Abstract
Rho GTPases are critical for mitosis progression and completion of cytokinesis. During mitosis, the GDP/GTP cycle of Rho GTPases is regulated by the exchange factor Ect2 and the GTPase activating protein MgcRacGAP which associates with the kinesin MKLP1 in the centralspindlin complex. We report here that expression of Ect2, MgcRacGAP, and MKLP1 is tightly regulated during cell cycle progression. These three genes share similar cell cycle-related signatures within their promoter regions: (i) cell cycle gene homology region (CHR) sites located at −20 to +40 nucleotides of their transcription start sites that are required for repression in G1, (ii) E2F binding elements, and (iii) tandem repeats of target sequences for the CUX1 transcription factor. CUX1 and E2F1 bind these three promoters upon S-phase entry, as demonstrated by chromatin immunoprecipitation, and regulate transcription of these genes, as established using promoter-luciferase reporter constructs and expression of activated or dominant negative transcription factors. Overexpression of either E2F1 or CUX1 increased the levels of the endogenous proteins whereas small interfering RNA knockdown of E2F1 or use of a dominant negative E2F1 reduced their expression levels. Thus, CUX1, E2F, and CHR elements provide the transcriptional controls that coordinate induction of Ect2, MgcRacGAP, and MKLP1 in S phase, leading to peak expression of these interacting proteins in G2/M, at the time they are required to regulate cytokinesis.
Small GTP-binding proteins of the Ras superfamily regulate a wide array of cellular functions and play critical roles in signal transduction, gene regulation, intracellular trafficking of vesicles, cell proliferation, and survival (37). Proteins in the Rho subfamily (Rho, Rac, and Cdc42) are best characterized for their effects on actin polymerization and cytoskeleton organization (15). Through their effects on the cytoskeleton, Rho GTPases are essential in cell morphology, cell migration and chemotaxis, and establishment of cell polarity (6, 18, 44). They also participate in several aspects of cell cycle progression. During mitosis, Cdc42 regulates spindle microtubule attachment to kinetochores in metaphase, whereas RhoA, which localizes at the cleavage furrow during telophase and ends up at the midbody at the abcission step, is involved in cytokinesis (25, 43, 59). Indeed, interfering with RhoA activity, by treating cells with Clostridium botulinum C3 exoenzyme, or with RhoA expression, by small interfering RNA (siRNA) silencing, prevents contraction of the actomyosin ring and leads to failure of cytokinesis and multinucleation in various cell types (25, 60).
During these steps, RhoA activity is regulated by the exchange factor Ect2 and the GTPase activating protein MgcRacGAP (hereafter, RacGAP) which colocalize with a kinesin known as MKLP1, Kif23, or kinesin-like protein 5 (35, 52, 61). MKLP1 associates with RacGAP in a complex known as centralspindlin and is thought to mediate recruitment of RacGAP to the mitotic spindle. Protein interactions between Ect2 and RacGAP lead to conformational changes that activate the exchange activity of Ect2 (20, 26).
During mitosis progression, dynamic protein interactions between Ect2, RacGAP, and MKLP1 are regulated by phosphorylation/dephosphorylation events mediated by the Cdk1 and Aurora B kinases (34, 36, 53). The dynamics and functions of this complex are evolutionarily conserved as the Drosophila orthologs (Pebble/RacGAP50/Pavarotti) and the Caenorhabditis elegans orthologs (LET-21/CYK4/ZEN4) also control cytokinesis with similar regulations.
Genes whose protein products play specific roles in cell cycling and exert their functions at defined steps of cycle progression are often regulated in a cell cycle-dependent manner (7, 14). These genes include not only the well-characterized mitotic cyclins and their associated kinases or inhibitors but also additional kinases (e.g., Plk-1 and Aurora) and components of the anaphase promoting complex/cyclosome (3, 56, 58).
Promoters for cell cycle-regulated genes contain binding sites for specific transcription factors (14). The E2F family contains eight identified members acting as transcriptional activators or repressors (11). E2F1 was initially characterized as an activator of genes expressed at the G1/S transition to regulate cell cycle progression but was also shown to regulate G2/M genes as well as the apoptosis pathway (19, 62). Besides its coactivator DP, E2F may interact with other transcription factors such as B-Myb, itself an E2F target in G1/S (24, 45, 62).
CUX1 (Cut homeobox 1) belongs to the family of Cut homeodomain-containing transcription factors that is present in all metazoans. In vertebrates, the protein was first identified as the CCAAT-displacement protein and has been variously called CDP, CUX, or CUTL1 (2, 41, 57). Whereas the CCAAT-displacement activity, which depends on rapid but transient interactions with DNA, appears to be constant throughout the cell cycle, a more stable interaction with DNA arises at the end of the G1 phase as the result of dephosphorylation by Cdc25A and proteolytic processing by a nuclear isoform of cathepsin L (8, 17, 38). The N-terminally truncated isoform thus generated, p110 CUX1, was shown to accelerate entry into S phase (46). The regulatory effect of p110 CUX1 was found to be promoter dependent: it repressed the p21cip and cyclin H genes but activated most genes including those coding for cyclin A2, DNA polymerase (Pol) α, and the Cdc25 proteins (8, 21, 46, 55).
Another signature of cell cycle-regulated genes is the frequent occurrence of cell cycle-dependent element/cycle homology region (CDE/CHR) sequences close to the transcription start site. Although factors binding to such elements have not yet been unambiguously characterized, their functionality has clearly been established by mutation or deletion analysis in several cell cycle genes such as cdc25C, Cyclin A, or aurora B (22, 27, 29, 30).
We report here that the mitotic complex genes Ect2, RacGAP, and MKLP1 are coordinately induced in S phase in proliferating T lymphocytes as well as in epithelial cells, depending upon activity of the CUX1 and E2F1 transcription factors. We have also identified CHR elements that mediate repression of these promoters in the G1 phase of the cell cycle.
MATERIALS AND METHODS
Cell lines and culture conditions.
The human interleukin-2 (IL-2)-dependent Kit 225 T-cell line was maintained in RPMI 1640 culture medium containing 2 mM l-glutamine, antibiotics, 2% sodium pyruvate, 10% fetal calf serum, and 0.5 nM recombinant human IL-2 (Proleukin; Chiron Corp., The Netherlands). Kit 225 cells were synchronized in G1 by culturing in the absence of IL-2 for 48 h, and cell cycle progression was induced by adding back IL-2 for various periods of time. The Hct 116 colon carcinoma cell line was cultured in McCoy medium supplemented with antibiotics and 10% fetal calf serum. For synchronization studies, Hct 116 cells were rendered quiescent by culturing for 6 to 7 days in 0.5% serum, after which complete medium was added.
For cell cycle inhibition studies, IL-2-deprived cells were set in culture with IL-2 and either hydroxyurea (100 μM) or a combination of rapamycin (5 nM) and LY294002 (5 μM). Cell DNA content was analyzed by flow cytometry following propidium iodide staining, and analysis of the fraction of cells in G1, S, or G2/M was performed using Modfit software.
Reverse transcription-PCR (RT-PCR) analysis and RNA ligase-mediated rapid amplification of cDNA ends (RLM-RACE).
Total cell RNA was extracted and purified according to standard procedures. Oligo(dT) first-strand cDNA was synthesized from 5 μg of total RNA using Superscript II reverse transcriptase (Invitrogen). Affymetrix array hybridization data were derived from our previously published study (40).
RLM-RACE was performed using a kit from Ambion, according to the manufacturer's instructions, on mRNA purified from S-phase synchronized Kit 225 cells. The RLM-RACE products were then cloned using a Strataclone PCR cloning kit (Stratagene), and randomly picked clones were sequenced. All primers used for RT-PCR or RLM-RACE are listed in File S3 in the supplemental material.
ChIP analysis.
Chromatin immunoprecipitation (ChIP) was performed as previously described (46) with minor modifications. Briefly, Kit 225 (107) cells that had been deprived of IL-2 were cross-linked in 1% formaldehyde at various time points after reseeding in IL-2-containing medium. Cells were lysed in hypo-osmotic buffer, and nuclei were isolated, washed, and lysed in radioimmunoprecipitation assay buffer. Nuclear extracts were sonicated on ice to generate DNA fragments of 250 to 750 bp. Immunoprecipitation and reversal of cross-linking were performed as previously described. Immunoprecipitated DNA was then amplified by PCR using primers listed in File S3 in the supplemental material, and the PCR products were resolved on 2% agarose gels.
Antibodies to RNA Pol II (sc-899) and E2F-1 (sc-251) were from Santa Cruz Biotechnology. The anti-CUX1 861 antibody has been previously described (21). An antihemagglutinin (anti-HA) tag antibody (clone 12CA5) was used as a control.
Western blotting.
Anti-RacGAP and anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH) antibodies were from Abcam (Cambridge, United Kingdom), and anti-Ect2, MKLP1, p27, and cyclin A were from Santa Cruz Biotechnology (Santa-Cruz). Horseradish peroxidase-conjugated anti-mouse and anti-rabbit secondary antibodies were purchased from GE Healthcare (Orsay, France) and Dako (Denmark) and were detected using enhanced chemiluminescence. Cell lysis and Western blot analysis were performed as previously described (1).
Plasmid constructs and siRNA.
The plasmid containing three copies of a gamma-activated sequence-luciferase (3×GAS-Luc) construct used to analyze STAT activation has been previously described (1). A plasmid carrying six copies of E2F-Luc (6×E2F-Luc) (39) was kindly provided by K. Helin (European Institute of Oncology, Milan, Italy), and a reporter construct containing the human CCNA (the fragment from nucleotide −1048 to +245) promoter (23) was provided by R. A. Padua (INSERM, Paris, France).
An active, truncated version of CUX1 was used for expression, together with the empty control pXJ42 vector, as described previously (54). The pcDNA3-E2F1 expression vector (10) was kindly provided by J. Nevins (Duke University Medical Center, Durham, NC). A dominant-negative (DN) version of this construct, coding for a C-terminally truncated protein containing amino acids 1 to 372, was derived by introducing a stop codon in position 1116 of the coding sequence.
Ect2, MKLP1, and RacGAP promoter constructs were obtained by cloning PCR-amplified promoter regions into the PGL-3 basic Luc reporter plasmid (Promega). Site-directed mutagenesis was performed using a QuikChange mutagenesis kit (Stratagene) according to the manufacturer's instructions. All constructs, whether wild type or mutated, were verified by DNA sequencing.
Control siRNA (AllStars Negative Control) and siRNA targeting E2F1 (Hs_E2F1_6 HP Validated siRNA) were obtained from Qiagen, S.A. (France).
Transient transfections and Luc assays.
Transfections of Kit 225 cells were performed by electroporation using a Gene Pulser apparatus (Bio-Rad Laboratories, Hercules CA) set at 250 V and 960 μF.
For Luc assays, IL-2-deprived Kit 225 cells (107) were washed in RPMI 1640 medium, resuspended in 150 μl of RPMI 1640 medium, electroporated with 5 μg of reporter plasmid DNA, and cultured with IL-2 or additional drugs. Because most of the normalizing internal control plasmids we have tested are often regulated either in the presence of IL-2 or by expression of transcription factors in Kit 225 cells, data were normalized by including a recombinant glutathione S-transferase (GST)-Renilla Luc fusion protein (0.6 μg) (unpublished data) in the electroporation mix.
Exponentially growing or serum-starved Hct 116 cells (2 × 105) were transfected using Jet polyethyleneimine (Ozyme) with 0.5 μg of promoter constructs and 0.05 μg of the normalizing pSRα-Renilla Luc reporter. For coexpression assays, CUX1 or E2F1 expression vectors were used at 5 μg in Kit 225 cells and 0.2 μg in Hct 116 cells. The total amounts of transfected DNA were kept constant by the addition of empty control vector. Cells were lysed in 25 μl of provided buffer to proceed to a dual Luc assay according to the manufacturer's instructions (Promega). Luc activity was measured on 5 μl of the lysates on a MicroLumat Plus LB 96 V luminometer (Berthold Technologies). Results were normalized by dividing firefly signals by Renilla signals. When needed, the remaining 20 μl of the cell lysates was used to assess expression of the desired proteins by immunoblotting.
RESULTS
Coordinated expression of Ect2, RacGAP, and MKLP1 during cell cycle progression.
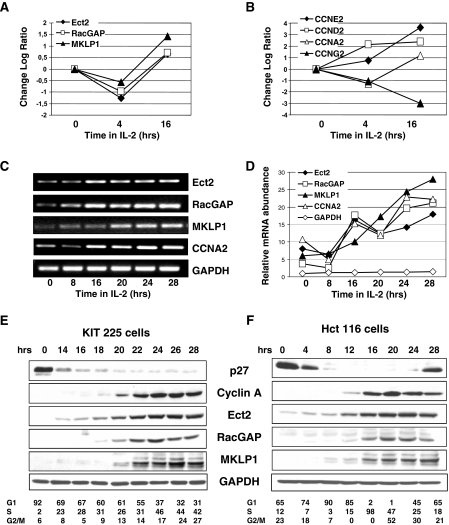
The Kit 225 cell line is a nontransformed CD4-positive human lymphocyte cell line that strictly depends on the growth factor IL-2 for proliferation. When deprived of IL-2, Kit 225 cells become arrested in the G1 phase of the cell cycle and reinitiate cell cycle progression synchronously upon IL-2 addition, as evidenced by variations in expression of the major cyclins (Fig. 1B) or by flow cytometry assessment for DNA contents at various time points. We previously reported, using DNA array analysis of gene expression in this cell line, that IL-2 regulates a number of genes that participate in the Rho GTPase pathways including GDP/GTP exchange factors and GTPase-activating proteins, such as Ect2 and RacGAP (40). We then observed that mRNA for MKLP1, a kinesin that associates with RacGAP during mitosis, undergoes variations that parallel those of Ect2 and RacGAP (Fig. 1A). RT-PCR analysis confirmed that, similarly to cyclin A2, expression of MKLP1, Ect2, and RacGAP increased between 16 and 20 h in culture with IL-2, reaching 20- to 25-fold higher levels as cells arrived in late S or G2/M (Fig. 1C and D). Western blot analysis (Fig. 1E) showed that Ect2, RacGAP, and MKLP1 were undetectable in G1-arrested cells, which express high levels of the cell cycle inhibitor p27. As cells entered S phase, displaying decreased expression of p27 concomitant with an increase in cyclin A2, the Ect2, RacGAP, and MKLP1 proteins became detectable and thereafter increased progressively to peak in late S phase or G2/M.
FIG. 1.
Cell cycle variations of expression of Ect2, RacGAP, and MKLP1. Kit 225 cells were arrested in G1 by culture for 48 h in the absence of IL-2 and were then restimulated with IL-2 for 4 or 16 h. mRNAs were prepared and hybridized to Affymetrix U133A DNA arrays as previously described. Shown are log plots of variations observed for Ect2, RacGAP, and MKLP1 (A) and selected cyclins (B) to illustrate cell cycle progression. (C) RT-PCR analysis of gene expression in G1-arrested Kit 225 cells (0) or cells restimulated for the indicated times following IL-2 addition. (D) mRNA quantification. Images of the ethidium bromide-stained gels shown in panel C were acquired using a Bio-Rad GelDoc 2000 apparatus and quantified with the Quantity-One software. mRNA signals for the indicated genes were normalized with the GAPDH signals of the corresponding time points. (E) Total cell extracts were obtained from G1-arrested Kit 225 cells (0) or cells cultured with IL-2 for the indicated times. Western blot analysis was performed with the indicated antibodies. Numbers shown underneath the figure represent the percentage of cells in G1, S, or G2/M following IL-2 addition as assessed by fluorescence-activated cell sorter analysis of cells stained with propidium iodide. (F) Serum-starved Hct 116 cells were stimulated to enter cell cycle progression by serum addition, and Western blot analysis was performed with the indicated antibodies. Numbers shown underneath the figure represent the percentage of cells in each phase of the cell cycle. The data shown here are representative of multiple experiments.
To exclude the possibility that such regulations might result from unique and unidentified features of Kit 225 cells, we assessed expression of these proteins in Hct 116 cells, an epithelial, adherent cell line derived from a colon carcinoma. As shown in Fig. 1F, Western blot analysis revealed cell cycle-dependent variations of Ect2, RacGAP, and MKLP1 expression in serum-stimulated Hct 116 cells similar to those observed in Kit 225 cells. Alternative methods of cell synchronization, including release from a nocodazole block or from a double thymidine block, confirmed that these variations were truly cell cycle dependent and not simply linked to cell proliferation or exit from quiescence (data not shown).
Thus, the Ect2, RacGAP, and MKLP1 proteins, which act together to regulate Rho GTPases during mitosis, are coordinately expressed: they are induced at the G1/S transition or early in S phase and peak in G2/M.
Characterization of transcription initiation sites and 5′ flanking regions.
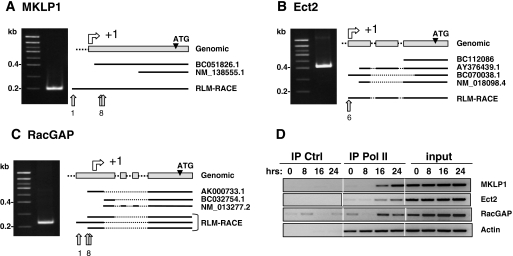
We then asked whether the Ect2, RacGAP, and MKLP1 genes could be regulated by common transcription pathways. Since their promoters had not been previously characterized, we first located their transcription start sites (TSS) and analyzed their 5′ flanking regions. Transcription initiation sites were identified by 5′ RLM-RACE. The 5′ ends of Ect2, RacGAP, and MKLP1 mRNAs, purified from Kit 225 cells, were amplified by PCR and cloned. Randomly chosen clones were then sequenced, and the nucleotide sequences obtained were aligned on the corresponding genomic sequences (Fig. 2A to C). The nucleotide sequences obtained were compared to mRNAs from expressed sequence tag data banks, and for the most part were found to be homologous to the 5′ end of the longest available expressed sequence tag. Most of the sequences obtained for Ect2 and MKLP1 were consistent with a single TSS. On the other hand, 5′ sequences for RacGAP were more divergent, suggesting the existence of multiple TSS located within a 100-nucleotide-long region. However, to simplify promoter numbering, an arbitrary +1 was assigned to correspond to the most frequent 5′ end identified in our experiments (see File S1 in the supplemental material).
FIG. 2.
5′ RLM-RACE determinations of MKLP1 (A), Ect2 (B), and RacGAP (C) promoter TSS. mRNAs were prepared from S-phase-synchronized Kit 225 cells. Shown to the left are results of 6% acrylamide gel electrophoresis of the RLM-RACE products. At right are schematic alignments of obtained sequences with GenBank references. Light-gray arrows indicate the most 5′ nucleotides. The number of independent clones that were sequenced is shown underneath. (D) Recruitment of RNA Pol II to proximal promoters upon IL-2 stimulation of Kit 225 cells as assessed by ChIP analysis using anti-Pol II or control (Ctrl; anti-HA) antibodies. Primers used for PCR amplification of ChIP products are listed in File S3 in the supplemental material. Input represents 2% of the starting material. IP, immunoprecipitation.
The occupancy of these regions by the RNA Pol transcription complex was confirmed by ChIP using antibodies directed against RNA Pol II on DNA prepared from synchronized Kit 225 cells (Fig. 2D). The immunoprecipitated DNA was amplified by PCR using oligonucleotides corresponding to sequences surrounding or lying just 5′ to the TSS (see File S3 in the supplemental material). These experiments demonstrated that, whereas the actin promoter was occupied by the RNA Pol II complex at all time points, the complex was recruited to the promoters of all three genes after approximately 16 h in culture with IL-2, i.e., when cells entered S phase.
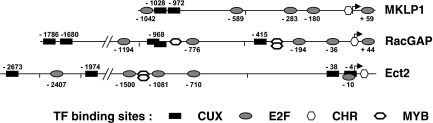
The nucleotide sequences immediately upstream of the transcription start sites were analyzed with the Genomatix suite (www.genomatix.de) and TSSW software (www.softberry.com). These analyses did not identify consensus TATA boxes in these three promoters. A schematic representation of the putative promoter regions for the three genes is shown in Fig. 3 and complete sequences are shown in File S2 in the supplemental material.
FIG. 3.
Sequence analysis of the 5′ flanking regions for the Ect2, RacGAP, and MKLP1 genes. Schematic organization of transcription factor (TF) binding sites for CUX1, E2F, and MYB and the CHR sites. Complete sequences and alignments with consensus TF binding sites are shown in Files S2 and S4 in the supplemental material.
Bioinformatics analysis revealed that these three genes share similar cell cycle-related signatures within their promoter regions including the following: (i) CHR binding elements located in the region −20 to +40 nucleotides of the TSS, (ii) a number of E2F binding sites, and (iii) tandem repeats of target sequences for the CUX1 transcription factor located in the region of nucleotides −1000 to −2500, 5′ of their TSS. Sequence comparison indicated that some of these features and their general organization are conserved in the corresponding regions of the mouse genes (data not shown), suggesting that they may play a role in transcription regulation. Binding sites for other transcription factors, such as SP1, MYB, or NF-Y, are also present; but although they may be relevant to cell cycle progression, they appear in random positions in these promoters, and their putative functionality has not been investigated.
Cloning and analysis of the Ect2, RacGAP, and MKLP1 promoters.
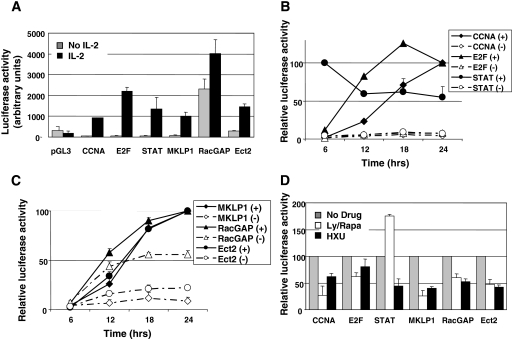
The signaling pathways which are triggered by IL-2 (16) result in activation of transcription factors that regulate cell survival and cell cycle progression. As shown in Fig. 4A, IL-2 activation of E2F and STAT5 can be assessed using synthetic 6×E2F or 3×GAS reporter plasmids, respectively, and cycle progression results in activation of a cyclin A promoter construct. Promoter regions of the Ect2, RacGAP, and MKLP1 genes were amplified by PCR from either human cell DNA (for MKLP1 and RacGAP) or bacterial artificial chromosome RP11-816B4 (for Ect2) and cloned into the pGL3-basic, promoterless Luc reporter plasmid. Initial experiments showed that when transiently transfected into Kit 225 cells, the MKLP1 and Ect2 promoter constructs did not drive reporter expression in the absence of IL-2. When, however, cells were cultured in IL-2-containing medium for 24 h after transfection, both promoters generated significant Luc activity (Fig. 4A). Although the RacGAP promoter construct appeared to be active in the absence of IL-2, Luc activity was further increased in IL-2-stimulated cells.
FIG. 4.
Cell cycle dependency of promoter activities. (A) IL-2-dependent Luc expression. Kit 225 cells were arrested in G1 by culturing for 48 h in the absence of IL-2, transfected with the following Lucreporter plasmids: empty pGL3-basic or pGL3 containing the indicated promoters; STAT and E2F represent the synthetic 3×GAS-Luc and 6×E2F-Luc reporters, respectively. Cells were further cultured for 24 h with or without IL-2. (B and C) Time-dependent reporter responses with (+) or without (−) IL-2. Luc activity was measured as relative light units at the indicated time points following transfection (time zero), and results are expressed as a percentage of maximum activity reached at 24 h, except for the STAT reporter, which peaked at 6 h. Actual relative increases in Luc between the IL-2-stimulated 24-h time point and the nonstimulated 6-h time point were as follows: CCNA, 47.7-fold; E2F, 66.6-fold; Ect2, 36.4-fold; RacGAP, 14.8-fold; and MKLP1, 25.9-fold. (D) Cells were treated as in panel A, and drugs were added following transfection. Ly/Rapa, LY294002 (5 μM) plus rapamycin (5 nM); HXU, hydroxyurea (100 μM). Results are expressed as a percentage of the IL-2 response in the absence of inhibitors. All experiments were repeated from three to six times, and the results shown are means ± standard deviations.
We then analyzed cell cycle variations in promoter activation using these constructs. Despite the reported short half-life of the firefly Luc protein, we were wary that Luc accumulation might mask cell cycle-dependent variations of promoter activities. However, as shown in Fig. 4B, the early and transient activation of STAT5 induced by IL-2 was faithfully reflected by the kinetics of activation of the 3×GAS reporter, with a high level of Luc activity that was detectable as early as 6 h following IL-2 stimulation and that decreased at later time points. In contrast, the 6×E2F reporter plasmid yielded Luc activity that accumulated significantly between the 6- and 12-h time points. Finally, the cyclin A2 promoter construct yielded detectable activity only starting at 18 h, the approximate time at which cells entered S phase. Luc activities generated by the Ect2 and MKLP1 promoter constructs were relatively low at the 6-h and 12-h time points and more than doubled between the 12-h and the 18-h time points (Fig. 4C). Consistent with a significant IL-2-independent response of the RacGAP promoter construct, Luc was produced during the first 6-h time interval, but the majority of the IL-2-stimulated Luc production, compared to the response obtained in the absence of IL-2, also occurred between 12 and 18 h. These results suggest that cell cycle progression is required for promoter regulation and that maximum activation occurs upon S phase entry.
To further assess the effect of the cell cycle on promoter activation, cells were stimulated with IL-2 in the presence of drugs known to inhibit cell cycle progression. Hydroxyurea was used to block cells in late G1 or at the G1/S transition and a combination of rapamycin with the phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 was used to block cells in early G1 as previously described (5, 40). Both treatments significantly reduced the level of Luc activity driven by the CCNA2 promoter, whereas, as predicted, hydroxyurea had little effect on the E2F reporter, and the LY294002-rapamycin combination increased, rather than inhibited, the response of the 3×GAS reporter construct. Taken together, the reporter constructs containing the Ect2, RacGAP, or MKLP1 promoters responded similarly to the CCNA2 promoter construct in that they were strongly repressed in the presence of the inhibitors, suggesting that induction of Ect2, RacGAP, and MKLP1 depends upon late signals possibly associated with S-phase entry.
The CHR elements are involved in promoter repression in G1.
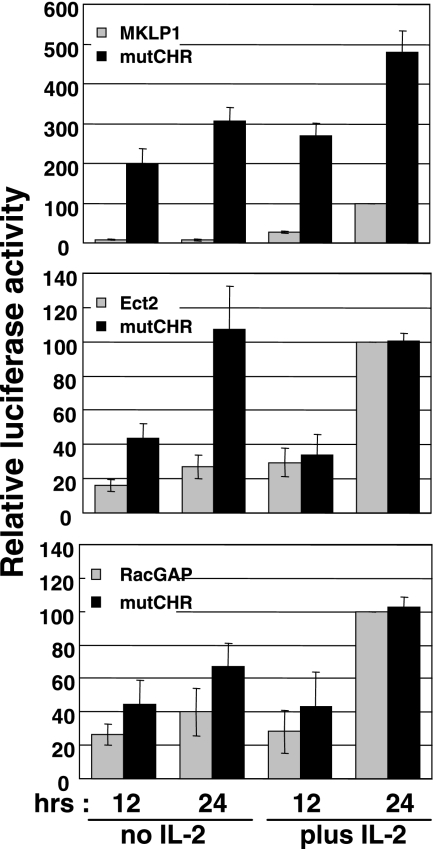
Since all three promoters contain CHR sequences close to the TSS, we next investigated their putative roles in promoter activity by introducing mutations in these sites. The CHR consensus (G/A)TTTGAA was mutated to (G/A)TTTGGG (mutated residues in bold) by site-directed mutagenesis. As shown in Fig. 5, replacement mutations of the CHR sequence in the MKLP1 promoter dramatically increased the production of Luc in response to IL-2, but more importantly, it revealed promoter activity in the absence of IL-2. Although the Ect2 and RacGAP promoter constructs responded in a slightly different way, their responses in the absence of IL-2 were also significantly increased when the CHR element was mutated. Thus, the CHR element appears to be essential for repression in the absence of IL-2 or in the G1 phase of the cell cycle, and one effect of IL-2 stimulation would be to relieve this repression. Consistent with this hypothesis, both the Ect2 and RacGAP promoters responded optimally after 24 h of IL-2 stimulation whether the CHR was mutated or not.
FIG. 5.
The CHR sites are required for repression of the MKLP1, Ect2, and RacGAP promoters in G1. CHR consensus TTTGAA sequences present in the proximal region of all three promoters were changed to TTTGGG (mutations are underlined) by site-directed mutagenesis. Promoter activities, with or without IL-2, were compared to that of wild-type constructs in Kit 225 cells. Results are expressed as a percentage of the response of the wild-type constructs at 24 h with IL-2 and are means ± standard deviations of three independent assays. mutCHR, mutated CHR.
Role of the CUX1 target sequences.
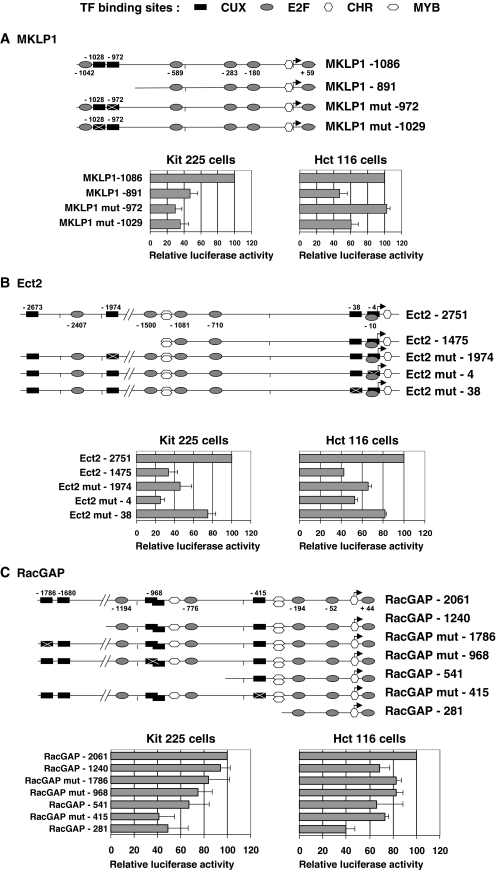
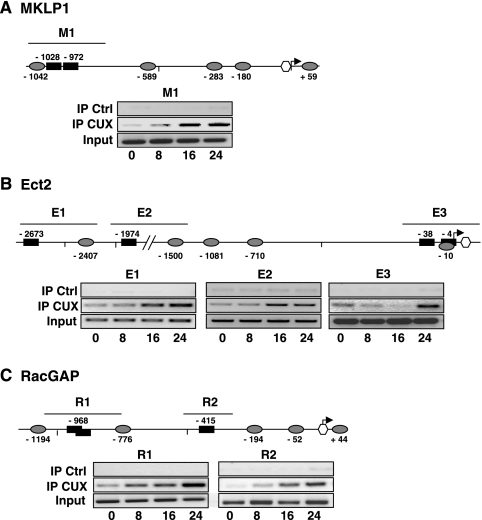
To analyze whether the predicted CUX1 target sequences might play a direct role in promoter activation, we first tested a series of 5′ deletions for each construct. As shown in Fig. 6, deleting the regions of the promoters that contain the CUX1 target sequences significantly reduced the level of Luc expression both in IL-2-stimulated Kit 225 cells and in serum-stimulated Hct 116 cells, suggesting that these elements are functionally important. This was further confirmed for the three promoters by introducing mutations in some of the predicted CUX1 target sequences. Similar to the deletion analysis, mutating either of the two CUX1 sequences reduced activity of the MKLP1 promoter by more than 50% in Kit 225 cells (Fig. 6A). Unexpectedly, mutation at the Cut site in position −972 seemed to have no effect when assayed in Hct 116 cells, but this has not been explored further. The Ect2 and RacGAP promoters each contain four CUX1 binding sequences, of which only three could be successfully mutated due to the unfavorable sequence environment of the fourth one. On both promoters, introducing a single mutation in any of the CUX1 sites significantly reduced activity of the full-length promoters, suggesting that these sites are important for their regulation (Fig. 6B and C). In contrast, analysis of the results shown in Fig. 6 with respect to the position of the Myb sites did not provide evidence for a critical role of Myb. We then asked whether recruitment of the CUX1 protein on these promoters could be detected by a ChIP approach. These experiments not only demonstrated that CUX1 bound all three promoters but also showed enrichment in bound target sequences as the Kit 225 cells progressed through the cell cycle (Fig. 7). Indeed, little or no binding, depending upon the specific region studied, was detectable in G1-arrested cells, and signals appeared and/or progressively increased in intensity with time to reach a maximum in cells that had been in culture with IL-2 for 24 h. Thus, these experiments clearly demonstrated recruitment of CUX1 to the regions of the MKLP1, Ect2, and RacGAP promoters that contain the putative target sequences identified by computer analysis.
FIG. 6.
Deletion/mutation analysis of reporter constructs. Deletions or mutations were introduced in each of the promoter constructs for MKLP1, Ect2, and RacGAP and assayed at 24 h posttransfection in IL-2-stimulated Kit 225 cells or serum-stimulated Hct 116 cells. Results are expressed as the percentage of the Luc activity produced by the longest construct for each promoter and are shown as means ± standard deviations calculated from three to four independent assays. Mut, mutant; TF, transcription factor.
FIG. 7.
CUX1 recruitment on the MKLP1, Ect2, and RacGAP promoters during cell cycle progression. ChIP was performed using anti-CUX1 or control (Ctrl; anti-HA) antibodies, as described in Materials and Methods, on nuclear extracts prepared at various time points from IL-2-stimulated Kit 225 cells. Positions of the PCR-amplified regions for each promoter are shown above the promoter scheme; primers used are listed in File S3 in the supplemental material. Input represents 2% of the starting material. The data are representative of at least two independent ChIP experiments. IP, immunoprecipitation.
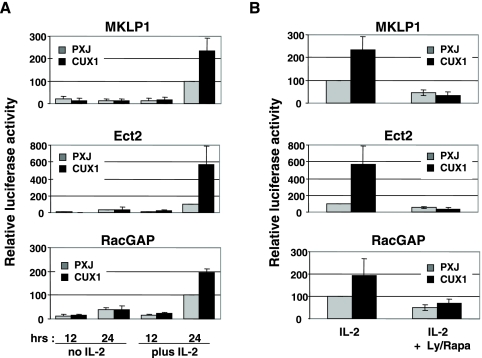
We then asked whether expression of a truncated form of CUX1 (p110 CUX1) that displays high transcriptional activity would modify Luc expression driven by these promoters. Kit 225 cells were cotransfected with the various reporter constructs together with an expression vector for a myc-tagged p110 CUX1 protein containing amino acids 878 to 1336 (54) or with an empty vector as a control. Activity of all three promoters increased by at least twofold when they were coexpressed with p110 CUX1. This was observed both in IL-2-stimulated Kit 225 cells (Fig. 8A) and in Hct 116 (see Fig. 10B). However, in Kit 225 cells, the effect of CUX1 expression was strictly dependent upon the presence of IL-2, indicating that IL-2-induced signals are required in T lymphocytes for promoter activation by p110 CUX1. To further investigate this point, we showed that when cells were cultured in the presence of IL-2 but blocked in cycle progression by addition of LY294002-rapamycin, expression of p110 CUX1 remained inefficient in boosting Luc production (Fig. 8B).
FIG. 8.
IL-2 is required for CUX1 to stimulate activity of the MKLP1, Ect2, and RacGAP promoters. (A) Kit 225 cells were cotransfected with the promoter constructs together with the empty pXJ vector or pXJ encoding p110 CUX1 and then cultured for an additional 12 or 24 h with or without IL-2. (B) Cells were treated as in panel A except that LY294002 (5 μM) and rapamycin (5 nM) (Ly/Rapa) were added to IL-2-stimulated cells to prevent cycle progression. Results are expressed as the percentage of the response obtained with IL-2 alone.
FIG. 10.
E2F1 is required for CUX1 activation of these promoters. Kit 225 cells (A) or Hct 116 cells (B) were cotransfected with the indicated reporter constructs together with empty vectors or vectors encoding p110 CUX1, E2F1, or a truncated DN-E2F1 protein. Results are expressed relative to the response of cells transfected with empty vectors and stimulated with IL-2 for 24 h. (C) Control blot for myc-CUX1 and HA-E2F1 expression in transfected cells.
E2F is required for CUX1 activation of these promoters.
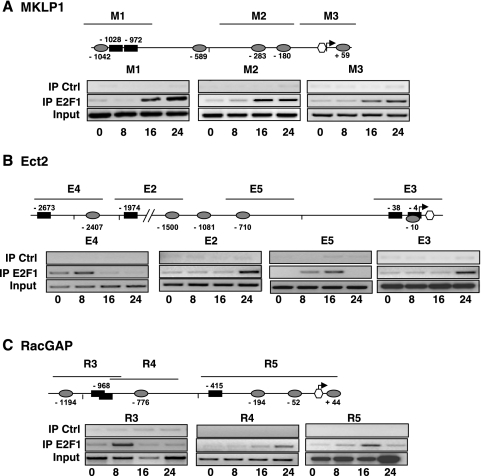
In T lymphocytes, one of the known downstream targets of the PI3K/mTOR pathway which is inhibited in the presence of LY294002-rapamycin, is the E2F family of transcription factors (4). Since computer analysis also revealed the presence of a number of E2F binding sites in all three promoters (Fig. 3), we performed a series of ChIP experiments to assess recruitment of E2F on these sites. A clear time-dependent recruitment of E2F1 on all three sites in the MKLP1 promoter (Fig. 9A) was observed. E2F1 was also recruited on all the target sequences present in the Ect2 and RacGAP promoters although individual sites showed variations in the time dependence of this recruitment (Fig. 9B and C).
FIG. 9.
E2F1 recruitment on the MKLP1 (A), Ect2 (B), and RacGAP (C) promoters during cell cycle progression. ChIP was performed using anti-E2F1 or control (Ctrl; anti-HA) antibodies on nuclear extracts prepared at various time points from IL-2-stimulated Kit 225 cells. Positions of the PCR-amplified regions are shown above the promoter scheme, and primers used are listed in File S3 in the supplemental material. The data are representative of at least two independent ChIP experiments. IP, immunoprecipitation.
We then assessed the effects of E2F on promoter activation by either expressing E2F1 or a truncated, DN form of E2F1 (DN-E2F). In these experiments we first noticed that Kit 225 cells were highly sensitive to apoptosis induced by forced expression of E2F1, which prevented a clear interpretation of the results. Yet we were able to show that DN-E2F completely abolished the IL-2 response of these promoters, demonstrating an absolute requirement for E2F (Fig. 10A). In addition, expression of the DN-E2F also inhibited Luc production generated by these promoter constructs in the presence of CUX1. On the other hand, overexpression of either E2F1 or CUX1 in Hct 116 cells increased Luc production by all three promoters. Some additive effect was observed when both transcription factors were coexpressed although no clear synergy was observed (Fig. 10B). Interestingly, expression of CUX1 did not modify the response of a synthetic 6×E2F reporter induced by E2F1 expression, suggesting that CUX1 does not interact with E2F1 independently of its target recognition sequences. It is of note that little or no endogenous E2F activity was detected in Hct 116 cells as assessed by the 6×E2F reporter. In keeping with this observation, expression of the DN-E2F did not significantly modify the response of these promoters in Hct 116 (not shown).
Modulating expression of CUX1 or E2F1 affects expression of Ect2, RacGAP, and MKLP1.
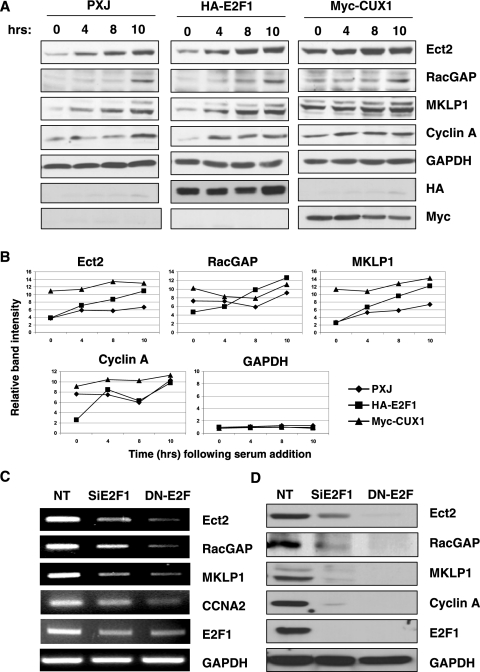
We then sought to confirm the role of E2F1 and CUX1 at the level of endogenous proteins by modulating their expressions. As stated above, overexpression of E2F1 could not be assessed in Kit 225 cells due to massive apoptosis, which was only partially prevented by the use of caspase inhibitors such as QVD-OPH (quinolone-Val-Asp-difluorophenoxy), and these experiments were therefore performed in Hct 116 cells (Fig. 11A). Expressing either E2F1 or CUX1 resulted in a more rapid and more potent induction of Ect2, MKLP1, and, to some extent, RacGAP compared to the empty control vector (Fig. 11B).
FIG. 11.
Modulating expression of CUX1 or E2F1 affects expression of Ect2, RacGAP, and MKLP1. (A) Serum-starved Hct 116 cells were transfected with empty vector (pXJ), HA-E2F1, or Myc-CUX1 expression plasmids. After 16 h, complete culture medium was added, and cells were collected at the indicated times. Cell lysates were prepared and analyzed by Western blotting with the indicated antibodies. (B) Densitometry scanning analysis of the gels shown above. Signals for the indicated proteins were normalized with the GAPDH signals of the corresponding time points. (C and D) Exponentially growing Kit 225 cells were transfected with nontargeting siRNA (NT), E2F1 siRNA (SiE2F1), or an expression plasmid for DN-E2F and cultured for 48 h in the absence of IL-2. Cells were then stimulated with IL-2 and harvested 20 h later to assess expression of the indicated genes by RT-PCR (C) and Western blotting (D).
In a reverse approach, we sought to knock down E2F activity, either through siRNA silencing or by expressing a DN-E2F. Of note, DN-E2F not only antagonized E2F activity but also affected expression of E2F1, most likely a result of E2F1's regulating its own promoter (9). As shown in Fig. 11C and D, interfering with E2F1 almost completely abolished expression of Ect2, RacGAP, and MKLP1 in Kit 225 cells, both at the RNA and protein levels. As anticipated, Cyclin A expression was also extinguished, leaving open the question of whether these results reflect direct effects of the absence of E2F1 at the promoter level or alterations in cell cycle progression. Of interest, these experiments did not provide any hint or suggestion that E2F1 and CUX1 regulate each other (data not shown). On the other hand, despite repeated attempts with multiple siRNA designs, we have thus far been unable to efficiently knock down CUX1 expression in either Kit 225 or Hct 116 cells; and in the absence of a DN form of CUX1, we cannot directly address the question of whether CUX1 is absolutely required for expression of these proteins.
DISCUSSION
The pathways controlled by Rho GTPases are crucial to mitosis progression and to the completion of cytokinesis, as evidenced by the drastic effects of RNA silencing of many of the proteins in these pathways including the Rho GTPases themselves, their regulators, or their effectors such as citron kinase or protein kinase N (12, 25, 47). Although other Rho regulators such as GEF-H1 or p190 RhoGAP have been implicated in cytokinesis, the current literature points to Ect2 and RacGAP as probably the major players in control of RhoA during mitosis. The work described here demonstrates that not only Ect2 and RacGAP but also the kinesin MKLP1, which acts to locate them to the spindle, are induced at the G1/S transition to allow maximal expression of the proteins in G2/M at the time their functions are required to regulate mitosis and cytokinesis.
We found that the promoters of Ect2, RacGAP, and MKLP1, like many of the genes that are regulated during cell cycle progression, contain salient features including critical CHR elements and E2F binding sites. We report here that E2F1 binds to these promoters at the time of G1/S transition and stimulates activity of these promoter constructs. More importantly, experiments using DN-E2F1 demonstrated that E2F activation is absolutely required to initiate transcription at these promoters. Overexpression of other members of the E2F family (E2F2, -3, or -4) gave inconsistent results depending on the promoter, and E2F4 recruitment could be demonstrated by ChIP on some, but not all, of the E2F binding sites (data not shown), suggesting that E2F1 is the major E2F family member involved in regulating these promoters. In this study we have not investigated the relationships that may exist between E2F family members and the presence and demonstrated influence of CHR sequences. Of interest, Ect2 has been found as a gene upregulated in Rb−/− cells (13). In addition, as this work was in progress, another publication suggested that Ect2 might also be regulated by a p53-dependent pathway (49). It is therefore of importance to note here that both Kit 225 and Hct 116 cells express wild-type p53.
We also show here that these genes are regulated by CUX1. Of interest, all three promoters contain at least one ATYRAT sequence, a CUX1 consensus site that was found to be overrepresented among cell cycle target genes identified by ChIP-microarray chip analysis (21). ChIP experiments demonstrated some binding of CUX1 on the three promoters in unstimulated cells, but we have no clue as to whether this may relate to a possible activity of CUX1 as a transcriptional repressor in G1. Most importantly, IL-2 stimulation of Kit 225 cells induced recruitment and a clear time-dependent increase in the amount of target-bound CUX1. We have observed that IL-2 stimulation does induce processing of CUX1 in Kit 225 cells and increases its DNA binding activity (unpublished data). A recombinant CUX1 processed isoform, however, was able to drive Luc expression by these promoters only in the presence of IL-2 (or serum, in the case of Hct 116 cells), suggesting that signals induced by the IL-2 receptor may be required to fully activate CUX1. Furthermore, in the presence of IL-2, a combination of a PI3K inhibitor and rapamycin was able to inhibit transcriptional activation by CUX1. This is likely a result of E2F inhibition, as suggested by a similar inhibitory effect of a DN mutant of E2F1 or siRNA silencing of E2F1. These observations are in accordance with previous findings showing that constitutive expression of p110 CUX1 was able to accelerate entry into S phase in the presence of serum but not in its absence (46). The dependence of CUX1 activity on IL-2 or other growth factors suggests that a downstream signal transduction pathway common to several growth factors functions to activate CUX1 or other transcription factors or cofactors that may cooperate with CUX1.
While several mechanisms of repression by CUX1 have been described (28, 31, 42), how CUX1 activates transcription is currently unclear as the protein does not appear to contain a trans-activation domain. Importantly, a mechanistic model should take into account the fact that CUX1 functions as a repressor on some promoters and as an activator on other promoters. These observations raise the possibility that CUX1 functions by promoting the recruitment of other factors via the formation of larger protein/DNA complexes. In support for this notion, we have recently shown that p110 CUX1 favors the recruitment of E2F1 to the promoters of some cell cycle-related genes (21). However, in the context of the three promoters studied in the present work, a clear synergy or interaction between CUX1 and E2F1 could not be demonstrated.
Besides its function in the cell cycle, CUX1 has also been reported to promote cell motility (32), a process which is controlled by Rho GTPases and in which Ect2 may be involved. Indeed, besides a role for Ect2 in mitosis, its Drosophila ortholog Pebble has been shown to regulate cell migration and the epithelial-mesenchymal transition (48, 50). These functions are in line with the transforming capacity of Ect2, which was first identified as an oncogene (33, 51). The deregulation of Ect2 expression that is observed in some tumors may thus result from alterations of CUX1 activity or of the p53/Rb/E2F pathway. Work currently in progress aims at exploring this hypothesis.
In conclusion, although our study did not directly address the GDP/GTP cycling of Rho GTPases during mitosis, it provides new insights in the regulation of their regulators by adding Ect2, RacGAP, and MKLP1 to the list of cell cycle-regulated genes and identifying them as common transcriptional targets of E2F1 and CUX1.
Supplementary Material
Acknowledgments
We thank the following colleagues for providing useful plasmids: K. Helin, for the 6×E2F reporter, R. A. Padua and C. Brechot for the CCNA reporter, and J. Nevins and L. Jakoi for E2F expression plasmids. We also thank M. David, O. Coqueret, J. Imbert, and J. Pierre for helpful discussions and critical reading of the manuscript; C. Crouin for technical help; and Martine Letourneur for advice.
This work was funded in part by INSERM, by grants from Ligue Nationale contre le Cancer (équipe labellisée 2006) and ANR grant BLAN06-2_135169 to J.B., and by grant MT-11590 from the Canadian Institute of Health Research of Canada to A.N. Exchanges between the Bertoglio and Nepveu laboratories were made possible through a grant from FRSQ and INSERM.
Footnotes
Published ahead of print on 17 November 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Arnaud, M., C. Crouin, C. Deon, D. Loyaux, and J. Bertoglio. 2004. Phosphorylation of Grb2-associated binder 2 on serine 623 by ERK MAPK regulates its association with the phosphatase SHP-2 and decreases STAT5 activation. J. Immunol. 1733962-3971. [DOI] [PubMed] [Google Scholar]
- 2.Barberis, A., G. Superti-Furga, and M. Busslinger. 1987. Mutually exclusive interaction of the CCAAT-binding factor and of a displacement protein with overlapping sequences of a histone gene promoter. Cell 50347-359. [DOI] [PubMed] [Google Scholar]
- 3.Botz, J., K. Zerfass-Thome, D. Spitkovsky, H. Delius, B. Vogt, M. Eilers, A. Hatzigeorgiou, and P. Jansen-Durr. 1996. Cell cycle regulation of the murine cyclin E gene depends on an E2F binding site in the promoter. Mol. Cell. Biol. 163401-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan, P., J. W. Babbage, G. Thomas, and D. Cantrell. 1999. p70 s6k integrates phosphatidylinositol 3-kinase and rapamycin-regulated signals for E2F regulation in T lymphocytes. Mol. Cell. Biol. 194729-4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breslin, E. M., P. C. White, A. M. Shore, M. Clement, and P. Brennan. 2005. LY294002 and rapamycin co-operate to inhibit T-cell proliferation. Br. J. Pharmacol. 144791-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bustelo, X. R., V. Sauzeau, and I. M. Berenjeno. 2007. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays 29356-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho, R. J., M. Huang, M. J. Campbell, H. Dong, L. Steinmetz, L. Sapinoso, G. Hampton, S. J. Elledge, R. W. Davis, and D. J. Lockhart. 2001. Transcriptional regulation and function during the human cell cycle. Nat. Genet. 2748-54. [DOI] [PubMed] [Google Scholar]
- 8.Coqueret, O., G. Berube, and A. Nepveu. 1998. The mammalian Cut homeodomain protein functions as a cell-cycle-dependent transcriptional repressor which downmodulates p21WAF1/CIP1/SDI1 in S phase. EMBO J. 174680-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeGregori, J., and D. G. Johnson. 2006. Distinct and overlapping roles for E2F family members in transcription, proliferation and apoptosis. Curr. Mol. Med. 6739-748. [DOI] [PubMed] [Google Scholar]
- 10.DeGregori, J., G. Leone, A. Miron, L. Jakoi, and J. R. Nevins. 1997. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc. Natl. Acad. Sci. USA 947245-7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimova, D. K., and N. J. Dyson. 2005. The E2F transcriptional network: old acquaintances with new faces. Oncogene 242810-2826. [DOI] [PubMed] [Google Scholar]
- 12.Echard, A., G. R. Hickson, E. Foley, and P. H. O'Farrell. 2004. Terminal cytokinesis events uncovered after an RNAi screen. Curr. Biol. 141685-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eguchi, T., T. Takaki, H. Itadani, and H. Kotani. 2007. RB silencing compromises the DNA damage-induced G2/M checkpoint and causes deregulated expression of the ECT2 oncogene. Oncogene 26509-520. [DOI] [PubMed] [Google Scholar]
- 14.Elkon, R., C. Linhart, R. Sharan, R. Shamir, and Y. Shiloh. 2003. Genome-wide in silico identification of transcriptional regulators controlling the cell cycle in human cells. Genome Res. 13773-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature 420629-635. [DOI] [PubMed] [Google Scholar]
- 16.Gesbert, F., M. Delespine-Carmagnat, and J. Bertoglio. 1998. Recent advances in the understanding of interleukin-2 signal transduction. J. Clin. Immunol. 18307-320. [DOI] [PubMed] [Google Scholar]
- 17.Goulet, B., A. Baruch, N. S. Moon, M. Poirier, L. L. Sansregret, A. Erickson, M. Bogyo, and A. Nepveu. 2004. A cathepsin L isoform that is devoid of a signal peptide localizes to the nucleus in S phase and processes the CDP/Cux transcription factor. Mol. Cell 14207-219. [DOI] [PubMed] [Google Scholar]
- 18.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279509-514. [DOI] [PubMed] [Google Scholar]
- 19.Hallstrom, T. C., S. Mori, and J. R. Nevins. 2008. An E2F1-dependent gene expression program that determines the balance between proliferation and cell death. Cancer Cell 1311-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hara, T., M. Abe, H. Inoue, L. R. Yu, T. D. Veenstra, Y. H. Kang, K. S. Lee, and T. Miki. 2006. Cytokinesis regulator ECT2 changes its conformation through phosphorylation at Thr-341 in G2/M phase. Oncogene 25566-578. [DOI] [PubMed] [Google Scholar]
- 21.Harada, R., C. Vadnais, L. Sansregret, L. Leduy, G. Berube, F. Robert, and A. Nepveu. 2008. Genome-wide location analysis and expression studies reveal a role for p110 CUX1 in the activation of DNA replication genes. Nucleic Acids Res. 36189-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haugwitz, U., M. Wasner, M. Wiedmann, K. Spiesbach, K. Rother, J. Mossner, and K. Engeland. 2002. A single cell cycle genes homology region (CHR) controls cell cycle-dependent transcription of the cdc25C phosphatase gene and is able to cooperate with E2F or Sp1/3 sites. Nucleic Acids Res. 301967-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henglein, B., X. Chenivesse, J. Wang, D. Eick, and C. Brechot. 1994. Structure and cell cycle-regulated transcription of the human cyclin A gene. Proc. Natl. Acad. Sci. USA 915490-5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwanaga, R., H. Komori, S. Ishida, N. Okamura, K. Nakayama, K. I. Nakayama, and K. Ohtani. 2006. Identification of novel E2F1 target genes regulated in cell cycle-dependent and independent manners. Oncogene 251786-1798. [DOI] [PubMed] [Google Scholar]
- 25.Kamijo, K., N. Ohara, M. Abe, T. Uchimura, H. Hosoya, J. S. Lee, and T. Miki. 2006. Dissecting the role of Rho-mediated signaling in contractile ring formation. Mol. Biol. Cell 1743-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim, J. E., D. D. Billadeau, and J. Chen. 2005. The tandem BRCT domains of Ect2 are required for both negative and positive regulation of Ect2 in cytokinesis. J. Biol. Chem. 2805733-5739. [DOI] [PubMed] [Google Scholar]
- 27.Kimura, M., C. Uchida, Y. Takano, M. Kitagawa, and Y. Okano. 2004. Cell cycle-dependent regulation of the human aurora B promoter. Biochem. Biophys. Res. Commun. 316930-936. [DOI] [PubMed] [Google Scholar]
- 28.Li, S., L. Moy, N. Pittman, G. Shue, B. Aufiero, E. J. Neufeld, N. S. LeLeiko, and M. J. Walsh. 1999. Transcriptional repression of the cystic fibrosis transmembrane conductance regulator gene, mediated by CCAAT displacement protein/cut homolog, is associated with histone deacetylation. J. Biol. Chem. 2747803-7815. [DOI] [PubMed] [Google Scholar]
- 29.Linhart, C., R. Elkon, Y. Shiloh, and R. Shamir. 2005. Deciphering transcriptional regulatory elements that encode specific cell cycle phasing by comparative genomics analysis. Cell Cycle 41788-1797. [DOI] [PubMed] [Google Scholar]
- 30.Liu, N., F. C. Lucibello, K. Engeland, and R. Muller. 1998. A new model of cell cycle-regulated transcription: repression of the cyclin A promoter by CDF-1 and anti-repression by E2F. Oncogene 162957-2963. [DOI] [PubMed] [Google Scholar]
- 31.Mailly, F., G. Berube, R. Harada, P. L. Mao, S. Phillips, and A. Nepveu. 1996. The human cut homeodomain protein can repress gene expression by two distinct mechanisms: active repression and competition for binding site occupancy. Mol. Cell. Biol. 165346-5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michl, P., A. R. Ramjaun, O. E. Pardo, P. H. Warne, M. Wagner, R. Poulsom, C. D'Arrigo, K. Ryder, A. Menke, T. Gress, and J. Downward. 2005. CUTL1 is a target of TGF(beta) signaling that enhances cancer cell motility and invasiveness. Cancer Cell 7521-532. [DOI] [PubMed] [Google Scholar]
- 33.Miki, T., C. L. Smith, J. E. Long, A. Eva, and T. P. Fleming. 1993. Oncogene ect2 is related to regulators of small GTP-binding proteins. Nature 362462-465. [DOI] [PubMed] [Google Scholar]
- 34.Minoshima, Y., T. Kawashima, K. Hirose, Y. Tonozuka, A. Kawajiri, Y. C. Bao, X. Deng, M. Tatsuka, S. Narumiya, W. S. May, Jr., T. Nosaka, K. Semba, T. Inoue, T. Satoh, M. Inagaki, and T. Kitamura. 2003. Phosphorylation by aurora B converts MgcRacGAP to a RhoGAP during cytokinesis. Dev. Cell 4549-560. [DOI] [PubMed] [Google Scholar]
- 35.Mishima, M., S. Kaitna, and M. Glotzer. 2002. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev. Cell 241-54. [DOI] [PubMed] [Google Scholar]
- 36.Mishima, M., V. Pavicic, U. Gruneberg, E. A. Nigg, and M. Glotzer. 2004. Cell cycle regulation of central spindle assembly. Nature 430908-913. [DOI] [PubMed] [Google Scholar]
- 37.Mitin, N., K. L. Rossman, and C. J. Der. 2005. Signaling interplay in Ras superfamily function. Curr. Biol. 15R563-R574. [DOI] [PubMed] [Google Scholar]
- 38.Moon, N. S., P. Premdas, M. Truscott, L. Leduy, G. Berube, and A. Nepveu. 2001. S phase-specific proteolytic cleavage is required to activate stable DNA binding by the CDP/Cut homeodomain protein. Mol. Cell. Biol. 216332-6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller, H., M. C. Moroni, E. Vigo, B. O. Petersen, J. Bartek, and K. Helin. 1997. Induction of S-phase entry by E2F transcription factors depends on their nuclear localization. Mol. Cell. Biol. 175508-5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mzali, R., L. Seguin, C. Liot, A. Auger, P. Pacaud, G. Loirand, C. Thibault, J. Pierre, and J. Bertoglio. 2005. Regulation of Rho signaling pathways in interleukin-2-stimulated human T-lymphocytes. FASEB J. 191911-1913. [DOI] [PubMed] [Google Scholar]
- 41.Neufeld, E. J., D. G. Skalnik, P. M. Lievens, and S. H. Orkin. 1992. Human CCAAT displacement protein is homologous to the Drosophila homeoprotein, cut. Nat. Genet. 150-55. [DOI] [PubMed] [Google Scholar]
- 42.Nishio, H., and M. J. Walsh. 2004. CCAAT displacement protein/cut homolog recruits G9a histone lysine methyltransferase to repress transcription. Proc. Natl. Acad. Sci. USA 10111257-11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Connell, C. B., S. P. Wheatley, S. Ahmed, and Y. L. Wang. 1999. The small GTP-binding protein rho regulates cortical activities in cultured cells during division. J. Cell Biol. 144305-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raftopoulou, M., and A. Hall. 2004. Cell migration: Rho GTPases lead the way. Dev. Biol. 26523-32. [DOI] [PubMed] [Google Scholar]
- 45.Ren, B., H. Cam, Y. Takahashi, T. Volkert, J. Terragni, R. A. Young, and B. D. Dynlacht. 2002. E2F integrates cell cycle progression with DNA repair, replication, and G2/M checkpoints. Genes Dev. 16245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sansregret, L., B. Goulet, R. Harada, B. Wilson, L. Leduy, J. Bertoglio, and A. Nepveu. 2006. The p110 isoform of the CDP/Cux transcription factor accelerates entry into S phase. Mol. Cell. Biol. 262441-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt, A., J. Durgan, A. Magalhaes, and A. Hall. 2007. Rho GTPases regulate PRK2/PKN2 to control entry into mitosis and exit from cytokinesis. EMBO J. 261624-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schumacher, S., T. Gryzik, S. Tannebaum, and H. A. Muller. 2004. The RhoGEF Pebble is required for cell shape changes during cell migration triggered by the Drosophila FGF receptor Heartless. Development 1312631-2640. [DOI] [PubMed] [Google Scholar]
- 49.Scoumanne, A., and X. Chen. 2006. The epithelial cell transforming sequence 2, a guanine nucleotide exchange factor for Rho GTPases, is repressed by p53 via protein methyltransferases and is required for G1-S transition. Cancer Res. 666271-6279. [DOI] [PubMed] [Google Scholar]
- 50.Smallhorn, M., M. J. Murray, and R. Saint. 2004. The epithelial-mesenchymal transition of the Drosophila mesoderm requires the Rho GTP exchange factor Pebble. Development 1312641-2651. [DOI] [PubMed] [Google Scholar]
- 51.Solski, P. A., R. S. Wilder, K. L. Rossman, J. Sondek, A. D. Cox, S. L. Campbell, and C. J. Der. 2004. Requirement for C-terminal sequences in regulation of Ect2 guanine nucleotide exchange specificity and transformation. J. Biol. Chem. 27925226-25233. [DOI] [PubMed] [Google Scholar]
- 52.Somers, W. G., and R. Saint. 2003. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev. Cell 429-39. [DOI] [PubMed] [Google Scholar]
- 53.Toure, A., R. Mzali, C. Liot, L. Seguin, L. Morin, C. Crouin, I. Chen-Yang, Y. G. Tsay, O. Dorseuil, G. Gacon, and J. Bertoglio. 2008. Phosphoregulation of MgcRacGAP in mitosis involves Aurora B and Cdk1 protein kinases and the PP2A phosphatase. FEBS Lett. 5821182-1188. [DOI] [PubMed] [Google Scholar]
- 54.Truscott, M., J. B. Denault, B. Goulet, L. Leduy, G. S. Salvesen, and A. Nepveu. 2007. Carboxyl-terminal proteolytic processing of CUX1 by a caspase enables transcriptional activation in proliferating cells. J. Biol. Chem. 28230216-30226. [DOI] [PubMed] [Google Scholar]
- 55.Truscott, M., L. Raynal, P. Premdas, B. Goulet, L. Leduy, G. Berube, and A. Nepveu. 2003. CDP/Cux stimulates transcription from the DNA polymerase alpha gene promoter. Mol. Cell. Biol. 233013-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uchiumi, T., D. L. Longo, and D. K. Ferris. 1997. Cell cycle regulation of the human polo-like kinase (PLK) promoter. J. Biol. Chem. 2729166-9174. [DOI] [PubMed] [Google Scholar]
- 57.Valarche, I., J. P. Tissier-Seta, M. R. Hirsch, S. Martinez, C. Goridis, and J. F. Brunet. 1993. The mouse homeodomain protein Phox2 regulates Ncam promoter activity in concert with Cux/CDP and is a putative determinant of neurotransmitter phenotype. Development 119881-896. [DOI] [PubMed] [Google Scholar]
- 58.Weinstein, J. 1997. Cell cycle-regulated expression, phosphorylation, and degradation of p55Cdc. A mammalian homolog of CDC20/Fizzy/slp1. J. Biol. Chem. 27228501-28511. [DOI] [PubMed] [Google Scholar]
- 59.Yasuda, S., F. Oceguera-Yanez, T. Kato, M. Okamoto, S. Yonemura, Y. Terada, T. Ishizaki, and S. Narumiya. 2004. Cdc42 and mDia3 regulate microtubule attachment to kinetochores. Nature 428767-771. [DOI] [PubMed] [Google Scholar]
- 60.Yoshizaki, H., Y. Ohba, M. C. Parrini, N. G. Dulyaninova, A. R. Bresnick, N. Mochizuki, and M. Matsuda. 2004. Cell type-specific regulation of RhoA activity during cytokinesis. J. Biol. Chem. 27944756-44762. [DOI] [PubMed] [Google Scholar]
- 61.Yuce, O., A. Piekny, and M. Glotzer. 2005. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J. Cell Biol. 170571-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu, W., P. H. Giangrande, and J. R. Nevins. 2004. E2Fs link the control of G1/S and G2/M transcription. EMBO J. 234615-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.