Abstract
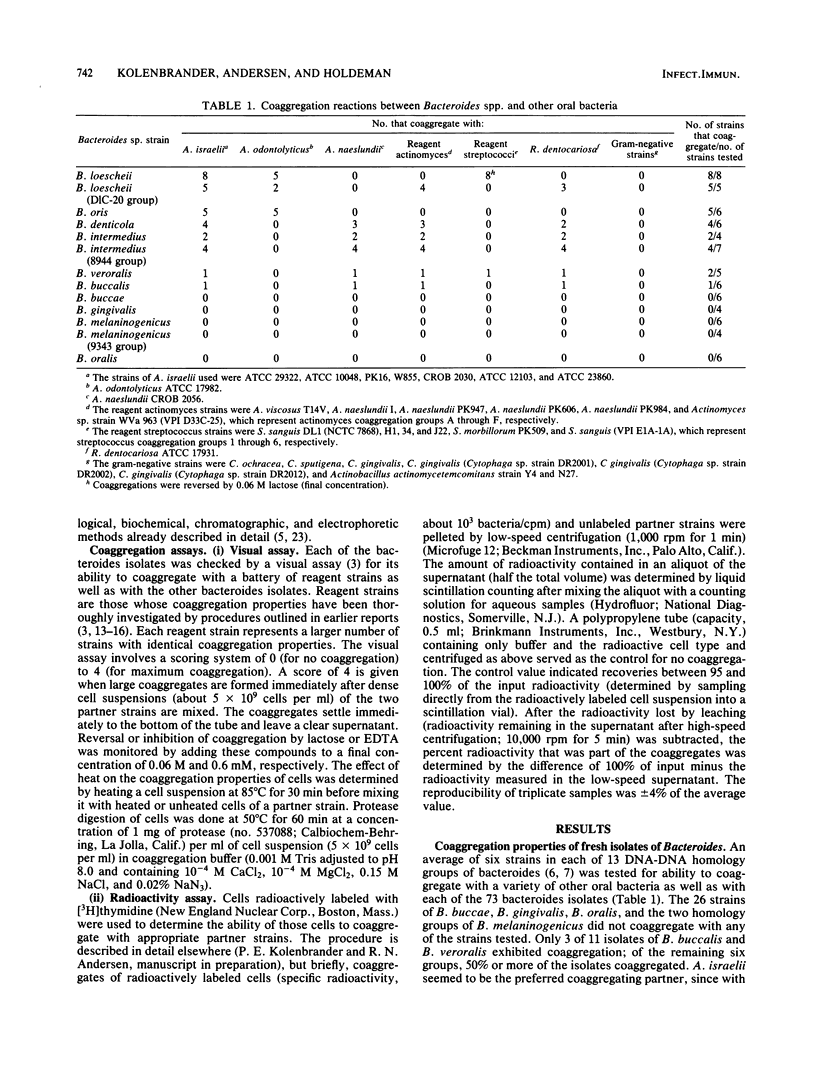
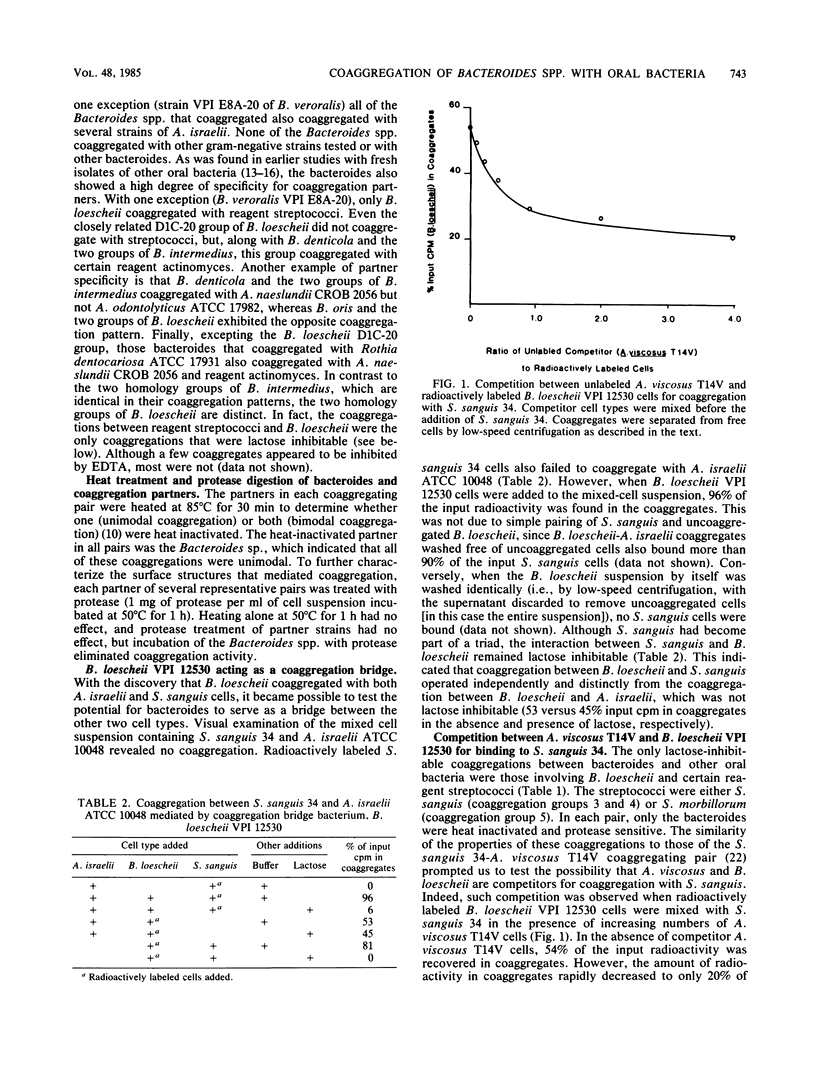
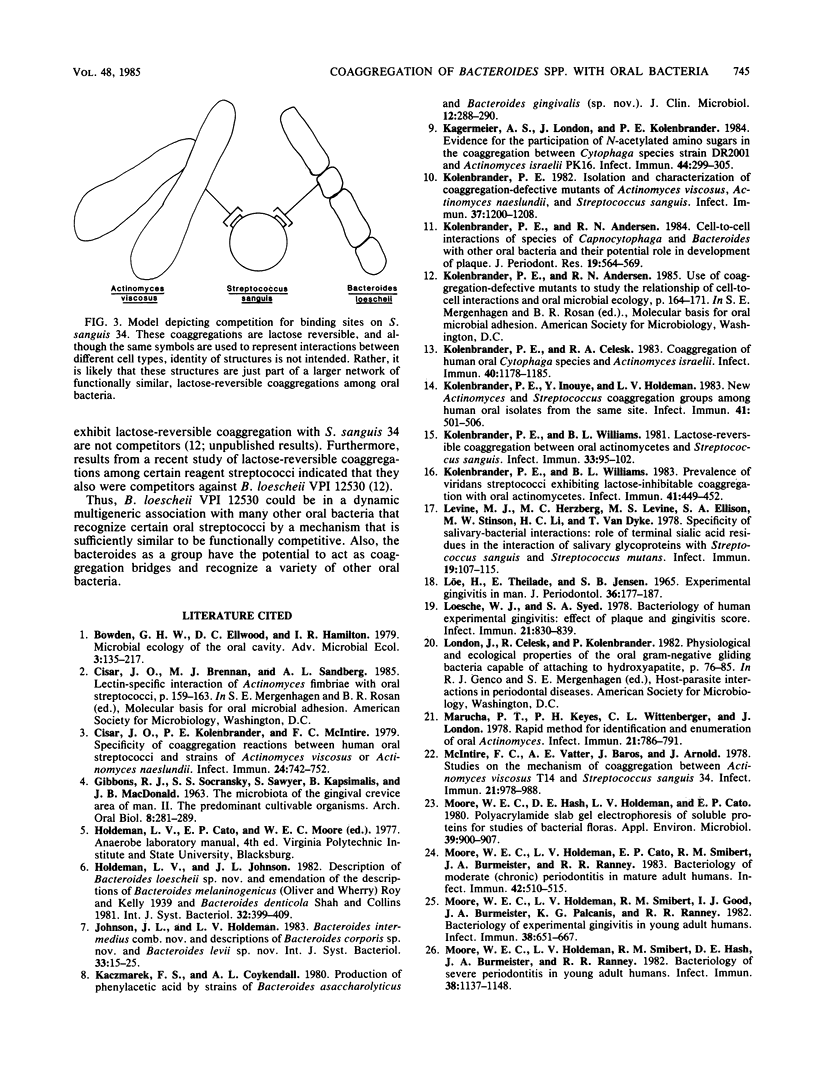
Seventy-three freshly isolated oral strains representing 10 Bacteroides spp. were tested for their ability to coaggregate with other oral gram-negative and gram-positive bacteria. None coaggregated with any of the gram-negative strains tested, which included Capnocytophaga gingivalis, C. ochracea, C. sputigena, and Actinobacillus actinomycetemcomitans. Strains of Bacteroides buccae, B. melaninogenicus, B. oralis, and B. gingivalis failed to coaggregate with any of the gram-positive strains tested. However, six Bacteroides spp. coaggregated with one or more species of gram-positive bacteria. Most isolates of B. buccalis, B. denticola, B. intermedius, B. loescheii, B. oris, and B. veroralis coaggregated with strains of Actinomyces israelii, A. viscosus, A. naeslundii, A. odontolyticus, Rothia dentocariosa, or Streptococcus sanguis. The strongest coaggregations involved B. denticola, B. loescheii, or B. oris; 22 of 25 strains coaggregated with A. israelii. Only B. loescheii interacted with certain strains of S. sanguis; these coaggregations were lactose inhibitable and were like coaggregations between A. viscosus and the same strains of S. sanguis. In fact, B. loescheii and A. viscosus were competitors for binding to S. sanguis. Many bacteroides also acted as coaggregation bridges by mediating coaggregations between two noncoaggregating cell types (e.g., S. sanguis and A. israelii). Evidence for binding-site competition and coaggregation bridging involving noncoaggregating cell types from three different genera provides support for the hypothesis that these intergeneric cell-to-cell interactions have an active role in bacterial colonization of the oral cavity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. J., SOCRANSKY S. S., SAWYER S., KAPSIMALIS B., MACDONALD J. B. The microbiota of the gingival crevice area of man. II. The predominant cultivable organisms. Arch Oral Biol. 1963 May-Jun;8:281–289. doi: 10.1016/0003-9969(63)90020-7. [DOI] [PubMed] [Google Scholar]
- Kaczmarek F. S., Coykendall A. L. Production of phenylacetic acid by strains of Bacteroides asaccharolyticus and Bacteroides gingivalis (sp. nov.). J Clin Microbiol. 1980 Aug;12(2):288–290. doi: 10.1128/jcm.12.2.288-290.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagermeier A. S., London J., Kolenbrander P. E. Evidence for the participation of N-acetylated amino sugars in the coaggregation between Cytophaga species strain DR2001 and Actinomyces israelii PK16. Infect Immun. 1984 May;44(2):299–305. doi: 10.1128/iai.44.2.299-305.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N. Cell to cell interactions of Capnocytophaga and Bacteroides species with other oral bacteria and their potential role in development of plaque. J Periodontal Res. 1984 Nov;19(6):564–569. doi: 10.1111/j.1600-0765.1984.tb01315.x. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Celesk R. A. Coaggregation of human oral Cytophaga species and Actinomyces israelii. Infect Immun. 1983 Jun;40(3):1178–1185. doi: 10.1128/iai.40.3.1178-1185.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Inouye Y., Holdeman L. V. New Actinomyces and Streptococcus coaggregation groups among human oral isolates from the same site. Infect Immun. 1983 Aug;41(2):501–506. doi: 10.1128/iai.41.2.501-506.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E. Isolation and characterization of coaggregation-defective mutants of Actinomyces viscosus, Actinomyces naeslundii, and Streptococcus sanguis. Infect Immun. 1982 Sep;37(3):1200–1208. doi: 10.1128/iai.37.3.1200-1208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Lactose-reversible coaggregation between oral actinomycetes and Streptococcus sanguis. Infect Immun. 1981 Jul;33(1):95–102. doi: 10.1128/iai.33.1.95-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Prevalence of viridans streptococci exhibiting lactose-inhibitable coaggregation with oral actinomycetes. Infect Immun. 1983 Aug;41(2):449–452. doi: 10.1128/iai.41.2.449-452.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOE H., THEILADE E., JENSEN S. B. EXPERIMENTAL GINGIVITIS IN MAN. J Periodontol. 1965 May-Jun;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A. Bacteriology of human experimental gingivitis: effect of plaque and gingivitis score. Infect Immun. 1978 Sep;21(3):830–839. doi: 10.1128/iai.21.3.830-839.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marucha P. T., Keyes P. H., Wittenberger C. L., London J. Rapid method for identification and enumeration of oral Actinomyces. Infect Immun. 1978 Sep;21(3):786–791. doi: 10.1128/iai.21.3.786-791.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Hash D. E., Holdeman L. V., Cato E. P. Polyacrylamide slab gel electrophoresis of soluble proteins for studies of bacterial floras. Appl Environ Microbiol. 1980 Apr;39(4):900–907. doi: 10.1128/aem.39.4.900-907.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Cato E. P., Smibert R. M., Burmeister J. A., Ranney R. R. Bacteriology of moderate (chronic) periodontitis in mature adult humans. Infect Immun. 1983 Nov;42(2):510–515. doi: 10.1128/iai.42.2.510-515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Good I. J., Burmeister J. A., Palcanis K. G., Ranney R. R. Bacteriology of experimental gingivitis in young adult humans. Infect Immun. 1982 Nov;38(2):651–667. doi: 10.1128/iai.38.2.651-667.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Hash D. E., Burmeister J. A., Ranney R. R. Bacteriology of severe periodontitis in young adult humans. Infect Immun. 1982 Dec;38(3):1137–1148. doi: 10.1128/iai.38.3.1137-1148.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. A., Levine M. J., Tabak L. A., Reddy M. S. Specificity of salivary-bacterial interactions: II. Evidence for a lectin on Streptococcus sanguis with specificity for a NeuAc alpha 2, 3Ga1 beta 1, 3Ga1NAc sequence. Biochem Biophys Res Commun. 1982 May 31;106(2):390–396. doi: 10.1016/0006-291x(82)91122-6. [DOI] [PubMed] [Google Scholar]
- SOCRANSKY S. S., GIBBONS R. J., DALE A. C., BORTNICK L., ROSENTHAL E., MACDONALD J. B. The microbiota of the gingival crevice area of man. I. Total microscopic and viable counts and counts of specific organisms. Arch Oral Biol. 1963 May-Jun;8:275–280. doi: 10.1016/0003-9969(63)90019-0. [DOI] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable microflora of advanced periodontitis. Scand J Dent Res. 1977 Jan-Feb;85(2):114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Socransky S. S., Manganiello A. D., Propas D., Oram V., van Houte J. Bacteriological studies of developing supragingival dental plaque. J Periodontal Res. 1977 Mar;12(2):90–106. doi: 10.1111/j.1600-0765.1977.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Syed S. A., Loesche W. J. Bacteriology of human experimental gingivitis: effect of plaque age. Infect Immun. 1978 Sep;21(3):821–829. doi: 10.1128/iai.21.3.821-829.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilade E., Wright W. H., Jensen S. B., Löe H. Experimental gingivitis in man. II. A longitudinal clinical and bacteriological investigation. J Periodontal Res. 1966;1:1–13. doi: 10.1111/j.1600-0765.1966.tb01842.x. [DOI] [PubMed] [Google Scholar]


