Abstract
The heterotrimeric G protein α subunit (Gα) is targeted to the cytoplasmic face of the plasma membrane through reversible lipid palmitoylation and relays signals from G-protein-coupled receptors (GPCRs) to its effectors. By screening 23 DHHC motif (Asp-His-His-Cys) palmitoyl acyl-transferases, we identified DHHC3 and DHHC7 as Gα palmitoylating enzymes. DHHC3 and DHHC7 robustly palmitoylated Gαq, Gαs, and Gαi2 in HEK293T cells. Knockdown of DHHC3 and DHHC7 decreased Gαq/11 palmitoylation and relocalized it from the plasma membrane into the cytoplasm. Photoconversion analysis revealed that Gαq rapidly shuttles between the plasma membrane and the Golgi apparatus, where DHHC3 specifically localizes. Fluorescence recovery after photobleaching studies showed that DHHC3 and DHHC7 are necessary for this continuous Gαq shuttling. Furthermore, DHHC3 and DHHC7 knockdown blocked the α1A-adrenergic receptor/Gαq/11-mediated signaling pathway. Together, our findings revealed that DHHC3 and DHHC7 regulate GPCR-mediated signal transduction by controlling Gα localization to the plasma membrane.
G-protein-coupled receptors (GPCRs) form the largest family of cell surface receptors, consisting of more than 700 members in humans. GPCRs respond to a variety of extracellular signals, including hormones and neurotransmitters, and are involved in various physiologic processes, such as smooth muscle contraction and synaptic transmission (20, 25). Heterotrimeric G proteins, composed of α, β, and γ subunits, transduce signals from GPCRs to their effectors and play a central role in the GPCR signaling pathway (13, 21, 24, 32). Although the Gα subunit seems to localize stably at the cytosolic face of the plasma membrane (PM), a recent report suggested that Gαo, a Gα isoform, shuttles rapidly between the PM and intracellular membranes (2). The PM targeting of Gα requires both interaction with the Gβγ complex and subsequent lipid palmitoylation of Gα (22). Thus, palmitoylation of Gα is a critical determinant of membrane targeting of the heterotrimer Gαβγ.
Protein palmitoylation is a common posttranslational modification with lipid palmitate and regulates protein trafficking and function (7, 18). Gα is a classic and representative palmitoyl substrate (19, 38), and recent studies revealed that protein palmitoylation modifies virtually almost all the components of G-protein signaling, including GPCRs, Gα subunits, several members of the RGS (regulators of G-protein signaling) family of GTPase-activating proteins, GPCR kinase GRK6, and some small GTPases (7, 33). This common lipid modification plays an important role in compartmentalizing G-protein signaling to the specific microdomain, such as membrane caveolae and lipid raft (26). The palmitoyl thioester bond is relatively labile, and palmitates on substrates turn over rapidly, allowing proteins to shuttle between the cytoplasm/intracellular organelles and the PM (2, 3, 27). For example, binding of isoproterenol to the β-adrenergic receptor markedly accelerates the depalmitoylation of the associated Gαs, shifting Gαs to the cytoplasm (37). This receptor activation-induced depalmitoylation was also observed in a major postsynaptic PSD-95 scaffold, which anchors the AMPA (alpha-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid)-type glutamate receptor at the excitatory postsynapse through stargazin (6). On glutamate receptor activation, accelerated depalmitoylation of PSD-95 dissociates PSD-95 from postsynaptic sites and causes AMPA receptor endocytosis (6). Thus, palmitate turnover on Gαs and PSD-95 is accelerated by receptor activation, contributing to downregulation of the signaling pathway. However, the enzymes that add palmitate to proteins (palmitoyl-acyl transferases [PATs]) and those that cleave the thioester bond (palmitoyl-protein thioesterases) were long elusive.
Recent genetic studies in Saccharomyces cerevisiae identified Erf2/Erf4 (1, 40) and Akr1 (29) as PATs for yeast Ras and yeast casein kinase 2, respectively. Erf2 and Akr1 have four- to six-pass transmembrane domains and share a common domain, referred to as a DHHC domain, a cysteine-rich domain with a conserved Asp-His-His-Cys signature motif. Because the DHHC domain is essential for the PAT activity, we isolated 23 mammalian DHHC domain-containing proteins (DHHC proteins) and developed a systematic screening method to identify the specific enzyme-substrate pairs (11, 12): DHHC2, -3, -7, and -15 for PSD-95 (11); DHHC21 for endothelial NO synthase (10); and DHHC3 and -7 for GABAA receptor γ2 subunit (9). Several other groups also reported that DHHC9 with GCP16 mediates palmitoylation toward H- and N-Ras (36) and that DHHC17, also known as HIP14, palmitoylates several neuronal proteins: huntingtin (14), SNAP-25, and CSP (14, 23, 35). However, the existence of PATs for Gα has been controversial because spontaneous palmitoylation of Gα could occur in vitro (4).
In this study, we screened the 23 DHHC clones to examine which DHHC proteins can palmitoylate Gα. We found that DHHC3 and -7 specifically and robustly palmitoylate Gα at the Golgi apparatus. Inhibition of DHHC3 and -7 reduces Gαq/11 palmitoylation levels and delocalizes it from the PM to the cytoplasm in HeLa cells and primary hippocampal neurons. Also, DHHC3 and -7 are necessary for the continuous Gαq shuttling between the Golgi apparatus and the PM. Finally, blocking DHHC3 and -7 inhibits the α1A-adrenergic receptor [α1A-AR]/Gαq-mediated signaling pathway, indicating that DHHC3 and -7 play an essential role in GPCR signaling by regulating Gα localization.
MATERIALS AND METHODS
Cell culture and transfection.
The drugs used were 2-bromohexadecanoic acid (2-bromopalmitate [2-BP]) (Fluka), cycloheximide (CHX; Sigma), phenylephrine (Sigma), and prazosin (Sigma). For transfection of plasmid DNA and small interfering RNA (siRNA) into HeLa or HEK293T cells, Lipofectamine Plus reagent and Lipofectamine 2000 (Invitrogen) were used, respectively. Cultured hippocampal neurons (2.5 × 104 cells) were seeded onto 12-mm coverslips in 24-well dishes. Neurons (DIV8) were transfected with pCAGGS-mCherry-miR (where mCherry is a fluorescent protein) vectors by Lipofectamine 2000.
Antibodies.
The antibodies used were rabbit polyclonal antibodies to Gαq/11 (Santa Cruz Biotechnology) and GODZ/DHHC3 (Abcam); mouse monoclonal antibodies to β-catenin (BD Biosciences), hemagglutinin ([HA] clone 12CA5 from Roche Applied Science and clone 16B12 from Covance), Lck (Chemicon), and GM130 (BD Biosciences); chicken polyclonal antibody to green fluorescent protein (GFP) (Chemicon); and rabbit monoclonal antibodies to CREB (Cell Signaling) and phospho-CREB Ser133 (pCREB) (Cell Signaling). Rabbit polyclonal antibodies to GFP and moesin were raised against glutathione S-transferase-GFP and glutathione S-transferase-moesin (amino acids 307 to 577), respectively.
Plasmid constructions.
Gαq-tagged with the fluorescent protein Dendra2 (Evrogen) (16) was made by replacing a GFP fragment of Gαq-GFP, which was well characterized (15). Gαq-Dendra2 stimulated phospholipase C in response to α1A-AR activation (data not shown); it was palmitoylated by DHHC3 and -7 (data not shown) and was localized at the PM (Fig. 1A) as effectively as Gαq-GFP. We concluded that Gαq-Dendra2 is functional as endogenous Gαq and Gαq-GFP. Gαi2-GFP was constructed by inserting enhanced GFP (EGFP) with an SGGGGS linker at both the N and C ends between 114A and 115G of Gαi2. pEF-Bos-HA-mouse DHHC (mDHHC) clones were described previously (11). Dendra2-DHHC3 and FLAG-DHHC3 were constructed by subcloning cDNA of DHHC3 into pDendra2-C and pCAGGS-FLAG, respectively. DHHC3 with the mutation C157S [DHHC3(C157S)], DHHC7(C160S), and Gαq with mutations of cysteines 9 and 10 to serine [Gαq (CS)] were generated using site-directed mutagenesis. To mark the Golgi compartment position, EGFP of galactosyl transferase (GalT)-EGFP (16, 34) was replaced with mCherry. α1A-AR (accession number NM013461) was cloned from mouse brain cDNA by reverse transcription-PCR (RT-PCR) and subcloned into pcDNA3.1 with sequence for a FLAG tag at the 5′ end. For knockdown of DHHC2 and -3 in hippocampal neurons, we used an miR-RNA interference (RNAi) system (Invitrogen). The following targeting sequences were used: DHHC2, GGTGAACAATTGTGTTGGATT; and DHHC3, TGAGACGGGAATAGAACAATT. After these oligonucleotides were subcloned into pcDNA6.2-EmGFP-miR (Invitrogen), EmGFP was replaced with mCherry, and the pre-microRNA expression cassette of pcDNA6.2-mCherry-miR was transferred to pCAGGS vector with β-actin promoter. The validity of knockdown vectors was confirmed by specific antibodies (data not shown). EGFP-CAAX, which encodes a polybasic region and CAAX motif of K-Ras, was generated by inserting a synthetic DNA fragment obtained by annealing the sense synthetic nucleotide 5′-GATCCAAGATGAGCAAAGATGGTAAAAAGAAGAAAAAGAAGTCAAAGACAAAGTGTGTAATTATGTAGA-3′ and antisense nucleotide 5′-GATCTCTACATAATTACACACTTTGTCTTTGACTTCTTTTTCTTCTTTTTACCATCTTTGCTCATCTTG-3′ into BamHI of pEGFP-C1. pcDNAI-Gαq-GFP, pcDNAI-Gβ1, and pcDNAI-Gγ2 were gifts of C. A. Berlot (Weis Center for Research) (15). pcDNA3-HA-Gαq, -HA-Gαs, and -EE-Gαi2 were provided by P. B. Wedegaertner (Thomas Jefferson University) (8). The Lck cDNA was provided by A. Weiss (University of California, San Francisco) and was subcloned into pcDNA3.1. pcDNA3-Gαs-GFP and cDNA of mCherry were provided by M. M. Rasenick (University of Illinois at Chicago) (39) and R. Y. Tsien (University of California, San Diego) (31), respectively.
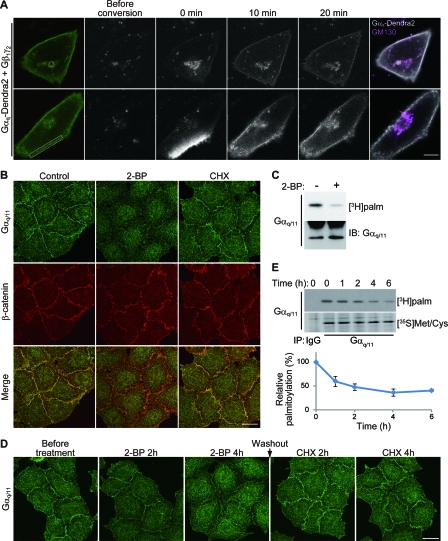
FIG. 1.
Dynamic palmitate turnover on Gαq/11. (A) Gαq shuttles between the PM and the Golgi apparatus. When Gαq-Dendra2, Gβ1, and Gγ2 were coexpressed in HeLa cells, Gαq-Dendra2 (green) was localized at the PM and the endomembrane. Gαq-Dendra2 in the endomembranes (upper) and a part of PM (lower) within the white regions was photoconverted by 405-nm laser. Converted Gαq-Dendra2 (gray scale) was monitored for 20 min. Cells were then immunostained with anti-GM130 (magenta) antibody (right). Scale bar, 10 μm. (B) Inhibition of palmitoylation causes detachment of Gαq/11 from the PM. HeLa cells were treated with 2-BP (100 μM) or CHX (20 μg/ml) for 4 h. The cells were then doubly stained with anti-Gαq/11 (green) and anti-β-catenin (red) antibodies. Scale bar, 20 μm. (C) 2-BP blocks palmitoylation of Gαq/11. HeLa cells were metabolically labeled with [3H]palmitate ([3H]palm) for 4 h either in the presence or absence of 2-BP. Gαq/11 was immunoisolated and subjected to fluorography (upper) or Western blotting (lower). IB, immunoblotting. (D) HeLa cells were treated with 2-BP for 4 h. Then, 2-BP was washed out, and protein synthesis was inhibited by 20 μg/ml CHX for 4 h. The cells were stained with anti-Gαq/11 antibody at the indicated times. The inhibition of palmitoylation for 4 h caused delocalization of Gαq/11 from the PM. The Gαq/11 dispersed by 2-BP came back to the PM again within 2 h after removal of 2-BP in the presence of CHX, indicating that the relocalization of Gαq/11 depends on palmitoylation and depalmitoylation. Scale bar, 20 μm. (E) Pulse-chase analysis of Gαq/11 palmitoylation. HeLa cells were labeled with [3H]palmitate or [35S]methionine-cysteine ([35S]Met/Cys) for 4 h. After incubation with chase medium for 0, 1, 2, 4, and 6 h, cells were lysed and subjected to IP with anti-Gαq/11 antibody. Immunoprecipitates were separated by SDS-PAGE, followed by fluorography. The ratio of [3H]palmitate to [35S]methionine-cysteine -labeled Gαq/11 was plotted in the graph. Error bars show ±SD (n = 3). IgG, control immunoglobulin G.
siRNAs.
All siRNAs were purchased from Qiagen (Vento, Netherlands): DHHC3, validated Hs_ZDHHC3_5_HP, and AllStars negative control siRNA. For human DHHC7, DHHC9, Gαq, and Gα11 knockdown, the following target sequences were used: DHHC7, AGGAAACGCTACGAAAGAATA; DHHC9, ATCGTCTATGTGGCCCTCAAA; Gαq, CAGGACACATCGTTCGATTTA and CAGGAATGCTATGATAGACGA; and Gα11, CCCGGGCATCCAGGAATGCTA and CCGCATCGCCACCTTGGGCTA.
Quantitative PCR.
Total RNA was extracted using TRIzol (Invitrogen), and cDNA was synthesized using a high-capacity cDNA RT kit (Applied Biosciences). Quantitative PCR was performed using an Applied Biosystems 7000 system (Applied Biosciences) and Power SYBR green PCR Master Mix (Applied Biosciences). Primers were the following: DHHC3, 5′-CTGTGCCATCGTTACCTGGTTTC-3′ and 5′-CTGCCCAGGCTTCAACTGTAAAC-3′; DHHC7, 5′-TGCAGACTTCGTGGTGACTTTCG-3′ and 5′-TGGGGCACTTGTAGATGACTTCC-3′; DHHC9, 5′-TATTTGCTGCCATGCTCTTC-3′ and 5′-GGAATCACTCCAGGGTCACT-3′; and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-GTATCGTGGAAGGACTCATGACC-3′ and 5′-GTTTTTCTAGACGGCAGGTCAGG-3′.
Metabolic labeling and pulse-chase assay.
For pulse-chase analysis, HeLa cells (5 × 105 per six-well plate) were preincubated with 1 ml of serum-, cysteine-, and methionine-free Dulbecco's modified Eagle's medium containing 5 mg/ml fatty acid-free bovine serum albumin for 30 min. The cells were then metabolically labeled for 4 h with a medium containing 50 μCi/ml [35S]methionine-cysteine (GE Healthcare) or 0.5 mCi/ml [3H]palmitate (Perkin Elmer). Cells were washed and incubated in Dulbecco's modified Eagle's medium containing 100 μM palmitate. At indicated time points (see Fig. 1E), labeled cells were subjected to anti-Gαq/11 immunoprecipitation (IP). Briefly, labeled cells were lysed with 100 μl of 1% sodium dodecyl sulfate (SDS)-containing IP buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1 mM EDTA, 1% Triton X-100, and 50 μg/ml phenylmethylsulfonyl fluoride). After a 5-min extraction, 900 μl of SDS-free IP buffer was added. After centrifugation at 10,000 × g for 10 min, the supernatants were incubated with 2 μg of antibody for 1 h and then incubated with 30 μl of protein A-Sepharose (GE Healthcare). The immunoprecipitates were separated by SDS-polyacrylamide gel electrophoresis (PAGE). Gels were silver stained (Daiichi) for 35S detection or treated with Amplify (GE Healthcare) for 3H detection. Dried gels were exposed to films at −80°C.
Screening of the candidate PATs was performed in HEK293T cells as described previously (11, 12). To detect palmitoylation of endogenous Gαq/11 in siRNA-treated cells, cells were metabolically labeled with 0.5 mCi/ml [3H]palmitate-containing medium for 4 h at 72 h after siRNA transfection. Cells were then lysed and subjected to anti-Gαq/11 IP, followed by fluorography. For hydroxylamine treatment, [3H]palmitate-labeled cells were collected and sonicated in phosphate-buffered saline and then mixed with an equal volume of 1 M hydroxylamine (NH2OH) (pH 7.0) or 1 M Tris-HCl (pH 7.0). After a 1-h incubation at room temperature, the cell lysates were subjected to fluorography and Western blotting.
In vitro PAT assay.
The PAT assay (final volume, 50 μl) was performed as described previously, with modification (11). Gαq-GFP and FLAG-DHHC3 were immunoprecipitated from transfected HEK293T cells using anti-GFP antibody and protein A-Sepharose and M2 anti-FLAG agarose beads (Sigma), respectively. Eluted FLAG-DHHC3 (50 nM) was added to Gαq-GFP (50 nM) immobilized beads in 24 mM morpholineethanesulfonic acid (pH 6.4), 8 mM Tris-HCl (pH 7.5), and 0.008% Triton X-100. The reaction was started by the addition of 0.5 μM [3H]palmitoyl-coenzyme A (CoA; 0.5 μCi) and incubated for 10 min at 30°C. The reaction was stopped by the addition of IP buffer and washed three times with ice-cold IP buffer. Then, the Gαq-GFP-containing beads were suspended in SDS sample buffer with 10 mM dithiothreitol. Samples were then resolved by SDS-PAGE, followed by fluorography and Western blotting.
Immunofluorescence analysis.
HeLa cells were seeded onto poly-d-lysine (10 μg/ml)-coated 12-mm glass slips (Fisher brand). Cells were fixed with 4% paraformaldehyde, 120 mM sucrose, and 100 mM HEPES (pH 7.4) for 10 min and permeabilized with 0.1% Triton X-100 for 10 min. For Gαq/11 staining, the cells were fixed with methanol for 5 min at −20°C. Cells were then stained with indicated antibodies. Fluorescent images were obtained using an LSM5 Exciter system (Carl Zeiss) with a Plan-Apochromat 63× objective.
Rescue experiment.
HeLa cells (4 × 103) were seeded onto poly-d-lysine-coated 12-mm glass slips. Cells on a slip were transfected with 10 pmol of siRNA and 10 ng of siDHHC3 (siRNA targeting DHHC3)-resistant GFP-mDHHC3 expression vector in 24-well plates. At 72 h after transfection, cells were fixed with 4% paraformaldehyde, permeabilized, and stained with antibodies to GFP, Gαq/11, and β-catenin. Because overexpressed DHHC3 sometimes mislocalizes substrate proteins at the Golgi apparatus, we limited the amount of plasmid to mildly express DHHC3. To quantitate the intensity of Gαq/11 at the PM and the cytosol, 20 GFP-DHHC3-expressing cells were randomly chosen. The regions of the PM and cytosol were traced by polyline drawing, and their mean intensities were measured using Zeiss ZEN software. The relative PM intensities were calculated by the ratio of mean intensities of the PM and cytoplasmic regions.
Living-cell imaging.
HeLa cells were seeded onto a poly-d-lysine-coated 35-mm glass-bottom dish (Iwaki) and observed at 37°C in a CO2 chamber (Tokai Hit). For photoconversion analysis, Gαq-Dendra2 at the Golgi apparatus or PM was converted with a 405-nm laser using an LSM5 Exciter system and Zeiss ZEN software. Images were obtained every 5 min. After live imaging, the cells were fixed and stained by anti-GM130 antibody. For fluorescence recovery after photobleaching (FRAP) analysis, Gαq-GFP at the GalT-mCherry-positive region was bleached with a 488-nm laser. Images were acquired every 10 s for 20 min with or without 2-BP, and the average intensities of the regions were plotted in the graph. Microscope control and all image analysis were performed with Carl Zeiss ZEN software. For total internal reflection fluorescence microscopy (TIRFM) imaging, transfected HeLa cells were observed at 37°C by an IX81 TIRF system (Olympus) with a Plan-Apochromat 100× TIRFM objective. Images were captured using an ImageEM charge-coupled-device camera (C9100-13; Hamamatsu). Fluorescent intensities from epifluorescence and TIRF images were analyzed using MetaMorph software (version 7.1; MDS Analytical Technologies).
α1A-AR-dependent CREB phosphorylation.
HeLa cells (1.5 × 105) in a 12-well plate were transfected with α1A-AR. At 24 h after transfection, cells were stimulated with 50 μM phenylephrine with or without prazosin for 5 min. Cells were then lysed with IP buffer, and proteins were precipitated by addition of 1/10 volume of 100% trichloroacetic acid. Pellets were rinsed with acetone three times, suspended in 200 μl of SDS sample buffer, and subjected to anti-CREB or anti-phospho-CREB (pCREB) immunoblotting. For Gαq/11 rescue experiments, siRNA-transfected cells were reseeded at 30 h after transfection and subjected to the second-round transfection of Gαq-HA and α1A-AR expression plasmids at 48 h after siRNA transfection. At 72 h after siRNA transfection, cells were stimulated with phenylephrine for 5 min. For DHHC3/DHHC7 knockdown experiments, cells were transfected with siRNA and α1A-AR, and stimulated with phenylephrine at 72 h after transfection.
For pCREB immunostaining of DHHC knockdown cells, HeLa cells were cotransfected with siRNA and a limited amount of GFP-DHHC3 expression vector as described in the paragraph “Rescue experiment” above. At 72 h after transfection, cells were stimulated with phenylephrine and fixed with 4% paraformaldehyde, permeabilized, and stained with anti-pCREB antibody.
Measurement of IP3 production.
HeLa cells (3 × 105) expressing α1A-AR were stimulated with phenylephrine (50 μM) for 10 min. Inositol triphosphate (IP3) was extracted from the cells by incubation with 1.7% HClO4 for 20 min on ice. Quantification of IP3 was performed using an IP3 3H biotrak assay system (GE Healthcare), which is based on competition between [3H]IP3 (the tracer) and unlabeled IP3 in samples for binding to an IP3-binding protein prepared from bovine adrenal cortex.
Statistical analysis.
The results are expressed as mean ± standard deviation (SD). Statistical comparisons between groups were done by a Student t test.
RESULTS
Dynamic palmitate cycling on Gαq.
Taking advantage of Gαq tagged with Dendra2, a green-to-red photoconvertible fluorescent protein, we first visualized the dynamic movement of Gαq in HeLa cells. When Gαq-Dendra2 was coexpressed with Gβ1 and Gγ2 subunits in HeLa cells, Gαq-Dendra2 was localized at the PM and weakly distributed in the endomembranes (Fig. 1A). Photoconverted Gαq-Dendra2 in the endomembranes was rapidly targeted to the PM within 10 min (Fig. 1A, upper panel). In contrast, photoconverted Gαq-Dendra2 in the PM region diffused throughout the PM, and some population of Gαq-Dendra2 accumulated in the GM130-labeled endomembranes (i.e., Golgi apparatus) in a retrograde manner (Fig. 1A, lower panel). These results indicate that Gαq dynamically shuttles between the PM and the Golgi apparatus. Consistently, similar shuttling between the PM and endomembranes was recently observed by H-Ras/N-Ras-GFP (27) and Gαo-GFP (2).
A previous study using GFP-tagged Gαq showed that palmitoylation of Gαq is essential for its membrane targeting (15). We examined whether palmitoylation of Gαq is a dynamic process in HeLa cells. Because the antibody against Gαq cross-reacts with Gα11, the closest isoform (90% identity), we describe them as Gαq/11. Treatment of the HeLa cells for 4 h with 2-BP, an inhibitor of protein palmitoylation, relocalized Gαq/11 from the PM at the cell-to-cell contact sites to the cytoplasm (Fig. 1B) and blocked palmitoylation of Gαq/11 (Fig. 1C). This treatment did not affect the PM localization of β-catenin, a component of adherence junction used as a control. The effect of 2-BP on Gαq/11 localization is not simply due to disrupting palmitoylation of newly synthesized Gαq/11 because CHX, an inhibitor of protein synthesis, did not affect the Gαq/11 localization at the PM (Fig. 1B). Gαq/11 dispersed by 2-BP came back to the PM again within 2 h after removal of 2-BP in the presence of CHX (Fig. 1D). Furthermore, the pulse-chase experiments with [3H]palmitate and [35S]methionine-cysteine revealed that palmitate on Gαq/11 turns over rapidly (Fig. 1E). The half-life of palmitate on Gαq/11 is approximately 2 h, whereas the half-life of Gαq/11 protein itself is much longer, about 35 h. These results suggest that the de-/repalmitoylation cycle on Gαq dynamically regulates Gαq subcellular localization.
Screening of Gα palmitoylating enzymes.
To understand the molecular mechanisms for dynamic regulation of Gαq localization, we screened the candidate Gαq-palmitoylating enzyme. We transfected individually 23 DHHC proteins (11) together with Gαq-GFP in HEK293T cells and assessed palmitoylation of Gαq-GFP by metabolic labeling with [3H]palmitate (Fig. 2A). Only DHHC3/GODZ and DHHC7/SERZ-β showed robust PAT activity toward Gαq. Western blotting with anti-HA antibody indicates that all transfected HA-DHHC clones express in HEK293T cells albeit at different levels. Because some DHHC proteins, such as DHHC16, -20, and especially -21 express at much lower levels than DHHC3 and -7, it is possible that the clones with lower levels of expression have PAT activity toward Gαq. To verify the possibility, we limited the amount of transfected DHHC3 and examined the PAT activity. The limited DHHC3, even at a 1:50 transfection ratio, still induced Gαq palmitoylation (Fig. 2B). Under these conditions, DHHC proteins except for DHHC21 were expressed at higher (or equivalent) levels than limited DHHC3 and showed no PAT activity toward Gαq (Fig. 2B). DHHC21 apparently showed the PAT activity toward Lck in spite of a very low expression level, whereas DHHC3 did not show activity toward Lck (Fig. 2C). These results indicate that the expression level of DHHC clones hardly affects our screening results.
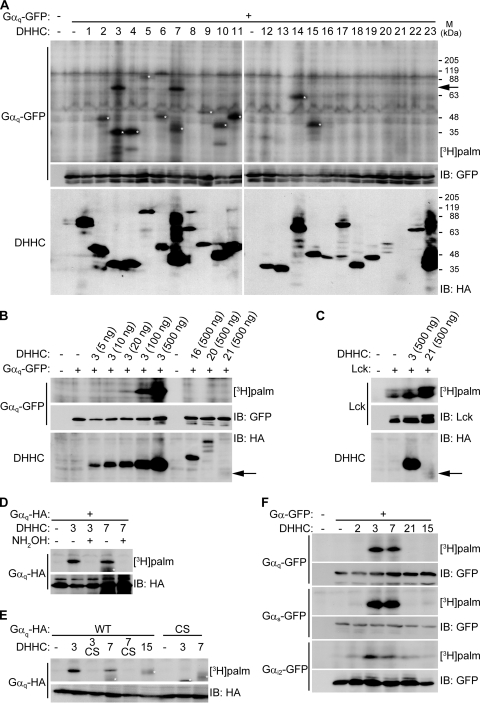
FIG. 2.
Screening of potential Gαq palmitoylating enzymes. (A) Individual HA-DHHC clones (0.5 μg plasmid) were transfected with Gαq-GFP (0.5 μg) into HEK293T cells. After metabolic labeling with [3H]palmitate, proteins were separated by SDS-PAGE, followed by fluorography and Western blotting with anti-GFP antibody for Gαq-GFP and anti-HA antibody for DHHC proteins. An arrow indicates the position of Gαq-GFP. White asterisks indicate autopalmitoylation of expressed DHHC proteins. Coexpression of DHHC3 or -7 robustly and specifically increased Gαq palmitoylation. Note that several DHHC proteins, such as DHHC16, -20, and -21, express at lower levels than DHHC3 and -7. M, molecular mass. (B) HEK293T cells were transfected with the indicated amount of DHHC proteins with Gαq-GFP and were labeled with [3H]palmitate. DHHC16 and -20 did not increase Gαq palmitoylation, whereas limited DHHC3 expression by 10 ng of plasmids (showing expression levels similar to DHHC16 and -20) still enhanced Gαq palmitoylation. An arrow indicates the position of DHHC21. (C) Although DHHC21 expressed at a lower level than DHHC3, DHHC21 has apparent PAT activity toward Lck. An arrow indicates the position of DHHC21. (D) Treatment of labeled cell lysates with 0.5 M hydroxylamine (NH2OH) but not 0.5 M Tris-HCl (−) released DHHC3- or DHHC7-mediated [3H]palmitate incorporated into Gαq-HA, indicating that DHHC3- or DHHC7-induced palmitoylation is mediated by a thioester bond. (E) The DHHC3(C157S) and DHHC7(C160S) mutations (shown as CS) abolished the palmitoylating activity. The mutations cysteines 9 and 10 in Gαq (CS) abolished its palmitoylation. Asterisks indicate the autopalmitoylation of DHHCs. (F) HEK293T cells cotransfected with indicated DHHC clones (2, 3, 7, 21, and 15) and Gα-GFP subfamily (αq, αs, and αi2) were metabolically labeled with [3H]palmitate. All Gα members were palmitoylated by DHHC3 and DHHC7. Gαi2 was also palmitoylated by DHHC2 and DHHC21 to a lesser extent. WT, wild type; IB, immunoblotting.
Gαq palmitoylation induced by DHHC3 and -7 is mediated by a labile thioester bond because the [3H]palmitate incorporated into Gαq was released with 0.5 M hydroxylamine treatment (Fig. 2D). We also found that the DHHC motif in DHHC3 and -7 was essential for Gαq palmitoylation because mutating a cysteine residue in the DHHC motif to serine in DHHC3 and -7 blocked their effects on Gαq palmitoylation (Fig. 2E). Furthermore, palmitoylation by DHHC3 and -7 required cysteines 9 and 10 of Gαq (Fig. 2E).
We next investigated whether other Gα subunits such as Gαs and Gαi2 are also palmitoylated by DHHC3 and -7. We systematically screened candidate PATs for Gαs and Gαi2 (data not shown) and found that DHHC proteins showed similar specificity for palmitoylation of Gαs and Gαq (Fig. 2F). However, Gαi2, which undergoes both myristoylation and palmitoylation, was palmitoylated by DHHC3 and -7 and, to a lesser extent, by DHHC2 and DHHC21 (Fig. 2F). Thus, DHHC3 and -7 are common candidate PATs for Gαq, Gαs, and Gαi2. We noted that DHHC3 and -7 show high conservation (87% identity) in the catalytic DHHC domain and form a subfamily in the phylogenetic tree of DHHC proteins (9, 11).
We next asked whether purified DHHC3 and -7 could directly palmitoylate purified Gαq in vitro. We immunoisolated FLAG-DHHC3 and Gαq-GFP from transfected HEK293T cells (Fig. 3A) and incubated them with [3H]palmitoyl-CoA. Purified DHHC3 apparently mediated incorporation of radiolabeled palmitate into wild-type Gαq-GFP (Fig. 3B). Under these conditions, spontaneous (nonenzymatic) palmitate transfer into Gαq-GFP was not observed (Fig. 3B). A similar result was obtained by using DHHC7 instead of DHHC3 (data not shown).
FIG. 3.
DHHC3 directly palmitoylates Gαq in vitro. (A) Immunopurified GFP-tagged wild-type (WT) Gαq and Gαq (CS) and FLAG-DHHC3 were stained with Coomassie brilliant blue (CBB). An arrowhead and an arrow indicate the positions of Gαq-GFP and FLAG-DHHC3, respectively. An asterisk indicates the nonspecific band. M, molecular mass. (B) Purified Gαq and DHHC3 were incubated in the presence of [3H]palmitoyl-CoA for 10 min at 30°C. Then, radiolabeled Gαq-GFP was evaluated by fluorography (upper) and immunoblotting (IB) with anti-GFP antibody (lower). DHHC3 palmitoylated wild-type Gαq but not Gαq (CS).
Knockdown of DHHC3 and -7 impairs Gαq palmitoylation and PM targeting.
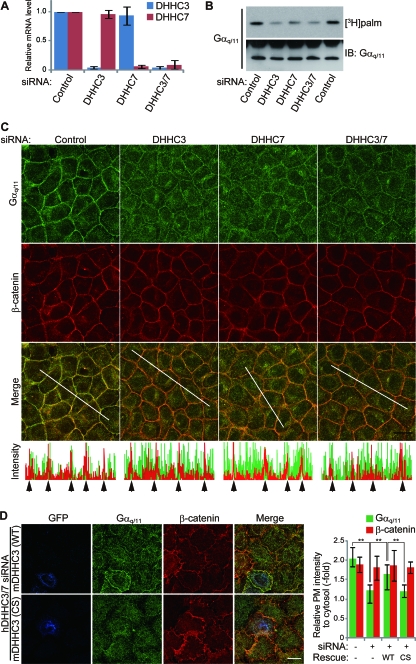
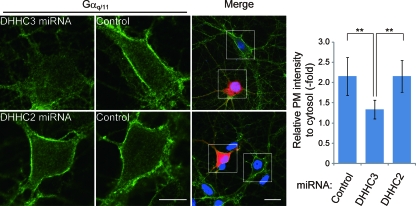
To determine whether DHHC3 and -7 are responsible for Gαq palmitoylation in cells, we knocked down DHHC3 and -7. Treatment of HeLa cells with siRNAs directed against human DHHC3 and/or human DHHC7 specifically reduced the expression of DHHC3 and/or -7 (validated by quantitative RT-PCR) (Fig. 4A). When DHHC3 and/or DHHC7 was knocked down in HEK293T cells, the incorporation of [3H]palmitate into endogenous Gαq/11 was markedly reduced compared to control siRNA-transfected cells (Fig. 4B). A similar result was obtained in HeLa cells (data not shown). Because palmitoylation of Gαq/11 was essential for its PM localization (15) (Fig. 1B and D), we performed immunofluorescence analysis of endogenous Gαq/11 in HeLa cells. Knockdown of DHHC3 and/or -7 delocalized Gαq/11 from the PM to the cytoplasm, whereas the intensity of β-catenin at the cell-to-cell contact sites did not change (Fig. 4C). The mislocalization of Gαq/11 to the cytoplasm by siRNAs to human DHHC3 and human DHHC7 was rescued by siDHHC3-resistant wild-type mDHHC3 (Fig. 4D). In contrast, the mislocalization of Gαq/11 was not rescued by the PAT-inactive mDHHC3(C157S) (Fig. 4D). We next examined the effect of DHHC3 knockdown on the Gαq/11 localization in primary hippocampal neurons, in which DHHC3 plays a more dominant role than DHHC7 (9). When DHHC3 was knocked down by vector-based RNAi, Gαq/11 at the PM was significantly reduced and relocalized into the cytoplasm, whereas DHHC2 knockdown did not affect the PM localization of Gαq/11 (Fig. 5).
FIG. 4.
DHHC3 and -7 are necessary for Gαq/11 palmitoylation and PM localization. (A) DHHC3 and -7 siRNAs downregulate targeted mRNA expressions. HeLa cells were transfected with a control or DHHC3 or -7 siRNA duplex. At 72 h after transfection, the expression of DHHC3 or -7 mRNA was quantitated by real-time RT-PCR. The expression of DHHC3 and -7 was normalized to that of GAPDH. Error bars show ±SD (n = 3). (B) HEK293T cells were transfected with the indicated siRNAs, and cells were labeled with [3H]palmitate for 4 h. Endogenous Gαq/11 was then immunoprecipitated, followed by fluorography and Western blotting. (C) HeLa cells were transfected with the indicated siRNAs and doubly stained with anti-Gαq/11 (green) and anti-β-catenin (red) antibodies. Note that knockdown of DHHC3 and/or DHHC7 specifically impairs PM localization of Gαq/11. Graphs indicate fluorescent intensity along white lines. Arrows mark the cell-to-cell contact sites along the white line. Scale bar, 20 μm. (D) siRNAs to human-specific DHHC3 and DHHC7 were cotransfected into HeLa cells together with wild-type (WT) GFP-mDHHC3 or catalytically inactive GFP-mDHHC3(C157S) [mDHHC3(CS)]. Cells were immunostained with anti-GFP (blue), anti-Gαq/11 (green), and anti-β-catenin (red) antibodies. The colors are pseudocolors. Scale bar, 20 μm. Relative fluorescence intensities of PM to cytosol are shown (right graph). Error bars show ±SD (n = 20). **, P < 0.01. IB, immunoblotting.
FIG. 5.
Depletion of DHHC3 expression impairs the PM targeting of Gαq/11 in hippocampal neurons. Rat hippocampal neurons (DIV8) were transfected with mCherry-miR RNA (to rat DHHC2 or -3) expression vectors (red). At 7 days after transfection, neurons were fixed and stained with anti-Gαq/11 antibody (green) and Hoechst (blue). Scale bars, 5 μm (high magnification) and 10 μm (low magnification). Graph shows ratio of fluorescence intensities of the PM to the cytosol. Error bars show ±SD (n = 12). **, P < 0.01. miRNA, microRNA.
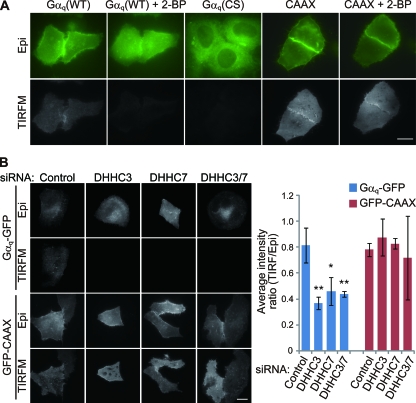
Next, to visualize Gαq at the cytoplasmic face of the PM with a high signal-to-noise ratio, we used TIRFM, which excites the molecules within 100-nm of the cover glass. When Gαq-GFP was expressed in HeLa cells, strong signals throughout the ventral surface of cells were detected by TIRFM (Fig. 6A). In contrast, the TIRFM signals of Gαq-GFP were apparently reduced when the cells were treated with 2-BP. The intensity of the palmitoylation-deficient mutant of Gαq, i.e., Gαq (CS)-GFP, was also lower than that of wild-type Gαq-GFP, suggesting that the signals visualized by TIRFM mainly reflect the membrane-bound palmitoylated Gαq-GFP. Supporting this, the intensity of GFP-K-Ras-CAAX (where A and X represent aliphatic and any residues, respectively), which targets to the PM by polybasic and prenylation sequences, did not change on 2-BP treatment. To examine the role of DHHC3 and/or -7 in the Gαq localization at the PM, we treated cells with siRNAs, observed them by TIRFM and epifluorescence microscopy, and measured the intensity ratio of TIRFM images to epifluorescence images. Knockdown of DHHC3 and/or -7 reduced significantly the intensity visualized by TIRFM (Fig. 6B). Under these conditions, GFP-K-Ras-CAAX intensity by TIRFM was not affected (Fig. 6B). Taken together, these results indicate that DHHC3 and/or -7 are authentic PATs for Gαq and are necessary for PM targeting of Gαq.
FIG. 6.
TIRFM imaging of membrane-bound palmitoylated Gαq. (A) HeLa cells were transfected with GFP-tagged Gαq (WT), palmitoylation-deficient Gαq (CS), or the C-terminal sequence of K-Ras including polybasic and prenylation sequences (GFP-CAAX). Cells were observed by epifluorescence microscopy (Epi) and TIRFM before and at 4 h after treatment with 2-BP. Gαq-GFP was clearly detected by TIRFM, and the intensity was reduced on 2-BP treatment. The intensity of Gαq (CS) was apparently weaker than that of Gαq (WT). Scale bar, 20 μm. (B) HeLa cells were transfected with control or DHHC3/DHHC7 siRNAs together with Gαq-GFP or GFP-CAAX. Scale bar, 20 μm. Relative fluorescence intensities of cell images from TIRFM compared to those of epifluorescence microscopy are indicated in the graph. Note that Gαq-GFP intensity visualized by TIRFM was reduced significantly in DHHC3 and DHHC7 knocked down cells. Error bars show ±SD (n = 5). **, P < 0.01; *, P < 0.05.
DHHC3 palmitoylates Gαq at the Golgi apparatus and drives PM-Golgi apparatus shuttling of Gαq.
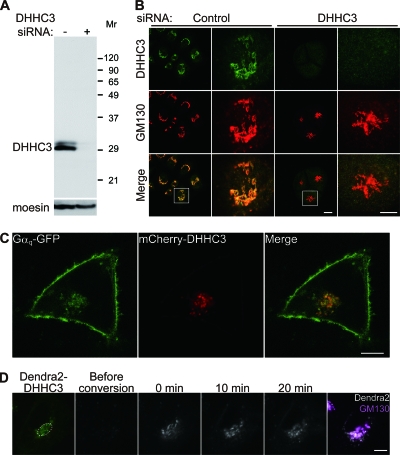
We next examined the cellular location of Gαq/11 palmitoylation. We first confirmed that our anti-DHHC3 antibody is specific because two bands detected by the DHHC3 antibody completely disappeared in the knocked down cell lysate (Fig. 7A). These two bands may contain (i) splicing variants as previously reported (17); (ii) differentially modified proteins by posttranslational modifications, such as phosphorylation, glycosylation, and palmitoylation; and (iii) degradation products. Consistent with previous observations in neurons (17), when HeLa cells were stained by this specific DHHC3 antibody, DHHC3 immunoreactivity occurred only at the GM130-labeled Golgi apparatus (Fig. 7B). The staining is specific because this signal disappeared in DHHC3 knocked down cells (Fig. 7B). When mCherry-DHHC3 was coexpressed with Gαq-GFP, some Gαq was colocalized with DHHC3 in the Golgi apparatus (Fig. 7C). Photoconversion analysis by Dendra2-DHHC3 showed that DHHC3 localizes stably at the Golgi apparatus (Fig. 7D), in contrast to Gαq (Fig. 1A). These results strongly suggest that DHHC3 palmitoylates Gαq at the Golgi apparatus. We also found that HA-tagged DHHC7 showed similar distribution to DHHC3 at the Golgi apparatus (data not shown).
FIG. 7.
Gαq and DHHC3 colocalize to the Golgi apparatus. (A) HeLa cells were transfected with control or DHHC3 siRNA. The lysates were then immunoblotted with anti-DHHC3 and anti-moesin antibodies, indicating that DHHC3 antibody specifically detects endogenous DHHC3. Molecular weights (in thousands) are shown at the right. (B) HeLa cells transfected with control or DHHC3 siRNA were immunostained with antibodies to DHHC3 (green) and Golgi marker GM130 (red). White boxes are magnified. The Golgi staining by DHHC3 antibody is specific because the signal disappeared in siDHHC3-treated cells. Scale bars, 20 μm (low magnification) and 10 μm (high magnification). (C) HeLa cells were cotransfected with Gαq-GFP and mCherry-DHHC3. Some population of Gαq was colocalized with DHHC3 at the Golgi apparatus. Scale bar, 20 μm. (D) Dendra2-DHHC3 (green) at the Golgi apparatus was photoconverted (gray scale). DHHC3 was stably localized at the Golgi apparatus, where GM130 was labeled (magenta). Scale bar, 10 μm.
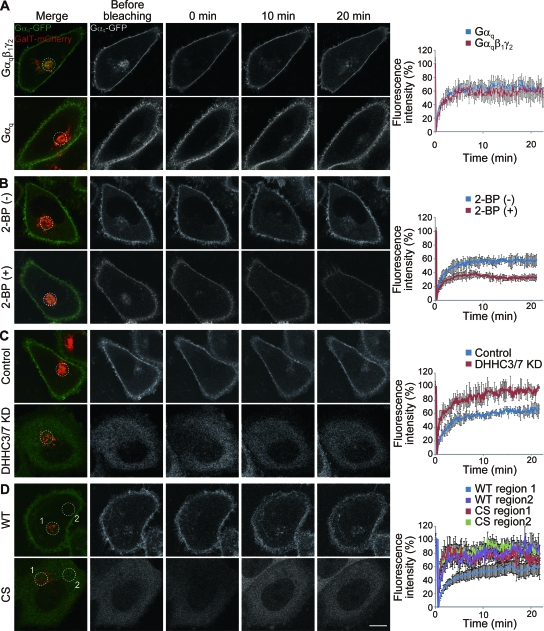
To examine the role of DHHC3 and -7 in the dynamic shuttling of Gαq, the Gαq dynamics were assessed by monitoring FRAP. To mark the Golgi apparatus, we coexpressed mCherry-tagged GalT (16, 34) together with Gαq-GFP with or without the β1 and γ2 subunits. After GFP fluorescence at the Golgi apparatus was bleached, Gαq-GFP fluorescence at the Golgi apparatus recovered within 20 min (64.6% ± 14.3%) (Fig. 8A). This newly arrived Gαq-GFP did not include newly synthesized Gαq-GFP because CHX did not affect the fluorescence recovery (data not shown). Because the fluorescence recovery of Gαq-GFP at the Golgi apparatus was not affected in the presence or absence of β1γ2 subunits (Fig. 8A), we expressed only Gαq-GFP in the following experiments. We next asked whether palmitoylation is involved in the retrograde PM-Golgi trafficking of Gαq-GFP. We found that inhibition of protein palmitoylation by 2-BP significantly reduced the fluorescence recovery at the GalT-positive Golgi region (33.3% ± 3.7%; P < 0.05 compared to control) (Fig. 8B), indicating that palmitoylation of Gαq at the Golgi apparatus is necessary for the retrograde trafficking from the PM to the Golgi apparatus. Gαq-GFP intensity in the cytoplasm and at the PM did not apparently change during the 20-min observation with 2-BP treatment. In contrast, the apparent delocalization of endogenous Gαq from the PM to the cytoplasm was observed at 4 h after 2-BP treatment (Fig. 1B). Judging from the half-life of palmitate on Gαq (about 2 h, based on Fig. 1E), these results are consistent. In fact, our longer observation confirmed that Gαq-GFP was relocalized from the PM to the cytoplasm at 4 h after 2-BP treatment (data not shown). When DHHC3 and -7 were knocked down by siRNAs, Gαq-GFP was apparently localized in the cytoplasm and not localized at the Golgi apparatus (Fig. 8C). The recovery of fluorescence around the GalT-positive Golgi region was rapid and nearly complete (94.6%± 4.8%) (Fig. 8C). Palmitoylation-deficient Gαq (CS)-GFP showed a similar cytoplasmic distribution and rapid recovery both around the GalT-positive region (Fig. 8D, CS region 1) and at cytoplasmic regions (Fig. 8D, CS region 2). This rapid recovery is not due to the enhanced retrograde PM-Golgi trafficking but simply to free diffusion of soluble cytoplasmic proteins. Taken together with the data presented in Fig. 1A, these results indicate that Gαq repalmitoylation by Golgi compartment-resident DHHC3/DHHC7 is necessary for temporal trapping of Gαq at the Golgi apparatus, leading to the constitutive Golgi compartment-PM shuttling.
FIG. 8.
Retrograde PM-Golgi trafficking of Gαq depends on Gαq palmitoylation by Golgi apparatus-resident DHHC3 and -7. (A) HeLa cells were transfected with Gαq-GFP (green and white) and GalT-mCherry (red) expression vectors with or without Gβ1γ2. The Golgi region (white circle) marked by GalT-mCherry was bleached, and then fluorescent recovery of Gαq-GFP was monitored by acquiring images every 10 s. Fluorescence intensities were plotted in graphs (right). (B) HeLa cells expressing Gαq-GFP (green and white) and GalT-mCherry (red) were treated with or without 2-BP for 30 min before FRAP analysis. The region (white circle) identified with GalT-mCherry was bleached, and then fluorescent recovery of Gαq-GFP was monitored. Treatment with 2-BP inhibited the recovery of fluorescence. (C) Knockdown (KD) of DHHC3 and -7 inhibited the Golgi and PM targeting of Gαq-GFP, resulting in the diffuse cytoplasmic localization. (D) Palmitoylation-deficient Gαq (CS)-GFP showed similar cytoplasmic distribution and rapid recovery both around the GalT-positive region (CS region 1) and at cytoplasmic region (CS region 2). The recovery of palmitoylation deficient Gαq (CS)-GFP was as rapid as that of DHHC3 and -7 knocked down cells in panel C. Scale bar, 10 μm. Error bars show ±SD (n = 5). WT, wild type.
DHHC3 and -7 are involved in the GPCR-mediated signaling pathway.
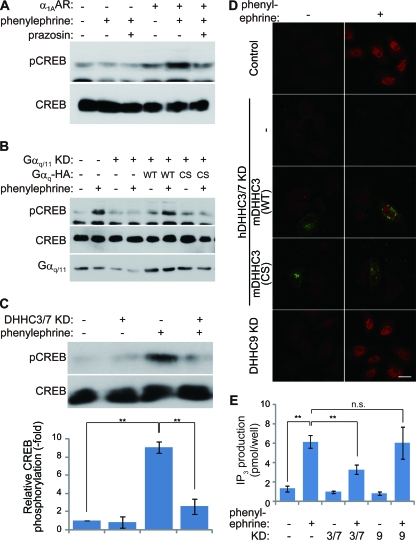
Next, we investigated whether DHHC3 and -7 are involved in the physiologic GPCR-mediated signal transduction. We selected α1A-AR as the Gαq/11-coupled receptor and monitored its downstream signaling by pCREB (28) and IP3 production. When HeLa cells transiently transfected with α1A-AR were stimulated with phenylephrine, an α1A-AR agonist, the phosphorylation level of CREB increased (Fig. 9A and D). This increase was completely blocked by the coapplication of prazosin, an α1A-AR antagonist (Fig. 9A). Knockdown of Gαq/11 inhibited α1A-AR activation-induced CREB phosphorylation (Fig. 9B), indicating that α1A-AR-mediated CREB phosphorylation is mediated by Gαq/11. Palmitoylation of Gαq/11 is necessary for the α1A-AR -mediated signaling pathway because wild-type Gαq (RNAi-resistant mouse Gαq), but not the palmitoylation-deficient Gαq (CS), rescued α1A-AR-mediated CREB phosphorylation (Fig. 9B). Furthermore, DHHC3 and -7 knockdown significantly blocked α1A-AR-mediated CREB phosphorylation (Fig. 9C and D). We also found that wild-type DHHC3 (RNAi-resistant mDHHC3) but not the PAT-inactive mDHHC3(C157S), rescued α1A-AR-mediated CREB phosphorylation (Fig. 9D). Finally, we examined whether DHHC3 and -7 are involved in the α1A-AR/Gq-induced phospholipase C activation by measuring IP3 production. When HeLa cells transiently transfected with α1A-AR were stimulated with phenylephrine, the IP3 production significantly increased (Fig. 9E). This increase was significantly blocked by knockdown of DHHC3 and -7 but not by knockdown of DHHC9. Thus, DHHC3 and DHHC7 play an essential role in the α1A-AR-mediated GPCR signaling pathway by Gαq palmitoylation.
FIG. 9.
DHHC3 and -7 are necessary for GPCR-mediated signal transduction through the Gαq palmitoylation. (A) α1A-AR activation leads to CREB phosphorylation. HeLa cells transiently transfected with α1A-AR were treated with 50 μM phenylephrine with or without 50 μM prazosin for 5 min. The cell lysates were treated with 10% trichloroacetic acid, and the resulting precipitates were subjected to immunoblotting with antibodies to CREB and Ser133 pCREB. (B to D) HeLa cells were transiently transfected with α1A-AR and indicated plasmids or siRNAs. (B) α1A-AR-mediated CREB phosphorylation requires palmitoylated Gαq. Knockdown (KD) of Gαq and Gα11 blocked α1A-AR-induced CREB phosphorylation, and RNAi-resistant Gαq (WT), but not palmitoylation-deficient Gαq (CS), rescued α1A-AR-mediated CREB phosphorylation. (C) Knocked down DHHC3 and DHHC7 (DHHC3/7 KD) inhibited α1A-AR-induced CREB phosphorylation, indicating that DHHC3 and -7 are essential for the GPCR-signaling pathway through Gαq palmitoylation. pCREB/CREB ratios are indicated in the graph. Error bars show ±SD (n = 3). **, P < 0.01. (D) HeLa cells expressing α1A-AR were treated with phenylephrine and stained with anti-pCREB antibody (red). Note that α1A-AR stimulation induced robust phosphorylation of CREB in the nucleus. The defect of CREB phosphorylation by knocked down human DHHC3 and human DHHC7 (hDHHC3/7 KD) was rescued by GFP-mDHHC3 (WT; green) but not catalytically inactive GFP-mDHHC3 (C157S) [mDHHC3 (CS)]. Scale bar, 20 μm. (E) The HeLa cells cotransfected with α1A-AR and the indicated siRNAs were treated with phenylephrine for 10 min, followed by IP3 extraction. Average IP3 production of three independent experiments is indicated in the graph. Agonist-induced IP3 production was specifically blocked by knockdown of DHHC3 and -7 (KD 3/7), indicating that DHHC3 and -7 are necessary for the α1A-AR-mediated GPCR signaling pathway. Error bars show ±SD (n = 3). **, P < 0.01; n.s., not significant (P = 0.20).
DISCUSSION
This study identified DHHC3 and DHHC7 as Gα palmitoylating enzymes that mediate palmitoyl transfer to Gαq, Gαs, and Gαi2. Golgi compartment-resident DHHC3 and -7 play essential roles in the PM-Golgi apparatus shuttling of Gαq. Furthermore we showed that DHHC3 and -7 are necessary for α1A-AR-mediated GPCR signaling pathway through Gαq targeting to the PM.
Identification of Gα palmitoylating enzymes has long been controversial. Some studies have suggested that Gα palmitoylation in cells might occur nonenzymatically because the formation of palmitoyl thioester linkage on proteins could occur spontaneously in vitro in the presence of a high concentration (10 to 20 μM) of palmitoyl-CoA (4). Recent systematic proteomic analysis revealed that DHHC family proteins are the main PATs that catalyze most of the protein palmitoylation in yeast because 29 of the 30 surveyed palmitoyl proteins were not palmitoylated in yeast lacking six of seven DHHC genes (30). In addition, our systematic screening analyses using 23 mammalian DHHC clones revealed that the palmitoylation levels of the more than 20 tested substrates were all enhanced by specific DHHC proteins (9, 10, 11, 12; also data not shown). These studies strongly suggest that members of the DHHC protein family mediate Gα palmitoylation. Our analyses showed decisively that DHHC3 and -7 are authentic Gα palmitoylating enzymes in vitro and in vivo. It is conceivable that similar approaches will be useful to identify PATs for other subfamilies of Gα, GPCRs, RGSs, and small GTPases.
By a knockdown approach, we found that DHHC3 and/or -7 knockdown reduces endogenous Gαq palmitoylation in HEK293T cells (Fig. 4B). This result raised a couple of questions. One may wonder why knockdown of DHHC3 and/or DHHC7 does not completely abolish Gαq/11 palmitoylation (Fig. 4B). Because our knockdown efficiency was about 85%, judging from our immunofluorescence analysis with anti-DHHC3 antibody, the remaining Gαq/11 palmitoylation may be largely derived from the nontransfected cells with siRNA or the cells in which DHHC3 and/or DHHC7 were partially knocked down. Although the existence of other PATs and nonenzymatic mechanisms (4) cannot be completely ruled out, our results strongly suggest that DHHC3 and DHHC7 represent major Gαq/11 palmitoylating enzymes. Double-knockout experiments of DHHC3 and DHHC7 will decisively demonstrate this issue. On the other hand, others may ask why knockdown of either DHHC3 or DHHC7 reduces Gαq palmitoylation (Fig. 4B) although the redundancy of DHHC3 and DHHC7 is expected. A straightforward possibility is that the total amount of DHHC3 and DHHC7 limits the palmitoylation of Gαq in the intact cells. Acute knockdown of DHHC3 or DHHC7 may not be fully compensated by the rest of DHHC. Alternatively, DHHC3 and DHHC7 may have synergistic effects on their PAT activity. In fact, DHHC3 and DHHC7 form homo-/heteromultimers (9). Further studies using the DHHC3/DHHC7 knockout cells/animals will be required to reveal the molecular redundancy issue.
In this study, we found that Gαq-GFP shuttles between the PM and Golgi apparatus within 10 min in HeLa cells. In contrast, Chisari and coworkers have recently shown that Gαo-GFP and also the β9-γ3 subunits cycle in a matter of seconds in CHO cells (2). These differences may depend on the cellular context. In CHO cells, the distance between the PM and the endomembrane looks much shorter than that in HeLa cells. Alternatively, the difference in acylation types of Gαq (dual palmitoylation) and Gαo (myristoylation and adjacent palmitoylation) may contribute to individual kinetics of shuttling. Because 16-carbon palmitate has higher membrane affinity than the 14-carbon myristate, dually palmitoylated Gαq may be more pronounced in PM localization than myristoylated/monopalmitoylated Gαo, leading to slower retrograde trafficking. In support of this idea, Rocks and colleagues reported that monopalmitoylated N-Ras displays a faster retrograde PM-Golgi apparatus trafficking than dually palmitoylated H-Ras (27). Direct comparison between Gαq and Gαo in the same cell type will address this question.
Wedegaertner and Bourne reported that activation of β-AR accelerates the palmitate cycling on Gαs (depalmitoylation and subsequent repalmitoylation of Gαs) and induces PM-to-cytosol translocation of Gαs (37). In contrast, another report showed that Gαq-GFP at the PM did not change on α2A-AR agonist stimulation in HEK293T cells (15). Our analysis in HeLa cells using FRAP and photoconversion methods showed that Gαq constitutively cycles between the PM and Golgi apparatus and that this cycling requires the palmitoylation of Gαq by Golgi apparatus-resident DHHC3 and -7. Although we attempted to decipher whether α1A-AR agonist stimulation affects Gα dynamic relocalization, our imaging resolution could not detect a significant difference (data not shown). These results imply that Gα dynamics depend on the subfamily of Gα and cellular/agonist contexts. Considering that H-Ras and N-Ras shuttle between the PM and Golgi apparatus through de-/repalmitoylation (27), such a constitutive cycling between the PM and intracellular organelles may be a general mechanism that allows cells to rapidly adjust to extracellular stimulation. To understand the whole picture of this shuttling mechanism, examination of the effect of acyl-protein thioesterase (5), a potential depalmitoylating enzyme, or identification of a novel depalmitoylating enzyme family still needs to be done.
We investigated whether DHHC3 and -7 activity is regulated by extracellular stimulation. We found that incorporation of [3H]palmitate into Gαq did not change when the cells were treated with an α1A-AR agonist (data not shown). Also, the localization of DHHC3 at the Golgi apparatus did not change with an α1A-AR agonist (data not shown). In contrast, DHHC21, one of the endothelial NO synthase PATs, was shown to be involved in the release of nitric oxide stimulated by both ionomycin and ATP, suggesting that DHHC21 is regulated downstream of calcium and ATP (10). These results imply that the individual DHHC proteins are regulated differently. Because protein palmitoylation modifies a wide variety of GPCR-related proteins and because GPCR signaling pathways play important roles in physiologic and pathological phenomena, the DHHC enzyme family may become an ideal therapeutic target.
Acknowledgments
We thank K. Kaibuchi (Nagoya University), S. Kobayashi (NIBB), T. Tabira, and K. Takahashi (NCGG) for sharing equipment; T. Watanabe (Nagoya University), S. Ishii (Tokyo University), and A. Dimitrov (Institut Curie) for valuable suggestions; and D. S. Bredt (Eli Lilly and Company), C. A. Berlot (Weis Center for Research), P. B. Wedegaertner (Thomas Jefferson University), A. Weiss (University of California, San Francisco), M. M. Rasenick (University of Illinois at Chicago), and R. Y. Tsien (University of California, San Diego) for providing plasmid vectors.
R.T. and J.N. are supported by the Japan Society for the Promotion of Science. Y.F. is supported by grants from the Human Frontier Science Program (HFSP-CDA) and MEXT (18700376). M.F. and F.P. are supported by grants from HFSP (Young Investigators). M.F. is supported by grants from MEXT (20670005, 20022043, 20054022, and 18687008).
Footnotes
Published ahead of print on 10 November 2008.
REFERENCES
- 1.Bartels, D. J., D. A. Mitchell, X. Dong, and R. J. Deschenes. 1999. Erf2, a novel gene product that affects the localization and palmitoylation of Ras2 in Saccharomyces cerevisiae. Mol. Cell. Biol. 196775-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chisari, M., D. K. Saini, V. Kalyanaraman, and N. Gautam. 2007. Shuttling of G protein subunits between the plasma membrane and intracellular membranes. J. Biol. Chem. 28224092-24098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drenan, R. M., C. A. Doupnik, M. P. Boyle, L. J. Muglia, J. E. Huettner, M. E. Linder, and K. J. Blumer. 2005. Palmitoylation regulates plasma membrane-nuclear shuttling of R7BP, a novel membrane anchor for the RGS7 family. J. Cell Biol. 169623-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duncan, J. A., and A. G. Gilman. 1996. Autoacylation of G protein α subunits. J. Biol. Chem. 27123594-23600. [DOI] [PubMed] [Google Scholar]
- 5.Duncan, J. A., and A. G. Gilman. 1998. A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein α subunits and p21RAS. J. Biol. Chem. 27315830-15837. [DOI] [PubMed] [Google Scholar]
- 6.El-Husseini, A., E. Schnell, S. Dakoji, N. Sweeney, Q. Zhou, O. Prange, C. Gauthier-Campbell, A. Aguilera-Moreno, R. A. Nicoll, and D. S. Bredt. 2002. Synaptic strength regulated by palmitate cycling on PSD-95. Cell 108849-863. [DOI] [PubMed] [Google Scholar]
- 7.El-Husseini, A. E.-D., and D. S. Bredt. 2002. Protein palmitoylation: a regulator of neuronal development and function. Nat. Rev. Neurosci. 3791-802. [DOI] [PubMed] [Google Scholar]
- 8.Evanko, D. S., M. M. Thiyagarajan, and P. B. Wedegaertner. 2000. Interaction with Gβγ is required for membrane targeting and palmitoylation of Gαs and Gαq. J. Biol. Chem. 2751327-1336. [DOI] [PubMed] [Google Scholar]
- 9.Fang, C., C. A. Deng, L., Keller, M. Fukata, Y. Fukata, G. Chen, and B. Luscher. 2006. GODZ-mediated palmitoylation of GABAA receptors is required for normal assembly and function of GABAergic inhibitory synapses. J. Neurosci. 2612758-12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Hernando, C., M. Fukata, P. N. Bernatchez, Y. Fukata, M. I. Lin, D. S. Bredt, and W. C. Sessa. 2006. Identification of Golgi-localized acyl transferases that palmitoylate and regulate endothelial nitric oxide synthase. J. Cell Biol. 174369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukata, M., Y. Fukata, H. Adesnik, R. A. Nicoll, and D. S. Bredt. 2004. Identification of PSD-95 palmitoylating enzymes. Neuron 44987-996. [DOI] [PubMed] [Google Scholar]
- 12.Fukata, Y., T. Iwanaga, and M. Fukata. 2006. Systematic screening for palmitoyl transferase activity of the DHHC protein family in mammalian cells. Methods 40177-182. [DOI] [PubMed] [Google Scholar]
- 13.Gilman, A. G. 1987. G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 56615-649. [DOI] [PubMed] [Google Scholar]
- 14.Huang, K., A. Yanai, R. Kang, P. Arstikaitis, R. R. Singaraja, M. Metzler, A. Mullard, B. Haigh, C. Gauthier-Campbell, C. A. Gutekunst, M. R. Hayden, and A. El-Husseini. 2004. Huntingtin-interacting protein HIP14 is a palmitoyl transferase involved in palmitoylation and trafficking of multiple neuronal proteins. Neuron 44977-986. [DOI] [PubMed] [Google Scholar]
- 15.Hughes, T. E., H. Zhang, D. E. Logothetis, and C. H. Berlot. 2001. Visualization of a functional Gαq-green fluorescent protein fusion in living cells. Association with the plasma membrane is disrupted by mutational activation and by elimination of palmitoylation sites, but not by activation mediated by receptors or AlF4. J. Biol. Chem. 2764227-4235. [DOI] [PubMed] [Google Scholar]
- 16.Jollivet, F., G. Raposo, A. Dimitrov, R. Sougrat, B. Goud, and F. Perez. 2007. Analysis of de novo Golgi complex formation after enzyme-based inactivation. Mol. Biol. Cell 184637-4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller, C. A., X. Yuan, P. Panzanelli, M. L. Martin, M. Alldred, M. Sassoe-Pognetto, and B. Luscher. 2004. The γ2 subunit of GABAA receptors is a substrate for palmitoylation by GODZ. J. Neurosci. 245881-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linder, M. E., and R. J. Deschenes. 2007. Palmitoylation: policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 874-84. [DOI] [PubMed] [Google Scholar]
- 19.Linder, M. E., P. Middleton, J. R. Hepler, R. Taussig, A. G. Gilman, and S. M. Mumby. 1993. Lipid modifications of G proteins: α subunits are palmitoylated. Proc. Natl. Acad. Sci. USA 903675-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luttrell, L. M. 2006. Transmembrane signaling by G protein-coupled receptors. Methods Mol. Biol. 3323-49. [DOI] [PubMed] [Google Scholar]
- 21.Malbon, C. C. 2005. G proteins in development. Nat. Rev. Mol. Cell Biol. 6689-701. [DOI] [PubMed] [Google Scholar]
- 22.Marrari, Y., M. Crouthamel, R. Irannejad, and P. B. Wedegaertner. 2007. Assembly and trafficking of heterotrimeric G proteins. Biochemistry 467665-7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohyama, T., P. Verstreken, C. V. Ly, T. Rosenmund, A. Rajan, A. C. Tien, C. Haueter, K. L. Schulze, and H. J. Bellen. 2007. Huntingtin-interacting protein 14, a palmitoyl transferase required for exocytosis and targeting of CSP to synaptic vesicles. J. Cell Biol. 1791481-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oldham, W. M., and H. E. Hamm. 2008. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 960-71. [DOI] [PubMed] [Google Scholar]
- 25.Pierce, K. L., R. T. Premont, and R. J. Lefkowitz. 2002. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3639-650. [DOI] [PubMed] [Google Scholar]
- 26.Prior, I. A., A. Harding, J. Yan, J. Sluimer, R. G. Parton, and J. F. Hancock. 2001. GTP-dependent segregation of H-Ras from lipid rafts is required for biological activity. Nat. Cell Biol. 3368-375. [DOI] [PubMed] [Google Scholar]
- 27.Rocks, O., A. Peyker, M. Kahms, P. J. Verveer, C. Koerner, M. Lumbierres, J. Kuhlmann, H. Waldmann, A. Wittinghofer, and P. I. Bastiaens. 2005. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science 3071746-1752. [DOI] [PubMed] [Google Scholar]
- 28.Rosethorne, E. M., S. R. Nahorski, and R. A. Challiss. 2008. Regulation of cyclic AMP response-element binding-protein (CREB) by Gq/11-protein-coupled receptors in human SH-SY5Y neuroblastoma cells. Biochem. Pharmacol. 75942-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roth, A. F., Y. Feng, L. Chen, and N. G. Davis. 2002. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J. Cell Biol. 15923-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roth, A. F., J. Wan, A. O. Bailey, B. Sun, J. A. Kuchar, W. N. Green, B. S. Phinney, J. R. Yates III, and N. G. Davis. 2006. Global analysis of protein palmitoylation in yeast. Cell 1251003-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaner, N. C., P. A. Steinbach, and R. Y. Tsien. 2005. A guide to choosing fluorescent proteins. Nat. Methods 2905-909. [DOI] [PubMed] [Google Scholar]
- 32.Simon, M. I., M. P. Strathmann, and N. Gautam. 1991. Diversity of G proteins in signal transduction. Science 252802-808. [DOI] [PubMed] [Google Scholar]
- 33.Smotrys, J. E., and M. E. Linder. 2004. Palmitoylation of intracellular signaling proteins: regulation and function. Annu. Rev. Biochem. 73559-587. [DOI] [PubMed] [Google Scholar]
- 34.Storrie, B., J. White, S. Rottger, E. H. Stelzer, T. Suganuma, and T. Nilsson. 1998. Recycling of Golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J. Cell Biol. 1431505-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stowers, R. S., and E. Y. Isacoff. 2007. Drosophila huntingtin-interacting protein 14 is a presynaptic protein required for photoreceptor synaptic transmission and expression of the palmitoylated proteins synaptosome-associated protein 25 and cysteine string protein. J. Neurosci. 2712874-12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swarthout, J. T., S. Lobo, L. Farh, M. R. Croke, W. K. Greentree, R. J. Deschenes, and M. E. Linder. 2005. DHHC9 and GCP16 constitute a human protein fatty acyltransferase with specificity for H- and N-Ras. J. Biol. Chem. 28031141-31148. [DOI] [PubMed] [Google Scholar]
- 37.Wedegaertner, P. B., and H. R. Bourne. 1994. Activation and depalmitoylation of Gsα. Cell 771063-1070. [DOI] [PubMed] [Google Scholar]
- 38.Wedegaertner, P. B., D. H. Chu, P. T. Wilson, M. J. Levis, and H. R. Bourne. 1993. Palmitoylation is required for signaling functions and membrane attachment of Gqα and Gsα. J. Biol. Chem. 26825001-25008. [PubMed] [Google Scholar]
- 39.Yu, J. Z., and M. M. Rasenick. 2002. Real-time visualization of a fluorescent Gαs: dissociation of the activated G protein from plasma membrane. Mol. Pharmacol. 61352-359. [DOI] [PubMed] [Google Scholar]
- 40.Zhao, L., S. Lobo, X. Dong, A. D. Ault, and R. J. Deschenes. 2002. Erf4p and Erf2p form an endoplasmic reticulum-associated complex involved in the plasma membrane localization of yeast Ras proteins. J. Biol. Chem. 27749352-49359. [DOI] [PubMed] [Google Scholar]