Abstract
Animals have evolved defense systems for surviving in a chemically diverse environment. Such systems should demonstrate plasticity, such as adaptive immunity, enabling a response to even unknown chemicals. The antioxidant transcription factor Nrf2 is activated in response to various electrophiles and induces cytoprotective enzymes that detoxify them. We report here the discovery of a multiple sensing mechanism for Nrf2 activation using zebrafish and 11 Nrf2-activating compounds. First, we showed that six of the compounds tested specifically target Cys-151 in Keap1, the ubiquitin ligase for Nrf2, while two compounds target Cys-273. Second, in addition to Nrf2 and Keap1, a third factor was deemed necessary for responding to three of the compounds. Finally, we isolated a zebrafish mutant defective in its response to seven compounds but not in response to the remaining four. These results led us to categorize Nrf2 activators into six classes and hypothesize that multiple sensing allows enhanced plasticity in the system.
Nrf2 is a transcription factor that transactivates cytoprotective genes through a common DNA regulatory element, called the antioxidant response element or electrophile response element (18, 24). Nrf2 target genes are multifarious and encode phase 2 detoxifying enzymes, antioxidant proteins, enzymes for glutathione biosynthesis, ABC transporters, scavenger receptors, transcription factors, proteases, chaperone proteins, and so forth (23). Under basal conditions, Nrf2 is rapidly degraded by proteasomes, and little induction of target genes is observed. This degradation is controlled by Keap1, an Nrf2-specific adaptor protein for the Cul3 ubiquitin ligase complex (12, 20). Nrf2-activating compounds block Keap1-dependent Nrf2 ubiquitination, leading to the stabilization and nuclear translocation of Nrf2 and subsequent induction of Nrf2 target genes.
A number of Nrf2 activators have been found but, interestingly, no common structures were identified among them (23). Talalay and coworkers classified Nrf2-activating compounds into the following 10 distinct classes based on their chemical structures (7): diphenols, Michael reaction acceptors, isothiocyanates, thiocarbamates, trivalent arsenicals, 1,2-dithiole-3-thiones, hydroperoxides, vicinal dimercaptans, heavy metals, and polyenes. A current pursuit is unraveling how cells detect these chemical compounds and transduce their signals into the activation of Nrf2. Keap1 has many highly reactive cysteine residues that have the potential to sense electrophilic Nrf2 activators by forming covalent adducts with them. We and others have therefore proposed the model that Nrf2-activating compounds directly modify the sulfhydryl groups of Keap1 cysteines by oxidation, reduction, or alkylation, which alters the conformation of Keap1 and ceases the ubiquitination of Nrf2 (7, 24). In fact, mass spectrometry (MS) studies revealed that some Nrf2-activating compounds can covalently react with cysteines in mouse or human Keap1. For example, dexamethasone 21-mesylate with Cys-257, -273, -288, -297, and -613 (2); iodoacetyl-N-biotinylhexylenediamine/biotinylated iodoacetamide (IBA/BIA) with Cys-196, -226, -241, -257, -288, and -319 (9) (in separate reports, Cys-151, -288, and -297 [4, 5] or Cys-13, -151, -257, -288, -297, -613, and -622 [31]); sulforaphane with Cys-77, -226, -249, -257, -489, -513, -518, and -583 (8); xanthohumol with Cys-151, -319, and -613 (27); isoliquiritigenin with Cys-151 and -226 (27); and 10-shogaol with Cis-151, -257, and -368 (27). Among these cysteine residues, Cys-151, -273, and -288 were demonstrated to be essential for regulating Nrf2 degradation in cell biological studies (21, 25, 33, 34, 43, 46), suggesting that these, and possibly other, cysteines are sensor sites for Nrf2 activators. These findings support the model of Nrf2 activation by adduct formation between Keap1 and activating compounds but at the same time raise several fundamental questions. For instance, which cysteines are the true in vivo sensor sites? Do different compounds target different cysteines? Does modifying different cysteines regulate Keap1 functions in similar or different manners? Are there any Nrf2-activating compounds that do not target Keap1 cysteines?
To address these questions, we systematically analyzed a variety of Nrf2 activators using zebrafish as an experimental system to compare sensor requirements under physiological conditions. We showed before that fish have a Keap1-based response system against electrophilic compounds, as in mammals (22, 38, 39). In contrast to the mammalian system, zebrafish possess two Keap1 molecules, Keap1a and Keap1b, both of which regulate Nrf2 degradation (26), but have different recognition specificities for Nrf2-activating compounds, as demonstrated here. We now report that the Keap1-Nrf2 system comprises discrete sensor sites, including the Keap1 cysteines Cys-151 and Cys-273, for a variety of Nrf2-activating compounds.
MATERIALS AND METHODS
Fish and chemical treatments.
Zebrafish embryos and larvae were obtained by natural mating. For induction studies, fish were placed in culture dishes (embryos and larvae) or plastic chambers (adult fish) containing 100 μM diethylmaleate (DEM; Wako), 2 μM ebselen (Ebs; a gift from K. Uchida), 40 μM 1,2-dithiole-3-thione (D3T; a gift from T. Kensler), 30 μM tert-butylhydroquinone (tBHQ; Sigma-Aldrich), 40 μM sulforaphane (SF; LKT laboratories), 3 μM 1,2-naphthoquinone (1,2-NQ; Tokyo Kasei Industries), 50 μM prostaglandin A2 (PGA2; Wako), 2.5 to 10 μM 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2; Cayman Chemical), 1 μM auranofin (AF; Alexis Biochemicals), 1 mM hydrogen peroxide (H2O2; Wako), or 40 μM cadmium chloride (CdCl2; Wako). In each case, chemical treatments were carried out for 6 h. The gene expressions of pi-class glutathione S-transferase (GST) gene (gstp1) and γ-glutamylcysteine synthetase heavy subunit gene (γgcsh) were analyzed by in situ hybridization or by reverse transcription-PCR (RT-PCR), as described previously (22, 38). Real-time RT-PCR was performed to quantitate gstp1 expression using an ABI Prism 7700 (Applied Biosystems) and probes labeled with a reporter fluorescent dye (TaqMan probe) as described previously (26).
Plasmid construction.
The plasmids pCS2FLkeap1a1bC1 and pCS2FLkeap1a1bC2 were generated by inserting the cDNA encoding amino acids 2 to 54 or amino acids 2 to 148, respectively, of zebrafish Keap1a into the HindIII and PmaCI or the HindIII and BglII sites, respectively, of pCS2FLkeap1b (26). Plasmid pCS2FLkeap1a1bC3 was generated by inserting the cDNA encoding amino acids 299 to 593 of zebrafish Keap1b into the BglII and XbaI sites of pCS2FLkeap1a (26). For constructing pCS2FLmKeap1, cDNAs encoding the regions from amino acids 2 to 7 and amino acids 8 to 624 were generated by annealing synthetic oligonucleotides (Hokkaido System Science) or by PCR, respectively, and inserted together into the HindIII and XbaI sites of pCS2FL (26). Point mutations were introduced by PCR into pCS2FLkeap1b to give plasmids pCS2FLkeap1bK124T, pCS2FLkeap1bC125S, and pCS2FLkeap1bC125W. To prepare pCS2FLmKeap1C151S, pCS2FLmKeap1C273S, and pCS2FLmKeap1C288S, Cys-to-Ser point mutations were introduced by PCR into pCS2FLmKeap1. All constructs were verified by DNA sequencing. Plasmids pCS2nrf2, pCS2keap1a, pCS2keap1b, and pCS2Nrf2NTnGFP were described previously (22, 26).
Injection of mRNA and morpholino oligonucleotides.
Synthetic capped RNA was made with an SP6 mMESSAGE mMACHINE in vitro transcription kit (Ambion) using linearized DNA of the pCS2 derivatives described above. Nrf2-morpholino oligonucleotide (nrf2MO) was described previously (22). mRNA or morpholino oligonucleotides were injected into yolk at the one-cell stage using an IM300 microinjector (Narishige). Green fluorescent protein (GFP) expression was examined under the GFP filter of a BZ-8000 microscope (Keyence).
Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) MS analysis.
Expression in Escherichia coli and purification of mouse Keap1 protein was performed according to the method of Dinkova-Kostova et al. (2). Mouse Keap1 was incubated with or without 100 μM 15d-PGJ2, 100 μM PGA2, or 500 μM DEM for 60 min at 25°C in buffer containing 20 mM Tris-HCl (pH 8.5). Trypsin-digested mouse Keap1 was mixed with dithiothreitol and trifluoroacetic acid. To improve the ionization efficiency of the MS, samples were purified with Zip-tip μC18 (Millipore) before MS analysis. Peptides were mixed with α-cyano-4-hydroxycinnamic acid (2.5 mg/ml) containing 50% acetonitrile and 0.1% trifluoroacetic acid and dried on stainless steel targets at room temperature. Analyses were performed using an AXIMA-TOF2 (Shimadzu) with a nitrogen laser, as described previously (14). All analyses took place in the positive ion mode, and the instrument was calibrated immediately prior to each series of studies.
Mutant screening.
Random mutations were generated by soaking male zebrafish of the AB strain in 3 mM N-ethyl-N-nitrosourea (ENU) for 1 h between 22 and 23°C and repeating this process four times at weekly intervals. The resulting males were crossed with AB females and bred to homozygosity over two generations, as previously described (3, 6).
RESULTS
A variety of Nrf2-activating compounds induce gstp1 expression in zebrafish.
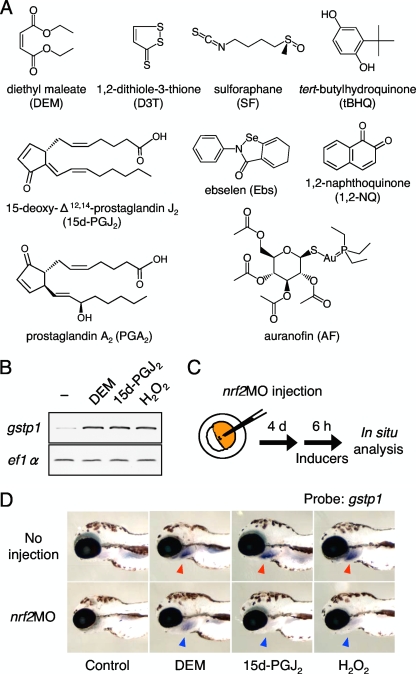
To determine whether zebrafish can respond to a wide variety of Nrf2 activators, as do mammalian cells, we analyzed the induction of gstp1 by 11 Nrf2-activating compounds in 4-day-old zebrafish larvae by whole-mount in situ hybridization. The compounds tested were DEM, D3T, SF, tBHQ, 1,2-NQ, Ebs, 15d-PGJ2, PGA2, H2O2, AF, and CdCl2 (Fig. 1A). All 11 compounds strongly induced gstp1 expression in gills, an organ of detoxification in fish (see Fig. S1 in the supplemental material). We also studied gstp1 induction in adult gill by conventional and real-time RT-PCR analyses and obtained the same results (Fig. 1B; also see Fig. S2 in the supplemental material). In the early larval stages, Nrf2 expression can be knocked down using morpholino antisense oligonucleotides (22), allowing us to elucidate the contribution of Nrf2 to each induction. One-cell stage embryos were injected with or without nrf2MO and raised to 4-day-old larvae before treatment with DEM, 15d-PGJ2, or H2O2 (Fig. 1C and D). The induction of gstp1 by these compounds was decreased by the Nrf2 knockdown, indicating that Nrf2 mediates gstp1 induction in all three cases. We concluded that lineups of the Nrf2-activating compounds are equivalent in fish and mammals, and thus it was worthwhile to study the chemical sensing mechanisms using zebrafish as a model.
FIG. 1.
Induction of zebrafish gstp1 by a variety of Nrf2 activators. (A) Structural representation of Nrf2-activating compounds. (B) Induction of gstp1 expression in adult gills was analyzed by RT-PCR after 6 h of treatment with compounds. The expression of ef1α was used to standardize the amount of cDNA. (C) Experimental scheme for zebrafish Nrf2 gene knockdown. (D) Induction of gstp1 expression in 4-day-old larvae. Strong gstp1 induction was observed in larvae treated with all three compounds, especially in the gills (red arrowheads). It was greatly reduced when nrf2MO was injected at the embryonic stage (blue arrowheads).
Classification of Nrf2-activating compounds based on the requirement of Keap1a, Keap1b, and other factors.
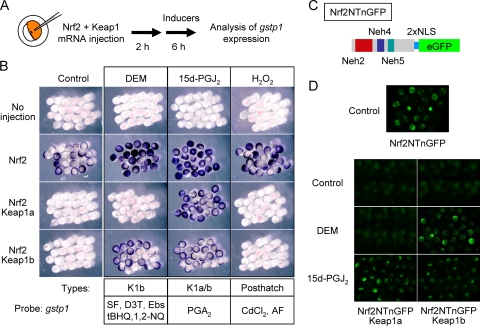
In contrast to cultured cells, it is easy to monitor Nrf2-induced transactivation of endogenous target genes in zebrafish embryos by mRNA injection assay (22), probably because of the absence of artificial oxidative stress and the very low expression of endogenous Nrf2 mRNA during embryonic development (see Fig. S3 in the supplemental material). Using this experimental system, we tried to decipher whether Keap1a and Keap1b have different specificities in the recognition of Nrf2-activating compounds. The Nrf2 repression activities of both Keap1 molecules were previously shown to be equivalent (26). No gstp1 induction was observed for any of the compounds tested in embryos without adding exogenous Nrf2 (Fig. 2B, no injection). Supplying exogenous Nrf2 by mRNA injection induced gstp1 expression in whole embryos (Fig. 2A and B, control). This induction was abolished by co-overexpressing either Keap1a or Keap1b. Embryos were coinjected with Nrf2 and either Keap1a or Keap1b, mRNAs were treated with DEM, 15d-PGJ2, or H2O2, and gstp1 expression was analyzed. The expression of gstp1 was induced by DEM treatment in embryos overexpressing Keap1b, but not Keap1a (Fig. 2B, DEM). Surprisingly, 15d-PGJ2 treatment cancelled suppression by both Keap1a and Keap1b (Fig. 2B, 15-PGJ2). The results suggest that Keap1a has a sensor site for 15d-PGJ2, but not for DEM, whereas Keap1b can sense both compounds. On the other hand, H2O2 was unable to cancel the suppression elicited by either Keap1 protein (Fig. 2B, H2O2). Since H2O2-induced gstp1 expression was Nrf2-dependent in larvae (Fig. 1D), factors additional to Nrf2 and Keap1 are required for sensing H2O2, and these factors are not expressed or functional at the embryonic stages. Both conventional and real-time RT-PCR using total RNA from whole embryos confirmed these differences among three compounds (see Fig. S4 and S5 in the supplemental material). We then systematically analyzed the specificities of the remaining eight Nrf2-activating compounds toward Keap1 molecules (see Fig. S5 in the supplemental material) and categorized them into three types: a Keap1b sensing type (K1b), a Keap1a or Keap1b sensing type (K1a/b), and a posthatch factor sensing type (posthatch) dependent on the putative factors missing at the embryonic stages (see Fig. 2B). SF, D3T, Ebs, tBHQ, and 1,2-NQ were grouped as K1b type, PGA2 is K1a/b type, and CdCl2 and AF are posthatch type.
FIG. 2.
Chemical-specific induction of gstp1 in zebrafish embryos. (A) Experimental scheme for gstp1 induction in zebrafish embryos. (B) Induction of gstp1 expression in 8-hour-old embryos. Nrf2 and Keap1 mRNAs were coinjected into embryos at the one-cell stage. After 2 h, embryos were treated with the compounds indicated for 6 h. The expression of gstp1 was analyzed by whole-mount in situ hybridization. (C) Structure of Nrf2-GFP fusion protein. The N-terminal half of Nrf2 protein and enhanced GFP (eGFP) protein were connected by two copies of simian virus 40 nuclear localizing signal (NLS). (D) Expression analysis of Nrf2-GFP fusion protein. Nrf2NTnGFP mRNA was injected with or without mRNA encoding Keap1 proteins into one-cell stage embryos. After 2 h, embryos were treated with the compounds indicated for 6 h, and GFP expression was analyzed.
To ascertain whether or not induction was based on cancellation of the Keap1-promoting Nrf2 degradation, we overexpressed an Nrf2 GFP fusion protein (Nrf2NTnGFP) in zebrafish embryos and tested its stability by monitoring GFP fluorescence after DEM or 15d-PGJ2 treatment (Fig. 2C and D). Nrf2NTnGFP contains the Neh2 domain, a degron for Keap1-dependent degradation of Nrf2, which has been shown to be functional not only in cultured cells but also in mouse and zebrafish (13, 26). GFP expression was almost undetectable in embryos overexpressing Nrf2NTnGFP with either Keap1a or Keap1b (Nrf2NTnGFP, 25.0%, n = 20; Nrf2NTnGFP+Keap1a, 2.4%, n = 41; Nrf2NTnGFP+Keap1b, 0%, n = 34). In Keap1b-overexpressing embryos, GFP expression was induced after DEM treatment, while it remained low in Keap1a-overexpressing embryos (Keap1a, 4.2%, n = 47; Keap1b, 67.6%, n = 34). In the case of 15d-PGJ2, both Keap1a- and Keap1b-overexpressing embryos showed strong GFP induction (Keap1a, 58.3%, n = 48; Keap1b, 58.6%, n = 29). The results were basically identical to those from experiments monitoring endogenous gstp1 induction, suggesting that the different DEM-responding activities of Keap1a and Keap1b in inducing gstp1 was due to their different activities in relieving Keap1-mediated Nrf2 degradation. In conclusion, we propose a model that the Keap1-Nrf2 system senses signals of a variety of electrophiles by disparate mechanisms, and yet all of the signals lead to Nrf2 activation.
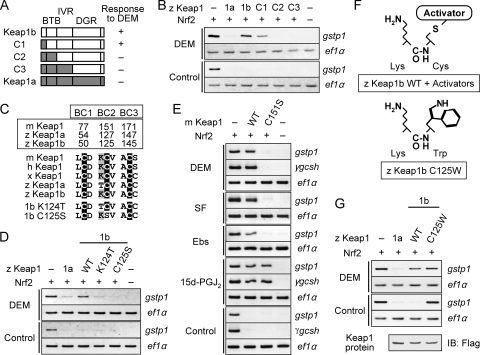
Identification of a sensor cysteine for K1b-type Nrf2-activating compounds.
Previous in vitro studies demonstrated that some cysteine residues in Keap1 bind directly to electrophilic Nrf2 activators and are presumed to function as electrophile sensors (2, 4, 5, 8, 9, 27, 31). The fact that some of the activators were specific for Keap1b gave us the unique opportunity to identify the putative sensor cysteine residues that rely on the molecular differences between Keap1a and Keap1b. We predicted that a sensor site for K1b-type compounds is present in Keap1b but not in Keap1a. To identify these sites systematically, we constructed chimeras of Keap1a and Keap1b proteins (Fig. 3A) and compared their DEM-responding activities by examining the induction of gstp1 expression by RT-PCR, since that can provide more quantitative data than in situ hybridization. Only embryos overexpressing C1 protein, but not C2 or C3 proteins, were responsive to DEM (Fig. 3B). These findings indicate that the BTB domain of Keap1b is important for sensing DEM. In the BTB domain, three cysteine residues are conserved among vertebrate Keap1 (Fig. 3C). We refer to these cysteine residues as BTB cysteines (BCs) to ease comparison among various Keap1 molecules. Although all three BCs are conserved in both Keap1a and Keap1b, the amino acid adjacent to BC2 is a threonine in Keap1a and a lysine in Keap1b and other vertebrate Keap1 proteins. It is known that adjacent basic residues stabilize the thiolate form of the cysteine residue, thus enhancing its reactivity (2, 29). Based on the fact that DEM could cancel the Nrf2 repression activity of Keap1b but not that of Keap1a, we predicted that BC2 is a sensor site for DEM and that the adjacent lysine residue in Keap1b and other Keap1 proteins is critical for its reactivity. In order to verify this possibility, we substituted the cysteine residue of Keap1b BC2 to serine (C125S) and its adjacent lysine to threonine (K124T) and examined the effects of these substitutions on the DEM-induced expression of gstp1 (Fig. 3D). Both K124T and C125S substitutions reduced the response of Keap1b to DEM, implying that BC2 is indeed a sensor site for DEM. Similar results were obtained by real-time RT-PCR (see Fig. S6 in the supplemental material).
FIG. 3.
Identification of a sensor cysteine for the K1b-type compounds. Induction of gstp1 was carried out as shown in Fig. 2A, and its expression was analyzed by RT-PCR. FLAG-tagged Keap1 proteins were used instead of nontagged proteins. The amount of mRNA was normalized to that of Keap1 protein with a comparable activity in repressing Nrf2-induced gstp1 expression in uninduced conditions, except for in panel G. The expression of ef1α was used to standardize the amount of cDNA. (A) Keap1a-Keap1b chimeric proteins used in the analysis. (B) DEM-induced expression of gstp1 in embryos overexpressing chimeric proteins. Note that gstp1 induction was observed in embryos overexpressing C1 but not C2 or C3 proteins. (C) Alignments of three cysteine residues and their adjacent amino acid residues in the BTB domains of various Keap1 proteins. (D) DEM-induced expression of gstp1 in embryos overexpressing Keap1bK124T and Keap1bC125S. (E) Electrophile-induced expression of gstp1 and γgcsh in embryos overexpressing mouse Keap1 and its C151S mutation. (F) Similarity between a Cys-to-Trp mutation and covalent binding of Nrf2-activating compounds to Cys. (G) DEM-induced expression of gstp1 in embryos overexpressing Keap1bC125W. The amount of Keap1 mRNA was standardized by the expression level of FLAG-tagged Keap1 protein analyzed by immunoblotting with anti-FLAG antibody.
We clarified that the function of BC2 in sensing DEM is conserved among vertebrate Keap1 proteins other than zebra fish. The corresponding BC2 residue of mouse Keap1 (Cys-151) was mutated to serine and the DEM-induced expression of gstp1 was assessed (Fig. 3E). As expected, a C151S mutation significantly reduced the activity of mouse Keap1 to respond to DEM without affecting its activity to repress Nrf2. This was also observed in the case of the DEM-induced expression of γgcsh. Other K1b-type compounds (SF, D3T, tBHQ, 1,2-NQ, and Ebs) gave identical results to those obtained with DEM (Fig. 3E and see Fig. S7 in the supplemental material), indicating that BC2 is a sensor site for K1b-type compounds. BC2 (Cys-151) has been shown to be required for sensing tBHQ and Ebs in cultured cells (33, 46) and tBHQ in transgenic rescue mice (45). The important point here is that BC1 and BC3 were not mutated, so we concluded that BC2 is the only in vivo sensing site for certain types of Nrf2-activating compounds.
It is natural to assume that K1b-type compounds bind directly to Keap1 BC2. Indeed, we previously demonstrated that Ebs bound to wild-type mouse Keap1 but not to Keap1C151S mutant molecules (33). In addition, MS studies revealed that some Nrf2-activating compounds react covalently with the BC2 (Cys-151) of mouse and human Keap1 proteins (4, 5, 27), although they also bind to many other cysteine residues in Keap1. It would be debatable to state that modification of BC2 acts solely as a trigger to terminate Nrf2 degradation owing to the difficulty in isolating Keap1 proteins with modification in only BC2. We overcame this obstacle by introducing a cysteine-to-tryptophan mutation in Keap1b BC2 (C125W) and analyzed its effects (Fig. 3F). Tryptophan (186 kDa) is 83 kDa larger than cysteine (103 kDa), so we expected that the Cys-to-Trp substitution would mimic the binding of DEM (172 kDa) or other K1b-type compounds to BC2. As a result, Keap1bC125W failed to suppress Nrf2-mediated gstp1 expression when the protein was overexpressed to a similar amount as wild-type Keap1b and Keap1a (Fig. 3G). This was not observed in the case of the C125S mutation, in which cysteine was substituted with an amino acid of comparable size (serine, 87 kDa) (see Fig. 3D). These results suggest that compounds binding to BC2 inhibit the Nrf2-repression activity of Keap1, probably due to the bulky mass on BC2 masking important regions of Keap1. For instance, the interaction between Keap1 and Cul3 would likely be perturbed, because the α-helix just after BC2 is believed to be critical for the BTB-Cul3 interaction (44). In fact, Rachakonda et al. (31) reported recently that BC2 adduction with IBA/BIA (383 kDa) disrupts the Keap1-Cul3 interaction. We hypothesize that BC2 adduction to Nrf2 activators may cease Nrf2 ubiquitination by disturbing the proper conformation of the Keap1-Cul3 complex. This hypothesis is based on the “hinge and latch” model that depicts strict regulation of Nrf2 ubiquitination (41).
Identification of sensor cysteines for K1a/b-type Nrf2-activating compounds.
We found that the BC2 mutation had no effect on sensing 15d-PGJ2 and PGA2 (Fig. 3E; also see Fig. S7 in the supplemental material), and so the sensor site for these K1a/b-type compounds is not BC2 and is different from the site for K1b-type compounds. Similar results were obtained by real-time RT-PCR (see Fig. S8 in the supplemental material). This is the first demonstration that different Nrf2-activating compounds target different sensor sites in the Keap1-Nrf2 system. Although detailed mapping of the 15d-PGJ2-sensor site has not yet been carried out, we assume that it is located within the Keap1 protein, since direct binding between 15d-PGJ2 and Keap1 has been demonstrated in cultured cells by us and others (11, 21, 25). To determine the sites in Keap1 modified by 15d-PGJ2, mouse Keap1 protein treated with or without 15d-PGJ2 was digested with trypsin and analyzed by MALDI-TOF MS. Peptide mass mapping by MALDI-TOF MS analysis of the trypsin fragments of native mouse Keap1 provided identification of the peptides accounting for ∼80% of the protein sequence (see Table S1 in the supplemental material). Compared to the calculated masses of the unmodified peptides, modified peptides P-1 and P-2 had an increased mass of +316 Da, corresponding to the addition of a single molecule of 15d-PGJ2 (Fig. 4A). The sequences and masses of the peptides were: P-1 (CHALTPR, m/z = 1,113.7) and P-2 (CEILQADAR, m/z = 1,334.8), indicating that mouse Keap1 is modified by 15d-PGJ2 at Cys-273 and Cys-288. Binding sites for PGA2 in the mouse Keap1 protein were also examined by MALDI-TOF MS analysis and identified as Cys-273, Cys-297, and Cys-489 (Fig. 4B; see Table S2 in the supplemental material). It was shown that Cys-273 is the only common binding site for 15d-PGJ2 and PGA2, suggesting that Cys-273 acts as a sensor cysteine for K1a/b-type compounds. We verified by MALDI-TOF MS analysis that the K1b-type compound DEM has a binding specificity for mouse Keap1 Cys-151, but not Cys-273 (Fig. 4B; see Table S3 in the supplemental material). This observation not only indicates the specificity of the analysis but also supports our conclusion that Cys-151 is the sensor site for K1b-type compounds.
FIG. 4.
Identification of sensor cysteines for the K1a/b-type compounds. (A) MS analysis of peptides from mouse Keap1 digested with trypsin after incubation with (right) or without (left) 15d-PGJ2. Keap1 protein (10 μg) was incubated at 25°C for 60 min in the absence (left) or presence (right) of 100 μM 15d-PGJ2 in a total volume of 10 μl containing 20 mM Tris-HCl (pH 8.5). (B) Modification sites in mouse Keap1 for Nrf2-activating compounds were determined by MS analysis. (C) Nrf2-induced expression of gstp1 in embryos overexpressing mKeap1C273S or mKeap1C288S. The amount of Keap1 mRNA was standardized by the expression level of FLAG-tagged Keap1 protein analyzed by immunoblotting using anti-FLAG antibody. (D) Electrophile-induced expression of gstp1 in embryos overexpressing mouse Keap1 and its C288S mutation.
Since MALDI-TOF MS pinpointed Cys-273 as the potential sensor site for K1a/b-type compounds, we examined the effects of Cys-to-Ser mutations at Cys-273 (C273S) and also Cys-288 (C288S) in mouse Keap1 on Nrf2 repression and the response to compounds. Mutant mKeap1C273S failed to suppress Nrf2-mediated gstp1 expression when the amount of protein overexpressed was similar to wild-type mouse Keap1 (Fig. 4C). This result is consistent with previous reports demonstrating that a Cys-273 mutation reduces the Nrf2-repression activity of mouse Keap1 in cultured cells (25, 43, 46) and in transgenic rescued mice (45). Basically identical effects were seen with the C273S mutation and the BC2 cysteine-to-tryptophan mutation (Fig. 3G), so we hypothesized that Cys-273 adducts with K1a/b-type compounds might disrupt the normal structure of the Keap1-Cul3 complex, as in BC2 adducts with K1b-type compounds. The mouse Keap1C288S mutation did not reduce Nrf2 repression (Fig. 4C), which was inconsistent with previous reports (25, 43, 45, 46), perhaps due to differences in experimental conditions. Since mKeap1C288S did not repress Nrf2 activity, we further analyzed its response to activators and found that the C288S mutation did not affect responses to either DEM or 15d-PGJ2 (Fig. 4D). This suggested that mouse Keap1 Cys-288 might not be required for Nrf2 repression or response to K1b- and K1a/b-type compounds, at least in this experimental condition. Although our present data do not exactly deny the possibility that Cys-288 is a sensor site for K1a/b-type compounds, it is plausible that Cys-273 is the major site for sensing such compounds.
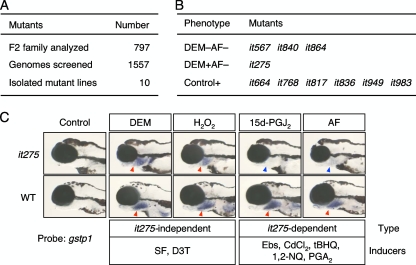
Isolation of a zebrafish mutant defective in responding to certain groups of Nrf2-activating compounds.
Our present findings suggest that additional unknown factors regulate the response to Nrf2-activating compounds. We therefore screened for mutant zebrafish with defects in gstp1 expression after treatment with or without the K1b-type compound DEM and posthatch-type compound AF. Screening makes it possible to identify factors contributing to regulation of the Keap1-Nrf2 system, for example, factors related to the response to posthatch-type compounds. In a large-scale genetic screen, we used induction of gstp1 expression in 5-day-old larvae. We examined the F3 progeny of males mutagenized with ENU for changes in gstp1 expression. After screening about 800 F2 families, 10 mutations were identified and confirmed in the subsequent generation (Fig. 5A). Mutants were classified into three groups based on their phenotypes (Fig. 5B): (i) DEM−AF− type mutants were defective in responding to both DEM and AF, (ii) DEM+AF− type mutants were defective in responding to AF but not DEM, and (iii) Control+ type mutants showed basal gstp1 expression in embryos without compound treatment. The only mutant classed as DEM+AF− type was it275. Eleven Nrf2-activating compounds were tested for induction of gstp1 expression in it275 larvae (Fig. 5C; also see Fig. S9 in the supplemental material). DEM, H2O2, SF, and D3T induced gstp1 expression in it275 larvae, whereas AF, 15d-PGJ2, Ebs, CdCl2, tBHQ, 1,2-NQ, and PGA2 did not. This was unexpected since K1b-type compounds that are supposed to bind directly to BC2 were divided into two classes. We speculated that the it275 gene product may play some roles in maintaining or enhancing electrophile activities. Although a candidate gene for the it275 mutation has not been identified, its gene locus was genetically mapped to chromosome 24, where no known factors related to the Keap1-Nrf2 system exist (unpublished results). Clarifying the roles and molecular nature of the it275 gene product will provide new insights into regulation of the Keap1-Nrf2 system.
FIG. 5.
Screen for zebrafish mutants with abnormal gstp1 expression. (A) Summary of zebrafish mutagenesis. (B) Phenotypic classes of mutations isolated. (C) Chemical-specific gstp1 induction in it275 larvae 4 days after fertilization. The strong gstp1 induction seen in the gills of wild-type fish (red arrowheads) treated with certain Nrf2-activating compounds was greatly reduced in it275 larvae (blue arrowheads).
DISCUSSION
In this study, we propose a classification system for Nrf2-activating compounds based on the combination of three mechanisms: (i) involvement of Keap1a and/or Keap1b, (ii) requirement for posthatch-specific factors, and (iii) it275 dependency. Our systematic approach classified Nrf2-activating compounds into six classes (Fig. 6A) and indicated that Keap1-Nrf2 responds to diverse chemical compounds via distinct molecular mechanisms constituting several molecular cascades or networks. It was interesting to find that compounds with similar structures belong to the same classes. For example, class 2 compounds tBHQ and 1,2-NQ are quinone derivatives, class 4 compounds 15d-PGJ2 and PGA2 are cyclopentenone prostaglandins and class 6 compounds AF and CdCl2 contain heavy metals. Our classification therefore reflects some structural features of the compounds. We assumed that compounds in the same class share target molecules and target cysteine residues and thus show similar Nrf2-activating properties, such as dynamics of gene induction, tissue and cell specificities, and age-dependent regulation, including larvae versus embryos. Thus, it is important to identify the it275 gene and posthatch factors to elucidate the molecular bases of such classifications.
FIG. 6.
Hypothetical models. (A) A cascade-based classification of Nrf2-activating compounds. Chemical compounds belonging to class 3 have not been identified. (B) A cysteine code hypothesis. Electrophilic compounds that target different sets of cysteines may produce distinct biological effects.
DEM and 15d-PGJ2 are both Michael reaction acceptors, so the discovery that their sensor sites in Keap1 are different was unexpected. We believe that the MS results are not in vitro artifacts for two reasons. First, the binding site for the K1b-type compounds DEM and 1,2-NQ was shown to be Cys-151, while the K1a/b-type compounds 15d-PGJ2 and PGA2 bound to Cys-273 using the same protein preparation (Fig. 4B) (unpublished results). Second, the Cys-to-Ser mutation in Cys-151 disrupted the ability of mouse Keap1 to respond to DEM and other K1b-type compounds, but not to 15d-PGJ2 or PGA2 (Fig. 3E; also see Fig. S7 in the supplemental material). Instead, we conjectured that the conformation formed by a cyclopentenone ring, double bonds, a carboxyl group, and other structures in 15d-PGJ2 is suitable for interacting with Cys-273, while obstructing any interaction with Cys-151. In other words, a strict intramolecular conformation of Nrf2-activating compounds may be required for targeting Cys-273, but not Cys-151. Since 15d-PGJ2 is an endogenous Nrf2 activator, it is possible that other endogenous Nrf2-activating compounds, such as 8-nitro-cGMP (35) and 4-hydroxynonenal (10), target Cys-273 or other non-Cys-151 cysteines for strict regulation. The target selection of a variety of Nrf2-activating compounds should be investigated comprehensively in the future.
The biological effects of Nrf2-activating compounds are considerably diverse and range from being therapeutic to toxic. It is hard to consider that Nrf2 activation solely contributes to all of these effects. Instead, the interesting possibility exists that each compound reacts with specific target proteins unrelated to the Keap1-Nrf2 system through specific cysteine residues. Indeed, the class 2 compound 1,2-NQ was reported to target Cys-121 in protein tyrosine phosphatase 1B (PTP1B) (14). Emitted as one of combustion products of fossil fuels and also formed in the atmosphere by photochemical reactions of aromatic hydrocarbons, 1,2-NQ is considered to create a variety of hazardous effects in vivo, including acute cytotoxicity, immunotoxicity, and carcinogenesis. Covalent attachment of 1,2-NQ to PTP1B reduced its activity and led to prolonged activation of epidermal growth factor receptor, followed by abnormal cell proliferation. We speculated that 1,2-NQ forms adducts to Cys-151 in Keap1, to Cys-121 in PTP1B, and to specific cysteines in other proteins to instigate the cooperative induction of a variety of adverse health effects (Fig. 6B). Deciphering the lineup of 1,2-NQ targets to understand its toxic effects on our health should prove worthwhile. Similarly, the class 4 compound 15d-PGJ2 has been shown to react with some proteins (19, 42), namely, by covalent binding to specific cysteine residues identified as, for example, Cys-179 of IKKβ (32), Cys-38 of NF-κB p65 (37), Cys-62 of NF-κB p50 (1), Cys269 of cJun (30), and Cys-285 of PPARγ (36). Protein functions are inhibited or activated by modification of these cysteines by 15d-PGJ2. It has been suggested that 15d-PGJ2, a naturally occurring derivative of prostaglandin D2, exerts anti-inflammatory effects in vivo (19, 42). The carrageenan-induced pleurisy model of acute lung injury was used to demonstrate the relationship between Nrf2 activation and anti-inflammation and that accumulation of 15d-PGJ2 mediates this relation (11). In the same manner, inhibition or activation of other target proteins are all related to anti-inflammation. We therefore assumed that cooperative adducts of 15d-PGJ2 to specific cysteines in proteins, including Keap1, are quite important for establishing anti-inflammatory states (Fig. 6B). As well as in anti-inflammation, Nrf2-activating compounds have a variety of other clinical applications, such as in cancer chemoprevention (SF) (17, 28, 40) and anti-rheumatism (AF) (15, 16). We now propose a “cysteine code” hypothesis that converts a set of cysteine modifications into specific biological effects. Breaking the cysteine code for each Nrf2-activating compound will serve to increase understanding of its therapeutic and/or toxic effects. Please note that 1,2-NQ, 15d-PGJ2, SF, and AF were grouped into different classes in our classification. We presume that compounds in the same class share similar “cysteine codes” that lead to analogous biological effects.
Supplementary Material
Acknowledgments
This study was supported by Grants-in-Aid from the Japan Science and Technology Corp. (ERATO) (M.Y.) and the Ministry of Education, Science, Sports, and Culture of Japan (M.K. and M.Y.).
We thank T. Kensler and K. Uchida for kind providing D3T and Ebs, respectively, and Y. Kishimoto for technical advice. We also thank T. Kinoshita, H. Niu, M. Oikawa, T. Shimokoube, and Y. Terashita for help in mutant screening and fish maintenance; R. Ide, T. Suzuki, M. Takeuchi, K. Tong, and T. Tsujita for help with experiments and for discussions; and K. Igarashi, H. Kakeya, A. Kobayashi, H. Motohashi, D. Sumi, and T. O'Connor for critical reading of the manuscript.
Footnotes
Published ahead of print on 11 November 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Cernuda-Morollón, E., E. Pineda-Molina, F. J. Cañada, and D. Pérez-Sala. 2001. 15-Deoxy-Δ12,14-prostaglandin J2 inhibition of NF-κB-DNA binding through covalent modification of the p50 subunit. J. Biol. Chem. 27635530-35536. [DOI] [PubMed] [Google Scholar]
- 2.Dinkova-Kostova, A. T., W. D. Holtzclaw, R. N. Cole, K. Itoh, N. Wakabayashi, Y. Katoh, M. Yamamoto, and P. Talalay. 2002. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 9911908-11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driever, W., L. Solnica-Krezel, A. F. Schier, S. C. Neuhauss, J. Malicki, D. L. Stemple, D. Y. Stainier, F. Zwartkruis, S. Abdelilah, Z. Rangini, J. Belak, and C. Boggs. 1996. A genetic screen for mutations affecting embryogenesis in zebrafish. Development 12337-46. [DOI] [PubMed] [Google Scholar]
- 4.Eggler, A. L., G. Liu, J. M. Pezzuto, R. B. van Breemen, and A. D. Mesecar. 2005. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc. Natl. Acad. Sci. USA 102:10070-10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eggler, A. L., Y. Luo, R. B. van Breemen, and A. D. Mesecar. 2007. Identification of the highly reactive cysteine 151 in the chemopreventive agent-sensor Keap1 protein is method-dependent. Chem. Res. Toxicol. 20:1878-1884. [DOI] [PubMed] [Google Scholar]
- 6.Haffter, P., M. Granato, M. Brand, M. C. Mullins, M. Hammerschmidt, D. A. Kane, J. Odenthal, F. J. van Eeden, Y. J. Jiang, C. P. Heisenberg, R. N. Kelsh, M. Furutani-Seiki, E. Vogelsang, D. Beuchle, U. Schach, C. Fabian, and C. Nüsslein-Volhard. 1996. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 1231-36. [DOI] [PubMed] [Google Scholar]
- 7.Holtzclaw, W. D., A. T. Dinkova-Kostova, and P. Talalay. 2004. Protection against electrophile and oxidative stress by induction of phase 2 genes: the quest for the elusive sensor that responds to inducers. Adv. Enzyme Regul. 44335-367. [DOI] [PubMed] [Google Scholar]
- 8.Hong, F., M. L. Freeman, and D. C. Liebler. 2005. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem. Res. Toxicol. 181917-1926. [DOI] [PubMed] [Google Scholar]
- 9.Hong, F., K. R. Sekhar, M. L. Freeman, and D. C. Liebler. 2005. Specific patterns of electrophile adduction trigger Keap1 ubiquitination and Nrf2 activation. J. Biol. Chem. 28031768-31775. [DOI] [PubMed] [Google Scholar]
- 10.Ishii, T., K. Itoh, E. Ruiz, D. S. Leake, H. Unoki, M. Yamamoto, and G. E. Mann. 2004. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ. Res. 94609-616. [DOI] [PubMed] [Google Scholar]
- 11.Itoh, K., M. Mochizuki, Y. Ishii, T. Ishii, T. Shibata, Y. Kawamoto, V. Kelly, K. Sekizawa, K. Uchida, and M. Yamamoto. 2004. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Δ12,14-prostaglandin J2. Mol. Cell. Biol. 2436-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itoh, K., N. Wakabayashi, Y. Katoh, T. Ishii, K. Igarashi, J. D. Engel, and M. Yamamoto. 1999. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1376-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itoh, K., N. Wakabayashi, Y. Katoh, T. Ishii, T. O'Connor, and M. Yamamoto. 2003. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells 8:379-391. [DOI] [PubMed] [Google Scholar]
- 14.Iwamoto, N., D. Sumi, T. Ishii, K. Uchida, A. K. Cho, J. R. Froines, and Y. Kumagai. 2007. Chemical knockdown of protein-tyrosine phosphatase 1B by 1,2-naphthoquinone through covalent modification causes persistent transactivation of epidermal growth factor receptor. J. Biol. Chem. 28233396-33404. [DOI] [PubMed] [Google Scholar]
- 15.Kataoka, K., H. Handa, and M. Nishizawa. 2001. Induction of cellular antioxidative stress genes through heterodimeric transcription factor Nrf2/small Maf by antirheumatic gold(I) compounds. J. Biol. Chem. 27634074-34081. [DOI] [PubMed] [Google Scholar]
- 16.Kean, W. F., L. Hart, and W. W. Buchanan. 1997. Auranofin. Br. J. Rheumatol. 36:560-572. [DOI] [PubMed] [Google Scholar]
- 17.Kensler, T. W., J. G. Chen, P. A. Egner, J. W. Fahey, L. P. Jacobson, K. K. Stephenson, L. Ye, J. L. Coady, J. B. Wang, Y. Wu, Y. Sun, Q. N. Zhang, B. C. Zhang, Y. R. Zhu, G. S. Qian, S. G. Carmella, S. S. Hecht, L. Benning, S. J. Gange, J. D. Groopman, and P. Talalay. 2005. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People's Republic of China. Cancer Epidemiol. Biomarkers Prev. 142605-2613. [DOI] [PubMed] [Google Scholar]
- 18.Kensler, T. W., N. Wakabayashi, and S. Biswal. 2007. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 4789-116. [DOI] [PubMed] [Google Scholar]
- 19.Kim, E. H., and Y. J. Surh. 2006. 15-deoxy-Δ12,14-prostaglandin J2 as a potential endogenous regulator of redox-sensitive transcription factors. Biochem. Pharmacol. 721516-1528. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi, A., M. I. Kang, H. Okawa, M. Ohtsuji, Y. Zenke, T. Chiba, K. Igarashi, and M. Yamamoto. 2004. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 247130-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi, A., M. I. Kang, Y. Watai, K. I. Tong, T. Shibata, K. Uchida, and M. Yamamoto. 2006. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell. Biol. 26221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi, M., K. Itoh, T. Suzuki, H. Osanai, K. Nishikawa, Y. Katoh, Y. Takagi, and M. Yamamoto. 2002. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells 7807-820. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi, M., and M. Yamamoto. 2005. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid. Redox Signal. 7385-394. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi, M., and M. Yamamoto. 2006. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzyme Regul. 46113-140. [DOI] [PubMed] [Google Scholar]
- 25.Levonen, A. L., A. Landar, A. Ramachandran, E. K. Ceaser, D. A. Dickinson, G. Zanoni, J. D. Morrow, and V. M. Darley-Usmar. 2004. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem. J. 378373-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, L., M. Kobayashi, H. Kaneko, Y. Nakajima-Takagi, Y. Nakayama, and M. Yamamoto. 2008. Molecular evolution of Keap1: two Keap1 molecules with distinctive IVR structures are conserved among fish. J. Biol. Chem. 2833248-3255. [DOI] [PubMed] [Google Scholar]
- 27.Luo, Y., A. L. Eggler, D. Liu, G. Liu, A. D. Mesecar, and R. B. van Breemen. 2007. Sites of alkylation of human Keap1 by natural chemoprevention agents. J. Am. Soc. Mass Spectrom. 182226-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McWalter, G. K., L. G. Higgins, L. I. McLellan, C. J. Henderson, L. Song, P. J. Thornalley, K. Itoh, M. Yamamoto, and J. D. Hayes. 2004. Transcription factor Nrf2 is essential for induction of NAD(P)H:quinone oxidoreductase 1, glutathione S-transferases, and glutamate cysteine ligase by broccoli seeds and isothiocyanates. J. Nutr. 134:3499S-3506S. [DOI] [PubMed] [Google Scholar]
- 29.Netto, L. E., M. A. de Oliveira, G. Monteiro, A. P. Demasi, J. R. Cussiol, K. F. Discola, M. Demasi, G. M. Silva, S. V. Alves, V. G. Faria, and B. B. Horta. 2007. Reactive cysteine in proteins: protein folding, antioxidant defense, redox signaling and more. Comp. Biochem. Physiol. C 146180-193. [DOI] [PubMed] [Google Scholar]
- 30.Pérez-Sala, D., E. Cernuda-Morollón, and F. J. Cañada. 2003. Molecular basis for the direct inhibition of AP-1 DNA binding by 15-deoxy-Δ12,14-prostaglandin J2. J. Biol. Chem. 27851251-51260. [DOI] [PubMed] [Google Scholar]
- 31.Rachakonda, G., Y. Xiong, K. R. Sekhar, S. L. Stamer, D. C. Liebler, and M. L. Freeman. 2008. Covalent modification at Cys151 dissociates the electrophile sensor Keap1 from the ubiquitin ligase CUL3. Chem. Res. Toxicol. 21705-710. [DOI] [PubMed] [Google Scholar]
- 32.Rossi, A., P. Kapahi, G. Natoli, T. Takahashi, Y. Chen, M. Karin, and M. G. Santoro. 2000. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature 403103-108. [DOI] [PubMed] [Google Scholar]
- 33.Sakurai, T., M. Kanayama, T. Shibata, K. Itoh, A. Kobayashi, M. Yamamoto, and K. Uchida. 2006. Ebselen, a seleno-organic antioxidant, as an electrophile. Chem. Res. Toxicol. 191196-1204. [DOI] [PubMed] [Google Scholar]
- 34.Satoh, T., S.-I. Okamoto, J. Cui, Y. Watanabe, K. Furuta, M. Suzuki, K. Tohyama, and S. A. Lipton. 2006. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic phase II inducers. Proc. Natl. Acad. Sci. USA 103768-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawa, T., M. H. Zaki, T. Okamoto, T. Akuta, Y. Tokutomi, S. Kim-Mitsuyama, H. Ihara, A. Kobayashi, M. Yamamoto, S. Fujii, H. Arimoto, and T. Akaike. 2007. Protein S-guanylation by the biological signal 8-nitroguanosine 3′,5′-cyclic monophosphate. Nat. Chem. Biol. 3727-735. [DOI] [PubMed] [Google Scholar]
- 36.Shiraki, T., N. Kamiya, S. Shiki, T. S. Kodama, A. Kakizuka, and H. Jingami. 2005. α,β-Unsaturated ketone is a core moiety of natural ligands for covalent binding to peroxisome proliferator-activated receptor γ. J. Biol. Chem. 28014145-14153. [DOI] [PubMed] [Google Scholar]
- 37.Straus, D. S., G. Pascual, M. Li, J. S. Welch, M. Ricote, C. H. Hsiang, L. L. Sengchanthalangsy, G. Ghosh, and C. K. Glass. 2000. 15-Deoxy-Δ12,14-prostaglandin J2 inhibits multiple steps in the NF-κB signaling pathway. Proc. Natl. Acad. Sci. USA 974844-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki, T., Y. Takagi, H. Osanai, L. Li, M. Takeuchi, Y. Katoh, M. Kobayashi, and M. Yamamoto. 2005. Pi-class glutathione S-transferase genes are regulated by Nrf2 through an evolutionarily conserved regulatory element in zebrafish. Biochem. J. 38865-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takagi, Y., M. Kobayashi, L. Li, T. Suzuki, K. Nishikawa, and M. Yamamoto. 2004. MafT, a new member of the small Maf protein family in zebrafish. Biochem. Biophys. Res. Commun. 32062-69. [DOI] [PubMed] [Google Scholar]
- 40.Talalay, P., A. T. Dinkova-Kostova, and W. D. Holtzclaw. 2003. Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Adv. Enzyme Regul. 43121-134. [DOI] [PubMed] [Google Scholar]
- 41.Tong, K. I., A. Kobayashi, F. Katsuoka, and M. Yamamoto. 2006. Two-site substrate recognition model for the Keap1-Nrf2 system: a hinge and latch mechanism. Biol. Chem. 3871311-1320. [DOI] [PubMed] [Google Scholar]
- 42.Uchida, K., and T. Shibata. 2008. 15-deoxy-Δ12,14-prostaglandin J2: an electrophilic trigger of cellular responses. Chem. Res. Toxicol. 21138-144. [DOI] [PubMed] [Google Scholar]
- 43.Wakabayashi, N., A. T. Dinkova-Kostova, W. D. Holtzclaw, M. I. Kang, A. Kobayashi, M. Yamamoto, T. W. Kensler, and P. Talalay. 2004. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. USA 1012040-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willems, A. R., M. Schwab, and M. Tyers. 2004. A hitchhiker's guide to the cullin ubiquitin ligases: SCF and its kin. Biochim. Biophys. Acta 1695133-170. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto, T., T. Suzuki, A. Kobayashi, J. Wakabayashi, J. Maher, H. Motohashi, and M. Yamamoto. 2008. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol. Cell. Biol. 282758-2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, D. D., and M. Hannink. 2003. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 238137-8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.