Abstract
Deficiency in both ATM and the DNA-dependent protein kinase catalytic subunit (DNA-PKcs) is synthetically lethal in developing mouse embryos. Using mice that phenocopy diverse aspects of Atm deficiency, we have analyzed the genetic requirements for embryonic lethality in the absence of functional DNA-PKcs. Similar to the loss of ATM, hypomorphic mutations of Mre11 (Mre11ATLD1) led to synthetic lethality when juxtaposed with DNA-PKcs deficiency (Prkdcscid). In contrast, the more moderate DNA double-strand break response defects associated with the Nbs1ΔB allele permitted viability of some Nbs1ΔB/ΔB Prkdcscid/scid embryos. Cell cultures from Nbs1ΔB/ΔB Prkdcscid/scid embryos displayed severe defects, including premature senescence, mitotic aberrations, sensitivity to ionizing radiation, altered checkpoint responses, and increased chromosome instability. The known functions of DNA-PKcs in the regulation of Artemis nuclease activity or nonhomologous end joining-mediated repair do not appear to underlie the severe genetic interaction. Our results reveal a role for DNA-PKcs in the maintenance of S/G2-phase chromosome stability and in the induction of cell cycle checkpoint responses.
The Mre11 complex, consisting of Mre11, Rad50, and Nbs1 (Xrs2 in Saccharomyces cerevisiae), is involved in diverse aspects of DNA double-strand break (DSB) metabolism. The Mre11 complex acts as a DSB sensor, mediates cell cycle checkpoint arrest and apoptosis, and promotes DSB repair (47, 48). The influence of the Mre11 complex on DSB responses is attributable partly to its influence on ataxia-telangiectasia mutated (ATM) kinase activity (29). ATM is a central signal transducer in the response to DSBs and is required for arrest throughout the cell cycle, as well as the efficient execution of apoptosis in response to many types of genotoxic stress (43).
The Mre11 complex is required for ATM activation and governs the phosphorylation of ATM substrates such as SMC1, Chk2, and BID (4, 6, 26, 47, 49, 51). The C terminus of Nbs1 interacts with ATM and plays an important role in facilitating a subset of these events, particularly those important for apoptosis (11, 14, 47, 58). However, ATM makes multiple functional contacts with members of the Mre11 complex. Nbs1, Mre11, and Rad50 are all ATM substrates, and many aspects of ATM checkpoint signaling are impaired by hypomorphic Mre11 and Nbs1 mutations that do not affect the ATM binding domain in the C terminus of Nbs1 (32, 36, 52, 54).
Several molecular and genetic observations support the view that the Mre11 complex's role in preserving genome stability is particularly relevant to the S and G2 phases of the cell cycle (3, 56). The complex, predominantly nucleoplasmic in G1 cells, becomes predominantly chromatin associated and colocalizes with PCNA throughout S phase (35, 38). This association is a likely prerequisite for the complex's influence on DNA damage signaling as well as DNA repair.
Cell cultures established with samples from patients with Nijmegen breakage syndrome (NBS1 hypomorphism) and ataxia-telangiectasia-like disorder (MRE11 hypomorphism) exhibit checkpoint defects in S phase and at the G2/M transition, while the G1/S transition is relatively unaffected. These checkpoint defects are correlated with reduced Mre11 complex chromatin association both in human cells and in mouse models of Nijmegen breakage syndrome and ataxia-telangiectasia-like disorder (5, 45, 49, 52). Chromosomal aberrations arising in these cells are predominantly chromatid type breaks, consistent with impaired metabolism of DNA replication-associated DNA breaks (49, 52).
Further supporting a predominant role for the Mre11 complex in S phase is the observation that its primary role in DSB repair is the promotion of recombination between sister chromatids (3, 24). Structural and genetic evidence that the Mre11 complex effects molecular bridging between DNA duplexes offers a mechanistic basis for this observation (10, 23, 53). Molecular bridging by the Mre11 complex may also contribute to its influence on nonhomologous end joining (NHEJ) (12, 34, 57). Collectively, these data strongly support the view that the Mre11 complex's checkpoint and DSB repair functions are manifested predominantly in the S and G2 phases of the cell cycle.
Although the Mre11 complex and ATM function in the same arm of the DNA damage response, ATM deficiency is lethal in hypomorphic Mre11 and Nbs1 mutants (Mre11ATLD1/ATLD1 and Nbs1ΔB/ΔB mice, respectively) (49, 52), suggesting that aspects of ATM function are Mre11 complex independent. ATM deficiency is also synthetically lethal with mutations in Prkdc, the gene encoding the catalytic subunit of the DNA-dependent protein kinase (DNA-PKcs) that is mutated in mice with severe combined immunodeficiency (Prkdcscid mice) (22, 42). DNA-PKcs is an ATM paralog required for NHEJ, which appears to be the predominant mode of DSB repair in G1 cells (16).
Defective NHEJ is unlikely to be the basis for the embryonic lethality of Prkdc−/− Atm−/− or Prkdcscid/scid Atm−/− mice, as loss of ATM rescues the late embryonic lethality of both DNA ligase IV (Lig4) and XRCC4 null embryos, which have more severe NHEJ defects than Prkdcscid mice abolished by the Atm−/− genotype (31, 42). These observations argue that the DNA-PKcs functions required for viability in the absence of ATM do not include NHEJ.
To address this issue, we crossed Mre11ATLD1/ATLD1 and Nbs1ΔB/ΔB mice with Prkdcscid/scid mice. As these Mre11 complex hypomorphs do not completely phenocopy ATM deficiency, we reasoned that double-mutant animals would be viable and thus provide a venue in which to examine the functional relationship between the Mre11 complex/ATM arm of the DNA damage response and DNA-PKcs. Whereas the Mre11ATLD1/ATLD1 mutation was synthetically lethal with the Prkdcscid/scid genotype, some Nbs1ΔB/ΔB Prkdcscid/scid mice were born, consistent with the more moderate DNA damage response defects associated with the Nbs1ΔB allele than with the Mre11ATLD1 allele (48). Nbs1ΔB/ΔB Prkdcscid/scid embryos were born at drastically reduced Mendelian ratios, displayed gross developmental defects, and were severely runted. Nbs1ΔB/ΔB Prkdcscid/scid cell cultures exhibited profound chromosome instability, growth defects, and increased sensitivity to ionizing radiation (IR). DNA repair defects associated with DNA-PKcs deficiency did not appear to underlie the observed phenotypic synergy. Rather, the data suggest a novel regulatory function of DNA-PKcs in the maintenance of chromosomal stability during the S and G2 phases of the cell cycle.
MATERIALS AND METHODS
Mice and genotyping.
Mre11ATLD1/ATLD1 and Nbs1ΔB/ΔB mice were generated as described previously (49, 52). Atm−/− mice were obtained from T. Wynshaw Boris, Smc12SA/2SA mice were obtained from M. Kastan, and Art−/− mice were obtained from F. Alt. Prkdcscid/scid mice were purchased from the Jackson Laboratory. All mice were maintained on a mixed 129SvEv/C57B6 background and genotyped by PCR (details are available upon request).
Antibodies, Western blotting, and immunofluorescence.
Antibodies for Nbs1 were described previously (52). Antibodies for Myc (Sloan-Kettering Institute Monoclonal Antibody Core Facility) and actin (Sigma) were purchased. Cell extracts were prepared in 150 mM TNG buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% Tween 20, 0.5% NP-40), run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, and transferred onto a polyvinylidene difluoride membrane (Millipore). Primary and secondary antibodies were incubated with the membrane in 5% dry milk in phosphate-buffered saline (PBS) with 0.05% Tween 20, and antibody detection was performed using enhanced chemiluminescence with a kit from GE Healthcare.
MEF derivation and culture.
Primary and transformed mouse embryonic fibroblasts (MEFs) were generated from appropriate crosses as described previously (50). For proliferation assays, passage 1 (p1) MEFs were seeded at 5 × 105 per well onto a six-well plate. Cells were trypsinized and counted every 3 days for a total of 12 days. Total proliferation was determined by calculating the number of cells that would have been recovered had all cells been replated at every counting.
Chromosome analysis and telomere fluorescence in situ hybridization (FISH).
For mitotic analysis, early-passage (p2 to p3) MEFs were grown on sterile microscope slides, fixed in PBS containing 4% paraformaldehyde and 2% sucrose, and permeabilized in a solution of 10 mM HEPES, pH 7.9, 50 mM NaCl, 3 mM MgCl2, 200 mM sucrose, and 0.5% Triton X-100. Cells were stained with DAPI (4′,6-diamidino-2-phenylindole), mounted in Prolong Antifade reagent (Molecular Probes), and scored for abnormalities.
For metaphase analysis, early-passage (p2 to p5) or simian virus 40 (SV40)-transformed ear fibroblasts (EFs) or MEFs were prepared as described previously (50). Metaphase spreads were treated with Giemsa stain (Sigma) and mounted in Permount. For fusion analysis, telomere FISH was performed as described previously (28) using a fluorescein isothiocyanate-conjugated peptide nucleic acid probe (fluorescein isothiocyanate-5′-CCCTAACCCTAACCCTAA-3′; Applied Biosystems).
DNA damage sensitivity assays and checkpoint analysis.
IR sensitivity assays were performed as described previously (50). Exponentially growing SV40-transformed MEFs were treated with mitomycin C (MMC; Sigma) for 2 h or with camptothecin (CPT; Sigma) for 24 h, washed twice with PBS, and analyzed for colony formation. Plates were stained with crystal violet, and colonies with more than 50 cells were counted. The numbers of colonies growing on plates that received treatment were normalized to the numbers on mock-treated plates. Intra-S-phase, G1/S, and G2/M checkpoint assays were performed as described previously (50).
Viral infection.
Plasmid containing the dominant negative Trf2ΔBΔM allele was a gift from T. de Lange. Adenovirus expressing green fluorescent protein (Ad-GFP) and adenovirus expressing Trf2ΔBΔM were purchased from the University of Iowa Gene Transfer Vector Core. MEFs were infected with adenovirus vectors at a multiplicity of infection of 100 for 16 h, after which time the cells were washed and cultured in fresh medium. Metaphase spreads for fusion analysis were prepared 40 h postinfection.
Statistical analysis.
Statistical analysis was performed using MStat software (provided by Norman Drinkwater, McArdle Laboratory for Cancer Research) or Excel (Microsoft).
RESULTS
Genetic analysis of synthetic lethality.
ATM influences DSB responses throughout the cell cycle, whereas defects in hypomorphic Mre11 complex mutants are restricted to the S and G2 phases. To determine if defects in S- and G2-phase DSB responses interacted genetically with Prkdc deficiency, we established intercrosses to generate Mre11ATLD1/ATLD1 Prkdcscid/scid and Nbs1ΔB/ΔB Prkdcscid/scid double mutants. No viable Mre11ATLD1/ATLD1 Prkdcscid/scid embryos were recovered, indicating synthetic lethality between Mre11ATLD1 and Prkdcscid (Table 1). Expected genotypic ratios were observed at embryonic day 3.5 (E3.5) and E10.5, but by E10.5, all double-mutant embryos were runted, displayed abnormal morphology, or were partially reabsorbed, and attempts to culture cells from these embryos were not successful (Table 1 and data not shown).
TABLE 1.
Mre11ATLD1/ATLD1 Prkdcscid/scid mutant data
| Genotype of father | Genotype of mother | Time point of assessment | Total no. of embryos | Total no. of pups | Expected no. of double mutants | Observed no. of double mutants | P valueb |
|---|---|---|---|---|---|---|---|
| Mre11+/ATLD1Prkdcscid/scid | Mre11+/ATLD1Prkdcscid/scid | E10.5 | 15 | 3.75 | 3a | ||
| E3.5 | 10 | 2.5 | 3 | ||||
| Mre11+/ATLD1Prkdc+/scid | Mre11+/ATLD1Prkdc+/scid | After birth | 203 | 12.7 | 0 | 2.0 × 10−6 | |
| Mre11+/ATLD1Prkdcscid/scid | Mre11+/ATLD1Prkdc+/scid | After birth | 35 | 4.4 | 0 | 9.4 × 10−3 | |
| Mre11+/ATLD1Prkdcscid/scid | Mre11+/ATLD1Prkdcscid/scid | After birth | 62 | 15.5 | 0 | 1.8 × 10−8 |
Embryos were partially reabsorbed or were abnormal or runted.
P values are based on binomial distribution.
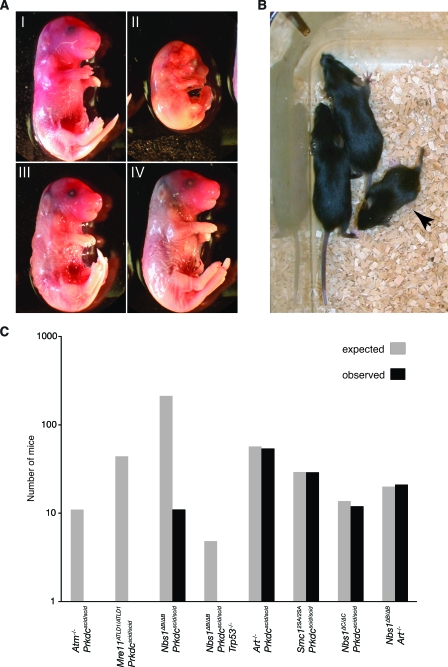
Embryonic lethality was also observed in Nbs1ΔB/ΔB Prkdcscid/scid mutants, but unlike the lethality of the Mre11ATLD1/ATLD1 Prkdcscid/scid genotype, this outcome was not fully penetrant, and double-mutant animals were born at about 5% of the expected frequency. Developmental defects were evident in double-mutant Nbs1ΔB/ΔB Prkdcscid/scid embryos (Fig. 1 A and B and data not shown; see also Table S1 in the supplemental material), and the rare double-mutant pups that survived to term were severely runted (Fig. 1B).
FIG. 1.
Genetic interactions with the Prkdcscid allele. (A) Embryos isolated from Nbs1+/ΔB Prkdcscid/scid breedings at E15.5. (I) Nbs1+/+ Prkdcscid/scid embryo. (II) Nbs1ΔB/ΔB Prkdcscid/scid embryo. (III and IV) Nbs1+/ΔB Prkdcscid/scid embryos. (B) Female Nbs1ΔB/ΔB Prkdcscid/scid pup (arrow) with Nbs1+/ΔB Prkdcscid/scid littermates at 1 month of age. All double mutants were runted, and 10 of 11 had a short, rigid, kinked tail. (C) Synthetic lethality was observed in Atm−/− Prkdcscid/scid and Mre11ATLD1/ATLD1 Prkdcscid/scid embryos. Partial synthetic lethality of the Nbs1ΔB and Prkdcscid alleles was observed, while Nbs1ΔB/ΔB Art−/−, Smc12SA/2SA Prkdcscid/scid, Nbs1ΔC/ΔC Prkdcscid/scid, and Art−/− Prkdcscid/scid double mutants were born at expected ratios. The loss of p53 did not eliminate the synthetic lethality of the Nbs1ΔB/ΔB Prkdcscid/scid genotype.
The observed phenotypic synergy may reflect an interaction between the intra-S-phase and G2/M checkpoint defects ensuing from Mre11 complex hypomorphism and the NHEJ defects associated with the Prkdcscid/scid genotype. Alternatively, synergy may result from the coincident reductions in DNA repair processes mediated by the Mre11 complex and DNA-PKcs. Given the cell cycle-restricted penetrance of the Nbs1ΔB/ΔB and Mre11ATLD1/ATLD1 phenotypes, either scenario implicitly requires that DNA-PKcs influence the DNA damage response in S and G2, in addition to fulfilling its role in G1 cells.
We tested this interpretation using Nbs1ΔC/ΔC and Smc12SA/2SA murine mutants, both of which exhibit defects in the intra-S-phase checkpoint but have functional G2/M checkpoint responses (27, 47). In contrast to the Nbs1ΔB/ΔB and Mre11ATLD1/ATLD1 mutations, the Nbs1ΔC/ΔC and Prkdcscid/scid mutations exhibited no synthetic genetic interaction in double-mutant mice; the expected number of double-mutant pups was obtained, and no developmental defects were evident (Fig. 1C). The Nbs1ΔC protein lacks a C-terminal domain implicated in interaction between ATM and the Mre11 complex (47). This interaction promotes the phosphorylation of the cohesin subunit SMC1 by ATM, a modification that is required for the full imposition of the intra-S-phase checkpoint (27). Intercrosses between Prkdcscid mice and mice expressing an allele of SMC1 (Smc12SA), lacking ATM consensus phosphorylation sites, were similarly unaffected. Smc12SA/2SA Prkdcscid/scid mice were born at the expected ratios and did not exhibit any overt developmental defects, with the exception of the immunodeficiency characteristic of Prkdcscid/scid mice (Fig. 1C and data not shown).
DNA-PKcs regulates the Artemis nuclease, which is required for hairpin opening during V(D)J recombination, as well as the repair of some IR-induced lesions (20, 33, 39, 41). ATM and the Mre11 complex have also been suggested previously to regulate Artemis in response to IR (39). Thus, a second potential mechanism for the observed synergy may be the additive effects of Mre11 complex hypomorphism and impaired NHEJ associated with the misregulation of the Artemis nuclease. If so, then Artemis deficiency in the context of Mre11 complex hypomorphism (i.e., in Nbs1ΔB/ΔB or Prkdcscid/scid mice) might produce a phenocopy of the Nbs1ΔB/ΔB Prkdcscid/scid phenotype. To test this, Nbs1ΔB/ΔB Art−/− and Prkdcscid/scid Art−/− mice were generated. Both Nbs1ΔB/ΔB Art−/− and Art−/− Prkdcscid/scid double-mutant mice were born at the expected ratios, without any overt phenotypic synergy (Fig. 1C).
Together, these data indicate that an Artemis-independent function of DNA-PKcs is required for the viability of mutants with defects in DNA damage responses in the S and G2 phases of the cell cycle. That the phenotypes of Nbs1ΔB/ΔB mice are mild relative to those of Atm−/− and Mre11ATLD1/ATLD1 mice may account for the partial (as opposed to complete) lethality of the Nbs1ΔB/ΔB Prkdcscid/scid genotype.
Defects in cellular growth and chromosomal stability.
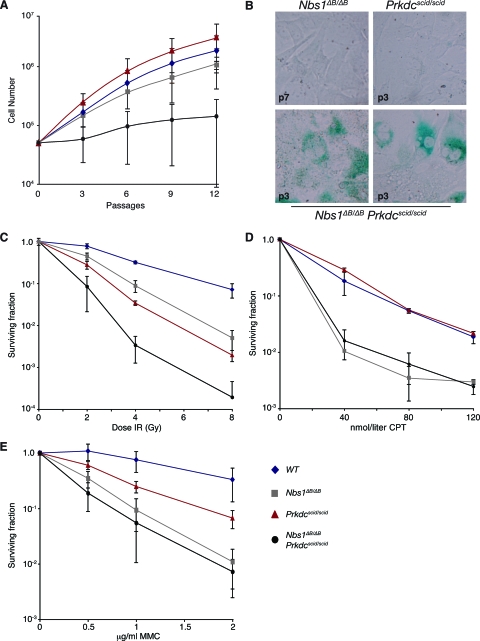
The synergy between the Nbs1ΔB and Prkdcscid alleles was evident at the level of cell growth, chromosomal instability, and sensitivity to IR in cell cultures from Nbs1ΔB/ΔB Prkdcscid/scid mouse specimens. Nbs1ΔB/ΔB Prkdcscid/scid MEFs exhibited a marked growth defect compared to either wild-type (WT) or single-mutant cultures (Fig. 2A). WT and Prkdcscid/scid MEFs displayed similar growth characteristics, whereas Nbs1ΔB/ΔB MEFs showed a slight reduction in proliferation (Fig. 2A). By p3, Nbs1ΔB/ΔB Prkdcscid/scid MEFs showed high levels of senescence-associated β-galactosidase (SA-β-Gal) activity compared to those of either single mutant, indicating that the growth defect was the result of premature senescence (Fig. 2B).
FIG. 2.
Analysis of cell growth and survival. (A) The cell growth of primary MEFs of the indicated genotypes was analyzed using a modified 3T3 protocol. Nbs1ΔB/ΔB Prkdcscid/scid cell cultures showed little proliferation compared to WT, Prkdcscid/scid, or Nbs1ΔB/ΔB cell cultures. (B) Early-passage MEFs were stained for SA-β-Gal activity. p3 Nbs1ΔB/ΔB Prkdcscid/scid cultures showed increased SA-β-Gal activity compared to p3 Prkdcscid/scid or p7 Nbs1ΔB/ΔB MEFs, where little activity is detectable. (C) SV40-transformed EFs of the indicated genotypes were exposed to IR and assessed for colony formation. Nbs1ΔB/ΔB Prkdcscid/scid EFs were acutely sensitive to IR-induced damage, and this sensitivity was also observed in MEF cultures (data not shown). Enhanced sensitivity in Nbs1ΔB/ΔB Art−/− cell cultures was not observed (see Fig. S3 in the supplemental material). (D and E) SV40-transformed MEFs were exposed to the indicated doses of CPT for 24 h (D) or to the DNA cross-linker MMC for 2 h (E), and survival was assessed by monitoring colony formation. Double-mutant Nbs1ΔB/ΔB Prkdcscid/scid MEFs did not display increased sensitivity to either agent compared to that of single-mutant Nbs1ΔB/ΔB MEFs.
Nbs1ΔB/ΔB Prkdcscid/scid EFs and MEFs showed increased sensitivity to IR compared to WT or single-mutant cell cultures (Fig. 2C and data not shown). In contrast, Nbs1ΔB/ΔB Prkdcscid/scid MEFs were not more sensitive than Nbs1ΔB/ΔB MEFs to agents such as CPT and MMC that induce breaks specifically during S phase (Fig. 2D and E).
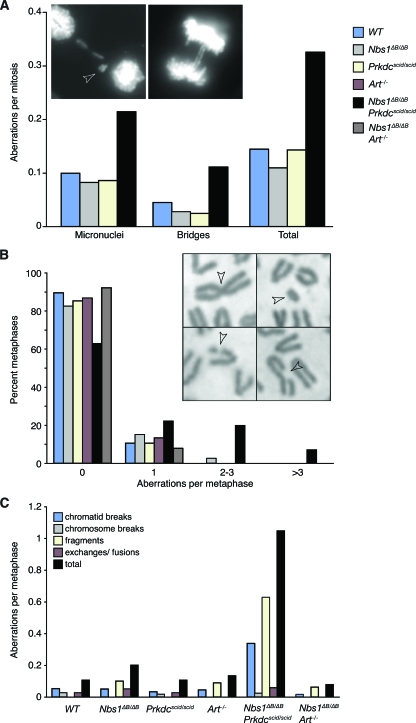
As cell cultures from Nbs1ΔB/ΔB Prkdcscid/scid embryos and ears were highly sensitive to IR and exhibited severe growth defects, we analyzed genomic stability in cell cultures derived from single and double mutants. Early-passage Nbs1ΔB/ΔB Prkdcscid/scid MEF cultures exhibited higher frequencies of chromosome bridges and micronuclei than any of the other lines tested (Fig. 3A); WT, Nbs1ΔB/ΔB, and Prkdcscid/scid MEFs each displayed low percentages of mitotic aberrations (Fig. 3A).
FIG. 3.
Genomic instability in fibroblast cultures. (A) Increased mitotic aberrations in DAPI-stained Nbs1ΔB/ΔB Prkdcscid/scid MEFs were observed. Examples of the aberrations scored, the micronucleus (left; arrowhead) and chromosome bridges (right), are shown. (B) Metaphase aberrations in early-passage (p3 to p5) EFs of the indicated genotypes were scored. Nbs1ΔB/ΔB Prkdcscid/scid double mutants showed increased spontaneous aberrations compared to other mutants, which were indistinguishable from the WT. Examples of a chromosome fusion (top left), a chromosome fragment (top right), and two chromatid breaks (bottom left and right) are shown and are indicated by arrowheads. (C) Aberrations in Nbs1ΔB/ΔB Prkdcscid/scid cultures were primarily chromatid breaks and fragments. Similar results were obtained in analyses of MEF cultures (see Fig. S1 in the supplemental material).
To further characterize the nature of the genomic instability, we analyzed aberrations in metaphase chromosome spreads of early-passage EF and MEF cultures. Chromosomal aberrations were evident in less than 20% of cells in WT, Nbs1ΔB/ΔB, and Prkdcscid/scid MEF and EF cultures, whereas more than 35 to 50% of Nbs1ΔB/ΔB Prkdcscid/scid cells exhibited spontaneous aberrations that were commonly manifested as chromatid breaks (Fig. 3B; see also Fig. S1 in the supplemental material), indicating that they arose during or after S phase.
Defects in cell growth, enhanced IR sensitivity, and increased chromosomal instability in Nbs1ΔB/ΔB Prkdcscid/scid cultures were not due to the loss of Artemis function, as Nbs1ΔB/ΔB Art−/− and single-mutant cell cultures grew at similar rates (see Fig. S2 in the supplemental material) and Nbs1ΔB/ΔB Art−/− cultures did not display increased chromosomal aberrations or IR sensitivity compared to single-mutant cultures (Fig. 3B; see Fig. S3 in the supplemental material) compared to WT cell cultures. These data indicate that DNA-PKcs plays an Artemis-independent role in the maintenance of chromosomal stability that is crucial in the context of impaired S- and G2-phase DSB signaling.
Influence of Nbs1 hypomorphism and DNA-PKcs deficiency on NHEJ.
Both the Mre11 complex and DNA-PK have been implicated in the NHEJ pathway of DSB repair. A potential explanation for the phenotypic synergy we observed between the Prkdcscid and Nbs1ΔB alleles was that NHEJ was more severely affected in Nbs1ΔB/ΔB Prkdcscid/scid double mutants than in single mutants. To address this possibility, we analyzed the end joining of fragments created by restriction enzyme- or RAG-induced breaks in plasmid substrates. WT, Nbs1ΔB/ΔB, Prkdcscid/scid, and Nbs1ΔB/ΔB Prkdcscid/scid cell cultures (see Fig. S4 in the supplemental material) were able to repair complementary or noncomplementary ends generated by restriction enzymes, and no reduction in the fidelity of joining was observed in any case (see Table S2 in the supplemental material). Nbs1ΔB/ΔB Prkdcscid/scid cells also retained the ability to rejoin fragments produced by RAG genes, as signal joints formed in Nbs1ΔB/ΔB Prkdcscid/scid cell cultures (L. Deriano, T. H. Stracker, A. Baker, J. H. J. Petrini, and D. B. Roth, submitted for publication).
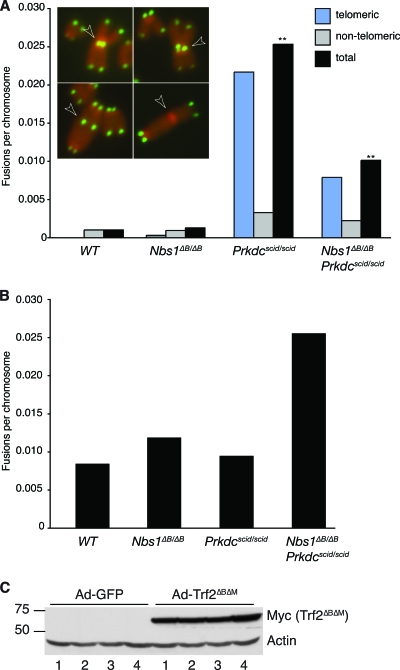
Prkdcscid/scid and Prkdc−/− fibroblasts have been reported previously to exhibit spontaneous leading-strand-specific telomere fusions that become more evident when cells are immortalized (1, 2, 18, 21). As we did not observe increased fusions in early-passage (p2 to p3) Prkdcscid/scid MEF or EF cultures (Fig. 3C; see also Fig. S1 in the supplemental material), we assessed chromosomal aberrations in SV40-immortalized cultures by using telomere-specific FISH. Whereas neither WT nor Nbs1ΔB/ΔB cultures showed an increase in fusions, a strong increase in multiple Prkdcscid/scid cultures was observed, with most metaphases harboring one or two telomere fusions (i.e., fusions that contain a telomeric FISH signal) (Fig. 4A). This phenotype was not due to impaired Artemis activity, as increased fusions were not observed in multiple primary or transformed Art−/− cultures (Fig. 3C and data not shown).
FIG. 4.
(A) Analysis of spontaneous chromosome fusions in SV40-transformed cultures of the indicated genotypes by telomere-specific FISH. Examples of chromosome fusions with telomeric sequence (top left and bottom left), a chromatid fusion involving telomeric sequence (top right), and a chromosome fusion without telomeric sequence (bottom right) are shown and are indicated by arrowheads. The total fusions per chromosome are shown and are further classified as telomeric or nontelomeric. Increased spontaneous fusions involving telomere sequence were observed in transformed Prkdcscid/scid MEFs. Increased fusions in Nbs1ΔB/ΔB Prkdcscid/scid cultures were also observed, but their frequency was significantly reduced compared to that in Prkdcscid/scid single mutants (**, P = 2.5e−9; Wilcoxon rank sum test). DAPI banding data indicating that fusions were nonclonal are presented in Table S3 in the supplemental material. (B) Telomeric fusions induced by Trf2ΔBΔM expression. Similar numbers of fusions were induced after Trf2ΔBΔM expression regardless of the genotype. Fusion numbers were normalized to those in cultures infected with Ad-GFP to account for spontaneous fusion levels. (C) Western blot analysis of the expression of Myc-tagged Trf2ΔBΔM in Ad-GFP- or Trf2ΔBΔM-expressing adenovirus (Ad-Trf2ΔBΔM)-infected MEFs. Lanes: 1, WT; 2, Nbs1ΔB/ΔB; 3, Prkdcscid/scid; and 4, Nbs1ΔB/ΔB Prkdcscid/scid. Actin was included as a control for protein loading.
Spontaneous fusions were observed more frequently in transformed Nbs1ΔB/ΔB Prkdcscid/scid cells than in WT or Nbs1ΔB/ΔB cells, but Nbs1ΔB/ΔB Prkdcscid/scid cultures showed a significant decrease in chromosome fusions compared to Prkdcscid/scid cultures (Fig. 4A and data not shown). To determine if the difference in the number of fusions we observed between Prkdcscid/scid and Nbs1ΔB/ΔB Prkdcscid/scid cell cultures reflected clonal events that had arisen prior to transformation, as opposed to an ongoing fusion process, we performed DAPI banding to identify the chromosomes involved. Chromosome fusions in Prkdcscid/scid cultures were nonclonal, indicating that chromosome breakage and rejoining were continually occurring in cultured cells (see Table S3 in the supplemental material).
We examined the ability of double-mutant cells to generate chromosome fusions by an alternative means. The expression of a dominant negative form of Trf2, Trf2ΔBΔM, compromises the shelterin complex that protects chromosome ends and results in chromosomal fusions dependent upon the NHEJ factors ligase 4 and Ku70 (7, 44). The expression of Trf2ΔBΔM from an adenovirus vector resulted in chromosomal fusions in WT, Nbs1ΔB/ΔB, Prkdcscid/scid, and Nbs1ΔB/ΔB Prkdcscid/scid SV40-transformed cell cultures (Fig. 4B). Although increased numbers of fusions were observed in Nbs1ΔB/ΔB Prkdcscid/scid MEFs compared to those in WT and single-mutant cells, this increase is not likely to reflect an increased repair capacity of double-mutant cells. Rather, it more likely reflects the fact that Nbs1ΔB/ΔB Prkdcscid/scid cells were more permissive of transduction with adenovirus vectors than the other cell types, resulting in increased Trf2ΔBΔM expression (Fig. 4C) and thereby increased numbers of uncapped telomeres. These data indicated that NHEJ functions required for the repair of nonhairpin-capped ends were largely intact in single-mutant or Nbs1ΔB/ΔB Prkdcscid/scid double-mutant backgrounds. The effect of the Nbs1ΔB allele on spontaneous telomere fusions that arise in DNA-PKcs-deficient cells may suggest a subtle influence of the Mre11 complex hypomorphism on some aspects of NHEJ.
Cell cycle checkpoint regulation.
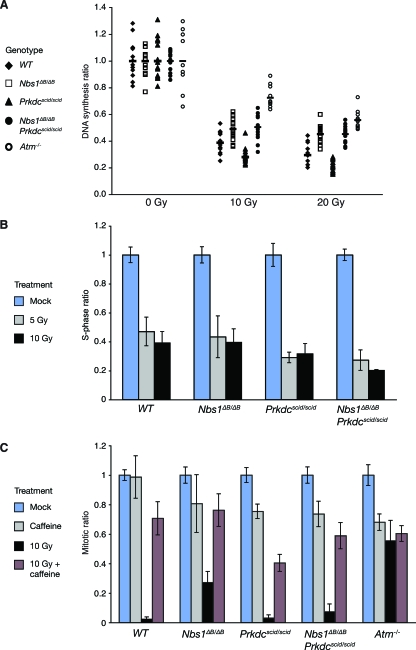
We have shown previously that cells from both Mre11ATLD1/ATLD1 and Nbs1ΔB/ΔB mutants exhibit intra-S-phase and G2/M cell cycle defects (49, 52). Checkpoint deficiency was not seen in Prkdcscid/scid mice. In fact, DNA-PKcs deficiency led to a more robust intra-S-phase checkpoint arrest than that in WT cells, as Prkdcscid/scid MEFs showed a 79% reduction in DNA synthesis after treatment with 20 Gy, compared to the 70% reduction in DNA synthesis in WT MEFs. The defective intra-S-phase checkpoint responses characteristic of Nbs1ΔB/ΔB or Smc12SA/2SA cell cultures were not influenced by the Prkdcscid allele in Nbs1ΔB/ΔB Prkdcscid/scid (Fig. 5A) or Smc12SA/2SA Prkdcscid/scid (data not shown) MEFs, indicating that enhanced S-phase arrest in Prkdcscid/scid MEFs is dependent upon Nbs1 and SMC1. As both Nbs1 and SMC1 are regulated by ATM during the imposition of this checkpoint, these data imply that DNA-PKcs may antagonize ATM enforcement of the intra-S-phase checkpoint.
FIG. 5.
Influence of DNA-PKcs on checkpoint induction. (A) The intra-S-phase checkpoint defect of Nbs1ΔB/ΔB mutants was indistinguishable from that of Nbs1ΔB/ΔB Prkdcscid/scid double mutants. Prkdcscid/scid MEFs showed significantly stronger arrest than WT MEFs (Wilcoxon rank sum test; at 10 Gy, P = 3.18e−2 [two sided], and at 20 Gy, P = 2.98e−5 [two sided]). Atm−/− cultures are shown for comparison. (B) G1/S checkpoint responses in early-passage MEFs. Cultures were irradiated with 5 or 10 Gy, and DNA synthesis levels were normalized to those in untreated cultures. Both Prkdcscid/scid and Nbs1ΔB/ΔB Prkdcscid/scid MEFs showed stronger G1/S checkpoint arrest than WT MEFs. Representative flow cytometry data are included in Fig. S5 in the supplemental material. (C) Deficient G2/M checkpoint arrest in Nbs1ΔB/ΔB mutants was rescued by the Prkdcscid/scid mutation. MEFs of the indicated genotypes were mock treated, incubated with 10 mM caffeine, exposed to 10 Gy of IR, or exposed to 10 Gy IR and treated with 10 mM caffeine, and their mitotic indices were determined 1 h posttreatment. Treatment with caffeine abolished the checkpoint response in all backgrounds. Representative flow cytometry data are included in Fig. S6 in the supplemental material.
Similar to the intra-S-phase checkpoint responses we observed, a more robust G1/S checkpoint response than WT has been reported in Prkdcscid/scid and Prkdc−/− MEFs (25). Early-passage Nbs1ΔB/ΔB MEF cultures exhibited normal G1/S-phase checkpoint arrest, showing a 57% reduction in bromodeoxyuridine (BrdU)-positive cells following exposure to 5 Gy of IR, comparable to the 54% reduction in BrdU-positive cells in WT cultures. The checkpoint responses in both Prkdcscid/scid and Nbs1ΔB/ΔB Prkdcscid/scid cultures were more pronounced than those in WT and Nbs1ΔB/ΔB cultures (Fig. 5B; see also Fig. S5 in the supplemental material), as these cultures showed 71 and 73% reductions in BrdU-positive cells, respectively, after IR exposure.
The G2/M checkpoint is dependent upon ATM and was defective in Nbs1ΔB/ΔB MEFs (Fig. 5C) (52, 55), whereas the G2/M checkpoint in WT and Prkdcscid/scid cells was normal. DNA-PKcs deficiency abolished the G2/M defect conferred by the Nbs1ΔB allele; Nbs1ΔB/ΔB Prkdcscid/scid MEFs and WT MEFs arrested to similar degrees (Fig. 5C; see also Fig. S6 in the supplemental material). Checkpoint arrest in Nbs1ΔB/ΔB Prkdcscid/scid cells correlated with a decrease in the activity of cyclin B-associated kinase activity (see Fig. S7 in the supplemental material). The initiation of G2/M checkpoint arrest is controlled by the activation of the ATM/ATR kinases, and the treatment of cells with checkpoint inhibitors, such as caffeine, blocks G2/M arrest. To determine whether checkpoint activation in Nbs1ΔB/ΔB Prkdcscid/scid MEFs was still responsive to caffeine, we treated cells with 10 mM caffeine to block ATM/ATR activity (Fig. 5C). In all cases, checkpoint induction was impaired, suggesting that the elimination of the Nbs1ΔB/ΔB defect by the Prkdcscid/scid mutation was due to enhanced signaling through caffeine-sensitive ATM/ATR pathways. These data suggest that DNA-PKcs exerts an inhibitory effect on the DNA damage response in S and G2, such that ATM and ATR-dependent checkpoint functions are more robust in DNA-PKcs-deficient cells than in WT cells.
DISCUSSION
The ATM arm of the DNA damage response influences recombinational DNA repair, DNA damage-dependent checkpoints, and apoptosis. Despite these broad influences, ATM is dispensable for organism and cell viability. However, ATM functions become essential for survival in the context of DNA-PKcs deficiency. In this study, we examined the mechanistic basis of this genetic interaction in Prkdcscid/scid mice. Mice expressing hypomorphic Mre11ATLD1 and Nbs1ΔB alleles, which partially phenocopy the Atm−/− phenotype, were used as proxies for animals with ATM deficiency. The Mre11ATLD1 and Nbs1ΔB alleles attenuate the intra-S-phase and G2/M checkpoints and are associated with various degrees of impaired ATM activity, the Nbs1ΔB phenotype being less severe than the Mre11ATLD1 phenotype (48).
Like Atm−/− Prkdcscid/scid mice, Mre11ATLD1/ATLD1 Prkdcscid/scid embryos were nonviable (Table 1). However, viable Nbs1ΔB/ΔB Prkdcscid/scid pups were recovered at roughly 5% of the predicted Mendelian ratio. Consistent with this reduction in embryonic viability, Nbs1ΔB/ΔB Prkdcscid/scid mice exhibited severe defects. The cellular phenotypes were similarly severe, with double-mutant MEFs exhibiting premature senescence accompanied by extensive chromatid instability, anaphase bridges, micronuclei, and hypersensitivity to γ-irradiation.
Genetic analysis.
The results of intercrosses between mice expressing the Prkdcscid and Smc12SA (Fig. 1C), Nbs1ΔC (Fig. 1C), or Chk2 null (46) alleles suggested that deficiencies in the intra-S-phase checkpoint and apoptosis are not major contributors to the synthetic lethality. It remains possible that the G2/M checkpoint or repair defects of the Nbs1ΔB phenotype or some combination of phenotypes underlies the severe genetic interaction with the Prkdcscid mutation.
Biochemical and genetic evidence suggests that the regulation of Artemis endonuclease activity is dependent on DNA-PKcs (20, 33, 41). The loss of Artemis in Nbs1ΔB/ΔB cell cultures did not cause increased sensitivity to IR (see Fig. S3 in the supplemental material), chromosomal aberrations (Fig. 3B), or cell growth defects (see Fig. S2 in the supplemental material). The birth of Art−/− Nbs1ΔB/ΔB and Atm−/− Art−/− mice at expected Mendelian ratios (Fig. 1C) (40) indicates that impaired Artemis function does not underlie the severe genetic interaction between DNA-PKcs and Mre11 complex hypomorphism (or ATM deficiency). This outcome also indicates that functions of DNA-PKcs in chromosome metabolism are not limited to Artemis regulation.
DSB repair and embryonic lethality.
The loss of Atm or Trp53 in a Lig4 or XRCC4 null background abolishes late embryonic lethality and neuronal apoptosis (15, 31, 42). The lack of synthetic lethality in Atm−/− Lig4−/− double mutants and the inability of p53 deficiency to rescue Atm−/− Prkdcscid/scid or Nbs1ΔB/ΔB Prkdcscid/scid embryos (Fig. 1C) indicate that deficient NHEJ is unlikely to underlie the severe genetic interaction between impaired ATM signaling and DNA-PKcs deficiency (19). Consistent with this, developmental analysis of Atm−/− Prkdcscid/scid and Lig4−/− embryos indicates that patterns of cell death in these backgrounds are temporally and mechanistically distinct (15, 19, 31).
Mice with a p53 null background that are doubly deficient in the homologous recombination (HR) factor Rad54 and either ligase IV or the DNA-PK holoenzyme component Ku80 are viable (9, 37). Similar to Nbs1ΔB/ΔB Prkdcscid/scid mice, these animals are born at a reduced frequency, are severely runted, and show increased IR sensitivity and high levels of chromatid breakage. As the Mre11 complex is involved in HR-mediated DSB repair, another explanation for the synthetic phenotype of Nbs1ΔB/ΔB Prkdcscid/scid mutants is the simultaneous impairment of HR and NHEJ. The inability of p53 deficiency to influence the survival of Nbs1ΔB/ΔB Prkdcscid/scid or Atm−/− Prkdcscid/scid animals, as well as the absence of increased chromosome breaks, which are observed in Rad54−/− Lig4−/− mice, makes this explanation unlikely (19, 37). This view is supported by the fact that embryonic lethality and severe cellular phenotypes were not observed previously in Rad54−/− Prkdcscid/scid mice or cell cultures (13). However, it is likely that the repair deficiencies of the Nbs1ΔB/ΔB phenotype are more severe than those of the Rad54−/− phenotype. The loss of Rad54 confers a mild HR defect on embryonic stem cells but not on MEFs, and Rad54−/− MEFs are not sensitive to IR (13). In contrast, Nbs1ΔB/ΔB MEFs are highly sensitive to IR and CPT, due potentially to checkpoint defects or problems in the HR process itself, in which the Mre11 complex plays important roles in end resection and molecular bridging (48, 52). It is therefore possible that DNA-PKcs-deficient cells can tolerate a certain level of HR deficiency that is exceeded by the hypomorphic Nbs1 mutation.
Influence of DNA-PKcs on checkpoint regulation.
The elimination of Nbs1ΔB checkpoint deficiency in G2/M, as well as the more robust induction of the G1/S and intra-S-phase checkpoints, in Prkdcscid/scid cell cultures suggests that DNA-PKcs negatively influences ATM/ATR-mediated checkpoint induction throughout the cell cycle. Both the S-phase and G2/M checkpoints in all genetic backgrounds were inhibited by caffeine, indicating that G2/M checkpoint initiation in Nbs1ΔB/ΔB Prkdcscid/scid cultures was dependent upon ATM/ATR pathways that would normally be inhibited by DNA-PKcs. To a large extent, the novel checkpoint functions of DNA-PKcs elucidated here would not have been evident in other studies: inquiries regarding the checkpoint role of DNA-PKcs were designed to assess a positive role in checkpoint activation rather than an inhibitory one.
In this regard, it is important to note that DNA repair per se can be viewed as an inhibitor of checkpoint induction; hence, the phenotypic synergy observed with the Nbs1ΔB/ΔB mutation may indicate that the DNA repair functions of DNA-PKcs are manifested in S-phase cells. Certain results make this possibility unappealing. For example, Prkdcscid/scid cells are not sensitive to S-phase-specific damaging agents such as CPT (Fig. 2D), nor do Nbs1ΔB/ΔB Prkdcscid/scid cells exhibit greater sensitivity than Nbs1ΔB/ΔB cells. Similarly, the end joining of uncapped telomeres or DSB fragments generated by restriction enzymes is not further impaired in Nbs1ΔB/ΔB Prkdcscid/scid cultures (Fig. 4B; see Fig. S4 and Table S2 in the supplemental material). If the rescue of the Nbs1ΔB/ΔB G2/M checkpoint by the Prkdcscid/scid mutation was attributable to impaired DSB repair associated with DNA-PKcs deficiency, subjecting Nbs1ΔB/ΔB cells to higher numbers of DSBs should produce a phenocopy of the double-mutant phenotype. No elimination of the Nbs1ΔB/ΔB checkpoint defects was evident at IR doses as high as 40 Gy (data not shown). Together, these data provide evidence for an unexpected role for DNA-PKcs in modulating ATM/ATR checkpoint signaling during S and G2.
Artemis- and NHEJ-independent role for DNA-PKcs in chromosomal stability.
The cellular functions of DNA-PKcs, the in vivo targets of its kinase activity, and the extent of its role in DNA repair remain largely unclear. The phenotypes of DNA-PKcs-deficient cell lines and animals are inconsistent with its playing an essential role in the end joining of fragments from clean DSBs, and DNA-PKcs-deficient cells show IR sensitivity that is intermediate between that of sensitive Lig4-deficient cells and that of mildly sensitive Artemis-deficient lines (39). Studies using DT40 knockout lines demonstrated that the IR sensitivity of DNA-PKcs null cell lines is highest in G1 and early S phases, suggesting a link between IR sensitivity and G1 repair deficiency (16). Concurrently, the levels of DNA-PKcs kinase activity and autophosphorylation, which is required for IR resistance, are high in G1 and low in S phase (8, 30). Finally, the loss of DNA-PKcs in embryonic stem cells, which cycle between S and M and rely primarily on HR, does not result in radiosensitivity (17).
Considering that the major functions of DNA-PKcs in radioresistance, Artemis regulation, and NHEJ-mediated repair are restricted largely to G1, the severe impact that DNA-PKcs deficiency had on embryonic development and chromosomal stability in the presence of hypomorphic Mre11 complex alleles was unexpected. Our data suggest that DNA-PKcs plays a regulatory role in maintaining chromosomal stability in S/G2 phase when ATM-Mre11 complex signaling is compromised. Further, this function is independent of the previously described roles of DNA-PKcs in DSB repair.
Although the G2/M damage response was enhanced in Nbs1ΔB/ΔB Prkdcscid/scid cell cultures compared to Nbs1ΔB/ΔB cell cultures, we observed increased levels of mitotic aberrations, such as chromosome bridges, as well as increased levels of spontaneous metaphase aberrations, including chromatid breaks and fragments, in Nbs1ΔB/ΔB Prkdcscid/scid cultures. These findings indicated that cells that escaped G2 arrest and entered mitosis harbored extensive DNA damage. We propose that DNA-PKcs opposes the functions of ATM/ATR in the regulation of cell cycle progression through a direct or indirect influence on cyclin-dependent kinase activity. The simultaneous impairment of ATM signaling (directly or indirectly via Mre11 complex mutations) and DNA-PKcs function upsets this regulatory balance, leading to cytotoxic/static chromosomal damage and the attrition of cells through the activation of p53-independent senescence or cell death pathways in Nbs1ΔB/ΔB Prkdcscid/scid cells. Our results suggest that the simultaneous targeting of ATM-Mre11 complex and DNA-PKcs signaling pathways is highly cytotoxic, even in the absence of p53 function, and may represent a viable strategy for chemotherapeutic approaches.
Supplementary Material
Acknowledgments
We thank Annalee Baker and Hussein Hussein for diligent maintenance of our animal colony; Titia de Lange, Andy Koff, and members of the Petrini and de Lange labs for helpful advice and discussions; and Rob Fisher and Claire Attwooll for critical reading of the manuscript.
T.H.S. was supported by an NIH National Research Service Award and is a Leukemia and Lymphoma Society special fellow, L.D. is a Leukemia and Lymphoma Society fellow, D.B.R. is supported by grants from the NIH, and J.H.J.P. is supported by NIH grants and the Jean and Joel Smilow Initiative.
Footnotes
Published ahead of print on 17 November 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bailey, S. M., M. N. Cornforth, A. Kurimasa, D. J. Chen, and E. H. Goodwin. 2001. Strand-specific postreplicative processing of mammalian telomeres. Science 2932462-2465. [DOI] [PubMed] [Google Scholar]
- 2.Bailey, S. M., J. Meyne, D. J. Chen, A. Kurimasa, G. C. Li, B. E. Lehnert, and E. H. Goodwin. 1999. DNA double-strand break repair proteins are required to cap the ends of mammalian chromosomes. Proc. Natl. Acad. Sci. USA 9614899-14904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bressan, D. A., B. K. Baxter, and J. H. Petrini. 1999. The Mre11-Rad50-Xrs2 protein complex facilitates homologous recombination-based double-strand break repair in Saccharomyces cerevisiae. Mol. Cell. Biol. 197681-7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buscemi, G., C. Savio, L. Zannini, F. Micciche, D. Masnada, M. Nakanishi, H. Tauchi, K. Komatsu, S. Mizutani, K. Khanna, P. Chen, P. Concannon, L. Chessa, and D. Delia. 2001. Chk2 activation dependence on Nbs1 after DNA damage. Mol. Cell. Biol. 215214-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carney, J. P., R. S. Maser, H. Olivares, E. M. Davis, M. Le Beau, J. R. Yates III, L. Hays, W. F. Morgan, and J. H. Petrini. 1998. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell 93477-486. [DOI] [PubMed] [Google Scholar]
- 6.Carson, C. T., R. A. Schwartz, T. H. Stracker, C. E. Lilley, D. V. Lee, and M. D. Weitzman. 2003. The Mre11 complex is required for ATM activation and the G(2)/M checkpoint. EMBO J. 226610-6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celli, G. B., E. L. Denchi, and T. de Lange. 2006. Ku70 stimulates fusion of dysfunctional telomeres yet protects chromosome ends from homologous recombination. Nat. Cell Biol. 8885-890. [DOI] [PubMed] [Google Scholar]
- 8.Chen, B. P., D. W. Chan, J. Kobayashi, S. Burma, A. Asaithamby, K. Morotomi-Yano, E. Botvinick, J. Qin, and D. J. Chen. 2005. Cell cycle dependence of DNA-dependent protein kinase phosphorylation in response to DNA double strand breaks. J. Biol. Chem. 28014709-14715. [DOI] [PubMed] [Google Scholar]
- 9.Couedel, C., K. D. Mills, M. Barchi, L. Shen, A. Olshen, R. D. Johnson, A. Nussenzweig, J. Essers, R. Kanaar, G. C. Li, F. W. Alt, and M. Jasin. 2004. Collaboration of homologous recombination and nonhomologous end-joining factors for the survival and integrity of mice and cells. Genes Dev. 181293-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jager, M., J. van Noort, D. C. van Gent, C. Dekker, R. Kanaar, and C. Wyman. 2001. Human Rad50/Mre11 is a flexible complex that can tether DNA ends. Mol. Cell 81129-1135. [DOI] [PubMed] [Google Scholar]
- 11.Difilippantonio, S., A. Celeste, M. J. Kruhlak, Y. Lee, M. J. Difilippantonio, L. Feigenbaum, S. P. Jackson, P. J. McKinnon, and A. Nussenzweig. 2007. Distinct domains in Nbs1 regulate irradiation-induced checkpoints and apoptosis. J. Exp. Med. 2041003-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Virgilio, M., and J. Gautier. 2005. Repair of double-strand breaks by nonhomologous end joining in the absence of Mre11. J. Cell Biol. 171765-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Essers, J., H. van Steeg, J. de Wit, S. M. Swagemakers, M. Vermeij, J. H. Hoeijmakers, and R. Kanaar. 2000. Homologous and non-homologous recombination differentially affect DNA damage repair in mice. EMBO J. 191703-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falck, J., J. Coates, and S. P. Jackson. 2005. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature 434605-611. [DOI] [PubMed] [Google Scholar]
- 15.Frank, K. M., N. E. Sharpless, Y. Gao, J. M. Sekiguchi, D. O. Ferguson, C. Zhu, J. P. Manis, J. Horner, R. A. DePinho, and F. W. Alt. 2000. DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Mol. Cell 5993-1002. [DOI] [PubMed] [Google Scholar]
- 16.Fukushima, T., M. Takata, C. Morrison, R. Araki, A. Fujimori, M. Abe, K. Tatsumi, M. Jasin, P. K. Dhar, E. Sonoda, T. Chiba, and S. Takeda. 2001. Genetic analysis of the DNA-dependent protein kinase reveals an inhibitory role of Ku in late S-G2 phase DNA double-strand break repair. J. Biol. Chem. 27644413-44418. [DOI] [PubMed] [Google Scholar]
- 17.Gao, Y., J. Chaudhuri, C. Zhu, L. Davidson, D. T. Weaver, and F. W. Alt. 1998. A targeted DNA-PKcs-null mutation reveals DNA-PK-independent functions for KU in V(D)J recombination. Immunity 9367-376. [DOI] [PubMed] [Google Scholar]
- 18.Gilley, D., H. Tanaka, M. P. Hande, A. Kurimasa, G. C. Li, M. Oshimura, and D. J. Chen. 2001. DNA-PKcs is critical for telomere capping. Proc. Natl. Acad. Sci. USA 9815084-15088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gladdy, R. A., L. M. Nutter, T. Kunath, J. S. Danska, and C. J. Guidos. 2006. p53-independent apoptosis disrupts early organogenesis in embryos lacking both ataxia-telangiectasia mutated and Prkdc. Mol. Cancer Res. 4311-318. [DOI] [PubMed] [Google Scholar]
- 20.Goodarzi, A. A., Y. Yu, E. Riballo, P. Douglas, S. A. Walker, R. Ye, C. Harer, C. Marchetti, N. Morrice, P. A. Jeggo, and S. P. Lees-Miller. 2006. DNA-PK autophosphorylation facilitates Artemis endonuclease activity. EMBO J. 253880-3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goytisolo, F. A., E. Samper, S. Edmonson, G. E. Taccioli, and M. A. Blasco. 2001. The absence of the DNA-dependent protein kinase catalytic subunit in mice results in anaphase bridges and in increased telomeric fusions with normal telomere length and G-strand overhang. Mol. Cell. Biol. 213642-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurley, K. E., and C. J. Kemp. 2001. Synthetic lethality between mutation in Atm and DNA-PK(cs) during murine embryogenesis. Curr. Biol. 11191-194. [DOI] [PubMed] [Google Scholar]
- 23.Hopfner, K. P., L. Craig, G. Moncalian, R. A. Zinkel, T. Usui, B. A. Owen, A. Karcher, B. Henderson, J. L. Bodmer, C. T. McMurray, J. P. Carney, J. H. Petrini, and J. A. Tainer. 2002. The Rad50 zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair. Nature 418562-566. [DOI] [PubMed] [Google Scholar]
- 24.Ivanov, E. L., V. G. Korolev, and F. Fabre. 1992. XRS2, a DNA repair gene of Saccharomyces cerevisiae, is needed for meiotic recombination. Genetics 132651-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jimenez, G. S., F. Bryntesson, M. I. Torres-Arzayus, A. Priestley, M. Beeche, S. Saito, K. Sakaguchi, E. Appella, P. A. Jeggo, G. E. Taccioli, G. M. Wahl, and M. Hubank. 1999. DNA-dependent protein kinase is not required for the p53-dependent response to DNA damage. Nature 40081-83. [DOI] [PubMed] [Google Scholar]
- 26.Kim, S.-T., B. Xu, and M. B. Kastan. 2002. Involvement of the cohesin protein Smc1, in Atm-dependent and independent responses to DNA damage. Genes Dev. 16560-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitagawa, R., C. J. Bakkenist, P. J. McKinnon, and M. B. Kastan. 2004. Phosphorylation of SMC1 is a critical downstream event in the ATM-NBS1-BRCA1 pathway. Genes Dev. 181423-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lansdorp, P. M., N. P. Verwoerd, F. M. van de Rijke, V. Dragowska, M. T. Little, R. W. Dirks, A. K. Raap, and H. J. Tanke. 1996. Heterogeneity in telomere length of human chromosomes. Hum. Mol. Genet. 5685-691. [DOI] [PubMed] [Google Scholar]
- 29.Lee, J. H., and T. T. Paull. 2007. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene 267741-7748. [DOI] [PubMed] [Google Scholar]
- 30.Lee, S. E., R. A. Mitchell, A. Chieng, and E. A. Hendrickson. 1997. Evidence for DNA-PK-dependent and -independent DNA double-strand break repair pathways in mammalian cells as a function of the cell cycle. Mol. Cell. Biol. 171425-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee, Y., D. E. Barnes, T. Lindahl, and P. J. McKinnon. 2000. Defective neurogenesis resulting from DNA ligase IV deficiency requires Atm. Genes Dev. 142576-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim, D. S., S. T. Kim, B. Xu, R. S. Maser, J. Lin, J. H. Petrini, and M. B. Kastan. 2000. ATM phosphorylates p95/nbs1 in an S-phase checkpoint pathway. Nature 404613-617. [DOI] [PubMed] [Google Scholar]
- 33.Ma, Y., U. Pannicke, K. Schwarz, and M. R. Lieber. 2002. Hairpin opening and overhang processing by an Artemis/DNA-dependent protein kinase complex in nonhomologous end joining and V(D)J recombination. Cell 108781-794. [DOI] [PubMed] [Google Scholar]
- 34.Manolis, K. G., E. R. Nimmo, E. Hartsuiker, A. M. Carr, P. A. Jeggo, and R. C. Allshire. 2001. Novel functional requirements for non-homologous DNA end joining in Schizosaccharomyces pombe. EMBO J. 20210-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maser, R. S., O. K. Mirzoeva, J. Wells, H. Olivares, B. R. Williams, R. Zinkel, P. J. Farnham, and J. H. J. Petrini. 2001. The MRE11 complex and DNA replication: linkage to E2F and sites of DNA synthesis. Mol. Cell. Biol. 216006-6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuoka, S., B. A. Ballif, A. Smogorzewska, E. R. McDonald III, K. E. Hurov, J. Luo, C. E. Bakalarski, Z. Zhao, N. Solimini, Y. Lerenthal, Y. Shiloh, S. P. Gygi, and S. J. Elledge. 2007. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 3161160-1166. [DOI] [PubMed] [Google Scholar]
- 37.Mills, K. D., D. O. Ferguson, J. Essers, M. Eckersdorff, R. Kanaar, and F. W. Alt. 2004. Rad54 and DNA ligase IV cooperate to maintain mammalian chromatid stability. Genes Dev. 181283-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mirzoeva, O. K., and J. H. Petrini. 2003. DNA replication-dependent nuclear dynamics of the Mre11 complex. Mol. Cancer Res. 1207-218. [PubMed] [Google Scholar]
- 39.Riballo, E., M. Kuhne, N. Rief, A. Doherty, G. C. Smith, M. J. Recio, C. Reis, K. Dahm, A. Fricke, A. Krempler, A. R. Parker, S. P. Jackson, A. Gennery, P. A. Jeggo, and M. Lobrich. 2004. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-H2AX foci. Mol. Cell 16715-724. [DOI] [PubMed] [Google Scholar]
- 40.Rooney, S., F. W. Alt, J. Sekiguchi, and J. P. Manis. 2005. Artemis-independent functions of DNA-dependent protein kinase in Ig heavy chain class switch recombination and development. Proc. Natl. Acad. Sci. USA 1022471-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rooney, S., J. Sekiguchi, C. Zhu, H. L. Cheng, J. Manis, S. Whitlow, J. DeVido, D. Foy, J. Chaudhuri, D. Lombard, and F. W. Alt. 2002. Leaky Scid phenotype associated with defective V(D)J coding end processing in Artemis-deficient mice. Mol. Cell 101379-1390. [DOI] [PubMed] [Google Scholar]
- 42.Sekiguchi, J., D. O. Ferguson, H. T. Chen, E. M. Yang, J. Earle, K. Frank, S. Whitlow, Y. Gu, Y. Xu, A. Nussenzweig, and F. W. Alt. 2001. Genetic interactions between ATM and the nonhomologous end-joining factors in genomic stability and development. Proc. Natl. Acad. Sci. USA 983243-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiloh, Y. 2003. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3155-168. [DOI] [PubMed] [Google Scholar]
- 44.Smogorzewska, A., J. Karlseder, H. Holtgreve-Grez, A. Jauch, and T. de Lange. 2002. DNA ligase IV-dependent NHEJ of deprotected mammalian telomeres in G1 and G2. Curr. Biol. 121635-1644. [DOI] [PubMed] [Google Scholar]
- 45.Stewart, G. S., R. S. Maser, T. Stankovic, D. A. Bressan, M. I. Kaplan, N. G. Jaspers, A. Raams, P. J. Byrd, J. H. Petrini, and A. M. Taylor. 1999. The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell 99577-587. [DOI] [PubMed] [Google Scholar]
- 46.Stracker, T. H., S. S. Couto, C. Cordon-Cardo, T. Matos, and J. H. Petrini. 2008. Chk2 suppresses the oncogenic potential of DNA replication-associated DNA damage. Mol. Cell 3121-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stracker, T. H., M. Morales, S. S. Couto, H. Hussein, and J. H. Petrini. 2007. The carboxy terminus of NBS1 is required for induction of apoptosis by the MRE11 complex. Nature 447218-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stracker, T. H., J. W. Theunissen, M. Morales, and J. H. Petrini. 2004. The Mre11 complex and the metabolism of chromosome breaks: the importance of communicating and holding things together. DNA Repair (Amsterdam) 3845-854. [DOI] [PubMed] [Google Scholar]
- 49.Theunissen, J. W., M. I. Kaplan, P. A. Hunt, B. R. Williams, D. O. Ferguson, F. W. Alt, and J. H. Petrini. 2003. Checkpoint failure and chromosomal instability without lymphomagenesis in Mre11(ATLD1/ATLD1) mice. Mol. Cell 121511-1523. [DOI] [PubMed] [Google Scholar]
- 50.Theunissen, J. W., and J. H. Petrini. 2006. Methods for studying the cellular response to DNA damage: influence of the Mre11 complex on chromosome metabolism. Methods Enzymol. 409251-284. [DOI] [PubMed] [Google Scholar]
- 51.Uziel, T., Y. Lerenthal, L. Moyal, Y. Andegeko, L. Mittelman, and Y. Shiloh. 2003. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 225612-5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams, B. R., O. K. Mirzoeva, W. F. Morgan, J. Lin, W. Dunnick, and J. H. Petrini. 2002. A murine model of Nijmegen breakage syndrome. Curr. Biol. 12648-653. [DOI] [PubMed] [Google Scholar]
- 53.Wiltzius, J. J., M. Hohl, J. C. Fleming, and J. H. Petrini. 2005. The Rad50 hook domain is a critical determinant of Mre11 complex functions. Nat. Struct. Mol. Biol. 12403-407. [DOI] [PubMed] [Google Scholar]
- 54.Wu, X., V. Ranganathan, D. S. Weisman, W. F. Heine, D. N. Ciccone, T. B. O'Neill, K. E. Crick, K. A. Pierce, W. S. Lane, G. Rathbun, D. M. Livingston, and D. T. Weaver. 2000. ATM phosphorylation of Nijmegen breakage syndrome protein is required in a DNA damage response. Nature 405477-482. [DOI] [PubMed] [Google Scholar]
- 55.Xu, B., S.-T. Kim, D.-S. Lim, and M. B. Kastan. 2002. Two molecularly distinct G2/M checkpoints are induced by ionizing irradiation. Mol. Cell. Biol. 221049-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamaguchi-Iwai, Y., E. Sonoda, M. S. Sasaki, C. Morrison, T. Haraguchi, Y. Hiraoka, Y. M. Yamashita, T. Yagi, M. Takata, C. Price, N. Kakazu, and S. Takeda. 1999. Mre11 is essential for the maintenance of chromosomal DNA in vertebrate cells. EMBO J. 186619-6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang, Y. G., A. Saidi, P. O. Frappart, W. Min, C. Barrucand, V. Dumon-Jones, J. Michelon, Z. Herceg, and Z. Q. Wang. 2006. Conditional deletion of Nbs1 in murine cells reveals its role in branching repair pathways of DNA double-strand breaks. EMBO J. 255527-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.You, Z., C. Chahwan, J. Bailis, T. Hunter, and P. Russell. 2005. ATM activation and its recruitment to damaged DNA require binding to the C terminus of Nbs1. Mol. Cell. Biol. 255363-5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.