Abstract
Reelin coordinates the movements of neurons during brain development by signaling through the Dab1 adaptor and Src family tyrosine kinases. Experiments with cultured neurons have shown that when Dab1 is phosphorylated on tyrosine, it activates Akt and provides a scaffold for assembling signaling complexes, including the paralogous Crk and CrkL adaptors. The roles of Akt and Dab1 complexes during development have been unclear. We have generated two Dab1 alleles, each lacking two out of the four putative tyrosine phosphorylation sites. Neither allele supports normal brain development, but each allele complements the other. Two tyrosines are required for Reelin to stimulate Dab1 phosphorylation at the other sites, to activate Akt, and to downregulate Dab1 levels. The other two tyrosines are required to stimulate a Crk/CrkL-C3G pathway. The absence of Crk/CrkL binding sites and C3G activation causes an unusual layering phenotype. These results show that Reelin-induced Akt stimulation and Dab1 turnover are not sufficient for normal development and suggest that Dab1 acts both as a kinase switch and as a scaffold for assembling signaling complexes in vivo.
The arrangement of layers of projection neurons in the mammalian brain is coordinated by the Reelin signaling pathway (7, 37). Reelin is a secreted protein that binds to two cell surface receptors, VLDLR and ApoER2, on migrating neurons. Reelin induces the phosphorylation of the intracellular Dab1 adaptor protein by the Src family tyrosine kinases (SFKs) Fyn and Src (2, 5, 8, 14, 19, 21). Dab1 phosphorylation and SFK activity are both critical for Reelin signaling, since a nonphosphorylated Dab1 mutant causes the same phenotype as the absence of Reelin or the absence of Src and Fyn (22, 28). However, a key unanswered question is how the phosphorylation of Dab1 by SFKs regulates neuron positioning.
Reelin has many effects on neurons, including activating the protein kinases Akt/PKB and mTor (6, 9, 24); inhibiting GSK3 and the phosphorylation of the microtubule-associated protein tau (6, 19); inducing the binding of Dab1 to Lis1, Crk, CrkL, and Nckβ (3, 4, 11, 23, 31); stimulating the polyubiquitination and proteasome-dependent degradation of Dab1 (1, 10, 15); increasing the tyrosine phosphorylation of the Rap1 guanine nucleotide exchange factor C3G (4); and stimulating GTP loading of Rap1 (4). Although Bock et al. and Jossin and colleagues previously provided evidence that SFKs, the proteasome, phosphatidylinositol 3 (PI3)-kinase, and Akt, but not mTor, are involved in cortical plate formation in slice cultures in vitro (9, 10, 24, 25), it remains unclear which of these Reelin-regulated events are required for normal brain development in vivo.
The mechanism by which Reelin stimulates the phosphorylation of Dab1 is unclear. Reelin may recruit SFKs and Dab1 to a common location where phosphorylation may occur. However, Reelin has also been reported to stimulate SFKs, dependent on Dab1 (5, 8). This stimulation is slight but suggests a positive-feedback loop in which Dab1 phosphorylation by SFKs stimulates the SFKs. It also raises the possibility that Dab1 may serve solely as a kinase switch: Dab1 could stimulate SFKs to phosphorylate other proteins that regulate neuron migrations. Alternatively, or in addition, Dab1 may serve as a scaffold for assembling signaling complexes containing Lis1, Crk, CrkL, Nckβ, and other proteins (3, 4, 11, 23, 31). Because all available mutations of Reelin, Reelin receptors, Dab1, or SFKs prevent both SFK activation and Dab1 phosphorylation, it has been unclear whether Dab1 acts solely as a kinase switch or whether its scaffolding function is also required for normal development. Here, we report that Dab1-dependent, Reelin-stimulated Akt kinase activity is insufficient for normal development and implicate Dab1-dependent protein-protein complexes in regulating neuronal migrations.
MATERIALS AND METHODS
Generation of mutant mice.
Targeting constructs were made as described previously for wild-type (WT) and 5F alleles of Dab1 p80 (22). AK7 (129Sv/Sor) embryonic stem (ES) cells (5 × 106 cells) were electroporated with 20 μg of either pDab1abKI or pDab1cdKI that had been linearized with XhoI. Cells were selected with G418, and homologous recombinants were detected using PCR as previously described (22). The neomycin cassette was excised using PGKCre, and ES cells were introduced into blastocysts. The altered loci were confirmed by PCR and Southern blotting. Chimeric mice were bred to C57BL/6 mice to obtain heterozygotes, which were maintained in a mixed 129Sv/C57BL6 (1:1) strain background. Two independent ES cell lines for dab1cd and one for dab1ab were used for the experiments shown. The dab1−/+ knockout strain was described previously (20).
Biochemistry.
Primary neuron culture and stimulation, immunoprecipitation, and Western blotting were described previously (15, 17). Transfection of 293T cells were performed using Lipofectamine Plus reagent according to the manufacturer's instructions. The following antibodies were used for biochemistry: anti-phosphotyrosine antibody 4G10 (Upstate Biotechnology); anti-Fyn antibody (FYN3), anti-C3G (C-19), and anti-CrkL (C-20) (Santa Cruz Biotechnology); anti-Crk (BD Transduction Laboratories); anti-Akt-pS473 and anti-Akt (Cell Signaling); Tuj1 anti-tubulin β3 (Covance); and affinity-purified anti-Dab1 (B3) (from B. W. Howell). Western blots were quantified using ImageJ software.
Immunohistochemistry.
Nissl staining of paraffin sections from mice at postnatal day 19 (P19) to P20 was done as described previously (17). Paraffin sections were also deparaffinized and stained with anti-calbindin (1:400; Chemicon). Immunohistochemistry of cryostat sections of the cerebral cortex was done as described previously (28) using mouse anti-chondroitin sulfate proteoglycan (CSPG) (1:100) (C-8035; Sigma), rabbit anti-Tbr1 (1:1,000) (obtained from Robert Hevner), guinea pig anti-Brn1 (1:500) (Robert McEvilly), rabbit anti-Cux1 (1:500) (sc-13024; Santa Cruz), and Ctip2 (1:1,000) (ab18465; Abcam).
RESULTS
With the goal of separating the biochemical functions of Dab1, we generated mouse “knock-in” alleles of dab1 encoding phosphorylation site mutants. SFKs phosphorylate the Dab1 protein at four sites in vitro (Fig. 1A) (22, 27). These sites are conserved across all vertebrates, implying that they are all functionally important. The sites may be classified by sequence: two YQXI sequences (Tyr185, a; Tyr198, b) and two YXVP sequences (Tyr220, c; Tyr232, d). The phosphorylation of the b, c, and d, but not a, sites has been detected in Reelin-stimulated neurons (4, 27). The c and d but not the b sites were previously shown to be important for regulating neuron migration in the developing neocortex (32). However, an important role for the b site may have been concealed if it is redundant with the homologous a site. It is also unclear whether Dab1 communicates with different downstream effectors through different phosphorylation sites. To investigate these questions in vivo, and to avoid possible redundancy effects, we engineered mice with double-phenylalanine substitutions at both the a and b sites (dab1ab allele) or both the c and d sites (dab1cd). These alleles were made using the same strategy described above to make dab1WT, which encodes the WT p80 splice form of Dab1, and dab15F, which encodes p80 with substitutions at the four known tyrosine phosphorylation sites plus Tyr200 (22). The dab1WT allele shows that other splice forms are not required for normal development and controls for transcription or splicing effects that may affect expression levels of the knock-in alleles (1). Heterozygous dab1ab/+ and dab1cd/+ mice were intercrossed to obtain homozygous knock-in embryos and pups for study.
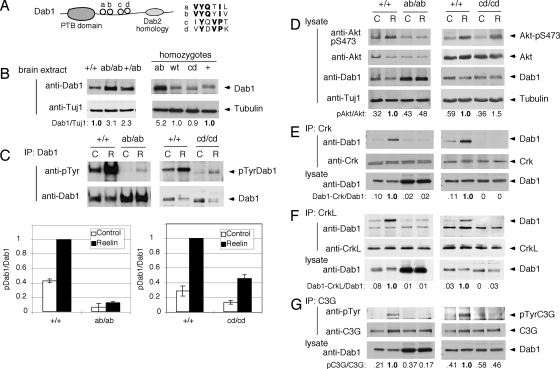
FIG. 1.
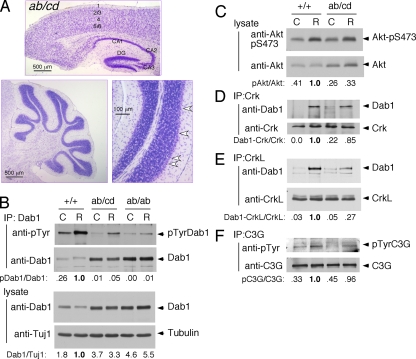
Effects of Dab1 phosphorylation site mutations on signal transduction. (A) Schematic of Dab1 protein structure showing the protein tyrosine-binding (PTB) domain and four tyrosine phosphorylation sites. (B) Expression level of Dab1 in embryonic brain. Protein extracts were prepared from E16.5 embryonic brains and analyzed directly by immunoblotting for Dab1 and tubulin. The samples on the left came from one litter, and those on the right were obtained from three different litters. The ratio of Dab1 to tubulin was calculated, with normalizing to +/+ brain extract. (C to G) Phosphorylation and binding events in Reelin-stimulated and control neurons. In each case, cortical neuron cultures were prepared from individual E16.5 embryos obtained from dab1ab/+ intercross or dab1cd/+ intercross timed matings. The embryos were then genotyped, and homozygous mutant and WT cultures were identified. Duplicate dishes were treated with control (C) or Reelin (R) for 15 min and then lysed. (C) Dab1 phosphorylation. Lysates were immunoprecipitated (IP) for Dab1 and immunoblotted for phosphotyrosine (4G10) and Dab1. Each graph shows means and ranges of the ratio of pDab1 to Dab1, normalized to the ratio in Reelin-stimulated dab1+/+ neurons, from two independent litters. (D) Akt phosphorylation. Lysates were analyzed using anti-Akt-pS473, anti-Akt, anti-Dab1 (B3), and Tuj1 anti-tubulin. The ratio of pS473Akt to Akt was calculated and normalized to the ratio in Reelin-stimulated dab1+/+ neurons. (E) Binding of CrkL to Dab1. CrkL immunoprecipitates were immunoblotted for Dab1 and CrkL. The lysates were also analyzed directly using Dab1 (B3). The ratio of Dab1 in the CrkL immunoprecipitate to total Dab1 was calculated. (F) Binding of Crk to Dab1 in neurons. The protocol is the same as that described above (E) except that Crk antibodies were used. (G) Phosphorylation of C3G in neurons. C3G immunoprecipitates were immunoblotted for anti-phosphotyrosine (4G10) and anti-C3G. The ratio of pC3G to C3G was calculated. Results in each panel are representative of two to three repeat experiments done using different litters of embryos.
Signaling functions of the ab and cd phosphorylation sites.
We examined the levels of phosphorylation and expression of Dab1 mutant proteins at embryonic day 16.5 (E16.5), when Reelin-dependent Dab1 tyrosine phosphorylation is high and Dab1 protein levels are downregulated by ubiquitin-mediated proteolysis. The Dab1ab protein level was increased approximately three- to fivefold in the dab1ab/ab homozygous cortex (Fig. 1B) or cultured neurons (Fig. 1C) relative to levels for WT Dab1 in dab1+/+ or dab1WT/WT controls, while the Dabcd protein level in the dab1cd/cd homozygous cortex was normal. The upregulation of Dab1ab was consistent with results obtained using overexpressed epitope-tagged Dab1 mutants, which showed that the ab but not cd sites are required for ubiquitin-mediated proteolysis (15).
Reelin stimulated the phosphorylation of both WT Dab1 and mutant Dab1cd proteins but only slightly increased the level of phosphorylation of Dab1ab (Fig. 1C). The basal and Reelin-induced phosphorylations of Dab1cd were reduced by approximately one-half, consistent with the absence of two phosphate acceptor sites (Fig. 1C). Presumably, the remaining phosphorylation occurs mostly at the ab sites. However, the strong inhibition of both basal and Reelin-induced phosphorylation of Dab1ab was unexpected (Fig. 1C). An epitope-tagged Dab1ab mutant is phosphorylated in response to Reelin when it is overexpressed in neurons (15) and is phosphorylated when coexpressed with active Src in tissue culture cells (22). This suggests that the ab mutant is not impaired as an SFK substrate, but the ab sites may be important to recruit or activate SFKs, and that if SFKs are not recruited or activated, the cd sites in the Dab1ab mutant protein are not phosphorylated.
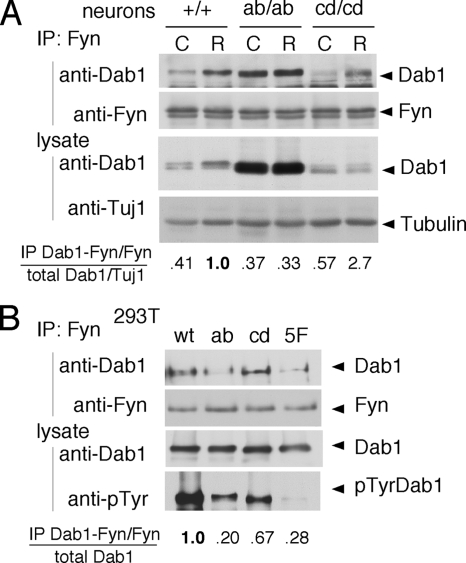
Reelin activates only a small fraction of the SFKs in neurons (2, 5, 8), and we were unsuccessful in directly measuring SFK activity in the current experiments. However, we reasoned that if the ab sites are important for SFK activation by Dab1, they may also be important for SFK binding to Dab1. We tested for Dab1 binding to SFKs by immunoprecipitation and immunoblotting. Reelin stimulated the binding of Fyn to normal Dab1 or mutant Dab1cd proteins by 2.5- to 4.5-fold but did not stimulate Fyn binding to mutant Dab1ab (Fig. 2A). However, high levels of mutant Dab1ab were constitutively bound to Fyn. This may be nonspecific binding that is detected because Dab1ab is expressed at higher levels. Indeed, when we normalized the quantity of Dab1 in Fyn immunoprecipitates for the level of Dab1 in the neuron lysates, we found that the fraction of mutant Dab1ab bound to Fyn was similar to the fraction of normal Dab1 bound to Fyn in unstimulated WT neurons and was not increased by Reelin (Fig. 2A). This suggests that the ab phosphorylation sites are needed for Reelin to stimulate the formation of a Dab1-Fyn complex.
FIG. 2.
Dab1ab sites bind to the Fyn domain. (A) Neuron cultures prepared from WT, dab1ab/ab, and dab1cd/cd embryos at E16.5 were treated with control (C) or Reelin (R) for 15 min. Fyn was immunoprecipitated (IP) from lysates and immunoblotted for Dab1 and Fyn. The level of Dab1 in the Fyn immunoprecipitate was normalized for slight variations in Fyn immunoprecipitation. The level of Dab1 in the lysate was normalized for slight variations in loading using Tuj1 antibody as a control. The ratio of Dab1 in the Fyn immunoprecipitate to total Dab1 was then calculated and normalized to the ratio in Reelin-stimulated dab1+/+ neurons. (B) 293T cells were transfected with WT or mutant Dab1-green fluorescent protein (GFP) fusion proteins together with WT Fyn. Lysates were immunoprecipitated with anti-Fyn and immunoblotted with anti-Dab1 antibody. The level of Dab1 in the Fyn immunoprecipitate was normalized for slight variations in Fyn immunoprecipitation. The ratio of Dab1-GFP in the Fyn immunoprecipitate to total Dab1-GFP was then calculated and normalized to that of WT Dab1-GFP.
To further test the roles of the ab sites in Fyn binding, WT or mutant Dab1 proteins were expressed together with Fyn in nonneuronal 293T cells (Fig. 2B). Fyn bound more WT Dab1 and Dab1cd than Dab1ab or Dab15F. We infer that the a or b phosphorylation site is needed to bind and presumably activate SFKs. The phosphorylation of overexpressed Dab1ab in WT neurons (15) may be explained if SFKs, recruited to or activated by endogenous Dab1 through its ab sites, can phosphorylate the cd sites of another Dab1 molecule. The possibility of trans-phosphorylation will be addressed below.
Consistent with the inability of Dab1ab to recruit SFKs, other Reelin-stimulated signaling effects were greatly reduced in dab1ab/ab homozygotes. The stimulation of Akt (Fig. 1D), binding of Dab1 to Crk and CrkL (Fig. 1E and F), and tyrosine phosphorylation of C3G (Fig. 1G) were all inhibited. In embryonic brain extracts, complexes of Crk with Dab1 but not Dab1ab were detected (see Fig. S1 in the supplemental material). By these biochemical criteria, Dab1ab is essentially inactive, presumably due to a lack of SFK recruitment and activation.
In contrast, Dab1cd supported two effects of Reelin: the above-mentioned Reelin-induced downregulation of Dab1 (Fig. 1B) and the Reelin-induced stimulation of Akt (Fig. 1D). This means that Akt and, presumably, PI3-kinase (9) are activated independently of the cd phosphorylation sites. However, Dab1cd did not bind to Crk or CrkL in Reelin-treated neurons (Fig. 1E and F) or to Crk in embryonic brain extracts (see Fig. S1 in the supplemental material), consistent with data from previously reported in vitro studies (4). In addition, Reelin did not induce C3G tyrosine phosphorylation in dab1cd neurons (Fig. 1G). Therefore, Reelin-induced C3G tyrosine phosphorylation requires more than just SFK recruitment to Dab1 and stimulation of Akt. The results suggest that WT Dab1 is capable of binding SFKs through the ab sites and a Crk/CrkL-C3G complex (36) through the cd sites, and this tetrameric complex may be needed for SFKs to phosphorylate C3G. However, we cannot exclude the possibility that the ab sites cooperate with the cd sites to bind Crk and CrkL and recruit C3G.
Abnormal development in the absence of Crk/CrkL binding sites.
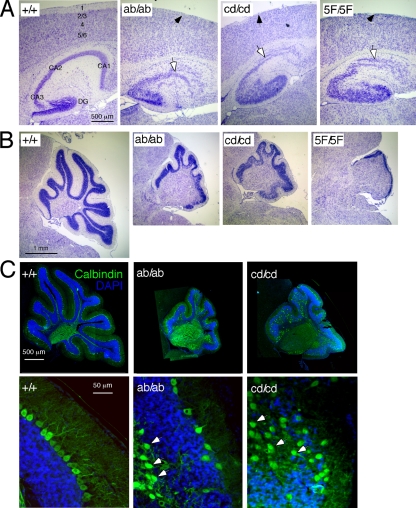
Superficially, both the dab1ab and dab1cd homozygous mutants exhibited similar postnatal phenotypes, with similar behavioral defects (see movies in the supplemental material), disorganized cerebral cortex and hippocampus, absent marginal zone, and reduced cerebellum size (Fig. 3A and B). In both cases, the cerebellum sizes were intermediate between the WT and dab15F/5F or dab1−/− mutants (Fig. 3B). Approximately one-third of the Purkinje cells were positioned correctly above the internal granule layer, while others remained in or below the internal granule layer (Fig. 3C, arrowheads), implying that the dab1ab and dab1cd alleles are both partially defective in the cerebellum. The phenotypes were highly penetrant.
FIG. 3.
Abnormal development of cortex, hippocampus, and cerebellum in Dab1 phosphorylation site mutants. (A) Abnormal development of cortex and hippocampus. The arrows indicate the abnormal marginal zone (black arrows) and CA1 region of the hippocampus (white arrows). DG, dentate gyrus. (B) Partial development of cerebellum in dab1ab and dab1cd homozygotes. Compared with the WT and the dab15F/5F mutant, a partial phenotype was seen in dab1ab and dab1cd homozygotes. Sagittal sections were prepared from P20 brains and stained with Nissl stain. (C) Purkinje cell migration defects in dab1ab and dab1cd mice. Sagittal sections of P20 cerebellum were stained with anti-calbindin and 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI). Some Purkinje cells migrated properly, but many were misplaced in clusters below or in the granule layer (arrowheads). Scale bars, 500 μm (A), 1 mm (B), and 50 μm (C).
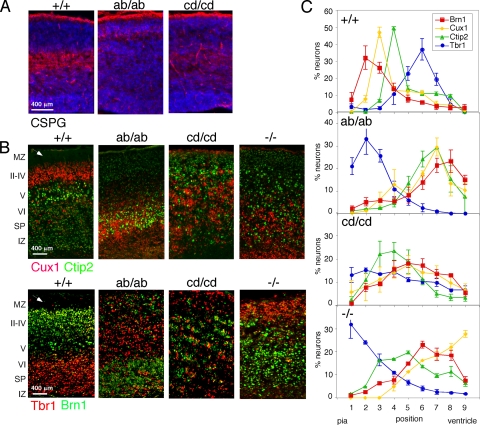
To characterize the neocortex in more detail, we used immunohistochemistry on embryonic or newborn brains (Fig. 4). The subplate, detected with CSPG antibodies, was poorly developed in both mutants at E16.5 (Fig. 4A), resembling dab1−/− (data not shown), consistent with the absent marginal zone (Fig. 3A) and implying that the invasion of the preplate by the first wave of cortical plate neurons requires both the ab and cd phosphorylation sites. However, layer-specific markers revealed significant differences between dab1ab/ab, dab1cd/cd, and dab1−/− newborn mouse brains (illustrated in Fig. 4B and quantified in Fig. 4C). In WT mice, neocortical layers were in the order they were generated, Tbr1→Ctip2→Cux1→Brn1, from bottom to top (18, 37). Reduced Reelin signaling causes cortical inversion in dab1−/− mutants (37), with the layer order Brn1/Cux1→Ctip2→Tbr1. The layer order in dab1ab/ab mutants was similar to that of dab1−/−, Brn1→Cux1/Ctip2→Tbr1, consistent with only slight Dab1 function. However, the cortex in dab1cd/cd mutants was distinct, with Cux1 neurons at the bottom, Brn1 and Ctip2 neurons above, and Tbr1 cells broadly distributed through the cortical plate. Compared with dab1ab/ab or dab1−/− populations, the later-born Ctip2, Cux1, and Brn1 dab1cd/cd populations had migrated further up through the cortical plate.
FIG. 4.
Abnormal preplate splitting and cortical lamination in dab1ab and dab1cd homozygous cortex. (A) E16.5 neocortex stained for CSPG (red) and DNA (blue). The subplate was intact in the WT but not in dab1ab or dab1cd mutants. (B) P1 cortex stained with Cux1 (red) and Ctip2 (green) or with Tbr1 (red) and Brn1 (green). The cortex was inverted in dab1ab/ab mice although not as severely as in dab1−/− mice. The dab1cd/cd phenotype was novel. Arrowheads point to the WT marginal zone, which is absent in the mutants. Scale bars in A and B are 400 μm. (C) Distributions of Tbr1+, Brn1+, Cux1+, and Ctip2+ cells in WT and mutant brains. Images were divided into nine equal-sized areas from the marginal zone (area 1) to the ventricular zone (area 9). The percentage of stained cells in each area was determined for each embryo. The graphs show means and standard errors of the results from three to four +/+, ab/ab, and cd/cd pups and two −/− pups.
Importantly, the cortical layering phenotypes of homozygous dab1cd/cd and hemizygous dab1cd/− animals were similar (see Fig. S2 in the supplemental material). In particular, the dab1cd/− cortex still lacked a marginal zone, unlike the cortex of animals hemizygous for two hypomorphic dab1 alleles (16, 17). This means that the dab1cd phenotype reflects qualitative, not quantitative, differences between Dab1cd and WT Dab1. The dab1cd phenotype suggests that the later-born dab1cd/cd but not dab1ab/ab or dab1−/− cortical neurons can migrate between mispositioned early neurons. Dab1 signaling through the cd sites may be more important in early than in late neurons or may regulate different modes of neuron migration.
The Dab1ab and Dab1cd mutants have complementary functions.
To test if the ab and cd phosphorylation sites have dependent or independent functions in Dab1 signaling, we crossed dab1ab/+ and dab1cd/+ animals and examined the progeny. dab1ab/cd pups were viable and completely normal in behavior. Their brains showed no morphological abnormalities. The cortical marginal zone was well developed, the hippocampus was normal, and the cerebellum was normal in size and well foliated (Fig. 5A). This implies that the Dab1ab and Dab1cd proteins have individual functions that are needed together for normal lamination. Indeed, when neurons were made from dab1ab/cd embryos, Reelin stimulated the phosphorylation of Dab1 (Fig. 5B). This suggests that Dab1ab may be trans-phosphorylated at the cd sites by complexes of Dab1cd and active SFKs. Even so, there was a substantial increase in overall Dab1 protein levels in dab1ab/cd neurons, presumably due to the lack of degradation of the Dab1ab protein (Fig. 5B). Reelin also stimulated Akt, induced Crk and CrkL binding to Dab1, and stimulated C3G tyrosine phosphorylation in neurons from dab1ab/cd embryos (Fig. 5C to F). This intragenic complementation, or mutual cosuppression, of both developmental and biochemical phenotypes may be explained if functions missing from Dab1ab can be supplied by Dab1cd and vice versa.
FIG. 5.
Intragenic complementation. (A) Normal development of cortex, hippocampus, and cerebellum of Dab1ab/cd mice. Brains (P20) were sectioned and stained with Nissl stain. Arrowheads indicate the normal migration of Purkinje cells. DG, dentate gyrus. (B to F) Phosphorylation and binding events in cultured neurons. dab1ab/+ and dab1cd/+ animals were crossed, and neuron cultures from individual E16.5 embryos were prepared. (B) Expression level and phosphorylation of Dab1 in neurons from dab1ab/cd and littermate dab1+/+ E16.5 embryos compared with dab1ab/ab embryos from another cross. Neuron cultures were treated with control (C) or Reelin (R) for 15 min. Cell lysates were immunoblotted with anti-Dab1 and anti-Tuj1 antibodies. The ratio of Dab1 to tubulin was calculated and normalized to the ratio in Reelin-stimulated dab1+/+ neurons. Lysates were also immunoprecipitated (IP) for Dab1 and immunoblotted with anti-phosphotyrosine (4G10) and Dab1 antibodies. The ratio of pDab1 to Dab1 was calculated and normalized to the ratio in Reelin-stimulated dab1+/+ neurons. (C) Activation of Akt by Reelin. Lysates were analyzed by using anti-Akt-pS473 and anti-Akt antibodies. The ratio of pAkt to Akt was calculated. (D) Binding of Crk to Dab1. Crk immunoprecipitates were immunoblotted for Dab1. The ratio of Dab1 to Crk in the immunoprecipitate was calculated. (E) Same as described above (D) but using CrkL antibodies. (F) Phosphorylation of C3G. C3G immunoprecipitates were immunoblotted with antibodies to phosphotyrosine (4G10) and C3G. The ratio of pC3G to C3G was calculated.
DISCUSSION
Dab1 as a kinase activator.
We found that Dab1 tyrosine phosphorylation sites have distinct signaling properties (Fig. 1). The removal of the cd sites does not prevent Reelin-induced binding to active SFKs, stimulation of Dab1 phosphorylation (at the ab sites), stimulation of Akt, and targeting of Dab1 for degradation but does prevent binding to Crk/CrkL and stimulation of C3G tyrosine phosphorylation. In contrast, the removal of the ab sites inhibited essentially all downstream signaling events, including, critically, the phosphorylation of the cd sites (Fig. 1). This was not expected. If the Dab1ab protein is overexpressed in neurons, it is phosphorylated in response to Reelin (15), suggesting that the ab mutations do not cause conformational changes that inhibit phosphorylation. However, we found that the Dab1ab mutant bound less Fyn (Fig. 2). This suggests the model shown in Fig. 6, in which the ab sites may be required to bind and stabilize active SFKs, which then “trans-phosphorylate” the cd sites in the same and possibly other Dab1 molecules and other cell proteins. This mechanism may allow endogenous Dab1 to stimulate SFKs and phosphorylate ectopically expressed Dab1ab at the cd sites. This would not be possible for dab1ab/ab neurons.
FIG. 6.
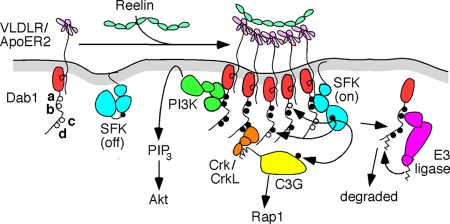
Proposed model for signal transduction by Dab1. Dab1 and SFKs are associated with the inner leaflet of the plasma membrane. Dab1 is also reversibly bound to the cytoplasmic tails of the Reelin receptors VLDLR and ApoER2. Oligomeric complexes of Reelin induce receptor clustering, and binding of active SFKs to Dab1 phosphorylated at the ab sites permits the phosphorylation of adjacent Dab1 molecules at all sites. ab-phosphorylated Dab1 stimulates Akt, possibly by recruiting PI3-kinase, and cd-phosphorylated Dab1 recruits Crk/CrkL-C3G complexes. Only C3G molecules that are associated with Dab1-Crk/CrkL are targets for phosphorylation and stimulate Rap1. After complexes dissociate, ab-phosphorylated Dab1 is targeted for ubiquitination and degradation. See the text and Fig. S3 in the supplemental material for details and references.
The ab phosphorylation sites resemble immunoreceptor tyrosine-based activation motifs found in multichain immune recognition receptors, which recruit SFKs, binding to the SFK SH2 domain and stabilizing the SFK in its active state (13, 35). By comparing Reelin signaling in the Dab1ab and Dab1cd mutants, we infer that the ab sites are sufficient to activate Akt and induce Dab1 ubiquitination and downregulation. Akt stimulation by Reelin requires PI3-kinase, and PI3-kinase-Dab1 complexes are induced by Reelin (9). The noncatalytic subunit of PI3-kinase contains two SH2 domains, which may bind to the ab sites, although the sequences are not optimal (33). The ab sites also bind to an E3 ubiquitin-protein ligase complex that targets Dab1 for degradation (15). Accordingly, the Dab1ab mutant is stable, but the Dab1cd mutant is downregulated to normal levels. In dab1ab/cd cells that contain both mutant proteins, only the Dab1cd protein is degraded, so the total level of the Dab1 protein is intermediate between those in WT and those in dab1ab homozygotes (Fig. 5). This result is consistent with a previously reported finding showing that nonphosphorylated molecules of Dab1 are not degraded (1). Presumably, the recognition of phosphorylated Dab1 by the E3 ligase would occur after SFK dissociation, since the same sites are needed to bind SFKs and the E3 ligase.
We did not test the effects of mutating the a and b phosphorylation sites separately, but Sanada et al. (32) previously found that the b site is dispensable for the Reelin-induced detachment of neurons from radial glia, while the c and d sites are each individually required. Our finding that the ab mutant is virtually inactive implies that the a site may take over if the b site is unavailable. Also, all four phosphorylation sites are required for overexpressed Dab1 to induce the differentiation of zebrafish retinal precursor cells (26). However, phosphorylation of the a site in neurons has not yet been confirmed.
Dab1 as a scaffold for signaling complexes.
We found that the Dab1 cd sites are needed to bind to Crk and CrkL in vivo (Fig. 1 and see Fig. S1 in the supplemental material). This result is consistent with in vitro and cell culture experiments which showed that the mutation of the cd sites inhibited Crk and CrkL binding and that the ab sites were dispensable (4, 11, 23). However, we also found that Crk and CrkL do not bind to Dab1ab in homozygous mutant neurons. While a direct role of the ab sites in Crk and CrkL binding cannot be excluded, we presume that Dab1ab does not bind Crk or CrkL because it is not phosphorylated at the cd sites.
Is this scaffolding important? The developmental defects of dab1cd/cd homozygotes imply that the Reelin-dependent activation of SFKs and Akt and other functions of the Dab1 ab sites are insufficient for normal development. This excludes the possibility that the sole function of Dab1 is to recruit or switch on SFKs and Akt in response to Reelin. Since the only known function of the cd sites is to form signaling complexes (3, 4, 23, 31), we suggest that the scaffolding of signaling complexes by Dab1 is also important for development (Fig. 6). In this regard, Dab1 again resembles the immunoreceptor tyrosine-based activation motifs of immune recognition receptors or the phosphorylated tails of growth factor receptor tyrosine kinases, which signal by forming complexes with cell proteins (35, 38).
Dab1 signaling complexes may be quite large. The lack of Reelin-stimulated tyrosine phosphorylation of C3G in dab1cd/cd homozygotes implies that C3G phosphorylation occurs only if C3G is bound via Crk or CrkL to Dab1. Thus, we hypothesize that Dab1-Crk/CrkL-C3G complexes are brought close to Dab1-SFK complexes by receptor clustering or that a single Dab1 molecule binds to an SFK and Crk/CrkL-C3G (Fig. 6). The proximity of C3G to an SFK would then promote C3G phosphorylation. Other proteins such as Nckβ and Lis1 may also be present and could be phosphorylated by the associated SFK. Which of the bound proteins is required for proper migration remains to be discovered, although a recent study of C3G mutant mice showed that C3G is needed for the normal migration of cortical neurons in vivo (39), suggesting that C3G phosphorylation may be crucial.
The scaffold effect of Dab1 may also underlie the observed intragenic complementation between the dab1ab and dab1cd alleles. Such complementation can occur if Dab1 has two parallel, independent functions and each allele retains one of the functions. Alternatively, each altered product may be labile but may stabilize the other. Our case is likely a hybrid of the two. We suspect that the ab and cd sites do have parallel functions, both required for normal development, but the functions are not independent (Fig. 6 and see Fig. S3 in the supplemental material). The phosphorylated Dab1cd protein recruits an active SFK, which then phosphorylates the cd sites on a nearby Dab1ab molecule (see Fig. S3 in the supplemental material). The phosphorylated ab sites stimulate Akt, and the phosphorylated cd sites recruit Crk/CrkL-C3G. This phosphorylation in trans implies a proximity of individual Dab1 molecules in Reelin-stimulated cells, which is consistent with Dab1 activation by receptor clustering and with clustering-induced phosphorylation (34).
In contrast, Dab1 ubiquitination and proteolysis likely occur after such complexes have dissociated. Nonphosphorylated Dab1 molecules are stable in neurons expressing a mixture of phosphorylated and nonphosphorylated Dab1 (1), and the intermediate level of Dab1 in dab1ab/cd brains implies that the degradation of phosphorylated Dab1cd did not induce the codestruction of the stable Dab1ab mutant (Fig. 5). Therefore, oligomeric Dab1 signaling complexes may dissociate before Dab1, phosphorylated at the ab sites, is subject to ubiquitination and degradation.
Developmental roles of Dab1 phosphorylation.
Dab1ab is only slightly phosphorylated, yet it partially rescues cerebellum development relative to that of Dab15F. The phenotype is reminiscent of src−/− fyn−/− double mutants in which Dab1 is phosphorylated at approximately 5 to 10% of the normal level (28). This implies that even very-low-level phosphorylation at the cd sites is sufficient for some correct positioning of Purkinje cells in the cerebellum. This is consistent with the finding that the cerebellum is more resistant to partial reductions in Dab1 signaling than are the cortex and hippocampus (16, 17). Less Dab1 activity may needed for Purkinje cell positioning than is needed for neocortical layering.
The cortical phenotype dab1cd/cd is distinct from dab1ab/ab or dab1−/− and the WT. Examination of dab1cd/− hemizygotes suggests that the dab1cd/cd phenotype is due to qualitative, and not quantitative, defects in signaling from the Dab1cd protein. The phenotype suggests that Dab1cd may support the movement of later-born cortical neurons but not early neurons. Neurons migrate by two principal modes, somal translocation and locomotion along radial glia (29). Somal translocation is more prevalent early in development. One hypothesis for layer formation during cortical development posits that Reelin regulates both modes of migration: it may stimulate somal translocation and terminate locomotion by stimulating neuron detachment from radial glia (12, 29). We speculate that Dab1cd may support Reelin-induced detachment but not somal translocation. A failure of somal translocation would explain why early cortical plate dab1cd neurons fail to split the preplate and why later neurons fail to rise high in the cortical plate. Although further experiments are needed to be sure, the results suggest that signaling from the phosphorylated Dab1 cd sites (or cd and ab sites together) may stimulate somal translocation, and, by subtraction, the Dab1 ab sites may be sufficient for Reelin-induced detachment from radial glia.
This hypothesis agrees with observations made previously by Sanada et al. (32). Using in utero microinjection, they found that a mutation of the Dab1 c or d site inhibited neuron movement to the top of the cortical plate and reduced the tangential distance between a neuron and its parental radial glial fiber from an average of 16 μm in the control to 7 μm in the mutant (32). Those authors interpreted this reduction in distance as blocked detachment, but since an attached neuron would be within 3 μm of its radial glial fiber and the average spacing between radial glial fibers is 8 to 10 μm (30), it is possible that larger distances between neurons and their parental radial glia result from somal translocation after detachment had occurred. Resolution of this question awaits other approaches such as real-time imaging.
Supplementary Material
Acknowledgments
We thank Esther Jhingan for expert technical assistance, Nanyang Jiang for blastocyst injections, Brian Howell and Lionel Arnaud for constructs, Robert McEvilly and Robert Hevner for generous gifts of antisera, Philippe Soriano for cryostat and microscope use, and members of the Cooper laboratory for interesting discussions. We greatly appreciate Yves Jossin for his thoughtful comments on the paper.
This work was supported by U.S. Public Health Service grant CA41072 from the National Cancer Institute.
Footnotes
Published ahead of print on 3 November 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Arnaud, L., B. A. Ballif, and J. A. Cooper. 2003. Regulation of protein tyrosine kinase signaling by substrate degradation during brain development. Mol. Cell. Biol. 239293-9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnaud, L., B. A. Ballif, E. Forster, and J. A. Cooper. 2003. Fyn tyrosine kinase is a critical regulator of Disabled-1 during brain development. Curr. Biol. 139-17. [DOI] [PubMed] [Google Scholar]
- 3.Assadi, A. H., G. Zhang, U. Beffert, R. S. McNeil, A. L. Renfro, S. Niu, C. C. Quattrocchi, B. A. Antalffy, M. Sheldon, D. D. Armstrong, A. Wynshaw-Boris, J. Herz, G. D'Arcangelo, and G. D. Clark. 2003. Interaction of reelin signaling and Lis1 in brain development. Nat. Genet. 35270-276. [DOI] [PubMed] [Google Scholar]
- 4.Ballif, B. A., L. Arnaud, W. T. Arthur, D. Guris, A. Imamoto, and J. A. Cooper. 2004. Activation of a Dab1/CrkL/C3G/Rap1 pathway in Reelin-stimulated neurons. Curr. Biol. 14606-610. [DOI] [PubMed] [Google Scholar]
- 5.Ballif, B. A., L. Arnaud, and J. A. Cooper. 2003. Tyrosine phosphorylation of disabled-1 is essential for Reelin-stimulated activation of Akt and Src family kinases. Brain Res. Mol. Brain Res. 117152-159. [DOI] [PubMed] [Google Scholar]
- 6.Beffert, U., G. Morfini, H. H. Bock, H. Reyna, S. T. Brady, and J. Herz. 2002. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J. Biol. Chem. 27749958-49964. [DOI] [PubMed] [Google Scholar]
- 7.Bielas, S., H. Higginbotham, H. Koizumi, T. Tanaka, and J. G. Gleeson. 2004. Cortical neuronal migration mutants suggest separate but intersecting pathways. Annu. Rev. Cell Dev. Biol. 20593-618. [DOI] [PubMed] [Google Scholar]
- 8.Bock, H. H., and J. Herz. 2003. Reelin activates SRC family tyrosine kinases in neurons. Curr. Biol. 1318-26. [DOI] [PubMed] [Google Scholar]
- 9.Bock, H. H., Y. Jossin, P. Liu, E. Forster, P. May, A. M. Goffinet, and J. Herz. 2003. Phosphatidylinositol 3-kinase interacts with the adaptor protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J. Biol. Chem. 27838772-38779. [DOI] [PubMed] [Google Scholar]
- 10.Bock, H. H., Y. Jossin, P. May, O. Bergner, and J. Herz. 2004. Apolipoprotein E receptors are required for reelin-induced proteasomal degradation of the neuronal adaptor protein Disabled-1. J. Biol. Chem. 27933471-33479. [DOI] [PubMed] [Google Scholar]
- 11.Chen, K., P. G. Ochalski, T. S. Tran, N. Sahir, M. Schubert, A. Pramatarova, and B. W. Howell. 2004. Interaction between Dab1 and CrkII is promoted by Reelin signaling. J. Cell Sci. 1174527-4536. [DOI] [PubMed] [Google Scholar]
- 12.Cooper, J. A. 2008. A mechanism for inside-out lamination in the neocortex. Trends Neurosci. 31113-119. [DOI] [PubMed] [Google Scholar]
- 13.Cooper, J. A., and H. Qian. 2008. A mechanism for SRC kinase-dependent signaling by noncatalytic receptors. Biochemistry 475681-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Arcangelo, G., R. Homayouni, L. Keshvara, D. S. Rice, M. Sheldon, and T. Curran. 1999. Reelin is a ligand for lipoprotein receptors. Neuron 24471-479. [DOI] [PubMed] [Google Scholar]
- 15.Feng, L., N. S. Allen, S. Simo, and J. A. Cooper. 2007. Cullin 5 regulates Dab1 protein levels and neuron positioning during cortical development. Genes Dev. 212717-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrick, T. M., and J. A. Cooper. 2004. High affinity binding of Dab1 to Reelin receptors promotes normal positioning of upper layer cortical plate neurons. Brain Res. Mol. Brain Res. 126121-128. [DOI] [PubMed] [Google Scholar]
- 17.Herrick, T. M., and J. A. Cooper. 2002. A hypomorphic allele of dab1 reveals regional differences in reelin-Dab1 signaling during brain development. Development 129787-796. [DOI] [PubMed] [Google Scholar]
- 18.Hevner, R. F., R. A. Daza, J. L. Rubenstein, H. Stunnenberg, J. F. Olavarria, and C. Englund. 2003. Beyond laminar fate: toward a molecular classification of cortical projection/pyramidal neurons. Dev. Neurosci. 25139-151. [DOI] [PubMed] [Google Scholar]
- 19.Hiesberger, T., M. Trommsdorff, B. W. Howell, A. Goffinet, M. C. Mumby, J. A. Cooper, and J. Herz. 1999. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron 24481-489. [DOI] [PubMed] [Google Scholar]
- 20.Howell, B. W., R. Hawkes, P. Soriano, and J. A. Cooper. 1997. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature (London) 389733-737. [DOI] [PubMed] [Google Scholar]
- 21.Howell, B. W., T. M. Herrick, and J. A. Cooper. 1999. Reelin-induced tyrosine phosphorylation of disabled 1 during neuronal positioning. Genes Dev. 13643-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howell, B. W., T. M. Herrick, J. D. Hildebrand, Y. Zhang, and J. A. Cooper. 2000. Dab1 tyrosine phosphorylation sites relay positional signals during mouse brain development. Curr. Biol. 10877-885. [DOI] [PubMed] [Google Scholar]
- 23.Huang, Y., S. Magdaleno, R. Hopkins, C. Slaughter, T. Curran, and L. Keshvara. 2004. Tyrosine phosphorylated Disabled 1 recruits Crk family adapter proteins. Biochem. Biophys. Res. Commun. 318204-212. [DOI] [PubMed] [Google Scholar]
- 24.Jossin, Y., and A. M. Goffinet. 2007. Reelin signals through phosphatidylinositol 3-kinase and Akt to control cortical development and through mTor to regulate dendritic growth. Mol. Cell. Biol. 277113-7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jossin, Y., M. Ogawa, C. Metin, F. Tissir, and A. M. Goffinet. 2003. Inhibition of SRC family kinases and non-classical protein kinases C induce a reeler-like malformation of cortical plate development. J. Neurosci. 239953-9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katyal, S., Z. Gao, E. Monckton, D. Glubrecht, and R. Godbout. 2007. Hierarchical disabled-1 tyrosine phosphorylation in Src family kinase activation and neurite formation. J. Mol. Biol. 368349-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keshvara, L., D. Benhayon, S. Magdaleno, and T. Curran. 2001. Identification of reelin-induced sites of tyrosyl phosphorylation on disabled 1. J. Biol. Chem. 27616008-16014. [DOI] [PubMed] [Google Scholar]
- 28.Kuo, G., L. Arnaud, P. Kronstad-O'Brien, and J. A. Cooper. 2005. Absence of Fyn and Src causes a reeler-like phenotype. J. Neurosci. 258578-8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nadarajah, B., J. E. Brunstrom, J. Grutzendler, R. O. Wong, and A. L. Pearlman. 2001. Two modes of radial migration in early development of the cerebral cortex. Nat. Neurosci. 4143-150. [DOI] [PubMed] [Google Scholar]
- 30.Pinto-Lord, M. C., P. Evrard, and V. S. Caviness, Jr. 1982. Obstructed neuronal migration along radial glial fibers in the neocortex of the reeler mouse: a Golgi-EM analysis. Brain Res. 256379-393. [DOI] [PubMed] [Google Scholar]
- 31.Pramatarova, A., P. G. Ochalski, K. Chen, A. Gropman, S. Myers, K. T. Min, and B. W. Howell. 2003. Nckβ interacts with tyrosine-phosphorylated disabled 1 and redistributes in Reelin-stimulated neurons. Mol. Cell. Biol. 237210-7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanada, K., A. Gupta, and L. H. Tsai. 2004. Disabled-1-regulated adhesion of migrating neurons to radial glial fiber contributes to neuronal positioning during early corticogenesis. Neuron 42197-211. [DOI] [PubMed] [Google Scholar]
- 33.Songyang, Z., S. E. Shoelson, M. Chaudhuri, G. Gish, T. Pawson, W. G. Haser, F. King, T. Roberts, S. Ratnofsky, R. J. Lechleider, B. G. Neel, R. B. Birge, J. E. Fajardo, M. M. Chou, H. Hanafusa, B. Schaffhausen, and L. C. Cantley. 1993. SH2 domains recognize specific phosphopeptide sequences. Cell 72767-778. [DOI] [PubMed] [Google Scholar]
- 34.Strasser, V., D. Fasching, C. Hauser, H. Mayer, H. H. Bock, T. Hiesberger, J. Herz, E. J. Weeber, J. D. Sweatt, A. Pramatarova, B. Howell, W. J. Schneider, and J. Nimpf. 2004. Receptor clustering is involved in Reelin signaling. Mol. Cell. Biol. 241378-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamir, I., and J. C. Cambier. 1998. Antigen receptor signaling: integration of protein tyrosine kinase functions. Oncogene 171353-1364. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka, S., T. Morishita, Y. Hashimoto, S. Hattori, S. Nakamura, M. Shibuya, K. Matuoka, T. Takenawa, T. Kurata, K. Nagashima, and M. Matsuda. 1994. C3G, a guanine nucleotide-releasing protein expressed ubiquitously, binds to the Src homology 3 domains of CRK and GRB2/ASH proteins. Proc. Natl. Acad. Sci. USA 913443-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tissir, F., and A. M. Goffinet. 2003. Reelin and brain development. Nat. Rev. Neurosci. 4496-505. [DOI] [PubMed] [Google Scholar]
- 38.Ullrich, A., and J. Schlessinger. 1990. Signal transduction by receptors with tyrosine kinase activity. Cell 61203-212. [DOI] [PubMed] [Google Scholar]
- 39.Voss, A. K., J. M. Britto, M. P. Dixon, B. N. Sheikh, C. Collin, S. S. Tan, and T. Thomas. 2008. C3G regulates cortical neuron migration, preplate splitting and radial glial cell attachment. Development 1352139-2149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.