroreflexes are abnormal in heart failure (7, 10). In contrast to heart failure, patients with ESRD have intact baroreflex sensitivity (2); however, the peripheral chemoreceptors are tonically sensitized (14).
We used the microneurography technique to make simultaneous measurements of MSNA and SSNA in patients with ESRD and normal controls to determine whether 1) sympathetic overactivity is also directed to skin, a body distribution that is independent of baroreflex and chemoreflex control, and 2) the pattern of sympathetic discharge in ESRD resembles other disease states characterized by chronic sympathetic overactivity.
MATERIALS AND METHODS
Subjects.
The study population consisted of 16 total participants (age range 21–59 yr): 8 ESRD patients and 8 healthy, age- and sex-matched controls. Six ESRD patients and six controls were male, and two ESRD patients and two controls were women. Exclusion criteria for all participants included smoking; illicit drug use; recent treatment with central α2-agonists; and major comorbid conditions, including diabetes, vascular disease, or any clinical evidence of heart disease determined by electrocardiogram (ECG), echocardiogram, stress test, and/or history. Of the subjects, 0 of 8 controls and 3 of 8 ESRD patients had hypertension that was controlled on an average of 2.3 medications. Antihypertensive medications included dihydropyridine calcium channel blocker (3), β1-selective β-blocker (2), β-blocker with α-blocking activity (1), and angiotensin receptor blocker (1). The etiology of ESRD was autosomal dominant polycystic kidney disease (ADPKD) in one, hypertension in one, lithium toxicity in one, shock in one, and unknown in four patients. All ESRD patients were on chronic maintenance hemodialysis at a frequency of 3 times per week, for ∼3.5 h at each session. None of the patients had problems with hemodynamic instability during dialysis. All subjects were being treated with stable doses of erythropoiesis stimulating agents with dialysis. Time on dialysis ranged from <1 mo to 14 yr, with an average duration of 2.9 yr. Written informed consent was obtained from each participant, and the study protocol was approved by the institutional review board of the University of Southern California.
Experimental procedures.
Arterial blood pressure was measured with an automated sphygmomanometer (Dynamap). Each data point of blood pressure was the mean of at least three consecutive readings. Heart rate was monitored with continuous ECG. Multiunit postganglionic sympathetic nerve activity directed to muscle (MSNA) and directed to skin (SSNA) were both obtained in each participant, and recorded directly from the peroneal nerve by microneurography, as previously described (28, 29). A tungsten microelectrode (tip diameter 5–15 μm) (Dept. of Bioengineering, University of Iowa, Ames, IA) was inserted into the nerve, and a reference microelectrode was inserted subcutaneously 1–2 cm from the recording electrode. The microelectrodes were connected to a preamplifier (gain 1,000), and an amplifier (gain 50-100). Nerve signals were filtered (700–2,000 Hz; model 662C-3, Nerve Traffic Analyzer, Dept. of Bioengineering, University of Iowa), rectified, and integrated (time constant 0.1 s) to obtain a mean voltage display of sympathetic nerve activity that was recorded by the Chart 5 Program (PowerLab 16sp, ADInstruments). Lead II of the ECG was recorded simultaneously with the neurogram. Sympathetic nerve activity directed to the muscle (MSNA) vs. skin (SSNA) was distinguished by previously described standards (4–6, 12, 22). The MSNA neurogram fulfilled the following criteria: 1) mild electrical stimulation caused a muscle contraction, but no paresthesia or pain; 2) nerve bursts were pulse synchronous and had a signal to noise ratio of > 3:1; 3) nerve activity increased with apnea and Valsalva, but it was not affected by a loud acoustic stimulus; and 4) afferent nerve signals were elicited with passive muscle stretch. The SSNA neurogram fulfilled the following criteria: 1) mild electrical stimulation caused paresthesias without causing a muscle contraction; 2) nerve bursts were not synchronous with the heart rate and were broad based, with a signal to noise ratio of >3:1; 3) nerve activity increased with loud acoustic stimulus; 4) afferent signals were elicited with light skin touching. A skin sympathetic nerve burst was identified if it occurred within a neurogram fulfilling these criteria and if it was broad based with an amplitude at least threefold greater than baseline noise. Sympathetic bursts were identified by visual inspection of nerve bursts by a single investigator without knowledge of the participant's status as patient or control. MSNA was expressed as burst frequency (bursts/min) and bursts per 100 heartbeats, and SSNA was expressed as burst frequency.
Experimental protocol.
All participants were studied in the early afternoon, after abstaining from food for 4 h and from exercise, caffeine, and alcohol for at least 12 h. All ESRD patients were studied on a nondialysis day, ∼24–30 h after the previous dialysis session. The study room was quiet, semidark, and temperate (∼21°C). Participants were placed in a supine position, and they were fitted with a blood pressure cuff on the upper arm for intermittent automatic blood pressure monitoring, and ECG patch electrodes for continuous heart rate recordings. Baseline blood pressure and heart rate were measured after 5 min of rest. The leg was positioned for microneurography, and the tungsten microelectrode was inserted and manipulated to obtain a satisfactory nerve recording. After 10 min of rest, sympathetic nerve activity to the muscle or skin distribution was recorded for 5–10 min. Afterward, the electrode was repositioned to record sympathetic nerve activity to the distribution that was not recorded earlier. Again, after 10 min of rest, the neurogram was recorded for 5–10 min. The order of MSNA and SSNA recordings was randomly obtained from each participant.
Data analysis.
Individual values were averaged for each group and expressed as means ± SE. Mean values were compared between the two groups using unpaired Student's t-test. Analysis of covariance was used to compare MSNA and SSNA between the two groups, adjusting for age. A P value of <0.05 was deemed statistically significant.
RESULTS
Age and body mass index did not differ significantly between ESRD and control participants (Table 1). The majority of both groups were men (men/women, 6/2 in ESRD, 6/2 in controls). ESRD patients tended to have higher systolic blood pressures (ESRD vs. controls, 136.6 ± 7.5 vs. 121.9 ± 4.2 mmHg; P = 0.11), but this difference did not reach statistical significance. Three of the eight ESRD patients also had hypertension, which was well controlled on two to three medications. Diastolic blood pressure and heart rate were not significantly different between the two groups.
Table 1.
Demographic, hemodynamic, and sympathetic activity values in end-stage renal disease and control participants
| ESRD | Control | P Value | |
|---|---|---|---|
| Age, yr | 39.0±5.6 | 31.9±3.1 | 0.29 |
| Body mass index, kg/m2 | 25.5±1.3 | 25.8±1.4 | 0.80 |
| Systolic BP, mmHg | 136.6±7.5 | 121.9±4.2 | 0.11 |
| Diastolic BP, mmHg | 77.8±3.3 | 71.8±3.4 | 0.23 |
| Heart rate, beats/min | 70.6±2.6 | 63.9±2.6 | 0.26 |
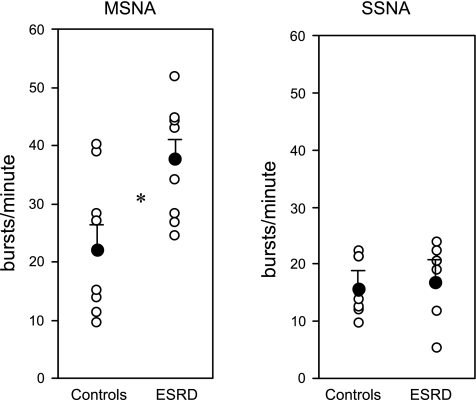
| MSNA, bursts/min | 37.3±3.6 | 23.1±4.4 | 0.025* |
| MSNA, bursts/100 heartbeats | 53.5±5.2 | 35.8±6.2 | 0.046* |
| SSNA, bursts/min | 17.6±2.2 | 16.1±1.7 | 0.61 |
Values are means ± SE. ESRD, end-stage renal disease; BP, blood pressure. Muscle sympathetic nerve activity (MSNA) is shown as both bursts per minute and bursts per 100 heartbeats. Skin sympathetic nerve activity (SSNA) is shown as bursts per minute.
Significant P values < 0.05.
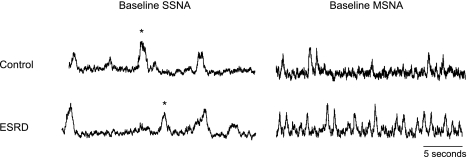
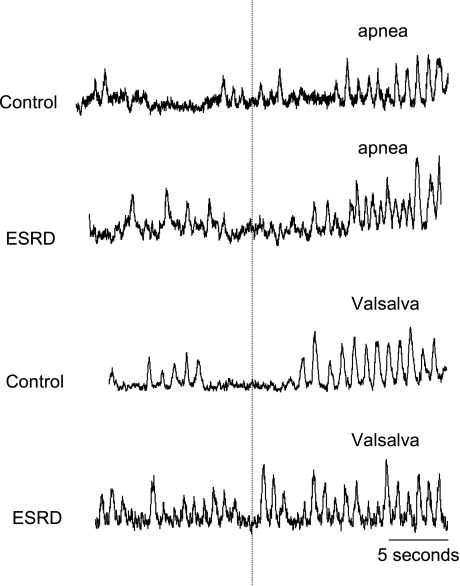
Figure 1 depicts baseline MSNA and SSNA neurograms from a representative ESRD and control participant. Figure 2 depicts examples of changes in MSNA during apnea and Valsalva maneuvers in ESRD and control participants. MSNA was significantly higher in ESRD patients compared with controls (ESRD vs. controls, 37.3 ± 3.6 vs. 23.1 ± 4.4 bursts/min; P = 0.025) (Table 1 and Fig. 3), and this difference remained significant after age adjustments. Results were similar when MSNA was analyzed as bursts per minute and bursts per 100 heartbeats (ESRD vs. controls, 53.5 ± 5.2 vs. 35.8 ± 6.2 bursts/100 heartbeats; P = 0.02). Conversely, SSNA was not significantly different between the two groups (ESRD vs. controls, 17.6 ± 2.2 vs. 16.1 ± 1.7 bursts/min; P = 0.61).
Fig. 1.
Neurograms depicting muscle sympathetic nerve activity (MSNA) and skin sympathetic nerve activity (SSNA) in a representative control and end-stage renal disease (ESRD) patient. *Loud acoustic stimulus.
Fig. 2.
MSNA during apnea and Valsalva maneuver. MSNA during 15–20 s of apnea and 15–20 s of Valsalva in ESRD and control participants is shown.
Fig. 3.
Sympathetic nerve activity directed to muscle and skin in ESRD and control participants. Sympathetic activity is compared between control participants and ESRD patients. MSNA and SSNA are shown as bustst/minute. Data are expressed as means ± SE. ○, Individual data points; •, means with SE. *Significant P values < 0.05.
DISCUSSION
We report the novel finding that although MSNA is chronically elevated in ESRD patients, SSNA is not elevated compared with healthy controls. Sympathetic activation does not appear to be uniform to all body distributions in ESRD, and it is selectively heightened to the muscle distribution, which is dependent on baroreflex and chemoreflex regulation.
Prior studies using microneurography to measure sympathetic nerve traffic to muscle have demonstrated chronic elevations of MSNA in patients with ESRD (3) and chronic kidney disease (16–18, 20, 24). In humans with ADPKD, MSNA was chronically elevated, regardless of renal function. However, increased MSNA was only apparent in hypertensive patients with ADPKD and not in normotensive patients. In fact, in most human studies to date that demonstrate chronic MSNA elevation in renal failure, the majority of the patients had hypertension as a comorbid condition. Interestingly, our study, in which the majority of the dialysis patients were normotensive, revealed significant increases in MSNA compared with healthy controls, despite comparable blood pressures. Although SNS activity directed to muscle was significantly elevated, we found that SNS activity directed to the skin was virtually identical between ESRD patients and controls.
The mechanisms underlying the differential distribution of SNS activity to the muscle vs. skin in this population can only be speculated in this study, but it could potentially reflect chronic chemoreceptor activation of central sympathetic discharge, which was demonstrated in a previous study (14). Chemoreceptors are stimulated experimentally by voluntary apneic maneuvers and increase MSNA selectively, without increasing SSNA. Peripheral chemoreflex activation of renal afferent nerves by intrarenal adenosine accumulation (15), one putative trigger for renal afferent activation of central sympathetic outflow, may potentially also cause nonuniform increases in sympathetic activity. In addition, other proposed contributors to SNS overactivity in renal failure, including high circulating levels of angiotensin II (17, 20, 24, 26), asymmetric dimethylarginine (21), leptin (27), insulin (30), and reactive oxygen species (13), may cause nonuniform increases in central sympathetic outflow.
We have found that patients with renal failure exhibit a similar pattern of SNS discharge as other disease states characterized by chronic sympathetic overactivity. MSNA is chronically elevated in patients with heart failure, obesity, and essential hypertension; however, SSNA is not elevated in these conditions compared with healthy controls (8, 23). The mechanisms underlying the nonuniform distribution of sympathetic activity in these conditions is not certain, but it is thought to be due to impaired baroreflex sensitivity. Baroreflex sensitivity has been shown to be abnormal in heart failure (7, 10) and obesity (9, 11) and could contribute to SNS overactivity and independent control of MSNA and SSNA in these conditions. One limitation of the present study is that we did not test baroreflexes in the ESRD patients. However, although some prior studies report abnormal baroreflex modulation of heart rate in renal failure, particularly in those prone to hemodynamic instability during dialysis (1, 19, 25), studies examining the cardiopulmonary and sinoaortic baroreflex regulation of sympathetic nerve activity in patients with ESRD (2) and chronic kidney disease (16, 20) have shown that sympathetic responses to baroreflex engagement are intact, albeit the baroreflex curve is shifted to the right. Thus abnormal arterial baroreflex sensitivity is unlikely to be the cause of chronic SNS overactivity or nonuniform distribution of sympathetic outflow in renal failure, and other mechanisms that result in activation and central partitioning of sympathetic outflow, must be considered.
PERSPECTIVES AND SIGNIFICANCE
We have found that although SNS activity directed to muscle is significantly elevated in ESRD patients, there is no elevation in SNS activity directed to skin, a body distribution that is free from baroreflex and chemoreflex control. In addition, we found that baseline MSNA was significantly higher than controls even in a predominantly normotensive group of dialysis patients. The differential distribution of SNS outflow to the muscle and skin in ESRD is similar to the pattern that has been observed in other disease states characterized by chronic SNS overactivation. Because sympathetic overactivity contributes to the profound increased risk of cardiovascular mortality in renal patients, further studies investigating sympathetic regulation, distribution, and potential therapeutic interventions are needed in the future.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants 9F32 DK-080995 (to J. Park), 1RO1 HL-071792 (to V. M. Campese), and 1RO1 HL-084525 (to H. R. Middlekauff) and by National Center for Research Resources /NIH Grant M01 RR-00043.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Agarwal A, Anand IS, Sakhuja V, Chugh KS. Effect of dialysis and renal transplantation on autonomic dysfunction in chronic renal failure. Kidney Int 40: 489–495, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Converse RL Jr, Jacobsen TN, Jost CM, Toto RD, Grayburn PA, Obregon TM, Fouad-Tarazi F, Victor RG. Paradoxical withdrawal of reflex vasoconstriction as a cause of hemodialysis-induced hypotension. J Clin Invest 90: 1657–1665, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Converse RL Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med 327: 1912–1918, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Delius W, Hagbarth KE, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand 84: 65–81, 1972. [DOI] [PubMed] [Google Scholar]
- 5.Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiol Scand 84: 177–186, 1972. [DOI] [PubMed] [Google Scholar]
- 6.Delius W, Hongell A, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol Scand 84: 82–94, 1972. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson DW, Berg WJ, Roach PJ, Oren RM, Mark AL. Effects of heart failure on baroreflex control of sympathetic neural activity. Am J Cardiol 69: 523–531, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Grassi G, Colombo M, Seravalle G, Spaziani D, Mancia G. Dissociation between muscle and skin sympathetic nerve activity in essential hypertension, obesity, and congestive heart failure. Hypertension 31: 64–67, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia G. Sympathetic activation in obese normotensive subjects. Hypertension 25: 560–563, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, Del Bo A, Sala C, Bolla GB, Pozzi M. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation 92: 3206–3211, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, Mancia G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation 97: 2037–2042, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Hagbarth KE, Hallin RG, Hongell A, Torebjork HE, Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand 84: 164–176, 1972. [DOI] [PubMed] [Google Scholar]
- 13.Hasdan G, Benchetrit S, Rashid G, Green J, Bernheim J, Rathaus M. Endothelial dysfunction and hypertension in 5/6 nephrectomized rats are mediated by vascular superoxide. Kidney Int 61: 586–590, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Hering D, Zdrojewski Z, Krol E, Kara T, Kucharska W, Somers VK, Rutkowski B, Narkiewicz K. Tonic chemoreflex activation contributes to the elevated muscle sympathetic nerve activity in patients with chronic renal failure. J Hypertens 25: 157–161, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Katholi RE, Whitlow PL, Hageman GR, Woods WT. Intrarenal adenosine produces hypertension by activating the sympathetic nervous system via the renal nerves in the dog. J Hypertens 2: 349–359, 1984. [PubMed] [Google Scholar]
- 16.Klein IH, Ligtenberg G, Neumann J, Oey PL, Koomans HA, Blankestijn PJ. Sympathetic nerve activity is inappropriately increased in chronic renal disease. J Am Soc Nephrol 14: 3239–3244, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Klein IH, Ligtenberg G, Oey PL, Koomans HA, Blankestijn PJ. Enalapril and losartan reduce sympathetic hyperactivity in patients with chronic renal failure. J Am Soc Nephrol 14: 425–430, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Klein IH, Ligtenberg G, Oey PL, Koomans HA, Blankestijn PJ. Sympathetic activity is increased in polycystic kidney disease and is associated with hypertension. J Am Soc Nephrol 12: 2427–2433, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Lazarus JM, Hampers CL, Lowrie EG, Merrill JP. Baroreceptor activity in normotensive and hypertensive uremic patients. Circulation 47: 1015–1021, 1973. [DOI] [PubMed] [Google Scholar]
- 20.Ligtenberg G, Blankestijn PJ, Oey PL, Klein IH, Dijkhorst-Oei LT, Boomsma F, Wieneke GH, van Huffelen AC, Koomans HA. Reduction of sympathetic hyperactivity by enalapril in patients with chronic renal failure. N Engl J Med 340: 1321–1328, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Mallamaci F, Tripepi G, Maas R, Malatino L, Boger R, Zoccali C. Analysis of the relationship between norepinephrine and asymmetric dimethyl arginine levels among patients with end-stage renal disease. J Am Soc Nephrol 15: 435–441, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Mano T, Iwase S, Toma S. Microneurography as a tool in clinical neurophysiology to investigate peripheral neural traffic in humans. Clin Neurophysiol 117: 2357–2384, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Middlekauff HR, Hamilton MA, Stevenson LW, Mark AL. Independent control of skin and muscle sympathetic nerve activity in patients with heart failure. Circulation 90: 1794–1798, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Neumann J, Ligtenberg G, Oey L, Koomans HA, Blankestijn PJ. Moxonidine normalizes sympathetic hyperactivity in patients with eprosartan-treated chronic renal failure. J Am Soc Nephrol 15: 2902–2907, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Pickering TG, Gribbin B, Oliver DO. Baroreflex sensitivity in patients on long-term haemodialysis. Clin Sci 43: 645–657, 1972. [DOI] [PubMed] [Google Scholar]
- 26.Reid IA Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am J Physiol Endocrinol Metab 262: E763–E778, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Sharma K, Considine RV, Michael B, Dunn SR, Weisberg LS, Kurnik BR, Kurnik PB, O'Connor J, Sinha M, Caro JF. Plasma leptin is partly cleared by the kidney and is elevated in hemodialysis patients. Kidney Int 51: 1980–1985, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Vallbo AB, Hagbarth KE, Wallin BG. Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. J Appl Physiol 96: 1262–1269, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Wallin BG, Fagius J. Peripheral sympathetic neural activity in conscious humans. Annu Rev Physiol 50: 565–576, 1988. [DOI] [PubMed] [Google Scholar]
- 30.Zoccali C, Tripepi G, Cambareri F, Catalano F, Finocchiaro P, Cutrupi S, Pizzini P, Testa A, Spoto B, Panuccio V, Enia G, Mallamaci F. Adipose tissue cytokines, insulin sensitivity, inflammation, and cardiovascular outcomes in end-stage renal disease patients. J Ren Nutr 15: 125–130, 2005. [DOI] [PubMed] [Google Scholar]