Abstract
The heat shock protein Hsp72 is expressed at the elevated levels in various human tumors, and its levels often correlate with poor prognosis. Previously we reported that knockdown of Hsp72 in certain cancer cells, but not in untransformed breast epithelial cells, triggers senescence via p53-dependent and p53-independent mechanisms. Here we demonstrate that the p53-dependent pathway controlled by Hsp72 depends on the oncogenic form of phosphatidylinositol 3-kinase (PI3K). Indeed, upon expression of the oncogenic PI3K, epithelial cells began responding to Hsp72 depletion by activating the p53 pathway. Moreover, in cancer cell lines, activation of the p53 pathway caused by depletion of Hsp72 was dependent on oncogenes that activate the PI3K pathway. On the other hand, the p53-independent senescence pathway controlled by Hsp72 was associated with the Ras oncogene. In this pathway, extracellular signal-regulated kinases (ERKs) were critical for senescence, and Hsp72 controlled the ERK-activating kinase cascade at the level of Raf-1. Importantly, upon Ras expression, untransformed cells started responding to knockdown of Hsp72 by constitutive activation of ERKs, culminating in senescence. Therefore, Hsp72 is intimately involved in suppression of at least two separate senescence signaling pathways that are regulated by distinct oncogenes in transformed cells, which explains why cancer cells become “addicted” to this heat shock protein.
Two recent reports demonstrated that tumorigenesis in several models strictly requires the heat shock transcription factor Hsf1. In first report, crossing of p53−/− mice with hsf−/− mice almost completely prevented lymphoma development but not the appearance of certain other cancers (34). In the second study, hsf1 knockout dramatically delayed overall development of various tumors and increased survival of p53 knock-in mutant (R172H) mice (15). Similarly, Hsf1 deficiency drastically delayed chemical skin carcinogenesis and increased survival from 30 to 90% (15). Since Hsf1 controls induction of heat shock proteins (Hsps), these findings suggest the importance of Hsps (e.g., Hsp72) for tumor development.
Originally, Hsp72 was described as a stress-inducible protein that protects cells from a variety of harmful conditions. Hsp72 and other members of the Hsp70 protein family function as molecular chaperones in protein folding, transport, and degradation, and overproduction of Hsp72 reduces stress-induced denaturation and aggregation of certain proteins (33, 45, 46). Compilation of these results has led to the common assumption that refolding and antiaggregating activities of Hsp72 determine its role in cellular protection against stresses. In addition, we and others have found that Hsp72 plays a distinct role in cell signaling, contributing to cell protection under conditions which activate apoptosis unrelated to protein damage, such as antineoplastic drugs, radiation, or tumor necrosis factor (3, 20, 23-25, 28, 36). Besides apoptosis, Hsp72 is apparently involved in protection of cells from other forms of cell death such as mitotic catastrophe (19, 39), autophagy (16, 37), and necrosis (26, 51), which also play an important role in tumor growth and resistance to antineoplastic therapy (1, 41) (31). Not surprisingly, there have been many reports that Hsp72 is present at elevated levels in human tumors, especially of epithelial origin (8, 13). Furthermore, in many types of cancer levels of Hsp72 closely correlate with the tumor grade and poor prognosis (13).
Previous data from this laboratory suggest that among possible mechanisms by which Hsp72 contributes to tumorigenesis, suppression of the senescence program may play the major role (52). Cellular senescence was originally described as a limit to the number of divisions that a normal cell can undergo. Senescence is a complex program with multiple end points that include growth arrest, enlargement of cells, extensive vacuolization, repression and derepression of certain sets of genes, secretion of various signaling molecules, inhibition of the heat shock response, and other manifestations (10, 11, 18, 21). The senescence program in normal cells is triggered by DNA damage that results from the telomere shortening or stressful treatments and is associated with activation of p53 and accumulation of the cell cycle inhibitor p21 and/or p16 (4, 18, 42, 47).
Importantly, the senescence program could also be triggered upon activation of certain oncogenes. For example, Ras or RAF oncogenes can trigger senescence via several mechanisms, both p53-dependent and p53-independent (2, 5, 6, 17, 18, 48). Activation of the phosphatidylinositol 3-kinase (PI3K) pathway by certain oncogenes in prostate, breast, and other cancers also can trigger senescence. Indeed, knockout of the phosphatase PTEN, which activates PI3K, causes senescence of prostate epithelial cells via activation of p53 (12). In line with this observation, expression of active Akt, a component of the PI3K pathway, also activates the p53 pathway and triggers cell senescence (35). Similarly, expression of the mutant constitutively active oncogenic form of PI3K (PIK3CA), also leads to activation of p53 and accumulation of p21 (30).
Based on these observations, a novel paradigm has emerged that the oncogene-induced senescence (along with apoptosis) provides a major break on neoplastic transformation. Indeed, multiple observations with animal models and human tumors indicate a massive process of cell senescence in early neoplastic lesions (7, 18). It appears that permanent activation of certain oncogenic pathways (e.g., Ras or PI3K pathways) causes senescence by default. Therefore, the proliferating cancer cells with activated oncogenes represent progeny of cells that have acquired mechanisms to suppress such senescence. In some cases, suppression of the oncogene-induced senescence could be associated with a negative feedback loop in regulation of the mitogenic signaling, while in other cases the suppression is associated with a specific block of the senescence program without affecting the mitogenic signaling (e.g., mutations that disable the p53 pathway). The latter mechanism appears to be very important, since mutations affecting components of the p53 pathway are very common in human cancers (about 50%). On the other hand, in many tumors the p53 pathway remains functional. Furthermore, certain senescence programs are p53 independent (see, e.g., references 5 and 17). Therefore, alternative mechanisms of suppression of the oncogene-induced senescence must operate in these tumors.
Here we hypothesized that Hsp72 is the major player in suppression of the oncogene-induced senescence. To test this possibility, we investigated whether Hsp72 becomes essential for suppression of the latent senescence programs upon expression of the major oncogenes. These studies uncovered a critical role of Hsp72 in the control of the PI3K-induced p53-dependent and Ras-induced p53-independent senescence programs.
MATERIALS AND METHODS
Cell cultures, treatments, and reagents.
MCF10A, HCT116, MDA-MB231, HEK293, DU-145, PC-3, BT20, Hs578T, PANC1, A549, and MCF-7 were from ATCC; OVCAR5 was from NCI. HCT116 p53−/− and HCT116 p21−/− cells were kindly provided by B. Vogelstein, HeLa cells were a kind gift from M. Borelli. MCF10A cells expressing PIK3CA were described before (30). MCF10A p21−/− cells were kindly provided by B. Park (27). MCF10A cells were cultivated in Dulbecco's modified Eagle's medium-F12 medium supplemented with 5% horse serum, hydrocortisone (500 ng/ml), insulin (10 μg/ml), cholera toxin (100 ng/ml), and epidermal growth factor ([20 ng/ml]). HCT116 cells were cultivated in 5× McCoy's medium supplemented with 10% fetal bovine serum (FBS); MDA-MB231, PC-3, and DU-145 were cultivated in RPMI 1640 supplemented with 10% FBS; all other cells were cultivated in Dulbecco's modified Eagle's medium supplemented with 10% FBS.
Recombinant retroviral vectors.
For knockout of Hsp72 expression, we used RNAi-Ready pSIREN-RetroQ vector from BD Biosciences. The vector is provided prelinearized for ligation with a double-stranded oligonucleotide encoding a hairpin small interfering RNA; it also contains a puromycin resistance gene for selection of stably expressing cells. The sequence of the human Hsp72 gene was selected as reported before (52): sh72-1 (start, 474), CAGGTGATCAACGACGGAGAC; and sh72-2 (start, 1961), GAAGGACGAGTTTGAGCACAA. sh72-2 was used in most experiments if not otherwise mentioned. As an unrelated (control) small interfering RNA, we used shLuciferase vector as reported before (39). The sequence of the human p21 gene that was selected as a target for shp21 is CCGCGACTGTGATGCGCTAAT. The sequence of the human p53 gene that was selected as the target for shp53 is GACUCCAGUGGUAAUCUAC. The corresponding double-stranded oligonucleotides were cloned into pLPCX-bsd-H1RNA as a vector.
H-RAS V12 and control (Babe) retroviral vectors were kindly provided by S. Lowe.
For production of retroviruses, we used 293T cells, which were cotransfected using GenePORTER (GTS, San Diego, CA) or Lipofectamine 2000 (Invitrogene) with plasmids expressing retroviral proteins Gag-Pol, G (vesicular stomatitis virus G protein pseudotype), and constructs of interest or enhanced green fluorescent protein (kindly provided by Jeng-Shin Lee, Harvard Medical School). At 48 h after transfection, supernatants containing the retrovirus were collected and frozen at −70°C. Cells were infected with supernatant diluted 2× and 10 μg/ml Polybrene overnight and washed, and selection with puromycin (0.5 μg/ml) or/and blastocidin (10 μg/ml) was started 48 h after infection. Retroviral vector expressing enhanced green fluorescent protein was used as a control for infection efficiency: usually 70 to 90% of cells were fluorescent 2 to 3 days after infection.
Immunoblotting and antibodies.
Cells were washed twice with PBS, aspirated, and lysed in lysis buffer (40 mM HEPES [pH 7.5], 50 mM KCl, 1% Triton X-100, 1 mM Na3VO4, 50 mM glycerophosphate, 50 mM NaF, 5 mM EDTA, 5 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, and 5 μg/ml each leupeptin, pepstatin A, and aprotinin). Total protein concentration was measured in supernatants with the Bio-Rad protein assay, after which they were diluted with lysis buffer to achieve equal protein concentrations in all samples.
The following antibodies were used: anti-β-actin from Sigma; anti-Hsp72 (SPA810) from Stressgen; anti-p53 (DO-1) from Santa Cruz; anti-p21 from BD PharMingen; and anti-phospho-p38 Thr180/Tyr182, anti-p-Ser473 Akt, anti-p-MEK1 and -2, anti-p-Ser338-Raf, anti-p-JNK, anti-phospho-extracellular signal-regulated kinase (p-ERK), and anti-ERK from Cell Signaling.
β-Galactosidase assay.
The β-galactosidase assay was performed using X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) at pH 6.0 as described previously (52).
Dephosphorylation assay.
Cells were washed two times with PBS and left in PBS supplemented with 5 μM rotenone and 10 mM 2-deoxyglucose to prevent further phosphorylation, as described previously (32). Cells were harvested at the indicated times.
Statistical analysis.
The data shown are means ± standard errors of the mean of three to four independent experiments. Statistical significance was assessed by Student's t test.
RESULTS
Hsp72 controls the PI3K-activated p53 pathway.
Previously, we have demonstrated that specific depletion of the major heat shock protein Hsp72 in several cancer cell lines (e.g., HCT116 and MCF7) activates the p53 pathway and triggers growth cessation and cell senescence (52). These data suggested that in certain cancers there is an endogenous mechanism of activation of the p53 pathway, which is latent because of the Hsp72-mediated control.
Here we tested if depletion of Hsp72 can cause these effects in untransformed MCF10A breast epithelial cells, naturally immortalized by inactivation of p16 (14). Accordingly, MCF10A cells were infected with retroviruses that express two different short hairpin (shRNAs) against Hsp72 (Fig. 1C), and cell senescence was monitored by the acidic β-galactosidase assay, as well as changes in cell shape and proliferation. In untransformed MCF10A cells, in contrast to transformed cells (e.g., HCT116 or MCF7) (52), depletion of the Hsp72 by either of the two shRNAs neither caused significant senescence nor triggered cell death or reduced cell number (Fig. 1A and B, wild-type [WT] columns). Furthermore, it did not lead to accumulation of the p53 target, senescence-associated protein p21 (Fig. 1C and E, WT), indicating the lack of activation of the p53 pathway. Therefore, untransformed epithelial cells are not sensitive to depletion of Hsp72, while proliferation of cancer lines is dependent on Hsp72 (52). In other words, transformed cells appear to become “addicted” to Hsp72.
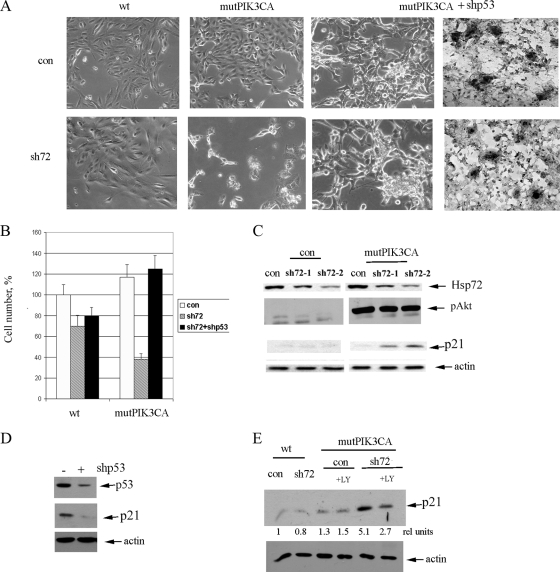
FIG. 1.
Expression of PIK3CA in MCF10A cells is necessary for accumulation of p21- and p53-dependent cell death upon depletion of Hsp72. (A) Bright-field microscopy of cells following expression of PIK3CA and/or depletion of Hsp72 and p53. The picture demonstrates that depletion of Hsp72 alone does not significantly affect control (con) cells but leads to growth inhibition and death of cells expressing PIK3CA. Importantly, knockdown of p53 reverses deleterious effects of Hsp72 depletion in PIK3CA-expressing cells and leads to extensive focus formation, as seen in the phase-contrast pictures (left panels) and following hematoxylin staining (right panels). (B) Cell numbers at day 4 postinfection upon expression of PIK3CA, sh72, and shp53 were quantified; sh72 reduces growth in PIK3CA-expressing cells much stronger than in control cells, and shp53 reverses the effects of sh72. (C) Effects of depletion of Hsp72 by sh72-1 or sh72-2 retrovirus in control and PIK3CA-expressing cells on p21. Levels of Hsp72, p21, and pAkt were measured by immunoblotting on day 5 postinfection with sh72 retroviruses. (D) Expression of p21 in PIK3CA-expressing cells is dependent on p53. p53 was depleted by infection of cells with shp53 retrovirus. (E) Depletion of Hsp72 leads to accumulation of p21 in PIK3CA-infected cells specifically. Accumulation of p21 is sensitive to the PI3K inhibitor LY294002 (LY).
Since activation of the p53 pathway and triggering senescence upon depletion of Hsp72 were specific for cancer cells and were not seen in untransformed epithelial cells, we proposed that these processes could require the permanent presence of active oncogenes. We decided to focus on the oncogenic PIK3CA, a mutant catalytic subunit of PI3K, because previously this oncogene was demonstrated to activate the p53 pathway in breast epithelial cells (30). Similarly, deletion of PTEN, a tumor suppressor regulating the PI3K pathway, was shown to upregulate the p53 pathway in cellular and animal models of prostate cancer (12, 30). Very importantly, both HCT116 and MCF7 cell lines, which are strictly “addicted” to Hsp72, carry oncogenic forms of PIK3CA (22). Accordingly, we hypothesized that Hsp72 can suppress the PI3K-activated p53 pathway, thus allowing cancer cells to proliferate.
To test the possibility that PI3K is responsible for maintaining the latent Hsp72-controlled p53-p21 pathway, we tested whether expression of recombinant PIK3CA oncogene can make untransformed cells sensitive to Hsp72 depletion. Accordingly, we expressed mutant PIK3CA (E545/K) in MCF10A cells using lentiviral infection (30). This expression led to a strong activation of Akt (phosphorylation of Ser473) (Fig. 1C) and little induction of p21 (Fig. 1C and E). On the other hand, PIK3CA-expressing MCF10A cells lost contact inhibition and started forming foci and colonies in soft agar (30), demonstrating signs of neoplastic transformation. Hsp72 was depleted in these cells using sh72-1 or sh72-2 viruses, and levels of p21 were monitored by immunoblotting. In contrast to parental MCF10A cells, depletion of Hsp72 in the PIK3CA-expressing MCF10A cells led to a more than fivefold accumulation of p21 (Fig. 1C and E). Expression of p21 required p53, since knockdown of p53 using shRNA retrovirus reduced levels of p21 (Fig. 1D). Furthermore, induction of p21 was sensitive to the PI3K inhibitor LY294002 (Fig. 1E). Therefore indeed, endogenous Hsp72 appears to keep under control the PIK3CA-activated p53 pathway.
Interestingly, unlike HCT116 or MCF7 cells (52), depletion of Hsp72 in PIK3CA-expressing MCF10A cells did not cause senescence, but rather severe growth inhibition and cell death (Fig. 1A and B). Of note, cell death was not associated with activation of caspase 3 and poly(ADP-ribose) polymerase (PARP) cleavage (not shown). Nevertheless, these effects were fully reversed by knockdown of p53 (Fig. 1A and B), indicating that cell demise in this system observed upon Hsp72 depletion is due to the PI3K-triggered activation of the p53 pathway. Furthermore, knockdown of p53 led to efficient transformation (focus formation) in both control and Hsp72-depleted cells expressing PIK3CA (Fig. 1A), but not in WT cells (not shown). It appears that in MCF10A cells the balance in activation of the p53 target genes is in favor of the death-promoting factors, while in HCT116 or MCF7 cells this balance is shifted toward senescence-related genes. Importantly, depletion of Hsp72 activated the p53-p21 pathway in this system selectively following expression of PIK3CA, indicating that the PIK3CA oncogene makes these cells “addicted” to Hsp72.
Control of the PI3K-p53 pathway by Hsp72 in tumor lines.
We further tested a possibility that the Hsp72-controlled activity of the p53-p21 pathway in tumor-derived cancer cell lines requires the presence of functional PIK3CA. Accordingly, we tested if accumulation of p53 and a subsequent buildup of p21 observed in cancer cells upon depletion of Hsp72 can be prevented by inhibition of PI3K. Of note, we previously demonstrated that in controlling the p53 pathway, Hsp72 regulates the activity of the p53 ubiquitin ligase Hdm2 (52).
For these experiments, we employed MCF7 cell line, which is known to carry a single known oncogene mutant PIK3CA (E545/K) (22). These cells were infected with shHsp72 retrovirus to deplete Hsp72, and on day 5 postinfection, the levels of Hsp72 were measured by immunoblotting to confirm the degree of depletion (Fig. 2A). Depletion of Hsp72 in MCF7 cells led to accumulation of p21 and caused typical signs of senescence, including growth inhibition, cell flattening, and appearance of acidic β-galactosidase activity (Fig. 2B and C). Furthermore, knockdown of p21 in MCF7 cells with shp21 retrovirus suppressed senescence caused by depletion of Hsp72 by more than 80% (Fig. 2B and C), indicating that in MCF7 cells the latent senescence almost entirely depends on the p53-p21 pathway.
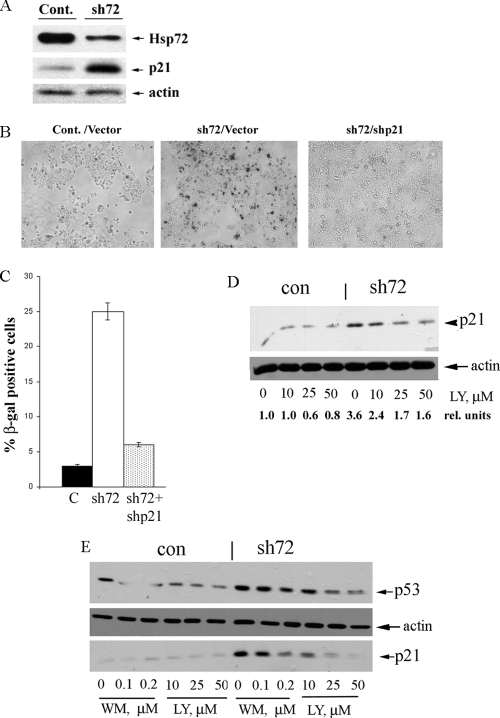
FIG. 2.
Depletion of Hsp72 leads to activation of the p53 pathway in cancer lines expressing oncogenic PIK3CA. (A) Depletion of Hsp72 and accumulation of p21 in MCF7 cells upon infection with sh72 retrovirus. Levels of Hsp72 and p21 were monitored by immunoblotting on day 5 postinfection. Cont., control. (B) Depletion of Hsp72 in MCF7 cells leads to cell senescence, which requires p21. p21 knockdown was done by infection with shp21 virus. Control and shp21 cells were infected with sh72 virus, and on day 6 postinfection, the cultures were stained for the acidic β-galactosidase activity. (C) Quantification of data from panel B. C, control. (D) Depletion of Hsp72 leads to accumulation of p21 in MCF7 cells, which is sensitive to the PI3K inhibitor LY294002 (LY). con, control; rel., relative. (E) Depletion of Hsp72 leads to accumulation of p53 and p21 in HCT116 cells. Accumulation of both p53 and p21 is sensitive to the PI3K inhibitors LY294002 (LY) and wortmannin (WM). Of note, accumulation of p21 in this system requires p53 (52).
Next we tested whether the PI3K activity is critical for activation of the p53-p21 pathway upon depletion of Hsp72. Accordingly, on day 5 postinfection with shHsp72, cells were treated with the PI3K inhibitor LY294002 for 16 h and levels of p21 were monitored. As seen in Fig. 2D, this PI3K inhibitor almost completely suppressed the buildup of p21 in Hsp72-depleted cells.
To further investigate the Hsp72-mediated control of the PI3K-dependent activation of the p53 pathway, we used HCT116 cells that also carry the oncogenic PI3K mutation (H1047R) and undergo senescence upon depletion of Hsp72 (52). Cells were infected with shHsp72 retrovirus to deplete Hsp72. On day 5 postinfection, when the level of p21 increased, cells were treated with PI3K inhibitors LY294002 (10 to 50 μM) or wortmannin (100 to 200 nM) at concentrations that inhibited the PI3K-dependent phosphorylation of Akt (not shown). After 16 h of incubation with these inhibitors, samples were taken, and levels of p53 and p21 were evaluated by immunoblotting with the corresponding antibodies. Both LY294002 and wortmannin suppressed the buildup of p53 and subsequent accumulation of p21 upon Hsp72 depletion (Fig. 2E). Interestingly, as with MCF7 cells (Fig. 2D), the background level of p21 was not affected by either LY294002 or wortmannin (Fig. 2E). This experiment clearly demonstrates that upon inhibition of PI3K, depletion of Hsp72 can no longer cause upregulation of p21. Considering that upregulation of p21 under these conditions is almost entirely p53 dependent (52; data not shown), these data indicate that in HCT116 cells the PI3K pathway is required for activation of the p53 pathway upon depletion of Hsp72. Therefore, permanent activity of PI3K in transformed HCT116 and MCF7 cells was essential for the latent activity of the p53-p21 pathway, which is normally suppressed by Hsp72.
These data together indicate that activity of the PIK3CA oncogene is both necessary and sufficient for activation of the p53-p21 pathway upon depletion of Hsp72. Therefore, Hsp72 appears to control the p53-p21 senescence pathway activated by the PIK3CA oncogene and thus allows cells to escape latent senescence/cell demise programs and to continue proliferating.
Correlation of Hsp72 levels with p53 status in cell lines and tumors.
The Hsp72-mediated control of p53 could represent a mechanism of suppression of senescence pathways that is alternative to mutations in p53, which are often found in tumors. A prediction of this hypothesis is that there might be a negative correlation between expression of Hsp72 and mutations in p53 in cancer lines and tumor biopsies.
To address this possibility, we measured levels of Hsp72 in several cancer cell lines of epithelial origin with normal or mutant p53. Among 11 analyzed common cancer lines, cells with normal p53 had higher levels of Hsp72 (e.g., MCF7 or HEK293), while cell lines with mutant p53 had lower levels of this chaperone (e.g., DU-145 or H1299) (Fig. 3A and B). Therefore, there was a clear trend suggesting that mutations in p53 and increased expression of Hsp72 are mutually exclusive. In spite of a limited number of tested cell lines, this finding was statistically significant (P < 0.01). We realized, however, that this negative correlation could be partially obscured, since Hsp72 is also involved in p53-independent senescence pathways (see below).
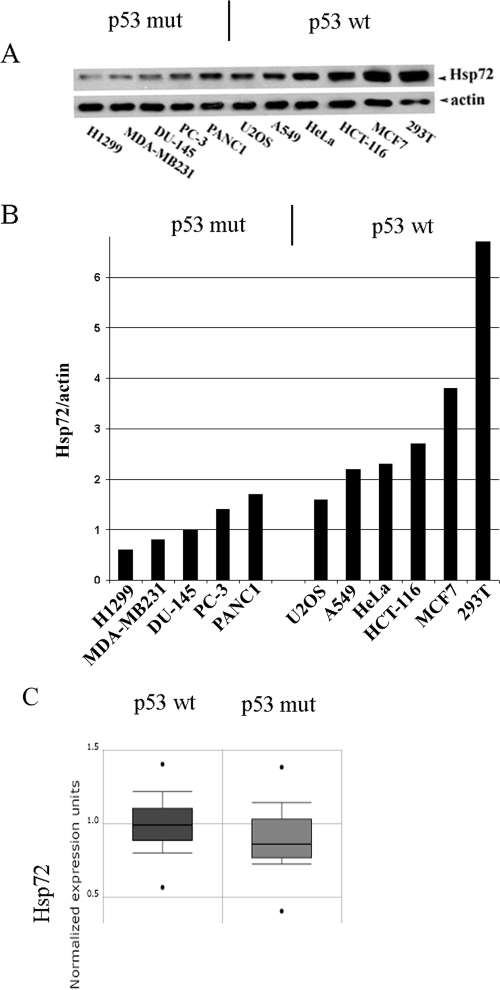
FIG. 3.
Reverse correlation between levels of Hsp72 and mutations in p53 in human cancer lines and biopsies. (A and B) Levels of Hsp72 in various tumor cell lines. The panels show the trend that lines with mutant (mut) p53 usually have lower levels of Hsp72. (C) Levels of Hsp72 (measured by gene arrays) in 179 (p53 wt) and 72 (p53 mut) breast cancer biopsies (data from www.oncomine.org). The box shows the interquartile range (25 to 75%), the whiskers represent the range 10 to 90%, and the asterisks indicate the minimum and the maximum. t test, −3.97; P value, 1.2E−4.
To further address the interaction of Hsp72 with the p53 pathway, we searched for the reverse correlation between expression of Hsp72 and mutations in p53 in cancer biopsies using the major cancer microarray data resource ONCOMINE (http://www.oncomine.org/). We specifically focused on breast cancer, since the PIK3CA oncogene is expressed in more than 25% of these tumors. Indeed, there was a significant (P < 0.02) difference between elevated expression of Hsp72 and mutations in p53 (Fig. 3C). Being statistically significant, the difference in expression was not big, most likely because Hsp72 can also suppress p53-independent senescence pathways in cancer cells (see below) and because we did not account for alternative ways of suppressing the p53 pathway, e.g., inactivation of Arf, or overexpression of Hdm2. The reverse correlation between the elevated expression of Hsp72 and mutations in p53 is consistent with the idea that in cancer lines and biopsies overexpression of Hsp72 and mutations in p53 represent alternative mechanisms that provide selective advantage to cancer cells by affecting the same p53 signaling pathway.
Hsp72 controls the Ras-stimulated ERK-dependent senescence pathway.
Our prior work indicated that Hsp72 can control both p53-dependent and p53-independent senescence pathways (52). For example, depletion of Hsp72 caused senescence in 40% of the WT HCT116 cells, and in 25% of the p53 knockout (KO) HCT116 cells (52), indicating that the p53-independent pathway significantly contributes to the overall senescence in this cell line. We suggested that a distinct oncogene can stimulate a p53-independent senescence pathway, which is also controlled by Hsp72. This oncogene could be Ras for the following reasons. (i) The oncogenic form of Ras (K-Ras G13D) is present in HCT116 cells, in addition to PIK3CA (22). (ii) K-Ras G13D, when expressed at high levels, triggers the p53-independent senescence in certain cell lines (5). (iii) Ras-activated senescence resulted from a constitutive activity of the ERK signaling cascade (5, 48), while Hsp72 can partially suppress the ERK pathway (44, 50). Therefore, we hypothesized that Hsp72 can keep the Ras-activated senescence pathway under control. Accordingly, we investigated whether upon expression of the oncogenic Ras, untransformed breast epithelium MCF10A cells start responding to Hsp72 depletion by enhancing the ERK signaling, culminating in the p53-independent senescence.
Importantly, as shown above (WT panels in Fig. 1A), depletion of Hsp72 in MCF10A cells does not trigger senescence. We tested whether depletion of Hsp72 can activate ERKs in MCF10A cells, and in line with the lack of senescence, no significant ERK activation was seen (Fig. 4A).
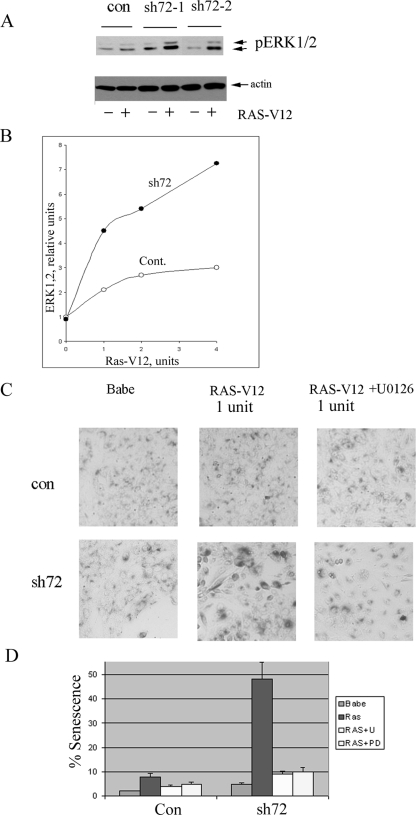
FIG. 4.
Upon expression of the oncogenic Ras, depletion of Hsp72 triggers senescence in MCF10A cells. (A) Hsp72 depletion enhances Ras-induced activation of ERKs. Cells were first infected with sh72 virus (sh72-1 or sh72-2), selected for 3 days, and then infected with Ras-expressing retrovirus at a concentration (1 U) that does not cause significant activation of ERKs and senescence on day 5 postinfection. ERK activation was measured by immunoblotting with p-ERK antibody. con, control. (B) Hsp72 depletion increases ERK activity at various concentrations of Ras virus. Cont., control. (C) Depletion of Hsp72 in MCF10A cells that express Ras causes senescence (enlarged, flattened, highly vacuolized cells with β-galactosidase staining), which is sensitive to the ERK inhibitor U0126. Addition of 5 μM of U0126 on day 2 postinfection with RAS (1 U) and incubation for 48 h relieve the effect. (D) Quantification of data from panel C.
To address the role of the oncogenic Ras in senescence triggered by downregulation of Hsp72, we expressed H-Ras G13D in MCF10A cells using a retroviral expression system. As with many other cell lines, high expression of the oncogenic Ras in MCF10A cells triggered senescence on its own (not shown). This senescence was p53 and p21 independent, since it was not suppressed by knocking down p53 by shRNA or knocking out p21 (not shown). To study the effects of depletion of Hsp72, we titrated down the Ras-expressing retrovirus to achieve a low infection that does not cause senescence in MCF10A cells. In fact, when these cells were infected with fourfold-diluted Ras virus, followed by selection of infected cells, we observed only a minor elevation of ERKs activity and no signs of senescence (Fig. 4A and C). Importantly, depletion of Hsp72 by either sh72-1 or sh72-2 retroviruses in these cells, in contrast to the parental MCF10A cells without Ras, caused stimulation of ERKs by up to fourfold (Fig. 4A and B) and senescence in about 50% of cells (Fig. 4C and D). (Incubation with inhibitors of the ERK pathway U0126 or PD98059 reversed senescence under these conditions [Fig. 4C and D].) Therefore, Hsp72 depletion stimulates Ras-dependent activation of the ERK pathway, triggering the p53-independent senescence.
Hsp72-mediated control of the ERK-dependent senescence in human cancer cells.
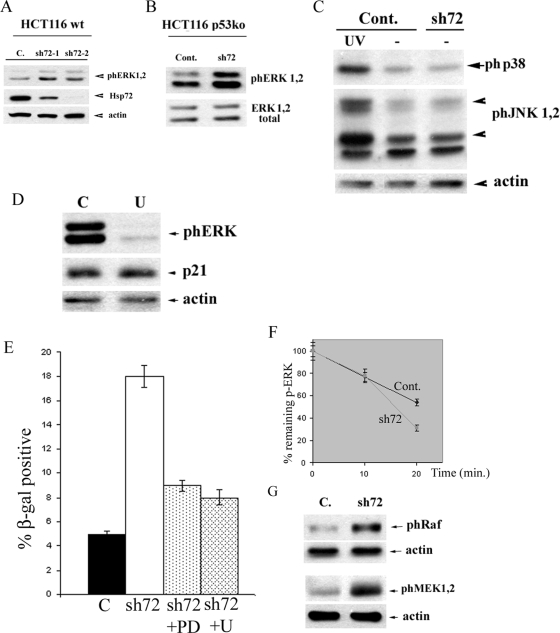
To address the role of Hsp72 in ERK-dependent senescence in human cancer cells, we have focused on the HCT116 line, which in addition to oncogenic PIK3CA, carries the oncogenic form of K-Ras (22). Using the HCT116 p53KO strain excluded the effects of the PI3K-p53 pathway. We first tested whether ERKs are activated upon depletion of Hsp72 in p53KO HCT116 cells. Hsp72 was depleted in these cells by sh72-1 or sh72-2 as described above, and activation of ERKs was monitored by immunoblotting at various time points. Figure 5A and B demonstrate that Hsp72 depletion indeed led to robust activation of both ERK1 and ERK2 isoforms. Importantly, activation of ERKs was quite specific since we did not observe significant activation of distinct mitogen-activated protein kinases p38 and JNK (Fig. 5C).
FIG. 5.
Depletion of Hs72 causes activation of ERKs and triggers the p53-independent senescence in HCT116 cells. (A and B) Depletion of Hsp72 activates ERKs in both WT (A) and p53KO (B) HCT116 cells. ERK activation was measured on day 6 postinfection with sh72-1 or sh72-2 by immunoblotting with p-ERK antibody. C. or Cont., control. (C) Depletion of Hsp72 does not activate either JNK or p38 in HCT116 cells. JNK and p38 activation was measured on day 5 postinfection by immunoblotting with p-JNK and p-p38 antibodies. (D) U0126 (U) inhibits activation of ERKs but does not affect levels of p21. (E) Inhibition of ERKs by U0128 (U) or PD98059 (PD) inhibits senescence caused by sh72 in the p53KO HCT116 cells. C, control. (F) Depletion of Hsp72 does not reduce the rate of ERK dephosphorylation. Dephosphorylation of ERKs was measured in control and sh72 HCT116 cells, as described in Materials and Methods. (G) Depletion of Hsp72 leads to activation of MEK1 and -2 and Raf-1. Phosphorylation of these proteins was measured with p-MEK and p-Raf antibodies.
As with MCF10A cells, to address the role of ERK activation in senescence of HCT116 cells observed upon depletion of Hsp72, we utilized specific inhibitors of the ERK pathway U0126 and PD98059. We found that in a range of 2 to 5 μM, U0126 strongly inhibits activation of ERKs (Fig. 5D) without causing significant growth inhibition (not shown). Of note, U0126 did not affect the levels of p21 (Fig. 5D). Similarly, PD98059 at concentrations of 10 to 20 μM inhibits ERKs without significantly suppressing cell growth (not shown). p53KO HCT116 cells were infected with sh72-2 retrovirus, and at day 4 postinfection treated with either U0126 (2 μM or 5 μM) or PD98059 (10 μM or 20 μM). After 48 h, cell senescence was monitored by the acidic β-galactosidase assay and counting the percentage of flattened cells. As seen in Fig. 5E, incubation with 2 μM of U0126 reduced the fraction of senescent cells from 18% to 9%. Strong suppression of senescence (down to 7%) was also seen with cells treated with PD98059 (Fig. 5E). Therefore, ERK activation appears to be critical for the p53-independent cell senescence of human cancer cells triggered by the Hsp72 depletion.
Downregulation of the ERK pathway by Hsp72 was described by several groups, including ours (44, 50). Indeed, in various systems Hsp72 either suppresses the upstream kinase of the pathway Raf (44) or promotes activity of ERK phosphatases MKP1 and MKP3 (50). Therefore, to dissect mechanisms of ERK activation in p53KO HCT116 cells upon depletion of Hsp72, we monitored the rate of the ERK dephosphorylation in vivo, using a previously developed method (50). ERKs were activated by the depletion of Hsp72 in HCT116 cells, as described above, and further phosphorylation by the MEK1 and -2 kinases was blocked (50). Samples were taken at different time points, and levels of ERK phosphorylation were monitored by immunoblotting. In shHsp72-infected cells, the half-life of phospho-ERKs was not slower (rather a little higher) than that in the control (Fig. 5F), indicating that changes in the rate of ERK dephosphorylation cannot account for ERK activation following depletion of Hsp72. These results suggested that ERK activation under these conditions results from stimulation of upstream kinases of the mitogen-activated protein kinase cascade. In fact, strong increase in the level of phospho-MEK1 and -2 was seen in the Hsp72-depleted cells (Fig. 5G). Since MEK1 and -2 are phosphorylated by Raf, the levels of MEK1 and -2 phosphorylation indicate enhanced Raf activity. Accordingly, depletion of Hsp72 significantly enhanced the activating phosphorylation (Ser339) of Raf-1 (Fig. 5G). Therefore, in HCT116 cells Hsp72 controls the pathway through regulation of the upstream kinase cascade rather than ERK phosphatases. These data together indicate that in the human cancer cell line Hsp72 can control the ERK signaling pathway leading to suppression of the p53-independent senescence.
We could not assess the role of the Ras oncogene in this pathway in a direct experiment since due to the phenomenon of “oncogene addiction,” shRNA-mediated depletion of Ras in these cells leads to a rapid death (not shown). Furthermore, Ras knockout HCT116 cells, although viable, may acquire additional adaptation that obscures the effects of Hsp72. Therefore, below we investigated the role of Ras in the correlative study using several cancer cell lines.
Hsp72 dependence of proliferation of cancer cell lines expressing PIK3CA and K-Ras oncogenes.
Based on the data described in previous sections, we proposed that Ras and PI3K pathways constitute two distinct oncogenic pathways regulated by Hsp72. To further address this issue, we investigated effects of depletion of Hsp72 in several cancer cell lines (mostly from the NCI60 collection) that express either PIK3CA or K-Ras oncogenes. Among these lines, besides HCT116 and MCF7, there were no lines that both express the PIK3CA oncogene and have normal p53. In MCF7, depletion of Hsp72 caused induction of p21 and senescence. On the other hand, in the BT20 breast cancer line, which expresses the PIK3CA oncogene but has mutant p53, neither induction of p21 nor senescence was seen upon Hsp72 depletion (Table 1). Importantly, in both MCF7 and BT20 cells we did not observe activation of ERKs. These findings are consistent with the idea that in cancer lines with oncogenes activating the PI3K pathway, depletion of Hsp72 leads to the p53-dependent senescence or apoptosis.
TABLE 1.
Effect of Hsp72 knockdown on p21 and ERK activation in tumor cell lines
| Cell line | Tissue type | Oncogene product | p53 type | Result fora:
|
||
|---|---|---|---|---|---|---|
| p21 increase | ERK activation | Senescence | ||||
| MCF7 | Breast | PIK3CA | WT | + | − | + |
| BT20 | Breast | PIK3CA | Mutant | − | − | − |
| HCT116 | Colon | PIK3CA + K-RAS | WT | + | + | + |
| KO | − | + | + | |||
| A549 | Lung | K-RAS | WT | − | + | + |
| OVCAR5 | Ovary | K-RAS | WTb | − | + | + |
| PANC1 | Pancreas | K-RAS | Mutant | −/+ | + | + |
| MDA-MB231 | Breast | K-RAS + B-RAF | Mutant | − | + | + |
| Hs578T | Breast | K-RAS | Mutant | − | + | + |
+, positive; −, negative.
Although according to information provided by NCI, the OVCAR5 line has normal p53 (22), we could detect neither p53 nor p21 under normal conditions or with Hsp72 depletion or UV irradiation (not shown).
Importantly, depletion of Hsp72 in cells expressing K-Ras oncogene, including A549, OVCAR5, MDA-MB231, and Hs578T, led to activation of ERKs but not of the p53/p21 pathway (judged by accumulation of p21). Under these conditions, all of these cells underwent senescence (Table 1). Of note, MDA-MB231, PANC1, and Hs578T cells have defective p53, clearly indicating that the Hsp72-controlled senescence in these cancer lines is independent of p53. Interestingly, in PANC1 cells which carry oncogenic Ras, depletion of Hsp72 while causing a robust activation of ERK also led to a minor but reproducible accumulation of p21 (Table 1). This accumulation possibly reflected the cross talk between the pathways. Therefore, in cancer lines with Ras oncogene, depletion of Hsp72 leads to stimulation of the ERK pathway and p53-independent senescence. Thus, Hsp72 can control distinct senescence pathways that are activated by different oncogenes.
DISCUSSION
Here, we attempted to understand a special role of Hsp72 in human cancer, which was previously proposed based on correlations between the expression levels of Hsp72 and various parameters of the disease, including poor prognosis (13). These earlier findings led to articulating a paradigm that Hsp72 provides a selective advantage to cancer cells, compared to normal cells, but mechanistic aspects of this advantage have not been explored. Based on our previous observation that depletion of Hsp72 triggers senescence programs in certain cancer lines, we suggested that this Hsp keeps the endogenous latent senescence programs under the control. This model, however, did not stipulate the nature of these senescence programs, and their specificity to cancer cells.
Here, we uncover the link between Hsp72 and the oncogene-induced latent senescence programs, thus explaining why cancer cells are specifically “addicted” to Hsp72. Indeed, we found that expression of the recombinant oncogenic forms of PI3K or Ras makes breast epithelial cell line MCF10A dependent on Hsp72. In contrast to parental MCF10A cells, these cells could no longer proliferate upon depletion of Hsp72 and underwent p53-independent senescence (in case of Ras expression) or p53-dependent death (in case of PIK3CA expression). These data indicate that Hsp72 plays an essential role in maintaining cell growth upon activation of oncogenic signaling pathways, specifically.
Detailed mechanisms of regulation of the p53 pathway by Hsp72 are not understood at present, although certain aspects of these regulatory mechanisms have been explored. For example, we have previously shown that depletion of Hsp72 regulates activity of Hdm2 ubiquitin ligase, which affects stability and activity of p53 (52). The mechanistic aspect of regulation of the ERK signaling cascade by Hsp72 is more developed. Since as we showed here the regulation is at the level of c-Raf, most likely it involves competition for Bag1, a regulatory protein that interacts with and inhibits both Hsp72 and c-Raf (44).
Interestingly, Hsp72 controls different latent senescence pathways upon expression of different oncogenes. In fact, it suppresses the p53-dependent senescence pathway in cells that express the oncogenic PI3K, and p53-independent ERK-dependent pathway in cells that express Ras. Furthermore, in a related study with both cell culture and xenograft models, we demonstrate that Hsp72 is critical for maintaining proliferation and formation of tumors by MCF10A cells transformed with Her2/NeuT oncogene, which causes activation of both p53-dependent and p53-independent senescence pathways (Le Meng et al., unpublished data). Therefore, Hsp72 plays a general role in control of various oncogene-activated senescence pathways. These findings appear to be relevant to cancer cell lines isolated from human tumors. Indeed, we observed that in cell lines that express PIK3CA oncogene, Hsp72 depletion triggered senescence via the p53 pathway, but did not activate ERK. In contrast, in cell lines expressing Ras, Hsp72 controlled the ERK-dependent pathways, but not p53 (Table 1). In HCT116 cells which carry both PIK3CA and Ras oncogenes, Hsp72 depletion triggered both p53 and ERK pathways (Table 1).
Although this work specifically focused on the role of Hsp72 in neoplastic transformation, at present it is clear that other Hsps are also important for cancer development and may play similar roles. For example, there have been several reports from this and other groups that downregulation of Hsp27 (38), Hsp70-2 (40), or mitochondrial Hsp70 (mortalin) (29) triggers p53-dependent senescence specifically in cancer cells (see reference 43 for review). Furthermore, Hsp27 is apparently involved in control of p53-independent senescence as well (38), and its knockdown, similar to Hsp72 knockdown, activated ERK pathway in RAS-transformed cells (unpublished data). It is not clear at the moment whether these chaperones cooperate with each other in suppressing the endogenous senescence programs and supporting proliferation of cancer cells, or they affect different steps of the senescence pathways. A possibility is that they control one of the common cofactors, like BAG3, which can cooperate with both Hsp70 and small Hsp family members (9). Independently of the mechanisms, it appears that upon neoplastic transformation the entire chaperone machinery becomes especially important, explaining the dependence of tumorigenesis on Hsf1 (15).
The nononcogenic “addiction” to Hsp72 by cancer cells suggests a novel approach toward drug design. Indeed, Hsp72 may be targeted to specifically prevent proliferation and eventually eliminate transformed cells. Accordingly, small molecules could be developed that target either Hsp72 or the entire heat shock response. Indeed, recently several inhibitors of the heat shock response have been developed and demonstrated significant anticancer effects both in cell culture and in xenograft models. Importantly, here we showed that depletion of Hsp72 could trigger senescence and growth suppression via both p53-dependent and p53-independent pathways, and this effect was seen with cancer cells with mutant p53 (e.g., BT-20 and MDA-MB231). Therefore, inhibitors of Hsp72 or the heat shock response potentially could have a broad specificity with cancers independently of p53 status.
There is a fundamental difference between mechanisms of action of the Hsp inhibitors and inhibitors of oncogenes, like inhibitory antibodies against Her2 (herceptin) or vascular endothelial growth factor or small molecules that inhibit the ERK signaling pathway (see reference 49 for review). Indeed, based on the findings in this work, it is clear that cell growth suppression by inhibition of Hsps or the entire heat shock response is dependent on high activity of the oncogenic signaling pathways. Furthermore, inhibition of Hsp72 enhances the oncogenic signaling, which results in triggering cell senescence or death. Therefore, potential inhibitors of Hsp72 or the heat shock response have an opposite activity from suppressors of oncogenic signaling pathways.
Acknowledgments
This work was supported by Public Health Service grant CA-081244 from the National Cancer Institute to M.S. and the Grunebaum Award to J.Y.
Footnotes
Published ahead of print on 10 November 2008.
REFERENCES
- 1.Amaravadi, R. K., and C. B. Thompson. 2007. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin. Cancer Res. 137271-7279. [DOI] [PubMed] [Google Scholar]
- 2.Bartek, J., J. Bartkova, and J. Lukas. 2007. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene 267773-7779. [DOI] [PubMed] [Google Scholar]
- 3.Bellmann, K., M. Jaattela, D. Wissing, V. Burkart, and H. Kolb. 1996. Heat shock protein hsp70 overexpression confers resistance against nitric oxide. FEBS Lett. 391185-188. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Porath, I., and R. A. Weinberg. 2005. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 37961-976. [DOI] [PubMed] [Google Scholar]
- 5.Bihani, T., A. Chicas, C. P.-K. Lo, and A. W. Lin. 2007. Dissecting the senescence-like program in tumor cells activated by Ras signaling. J. Biol. Chem. 2822666-2675. [DOI] [PubMed] [Google Scholar]
- 6.Bihani, T., D. X. Mason, T. J. Jackson, S. C. Chen, B. Boettner, and A. W. Lin. 2004. Differential oncogenic Ras signaling and senescence in tumor cells. Cell Cycle 31201-1207. [PubMed] [Google Scholar]
- 7.Braig, M., and C. A. Schmitt. 2006. Oncogene-induced senescence: putting the brakes on tumor development. Cancer Res. 662881-2884. [DOI] [PubMed] [Google Scholar]
- 8.Calderwood, S. K., M. A. Khaleque, D. B. Sawyer, and D. R. Ciocca. 2006. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem. Sci. 31164-172. [DOI] [PubMed] [Google Scholar]
- 9.Carra, S., S. J. Seguin, and J. Landry. 2008. HspB8 and Bag3: a new chaperone complex targeting misfolded proteins to macroautophagy. Autophagy 4237-239. [DOI] [PubMed] [Google Scholar]
- 10.Chang, B.-D., M. E. Swift, M. Shen, J. Fang, E. V. Broude, and I. B. Roninson. 2002. Molecular determinants of terminal growth arrest induced in tumor cells by a chemotherapeutic agent. Proc. Natl. Acad. Sci. USA 99389-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, J., and M. S. Goligorsky. 2006. Premature senescence of endothelial cells: Methuselah's dilemma. Am. J. Physiol. Heart Circ. Physiol. 290H1729-H1739. [DOI] [PubMed] [Google Scholar]
- 12.Chen, Z., L. C. Trotman, D. Shaffer, H.-K. Lin, Z. A. Dotan, M. Niki, J. A. Koutcher, H. I. Scher, T. Ludwig, W. Gerald, C. Cordon-Cardo, and P. Paolo Pandolfi. 2005. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436725-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciocca, D. R., and S. K. Calderwood. 2005. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 1086-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowell, J. K., J. LaDuca, M. R. Rossi, T. Burkhardt, N. J. Nowak, and S.-i. Matsui. 2005. Molecular characterization of the t(3;9) associated with immortalization in the MCF10A cell line. Cancer Genet. Cytogenet. 16323-29. [DOI] [PubMed] [Google Scholar]
- 15.Dai, C., L. Whitesell, A. B. Rogers, and S. Lindquist. 2007. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell 1301005-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daugaard, M., T. Kirkegaard-Sorensen, M. S. Ostenfeld, M. Aaboe, M. Hoyer-Hansen, T. F. Orntoft, M. Rohde, and M. Jaattela. 2007. Lens epithelium-derived growth factor is an Hsp70-2 regulated guardian of lysosomal stability in human cancer. Cancer Res. 672559-2567. [DOI] [PubMed] [Google Scholar]
- 17.Denoyelle, C., G. Abou-Rjaily, V. Bezrookove, M. Verhaegen, T. M. Johnson, D. R. Fullen, J. N. Pointer, S. B. Gruber, L. D. Su, M. A. Nikiforov, R. J. Kaufman, B. C. Bastian, and M. S. Soengas. 2006. Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nature 81053-1063. [DOI] [PubMed] [Google Scholar]
- 18.Di Micco, R., M. Fumagalli, and F. d'Adda di Fagagna. 2007. Breaking news: high-speed race ends in arrest—how oncogenes induce senescence. Trends Cell Biol. 17529-536. [DOI] [PubMed] [Google Scholar]
- 19.Gabai, V. L., K. R. Budagova, and M. Y. Sherman. 2005. Increased expression of the major heat shock protein Hsp72 in human prostate carcinoma cells is dispensable for their viability but confers resistance to a variety of anticancer agents. Oncogene 243328-3338. [DOI] [PubMed] [Google Scholar]
- 20.Gabai, V. L., A. B. Meriin, D. D. Mosser, A. W. Caron, S. Rits, V. I. Shifrin, and M. Y. Sherman. 1997. HSP70 prevent activation of stress kinases: a novel pathway of cellular thermotolerance. J. Biol. Chem. 27218033-18037. [DOI] [PubMed] [Google Scholar]
- 21.Heydari, A. R., R. Takahashi, A. Gutsmann, S. You, and A. Richardson. 1994. Hsp70 and aging. Experientia 501092-1098. [DOI] [PubMed] [Google Scholar]
- 22.Ikediobi, O. N., H. Davies, G. Bignell, S. Edkins, C. Stevens, S. O'Meara, T. Santarius, T. Avis, S. Barthorpe, L. Brackenbury, G. Buck, A. Butler, J. Clements, J. Cole, E. Dicks, S. Forbes, K. Gray, K. Halliday, R. Harrison, K. Hills, J. Hinton, C. Hunter, A. Jenkinson, D. Jones, V. Kosmidou, R. Lugg, A. Menzies, T. Mironenko, A. Parker, J. Perry, K. Raine, D. Richardson, R. Shepherd, A. Small, R. Smith, H. Solomon, P. Stephens, J. Teague, C. Tofts, J. Varian, T. Webb, S. West, S. Widaa, A. Yates, W. Reinhold, J. N. Weinstein, M. R. Stratton, P. A. Futreal, and R. Wooster. 2006. Mutation analysis of 24 known cancer genes in the NCI-60 cell line set. Mol. Cancer Ther. 52606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaatella, M., D. Wissing, P. A. Bauer, and G. C. Li. 1992. Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J. 113507-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaattela, M. 1999. Escaping cell death: survival proteins in cancer. Exp. Cell Res. 24830-43. [DOI] [PubMed] [Google Scholar]
- 25.Jaattela, M. 1993. Overexpression of major heat shock protein hsp70 inhibits tumor necrosis factor-induced activation of phospholipase A2. J. Immunol. 1514286-4294. [PubMed] [Google Scholar]
- 26.Kabakov, A. E., and V. L. Gabai. 1997. Heat shock proteins and cytoprotection: ATP-deprived mammalian cells. R. G. Landes Co., Austin, TX.
- 27.Karakas, B., A. Weeraratna, A. Abukhdeir, B. G. Blair, H. Konishi, S. Arena, K. Becker, W. Wood III, P. Argani, A. M. De Marzo, K. E. Bachman, and B. H. Park. 2006. Interleukin-1 alpha mediates the growth proliferative effects of transforming growth factor-beta in p21 null MCF-10A human mammary epithelial cells. Oncogene 255561-5569. [DOI] [PubMed] [Google Scholar]
- 28.Karlseder, J., D. Wissing, G. Holzer, L. Orel, G. Sliutz, H. Auer, M. Jaattela, and M. M. Simon. 1996. HSP70 overexpression mediates the escape of a doxorubicin-induced G2 cell cycle arrest. Biochem. Biophys. Res. Commun. 220153-159. [DOI] [PubMed] [Google Scholar]
- 29.Kaul, S. C., S. Aida, T. Yaguchi, K. Kaur, and R. Wadhwa. 2005. Activation of wild type p53 function by its mortalin-binding, cytoplasmically localizing carboxyl terminus peptides J. Biol. Chem. 28039373-39379. [DOI] [PubMed] [Google Scholar]
- 30.Kim, J.-S., C. Lee, C. L. Bonifant, H. Ressom, and T. Waldman. 2007. Activation of p53-dependent growth suppression in human cells by mutations in PTEN or PIK3CA. Mol. Cell. Biol. 27662-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathew, R., V. Karantza-Wadsworth, and E. White. 2007. Role of autophagy in cancer. Nat. Rev. Cancer 7961-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meriin, A. B., J. A. Yaglom, V. L. Gabai, D. D. Mosser, L. Zon, and M. Y. Sherman. 1999. Protein-damaging stresses activate c-Jun N-terminal kinase via inhibition of its dephosphorylation: a novel pathway controlled by Hsp72. Mol. Cell. Biol. 192547-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michels, A. A., B. Kanon, A. W. Konings, K. Ohtsuka, O. Bensaude, and H. H. Kampinga. 1997. Hsp70 and Hsp40 chaperone activities in the cytoplasm and the nucleus of mammalian cells. J. Biol. Chem. 27233283-33289. [DOI] [PubMed] [Google Scholar]
- 34.Min, J.-N., L. Huang, D. B. Zimonjic, D. Moskophidis, and N. F. Mivechi. 2007. Selective suppression of lymphomas by functional loss of Hsf1 in a p53-deficient mouse model for spontaneous tumors. Oncogene 265086-5097. [DOI] [PubMed] [Google Scholar]
- 35.Miyauchi, H., T. Minamino, K. Tateno, T. Kunieda, H. Toko, and I. Komuro. 2004. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J. 23212-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosser, D. D., A. W. Caron, L. Bourget, C. Denis-Larose, and B. Massie. 1997. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell. Biol. 175317-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nylandsted, J., M. Gyrd-Hansen, A. Danielewicz, N. Fehrenbacher, U. Lademann, M. Hoyer-Hansen, E. Weber, G. Multhoff, M. Rohde, and M. Jaattela. 2004. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J. Exp. Med. 200425-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Callaghan-Sunol, C., V. L. Gabai, and M. Y. Sherman. 2007. Hsp27 modulates p53 signaling and suppresses cellular senescence. Cancer Res. 6711779-11788. [DOI] [PubMed] [Google Scholar]
- 39.O'Callaghan-Sunol, C., and V. L. Gabai. 2007. Involvement of heat shock proteins in protection of tumor cells from genotoxic stresses, p. 169-190. In S. Calderwood, M. Y. Sherman, and D. R. Ciocca (ed.), Heat shock proteins in cancer. Springer, Berlin, Germany.
- 40.Rohde, M., M. Daugaard, M. H. Jensen, K. Helin, J. Nylandsted, and M. Jaattela. 2005. Members of the heat-shock protein 70 family promote cancer cell growth by distinct mechanisms. Genes Dev. 19570-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roninson, I. B., E. V. Broude, and B.-D. Chang. 2001. If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist. Updates 4303-313. [DOI] [PubMed] [Google Scholar]
- 42.Schmitt, C. A., J. S. Fridman, M. Yang, S. Lee, E. Baranov, R. M. Hoffman, and S. W. Lowe. 2002. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 109335-346. [DOI] [PubMed] [Google Scholar]
- 43.Sherman, M., V. Gabai, C. O'Callaghan, and J. Yaglom. 2007. Molecular chaperones regulate p53 and suppress senescence programs. FEBS Lett. 5813711-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song, J., M. Takeda, and R. I. Morimoto. 2001. Bag1-Hsp70 mediates a physiological stress signalling pathway that regulates Raf-1/ERK and cell growth. Nat. Cell Biol. 3276-282. [DOI] [PubMed] [Google Scholar]
- 45.Stege, G. J., J. F. Brunsting, H. H. Kampinga, and A. W. Konings. 1995. Thermotolerance and nuclear protein aggregation: protection against initial damage or better recovery? J. Cell. Physiol. 164579-586. [DOI] [PubMed] [Google Scholar]
- 46.Stege, G. J., H. H. Kampinga, and A. W. Konings. 1995. Heat-induced intranuclear protein aggregation and thermal radiosensitization. Int. J. Radiat. Biol. 67203-209. [DOI] [PubMed] [Google Scholar]
- 47.von Zglinicki, T., G. Saretzki, J. Ladhoff, F. d'Adda di Fagagna, and S. P. Jackson. 2005. Human cell senescence as a DNA damage response. Mech. Ageing Dev. 126111-117. [DOI] [PubMed] [Google Scholar]
- 48.Wang, W., J. X. Chen, R. Liao, Q. Deng, J. J. Zhou, S. Huang, and P. Sun. 2002. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol. Cell. Biol. 223389-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinstein, I. B., A. Joe, and D. Felsher. 2008. Oncogene addiction. Cancer Res. 683077-3080. [DOI] [PubMed] [Google Scholar]
- 50.Yaglom, J., C. O'Callaghan-Sunol, V. Gabai, and M. Y. Sherman. 2003. Inactivation of dual-specificity phosphatases is involved in the regulation of extracellular signal-regulated kinases by heat shock and Hsp72. Mol. Cell. Biol. 233813-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yaglom, J. A., D. Ekhterae, V. L. Gabai, and M. Y. Sherman. 2003. Regulation of necrosis of H9C2 myogenic cells upon transient energy deprivation: rapid DE-energization of mitochondria precedes necrosis and is controlled by reactive oxygen species, stress-kinase JNK, HSP72 and ARC. J. Biol. Chem. 27850483-50496. [DOI] [PubMed] [Google Scholar]
- 52.Yaglom, J. A., V. L. Gabai, and M. Y. Sherman. 2007. High levels of heat shock protein Hsp72 in cancer cells suppress default senescence pathways. Cancer Res. 672373-2381. [DOI] [PubMed] [Google Scholar]