Abstract
The TAR RNA binding Protein, TRBP, inhibits the activity of the interferon-induced protein kinase R (PKR), whereas the PKR activator, PACT, activates its function. TRBP and PACT also bind to each other through their double-stranded RNA binding domains (dsRBDs) and their Medipal domains, which may influence their activity on PKR. In a human immunodeficiency virus (HIV) long terminal repeat-luciferase assay, PACT unexpectedly reversed PKR-mediated inhibition of gene expression. In a translation inhibition assay in HeLa cells, PACT lacking the 13 C-terminal amino acids (PACTΔ13), but not full-length PACT, activated PKR and enhanced interferon-mediated repression. In contrast, in the astrocytic U251MG cells that express low TRBP levels, both proteins activate PKR, but PACTΔ13 is stronger. Immunoprecipitation assays and yeast two-hybrid assays show that TRBP and PACTΔ13 interact very weakly due to a loss of binding in the Medipal domain. PACT-induced PKR phosphorylation was restored in Tarbp2−/− murine tail fibroblasts and in HEK293T or HeLa cells when TRBP expression was reduced by RNA interference. In HEK293T and HeLa cells, arsenite, peroxide, and serum starvation-mediated stresses dissociated the TRBP-PACT interaction and increased PACT-induced PKR activation, demonstrating the relevance of this control in a physiological context. Our results demonstrate that in cells, TRBP controls PACT activation of PKR, an activity that is reversed by stress.
One of the early cellular responses to viral infection is the production of the interferon (IFN)-induced double-stranded RNA (dsRNA)-activated protein kinase R (PKR) (16, 56). PKR is a dsRNA binding protein (dsRBP) with a kinase domain that becomes activated upon phosphorylation. Activation of PKR leads to the phosphorylation of the α subunit of the eukaryotic translation initiation factor 2 (eIF2α) and a decrease in the rate of translational initiation of both viral and cellular mRNAs (27). Many viral and cellular factors regulate its activation (28, 29). PKR is activated by viral RNAs like human immunodeficiency virus (HIV) transactivation response (TAR), reovirus S1, and hepatitis delta virus RNAs (8, 55, 68). To counteract PKR activation, viruses encode several PKR inhibitors like influenza NS1, vaccinia E3L and K3L, reovirus σ3, herpesvirus Us11, rotavirus NSP3, hepatitis C virus NS5A and E2, and HIV type 1 (HIV-1) Tat proteins (10, 19, 20, 26, 29, 38, 45, 74). Large amounts of the viral HIV TAR, Epstein-Barr virus EBER, and adenovirus VAI RNAs also inhibit its activity (29). Several cellular factors (p58IPK, ribosomal protein L18, the autoantigen La, the TAR RNA binding protein TRBP, the PKR activator PACT, NF-90, Hsp90, nucleophosmin, Mda7, ADAR1, and hDUS2) bind to PKR and may control its activation during cellular processes (17, 22, 40, 44, 51, 58, 59, 62, 63, 65, 72). Among these, TRBP inhibits, whereas PACT enhances PKR function on translation, cell growth, and viral expression (5, 17, 64, 65).
TRBP1 and TRBP2 are cellular proteins that were originally described through their ability to bind the HIV-1 TAR RNA (1, 31, 32). TRBP2 has 21 additional amino acids (aa) in its N-terminal end, but both forms of the protein stimulate the expression of the HIV-1 long terminal repeat (LTR) in human and murine cells (4, 17, 24). TRBP is a dsRBP with two dsRNA binding domains (dsRBDs) (13, 71), a KR-helix motif within dsRBD2 (21, 25, 30), and a C-terminal domain that mediates protein-protein interactions and that we referred to as the Medipal domain because it binds Merlin, Dicer, and PACT (37, 46, 48). TRBP binds directly to PKR through the dsRBDs and blocks PKR's inhibitory effects on translation, Saccharomyces cerevisiae growth, and HIV expression and replication (5, 15, 17, 60, 61). The protein also increases translation from structured RNAs (23). Human astrocytes infected with HIV have a heightened PKR response due to low endogenous TRBP expression (2, 3, 60). During development, TRBP and the protamine RNA binding protein (PRBP), its murine homolog, have a function in spermatogenesis and growth control (50, 75). The protein has an oncogenic potential due to its inhibition of PKR (5). The tumor suppressor Merlin binds to TRBP and induces its ubiquitination and degradation, which reverses its oncogenicity (48, 49). TRBP also interacts with Dicer and is required for RNA interference (RNAi), suggesting a cross talk between the PKR and the RNAi pathways (14, 37).
PACT is a human cellular protein that heterodimerizes with PKR through its dsRBDs and its C terminus, also called the Medipal domain by homology with TRBP (46, 65). It activates PKR in vitro and in vivo in the absence of dsRNA. Mammalian cells transfected with PACT show an enhanced phosphorylation of PKR and eIF2α, which induces an increase in PKR-mediated translational inhibition (65). PKR activator X (RAX), the mouse homolog of PACT, regulates PKR activation upon phosphorylation in response to interleukin-3 (IL-3) deprivation and stress treatments (41). RAX and PACT are proapoptotic proteins that induce apoptosis by PKR activation (64). The two dsRBDs of PACT bind PKR, and its Medipal domain is essential for the activation of this kinase (36, 39, 66). In contrast to PACT enhancement of PKR inhibition of translation, in the absence of IFN, PACT activates translation in cells that express TRBP but inhibits translation in cells where TRBP-PACT heterodimers cannot be formed (46, 54).
Interestingly, TRBP and PACT have 40% similarity (24, 65), they interact with each other in their dsRBDs and their Medipal domains (46), and they both regulate the RNAi pathway (43, 52). Because they have opposite effects on the control of translation by PKR, it seems very likely that the balance between TRBP and PACT and their mode of interaction can regulate PKR function, but the interplay between these two proteins on PKR activation has not been investigated. In this study, we reveal the importance of the Medipal domain of PACT in the regulation of the activation of PKR through the comparison of a truncated version of PACT with full-length PACT. Importantly, we demonstrate that PACT activates PKR only when TRBP concentration is low or during stress due to a dissociation of the TRBP-PACT complex. Our results support the notion that TRBP regulates the activation of PKR by controlling its accessibility to PACT, an activity inhibited by stress treatment.
MATERIALS AND METHODS
Plasmid constructions.
pcDNA3-TRBP1 and pcDNA3-TRBP2 have been described previously (24). pCMV2-Flag-PACT (where CMV is cytomegalovirus) was previously described (46). To construct pCMV2-Flag-PACT with a deletion of 13 C-terminal amino acids of PACT (pCMV2-Flag-PACTΔ13), one nucleotide was removed by creating a mutated PCR product that was inserted into the EcoRI and SalI sites in PACT. The original Flag-PACT305 (PACT truncated at residue 300 plus 5 unrelated amino acids) (65) was renamed Flag-PACTΔ13-pCB6+. The wild-type (wt) Flag-PACT clone in the same vector was created by subcloning a Flag-PACT insert into the pCB6+ vector; this construct is designated Flag-PACT-pCB6+.
TRBP-C (Medipal domain) and PACT-C fragments in pGADGH, pGBT9, pGBT-Tat, and pGADGH-cyclin T1 (CycT) have been described previously (4, 17, 46). PGBT9-PACT-CΔ13 PACT was cloned by cutting the DraIII-SalI fragment from pCMV2-Flag-PACTΔ13 and inserting it into PGBT9-PACT-C cut with the same enzymes.
Yeast two-hybrid assay.
Yeast expression plasmids were introduced into the yeast reporter strain SFY526. The double transformants were selected and screened for β-galactosidase activity as described previously (46).
Cells and transfections.
Human HeLa, astrocytic U251MG (3), and HEK293T cells as well as monkey embryonic kidney cells COS-7, murine fibroblasts NIH 3T3 (ATCC), murine embryonic fibroblasts (MEFs), and PKR−/− MEFs (73) were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (HyClone), 2 mM l-glutamine and 1% penicillin-streptomycin (Invitrogen). Human lymphocytic Jurkat T-cells (ATCC) were maintained in RPMI 1640 medium (Invitrogen) supplemented similarly. Primary tail embryonic fibroblasts (TEFs) were isolated from 1- to 3-month-old Tarbp2 mutant and heterozygous littermates. About 1-cm tail tips were minced and subsequently digested with 0.5 mg/ml collagenase (from Clostridium histolyticum; Sigma) in DMEM for 1 h with modest stirring at 37°C. After collection by centrifugation at 1,200 rpm for 5 min, cells were cultured in DMEM supplemented with 2 mM l-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin (Invitrogen), and 16% fetal bovine serum (HyClone).
When indicated (see Fig. 2B, C, and D; 3B; 4A; and 5A), the cells were treated with 100 U/ml of human IFN-α (Sigma) or IFN-β (R&D Systems) at 24 h and harvested 48 h posttransfection. To verify the IFN response, HeLa or Jurkat cells were treated with 1,000 U/ml of human IFN-α2b or -β (R&D Systems) (see Fig. 2A). Cells were harvested 24 h after treatment and assayed by immunoblotting using phospho-specific anti-Stat1α (anti-P-Stat1α) antibodies (sc-417; Santa Cruz). Stress treatment was performed by incubating the cells for 1 h in 0.5 mM sodium arsenite, NaAsO2 (Sigma), for 1 h in 10 mM hydrogen peroxide, H2O2 (Sigma), or in medium with 0.1% serum (serum starvation) for 24 h immediately before cell lysis (42).
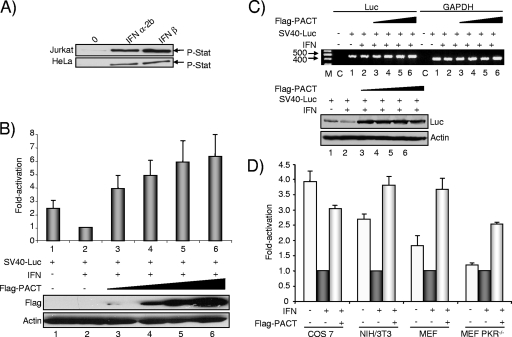
FIG. 2.
PACT overexpression reverses IFN-induced inhibition of SV40 promoter expression in HeLa, COS-7, NIH 3T3, and MEF cells. (A) Jurkat and HeLa cells are activated by IFN treatment. Jurkat or HeLa cells were incubated without IFN (0) or with 1,000 U/ml of IFN-α2b or IFN-β as indicated. Fifty micrograms of total protein was separated by SDS-PAGE and blotted with anti-P-Stat1 antibodies. (B) PACT reverses IFN-mediated SV40-Luc repression in HeLa cells. HeLa cells were transfected with 0.8 μg of SV40-Luc and 0.1 (lane 3), 0.2 (lane 4), 0.4 (lane 5), of 0.8 (lane 6) μg of pCMV-Flag-PACT. pCMV-Flag was added to reach the same amount of transfected DNA. Cells (lanes 2 to 6) were stimulated with 100 U/ml IFN-β as indicated. The Luc level was normalized to 1 for cells treated with IFN. Fifty micrograms of whole-cell extracts (lanes 1 to 6) was subjected to SDS-PAGE and blotted with anti-Flag and antiactin antibodies as indicated. (C) PACT mildly increases the RNA level of an SV40-Luc reporter gene inhibited by IFN. Cells were transfected as described in panel B (lanes 1 to 6). RT-PCR (top) was performed on RNA extracted from the transfected cells as indicated in Materials and Methods. Lane M, molecular weight markers; lane C, control with no cDNA. The Luc- and the GAPDH-amplified DNAs are indicated on top. Whole-cell extracts (200 μg) were subjected to SDS-PAGE and blotted (bottom) with anti-Luc and anti-actin antibodies as indicated. (D) PACT reverses IFN-mediated SV40-Luc repression in COS-7, NIH 3T3, and MEF cells. COS-7, NIH 3T3, MEF, and PKR−/− MEF cells were transfected as described in panel B with 0.8 μg of SV40-Luc plasmid (all) and 0.2 μg of pCMV-Flag-PACT (Flag-PACT) as indicated. Cells were treated with 100 U/ml IFN-β as indicated. The Luc level was normalized to 1 for cells treated with IFN (black bars) compared to untreated cells (open bars). The activation is calculated as the ratio between Luc expression in the presence of PACT versus in the absence of PACT in the IFN-treated cells (gray bars). Graphs represent the averages of three to five independent transfections ± standard error of the mean.
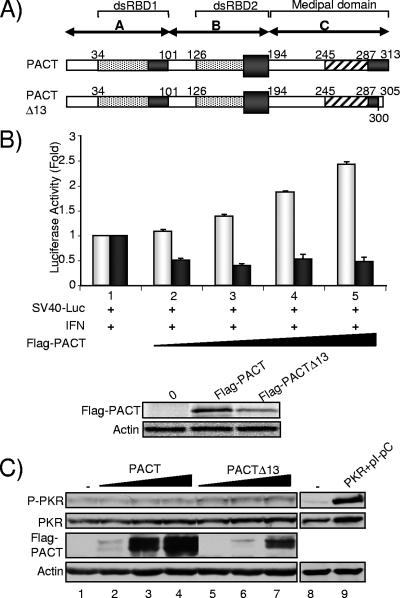
FIG. 3.
PACT activation of PKR in HeLa cells requires deletion of 13 amino acids (Δ13) and IFN induction. (A) Schematic representation of PACT and PACTΔ13 and their domains. Domain C is also called the Medipal domain of PACT by homology with TRBP (46). (B) Translation inhibition assay with PACT and PACTΔ13. (Top) HeLa cells were transfected with 800 ng of pGL2C (Promega) encoding Luc (SV40-Luc) and without plasmid (lane 1) or with 0.15 μg (lane 2), 0.3 μg (lane 3), 0.45 μg (lane 4), or 0.6 μg (lane 5) of Flag-PACT-pCB6+ (light bars) or Flag-PACTΔ13-pCB6+ (dark bars). At 24 h posttransfection, cells were treated with 100 U/ml of IFN-β for 24 h and then harvested for Luc activity measurement. Luc activity was normalized for the total protein present in the extract, and the error bars represent the standard error calculated from six independent values. (Bottom) Extracts from lane 1 (no PACT) above or lane 5 (PACT or PACTΔ13) were separated by SDS-PAGE and blotted with an anti-Flag or antiactin antibody. (C) PACT or PACTΔ13 does not activate PKR in the absence of IFN. HeLa cells were transfected alone (lanes 1, 8, and 9) or with 0.1 (lanes 2 and 5), 0.5 (lanes 3 and 6), and 1 (lanes 4 and 7) μg of pCMV2-Flag-PACT or pCMV2-Flag-PACTΔ13, as indicated, or with 2 μg of pcDNA1-PKR and 0.02 μg of poly(I)·poly(C) (lane 9). Whole-cell extracts (200 μg) were subjected to SDS-PAGE and blotted with anti-P-PKR, anti-PKR, anti-Flag, and antiactin antibodies as indicated.
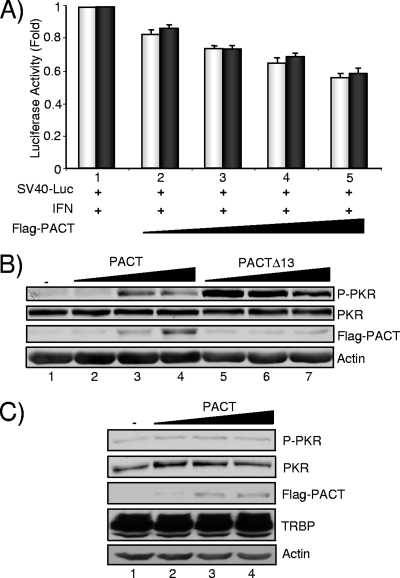
FIG. 4.
PACT and PACTΔ13 induce phosphorylation of PKR in astrocytes. (A) Translation inhibition assay with PACT and PACTΔ13. U251MG cells were transfected with 800 ng of pGL2C (SV40-Luc) without plasmid (lane 1) or with 0.15 μg (lane 2), 0.3 μg (lane 3), 0.45 μg (lane 4), or 0.6 μg (lane 5) of Flag-PACT-pCB6+ (light bars) or Flag-PACTΔ13-pCB6+ (dark bars). Cells were treated with IFN-β and harvested as described in the legend of Fig. 3B. Luc activity was normalized for the total protein present in the extract, and the error bars represent the standard error calculated from two independent experiments performed in triplicate. (B) PACT and PACTΔ13 activate PKR in astrocytes. U251MG cells were transfected alone (lane 1) or with 0.1 (lanes 2 and 5), 0.5 (lanes 3 and 6), and 1 (lanes 4 and 7) μg of pCMV2-Flag-PACT or pCMV2-Flag-PACTΔ13 as indicated. Whole-cell extracts (200 μg) were subjected to SDS-PAGE and blotted with anti-P-PKR, anti-PKR, anti-Flag, and antiactin antibodies as indicated. (C) PACT-induced phosphorylation of PKR in astrocytes is suppressed by TRBP2 overexpression. U251MG cells that overexpress pcDNA3-TRBP2 were transfected alone (lane 1) or with 0.1 (lane 2), 0.5 (lane 3), and 1 (lane 4) μg of pCMV2-Flag-PACT. Whole-cell extracts (150 μg) were subjected to SDS-PAGE and blotted with anti-P-PKR, anti-PKR, anti-Flag, anti-TRBP672, and antiactin antibodies as indicated.
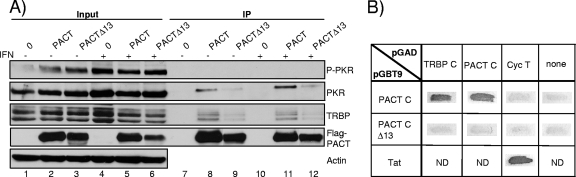
FIG. 5.
PACTΔ13 weakly interacts with either TRBP or PKR. (A) PACTΔ13 interacts weakly with TRBP and PKR. HeLa cells, not treated (lanes 1 to 3 and 7 to 9) or treated by IFN-α (lanes 4 to 6 and 10 to 12), were transfected alone (lanes 1 and 7) or with 10 μg of pCMV2-Flag-PACT (lanes 2, 5, 8, and 11) or 10 μg of pCMV2-Flag-PACTΔ13 (lanes 3, 6, 9, and 12). IP was performed with 1.5 mg of protein and anti-Flag antibody. A total of 200 μg of proteins from each lysate (input; lanes 1 to 6) and the immunoprecipitated complexes (lanes 7 to 12) was run on a 12% SDS-PAGE gel and blotted using anti-P-PKR, anti-PKR, anti-TRBP672, anti-Flag, and antiactin antibodies. (B) The TRBP Medipal domain does not bind PACT-CΔ13. Shown is the two-hybrid assay for the TRBP-C and PACT-C fragments with the PACT-C and PACT-CΔ13 fragments in the indicated vectors. Tat-CycT interaction is a positive control. CycT is a negative control for PACT-C and PACT-CΔ13.
For transfections, cells were plated in 12- or 6-well plates to obtain cell lysates or in 100-mm plates for immunoprecipitations (IPs). Transfections were performed using FuGENE 6 Reagent (Roche) at a 1:3 DNA-to-FuGENE ratio, and cells were lysed 48 h posttransfection for luciferase (Luc) assay, immunoblotting, or IP. For Luc assays, the cells were washed three times with phosphate-buffered saline (PBS) and lysed in 100 μl of Luc assay lysis buffer (Promega). Luc expression was measured by adding 50 μl of the substrate (Promega) to 20 μl of cell lysate. Luminescence was measured as previously described (17). Cells were cotransfected with pEGFP-N3 (Clontech) plasmid to measure transfection efficiency. Cells expressing enhanced green fluorescent protein (EGFP), and the total number of cells was counted using an inverted microscope. The ratio between EGFP-expressing cells and the total number of cells provided the transfection efficiency. Transfections of HeLa and 293T cells with small interfering RNAs (siRNAs) were performed by FuGENE 6 as above using a 1:3 RNA-to-FuGENE ratio 24 h prior to the transfection of the Flag-PACT plasmid. The siRNA targeting nucleotide 571 of TRBP (siRNA571) (15) and the nonspecific sequence (Qiagen) were synthesized using a Silencer siRNA construction kit (Ambion). The U251MG stable cell line overexpressing TRBP2 was obtained after transfection by the calcium phosphate precipitation method with pcDNA3-TRBP2 vector. Selection was performed in supplemented DMEM containing 0.5 mg/ml of G418 (Invitrogen).
Translation inhibition assay and IFN treatment.
A translation inhibition assay was performed as described previously (65). HeLa or U251MG cells were transfected with 800 ng of the pGL2-control reporter plasmid (Promega) and the Flag-PACT-pCB6+ or the Flag-PACTΔ13-pCB6+ clones. The cells were treated with 100 U/ml of human IFN-β (R&D Systems) at 24 h and harvested 48 h posttransfection. Luc activity was determined as described above.
Immunoblotting.
Cell lysates were prepared, separated, and transferred for immunoblotting as previously described (46). The membrane was blocked for 1 h in 5% nonfat milk and 0.05% Tris-buffered saline-Tween 20 (TBST) (70) or 5% bovine serum albumin (BSA) and 0.1% TBST for anti-PKR and anti-PKR-pT451 (PKR peptide phosphorylated at threonine 451). The membranes were incubated overnight at 4°C with anti-PRBP (50), anti-TRBP672 (2) or anti-TRBPjbx (raised in rabbits against the same peptides as TRBP672) antibodies at a 1/500 dilution; or with anti-PACTeg2 (raised in rabbits against PACT peptides) at a 1/1,000 dilution; or with 1 μg/ml of anti-Flag (Sigma), anti-P-Stat1α, or 2 μg/ml of anti-Luc (Sigma) monoclonal antibodies in 5% milk/PBST. Membranes were incubated for 1 h at room temperature with a monoclonal antiactin antibody (Chemicon) at a 1/10,000 dilution. For probing PKR and phosphorylated PKR (P-PKR), membranes were incubated overnight at 4°C with monoclonal anti-PKR 71/10, obtained through Ara Hovanessian (47, 57) or polyclonal anti-PKR-pT451 (Biosource) at a 1/1,000 dilution in 3% BSA-TBST. After five washes in TBST, membranes were incubated with peroxidase-conjugated secondary goat anti-rabbit antibody (Amersham) for TRBP, P-PKR, and PRBP and goat anti-mouse (Amersham) for Flag, Luc, Stat1, PKR, and actin at a 1/5,000 dilution. The bands were visualized as described previously (4). For anti-PKR, exposure time was 5 min with extracts from human cells and overnight with extracts from murine cells.
Coimmunoprecipitation.
At 48 h posttransfection, cells were washed twice with PBS and lysed in the cold lysis buffer with protease and phosphatase inhibitors (46). For each IP, 50 μl of protein G-agarose Fast Flow compact beads (Sigma) was washed twice with TNEN buffer (50 mM Tris-HCl [pH 7.4], 100 mM NaCl, 1 mM EDTA [pH 8], 0.5% NP-40 [Sigma]) and 1% BSA and left rotating at 4°C for 2 h with 1 μg of anti-Flag antibody. Cell extract (1.5 mg) was added to the beads for overnight incubation at 4°C. The beads were washed three times with 1 ml of cold lysis buffer and five times with 1 ml of cold PBS and resuspended in sodium dodecyl sulfate (SDS) loading dye. Bound proteins were eluted by boiling the beads for 5 min and fractionated by 10% SDS-polyacrylamide gel electrophoresis (PAGE). The immunoprecipitates were analyzed by Western blot analysis using the anti-PKR-pT451, anti-PKR, anti-TRBP672, anti-TRBPjbx, anti-Flag, or antiactin antibodies.
RNA analysis.
Total RNA was extracted from adult testes and cultured tail fibroblast cells by guanidinium isothiocyanate, followed by lithium chloride precipitation, as previously described (12). Fifteen micrograms of each RNA sample was separated on a 1.5% agarose gel containing 2.2 M formaldehyde and then transferred to a nylon membrane and hybridized as previously described (9). Probes were prepared from a 1.5-kb Tarbp2 cDNA fragment by random hexamer labeling.
For semiquantitative reverse transcription-PCR (RT-PCR), total RNA was extracted 24 h posttransfection with Trizol isolation reagent (Invitrogen) and treated by DNase I (GE). Luc and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNAs were reverse transcribed from 5 μg of total RNA using 30 ng of Luc antisense (5′-CGTCTACATCGACTGAAATCCC-3′) and GAPDH antisense (5′-CCAAAGTTGTCATGGATGACC-3′) specific primers in a 25-μl reaction mixture containing 30 U of RNAguard (GE), 1 mM each of the deoxynucleoside triphosphates, 10 mM dithiothreitol, and 300 U of Superscript II (Invitrogen). Incubation was performed at 42°C for 1 h, and 5 μl (for Luc) or 2 μl (for GAPDH) of the resulting reaction mixture containing the single-strand cDNA template was used for PCR amplification. Conditions for amplifications were the following: 94°C for 5 min and 25 cycles of 94°C for 45 s, 55°C for 45 s, and 72°C for 2 min, followed by a 5-min incubation at 72°C. PCR amplifications were performed in a 100-μl reaction mixture containing 250 ng of each Luc sense primer (5′-CTATCCTCTAGAGGATGGAACC-3′) or GAPDH sense primer(5′-CCTTCATTGACCTCAACTACAT-3′), 2.5 U of Taq DNA polymerase (Invitrogen), 1.5 mM MgCl2, 0.2 mM each of the deoxynucleoside triphosphates, and 1× Taq buffer (Invitrogen). The products were fractionated on a 1.5% agarose gel. The Luc and GAPDH sets of primers identify a 456-nucleotide (nt) and a 400-nt band, respectively. All reactions with Luc primers were also run in the absence of Superscript II and verified for an absence of band. The GAPDH set of primers identifies a 400-nt band amplified by RT-PCR from the mRNA and a 800-nt band amplified from the genomic DNA (18). All gels were verified for the absence of an 800-nt band with GAPDH primers.
RESULTS
PACT overexpression reverses PKR-mediated inhibition of HIV-1 LTR expression.
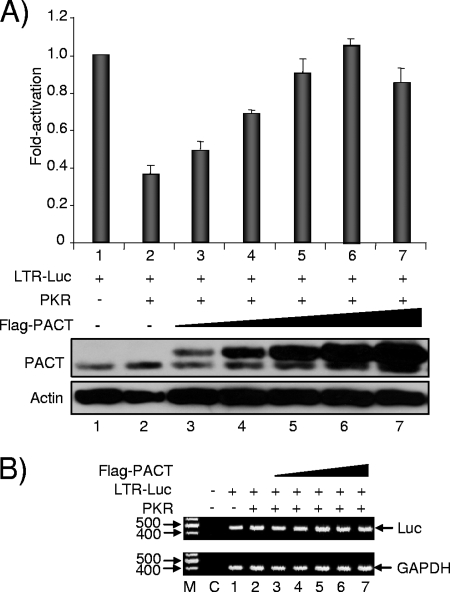
TRBP activates HIV-1 LTR expression by two mechanisms: by a release of the translation inhibition due to the TAR structure and by PKR inhibition (5, 17, 23). In contrast, PACT was reported to inhibit translation through the activation of PKR (65, 66). We therefore expected that PACT's activity would be opposite that of TRBP on PKR-mediated translation inhibition. As TRBP was previously assayed on the HIV-1 LTR repressed by PKR (17), we used the same assay to test for PACT activity. Surprisingly, under conditions where PKR alone inhibits HIV-1 LTR expression by two- to threefold, the addition of PACT restored HIV-1 LTR expression in a dose-dependent manner (Fig. 1A). We verified the amount of PACT protein produced before and after transfection using an antibody that recognizes both the endogenous and the transfected Flag-tagged forms. Although the recognition of both forms by the antibody may vary slightly, the result suggests that endogenous PACT is weakly expressed and might be limiting in the cell (Fig. 1A, bottom). To determine if part of PACT activity occurs at the RNA level, we performed a semiquantitative RT-PCR on the Luc mRNA and found no change in RNA concentration under these conditions, suggesting that the main activity of PACT on LTR-driven gene expression is at the posttranscriptional level (Fig. 1B). This result suggests that PACT may reverse the PKR-induced inhibition of the HIV-1 LTR, similarly to TRBP (17).
FIG. 1.
PACT overexpression reverses PKR-mediated inhibition of HIV-1 LTR expression. (A) PACT reverses PKR activity on HIV-1 LTR expression in HeLa cells. HeLa cells were transfected with 0.05 μg of pGL2-LTR-Luc (lanes 1 to 7) and 1 μg of pcDNA1-PKRwt (PKR) as indicated. Cotransfection was performed with 0.05 (lane 3), 0.1 (lane 4), 0.25 (lane 5), 0.5 (lane 6), or 0.95 (lane 7) μg of pCMV-Flag-PACT. In each experiment the empty vector (pCMV-Flag) was added to reach the same amount of transfected DNA. The activation is the ratio between the Luc level in the presence of PACT and/or PKR versus LTR-Luc alone. Whole-cell extracts (150 μg) (lanes 1 to 7) were subjected to SDS-PAGE and blotted with anti-PACTeg2 and antiactin antibodies as indicated. The lower band of PACT is the endogenous form, whereas the upper band is the transfected Flag-PACT. The graph represents the average of three independent transfections ± standard error of the means. (B) PACT does not increase the RNA level of an LTR-Luc reporter gene inhibited by PKR. Cells were transfected as described in panel A. RT-PCR was performed on RNA extracted from the transfected cells as indicated in Materials and Methods. Lane M, molecular weight markers; lane C, control with no cDNA. The Luc- and the GAPDH-amplified DNAs are indicated on the right.
PACT overexpression reverses IFN-induced inhibition of SV40-promoter expression in HeLa, COS-7, NIH 3T3, and MEF cells.
To rule out a specific activity of PACT on the HIV-1 promoter or on TAR, a simian virus 40 (SV40)-driven Luc gene assay with IFN as a PKR inducer was used as described previously (39, 65, 66). The phosphorylation of Stat1 in response to IFN-α and -β induction in HeLa cells and in the control Jurkat cells shows a functional IFN pathway in our cells (Fig. 2A). We assayed PACT activity on SV40-Luc expression after IFN induction (Fig. 2B). Surprisingly, in this context PACT also reversed the IFN inhibition and increased the Luc expression 2.6- and 6.2-fold over the nonrepressed and IFN-repressed activities, respectively. Semiquantitative RT-PCR showed that this increase may be partly ascribed (twofold or less) to an increase of the mRNA level at the highest PACT concentrations (Fig. 2C, top, lanes 4 to 6) but that the main activity of PACT is at the translational level (at least threefold). A Western blot showing Luc protein expression in the same experiment confirms that IFN decreases its expression, whereas small amounts of PACT increase only translation (Fig. 2C, bottom, lane 3). To confirm this activation in other cells and to determine which part of the translational increase may be due to PKR inhibition, we repeated the same experiment in COS-7 monkey cells and in NIH 3T3, MEFs, and PKR−/− MEFs murine cells (Fig. 2D). In each cell line, except in the PKR−/− MEF cells, SV40-Luc expression was inhibited by IFN, suggesting that PKR mediates most of IFN activity in these assays. In each case, PACT increased Luc expression three- to fourfold, but the lowest increase was found in PKR−/− cells, with 2.5-fold. Overall, we estimate that despite a partial (twofold) activation at the RNA level, increase in Luc expression is partly (threefold) due to PKR inhibition. These results suggest that PACT inhibits PKR activity in all cells that express the protein.
Efficient activation of PKR by PACTΔ13 but not by PACT in HeLa cells.
To better understand and to explain the discrepancies between our results and previous ones, we analyzed previous reports showing that PACT activates PKR by translation inhibition and by in vitro kinase assays (36, 39, 65, 66). All the results were obtained using a Flag-PACT expression vector generated from the original pGAD10-PACT (65). It was shown in a subsequent report that this clone contains an accidental mutation in its 3′ nucleotide end that induces a frameshift, removing 13 aa from the native PACT (66). As a consequence, all previous and subsequent assays were done with a Flag-PACT clone that expresses 300 aa from PACT and five unrelated amino acids (KLCSI) (Fig. 3A). It was assumed that the mutation had no consequence on PACT activity, and this clone was named wtPACT305 (66). Here, we will refer to this clone as PACTΔ13 to take into account the 13-aa deletion. In contrast to PACTΔ13, RAX, the murine homolog of PACT that also has 313 aa, did not activate PKR directly but only after IL-3 deprivation or stress induction (41). Consistent with these data, the human endogenous PACT also activated PKR upon stress (64). Because these data suggest that the ability of PACT to activate PKR is regulated by its Medipal domain containing the last 13 aa, we decided to directly compare the activity of the native PACT and truncated PACTΔ13 proteins under the same experimental conditions. We first constructed Flag-PACT in the same vector (pCB6+) as the original Flag-PACTΔ13 (65) and compared the activity of the constructs in the same translation inhibition assay (Fig. 3B). In agreement with previous assays, Flag-PACTΔ13-pCB6+ led to an additional twofold inhibition of translation following IFN induction. In contrast, the Flag-PACT-pCB6+ plasmid induced an increase in Luc expression similar to the one shown in Fig. 2B. The different activity of the two forms cannot be explained by their differences in expression as expression is affected in opposite ways (Fig. 3B, bottom). Because endogenous PACT is not truncated, this result raised the question of whether PACT induces PKR activation in cells in the absence of any cellular stress. To address this issue, we compared Flag-PACTΔ13 and Flag-PACT directly on PKR activation in the absence of IFN induction (Fig. 3C). Increasing amounts of the PACT-expressing vectors showed a limited activation, not dose dependent, with pCMV2-Flag-PACTΔ13 (lanes 5 to 7) and none with pCMV2-Flag-PACT (lanes 2 to 4) in HeLa cells, whereas a control-transfected PKR with poly(I)·poly(C) became activated in these cells (lane 9).
PACT and PACTΔ13 activate PKR in astrocytic cells.
The above results reveal a clear difference between the ability of PACT and PACTΔ13 to modulate translation and possibly PKR activation. As PACT can form heterodimers with TRBP (43, 46), the next question was whether PACT activity on PKR was dependent on the presence of TRBP. We approached this issue by measuring PACT activity in the relative absence of TRBP. Astrocytes are cells that express a very low level of TRBP1 and TRBP2 due to weak promoter expression (2, 3). As a consequence, in these cells TRBP-PACT interaction cannot be observed with Flag-PACT overexpression (46). We compared the activity of PACT and PACTΔ13 in the astrocytic cell line U251MG using the same translation inhibition assay shown in Fig. 3B. In these cells, PACT and PACTΔ13 induced a similar decrease in Luc activity of nearly twofold, suggesting an increase in the inhibition of translation mediated by PKR (Fig. 4A). We next compared PACT and PACTΔ13 activity on PKR activation in the same cell line in the absence of IFN (Fig. 4B). In contrast to HeLa cells, both PACT and PACTΔ13 activated PKR with a stronger phosphorylation induced by PACTΔ13 at low concentrations. This result suggests that efficient activation of PKR by PACT occurs only in cells that express a small amount of TRBP and that the truncation in the Medipal domain of PACT enhances this activity. To rule out that other factors unrelated to TRBP promote PKR phosphorylation in astrocytes, we performed this assay in U251MG cells stably transfected with pcDNA3-TRBP2 and observed no PKR activation (Fig. 4C). We concluded that the addition of TRBP2 likely prevents PKR activation by PACT in U251MG cells.
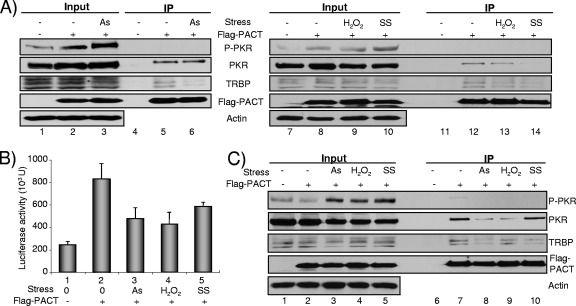
PACTΔ13 weakly interacts with either TRBP or PKR.
We have shown that Flag-PACT interacts with endogenous TRBP proteins in HeLa cells in the absence of RNA (46). To determine if the difference between PACT and PACTΔ13 activities on PKR activation could be due to a difference in interactions, we evaluated the binding of PACT to TRBP and PKR by IP (Fig. 5A). The protein expression in the input shows that PKR is induced and activated after IFN treatment (Fig. 5A, lanes 4 to 6). IP results show that PACTΔ13 has a decreased affinity for both PKR and TRBP compared to PACT and that the IFN induction does not change these interactions (Fig. 5A, lanes 9 and 12 compared to lanes 8 and 11). In addition, no interaction was obtained between PACT and P-PKR (Fig. 5A, lanes 8, 9, 11, and 12). Because TRBP and PACT bind through their Medipal domains (46) and because the 13-aa truncation of PACTΔ13 is within this domain, it was possible that this truncation could affect the formation of TRBP-PACT heterodimers. We therefore created this truncation in pGBT9-PACT-C (pGBT9-PACT-CΔ13) and assessed the interaction of this construct with TRBP-C and PACT-C fragments (Fig. 5B). The interaction was observed only with PACT-C and not with PACT-CΔ13, indicating that the truncated form of PACT has lost the ability to interact with TRBP Medipal and to homodimerize in this domain.
PACT activates PKR in Tarbp2−/− cells.
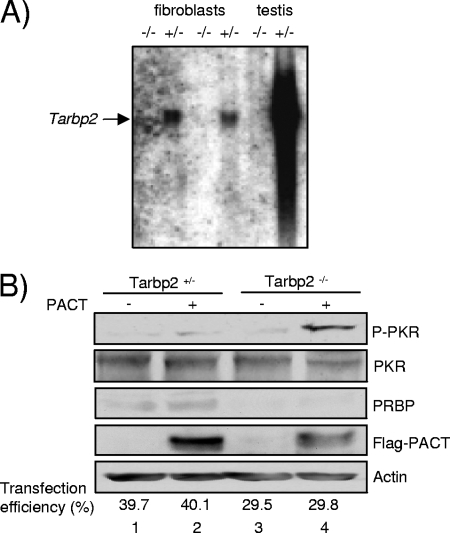
To further elucidate the role of TRBP in PKR activation by PACT, we next asked if a complete absence of TRBP would restore PACT activation of PKR in cells. We addressed this question with murine cells from mice that have a targeted disruption in the Tarbp2 gene (75). TEFs were generated from the corresponding Tarbp2+/− and Tarbp2−/− mice. The Tarbp2−/− TEFs expressed no Tarbp2 mRNA (Fig. 6A) and no PRBP protein, the TRBP murine homolog (Fig. 6B). The homozygous cells grew at the same rate as the heterozygous cells, and no difference was observed for eIF2α and PKR phosphorylation between the two cell types (see Fig. S1 in the supplemental material). In contrast, after transfection with a PACT-expressing vector, PKR became activated only in the Tarbp2−/− cells (Fig. 6B), indicating that the absence of PRBP allows PKR activation by PACT in murine cells. The low transfection efficiency in the Tarbp2−/− cells compared to Tarbp2+/− cells shows that the real increase of PKR phosphorylation by PACT is even higher than what is observed.
FIG. 6.
PACT activates PKR in Tarbp2−/− cells. (A) Northern blot for Tarbp2 mRNA. A blot with equal amounts of RNA from Tarbp2+/− (+/−) and Tarbp2−/− (−/−) fibroblasts (TEFs) and testis was probed with a 1.5-kb Tarbp2 cDNA fragment as indicated. (B) PACT-induced PKR activation in Tarbp2−/− cells. Tarbp2+/− and Tarbp2−/− TEFs were transfected with 2 μg of pCMV2-Flag (lanes 1 and 3) or pCMV2-Flag-PACT (lanes 2 and 4) and 2 μg of pEGFP-N3 (lanes 1 to 4). Whole-cell extracts (200 μg) were subjected to SDS-PAGE and blotted with anti-P-PKR, anti-PKR, anti-PRBP, anti-Flag, and antiactin antibodies as indicated. Transfection efficiency is the average of the percentage of EGFP-expressing cells versus total number of cells in three representative experiments.
PACT activity on PKR activation is restored in cells when TRBP expression is decreased by RNAi.
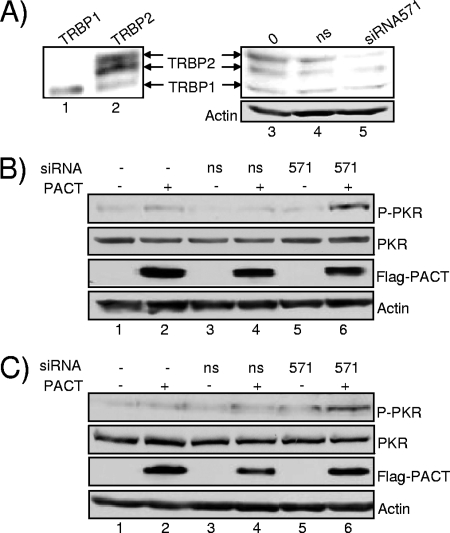
To further demonstrate that TRBP prevents PKR activation by PACT in human cells, we decreased TRBP expression by RNAi. Cells were transfected with an siRNA that targets TRBP1 and TRBP2 mRNAs (siRNA571) and was demonstrated to decrease TRBPs expression and HIV-1 replication (15). HeLa and HEK293T cells were transfected with TRBP siRNA571 with and without PACT, and PKR phosphorylation was examined in both cell lines (Fig. 7). Only cells transfected with TRBP siRNA571 and PACT showed increased PKR phosphorylation, demonstrating that the sole inhibition of TRBP expression restores PACT activity.
FIG. 7.
PACT activity on PKR activation is restored in cells when TRBP expression is decreased by RNAi. (A) RNAi against TRBP. HeLa cells were transfected with 2 μg of pcDNA3-TRBP1 (lane 1) or pcDNA3-TRBP2 (lane 2) without siRNAs (lane 3) or with 80 nM nonspecific siRNA (lane 4) or TRBP siRNA571 (lane 5). Whole-cell extracts in the amount of 25 (lanes 1 and 2) or 200 μg (lanes 3 to 5) were subjected to SDS-PAGE and blotted with anti-TRBP672 or antiactin antibodies as indicated. (B and C) siRNAs against TRBP restore PACT activation of PKR. HeLa (B) or HEK293T (C) cells were transfected without siRNAs (lanes 1 and 2) or with 80 nM nonspecific siRNA (lanes 3 and 4) or 80 nM siRNA571 (lanes 5 and 6) 24 h prior to transfection with 2 μg of pCMV2-Flag (lanes 1, 3, and 5) or pCMV2-Flag-PACT (lanes 2, 4, and 6). Whole-cell extracts (200 μg) were subjected to SDS-PAGE and blotted with anti-P-PKR, anti-PKR, anti-Flag, and antiactin antibodies as indicated. ns, nonspecific.
A stress activates PKR.
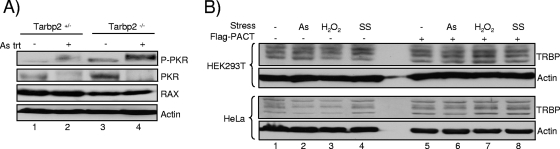
PACT and RAX are stress-activated proteins (41, 64). Therefore, the next question was whether a stress can dissociate the TRBP-PACT interaction and consequently enhance PKR phosphorylation. We first verified if TRBP could influence PKR activation mediated by an arsenite stress. We treated the Tarbp2+/− and the Tarbp2−/− cells with arsenite and verified PKR phosphorylation immediately after the treatment had been applied (Fig. 8A). We observed that PKR disappears from the stressed cells and that the phosphorylated form appears. This effect was much stronger in the Tarbp2−/− cells than in the Tarbp2+/− cells, suggesting a role for TRBP in the inhibition of PKR activation. We next verified if a stress could change the amount of TRBP in human cells in the presence or in the absence of transfected PACT. Although some decrease occurs in the absence of PACT, a stress mediated by arsenite, hydrogen peroxide, or serum starvation does not change the overall TRBP concentration in HeLa or HEK293T cells when PACT is overexpressed (Fig. 8B).
FIG. 8.
Stresses activate PKR but do not modify TRBP levels significantly. (A) Stress mediated by arsenite treatment induces higher PKR phosphorylation in Tarbp2−/− cells. Tarbp2+/− and Tarbp2−/− TEFs were either untreated (lanes 1 and 3) or treated with 0.5 mM sodium arsenite (As) for 1 h (lanes 2 and 4). Whole-cell extracts (200 μg) were subjected to SDS-PAGE and blotted with anti-P-PKR, anti-PKR, anti-PACTeg2, and antiactin antibodies as indicated. RAX, the murine homolog of PACT, was detected with the anti-PACT. The lower-migrating band revealed with anti-P-PKR in the absence of sodium arsenite treatment (lanes 1 and 3) corresponds to the size of PKR shown just below and might represent a different phosphorylated form in murine cells. (B) Stresses do not modify TRBP levels significantly. HEK293T cells were transfected without plasmid (lanes 1 to 4) or with 2 μg of pCMV2-Flag-PACT (lanes 5 to 8) and not treated (lanes 1 and 5) or treated with 0.5 mM sodium arsenite (As) for 1 h (lanes 2 and 6) or 10 mM hydrogen peroxide (H2O2) for 1 h or subjected to serum starvation (SS) for 24 h. A total of 150 μg of proteins from each lysate was run on a 10% SDS-PAGE gel and blotted using anti-TRBPjbx and antiactin antibodies.
A stress dissociates TRBP-PACT interaction.
We next verified if a stress could enhance PKR activation and change the TRBP-PACT interaction in human cells transfected with Flag-PACT (Fig. 9). HEK293T cells were first stressed with arsenite. We observed that the arsenite stress induced a loss of TRBP-PACT but not the PKR-PACT interaction and that this characteristic correlates with enhanced PKR phosphorylation (Fig. 9A, lanes 3 and 6). The same cells were treated with hydrogen peroxide or subjected to serum starvation and were evaluated similarly. We also found an increased PKR phosphorylation and a decreased TRBP-PACT interaction with a stronger effect after serum starvation (Fig. 9A, lanes 10 and 14) than after hydrogen peroxide treatment (lanes 9 and 13). The effect of stresses was also evaluated in the LTR-Luc assay as shown in Fig. 1 (Fig. 9B). We observed that each stress decreases the Luc expression that was increased by PACT, suggesting a reversion of PACT activation of LTR-mediated expression. To determine if the effect of stress can also be observed in another human cell line that expresses a similar level of TRBP, we tested HeLa cells using the same assay (Fig. 9C). In these cells, all stresses induced PKR phosphorylation, with a concomitant small decrease in the total amount of PKR. The IP shows a decreased affinity between TRBP and PACT when the cells are stressed. The effect was stronger with arsenite and serum starvation (Fig. 9C, lanes 8 and 10). Similarly to the result shown in Fig. 5, in all the IPs, PACT did not interact with P-PKR.
FIG. 9.
Stresses dissociate TRBP-PACT interaction and decrease PACT-induced LTR-Luc expression. (A) Stresses dissociate the TRBP-PACT interaction in HEK293T cells. HEK293T cells were transfected alone (lanes 1, 4, 7, and 11) or with 2 μg of pCMV2-Flag-PACT (lanes 2, 3, 5, 6, 8 to 10, and 12 to 14) and not treated (lanes 1, 2, 4, 5, 7, 8, 11, and 12) or treated with 0.5 mM sodium arsenite (As) for 1 h (lanes 3 and 6) or 10 mM hydrogen peroxide (H2O2) for 1 h (lanes 9 and 13) or subjected to serum starvation (SS) for 24 h (lanes 10 and 14). IP was performed with 1.5 mg of protein and anti-Flag antibody. Proteins (150 μg) from each lysate (input; lanes 1 to 3 and 7 to 10) and the immunoprecipitated complexes (lanes 4 to 6 and 11 to 14) were run on a 10% SDS-PAGE gel and blotted using anti-P-PKR, anti-PKR, anti-TRBPjbx, anti-Flag, and antiactin antibodies. Anti-TRBP672 and anti-TRBPjbx were both tested and gave the same results. (B) Stresses decrease PACT-induced LTR-Luc expression. HEK293T cells were transfected alone (lane 1) or with 2 μg of pCMV2-Flag-PACT (lanes 2 to 5) and not treated (lanes 1 and 2) or treated with 0.5 mM sodium arsenite (lane 3) or 10 mM H2O2 (lane 4) or subjected to SS (lane 5) as described in panel A. Bars represent the amount of firefly Luc activity per μg of total protein and are the average of three independent experiments (± standard error of the mean). (C) Stresses dissociate the TRBP-PACT interaction in HeLa cells. HeLa cells were transfected alone (lanes 1 and 6) or with 2 μg of pCMV2-Flag-PACT (lanes 2 to 5 and 7 to 10) and not treated (lanes 1, 2, 6, and 7) or treated with 0.5 mM sodium arsenite (lanes 3 and 8) or 10 mM H2O2 (lanes 4 and 9) or subjected to SS (lanes 5 and 10) as described in panel A. IP was performed with 1.5 mg of protein and anti-Flag antibody. Proteins (150 μg) from each lysate (input; lanes 1 to 5) and the immunoprecipitated complexes (lanes 6 to 10) were run and blotted as described in panel A.
DISCUSSION
PKR becomes activated in vivo and in vitro in response to virus infection, dsRNA, heparin, or PACT (27-29). Activated PKR inhibits viral and cellular translation, which leads to inhibition of viral replication and cell growth. Because of this powerful activity, viruses and cells have evolved means to regulate PKR function either by competing with dsRNA or by direct binding to PKR to prevent its activation. TRBP strongly inhibits PKR both by sequestering dsRNA and by binding PKR directly through its dsRBDs (5, 17, 61). Thus far, PACT/RAX is the only known dsRBP that activates PKR (41, 65). Because TRBP and PACT are highly similar proteins that have opposite effects on PKR and can interact, the expression levels of one protein could influence the activity of the other.
Human PACT and murine RAX are strongly homologous and differ by only 6 aa. Although they have not been identified in the same system, their activity is expected to be conserved. However, some important differences have been found in the conditions they require to activate PKR. For instance, transfected human PACT directly activates PKR, as shown by in vitro kinase assays and by a translation inhibition assay in IFN-treated cells (36, 39, 65, 66), but it activates translation in the absence of IFN (46, 54). Murine RAX does not activate PKR directly but only after a stress or IL-3 deprivation (6, 7, 41). Interestingly, endogenous PACT behaves like RAX and activates PKR only after a stress treatment (64). Because we did not find PKR activation by our PACT construct in assays similar to those performed previously (36, 39, 65, 66) (Fig. 2), we hypothesized that this discrepancy may be due to a mutation that was accidentally introduced in all previous clones and produced a deleted protein that lacks the 13 C-terminal amino acids of PACT (66). Indeed, when we used the same constructs and the same translation inhibition assay with the truncated PACTΔ13 and the nontruncated PACT, only PACTΔ13 increased the IFN-induced translation inhibition (Fig. 3B). Furthermore, when the two forms of PACT were tested for their direct activation of PKR in HeLa cells, only a small amount of P-PKR was revealed with PACTΔ13 suggesting that, like RAX, PACT does not activate PKR directly in HeLa, COS-7, NIH 3T3, and MEF cells (Fig. 1 to 3). Interestingly, a small part of the increased expression of the SV40-driven Luc gene can be ascribed to an increase in mRNA levels, suggesting multiple activities for PACT that can also act to increase transcription, splicing, or stability of the mRNA in addition to translation enhancement. Our results suggest that IFN activity only decreases translation and that low levels of PACT expression only increase translation, whereas a higher PACT concentration contributes to increased mRNA levels (Fig. 2C). Further studies will be required to elucidate which component of PACT mediates mRNA increase and whether it is similar in TRBP. Astrocytic cells are a model for PKR activation because they express a low level of TRBP proteins, thereby preventing PKR squelching activity (1-3). As a consequence, when HIV molecular clones are transfected in these cell lines, hyperactivation of PKR occurs, which blocks the translation of HIV proteins (34, 60). This activity contributes to the block to HIV replication in these cells (35), and, similarly, TRBP inactivation by siRNAs inhibits HIV replication (15). When PACT activity on HIV-1 LTR expression was compared between HeLa and U251MG cells, we found that it inhibited translation only in the astrocytic cell line (46). Therefore, PACT may also contribute to the block of HIV replication in astrocytes. We show here that both PACT and PACTΔ13 induce PKR phosphorylation more efficiently in astrocytes than in other cells, pointing to a role of TRBP in controlling PACT function (Fig. 4).
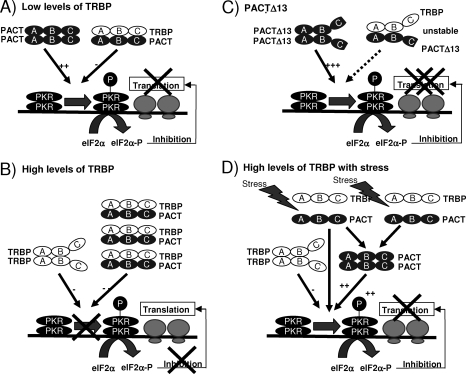
The puzzling difference in the activity between PACT and PACTΔ13 along with our studies indicating that TRBP and PACT interact through three domains (46) suggested that the interaction of these two forms of PACT with TRBP may be different. Indeed, PACTΔ13 showed a decreased affinity for TRBP compared to its wt counterpart, and this is due to the lack of binding of the Medipal domain (Fig. 5). Because a decreased interaction between PACT and TRBP results in increased PKR activation, we expected to see increased binding of PACTΔ13 to PKR, but instead, we also observed a weak interaction. There are two possible explanations for this observation: (i) a tight interaction between PACT and PKR may not be necessary for PKR activation, and weak binding may favor PKR phosphorylation; (ii) once phosphorylated, PKR may loose its affinity for PACT and gain affinity for its substrates. Indeed, the presence of P-PKR could not be detected in the proteins immunoprecipitated with anti-Flag-PACT, supporting the second hypothesis (Fig. 5 and 9). Our data favor the model in which PACTΔ13 could be a preactivated form of PACT due to the lack of homodimerization and heterodimerization with TRBP in the Medipal domain. This form of PACT (as a dimer or a monomer) would be available for efficient and rapid activation of PKR (Fig. 10).
FIG. 10.
Model for the controlled activation of PKR by TRBP, PACT, and stress. (A) PKR activation in cells with low levels of TRBP. In cells where TRBP concentration is low, PACT can activate PKR, which in turn phosphorylates eIF2α (eIF2α-P). eIF2α-P blocks viral and cellular translation initiation. (B) Absence of PKR activation in cells with high levels of TRBP. When TRBP concentration is high, PACT forms heterodimers with TRBP that inhibit PKR phosphorylation (P) and overcome its translation block. PACT is unable to activate PKR and, therefore, mRNA translation remains active. (C) Overexpression of PACTΔ13 strongly activates PKR. When PACTΔ13 is overexpressed in cells, the heterodimer with TRBP is unstable. As a consequence, PACTΔ13 strongly activates PKR. P-PKR has a decreased affinity for PACTΔ13 and becomes available to activate eIF2α, which blocks translation initiation. (D) Stress treatment dissociates TRBP-PACT interaction and activates PKR. A stress treatment induces a separation between TRBP and PACT. Therefore, PACT becomes available for PKR activation either as a monomer or as a dimer. In all panels, A, B, and C represent regions that encompass dsRBD1, dsRBD2, and the Medipal domains, respectively.
Because PACTΔ13 does not exist in cells, we evaluated the control of PACT activity by TRBP to determine if this interaction prevents PACT function. In murine and human cells with no or low TRBP, PACT function on PKR was restored, strongly suggesting that TRBP exerts an indirect inhibition of the kinase by preventing its activation by PACT (Fig. 6 and 7). The increased PKR activation by stress in Tarbp2−/− cells (Fig. 8), the dissociation of the TRBP-PACT interaction with various stresses concomitant to PKR activation, and the decrease in PACT-induced Luc expression (Fig. 9) support this hypothesis. The fact that these effects sometimes appear partial is likely due to the rapid reversibility of the stress effect, which is faster than the time frame of the experiments. Indeed, none of these effects were observed after a 24-h cell recovery after the stress (data not shown). Stresses also sometimes induce a mild decrease in TRBP levels (Fig. 8B and 9A and C, input), which also contributes to the heterodimer dissociation and PACT availability for PKR activation. Taken together, our results strongly suggest that TRBP-PACT interaction controls PACT activation of PKR and that endogenous PACT is a stress-activated protein upon its dissociation from TRBP. The heterodimer is likely an inactive storage form for PACT when TRBP concentration is high. In cells expressing low levels of TRBP, when TRBP expression is inhibited, or when TRBP-PACT complex is dissociated by stress, PACT likely forms homodimers or monomers that activate PKR and mediate translation inhibition (Fig. 10). These results also explain that cotransfection of PACT and PKR in yeast cells leads to growth arrest, which is likely due to PKR activation because no TRBP homolog exists in yeast (46).
TRBP has been shown to have an oncogenic potential due to its interaction with PKR (5), and the tumor suppressor Merlin induces its degradation by ubiquitination (49). TRBP-PACT dissociation induced by stresses may also increase TRBP availability for degradation by Merlin, which would increase PKR activation both directly and indirectly by liberating PACT function. TRBP and PACT are involved in various mechanisms and functions including cell response to viral infection (15, 33, 53, 67), development (69, 75), cancer (48), and RNAi (11, 37, 43, 52). The elucidation of the control of one protein on the other one will have important functional implications for understanding the regulation of these functions.
Supplementary Material
Acknowledgments
We thank M. Gale, Jr., and M. G. Katze for the gifts of the PKR control extracts of the transgenic CD4+ T lymphocytes and the eIF2α antibody. We are grateful to B. K. Kwieciszewski for excellent technical assistance with the Tarbp2−/− cells. We also thank A. Mouland, S. Lainé, and M. Gale, Jr., for helpful discussions and G. Clerzius, S. Daniels, J.-F. Gélinas, and R. Scarborough for comments on the manuscript.
This work was supported by grant MOP77747 from the Canadian Institutes for Health Research (CIHR) (to A.G.), by grants 0555503U from American Heart Association and HL63359 from National Institutes of Health (NIH) (to R.C.P.), and by a grant from the NIH (HD27215) (to R.E.B.). S.B. was a postdoctoral fellow from CIHR. A.H.F.M.P. was supported by the Mellon Foundation and a TALENT fellowship from The Netherlands Organization for Scientific Research. A.G. is a recipient of a Hugh and Helen McPherson Memorial Salary Award.
Footnotes
Published ahead of print on 20 October 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bannwarth, S., and A. Gatignol. 2005. HIV-1 TAR RNA: the target of molecular interactions between the virus and its host. Curr. HIV Res. 361-71. [DOI] [PubMed] [Google Scholar]
- 2.Bannwarth, S., S. Lainé, A. Daher, N. Grandvaux, G. Clerzius, A. C. Leblanc, J. Hiscott, and A. Gatignol. 2006. Cell-specific regulation of TRBP1 promoter by NF-Y transcription factor in lymphocytes and astrocytes. J. Mol. Biol. 355898-910. [DOI] [PubMed] [Google Scholar]
- 3.Bannwarth, S., L. Talakoub, F. Letourneur, M. Duarte, D. F. Purcell, J. Hiscott, and A. Gatignol. 2001. Organization of the human tarbp2 gene reveals two promoters that are repressed in an astrocytic cell line. J. Biol. Chem. 27648803-48813. [DOI] [PubMed] [Google Scholar]
- 4.Battisti, P. L., A. Daher, S. Bannwarth, J. Voortman, K. W. Peden, J. Hiscott, A. J. Mouland, R. Benarous, and A. Gatignol. 2003. Additive activity between the trans-activation response RNA-binding protein, TRBP2, and cyclin T1 on HIV type 1 expression and viral production in murine cells. AIDS Res. Hum. Retrovir. 19767-778. [DOI] [PubMed] [Google Scholar]
- 5.Benkirane, M., C. Neuveut, R. F. Chun, S. M. Smith, C. E. Samuel, A. Gatignol, and K. T. Jeang. 1997. Oncogenic potential of TAR RNA binding protein TRBP and its regulatory interaction with RNA-dependent protein kinase PKR. EMBO J. 16611-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett, R. L., W. L. Blalock, D. M. Abtahi, Y. Pan, S. A. Moyer, and W. S. May. 2006. RAX, the PKR activator, sensitizes cells to inflammatory cytokines, serum withdrawal, chemotherapy, and viral infection. Blood 108821-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett, R. L., W. L. Blalock, and W. S. May. 2004. Serine 18 phosphorylation of RAX, the PKR activator, is required for PKR activation and consequent translation inhibition. J. Biol. Chem. 27942687-42693. [DOI] [PubMed] [Google Scholar]
- 8.Bischoff, J. R., and C. E. Samuel. 1989. Mechanism of interferon action. Activation of the human P1/eIF-2 alpha protein kinase by individual reovirus s-class mRNAs: s1 mRNA is a potent activator relative to s4 mRNA. Virology 172106-115. [DOI] [PubMed] [Google Scholar]
- 9.Braun, R. E., J. J. Peschon, R. R. Behringer, R. L. Brinster, and R. D. Palmiter. 1989. Protamine 3′-untranslated sequences regulate temporal translational control and subcellular localization of growth hormone in spermatids of transgenic mice. Genes Dev. 3793-802. [DOI] [PubMed] [Google Scholar]
- 10.Cai, R., B. Carpick, R. F. Chun, K. T. Jeang, and B. R. Williams. 2000. HIV-I TAT inhibits PKR activity by both RNA-dependent and RNA-independent mechanisms. Arch. Biochem. Biophys. 373361-367. [DOI] [PubMed] [Google Scholar]
- 11.Castanotto, D., K. Sakurai, R. Lingeman, H. Li, L. Shively, L. Aagaard, H. Soifer, A. Gatignol, A. Riggs, and J. J. Rossi. 2007. Combinatorial delivery of small interfering RNAs reduces RNAi efficacy by selective incorporation into RISC. Nucleic Acids Res. 355154-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cathala, G., J. F. Savouret, B. Mendez, B. L. West, M. Karin, J. A. Martial, and J. D. Baxter. 1983. A method for isolation of intact, translationally active ribonucleic acid. DNA 2329-335. [DOI] [PubMed] [Google Scholar]
- 13.Chang, K. Y., and A. Ramos. 2005. The double-stranded RNA-binding motif, a versatile macromolecular docking platform. FEBS J. 2722109-2117. [DOI] [PubMed] [Google Scholar]
- 14.Chendrimada, T. P., R. I. Gregory, E. Kumaraswamy, J. Norman, N. Cooch, K. Nishikura, and R. Shiekhattar. 2005. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 436740-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christensen, H. S., A. Daher, K. J. Soye, L. B. Frankel, M. R. Alexander, S. Lainé, S. Bannwarth, C. L. Ong, S. W. Chung, S. M. Campbell, D. F. Purcell, and A. Gatignol. 2007. Small interfering RNAs against the TAR RNA binding protein, TRBP, a Dicer cofactor, inhibit human immunodeficiency virus type 1 long terminal repeat expression and viral production. J. Virol. 815121-5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clemens, M. J., J. W. Hershey, A. C. Hovanessian, B. C. Jacobs, M. G. Katze, R. J. Kaufman, P. Lengyel, C. E. Samuel, G. C. Sen, and B. R. Williams. 1993. PKR: proposed nomenclature for the RNA-dependent protein kinase induced by interferon. J. Interferon Res. 13241. [DOI] [PubMed] [Google Scholar]
- 17.Daher, A., M. Longuet, D. Dorin, F. Bois, E. Segeral, S. Bannwarth, P. L. Battisti, D. F. Purcell, R. Benarous, C. Vaquero, E. F. Meurs, and A. Gatignol. 2001. Two dimerization domains in the trans-activation response RNA-binding protein (TRBP) individually reverse the protein kinase R inhibition of HIV-1 long terminal repeat expression. J. Biol. Chem. 27633899-33905. [DOI] [PubMed] [Google Scholar]
- 18.Daher, A., M. Varin, Y. Lamontagne, and D. Oth. 1998. Effect of pre-conceptional external or internal irradiation of N5 male mice and the risk of leukemia in their offspring. Carcinogenesis 191553-1558. [DOI] [PubMed] [Google Scholar]
- 19.Dauber, B., J. Schneider, and T. Wolff. 2006. Double-stranded RNA binding of influenza B virus nonstructural NS1 protein inhibits protein kinase R but is not essential to antagonize production of alpha/beta interferon. J. Virol. 8011667-11677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies, M. V., H. W. Chang, B. L. Jacobs, and R. J. Kaufman. 1993. The E3L and K3L vaccinia virus gene products stimulate translation through inhibition of the double-stranded RNA-dependent protein kinase by different mechanisms. J. Virol. 671688-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daviet, L., M. Erard, D. Dorin, M. Duarte, C. Vaquero, and A. Gatignol. 2000. Analysis of a binding difference between the two dsRNA-binding domains in TRBP reveals the modular function of a KR-helix motif. Eur. J. Biochem. 2672419-2431. [DOI] [PubMed] [Google Scholar]
- 22.Donze, O., T. Abbas-Terki, and D. Picard. 2001. The Hsp90 chaperone complex is both a facilitator and a repressor of the dsRNA-dependent kinase PKR. EMBO J. 203771-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorin, D., M. C. Bonnet, S. Bannwarth, A. Gatignol, E. F. Meurs, and C. Vaquero. 2003. The TAR RNA-binding protein, TRBP, stimulates the expression of TAR-containing RNAs in vitro and in vivo independently of its ability to inhibit the dsRNA-dependent kinase PKR. J. Biol. Chem. 2784440-4448. [DOI] [PubMed] [Google Scholar]
- 24.Duarte, M., K. Graham, A. Daher, P. L. Battisti, S. Bannwarth, E. Segeral, K. T. Jeang, and A. Gatignol. 2000. Characterization of TRBP1 and TRBP2. Stable stem-loop structure at the 5′ end of TRBP2 mRNA resembles HIV-1 TAR and is not found in its processed pseudogene. J. Biomed. Sci. 7494-506. [DOI] [PubMed] [Google Scholar]
- 25.Erard, M., D. G. Barker, F. Amalric, K. T. Jeang, and A. Gatignol. 1998. An Arg/Lys-rich core peptide mimics TRBP binding to the HIV-1 TAR RNA upper-stem/loop. J. Mol. Biol. 2791085-1099. [DOI] [PubMed] [Google Scholar]
- 26.Gale, M., Jr., C. M. Blakely, B. Kwieciszewski, S. L. Tan, M. Dossett, N. M. Tang, M. J. Korth, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1998. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol. Cell. Biol. 185208-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gale, M., Jr., S. L. Tan, and M. G. Katze. 2000. Translational control of viral gene expression in eukaryotes. Microbiol. Mol. Biol. Rev. 64239-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia, M. A., J. Gil, I. Ventoso, S. Guerra, E. Domingo, C. Rivas, and M. Esteban. 2006. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. 701032-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia, M. A., E. F. Meurs, and M. Esteban. 2007. The dsRNA protein kinase PKR: virus and cell control. Biochimie 89799-811. [DOI] [PubMed] [Google Scholar]
- 30.Gatignol, A., C. Buckler, and K. T. Jeang. 1993. Relatedness of an RNA-binding motif in human immunodeficiency virus type 1 TAR RNA-binding protein TRBP to human P1/dsI kinase and Drosophila Staufen. Mol. Cell. Biol. 132193-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gatignol, A., A. Buckler-White, B. Berkhout, and K. T. Jeang. 1991. Characterization of a human TAR RNA-binding protein that activates the HIV-1 LTR. Science 2511597-1600. [DOI] [PubMed] [Google Scholar]
- 32.Gatignol, A., M. Duarte, L. Daviet, Y. N. Chang, and K. T. Jeang. 1996. Sequential steps in Tat trans-activation of HIV-1 mediated through cellular DNA, RNA, and protein binding factors. Gene Expr. 5217-228. [PMC free article] [PubMed] [Google Scholar]
- 33.Gatignol, A., S. Lainé, and G. Clerzius. 2005. Dual role of TRBP in HIV replication and RNA interference: viral diversion of a cellular pathway or evasion from antiviral immunity? Retrovirology 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorry, P. R., J. L. Howard, M. J. Churchill, J. L. Anderson, A. Cunningham, D. Adrian, D. A. McPhee, and D. F. Purcell. 1999. Diminished production of human immunodeficiency virus type 1 in astrocytes results from inefficient translation of gag, env, and nef mRNAs despite efficient expression of Tat and Rev. J. Virol. 73352-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorry, P. R., C. Ong, J. Thorpe, S. Bannwarth, K. A. Thompson, A. Gatignol, S. L. Vesselingh, and D. F. Purcell. 2003. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr. HIV Res. 1463-473. [DOI] [PubMed] [Google Scholar]
- 36.Gupta, V., X. Huang, and R. C. Patel. 2003. The carboxy-terminal, M3 motifs of PACT and TRBP have opposite effects on PKR activity. Virology 315283-291. [DOI] [PubMed] [Google Scholar]
- 37.Haase, A. D., L. Jaskiewicz, H. Zhang, S. Lainé, R. Sack, A. Gatignol, and W. Filipowicz. 2005. TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO Rep. 6961-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hatada, E., S. Saito, and R. Fukuda. 1999. Mutant influenza viruses with a defective NS1 protein cannot block the activation of PKR in infected cells. J. Virol. 732425-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang, X., B. Hutchins, and R. C. Patel. 2002. The C-terminal, third conserved motif of the protein activator PACT plays an essential role in the activation of double-stranded-RNA-dependent protein kinase (PKR). Biochem. J. 366175-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itahana, K., K. P. Bhat, A. Jin, Y. Itahana, D. Hawke, R. Kobayashi, and Y. Zhang. 2003. Tumor suppressor ARF degrades B23, a nucleolar protein involved in ribosome biogenesis and cell proliferation. Mol. Cell 121151-1164. [DOI] [PubMed] [Google Scholar]
- 41.Ito, T., M. Yang, and W. S. May. 1999. RAX, a cellular activator for double-stranded RNA-dependent protein kinase during stress signaling. J. Biol. Chem. 27415427-15432. [DOI] [PubMed] [Google Scholar]
- 42.Kedersha, N., and P. Anderson. 2007. Mammalian stress granules and processing bodies. Methods Enzymol. 43161-81. [DOI] [PubMed] [Google Scholar]
- 43.Kok, K. H., M. H. Ng, Y. P. Ching, and D. Y. Jin. 2007. Human TRBP and PACT directly interact with each other and associate with Dicer to facilitate the production of small interfering RNA. J. Biol. Chem. 28217649-17657. [DOI] [PubMed] [Google Scholar]
- 44.Kumar, K. U., S. P. Srivastava, and R. J. Kaufman. 1999. Double-stranded RNA-activated protein kinase (PKR) is negatively regulated by 60S ribosomal subunit protein L18. Mol. Cell. Biol. 191116-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langland, J. O., S. Pettiford, B. Jiang, and B. L. Jacobs. 1994. Products of the porcine group C rotavirus NSP3 gene bind specifically to double-stranded RNA and inhibit activation of the interferon-induced protein kinase PKR. J. Virol. 683821-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laraki, G., G. Clerzius, A. Daher, C. Melendez-Peña, S. Daniels, and A. Gatignol. 2008. Interactions between the double-stranded RNA-binding proteins TRBP and PACT define the Medipal domain that mediates protein-protein interactions. RNA Biol. 592-103. [DOI] [PubMed] [Google Scholar]
- 47.Laurent, A. G., B. Krust, J. Galabru, J. Svab, and A. G. Hovanessian. 1985. Monoclonal antibodies to an interferon-induced Mr 68,000 protein and their use for the detection of double-stranded RNA-dependent protein kinase in human cells. Proc. Natl. Acad. Sci. USA 824341-4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee, J. Y., H. Kim, C. H. Ryu, J. Y. Kim, B. H. Choi, Y. Lim, P. W. Huh, Y. H. Kim, K. H. Lee, T. Y. Jun, H. K. Rha, J. K. Kang, and C. R. Choi. 2004. Merlin, a tumor suppressor, interacts with transactivation-responsive RNA-binding protein and inhibits its oncogenic activity. J. Biol. Chem. 27930265-30273. [DOI] [PubMed] [Google Scholar]
- 49.Lee, J. Y., H. J. Moon, W. K. Lee, H. J. Chun, C. W. Han, Y. W. Jeon, Y. Lim, Y. H. Kim, T. P. Yao, K. H. Lee, T. Y. Jun, H. K. Rha, and J. K. Kang. 2006. Merlin facilitates ubiquitination and degradation of transactivation-responsive RNA-binding protein. Oncogene 251143-1152. [DOI] [PubMed] [Google Scholar]
- 50.Lee, K., M. A. Fajardo, and R. E. Braun. 1996. A testis cytoplasmic RNA-binding protein that has the properties of a translational repressor. Mol. Cell. Biol. 163023-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee, T. G., J. Tomita, A. G. Hovanessian, and M. G. Katze. 1992. Characterization and regulation of the 58,000-dalton cellular inhibitor of the interferon-induced, dsRNA-activated protein kinase. J. Biol. Chem. 26714238-14243. [PubMed] [Google Scholar]
- 52.Lee, Y., I. Hur, S. Y. Park, Y. K. Kim, M. R. Suh, and V. N. Kim. 2006. The role of PACT in the RNA silencing pathway. EMBO J. 25522-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li, S., J. Y. Min, R. M. Krug, and G. C. Sen. 2006. Binding of the influenza A virus NS1 protein to PKR mediates the inhibition of its activation by either PACT or double-stranded RNA. Virology 34913-21. [DOI] [PubMed] [Google Scholar]
- 54.Li, S., and G. C. Sen. 2003. PACT-mediated enhancement of reporter gene expression at the translational level. J. Interferon Cytokine Res. 23689-697. [DOI] [PubMed] [Google Scholar]
- 55.Maitra, R. K., N. A. McMillan, S. Desai, J. McSwiggen, A. G. Hovanessian, G. Sen, B. R. Williams, and R. H. Silverman. 1994. HIV-1 TAR RNA has an intrinsic ability to activate interferon-inducible enzymes. Virology 204823-827. [DOI] [PubMed] [Google Scholar]
- 56.Meurs, E., K. Chong, J. Galabru, N. S. Thomas, I. M. Kerr, B. R. Williams, and A. G. Hovanessian. 1990. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell 62379-390. [DOI] [PubMed] [Google Scholar]
- 57.Meurs, E. F., Y. Watanabe, S. Kadereit, G. N. Barber, M. G. Katze, K. Chong, B. R. Williams, and A. G. Hovanessian. 1992. Constitutive expression of human double-stranded RNA-activated p68 kinase in murine cells mediates phosphorylation of eukaryotic initiation factor 2 and partial resistance to encephalomyocarditis virus growth. J. Virol. 665805-5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mittelstadt, M., A. Frump, T. Khuu, V. Fowlkes, I. Handy, C. V. Patel, and R. C. Patel. 2008. Interaction of human tRNA-dihydrouridine synthase-2 with interferon-induced protein kinase PKR. Nucleic Acids Res. 36998-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nie, Y., G. L. Hammond, and J. H. Yang. 2007. Double-stranded RNA deaminase ADAR1 increases host susceptibility to virus infection. J. Virol. 81917-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ong, C. L., J. C. Thorpe, P. R. Gorry, S. Bannwarth, A. Jaworowski, J. L. Howard, S. Chung, S. Campbell, H. S. Christensen, G. Clerzius, A. J. Mouland, A. Gatignol, and D. F. Purcell. 2005. Low TRBP levels support an innate human immunodeficiency virus type 1 resistance in astrocytes by enhancing the PKR antiviral response. J. Virol. 7912763-12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park, H., M. V. Davies, J. O. Langland, H. W. Chang, Y. S. Nam, J. Tartaglia, E. Paoletti, B. L. Jacobs, R. J. Kaufman, and S. Venkatesan. 1994. TAR RNA-binding protein is an inhibitor of the interferon-induced protein kinase PKR. Proc. Natl. Acad. Sci. USA 914713-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parker, L. M., I. Fierro-Monti, and M. B. Mathews. 2001. Nuclear factor 90 is a substrate and regulator of the eukaryotic initiation factor 2 kinase double-stranded RNA-activated protein kinase. J. Biol. Chem. 27632522-32530. [DOI] [PubMed] [Google Scholar]
- 63.Pataer, A., S. A. Vorburger, S. Chada, S. Balachandran, G. N. Barber, J. A. Roth, K. K. Hunt, and S. G. Swisher. 2005. Melanoma differentiation-associated gene-7 protein physically associates with the double-stranded RNA-activated protein kinase PKR. Mol. Ther. 11717-723. [DOI] [PubMed] [Google Scholar]
- 64.Patel, C. V., I. Handy, T. Goldsmith, and R. C. Patel. 2000. PACT, a stress-modulated cellular activator of interferon-induced double-stranded RNA-activated protein kinase, PKR. J. Biol. Chem. 27537993-37998. [DOI] [PubMed] [Google Scholar]
- 65.Patel, R. C., and G. C. Sen. 1998. PACT, a protein activator of the interferon-induced protein kinase, PKR. EMBO J. 174379-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peters, G. A., R. Hartmann, J. Qin, and G. C. Sen. 2001. Modular structure of PACT: distinct domains for binding and activating PKR. Mol. Cell. Biol. 211908-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peters, G. A., D. Khoo, I. Mohr, and G. C. Sen. 2002. Inhibition of PACT-mediated activation of PKR by the herpes simplex virus type 1 Us11 protein. J. Virol. 7611054-11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robertson, H. D., L. Manche, and M. B. Mathews. 1996. Paradoxical interactions between human delta hepatitis agent RNA and the cellular protein kinase PKR. J. Virol. 705611-5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rowe, T. M., M. Rizzi, K. Hirose, G. A. Peters, and G. C. Sen. 2006. A role of the double-stranded RNA-binding protein PACT in mouse ear development and hearing. Proc. Natl. Acad. Sci. USA 1035823-5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 71.Saunders, L. R., and G. N. Barber. 2003. The dsRNA binding protein family: critical roles, diverse cellular functions. FASEB J. 17961-983. [DOI] [PubMed] [Google Scholar]
- 72.Xiao, Q., T. V. Sharp, I. W. Jeffrey, M. C. James, G. J. Pruijn, W. J. van Venrooij, and M. J. Clemens. 1994. The La antigen inhibits the activation of the interferon-inducible protein kinase PKR by sequestering and unwinding double-stranded RNA. Nucleic Acids Res. 222512-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang, Y. L., L. F. Reis, J. Pavlovic, A. Aguzzi, R. Schafer, A. Kumar, B. R. Williams, M. Aguet, and C. Weissmann. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 146095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yue, Z., and A. J. Shatkin. 1997. Double-stranded RNA-dependent protein kinase (PKR) is regulated by reovirus structural proteins. Virology 234364-371. [DOI] [PubMed] [Google Scholar]
- 75.Zhong, J., A. H. Peters, K. Lee, and R. E. Braun. 1999. A double-stranded RNA binding protein required for activation of repressed messages in mammalian germ cells. Nat. Genet. 22171-174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.