Abstract
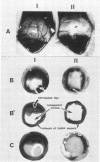
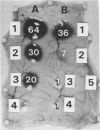
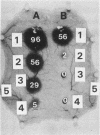
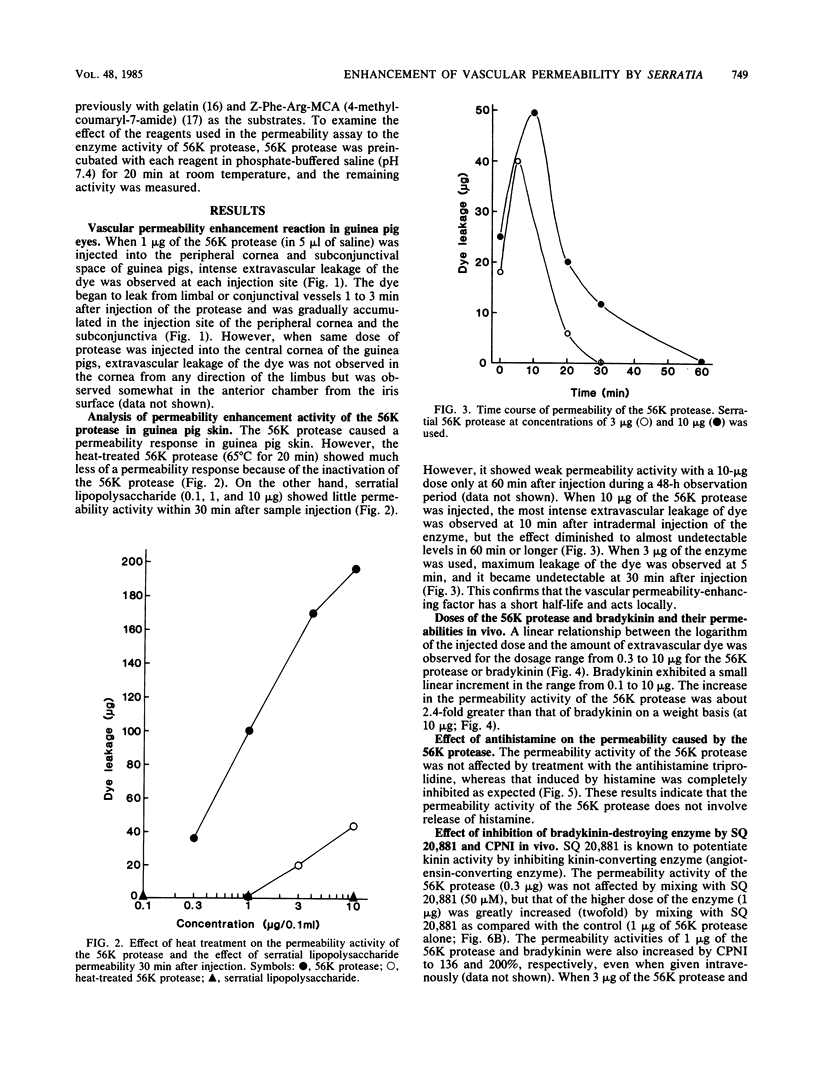
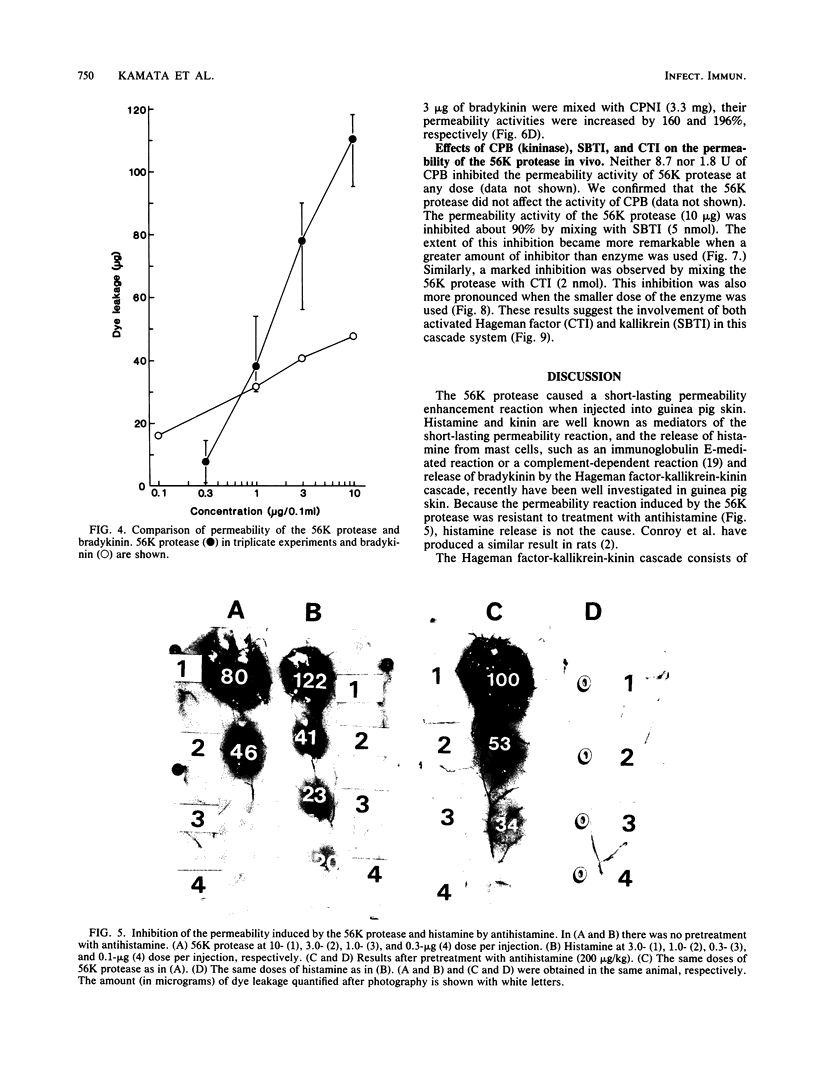
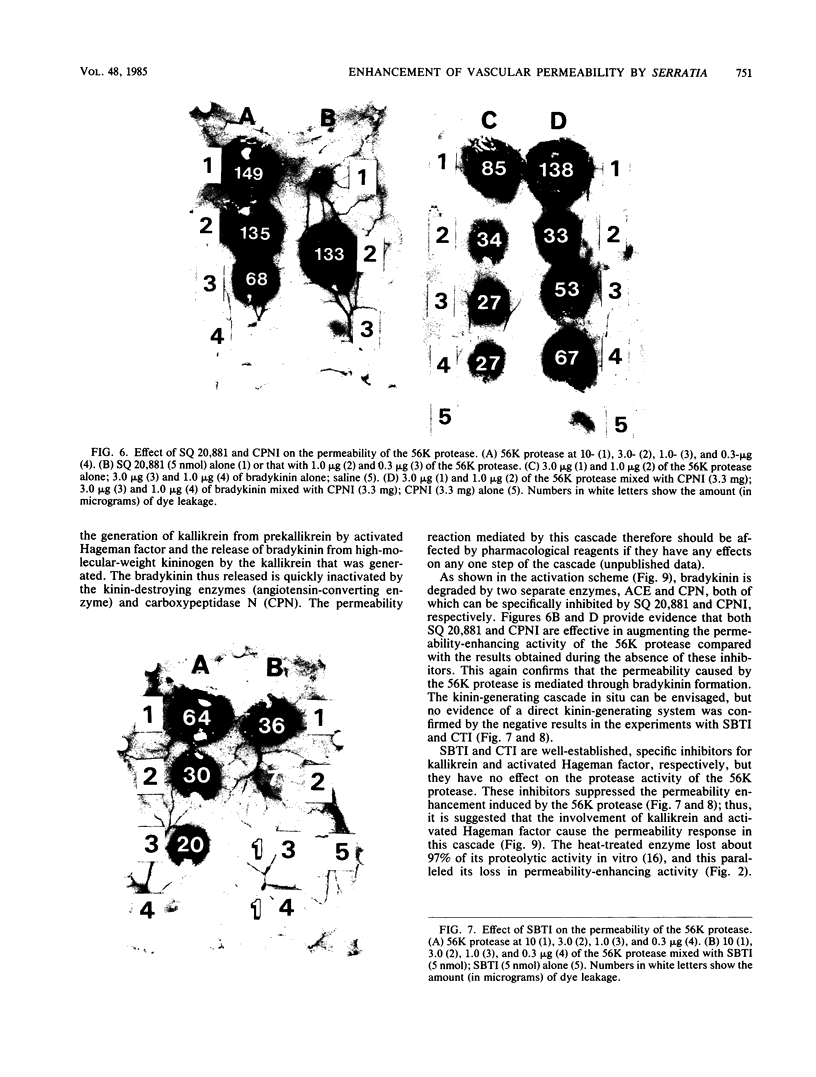
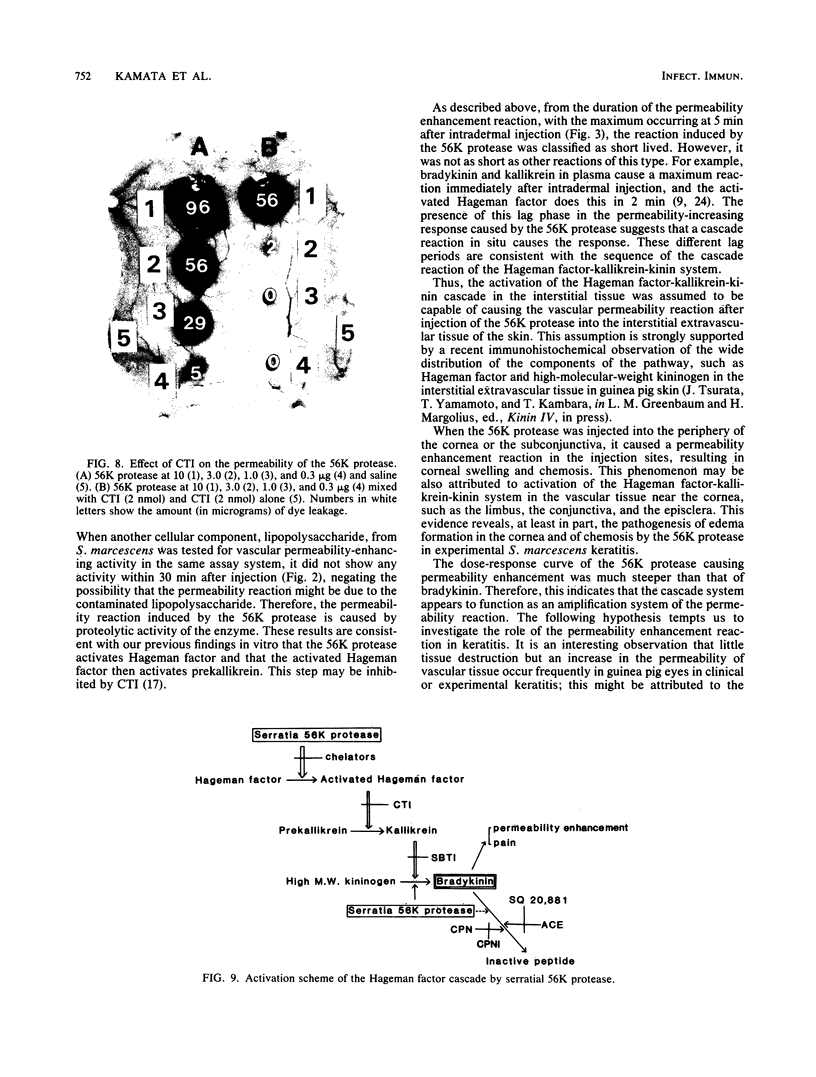
The 56-kilodalton (56K) protease isolated from a culture filtrate of Serratia marcescens caused vascular permeability enhancement followed by edema formation when injected into guinea pig peripheral corneas and subconjunctival space or skin. The character and the mechanism of permeability enhancement were analyzed in vivo. The enhancement was maximum at 5 to 10 min. The permeability reaction increased exponentially by the amount of enzyme used. The enhancement of permeability induced by the 56K protease was not affected by treatment with an antihistamine but was greatly augmented by simultaneous injection of a kinin potentiator, Glu-Trp-Pro-Arg-Pro-Gln-Ile-Pro-Pro-OH (SQ 20,881). Furthermore, the permeability activity of the protease, but not the amidolytic activity, was inhibited by soybean trypsin inhibitor, a well-known inhibitor of plasma kallikrein, as well as by corn trypsin inhibitor, the best inhibitor of activated Hageman factor. Results of these in vivo studies indicate that the permeability-enhancing reaction induced by the 56K protease is caused by activation of the Hageman factor-dependent pathway in the tissue. The permeability-increasing activity of the 56K protease was parallel with the enzyme activity. Serratial lipopolysaccharide did not produce a permeability enhancement reaction within 30 min when injected into guinea pig skin. These results are consistent with the results of recent in vitro experiments in which activation of the purified Hageman factor but not of prekallikrein by the 56K protease was elucidated (Matsumoto et al., J. Biochem. (Tokyo) 96:739-749, 1984). Thus, the molecular mechanism described above appears to be operative in the pathogenesis of corneal edema and chemosis, which is induced by S. marcescens, in addition to the direct tissue destruction by the protease.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlee W. E., Burns R. P., Oden M. Serratia marcescens keratoconjunctivitis. Am J Ophthalmol. 1970 Jul;70(1):31–33. doi: 10.1016/0002-9394(70)90664-1. [DOI] [PubMed] [Google Scholar]
- Conroy M. C., Bander N. H., Lepow I. H. Effects in the rat of intradermal injection of purified proteinases from streptococcus and Serratia marcescens. Proc Soc Exp Biol Med. 1975 Dec;150(3):801–806. doi: 10.3181/00379727-150-39128. [DOI] [PubMed] [Google Scholar]
- Davis J. T., Foltz E., Blakemore W. S. Serratia marcescens. A pathogen of increasing clinical importance. JAMA. 1970 Dec 21;214(12):2190–2192. doi: 10.1001/jama.214.12.2190. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Richardson S. H. Adenosine diphosphate-ribosylation of adenylate cyclase catalyzed by heat-labile enterotoxin of Escherichia coli: comparison with cholera toxin. J Infect Dis. 1980 Jan;141(1):64–70. doi: 10.1093/infdis/141.1.64. [DOI] [PubMed] [Google Scholar]
- Hojima Y., Pierce J. V., Pisano J. J. Hageman factor fragment inhibitor in corn seeds: purification and characterization. Thromb Res. 1980 Oct 15;20(2):149–162. doi: 10.1016/0049-3848(80)90381-3. [DOI] [PubMed] [Google Scholar]
- Homma J. Y. Roles of exoenzymes and exotoxin in the pathogenicity of Pseudomonas aeruginosa and the development of a new vaccine. Jpn J Exp Med. 1980 Jun;50(3):149–165. [PubMed] [Google Scholar]
- Hughes J. M., Murad F., Chang B., Guerrant R. L. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature. 1978 Feb 23;271(5647):755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- Imamura T., Yamamoto T., Kambara T. Guinea pig plasma kallikrein as a vascular permeability enhancement factor. Its dependence on kinin generation and regulation mechanisms in vivo. Am J Pathol. 1984 Apr;115(1):92–101. [PMC free article] [PubMed] [Google Scholar]
- Ishimatsu T., Yamamoto T., Kozono K., Kambara T. Guinea pig macroalbumin. A major inhibitor of activated Hageman factor in plasma with an alpha 2-macroglobulin-like nature. Am J Pathol. 1984 Apr;115(1):57–69. [PMC free article] [PubMed] [Google Scholar]
- Lass J. H., Haaf J., Foster C. S., Belcher C. Visual outcome in eight cases of Serratia marcescens keratitis. Am J Ophthalmol. 1981 Sep;92(3):384–390. doi: 10.1016/0002-9394(81)90529-8. [DOI] [PubMed] [Google Scholar]
- Lyerly D. M., Kreger A. S. Importance of serratia protease in the pathogenesis of experimental Serratia marcescens pneumonia. Infect Immun. 1983 Apr;40(1):113–119. doi: 10.1128/iai.40.1.113-119.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D., Gray L., Kreger A. Characterization of rabbit corneal damage produced by Serratia keratitis and by a serratia protease. Infect Immun. 1981 Sep;33(3):927–932. doi: 10.1128/iai.33.3.927-932.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D., Kreger A. Purification and characterization of a Serratia marcescens metalloprotease. Infect Immun. 1979 May;24(2):411–421. doi: 10.1128/iai.24.2.411-421.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Maeda H., Takata K., Kamata R., Okamura R. Purification and characterization of four proteases from a clinical isolate of Serratia marcescens kums 3958. J Bacteriol. 1984 Jan;157(1):225–232. doi: 10.1128/jb.157.1.225-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Yamamoto T., Kamata R., Maeda H. Pathogenesis of serratial infection: activation of the Hageman factor-prekallikrein cascade by serratial protease. J Biochem. 1984 Sep;96(3):739–749. doi: 10.1093/oxfordjournals.jbchem.a134892. [DOI] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Bacterial toxins: cellular mechanisms of action. Microbiol Rev. 1984 Sep;48(3):199–221. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyman G. A., Sathar M. L., May D. R. Intraocular gentamicin as intraoperative prophylaxis in South India eye camps. Br J Ophthalmol. 1977 Apr;61(4):260–262. doi: 10.1136/bjo.61.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton W. C., 3rd, Eiferman R. A., Snyder J. W., Melo J. C., Raff M. J. Serratia keratitis transmitted by contaminated eyedroppers. Am J Ophthalmol. 1982 Jun;93(6):723–726. doi: 10.1016/0002-9394(82)90467-6. [DOI] [PubMed] [Google Scholar]
- Tokunaga T., Fujino T., Yamasoto K., Ikeda A. [A case of serpiginous corneal ulcer caused by Serratia of Enterobacteriaceae]. Nihon Ganka Kiyo. 1969 Sep;20(9):862–868. [PubMed] [Google Scholar]
- Udaka K., Takeuchi Y., Movat H. Z. Simple method for quantitation of enhanced vascular permeability. Proc Soc Exp Biol Med. 1970 Apr;133(4):1384–1387. doi: 10.3181/00379727-133-34695. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Cochrane C. G. A protease-like permeability factor in guinea pig skin: immunologic identity with plasma Hageman factor. Am J Pathol. 1982 May;107(2):127–134. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Cochrane C. G. Guinea pig Hageman factor as a vascular permeability enhancement factor. Am J Pathol. 1981 Nov;105(2):164–175. [PMC free article] [PubMed] [Google Scholar]